AN ABSTRACT OF THE THESIS OF May 6, 1975 Anthropology, Psychology, Statistics
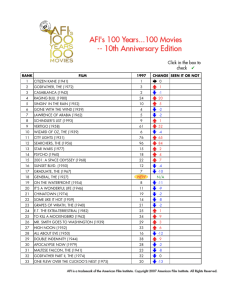
advertisement

AN ABSTRACT OF THE THESIS OF for the degree Dean Yates in Interdisciplinary Studies: Anthropology, Psychology, Statistics Title: Master of Arts presented on May 6, 1975 THE REFLECTION OF CULTURE IN BIOLOGICAL EVOLUTION Redacted for Privacy Abstract approved: Kenneth L. Beals Kelso's theory of Biocultural Evolution states that as culture evolves, the variation between groups diminishes while the variation within groups increases. index and nasal index. The theory is tested with stature, cephalic Means and standard deviations for these traits are examined for 237 ethnic groups. The groups are organized accord- ing to their stage of social organization: band, tribe, chiefdom and state. The theory is not verified for polygenic inheritance. Problems in the investigation of biocultural evolution are discussed, The Reflection of Culture in Biological Evolution by Dean Arnold Yates A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Master of Arts Commencement June 1975 APPROVED: Redacted for Privacy Beals, Assistant Professor of Anthropology Redacted for Privacy Donna F. Cruse, Assistant Professor of Psychology Redacted for Privacy Mogen. Petersen, Professor of Statistics Redacted for Privacy `Lavern J. Weber'/,' Chairman of Interdisciplinary Studies Redacted for Privacy Emery N. Castle, Dean of Graduate School Date thesis is presented Typed by Becky Gray for May 6, 1975 Dean Arnold Yates ACKNOWLEDGEMENTS I would like to extend my thanks to the faculty of the department Special of Anthropology for their collective help and responsiveness. thanks are due Dr. Ken Beals for his direction to Dr. Tom Hogg and Dr. Robert Johnson for their personal advise and goading, and to Dr. Courtland Smith for his financial support and technical aid. I would like further to thank Dr. Robert Petersen of the Statistics department for his help and patience, and Dr. Donna Cruse of Psychology for her critical commentary and moral support. Most of all I want to thank my wife Sharon for all her time and effort, patience and concern that went into the making of this project. TABLE OF CONTENTS Introduction Biocultural Evolution Purpose of the Study Prior Work II. III. Methods and Procedures Polygenic Traits Heterozygosity Stature Cephalic Index Nasal Index Social Organization Geographic Areas Data Collection Social Data Analysis Results Question One Question Two Question Three Question Four IV. Conclusions and Discussion Conclusions Discussion Bibliography Appendix I Appendix II 1 3' 7 8 11 11 12 13 14 15 16 19 20 22 23 25 25 26 28 29 39 39 41 45 52 70 LIST OF ILLUSTRATIONS Figures 1. Heterographic areas of the world (after Beals 1975) Page 21 Tables The correlation between stature, cephalic index, and nasal index, with mean heterozygosity 25 2. The rankings used in the calculation of the Spearman rs 27 3. Standard deviations of the four social levels for stature, cephalic index and nasal index 28 Rankings used in the construction of the Spearman rs using variances of mean trait values (by geographic area) and mean social organization 30 Rankings used in constructing the Spearman rs using standard deviations from within each geographic area, and the corresponding mean socia., organization of those groups ... 32 Variance of mean stature among bands, tribes, chiefdoms, and states in heterographic areas 33 Variance of mean cephalic index among bands, tribes, chiefdoms, and states in heterographic areas 34 Variance of mean nasal index among bands, tribes, chiefdoms, and states in heterographic areas 35 F tests on variances between the four levels, using variances of the total data 37 1. 4. 5. 6. 7. 8. 9. I. INTRODUCTION THE REFLECTION OF CULTURE IN BIOLOGICAL EVOLUTION Human variation is not simply a matter of genetic recombination. The mechanism of transferal of variation is purely biological. will not change. That But we recognize that something other than the urge to reproduce draws us to a particular mate. Variety is in itself an influence, but there are social considerations that control physical feelings and directives. At times these considerations seem to add little to the efficient working of the population, yet they remain and guide daily actions. We recognize that these social considerations are commonly refered to as culture. Dillon (1973:294) calls culture the "dual combin- ation of mind and hands production of things." a cooperative arrangement that results in the Though it expresses the nature of culture, it is far too incomplete. Culture is a part of all of us. As Pearson (1974:166) suggests, "Culture is that part of us that expresses itself in behavior." If we see human behavior as inextricable from biology, we may feel that evolution of one somehow inevitably affects the other in a like manner. As an expression of behavior, culture influences mating in numerous ways. This influence may come in the form of acceptable mating systems. 1 1 Mating system refers to the mechanisms used by a group for the selecSuch systems contain rules governing the marital elition of a mate. gibility of each individual relative to others in the group, as well as the pattern of selection, actual marriage, and residence requirements. 2 Such systems may vary depending on size and density of the population, The general result and the degree of opportunity for gene exchange. is that human groups are much different from randomly mating units (Oliver and Howells 1957; Shields and Gottesman 1971). This is an acceptable concept. terms of evolution. Campbell draws it together in He states (1966:289) that cultural evolution is interdependent of all other human activities. As a behavioral item, culture is as subject to a selection process as are biological units. Evolution is opportunistic, and any novel behavior pattern with a selective advantage will under appropriate circumstances, be incorporated into the behavior of the evolving deme. Harris (1971) shows that where cultural evolution operates in a manner analogous to biological evolution it is not bounded by the same mechanical constraints. Innovation, which might be considered as a cultural counterpart of the gene, does not depend on the rate of reproduction of a particular group. Its adaptive value is relative to the group as a whole, rather than to an individual. Importantly, cultural items are not constrained like biological characters in that they are accepted and travel between individuals within the same generation. The point is that culture is a survival mechanism which does not solely rely on breeding success or differential survival. Accumula- tion of successful cultural items increases a population's ability to survive and grow in size and density (Harris 1971). Human evolution is ceasing to be a biological process in a survival sense (Dillon 1973; Pearson 1974). will not maintain the population. But, logically culture alone Culture should be seen as one of 3 several mechanisms of the process of evolution. This is currently the case. Human evolution is viewed as an interplay of several parameters. Biocultural Evolution, the term which now identifies the human evolutionary process, suggests that evolution revolves around the elements, biology, culture, and environment (Kelso 1974; Pearson 1974; Campbel 1966). In Pearson's words (1974), this system is a triangle of interactions of these three elements. As biology and culture now operate to modify the environment, both elements must modify themselves to adjust to the new environment they produced. The rate of adaptive change is maintained by the ability to respond culturally to the change in the environment. But biological change as part of the overall adaptive scheme can only progress at the rate of the population's reproductive capacity (Campbell 1966). Biocultural Evolution The purpose of this study is to discover what relationship exists between biocultural evolution and human variation. Kelso (1974) in explaining this relationship describes the process of evolution in terms of increasing efficiency in the exploitation of environments. Increasing efficiency gradually led to the increase in species density, the number of separate populations, contact between groups, and increased migration. The processes affecting human genetic variability can be examined by inspecting current information on human prehistory (Kelso 1974). 4 We would find that during the Paleolithic period (roughly 3,000,000 B. P.. to 15,000 B. P. ) the number of humans was small, Population of the earth two million years ago was about 125,000 individuals, rising to roughly three million by 25,000 B, C. B. C., the figure stood at 86.5 million (F.C. Howells 1970). At 6,000 This growth in population was irregular, moving in rapid expansions that were the result of cultural innovation.2 (The most significant of these was the domestication of plants and animals, between 12,000 and 6,000 B.C.) Because populations were small, they were especially susceptable to the effects of inbreeding and genetic drift (Dobzhansky 1962; Handler 1970).3 Inbreeding, the higher likelihood of close relatives marrying one another, is a well developed concept of population genetics. effect of inbreeding is two-fold. The There is a decline in genetic varia- bility, and there is an increase in homozygosity, the increase in the number of loci where corresponding alleles are the same (Handler 1970). Genetic drift is the change in the genetic make-up of small 2 Barnett (No Date) describes innovations as the recombination of two or more previously existing elements into a new element with some features that are unique to itself. The primary acts essential to innovation are mental ability. The process of innovation itself is a mechanism of adaptation. 3 It must be pointed out here that others, notably Birdsell (1972:384) and W.W. Howells (1966:624), take exception with the argument that inbreeding results in the reduction of heterozygosity in natural populations. Yet these sources also note that in certain instances inbreeding is important to the study of the genetics of families. 5 populations that are the result of chance occurances. This type of change has the greatest effect in small populations, the kind found early in human evolution (Kelso 1974). rection to the change. There is no predictable di- It is simply the result of chance deviations between generations that are not found in large interbreeding units (Dillon 1973; Dobzhansky 1962). There are two consequences to this situation. homogenization within the group. The first is the Inbreeding results in a decrease in variability within the population; individuals become similar to each other. The second consequence deals with the difference between groups. Kelso (1974) suggests that variability between groups has become greater because the accidental changes in genetic strucutre, which are chance occurances, serve to randomize these differences. Examination of recorded history is proof of the rapid development of culture. This process has led to the adaptive success of humans at the species level. mation of world population. The best example of this is the estiBy the year 2000 A.D. that figure should be almost seven billion (Pearson 1974). The increase in cultural complexity and the resulting exploitive efficiency has brought about a drastic change in size and density of human populations. On the basis of numbers alone it is possible to assume that isolation would be less likely to occur now. A breakdown 6 4 of isolation mechanisms, migration , the result of increased transportation, and communication, brings with it changes in variability. It is expected that contact and the introduction of new genetic materials causes populations to become more variable within themselves. At the same time they would become more alike between each other. Since this reduction of isolation and change in variation are thought of as products of cultural growth, Kelso (1974: 328) has postulated a relationship between cultural evolution and human variation. He has related this in terms of a biocultural hypothesis: "As culture evolves, the biological variation within human populations increases..." and "As culture evolves, the biological variability between human populations declines." This hypothesis suggests that biocultural research is a well known product. 4 Though the relationship between culture and biology The breakdown of isolation is in part attributed to incest avoidance One explanation for these mechanisms has been and exogamous marriage. that they avoid the deleterious physical effects of inbreeding. Harris (1971) and Livingstone (1969) deny the idea that close inbreeding results in the accumulation of any dangerous recessive genes. They maintain that once a population overcomes a higher rate of homozygosity, it will reach a genetic equilibrium that involves a lower number of fatal The point they make is that there is no clearcut genetic adgenes. vantage in the avoidance of inbreeding using these mechanisms. A solely biological theory for incest avoidance and exogamy are dfficult to reconcile in view of present marriage practices, e.g., cross (Harris 1971:287) Livingcousin versus parallel cousin marriage. stone (1969:48-49) suggests that incest avoidance and exogamy are cultural developments, relevant more to the development of lanaguage than to any genetic characteristic. 7 has been debated for numerous years (Gerard, et. al. 1956; Oliver and Howells 1957) little research has been undertaken to examine this relationship in depth (Beals 1975; Kelso 1974). It appears that only in the past few years that the direction of study of related materials has begun in earnest (W.W. Howells 1970). Purpose The purpose of this study is to determine if a relationship exists between several physical characteristics and cultural evolution. Beals (1974) has used blood group heterozygosity, a trait of known inheritance, to demonstrate that an interchange relationship between culture and biology exists. This present study then will use several traits of unknown inheritance (stature, cephalic index, and nasal index) in a similar manner to test the applicability of Beals' results to phenotypic features. The biocultural hypothesis can be viewed as a four-part question. These questions relate results obtained by Beals to the above designated traits, as well as organizing the examination of the hypothesis. Questions: 1. Do traits of unknown inheritance (in particular stature, cephalic index, and nasal index) correlate with blood group heterozygosity? 2. Do these traits show any strong correlation to an increase in cultural complexity? 3. Does variation of the individual traits increase within 8 groups as cultural complexity increases? 4. Does variation of the individual traits decrease between groups as cultural complexity increases? Answering these four questions should provide necessary information for determining the adequacy of the hypothesis of biocultural evolution. Prior Work Previous work in this regard is strictly limited (Beals 1975; Kelso 1974). Initial attempts of comparison of cultural evolution with its biological counterpart seems to have gotten underway only about 20 years ago (Gerard, et. al. 1956; Oliver and Howells 1957). At best these attempts only draw analogies between culture and biology, providing a workable model of cultural evolution, and precedent for biocultural study. Incipient work in the comparison of cultural phenomena and biological characteristics has begun in a degree with the extensive examination of culture change vis-a-vis blood group change. Two illus- trations include articles by Spielman, Migliazza, and Neel (1974), dealing with the relationship of language change and blood group characters of the Yanomama. Also, the above mentioned work by Beals (1975) comparing change in social levels of human populations with change in blood group heterozygosity. Spielman, et. al, Spielman and his co-workers (1974) set out to compare biological 9 and linguistic divergence among Yanomama Indians of Brazil. The bio- logical component consisted of polymorphic blood group frequencies.5 The linguistic components were seven distinct dialects of the Yanomama language. Analysis of the data used a generalized distance approach, where data were assigned positions in a multidimensional space. Dis- tances calculated between data points (biological and linguistic) were used as a basis of comparison for divergence. The examination showed a significant correspondance between These results were linguistic change and genetic differentiation. used with genetic, linguistic, and historical information about the Yanomama for the construction of a time depth scale of genetic microdifferentiation. The point of the analysis was to show that linguis- tic data inferences could potentially provide conclusions about genetic processes (Spielman, Migliazza, and Neel 1974). Beals Beals (1975) has approached the concept of biocultural evolution more directly. Using some basic assumptions found in Kelso (1974) he has attempted to determine the interrelationship of culture and biology based on the respective levels of social organization of individual groups, and their corresponding heterozygous 6 frequencies calculated 5 Polymorphic blood group frequencies refer to the frequency of occurance of several distinctive blood group phenotypes. 6 This refers to polymorphic blood group frequencies. Heterozygous meaning that the individual contains two different alleles at a given locus. 10 from blood group information. The project examined 252 world populations and demonstrated that a significant relationship exists between the increase in social complexity and increase in frequency of heterozygosity. The increase in heterozygosity has shown to be approximately three percent between each of four levels of social evolution designated in the article. What these articles demonstrate is that biocultural evolution may be measurable if the proper cultural and physical parameters are examined together. There is a need to check other physical parameters besides blood frequencies in order to determine how extensive the apparent relationship is. Blood groups represent traits of known inheritance, and a reasonable extension of the examination would be to use polygenic traits of unknown inheritance. As this is what the present study does, the expectation is that culture in terms of its designated identifiers, will be reflected in biological differences. 11 II. METHODS AND PROCEDURES In answering the questions posed by the biocultural hypothesis, there are several variables and procedures that must first be defined and described more fully. The information below describes the procedures used in ordering and collecting data. It also gives a working definition of all the variables, biological and cultural, that are important to this study. Polygenic Traits The four physical traits of concern are described as polygenic which are called by Birdsell (1972:455) "genetically compli- traits, cated characteristics (that) are the expression of numbers of genes located at more than one locus." The indication is that no analysis (in real situations) of these characteristics has been undertaken on humans. 7 A polygenic characteristic involves two or more loci and falls into a category of continuous phenotypic variation. This variation, as well as being resultant from genetic materials, is at times influenced by the environment. Below, is a brief description and ex- planation of the four main polygenic characters used in this study. 7Birdsell (1972) has stated that up to 1972, no polygenic characters had been analysed in humans. This is due to the difficulty in analysis in real situations. Study has been limited to mathematical problems. 12 Heterozygosity Heterozygosity, or the containing in a genotype of two differing allelic forms of a gene, is a convenient measure of biological variation of a population. The amount of variation may be measured by the frequency of the heterozygosity contained within a population (Beals 1975). Lewontin (1967) suggests that the degree of genetic heterozygosity of a population is fundamental to any investigation of population genetics. When determining a measure of heterozygosity, traits used must necessarily have several qualities (Beals 1975). First there must be a commonality of the trait in a large number of people. it must be consistent through time in the individual, Second, Last it should be easy to detect and examine for heterozygosity. The obvious choices for such traits are blood groups, dividual has a definite blood type in a variety of systems. Each inBlood types are constant and are easily detectable by a laboratory test. Because inheritance is known and the number of alleles small, prediction of a frequency is a simple counting procedure. Heterozygosity as a measure of variability becomes useful and im- portant as a benchmark for comparing variation of other physical traits. 8 Further it provides stable data for correlation of non- physical characteristics to biology. 8 Because other traits may be of unknown inheritance, and the number of alleles large, calculation of a frequency of heterozygosity is not possible. Therefore the correlation of a more complex trait with blood heterozygosity becomes an important part of polygenic analysis. 13 Values for blood heterozygosity are found in Beals (1975) for 252 populations, Of these populations, the values of 64 are adapt- able for use in this paper. Stature Birdsell (1972) states that among mammals, humans have far more variation in body size than most. Stature is a characteristic that shows a continuous range of variation, running from less than 150 centi- meters to just more than 180 centimeters (Barnett 1971). Wide variation in stature occurs even among close neighbors, though there is little indication why this is so. It may be true that much of the variation can be attributed to the environment (Birdsell 1972; Beals and Hoijer 1961). Kelso (1974) however, indicates that some studies of identical twins shows that for the most part stature is under the control of genetic components. This results in conflict, though it is unlikely that it will soon be resolved. Birdsell (1972) claims that little if any polygenic analysis of humans has yet been done. Barnett (1971:86) illustrates the conflict and its seemingly impossible solution when he states that, ... a good deal of this variation (in stature) reflects differences of environment. But in so far as it is genetically determined, the genes responsible are probably to be numbered in the hundreds, and each has only a small effect. In response to environmental effects on stature, it may be worthwhile to note that though there is a scarcity of data on prehistorical 14 populations, what data is available tends to indicate that little change in stature has occurred since the development of the human species (Beals and Hoijer 1965). Cephalic Index The cephalic index is a measure of relative head shape. It is easily calculated by dividing the head's maximum breadth by its maximum length. The figure is normally multiplied by 100 for convenience. Kelso (1974) points out that this index is useful in that it expresses relative, as opposed to absolute differences in head shape. These are: 1) The index is usually divided into three groups. brachycephalic, or short or broad; 2) mesocephalic, or intermediate shape; and 3) dolichocephalic, or long or narrow. Barnett (1971) points out that these classifications are very arbitrary, Election of figures is generally a matter of convenience. Units for the three categories vary slightly but are generally as follows: more than 80 is brachycephalic; 75 to 80 is mesocephalic; and less than 75 is dolichocephalic (Barnett 1971; Beals and Hoijer 1965). Available data seems to demonstrate that the dolichocephalic head is more primitive in form than the others. But, there is no clear significance to this if it is true (Beals and Hoijer 1965). .cance of the variation is yet unclear. Signifi- Beals and Hoijer (1965) feel that the environment might be most significant in causing variation, . though they admit that index size may indeed be determined by a large number of genes. (This is related to the large number of features of the bones that make up the head.) Kelso (1974) on the other hand, 15 cites twin studies indicating that the majority of variations are due to genetic factors. His conclusions are based on high heritability coefficients Nasal Index The fourth measurement is the nasal index; relative nose shape, As in the cephalic index, the nasal index is calculated by dividing the width of the nose (Maximum width between the alae) by the maximum length of the nose (from the suture of the nasal bones on top, to the nasal septum at the bottom). This is also usually multiplied by 100 for convenience. Nasal index is divided into three categories, these being: 1) platyrrhine, or broad flat nose; 2) mesorrhine, or intermediate nose; and 3) leptorrhine, or long narrow nose. Measurements (on living sub- jects) are: above 85 are platyrrhine; 70 to 85 are mesorrhine; and below 70 are leptorrhine. Barnett (1971) feels the platyrrhine nose represents a more primitive nose form, the emergence of the leptorrhine nose a result of natural selection. This selection, reflecting an advantage in colder climates, where a longer nose better warms the air before it reaches the lungs. But, Beals and Hoijer (1965) show that while there is probably some effect on nose form by temperature and humidity, correlations are not high. The conclusion is that hereditary factors 9Heritability is the proportion of the total phenotypic variation (of a trait) that is due to additive genotypic variation. The coefficient is a representative measure of the degree to which a trait is inherited. 16 play a large role in determining nose size and form, Social Organization Social organization is the cultural characteristic of concern to this study, It is better suited than other cultural traits because it is common to all human populations, and it includes several of the other parameters often used as cultural identifiers. 10 In order to reduce inconsistancies of judgment, the criteria for social organization were taken from Service's Primitive Social Organization (1962). As a recognized authority in this subject, Service's criteria provides an operational definition of social organization for this study. According to Service social organization is broken down into four units. These units from simple to complex take into account the inte- grative elements found to be most distinctive of a particular level, Service designates these four as Bands, Tribes, Chiefdoms, and States, The levels are described below in terms of their most familiar features (Service 1962; Haviland 1974). Bands Bands are at times considered the oldest form of social organization. Regardless of that, they are the least complex form. The gen- eral characteristics are usually alike regardless of world locality, environment, or cultural features (Haviland 1974). 10 For example, subsistence patterns,politicalorganization,kinstructurel class structure, population size and density, and levels of productivity. 17 A band is a small autonomous unit that is made up of a few family groups that are integrated by a kinship structure. Generally they are hunters and gatherers, and at times are nomadic. They are small in size and density, this being a reflection of methods of substance they employ. Bands have no special economic groups, no formal political structure, no specialized labor, no authoritative unit or tank. They operate around a sort of informal authority and have a mutual dependence between individuals for subsistance and survival. Tribes Tribes, like bands are autonomous units, usually made up of more than one smaller unit. These smaller units are also kin based sets. Thus the tribe is in part integrated on a kin basis. Tribal economics typically centers on some type of horticulture, agriculture, or herding. A larger food production provides support for a larger, denser population. As in the band, leadership is informal. by pantribal sodalities. The group is integrated Such factors generally include kin groups such as clans as well as age grade associations, secret societies, ceremonial groups, or territorial groups. in causing the tribe to be self-regulating, These factors have a role Whereas leadership is normally informal, the activities of the pantribal groups often have institutional leaders whose functions are group and tribal regulation. 18 Chiefdoms This next step is a more advanced one. It is an intermediate stage of social evolution that separates simple groups from the most complex. Chiefdoms are much larger and denser in population. They are marked by specialization of production, and have organized central agencies that redistribute the products of the specialized labor. The agencies are responsible for the control of social, economic, and religious activity. These attributes allow the chiefdom to expand by internal growth, or by the absorbtion of outside groups. An important characteristic that typically marks the chiefdom is the emergence of two features. First is the development of a ranked, hierarchical class system with status usually marked by descent group. Second is the emergence of a ruling class of persons. Authority is usually placed in the person of a chief who is a true authority figure. The position is not necessarily hereditary. His purpose is as an articulative and control mechanism of the redistributional process. State The last of the levels of social integration is the state, It is made up as was each prior level of more complex social and economic activities. Generally, it is marked by the growth of urban centers surrounded by a rural population. political classes. There are divisions of social and Often they are marked by a superior technology. However, the most distinctive feature of the state is the concept 19 of a permanent government with which a state can augment legitimized force to regulate its people and its affairs with other groups. This authority is reflected in a rigid system of laws, administrated by a central authority which controls the delegation of power to maintain order. Geographic Areas Once data from all parts of the world are collected need for some type of organization of its comparison. there is a What is wanted is a way to demonstrate whether the hypothesized pattern operates by comparing units from within the total world sample. That is, across the world do similar groups display the same characteristic variations, and does variation between differing groups follow the hypothesized pattern from unit to unit. One method of breaking down the world into classes is to do it on the basis of geographical units such as continental land masses. On review of the literature it is found that this type of delimitation is frequent. Human groups can be broken into what are refered to as "geographical races." These races are defined as collections of simi- lar, and generally contiguous local populations which are set off by major physical barriers and migration patterns that reduced transmission of genetic materials in given directions (Beals and Hoijer 1965; Pearson 1974). Such barriers consist of oceans, deserts, and mountains. The number of such regions ranges from four to ten according to 20 one source (Beals and Hoijer 1965), Stanley Garn (1961) has con- structed nine categories of geographical races which closely follow 11 the major continental masses, and in part represent cultural regions. The break- This paper goes farther in breaking down these areas, down is constructed by Beals (1975) who has divided the world into 13 units which he has designated as "heterographic areas." This method further divides Garn's units more finely by taking into consideration the major deserts of Africa, the ocean separation of Asiatic regions, Figure 1 and the isthmus that separates North from South America. gives the outline and designations for each of these units. Data Collection Data were collected primarily from Biasutti's (1959) Razze e i Populi Della Terra. This source is exceptional. It is a compilation of data on a wide variety of human groups throughout the world. Biasutti presents his data by geographical areas. There are six areas including, Africa, Asia, Europe, Oceania, North America and South America. Throughout the volumes are a number of tables which give mean measurements of stature, cephalic index, and nasal index. Further, the numbers of observations and original sources are included. Because Biasutti is rich in anthropometric data, it is a logical source. But in a number of cases information is missing. Such as the 11 In particular this refers to the areas of the Pacific Ocean. Island groups that may loosely be called Oceania, are often separated into cultural units of Melanesia, Micronesia, and Polynesia. Figure 1. Heterographic areas of the world (after Beals, 1975). KEY Europe 2. Afro-Asia 3. Africa 4. Kalahari 5. India 6. Asia 1. Indonesia 8. Micronesia 9. Melanesia 10. Australia 11. Polynesia 12. North America 13. South America 7. 22 There are two drawbacks also. sample size or some of the measurements. One is that Biasutti offers no tabular data for European groups. Sec- ond, there are no standard deviations accompanying the tabular measurements. Some of this information is scattered through the text, but is difficult to accureately determine. Because of this, the source is supplemented by a variety of other sources to provide more measurements in certain geographical units as well as to provide some of the standard deviations which will be necessary for examining variation within groups. 12 From the combined sources, 237 usable populations were assembled together. The use of any single population was limited by the avail- ability of social information necessary for proper categorization, and the amoung of anthropometric data. If too much information was lacking, the group was dropped from the analysis. Social Data The designation of social organization according to Service were obtained from a variety of sources. Only a small number of these designations were provided by Service making it necessary to use other ethnographic materials. The designation of social organization was made by the author on the basis of the ethnographic literature. Designations that were not determinable as well as checking of the designations were provided by 12 Because the sources are too numerous to list in the text of this paper, the reader will find a complete list of the sources by population in appendix 1. 23 Drs. Beals, Smith and Hogg of the Oregon State University Department of Anthropology. Analysis Groups have been arranged within the above defined geographical units. Breaking the data up into units by geography provided a basis for comparison of differences in variation of social levels across the world. If the proposed hypothesis is true, differences should be demonstrated in the parts as well as the whole. Each social level is arbitrarily designed by a number. Bands are This one, tribes are two, chiefdoms are three, and states are four. is necessary in order to make group calculations and correlations that require the social data. The analysis of data was done by computer. ever, was limited. The actual work how- The information required amounted to several cor- relations, computation of variances of measurements, and the computations of variances of the social levels by area. The computer computations were done using the Statistical Interactive Programming System (SIPS) (Guthrie, D., D. Avery and K. Avery 1973) at Oregon State University. The system allows the examination of data requiring only the use of a computer terminal and a basic knowledge of the command system. Testing data on within group variance utilized the available standard deviations found throughout the literature. The number was small enough that the process of finding weighted standard deviations 24 for social levels was easily done by hand calculator. Ths first test is a correlation of stature, cephalic index, and nasal index with heterozygosity. This examination is to determine how closely these three polygenic traits follow the patterned change of heterozygosity found by Beals (1975). Test two uses the Spearman rs to rank values of population by social level to discover whether traits show correlation to increased social organization. The next test examines the standard deviations available to check the expected increase in within group variation. The Spearman rs is used as a check. The final test is to check for the expected decline in variation between levels of social organization. responding 13 geographical units. Data is broken into its cor- Examination of data used an F test to determine significant differences between levels over the 13 units. There was an examination of the direction of increase or decline in variability by checking rise and fall of variation between social levels. A rank order test of the total population was done also using the Spearman rs. 25 III. RESULTS Question One Question one checks trait correlation with blood group heteroThe results are found in Table 1. zygosity. Table 1. The correlation between stature, cephalic index, and nasal index, with mean heterozygosity. r n stature 64 0.181 cephalic index 62 0.264 nasal index 59 -0.545 Stature. The slight positive correlation between stature and hetero- zygosity is below a significant figure (0.244 at the five percent level). The statistic indicates that there is little correlation. Cephalic Index. The value of the correlation between cephalic index and heterozygosity is significant, though just barely at the five percent level (r = 0.244, n = 65). The index rises with heterozygosity in a positive direction. Nasal index. relation. In this case there is a reasonably strong negative cor- As mean heterozygosity rises the nasal index decreases. This is expected. Kelso (1974) has found that a similar negative cor- relation between latitude and nasal index occurs. As one moves away from the equator the nasal index becomes smaller. In observing the 26 data, this trend is also found. The broadest noses are found in Austrailia and the Kalahari desert in southern Africa. The narrowest are found in Europe and northern Asia.13 Question Two Question two examines trait correlation with increasing social complexity. Question two is similar to question one. However, social organization does not allow a correlation with the three polygenic traits. It cannot be assumed that social organization is a normally distributed variable. This is based on the fact that the scale of social organization is strictly arbitrary (non-random) and is not a continuously measureable numerical variable (Mendenhall 1971; Siegel 1956). Since strict assumptions cannot be made about the populations, analysis is done with a nonparametric device, This allows fewer qualifications to be made about a population and relies on counting rather than on numerical values (Siegel 1956). The particular nonparametric device is the Spearman rs (Mendenhall 1971), a rank order correlation coefficient. This simple test uses the squares of differences between two rankings, The two rank- ings are the expected or judged rank compared to the ranking from a test score. 13 We note from the data that the occurance of greater heterozygosity follows a similar pattern. Beals (1975) has indicated that this distribution of mean heterozygosity becomes larger with increasing levels of social organization; the less complex are more often found in equatorial areas. 27 In this case rank will correspond to level of social organization xi. Test rank, yi, corresponds to the order of variances of means cal- culated from the world sample. Table 2. The rankings used in the calculation of the Spearman rs. Nasal Index Cephalic Index Stature Level Table 2 illustrates the rankings. x. Yl . xi y. 1 1 4 1 2 2 2 2 1 2 3 tribe 3 3 3 3 3 1 band 4 4 4 2 4 4 xi y. state 1 chief Using the formula supplied in Mendenhall (19717389), rs=1-6Edi2 n (where d. = x.-y.) (n2-1) we find that the correlations are 1.0 for stature, -0.4 for cephalic index, and 0.4 for nasal index. But this correlation relies only on four categories, and as might be expected, only a perfect ordering of the units would result in a significant correlation.14 In this case then only stature is signi- ficant. 14 it is found that with 0n examining a table of critical values for r an n value as small as four, any value less tha8 1,0 is not significant at either the five percent or one percent levels. For the values corresponding to nasal and cephalic indices, n must equal 18 to be significant at the five percent level. , 28 Question Three Question three examines the within group variance increase in relation to social organization. Determining increasing variation within groups with rising social organization required the use of standard deviations from individual samples. Seventy-eight standard deviations were located for stature, while 67 were found for cephalic index, and 58 for nasal index. The units of examination were the weighted standard deviations for each level of social organization, state, chiefdom, tribe, and These were used to compare each level of organization in order band. to check for any increase in variance within each level. Because the standard deviations came from groups of differing sample size they were weighted by these respective sample sizes. Table 3 gives the resulting standard deviations of the groups for the three variables. Table 3. Standard deviations of the four social levels for stature, cephalic index, and nasal index. Trait Band Tribe Chiefdom State stature 5.90 5.93 5.59 6.07 cephalic index 3.03 3.39 3.48 3.19 nasal index 8.12 7.55 7.07 7.68 Again, if the Spearman rs is used, the correlation must be perfect to be significant because of the small number of units, The ex- pected pattern would have the smallest standard deviations in the band 29 level, with the value growing larger at each successive step. Table 3 shows that this pattern does not exist. Question Four Question four examines the decrease in variation between groups. This examination relies on the calculations of variance from means of the three polygenic variables. Calculations were made using only those means whose sample size were greater than or equal to 20. This was done to insure that each mean came from an approximately normally distributed sample, reducing the need to weight each measurement by sample size. Here, a rank correlation coefficient (Spearman rs) was used to determine change. This was done for the data as they were broken in- to geographic units. Two different correlations were made. The first of these used mean social organization of the geographic units, and the variances calculated from mean trait figures of each unit. There are 13 cases; the rankings of social organization and variances are shown in Table 4. The results show a correlation of 0.386 for stature, -0.609 for cephalic index, and 0.397 for nasal index. and nasal index are not significant. The values for stature The figure for cephalic index is significant at the 0.025 level. (The critical value is 0.566 for thirteen cases at that level.) The negative value of the head shape's correlation indicates a decrease in variation between levels as the rankings for mean social 30 Table 4. Rankings used in the construction of the Spearman r using variances of mean trait values (by geographic area)sand mean social organization. Cephalic Index Stature xi Y- xi yi Nasal Index yi xi 7 13 2 5 6 11 3 13 4 1 11 1 2 11 5 8 5 12 12 7 12 10 12 9 11 13 11 1 11 10 Melanesia 8 12 8 7 8 7 Micronesia 3.5 5 3.5 3.5 3 North America 9 8 9 9 9 8 Polynesia 3.5 1 3.5 6 3.5 1 Africa 7 9 7 Afro-Asia 2 4 2 Asia 6 6 6 13 2 13 Eruope 1 3 India 5 Indonesia Kalahari Australia South America 10 10 10 4.5 13 4.5 12 2 10 6 31 organization and variance are ordered from highest to lowest, Thus, a negative value indicates that as social organization is high, the corresponding variation is low. The second correlation uses only groups which have standard deviation values for the three traits. geographic unit as before. Groups were separated by For each unit a mean social organization and average variance were calculated. 15 Rankings used in this cor- relation are found in Table 5. The values were correlated in the same manner as the previous The resulting correlations were 0.041 for stature, -0.375 for test. cephalic index, and 0.435 for nasal index, significant. None of these figures are Notice however, that for cephalic index the figure is again negative, indicating the expected decrease in variation with increasing social organization as proposed by the hypothesis. We can look at the units in other ways to indicate the difference in variation from bands to states. The total data were broken up into the 13 geographical areas by social organization level and a variance calculated for each social level (based on mean trait values) where data were available. Tables 6,7, and 8 illustrate this for stature, cephalic index, and nasal index respectively. Looking at each table (using figures that are calculated with n 1 5This test is suggested by Beals as a further step in analyzing the He has indicated that this difference in variation between groups. method of analysis is an appropriate measure of the degree of difference in variation between groups of differing social organization. 32 Table 5. Rankings used in constructing the Spearman r, using standard deviations from within each geographic area,aand the corresponding mean social organization of those groups. Stature Cephalic Index Nasal Index xi yi xi yi x. y. Africa 7 10 9 3 9 8 Afro-Asia 2.5 8 2 2 2 5 Asia 2.5 6 3 9 3 11 10.5 7 Australia 10.5 1 10.5 10 Eruope 1 9 1 8 1 3 India 3 5 5 11 5 4 Kalahari 10.5 11 10.5 6 10.5 9 Micronesia 3 1 5 5 5 1 North America 9 2 8 7 8 7 Polynesia 3 4 7 4 7 2 South America 8 3 5 10 5 6 33 Table 6. Variance of mean stature among bands, tribes, chiefdoms, and states, in heterographic areas. N Band Africa Australia 5.75 State 9.75 11 12.70 10 15.25 7 3.86 7 23.46 9 6.33 19 9.94 N 20 49.75 24 13 8 N Tribe Afri-Asia Asia Chiefdom N 4.42 -- - Europe India Mw Indonesia 7 -16.38 8 22.84 - Kalahari Melanesia 7 37.99 Micronesia -- 8 11.45 17 30.18 7 1.26 88 28.67 INN 010 North America 7 4.23 13 28.83 Polynesia South America Total World 30 62.57 8 32.23 67 49.53 MO II= 52 18.27 34 Table 7. Variance of mean cephalic index among bands, tribes, chiefdoms, and states in heterographic areas. N Africa Band MN MN Afro-Asia -- Asia -- Australia 8 Europe - 7 13 - 6.56 4.05 24 3.68 11 4.89 10 7.36 7 27.19 7 5.39 9 6.00 18 10.18 -- Micronesia - -- 13 12.55 7 2.08 Polynesia - -. South America 13.93 7.55 - 8 10.67 - 17 9.79 6.41 - 10.21 North America 8 -- 7 19 State .. Melanesia Total World N -- - 5.08 -- Kalahari 20 Chiefdom N - .... India Indonesia 2.54 Tribe N - -- 7 8 2.89 - 67 14.04 88 -- 1.11 - __ 51 15.82 1= MO 11.68 35 Table 8. Variance of mean nasal index among bands, tribes, chiefdoms, and states in heterographic areas. N Band Africa -- Afro-Asia -- Asia -- Australia 8 Europe - India - Indonesia 6 Kalahari - 20.42 Chiefdom N States 22 177.18 9 75.52 10 39.08 5 34.85 7 43,89 8 87.44 16 11.98 N Tribe N 19 52.56 -- 8 - 24,76 -- -8 56.82 33.93 -- - -- Melanesia 5 13.28 -- Micronesia - North America 7 Polynesia - South America Total World 65.49 153.50 58.77 -- -- VW Mt 28 11 8 37.97 57 56.75 16 14.96 7 8.36 - .11M, 79 113.16 44 103.16 36 greater than or equal to five) one finds the number of cases from the total where variance decreases with increasing social organization. In stature, of seven cases where decrease can occur, it does three times while increasing four times, For cephalic index, of seven cases, decrease occurs in two and increases in.five. For the nasal index, again for seven cases, the decrease is four and the increase is three. These figures (with decrease occurring nine times and increasing 12 times) indicate that the pattern of decrease is little more than a chance happening. The data were examined using an F test on the total (world) figures to see if there were any significant differences in the variances of the four levels. The test checks the three traits to determine if differences occur between variances of bands and tribes, tribes and chiefdoms, and chiefdoms and states. A two tailed test was used, since the con- cern was only with a difference between the two variances of each case. The critical F values were found using the most conservative degrees of freedom. Each of the nine tests used the ten percent significance level, with any test found significant at that level being tested as well as the two percent level. Table 9 illustrates the information used, and the results. The results indicate that in three of the nine cases the differences are significant at the ten percent level. These are between tribes and chiefdoms for stature, and between bands and tribes and tribes and chiefdoms for nasal index. Further tests using the two 37 Table 9. F tests on variances between the four levels, using variances of the total data. Cephalic Index Nasal Index 30,60 60,30 24,60 Tribe v. Chiefdom 60,60 60,60 60,60 Chiefdom v. State 60,40 40,60 60,40 Band v. Tribe 1.65 1.74 1.74 Tribe v. Chiefdom 1.53 1.53 1.53 Chiefdom v. State 1.64 1.59 1.64 Band v. Tribe 1.263 1.007 2.704** Tribe v. Chiefdom 1.727* 1.202 1.994** Chiefdom v. State 1.569 1.354 1.090 Degrees of Freedom Stature Band v. Tribe Critical F Test Statistics * ** Significant at ten percent level. Significant at two percent level. percent significance level finds that only the two tests for nasal index remain significant. Note that in stature where a relation seems to exist, there is only one significant difference between the variances of the successive steps. Further, in nasal index where there was no apparent relation, there are significant differences in two of the three 38 steps (at the two percent significance level). 16 There is no indica- tion of significant difference between any of the three steps for cephalic index. 16 The difference between bands and tribes shows a decrease from bands to tribes. But the difference between tribes and chiefdoms shows an increase between the two. This represents significant changes in variation between groups apparently going in the opposite direction. 39 IV. CONCLUSION AND DISCUSSION Conclusions The above study has attempted to determine if any relationship exists between several measurable physical traits and cultural evolution which is expressed in terms of social organization. The study is an extension of previous work by Beals (1975) which demonstrated a measurable relationship vis-a-vis blood group heterozygosity. The hypothesis is that as culture continues to evolve in human populations, the biological variation of these populations will increase within groups and decrease between groups. Testing examines four items. First, did the traits show a good correlation to blood group heterozygosity? Was there a correlation Did variation between the traits and levels of social organization? within groups rise with social levels? And last, was there a decline in variation between groups with a corresponding rise in social levels? Correlation of blood group heterozygosity with polygenic characters reveals only one significant correlation, that with nasal index. Yet, while that correlation was high, it fails to explain better than 2 70 percent of the variation (r = 0.297) in the correlation. Due to the nature of the variables a correlation of social organization with the polygenic characters is unreliable. A rank order analysis does indicate however, that a good correlation exists for 40 stature while none occur for the remaining indices. Rank order correlation indicates further that there is no significant pattern of increase for variation within groups as social level goes up. A casual examination of weighted standard deviations reveals no apparent pattern of increase. The answer to the question concerning decline in variation between groups is not conclusive. The several tests for difference between variations and the direction of change overall yield no significant results. The exception is in the first correlation which produced a significant figure for cephalic index. This indicates a decline in variation with a rise in social organization for that particular trait. Further testing however, does not support the same trend. The results allow some general conclusions. These might be best viewed in terms of the four questions presented in Chapter one. Stature Stature reveals no definite relationship to heterozygosity. There is a significant relationship to an increase in social organization. strated. A variation increase within groups for stature is not demonDecrease in variation between groups for stature is not supported by the data. Cephalic Index Cephalic index does correlate with heterozygosity. not correlate with an increase in cultural complexity. But it does Increased 41 variation within groups is not observed. There is a significant correlation with decline in between group variation. Remaining tests however, do not support this further. Nasal Index Nasal index does significantly correlate with heterozygosity, though the variation explained by the correlation is not high. index does not correlate strongly with an increase in social zation. The organi- For increasing variation within groups, nasal index offers no conclusive evidence. There is no significant indication that de- cline in between group variation occurs with increasing social organization for this trait. The results indicate that this set of data supplies no proof strong enough to support the proposed hypothesis of biocultural evolution. It must be concluded that for these three traits the hypo- thesis is probably not correct. Discussion No particular population fits a typological definition of social organization. It is true that most groups fall generally into one of several broad categories such as those supplied by Service (1962). The problem in adopting any such typology has been to account for those groups which fall somewhere outside the defined categories. For example the apparent complexity of a group may differ from its actual structure. In such a case, groups may relate socially on a low level, like the tribal level. But the same groups may function 42 politically on a much higher level. An attempt to resolve the situation might be to extend scale to give broader definitions of social organization. Broadening might re- suit from extending an arbitrary scale to ten or more units. categorization would increase the flexibility greatly. A decimal 17 But, while this may extend the categorization and make social organization a more continuously measurable variable, the effort in itself would be inhibitive. Standardizaion of a finely graded social scale would be slow in developing and scaling of individual groups would be a large undertaking. And, there is no guarantee that such elaboration would solve the problem. The system would remain arbitrary and com- plexity would increase the error in decision making. The answer may be a cultural designator easily defined within each group, and relatively consistant. Such an item might be a scale of energy capture (White 1949), or degree of political force. But these alternatives may well be subject to the same objections. Overall there may be no separately measurable cultural item. Yet, as the cultural item may be enigmatic, perhaps physical characteristics offer the most problem to a study of this nature. Oliver and Howells (1957) reveal with special interest to this study, that the principles of genetics largely relate themselves to the actions of single gene pairs. POlygenic traits, subject to control of various loci, are not well described by these principles. 17 Such ideas are expressed by Hogg, Oregon State University (personal communication). 43 For traits governed by a single gene pair, heterozygosity has a definite meaning. For polygenic traits the concern is not one of relative heterozygosity, but rather the variety of combinations of these genes (Oliver and Howells, 1957). For example, inbreeding as applied to humans, will not affect polygenes in the manner of a single gene pair, because variation deals with entire chromosomes of differing pairs. Noting this, and the fact that the estimate of human gene-loci is in the neighborhood of 20,000 42,000, the effect of inbreeding on polygenes would not result in a reduction of heterozygosity (Oliver and Howells, 1957). Other complications in the study arise from a lack of control over human data. Aside from substandard procedures and measurement errors that limit the value of anthropometric data (Comas 1971), the problem of the specific expression of environment and diet on traits must be considered. The effect of these outside variables is not re- flected to the same degree for each characteristic. The study may indicate the relative effect of environmental factors on the specific traits. Stature is the only trait that tends to display any respectable pattern relative to social organization. Perhaps this is a result of stature's high heritability. (1963) confirms this heritability. Hiernaux But, he also points out that the heritability of cephalic index is also extremely high. Thus there is still no significant outcome in the results. The concept of biocultural evolution may have been directly measurable during the Paleolithic. During that period, human groups 44 may have been more homogeneous in their subsistance patterns, and an innovation that dramatically increased efficiency could have given a definite survival advantage to its innovators (W.W. Howells 1970). But cultural complexity is now at the point that each new addition does not dramatically increase the chances of survival, but rather the exploitation of resources. Such complexity, and present world population, is limiting the examination of the biocultural hypothesis. The present example is the examination of micro-evolu- tion of today's less complex human groups. What we remain with is an understanding of the potential for human variation and the concept of human adaptation in terms of culture. We retain the feeling that the changes in culture and biologi- cal variation are tied to each other. feeling remains unresolved. But with the present data this Solution to the problem rests on a future determination of the working of complex genetic characters and of the existance of a more suitable measure of cultural evolution. 45 Bibliography Andrew, J.M. IV In Studies in the Evolutionary Trends in Body Build. 1943 C.S. Coon and J.M. Andrews IV, Anthropology of Oceania and Asia. Papers, Peabody Museum of Archeology and Ethnology 20:102eds. Cambridge: Harvard University Press. 121. Barnett, N.G. No Date Oregon. The Innovative Process. (Mimeographed) Barnett, S.A. The Human Species: A Biology of Man. 1971 and Row Publishers. New York: Beals, K.L. Genetic Variation and Cultural Evolution. 1975 Anthropologist (in press). Beals, R.L, and Harry Hoijer An Introduction to Anthropology. 1965 The McMillan Company. York: Biasutti, Renato Razze e i Populi Della Terra. 1959 Turin: Unione Tipografic-Editrice. Birdsell, J.B. Human Evolution. 1972 Harper American Third Edition, Second Edition. New 1-4. Chicago: Rand McNally and Company. Boas, Franz Anthropometry of Shoshonian Tribes. gist N.S. 1:751-758. 1899 University of American Anthropolo- Changes in Bodily Form of Descendants of Immigrants. Reports of the Immigrations Commission. Senate Documents 64 (208). Washington D.C.: Government Printing Office. 1911 Bowles, G.T. In Linguistic and Racial Aspects of the Munda Problem, 1943 C.S. Coon and and Asia. Studies in the Anthropology of Oceania Papers, Peabody Museum of Archeology and J.M. Andrews IV, eds. Cambridge: Harvard University Press. Ethnology 20:81-101. Campbell, Bernard G. Human Evolution. 1966 Chicago: Aldine Publishing Company. 46 Comas, Juan Anthropometric Studies in Latin American Indian Populations. In The Ongoing Evolution of Latin American Populations. F.M. Charles C. Thomas. Salzano, Ed. Springfield, III.: 1971 Coon, C,S. Harvard African Studies. 9. Cambridge: Tribes of the Riff. Harvard University Press, 1931 1939 The Races of Europe. New York: The Macmillan Company. Craig, J.I. Anthropometry of Modern Egyptians. 1911 Biometrika 8:66-78. DaRocha, F.J. and F,M, Salzano Anthropometric Studies in Brazilian Cayapo Indians. 1972 American Journal of Physical Anthropology N.S. 36:95-102, Davenport, C.B. and A.G, Love The Medical Department of the U.S. Army in the World War 1921 Washington, D.C.: Government Printing Office. 15(1). Dillon, L.S. 1973 Evolution: Moss Company. Concepts and Consequences, Dobzhansky, Theodosius Mankind Evolving. 1962 St. Louis: The C.V. New Haven: Yale University Press. Field, Henry Contributions to the Anthropometry of the Caucasus. Peabody Museum of American Archeology and Ethnology 48(1). Cambridge: Harvard University Press. 1953 Papers, An Anthropological Reconnaissance in the Near East, 1950. Papers, Peabody Museum of American Archeology and Ethnology Cambridge: Harvard University Press, 48(2). 1956 An Anthropological Reconnaissance in West Pakistan, 1955. Papers, Peabody Museum of American Archeology and Ethnology 52. Cambridge: Harvard University Press. 1959 Fitzsimmons, Thomas, et.al, USSR: Its People; Its Society; Its Culture, 1960 Human Relations Area File. Garn, Stanley M, Human Races, 1961 Springfield, New Haven: Charles C, Thomas. 47 Gerard, R.W., Clyde Kluckholn, and Anatol Rapoport Biological and Cultural Evolution: Some Analogies and 1956 Explorations, Behavioral Science 1(1):6-34. Guthrie, D., C. Avery and K. Avery Statistical Interactive Programming System (*SIPS): 1973 Corvallis, User's Reference Manual. Technical Report No. 36. Oregon: Oregon State University Press, Hambly, W.D. Field Museum of Natural Anthropometry of the Ovimbundu. 1938 History: Anthropological Series 25(2):23-79. Handler, Phillip, Ed. Biology and the Future of Man. 1970 sity Press, Harris, Marvin Culture, Man, and Nature. 1971 New York: Oxford Univer- New York: Thomas Y. Crowell Company. Hastuck, M.M. and G.M. Mourant 1929 Measurement of Macedonian Men, Haviland, W.A. 1974 Anthropology. Biometrika 21:322-336. New York: Holt, Rinehart and Winston, Inc. Hiernaux, Jean Heredity and Environment: Their Influence on Human Morphol1963 A Comparison of Two Independant Lines of Study. American ogy. Journal of Physical Anthropology N.S. 21:575-589. Bruxelles: La Diversite' Humaine en Afrique Subsaharienne. 1968 Editions de L'Institut de Sociologie, Universite' Libre de Bruxelles, Hogg, T.C. 1975 Personal Communication Howells, F.C. 1970 Early Man. New York: Time-Life Books. Howells, W.W. Anthropometry and Blood Types in Fiji and the Solomon 1933 Islands. Anthropological Papers of the American Museum of Natural History 33(4). New York: Museum Publication. 48 1937 Anthropometry of the Natives of Arnhemland and the Australian Race Problem. Papers, Peabody Museum of American Archeology and Ethnology 15(1). Cambridge: Harvard University Press. 1966 Variability in Family Lines Vs. Population Variability. Annals of the New York Academy of Science 134(2):624-631. 1970 Recent Physical Anthropology. The Annals of the American Academy of Political and Social Sciences 389:116-126. Hrdlicka, Ales 1925 The Old Americans: A Physiological Profile. 1970 Reprint Edition. New York: Arno Press and the New York Times. Hsia, Hsiao, Ed. 1960 China: Its People; Its Society; Its Culture. Human Relations Area File. New Haven: Hunt, E.E. 1950 A View of Somatology and Serology in Micronesia, Journal of Physical Anthropology N.S. 8:157-183. Kelso, A.J. 1974 Physical Anthropology. Lippincott Company. Second Edition, American New York: J.B. Labar, Frank M. et. al. 1964 Ethnic Groups of Mainland Southeast Asia. New Haven: Human Relations Area File. Lessa, W.A. and Tracey Lay 1953 The Somatology of Ulithi Atoll. Physical Anthropology N.S. 11:405-412. American Journal of Lewontin, R.C. 1967 An Estimate of Average Heterozygosity in Man, Journal of Human Genetics 19:681-685. American Livingstone, Frank B. 1969 Genetics, Ecology and the Origins of Incest and Exogamy. Current Anthropology 10(1):45-62. MacDonnell, W.R. 1901-1902 On Criminal Anthropometry and the Identification of Criminals. Biometrika 1:177-227. Mahalanobis, P.C. 1930 A Statistical Study of Certain Anthropometric Measurements From Sweden. Biometrika 22:94-108. 49 A Revision of Risley's Anthropometric Data Relating to 1933 the Tribes and Castes of Bengal. Sanhya" 1:76-105. Mahalanobis, P.C. and D.N. Majumdar, and C.R. Rao Anthropometric Survey of the United Provinces, 1941: A 1949 Sanhya 9:90 -324. Statistical Study. Martin, Rudolf, and Karl Sailer Lehrbuch der Anthropologie. 1962 Stuttgart: Gustaf Fisher. Third Edition, Band II. McKennan, Robert A. The Physical Anthropology of Two Alaskan Athabaskan 1964 American Journal of Physical Anthropology N.S. 22:43-52. Groups. McVey, R.T., Ed, Indonesia. Southeast Asian Studies, Yale University. 1963 Human Realtions Area File. New Haven: Mendenhall, William Introduction to Probability and Statistics, 1971 Belmont, Cal.: Duxbury Press. Mendes-Correa, A.A. Origins of the Portugese. 1919 Anthropology 2:117-145. Third Edition. American Journal of Physical Murdock, G.P. Africa: Its People and Their Culture History. 1959 McGraw-Hill. New York: Neel, J.V., et. al. Studies on the Xavante Indians of the Brazillian Mato 1964 American Journal of Human Genetics 16:52-140. Grosso. Oliver, D.L. and W.W. Howells Micro-Evolution: Cultural Elements in Physical Evolution. 1957 American Anthropologist 59:965-978. Pearson, Roger Introduction to Anthropology. 1971 and Winston, Inc. New York: Holt, Rinehart Peterson, Roger Exercises in Statistical Inference. Corvallis, Ore.: 1972 Oregon State University Bookstores Inc., Publishers, Schuster, E. First Results from the Oxford Anthropometric Labor1911-1913 Biometrika 8:40-51. atory. 50 Service, E.R. Primitive Social Organization. 1962 Random House. Second Edition. New York: Shanklin, W.M. Anthropometry of the Akeydat and the Maualy Bedouin. 1936 ican Journal of Physical Anthropology 21:217-252. Amer- Shapiro, H.L. The Physical Characteristics of the Ontong Javanese: A 1933 Contribution to the Study of the Non-Melanesian Elements in MelanAnthropological Papers of the American Museum of Natural esia. History 33(3). New York: Museum Publication. Shapiro, H.L. and P.H. Buck The Physical Characteristics of the Cook Islanders. 1936 Memoirs of the Bernice P. Bishop Museum 12(1). Honolulu: B.P. Bishop Museum Press. Shields, James and I.I. Gottesman Man, Mind, and Heredity. 1971 Baltimore: The Johns Hopkins Press. Shirokogoroff, S.M. Anthropology of North China. Oosterhout N.B., The Nether1966 lands: Royal Asiatic Society, Anthropological Publications. Siegel, Sidney Nonparametric Statistics for the Behavioral Sciences. 1956 York: McGraw-Hill Book Company, Inc, New Smithsonian Institute Handbook of South American Indians. Bureau of American 1959 Ethnology 143(1-7). Washington, D.C.: Government Printing Office. Spielman, R.S., E.C. Migliazza, and J.V, Neel Regional Linguistic and Genetic Differences Among Yanomama 1974 Science 184:637-644. Indians. Steggerda, Morris 1932 Physical Measurement of Dutch Men and Women. Journal of Physical Anthropology 16:309-337. American Sullivan, L.R. 1920 Anthropometry of Siouan Tribes. Anthropological Papers of the American Museum of Natural History 23(3):81-174. New York: The American Museum of Natural History Press. 51 Marquesan Somatology with Comparative Notes on Samoa and 1923 Memoirs of the Bernice P. Bishop Museum 9(2):141-249. Tonga. Honolulu: B.P. Bishop Museum Press. Memoirs of the Observations on Hawaiian Somatology. Honolulu: B.P. Bishop Bernice P. Bishop Museum 9(4):269-342. Museum Press. 1927 White, Leslie A. The Science of Culture: A Study of Man and Civilization. 1949 New York: Farrar, Straus and Giroux. APPENDICES Appendix 1. Group The table below is a list of the data used in the above study. It records, by geographic area, the social organization (S. 0.) of each group, with the corresponding mean measurements of stature (St.), cephalic index (C. I.), nasal index (N. I.), and Heterozygosity (H.) of that group. The table also contains the sample sizes (N.) for each measurement, and gives the source from which the measurements were taken. (Heterozygosity based on ABO blood system.) Sources S.O. N. St. N. C.I. N. N.I. Ababda 3 62 163.6 62 73.7 62 75.1 Agni 4 171 167.5 171 76.3 Amhara 4 83 168.6 83 74.9 Antandroy 3 700 167.0 700 78.0 Ashanti 4 77 165.3 77 78.8 77 84.7 Azande 4 217 170.1 217 78.2 217 82.5 Hiernaux 1968 Bagiuni 2 28 165.4 28 75.6 28 75.8 Biasutti 1959 Bakongo 4 57 162.0 57 75.6 57 98.2 Biasutti 1959 Balese 2 36 158.5 36 76.3 36 95.1 Biasutti 1959 Bamun 3 71 171.5 71 79.8 71 89.2 Hiernaux 1968 Bangala 2 70 168.5 70 77.0 70 94.7 Biasutti 1959 H. Africa Biasutti 1959 Biasutti 1959 83 75.7 29.3 Biasutti 1959; Beals 1975 Biasutti 1959 27.3 Biasutti 1959; Beals 1975 N N.) Group S.O. N. St. N. C.I. N. N.I. Bapoto 2 26 163.9 26 78.0 26 90.0 Biasutti 1959 Basoko 2 24 165.6 24 81.3 24 95.2 Biasutti 1959 Basonghe 3 42 166.8 42 78.3 42 92.6 Biasutti 1959 Bateke 2 56 163.3 57 74.9 56 97.1 Hiernaux 1968 Batonga 3 23 171.2 23 72.3 23 90.9 Batwa 1 100 168.8 100 78.4 100 90.9 Hiernaux 1968 Baya 3 26 166.5 26 77.0 26 90.7 Biasutti 1959 Bon (Gelib) 1 27 169.4 27 72.8 27 83.0 Biasutti 1959 Cunama 3 60 165.4 60 74.9 60 70.2 Biasutti 1959 Dagari 3 71 169.0 71 75.3 39 107.8 Hiernaux 1968; Biasutti 1959 Dembo 2 100 160.0 100 74.3 100 92.6 Biasutti 1959 Dinka 2 63 183.9 49 71.7 60 92.0 Hiernaux 1968 Duala 3 75 169.1 73 78.9 74 87.8 Hiernaux 1968 Efe 1 111 143.0 111 79.4 111 105.7 Biasutti 1959 Ekoi 3 20 166.9 20 75.3 20 92.6 Biasutti 1959 Falasha 3 58 168.0 58 77.4 58 63.0 H. 19.9 26.3 Sources Biasutti 1959; Beals 1975 Biasutti 1959 vi co S.O. N. St. N. C.I. N. N.I. H. Sources Fang 2 91 166.4 91 79.3 91 91.4 -- Hiernaux 1968; Beals 1975 Galla (Oromo) 3 49 171.0 49 77.6 49 69.0 26.6 Hiernaux 1968; Beals 1975 Ganda 4 288 167.3 288 73.5 288 85.3 Biasutti 1959 Hutu 3 254 167.4 254 75.2 254 82.5 Hiernaux 1968 Ibo (Ika) 2 173 163.3 173 75.4 Kamasaia (Eltuken) 2 20 171.9 20 74.2 20 86.0 Kamba 2 128 165.7 128 76.5 128 86.5 24.4 Biasutti 1959; Hiernaux 1968; Beals 1975 Kikuyu 2 384 164.0 384 76.0 384 87.1 24.7 Biasutti 1959; Hiernaux 1968; Beals 1975 Kran 3 105 164.9 105 75.3 105 95.8 19.0 Hiernaux 1968; Beals 1975 Kru 3 40 165.4 40 75.8 40 98.9 19.0 Hiernaux 1968; Beals 1975 Logo 2 146 166.2 146 76.7 146 83.7 Biasutti 1959 Lotuko 2 34 178.0 34 73.3 34 84.6 Biasutti 1959 Group -- Hiernaux 1968 Biasutti 1959; Hiernaux 1968 Group. S.O. Luena 3 101 Makua (Niassa) 3 Masai Sources N. C.I. N. N.I. 168.5 101 77.0 101 97.4 Biasutti 1959 25 164.1 25 74.7 25 94.1 Biasutti 1959 2 147 172.7 148 72.8 149 72.0 Hiernaux 1968 Moru 2 20 170.7 20 77.0 20 86.3 Mossi 3 476 171.4 476 74.1 94 104.6 Mundang 3 26 175.3 26 74.0 26 97.0 Nuba (Eliri) 4 32 173.0 32 76.4 32 92.4 Biasutti 1959 Nuer 2 51 184.9 51 70.1 51 86.9 Hiernaux 1968 Nyoro 4 47 166.4 47 74.9 47 90.7 Hiernaux 1968 Ovimbundu 3 53 168.7 53 73.1 53 87.9 Hambly 1938 Rahawein 2 29 169.8 29 74.4 29 73.1 Biasutti 1959 Sandawi 1 100 164.6 100 75.2 100 80.2 Hiernaux 1968 Swahili 2 114 164.9 114 77.0 114 84.7 Hiernaux 1968 Somali (Hauiya) 3 42 173.2 42 74.5 42 65.0 28.0 Biasutti 1959; Beals 1975 Tigre 3 85 164.0 85 73.9 85 63.2 27.6 Hiernaux 1968; Beals 1975 N. St. H -- Biasutti 1959 22.0 Biasutti 1959; Hiernaux 1968; Beals 1975 Biasutti 1959 °I un Sources S.O. N. St. N. C.I. N. N.I. Tikkar 4 21 168.4 21 80.1 21 93.4 Biasutti 1959 Tussi 4 119 175.2 119 72.9 119 71.1 Hiernaux 1968 Wolof 3 257 172.4 227 75.0 Hiernaux 1968 Yoruba 4 156 168.0 156 75.1 Hiernaux 1968 Zulu 4 106 166.1 Arab (Yemen) 3 41 161.3 41 80.1 41 65.3 Arab (Kish) 3 164 169.6 164 76.6 164 72.8 Biasutti 1959 Armenian 4 114 165.5 114 85.2 114 61.5 Biasutti 1959 Assyrian 4 360 168.9 360 84.0 360 65.1 Field 1956 Bakhtari 3 150 162.2 150 81.7 150 67.7 Field 1956 Barabra 3 89 89 76.6 89 80.4 Biasutti 1959 Bedouin (Ruala) 3 270 161.9 270 75.0 270 63.7 Bedouin (Akeydat) 3 120 168.5 120 76.4 116 66.0 Shanklin 1936 Bedouin (Mauley) 3 176 170.1 175 77.3 175 66.3 Shanklin 1936 Group H Hiernaux 1968 Afro-Asia 164 28.8 30.3 Biasutti 1959; Beals 1975 Biasutti 1959; Beals 1975 Group Berber S.O. N. St. N. C.I. N. N.I. H. 3 73 165.2 73 77.3 73 63.8 27.5 Sources Coon 1931; Beals 1975 Craig 1911 Egyptian (Cairo) 4 802 165.8 799 75.9 Egyptian (Kharga) 4 150 163.8 150 74.8 150 76.6 34.6 Kurd 3 50 167.3 50 78.7 50 56.4 -- Riffian 4 529 168.6 529 75.0 Taureg 3 143 172.5 143 71.8 143 66.5 Tunisian 4 49 165.0 49 80.8 49 71.9 Biasutti 1959 Turk 4 200 167.9 200 87.2 200 67.2 Hastuck 1929 Ainu 3 91 156.7 91 77.3 55 83.4 Beltir 2 78 160.7 78 79.6 78 78.0 106 163.7 106 82.0 106 70.0 Biasutti 1959; Beals 1975 Field 1956 Biasutti 1959 29.6 Biasutti 1959; Beals 1975 Asia Burmese (North) 34.7 Biasutti 1959; Beals 1975 Biasutti 1959 29.0 Biasutti 1959; Beals 1975 Caren (Puo) 2 99 160.9 99 82.5 99 88.5 Biasutti 1959 Chagar-Mongol 3 52 164.0 52 81.7 52 71.7 Biasutti 1959 gL0112 S.O. N. St. N. C.I. N. N.I. H. Sources -- Shirokogoroff 1966 Chinese (Cih-li) 4 114 167.9 113 79.9 113 90.0 Chinese (Se-civan) 4 100 161.1 100 79.3 100 72.9 Chinese (Shan tung) 4 185 166.6 184 78.5 183 89.9 Chukchee 2 162 162.5 162 82.0 -- Japanese 4 6000 161.9 6000 80.8 - Jenessei 2 104 158.7 104 83.1 104 76.3 Biasutti 1959 Kachin 3 60 161.5 60 79.0 60 68.3 Biasutti 1959 Kamchadal 2 63 160.1 63 78.5 Korean 4 142 162.9 141 83.7 141 Koryak 2 197 159.9 197 80.0 ..... Lollo 3 29 167.5 29 79.4 29 75.9 Biasutti 1959 Man 2 82 158.3 82 78.2 82 82.5 Biasutti 1959 Miao 3 44 158.5 44 79.9 44 69.3 Biasutti 1959 Moi 3 30 159.4 30 79.8 30 77.6 Biasutti 1959 Osete (North) 3 106 169.8 106 84.6 106 63.6 Field 1953 Biasutti 1959 30.9 Shirokogoroff 1966; Beals 1975 Biasutti 1959 37.0 Biasutti 1959; Beals 1975 Biasutti 1959 92.5 .,, 35.0 Shirokogoroff 1966; Beals 1975 Biasutti 1959 TL0±21 S.O. N. St. N. C.I. N. N.I. H. Sources Ostyak 2 127 156.8 127 80.7 127 76.5 Samoyed 2 54 156.8 54 83.3 54 77.0 Selkup 2 29 158.1 29 82.1 29. 86.7 Thai (Central) 4 952 163.4 952 85.7 821 76.4 Tibetan 4 108 163.3 108 82.2 108 73.9 Biasutti 1959 Tungus 2 116 158.0 116 85.1 116 76.6 Biasutti 1959 Vietnamese 4 41 159.9 41 84.5 41 72.4 Biasutti 1959 Yakut 2 207 162.4 207 82.7 -- Biasutti 1959 Yukaghir 2 70 156.0 70 80.4 Arnhamland N.E. 1 77 167.0 77 70.4 77 103.9 Howells 1937 Arnhamland N.W. 1 98 169.6 98 73.0 98 98.1 Howells 1937 Central Aranda 1 20 166.3 20 74.7 20 95.3 Central-Meridian Aranda 1 42 165.6 42 74.3 72 94.8 Biasutti 1959 Central Northern 1 21 172.5 21 72.1 21 106.6 Biasutti 1959 Melville-Bathurst 1 28 166.8 28 70.5 28 103.6 Howells 1937 Biasutti 1959 -- Biasutti 1959 Biasutti 1959 26.3 Shirokogoroff 1966; Beals 1975 Biasutti 1959 Australia Is. 26.4 Biasutti 1959; Beals 1975 Group S.O. N. St. N. C.I. N. N.I. H. Sources Northern 1 62 170.5 62 72.8 62 95.8 Biasutti 1959 Victoria River 1 28 169.3 28 73.5 28 98.5 Howells 1937 Bulgarian 4 100 167.9 100 83.3 100 65.8 Hastuck 1929 Danish 4 18,727 169.0 2000 80.6 Dutch 4 70 173.2 70 79.3 English 4 4202 172.1 3000 77.2 French 4 1457 168.6 56 79.8 German 4 7077 172.0 QM. 200 167.3 200 Europe Greek 40 62.5 34.4 Hrdlicka 1925; Coon 1939; Beals 1975 35.7 Steggerda 1932; Beals 1975 33.6 Davenport 1921; Beals 1975 1000 67.3 32.4 Hrdlicka 1925; Davenport 1921; Beals 1975 38 63.5 34.7 Hrdlicka 1925; Davenport 1921; Beals 1975 85.9 200 68.4 Hastuck 1929 Boas 1911; Hrdlicka 1925 411. Hungarian 4 143 166.1 143 84.2 50 70.4 Irish 4 6164 171.4 35 78.0 102 64.3 31.4 Hrdlicka 1925; B evarM51921; CD Group S.O. N. St. N. C.I. N. N.I. H. Sources Italian 4 3519 165.7 50 84.3 50 67.7 33.8 Hrdlicka 1925; Davenport 1921; Beals 1975 Norwegian 4 11,774 172.4 11,761 79.0 77 61.0 34.3 Coon 1939; Beals 1975 Polish 4 2408 169.4 113 82.3 50 70.6 36.0 Hrdlicka 1925; Boas 1911; Davenport 1921; Beals 1975 Portugese 4 1444 164.5 1444 76.3 1444 65.1 Mendes-Correa 1919 Rumanian 4 50 169.8 50 85.9 50 69.0 Hrdlicka 1925 Russian 4 122 168.6 50 83.5 50 71.9 Hrdlicka 1925 Scott 4 2074 172.5 493 78.8 Spanish 4 6072 162.0 206 79.1 206 61.6 36.3 Coon 1931; Beals 1975 Swedish 4 46,983 172.2 46,983 77.7 260 62.7 33.1 Mahalanobis 1930; Coon 1939; Beals 1975 Yugoslavian 4 50 171.6 50 85.2 50 69.0 Davenport 1921; Schuster 19111913 Hrdlicka 1925 Sources S.O. N. St. N. C.I. N. N.I. Baluchi 3 85 168.2 85 81.6 85 68.7 Bhil 3 186 162.9 186 75.6 187 77.5 Brahuis 3 150 169.9 151 78.0 147 69.5 Field 1959 Chenchus 2 23 165.0 23 72.9 23 81.4 Biasutti 1959 Coorg 3 287 167.1 287 80.6 287 65.2 Biasutti 1959 Kadar 2 25 157.7 25 72.9 25 89.8 Biasutti 1959 Kashmiri 4 50 166.0 50 76.2 50 66.5 Biasutti 1959 Khasi 3 71 158.2 71 77.1 71 73.6 Bowles 1943 Kui 3 71 159.7 71 76.2 71 79.3 Biasutti 1959 Kurumbar 2 30 156.2 30 77.6 30 90.7 Male 2 100 157.7 100 74.9 100 94.7 Mahalanobis 1933 Rajput 4 420 174.8 420 72.4 420 71.6 Biasutti 1959 Santal 3 53 159.4 53 76.2 53 82.2 Biasutti 1959 Sikh 4 76 172.1 76 73.8 76 64.8 Biasutti 1959 Grou H. India Field 1959 32.0 26.9 Mahalanobis et al 1949; Beals 1975 Biasutti 1959; Beals 1975 01 na gy:2142 S.O. Toda 3 N. 22 St. N. C.I. N. N.I. H. 168.9 22 73.1 22 76.0 32.9 147.5 97 82.1 97 77.8 149.3 30 83.1 30 88.2 154.0 104 77.6 161.8 59 73.2 59 80.6 158.7 31 80.0 31 90.2 154.6 27 77.8 -- 152.0 78 78.5 78 90.2 153.6 53 77,8 53 83.3 157,3 25 82.2 25 100.2 155.8 21 76.3 21 109.2 24.5 Biasutti 1959; Beals 1975 164.6 101 75.6 101 93.3 20.5 Hiernaux 1968; Beals 1975 177.0 57 75.1 48 93.4 Sources Biasutti 1959 Indonesia Aeta Andamanese Igorot 97 1 30 3 Krunesi (Flores) Kubu 1 3 104 59 1 31 Loinang 1 27 Sakai (Ple) 1 78 Semang (Jahai) Toala 1 1 53 25 Biasutti 1959 20.9 -- Biasutti 1959; Beals 1975 Biasutti 1959 -- Biasutti 1959 Biasutti 1959 Biasutti 1959 Biasutti 1959 -- Biasutti 1959 Biasutti 1959 Kalahari Bushman (Auni) Bushman (Kung) Hottentot (Korana) 1 1 2 21 101 66 Hiernaux 1969 Group S.O. N. St. N. C.I. N. N.I. 2 73 162.4 74 72.9 74 100.1 Biak (Mafur) 2 28 160.1 28 72.9 28 88.9 Jakumul 2 100 158.2 100 73.5 100 86.8 Biasutti 1959 Kiwai 2 50 162,5 50 82.0 50 81.0 Biasutti 1959 Loyalty 3 87 167.7 87 72.5 87 91.5 Shapiro 1933 Monte Hagen (Papuan) 2 53 156.0 53 76.0 New Caledonian 3 250 166.4 250 76.5 Santa Cruz 3 34 160.3 34 76.5 Solomon 3 85 160.2 85 76.8 Hottentot (Nama) H. Sources 24.0 Hiernaux 1968; Beals 1975 -- Biasutti 1959 Melanesia Biasutti 1959 250 85 99.3 25.0 Biasutti 1959; Beals 1975 -- -- Biasutti 1959 87.1 -- W.W. Howells 1933 Tapiro (Papuan) 2 22 144.9 22 79.4 22 81.4 Biasutti 1959 Torricelli (Papuan) 2 30 151.9 30 77.7 30 87.4 Biasutti 1959 Wahgi (Papuan) 2 31 161.0 31 77.0 Biasutti 1959 Group S.O. N. St. N. C.I. N. N.I. 33 85.7 H. Sources Micronesia Kapingamaringa 3 33 171.1 33 78.5 Mortlock 3 59 163.5 59 73.6 Palua 3 134 160.9 134 80.9 Ponape 3 150 162.1 150 73.7 Saipan 3 186 162.9 186 80.1 -- Truk 3 164 161.6 164 73.4 164 77.7 Hunt 1950 Ulithi 3 56 163.5 56 74.6 56 82.0 Lessa and Lay Biasutti 1959 Biasutti 1959 a ..... 21.2 Biasutti 1959; Beals 1975 Biasutti 1959 -- Biasutti 1959 1953 Yap 3 347 160.3 347 79.6 Apache (San Carlos) 2 43 169.6 43 84.9 Aztec 4 100 159.0 100 78.9 100 80.5 Chilcotin 2 36 165.0 36 85.9 36 74.2 35.1 Biasutti 1959; Beals 1975 Chippewa 2 44 166.4 44 79.3 44 71.9 28.8 Biasutti 1959; Beals 1975 347 76.2 25.0 Hunt 1950; Beals 1975 North America WO V= Biasutti 1959 Comas 1971 un Group S.O. Choco N. St. N. C.I. N. N.I. H. 2 100 156.2 100 80.5 100 80.3 29.1 Comas 1971; Beals 1975 Chontal (Hoka) 3 80 159.8 80 83.2 80 77.2 24.2 Biasutti 1959; Beals 1975 Creek 3 33 171,4 33 78.8 33 76.5 Biasutti 1959 Cuna 3 20 154.9 20 85.7 20 70.4 Biasutti 1959 Delaware 3 126 171.5 126 79.8 Eskimo (Lorenz) 1 63 163.3 63 79.7 63 71.8 Eskimo (Seward) 1 40 165.4 40 78.0 40 71.1 Hausteco 3 100 157.2 100 84.3 100 71.3 Iroquois 2 97 173.0 94 79.5 Kutchin (Chandalar) 1 44 164.6 44 79.5 44 67.9 Kwakuital 3 40 164.0 40 83.8 40 76.2 Biasutti 1959 Maidu 2 43 163.0 43 79,0 43 85.0 Biasutti 1959 Mame 3 61 156.0 61 78.8 61 74.5 Biasutti 1959 Maya 4 77 155.1 77 85.0 50 68.5 Sources Biasutti 1959 -- Biasutti 1959 Biasutti 1959 30.2 Biasutti 1959; Beals 1975 Biasutti 1959 23.6 31.8 McKennan 1964; Beals 1975 Comas 1971; Beals 1975 Group S.O. N. St. N. C.I. N. N.I. H. Sources Mixtec 3 148 154.7 155 81.9 155 78.4 Comas 1971 Mohave 2 45 171.0 45 89.0 45 82.0 Biasutti 1959 Naskapi 1 41 166.2 41 80.6 41 73.0 20.4 Biasutti 1959; Beals 1975 Otomi 3 284 158.1 284 80.1 111 78.9 20.5 Comas 1971; Beals 1975 Papago 2 219 168.8 219 80.5 217 93.6 Pima 2 77 169.6 77 78.7 77 81.7 Pomo 2 32 164.0 32 80.0 32 91.0 Shoshoni-Ute 1 109 166.1 73 79.7 108 81.1 Sioux 2 537 172.4 537 79.6 536 68.8 Sullivan 1920 Tanada 1 33 169.9 33 82.5 33 70.8 McKennan 1964 Tarascans 3 116 159.9 116 76.7 100 82.6 Comas 1971 Totonac 3 100 158.0 100 88,2 100 73.0 Comas 1971 Trique 3 101 156.4 101 80.6 101 83.0 Comas 1971 Tsimshian 3 20 167.0 20 83.5 20 79.5 Biasutti 1959 Tzotzil 3 100 155.7 100 78.8 100 77.9 Comas 1971 Comas 1971 28.3 Comas 1971; Beals 1975 Biasutti 1959 24.0 Boas 1899; Beals 1975 Group S.O. N. St. N. C.I. N. N.I. H. Sources Yaqui 1 100 166.7 100 81.3 100 90.2 Comas 1971 Yuki 2 65 157.0 65 76.0 65 87.0 Biasutti 1959 Zapotec 3 50 155.4 50 81.1 50 78.3 Zendal 3 100 155.7 100 76.8 100 83.8 Biasutti 1959 Zoke 3 100 160.0 100 80.2 100 77.4 Biasutti 1959 Zuni 2 106 163.5 106 83.3 106 81.9 Biasutti 1959 Cook 3 416 170.8 416 82.8 416 75.4 Hawaii 3 205 169,5 206 84.0 175 78.4 Sullivan 1927 Marquesan 3 79 170,3 79 79.4 79 81.9 Sullivan 1923 Moari 3 424 170.6 424 77.7 424 75.9 Samoa 3 70 171.7 70 81.3 70 73,6 Sullivan 1923 Tahiti 3 85 171.4 85 84.9 85 80.3 Biasutti 1959 Tonga 3 117 173.0 117 81.1 117 77.6 30.0 Comas 1971; Beals 1975 Polynesia 32.8 32.8 35.2 Shapiro and Buck 1936; Beals 1975 Shapiro and Buck 1936; Beals 1975 Sullivan 1923; Beals 1975 Group S.O. N. St. N. C.I. N. N.I. Arawak 3 32 159.2 32 80.3 32 84.9 Comas 1971 Aueto 2 24 158.1 24 80.2 24 69.5 Biasutti 1959 Aymara 4 132 161.9 132 80.8 112 67.8 Bororo 2 20. 173.7 20 81.2 20 86.7 Biasutti 1959 Carib 2 104 156.8 104 80.2 104 71.0 Comas 1971 Cayapo 2 130 165.4 130 81.1 130 81.4 Chiriguano 3 40 163.4 40 80.2 40 79,8 Choroti 2 20 161.6 20 77.6 20 79,8 Nahuqua 2 65 161.8 65 79.5 65 75.4 Biasutti 1959 Ona 1 20 176.0 20 79.6 20 71.3 Biasutti 1959 Yahgan 1 67 158.1 -- -- Xavante 2 24 168.1 24 79.3 H. Sources South America 24 76.4 32.2 23.8 Comas 1971; Beals 1975 DaRocha & Salzano 1972; Beals 1975 Biasutti 1959 29.6 39.3 Biasutti 1959; Beals 1975 Comas 1971; Beals 1975 Neel et al 1964 70 Appendix 2. Standard Deviations of groups included These groups are arranged in the data. according to their respective level of social organization. Stature N. C.I. N. N.I. 5.64 77 2.85 77 10.12 98 5.94 98 2.88 98 9.68 Batna 100 5.99 100 2.78 100 7.60 Bushman (Kung) 101 6.40 101 3.32 101 8.84 Kutchin 44 5.26 44. 2.27 44 7.46 Melville Island 28 4.56 28 2.25 28 11.00 100 6.01 100 2.66 100 6.65 33 4.74 33 2.35 33 6.47 109 5.92 28 6.09 28 3.54 28 7.96 67 5.23 100 6.57 100 3.96 100 5.15 Arawak 32 5.88 32 3.15 32 8.28 Bateke 56 6.58 57 2.84 56 9.25 Carib 104 6.09 104 3.09 104 6.24 Cayapo 130 5.10 Dinka 63 6.11 Fang 91 5.85 91 5.42 128 5.66 128 2.80 Bands N. Arnhamland N.E. 77 Arnhamland N.W. Sandawi Tanada Ute Victoria Yahgan Yaqui River -- Tribes Kamba --91 12.32 71 N.I. Tribes N. Stature N. C.I. N. Arawak 32 5.88 32 3.15 32 8.28 Bateke 56 6.58 57 2.84 56 9.25 Carib 104 6.09 104 3.09 104 '6.24 Cayapo 130 5.10 .... Dinka 63 6.11 Fang 91 5.85 Kamba 128 Kikuyu Masai -- -- M MI .N. .M 91 5.42 91 5.66 128 2.80 -- 384 6.25 384 3.30 147 7.59 148 3.33 149 8.48 51 7.09 51 2.83 51 8.91 Papago 219 4.95 219 3.57 217 5.10 Sioux 537 5.65 537 3.20 536 7.05 Swaheli 114 5.79 114 3.65 114 6.86 Xavante 24 4.60 24 2.90 24 6.60 Baluchi 85 6.08 85 4.48 85 7.73 Bamun 71 6.50 71 2.92 56 8.06 Bedouin (Akeydat) 120 6.48 120 2.78 116 6.84 Bedouin (Maualy) 176 6.18 175 2.66 175 6.04 73 5.43 73 3.23 73 6.74 Bhil 186 5.48 186 2.82 187 7.43 Brahuis 150 5.77 151 4.18 147 6.59 Cook 416 5.70 416 4.20 416 6.70 Nuer -12.32 Chiefdoms Berber 72 Duala 75 6.50 73 3.21 74 3.06 Galla 49 5.01 49 2.81 49 7.66 Hawaii 205 6.03 206 3.66 175 7.24 Huasteco 100 5.03 100 4.20 100 6.93 Hutu 254 6.67 254 2.97 254 7.41 Khasi 71 4.86 71 3.69 71 5.32 Marquesan 79 5.12 79 3.52 79 7.75 Mixtec 148 4.92 155 2.88 155 7.20 Otomi 284 5.28 284 3.54 111 7.64 Ovimbundu 53 7.24 53 2.52 53 8.04 Samoan 70 5.25 70 3.53 70 5.86 Tarascans 116 4.98 -- Tongan 117 5.21 117 3.14 117 7.58 Totonac 101 4.74 100 4.10 100 7.80 Trique 101 4.43 101 3.45 101 9.08 Tzotzil 100 5.10 100 3.13 100 6.97 Ulithian 56 4.23 56 3.29 56 5.99 Zapotec 50 4.90 50 3.40 50 /7.50 Aymara 132 4.75 132 3.15 -- Bulgarian 100 5.70 100 4.01 100 5.88 Chinese 114 6.32 113 3.74 113 9.19 Chinese 185 5.96 184 3.74 183 10.26 70 7.24 70 3.85 -- -- States Dutch -- 73 802 6.03 799 2.95 -- English 4,202 6.62 3,000 2.14 so Ow French 1,457 6.50 56 3.84 German 7,077 6.61 Greek 200 5.79 200 3.97 200 Hungarian 143 6.50 143 3.20 -- Irish 6,164 *6.31 Italian 3,519 6.06 142 4.77 77 5.25 11,774 5.88 47 Egyptian -- 8.66 'MP 1m. 141 11.63 dm, MM 50 5.95 11,761 3.44 77 6.27 6.22 47 3.48 47 6.21 2,408 6.12 113 3.60 Thai 952 5.44 -- -- -- Turkish 200 6.10 200 3.50 200 8.11 Tussi 119 6.22 119 2.61 119 6.90 Scottish 2,074 6.39 493 2.79 M MD Swedish 46,983 5.93 46,983 3.14 260 106 6.10 Korean Maya Norwegian Nyoro Polish Zulu 141 OP ON 4.15 -- MN 01 NM 5.56 40 NO