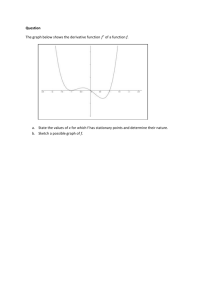
Discovery, taxonomic distribution, and phenotypic
advertisement

Discovery, taxonomic distribution, and phenotypic characterization of a gene required for 3-methylhopanoid production The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Welander, P. V., and R. E. Summons. “Discovery, Taxonomic Distribution, and Phenotypic Characterization of a Gene Required for 3-methylhopanoid Production.” Proceedings of the National Academy of Sciences 109.32 (2012): 12905–12910. CrossRef. Web. As Published http://dx.doi.org/10.1073/pnas.1208255109 Publisher National Academy of Sciences (U.S.) Version Final published version Accessed Wed May 25 22:06:22 EDT 2016 Citable Link http://hdl.handle.net/1721.1/77589 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Detailed Terms Discovery, taxonomic distribution, and phenotypic characterization of a gene required for 3-methylhopanoid production Paula V. Welander1 and Roger E. Summons Department of Earth, Atmospheric, and Planetary Sciences, Massachusetts Institute of Technology, 77 Massachusetts Avenue, E25-629, Cambridge, MA 02139 radical SAM ∣ bacteriohopanepolyols ∣ molecular markers H opanoids are pentacyclic triterpenoid lipids produced by a variety of bacteria that are often utilized as geological proxies or biomarkers for certain bacterial species and their metabolisms. Among the various hopanoid structures produced by bacteria (1), those methylated at the C-3 position and those with a penta- and hexafunctionalized amino polar side group are thought to be primarily produced by Type I and Type X methanotrophs (Fig. 1A) (2). As such, the occurrence of these hopanoids in conjunction with their significant 13 C-depletion in modern ecosystems are often utilized as an indicator of methanotrophic communities (3). In particular, environmental lipid analyses have uncovered the existence of aerobic methanotrophy in a variety of environments including, for example, the surface sediments of an active marine mud volcano in the Barents Sea and in the oxic-anoxic transition zone of the Black Sea water column (4, 5). Furthermore, the recalcitrant nature of hopanoid hydrocarbons allows for their preservation in ancient sediments, which may provide evidence for aerobic metabolisms deep in Earth’s history. Although the functionalized amino side group is lost over time, methylation of the A-ring is retained (6). Thus, the presence of C-3 methylated hopanes in sediments 2.5–2.7 billion years old has been used as one of several lines of molecular and isotopic evidence for Neoarchean aerobiosis (7–11). The effectiveness of these specific hopanoids as indicators for aerobic methanotrophy rests partly on the premise that the www.pnas.org/cgi/doi/10.1073/pnas.1208255109 majority of C-3 methylated hopanoid producers are aerobic methanotrophs. However, the production of 3-methylhopanoids has also been demonstrated in the acetic acid bacteria (12) indicating that the taxonomic distribution of 3-methylhopanoids is not restricted to methanotrophs. Furthermore, recent studies utilizing molecular approaches to identify hopanoid biosynthesis genes in sequenced genomes have highlighted that the diversity of bacteria capable of producing a specific hopanoid structure could be underestimated (13–15). These studies have also shown that a more precise interpretation of hopane hydrocarbon signatures in both ancient and modern ecosystems requires not only a grasp of the taxonomic distribution of methylhopanoid producers but also a deeper understanding of their physiological function in extant bacteria (16, 17). To this end, we employed a combination of microbial genetics, microbial physiology, and bioinformatics analysis to begin to understand the biosynthesis and function of C-3 methylated hopanoids in Methylococcus capsulatus. A genetic system for constructing unmarked in-frame deletion mutants was utilized to identify a methylase required for the production of 3-methylhopanoids. Bioinformatics analysis of this methylase revealed a diverse taxonomic distribution beyond the methanotrophic and acetic acid bacteria. Furthermore, phenotypic analysis of the C-3 methylase mutant uncovered a potential role for 3-methylhopanoids in late stationary phase survival. These studies highlight the power of combining gene discovery with bioinformatics and physiological analyses to potentially enhance our understanding of biomarker signatures in the rock record. Results and Discussion Identification of a C-3 Methylase in the M. capsulatus Genome. To identify a protein required for the methylation of hopanoids at the C-3 position, the genome of M. capsulatus was examined for possible C-3 methylase candidates utilizing search criteria based on two previous findings. First, bacterial feeding studies done with labeled methionine have posited that S-adenosylmethionine (AdoMet) is a potential methyl donor in the biosynthesis of both 2-methyl and 3-methylhopanoids (12). Second, it was recently discovered that a B-12 binding radical AdoMet protein, HpnP, is required for the production of 2methylhopanoids in the α-Proteobacterium Rhodopseudomonas palustris (14). Accordingly, we hypothesized that the methylase responsible for 3-methylhopanoid production was also a radical AdoMet protein possibly containing a B-12 binding domain. Author contributions: P.V.W. designed research; P.V.W. performed research; R.E.S. contributed new reagents/analytic tools; P.V.W. and R.E.S. analyzed data; and P.V.W. and R.E.S. wrote the paper. The authors declare no conflict of interest. This article is a PNAS Direct Submission. 1 To whom correspondence should be addressed. E-mail: welander@mit.edu. This article contains supporting information online at www.pnas.org/lookup/suppl/ doi:10.1073/pnas.1208255109/-/DCSupplemental. PNAS ∣ August 7, 2012 ∣ vol. 109 ∣ no. 32 ∣ 12905–12910 MICROBIOLOGY Hopanoids methylated at the C-3 position are a subset of bacterial triterpenoids that are readily preserved in modern and ancient sediments and in petroleum. The production of 3-methylhopanoids by extant aerobic methanotrophs and their common occurrence in modern and fossil methane seep communities, in conjunction with carbon isotope analysis, has led to their use as biomarker proxies for aerobic methanotrophy. In addition, these lipids are also produced by aerobic acetic acid bacteria and, lacking carbon isotope analysis, are more generally used as indicators for aerobiosis in ancient ecosystems. However, recent genetic studies have brought into question our current understanding of the taxonomic diversity of methylhopanoid-producing bacteria and have highlighted that a proper interpretation of methylhopanes in the rock record requires a deeper understanding of their cellular function. In this study, we identified and deleted a gene, hpnR, required for methylation of hopanoids at the C-3 position in the obligate methanotroph Methylococcus capsulatus strain Bath. Bioinformatics analysis revealed that the taxonomic distribution of HpnR extends beyond methanotrophic and acetic acid bacteria. Phenotypic analysis of the M. capsulatus hpnR deletion mutant demonstrated a potential physiological role for 3-methylhopanoids; they appear to be required for the maintenance of intracytoplasmic membranes and cell survival in late stationary phase. Therefore, 3-methylhopanoids may prove more useful as proxies for specific environmental conditions encountered during stationary phase rather than a particular bacterial group. EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES Edited by John M. Hayes, Woods Hole Oceanographic Institution, Berkeley, CA, and approved July 2, 2012 (received for review May 15, 2012) OH A OH OH OH OH A NH2 I 3 R = H aminobacteriohopanepentol (I) R = CH 3 3-methylaminobacteriohopanepentol (III) OH OH OH OH NH2 3 R II Relative Intensity R III IV R = H aminobacteriohopanetetrol (II) R = CH3 3-methylaminobacteriohopanetetrol (IV) B rfA O rfB 18 35 07 07 36 O nR hp 38 07 yf iA hy p 40 39 07 07 07 41 rp oN ISMca3 20 22 24 26 28 30 32 24 26 28 30 32 30 32 B 1 kb Using InterPro (http://www.ebi.ac.uk/interpro/), an integrated database of predictive protein signatures, 21 proteins with a radical AdoMet motif were identified in the M. capsulatus genome. These 21 proteins were queried against the Acetobacter pasterurianus genome, an acetic acid bacterium known to produce 3-methylhopanoids (12). Of these 21 proteins, MCA0738 was the only protein annotated as a B-12 binding radical AdoMet that also had a homologue in A. pasterurianus (e-value of 0). A Basic Local Alignment Search Tool (BLAST) search of MCA0738 revealed a homologue in other acetic acid bacterial genomes. Although the MCA0738 gene was not surrounded by other hopanoid biosynthesis genes on the chromosome (Fig. 1B), the occurrence of this particular gene in both M. capsulatus and all sequenced acetic acid bacterial genomes made it an attractive candidate for encoding the C-3 methylase. To determine if MCA0738 did encode for a C-3 methylase, an unmarked in-frame deletion of MCA0738 was attempted by adapting a counter selection protocol that has been used in a variety of bacterial species (18, 19). This allelic exchange method involves integrating a suicide plasmid at the locus of interest by homologous recombination and subsequently excising the plasmid from the chromosome, which can result in the deletion of the gene of interest (Fig. S1). To delete MCA0738, a deletion plasmid containing a replacement allele missing the MCA0738 gene was transferred into M. capsulatus via conjugation and integrated onto the chromosome by homologous recombination. The plasmid was forced to excise from the chromosome through nonselective growth and several potential deletion colonies were screened by PCR for deletion of MCA0738. One strain was found to be devoid of this gene and was picked for further characterization (Fig. S1). To verify that MCA0738 was required for C-3 methylation, a total lipid extract (TLE) was isolated from the MCA0738 deletion mutant and analyzed for its complement of bacteriohopanepolyols. As shown in Fig. 2, the MCA0738 deletion mutant is able to produce both the desmethyl aminobacteriohopanepentol and aminobacteriohopanetetrol but not their C-3 methylated counterparts as confirmed by detailed mass spectral analysis (Fig. S2). Furthermore, introduction of a copy of the MCA0738 gene on a self-replicating plasmid into the deletion strain restores production of the methylated hopanoids (Fig. 2). These data in12906 ∣ www.pnas.org/cgi/doi/10.1073/pnas.1208255109 II Relative Intensity I 18 20 22 C I Relative Intensity Fig. 1. Identification of a putative 3-methylhopanoid methylase in M. capsulatus. (A) The 3-methyl and desmethyl aminobacteriohopanepolyols produced by M. capsulatus. The roman numerals in parenthesis correspond to the structures identified in Fig. 2. (B) Genomic context of the C-3 methylase gene hpnR (MCA0738). Upstream of the gene there is a hypothetical protein (hyp), a putative Sigma-54 modulation protein (yfiA), and the RNA polymerase factor Sigma-54 (rpoN). Downstream are two genes that are annotated as ISMca3 transposase genes (OrfA and OrfB). II III IV 18 20 22 24 26 28 Time (min) Fig. 2. Deletion of hpnR results in loss of 3-methylhopanoid production. LC-MS extracted ion chromatograms of acetylated total lipid extracts from (A) wild type M. capsulatus, (B) ΔhpnR, and (C) ΔhpnR complemented with a copy of hpnR on a self-replicating plasmid (pPVW100). The chromatograms are a combination of ions m/z 830 (I, aminobacteriohopanepentol), 772 (II, aminobacteriohopanetetrol), 844 (III, 3-methylaminobacteriohopanepentol), and 786 (IV, 3-methylaminobacteriohopanetetrol). Hopanoids were identified based on their mass spectra shown in Fig. S2. dicate that MCA0738 is the only gene required for C-3 methylation of hopanoids in M. capsulatus and we propose to rename this locus hpnR based on a previously established nomenclature in Zymomonas mobilis (20). Identification of Putative HpnR Homologues. The radical AdoMet protein family encompasses a diverse set of proteins that catalyze a variety of biochemical reactions. The proteins in this family are primarily identified by the short amino acid sequence motif CxxxCxxC. As a result, BLASTanalyses of HpnR return a variety of radical AdoMet proteins that may or may not be involved in 3-methylhopanoid biosynthesis. To determine which of these Welander and Summons 1.00 0.1 Fig. 3. Maximum likelihood phylogenetic tree of putative HpnR sequences. A total of 192 radical AdoMet proteins were used to generate the tree: Fiftytwo HpnR sequences plus 140 radical AdoMets with an e-value less than e −17 when queried against the M. capsulatus HpnR sequence. The tree was rooted by using the 140 sequences as an out group to the 52 HpnR sequences shown. The full unrooted tree is shown in Fig. S3. Welander and Summons 3-Methylhopanoids Play a Role in Late Stationary Phase Survival. The diverse and sporadic distribution of HpnR introduces further ambiguity in our ability to correlate specific bacterial taxa to 3-methylhopanes in the environment. As a result, a proper analysis of the presence of 3-methylhopanes in the rock record needs to move beyond simply understanding which organisms produce these molecules. A more nuanced interpretation may be achieved if we better understand the physiological function of 3-methylhopanoids in bacteria as well as the environmental factors that induce their production in the cell. To this end, we have begun physiological characterization of the M. capsulatus hpnR mutant to identify any potential phenotype(s) associated with the loss of 3-methylhopanoid production. PNAS ∣ August 7, 2012 ∣ vol. 109 ∣ no. 32 ∣ 12907 EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES Candidatus Koribacter Candidatus Methylomirabilis oxyfera Nitrococcus mobilis 1.00 Frankia sp. EAN1 Frankia sp. CcI3 0.50 0.99 Streptomyces sp. e14 Streptomyces chartreusis 1.00 1.00 Streptomyces griseoflavus Streptomyces ghanaensis 0.98 Streptomyces xinghaiensis 0.72 Streptomyces fradiae 1.00 Streptomyces pristinaespiralis 0.45 0.81 1.00 Streptomyces cattleya DSM 46488 1.00 Streptomyces cattleya NRRL 8057 Nitrosococcus halophilus 1.00 Nitrosococcus watsoni 1.00 Nitrosococcus oceani AFC27 0.95 Nitrosococcus oceani ATCC 19707 Methylococcus capsulatus 0.99 1.00 Methylomicrobium alcaliphilum Methylomicrobium album Soil Metagenome AAFX01006490 0.89 0.95 Leptospirillum ferrooxidans Mine Drainage Metagenome ACXJ01008692.1 0.99 1.00 Leptospirillum sp. Group II 0.60 Leptospirillum rubarum Mine Drainage Metagenome ACXJ01008849.1 0.81 Burkholderia sp. H160 1.00 Burkholderia xenovorans 0.90 Burkholderia phymatum 0.96 Methylobacterium nodulans Gluconacetobacter diazotrophicus 0.55 Gluconacetobacter hansenii Gluconacetobacter sp. SXCC1 0.89 1.00 Gluconacetobacter xylinus Gluconacetobacter oboediens Gluconacetobacter europaeus LMG 18494 1.00 Gluconacetobacter europaeus LMG 18890 Gluconacetobacter europaeus 5P3 Acetobacter aceti 0.15 Gluconobacter oxydans Gluconobacter morbifer Acetobacter tropicalis 0.97 Acetobacter pomorum 0.98 Acetobacter pasteurianus IFO 3283-01 1.00 Acetobacter pasteurianus IFO 3283-01-42C Acetobacter pasteurianus IFO 3283-03 Acetobacter pasteurianus IFO 3283-07 Acetobacter pasteurianus IFO 3283-12 Acetobacter pasteurianus IFO 3283-22 Acetobacter pasteurianus IFO 3283-26 Acetobacter pasteurianus IFO 3283-32 0.89 copy of this protein suggesting that only a subset of methanotrophs may be capable of producing 3-methylhopanoids. The inconsistent distribution of HpnR among methanotrophs is also observed in the genomes of other hopanoid-producing bacterial genera. For example, 38 Burkholderia, 43 Streptomyces, and 8 Methylobacterium genomes have been sequenced to date. All of these genomes, except for Burkholderia pseudomallei MSHR346, contain a copy of the squalene hopene cyclase gene and at least one species from each of these groups has been shown to produce hopanoids. Yet, only three Burkholderia, eight Streptomyces, and one Methylobacterium contain the HpnR methylase suggesting that only a subset of species from certain genera may be able to produce 3-methylhopanoids. This observation is particularly critical when we consider that our current understanding of which bacteria produce certain hopanoid molecules is often based on lipid analysis of a few culturable species of a certain genus. The sporadic phylogenetic distribution of HpnR also suggests a potentially complex evolutionary history of this methylase. Two evolutionary scenarios seem plausible: either HpnR was present in the ancestor of all HpnR-containing bacteria and was repeatedly lost or it could have been acquired through horizontal gene transfer (HGT). For most of these taxa, the HpnR phylogeny is congruent with that of the species phylogeny based on 16S rRNA sequence (22). This consistency between the HpnR phylogeny and species phylogeny is evident for the acetic acid bacterial clade suggesting a vertical descent within this group. However, the Methylobacteium nodulans and Nitrococcus mobilis HpnR sequences tend to cluster outside their expected species phylogeny clade suggesting acquisition through HGT in these organisms. Thus, the evolutionary history of this protein remains unclear and more robust analyses are needed to better resolve it. Given the taxonomic diversity of potential 3-methylhopanoid producers uncovered by our analysis, the detection of 3-methylhopanoids in both modern and ancient sediments cannot be attributed specifically to aerobic methanotrophic bacteria without other lines of evidence (e.g., carbon isotope data). However, all of the bacterial species that contain an HpnR homologue in their genomes utilize a form of aerobic metabolism. Thus, it seems reasonable to continue to employ 3-methylhopanes in the rock record as indicators for the existence of aerobic metabolisms deep in time. The one organism that might be considered an exception to this rule is Candidatus Methylomirabilis oxyfera which was isolated from anoxic sediments and grows anaerobically by coupling nitrite reduction to methane oxidation (23). Although M. oxyfera is an anaerobe, it is also an oxygenic organism as it produces its own supply of oxygen through the dismutation of nitrite. It subsequently uses this oxygen for the oxidation of methane through the same methanotrophic pathway utilized by other aerobic methanotrophs (23). Whereas M. oxyfera is encountered in anaerobic environments, it still requires oxygen for its metabolism. Therefore, the distribution of the C-3 methylase in oxygen-demanding bacteria seems robust for now and can be seen as further evidence for the use of 3-methylhopanes as proxies for the occurrence of aerobic metabolisms in ancient environments. MICROBIOLOGY radical AdoMet proteins are genuine C-3 methylases, we constructed an unrooted maximum likelihood tree of 192 radical AdoMet proteins retrieved through a protein BLAST search of the M. capsulatus HpnR sequence against the Kyoto Encyclopedia of Genes and Genomes (KEGG) and National Center for Biotechnology Information (NCBI) databases (e-value cut-off < e −17 ). This analysis shows that those radical AdoMet proteins with an e-value lower than e −100 cluster together (Fig. S3). Within this clade, we find HpnR from M. capsulatus and homologues from the acetic acid bacteria, the only currently known producers of 3-methylhopanoids (Fig. 3). Further, all of the strains in this clade also contain a copy of the squalene hopene cyclase gene, which is required for hopanoid biosynthesis, as well as several other hopanoid biosynthesis genes in their genomes (17, 21). Therefore, it seems reasonable to propose that the cutoff for a bona fide C-3 methylase is a value lower than e −100 . Using this criteria, there are 52 putative homologues of HpnR in the genomic and metagenomic databases (Fig. 3). The species that contain these HpnR homologues are from a diverse set of bacterial phyla: 33 strains of Proteobacteria (22 α-, 3 β-, and 8 γ-Proteobacteria), 11 Actinobacteria, 3 Nitrospirae, 1 Acidobacterium, 1 candidate NC10 phylum organism, and 3 metagenomic sequences. In agreement with our current understanding of 3methylhopanoid distribution in bacteria, all 21 of the partially or completed acetic acid bacterial genomes have an HpnR homologue. However, only three of the nine methanotrophic genomes sequenced to date (two complete and seven incomplete) have a A previous study in M. capsulatus demonstrated that 3-methylhopanoids accumulate preferentially in stationary phase cells (24). To test whether 3-methylhopanoids play a role in stationary phase physiology, we grew both the wild type and ΔhpnR strains in batch culture for fourteen days. Cultures were supplemented with methane only at the point of inoculation (day 0) to ensure that they would become nutrient-limited and enter stationary phase. Cell growth was monitored by following the OD at 600 nm. Under these growth conditions, it was determined that the cells were entering stationary phase and ceased oxidizing methane on day 2 of growth (Fig. S4). Given that the methane in the headspace was not depleted (Fig. S4), we presumed that the cessation of growth on day 2 resulted from the depletion of oxygen. These experiments also demonstrated that ΔhpnR cells exhibited similar growth characteristics to the wild type strain during exponential growth. However, upon entering stationary phase, the methylase mutant seemed to experience a larger drop in OD than the wild type indicating a potential loss in viability under these conditions. To better assess cell viability in stationary phase, the number of cfu on day 2, 7, and 14 of growth were determined. As shown in Fig. 4, the wild type strain maintains the same number of cfu throughout stationary phase. The ΔhpnR strain sustains a similar number of viable cells on day 2 and 7, even though the observed drop in OD occurs immediately upon entering stationary phase at day 2. However, the number of cfu drops approximately six orders of magnitude on day 14 suggesting a role for 3-methylhopanoids in late stationary phase (Fig. 4). To more directly show that the lack of 3-methylhopanoids was responsible for this reduction in viability, we also determined the cfu values for the ΔhpnR strain complemented with a copy of the methylase gene (Fig. 4; ΔhpnR þ pPVW100). The overall cfu values for the complemented strain were approximately two orders of magnitude lower than the wild type on each day tested. This reduction in cfu was most likely a result of the sluggish growth observed in the presence of the kanamycin antibiotic necessary to maintain the complementing plasmid. Nevertheless, reinstating 3-methylhopanoid production in the ΔhpnR strain did result in sustained cell viability through day 14. 3-Methylhopanoids and Intracytoplasmic Membrane Formation in Late Stationary Phase. The decreased viability of the methylase mutant suggests that it may be deficient in mechanisms necessary to cope with the stresses encountered in stationary phase. M. capsulatus is one of several methanotrophs that are capable of forming Azotobacter-like cyst structures. Cyst formation has been shown to be important in enhancing the survival of a bacterium under adverse environmental conditions such as those experienced in stationary phase (25). Accordingly, we hypothesized that Colony Forming Units (cfu) 1.0E+09 1.0E+08 1.0E+07 1.0E+06 1.0E+05 1.0E+04 1.0E+03 1.0E+02 1.0E+01 1.0E+00 Day 2 Day 7 Day 14 wild type Fig. 4. Deletion of hpnR results in decreased survival in late stationary phase. The cfu for M. capsulatus strains were determined on day 2, 7, and 14 of growth by spot plating serial dilutions on NMS agar plates. Each bar represents the average cfu of three separate experiments and the error bars are standard deviations. 12908 ∣ www.pnas.org/cgi/doi/10.1073/pnas.1208255109 the lack of 3-methylhopanoids in the methylase mutant may result in inadequate cyst formation which, in turn, compromises viability during prolonged stationary phase incubation. To test this hypothesis, transmission electron microscopy (TEM) of both wild type and mutant cells on day 2 and day 14 of growth were analyzed for the formation of cyst-like structures. As shown in Fig. 5, no cyst-like cells were found to be produced either by the wild type or the methylase mutant throughout stationary phase suggesting that 3-methylhopanoids play a role in stationary phase survival independent of cyst formation. On the other hand, the images revealed formation of extensive intracytoplasmic membranes (ICM) in late stationary phase. In particular, both the wild type and ΔhpnR were capable of forming these membranes in early stationary phase (day 2). But by day 14, the wild type had significantly more ICM present than on day 2 whereas the 3-methylhopanoid deletion mutant was no longer producing these membranes. The ability of the methylase mutant to produce lamellar membranes upon entering stationary phase but not deep into stationary phase suggests that 3-methylhopanoids play a role in maintaining these membranes rather than a role in forming them. Complementation of the deletion strain with the hpnR gene partially restored the production of ICM on day 14 further indicating that 3-methylhopanoids may be important in maintaining ICM formation during stationary phase. The decreased viability of the methylase mutant along with its inability to maintain ICM formation leads us to speculate that ICM maintenance is necessary for survival in late stationary phase. Based on our physiological data, we presume that the cells in our cultures are limited for oxygen rather than methane (Fig. S4). Previous studies have shown that ICM formation and methane oxidation rates in methanotrophs increase under similar high methane/ low oxygen growth conditions (26). Because the methane monooxygenase is a membrane-bound enzyme localized to the ICM it is thought that the increase in ICM formation allows for increased methane oxidation at low oxygen levels. This strategy may then aid survival in low oxygen environments encountered in nature. Interestingly, these high methane/low oxygen growth conditions are reminiscent of the modern and ancient seafloor methane seeps in which 3-methylhopanes and 3-methylhopanoidproducing methanotrophs have been detected (4, 27). The physical conditions at these seafloor methane seeps are quite transitory, particularly in terms of the availability of methane and oxygen (28). The methanotrophs in these communities must be adapted to survive persistent low oxygen levels as well as to respond rapidly to methane pulses (28). Thus, our late stationary phase studies could be demonstrating a role for ICMs and 3methylhopanoids in the persistence of certain methanotrophic communities in their natural environments. This hypothesis is particularly appealing when we consider a recent study on the fate of spilled methane from the 2010 Deepwater Horizon oil spill (29). In this study, the idea was put forward that aerobic methanotrophic communities may act as dynamic biofilters of largescale methane inputs into the ocean (29). These methanotrophic communities persist for the most part in nutrient-limited environments yet seem poised to respond to sudden influxes of methane. Some of the methanotrophs identified from this potential methanotrophic bloom after the Deepwater Horizon disaster were γ-Proteobacteria of the Methylococcaceae family that is known to contain 3-methylhopanoid-producing methanotrophic species such as M. capsulatus. Therefore, it is possible that the ability to survive in these transient methanotrophic environments may be linked to 3-methylhopanoid production. The observations presented here point to a potential role of 3-methylhopanoids (and ICM formation) in cell viability under nutrient-limited conditions. These findings are pertinent given that low nutrients and harsh conditions are known to be ubiquitous in natural environments and as a result, microbes in nature are thought to persist in a type of stationary phase Welander and Summons Day 2 wild type ∆hpnR ∆hpnR + pPVW100 ∆hpnR ∆hpnR + pPVW100 Day 14 wild type Fig. 5. Deletion of 3-methylhopanoid production results in reduced intracytoplasmic membranes during stationary phase. TEM images show ICM formation (black arrows) by all cells on day 2 of growth. On day 14 of growth the ΔhpnR mutant has significantly lower ICM formation than the wild type and complemented strains (ΔhpnR þ pPVW100). (Black scale, 0.5 μ.) Bacterial Strains, Media, and Growth Conditions. Bacterial strains used in this study are listed in Table S1. Escherichia coli strains were grown in lysogeny broth (LB) and M. capsulatus strains were grown in nitrate minimal salts (NMS) medium supplemented with 10 μM CuSO4 (31) at 37 °C while shaking at 250 rpm. M. capsulatus batch cultures were sealed in serum vials without removing the ambient air and given methane: carbon dioxide mix (95∶5) at 60 kPa over ambient pressure. For growth on solid medium, LB or NMS was solidified with 1.5% agar and supplemented, if necessary, with 15 μg∕mL gentamicin (Gm), 50 μg∕mL kanamycin (Km), 600 μM diaminopimelic acid (DAP), or 5% sucrose. M. capsulatus plates were incubated in Vacu-Quik Jars (Almore International, Inc.) and supplied with methane: carbon dioxide mix at 20 kPa over ambient pressure. Additional details are described in SI Materials and Methods. Bioinformatics Analysis. InterPro (http://www.ebi.ac.uk/interpro) was used to identify putative radical AdoMet proteins in the M. capsulatus genome. HpnR homologues were identified in the KEGG and the NCBI databases by a translated Basic Local Assignment Search Tool (TBLASTN) (33) and were aligned using the Multiple Sequence Comparison by Log-Expectation (MUSCLE) program (34). Maximum likelihood trees were constructed by phylogenetic estimation using maximum likelihood (PhyML) (35) using the LG þ gamma model, four gamma rate categories, ten random starting trees, Nearest Neighbor Interchange (NNI) branch swapping, and substitution parameters estimated from the data. The HpnR tree was generated and edited by importing the resulting PhyML tree into the Interactive Tree of Life tool (iTOL) (http:// itol.embl.de/) (36). DNA Methods, Transformation, and Mutant Construction. All plasmid constructs and the sequences of oligonucleotide primers used in this study are described in Table S1. Construction of the hpnR deletion mutant and complemented strain is described in SI Materials and Methods. DNA sequences of all cloning intermediates were confirmed by sequencing at the GENEWIZ Boston Laboratory. E. coli strains were transformed by electroporation. Plasmids were mobilized from E. coli BW20767 into M. capsulatus by conjugation as described in SI Materials and Methods. ACKNOWLEDGMENTS. We thank Dr. Florence Schubotz for technical advice and assistance, Prof. Tanja Bosak and Prof. Shuhei Ono for providing lab space and equipment, and Prof. Martin Klotz for his generous gift of Methylococcus capsulatus strain Bath. This work was supported by the National Science Foundation (NSF) Program on Emerging Trends in Biogeochemical Cycles (OCE-0849940) supported, in turn, by NSF programs in Chemical Oceanography and Geobiology-Low Temperature Geochemistry (R.E.S.), and a National Aeronautics and Space Administration Postdoctoral Program Fellowship (P.V.W.). 1. Sahm H, Rohmer M, Bringer-Meyer S, Sprenger GA, Welle R (1993) Biochemistry and physiology of hopanoids in bacteria. Adv Microb Physiol 35:247–273. 2. Zundel M, Rohmer M (1985) Prokaryotic triterpenoids. 1. 3 β-Methylhopanoids from Acetobacter species and Methylococcus capsulatus. Eur J Biochem 150:23–27. 3. Talbot HM, Watson DF, Pearson EJ, Farrimond P (2003) Diverse biohopanoid compositions of non-marine sediments. Org Geochem 34:1353–1371. 4. Elvert M, Niemann H (2008) Occurrence of unusual steroids and hopanoids derived from aerobic methanotrophs at an active marine mud volcano. Org Geochem 39:167–177. 5. Blumenberg M, Seifert R, Michaelis W (2007) Aerobic methanotrophy in the oxic-anoxic transition zone of the Black Sea water column. Org Geochem 38:84–91. 6. Summons RE, Jahnke LL (1992) Hopenes and hopanes methylated in ring-A: Correlation of the hopanoids of extant methylotrophic bacteria with their fossil analogues. Biomarkers in Sediments and Petroleum, eds JM Moldowan, P Albrecht, and RP Philp (Prentice Hall, Englewood Cliffs, NJ), pp 189–200. 7. Eigenbrode JL, Freeman KH, Summons RE (2008) Methylhopane biomarker hydrocarbons in Hamersley Province sediments provide evidence for Neoarchean aerobiosis. Earth Planet Sci Lett 273:323–331. 8. Waldbauer JR, Sherman LS, Sumner DY, Summons RE (2009) Late Archean molecular fossils from the Transvaal Supergroup record the antiquity of microbial diversity and aerobiosis. Precambrian Res 169:28–47. 9. Hayes JM (1983) Earth’s Earliest Biosphere: Its Origin and Evolution, ed JW Schopf (Princeton University Press, Princeton), pp 291–301. Welander and Summons PNAS ∣ August 7, 2012 ∣ vol. 109 ∣ no. 32 ∣ 12909 EARTH, ATMOSPHERIC, AND PLANETARY SCIENCES Materials and Methods Analysis of Hopanoid Production. M. capsulatus strains were grown in 50 mL NMS at 37 °C for 3 d. Lipids were extracted and analyzed by liquid chromatography-mass spectrometry (LC-MS) as previously described (17, 21). The LCMS system comprises a 1200 Series HPLC (Agilent Technologies) equipped with an autosampler and a binary pump linked to a Q-TOF 6520 mass spectrometer (Agilent Technologies) via an atmospheric pressure chemical ionization interface (Agilent Technologies). Hopanoids were identified on the basis of accurate mass measurements of their protonated molecular ions, fragmentation patterns in MS-MS mode, and by comparison of relative retention time and the mass spectra with published data (32). MICROBIOLOGY (30). We are currently pursuing molecular and physiological studies to better pinpoint the factors that induce ICM formation in stationary phase, the potential role of 3-methylhopanoids in ICM maintenance, and how 3-methylhopanoids this might aid cell viability. If a relationship between 3-methylhopanoids and cell viability during stationary phase can be further established, then 3methylhopanoids have the potential to be proxies for the particular environmental stressors (e.g., low oxygen) encountered during stationary phase. 10. Summons RE, Bradley AS, Jahnke LL, Waldbauer JR (2006) Steroids, triterpenoids and molecular oxygen. Philos Trans R Soc Lond 361:951–968. 11. Waldbauer JR, Newman DK, Summons RE (2011) Microaerobic steroid biosynthesis and the molecular fossil record of Archean life. Proc Natl Acad Sci USA 108:13409–13414. 12. Zundel M, Rohmer M (1985) Prokaryotic triterpenoids. 3. The biosynthesis of 2 β-methylhopanoids and 3 β-methylhopanoids of Methylobacterium organophilum and Acetobacter pasteurianus ssp. pasteurianus. Eur J Biochem 150:35–39. 13. Bradley AS, Pearson A, Saenz JP, Marx CJ (2010) Adenosylhopane: The first intermediate in hopanoid side chain biosynthesis. Org Geochem 41:1075–1081. 14. Welander PV, Coleman ML, Sessions AL, Summons RE, Newman DK (2010) Identification of a methylase required for 2-methylhopanoid production and implications for the interpretation of sedimentary hopanes. Proc Natl Acad Sci USA 107:8537–8542. 15. Pearson A, Flood Page SR, Jorgenson TL, Fischer WW, Higgins MB (2007) Novel hopanoid cyclases from the environment. Environ Microbiol 9:2175–2188. 16. Doughty DM, Hunter RC, Summons RE, Newman DK (2009) 2-Methylhopanoids are maximally produced in akinetes of Nostoc punctiforme: Geobiological implications. Geobiology 7:524–532. 17. Welander PV, et al. (2009) Hopanoids play a role in membrane integrity and pH homeostasis in Rhodopseudomonas palustris TIE-1. J Bacteriol 191:6145–6156. 18. Ried JL, Collmer A (1987) An nptI-sacB-sacR cartridge for constructing directed, unmarked mutations in Gram-negative bacteria by marker exchange-eviction mutagenesis. Gene 57:239–246. 19. Csaki R, Hanczar T, Bodrossy L, Murrell JC, Kovacs KL (2001) Molecular characterization of structural genes coding for a membrane bound hydrogenase in Methylococcus capsulatus (Bath). FEMS Microbiol Lett 205:203–207. 20. Perzl M, et al. (1998) Cloning of conserved genes from Zymomonas mobilis and Bradyrhizobium japonicum that function in the biosynthesis of hopanoid lipids. Biochim Biophys Acta 1393:108–118. 21. Welander PV, Doughty DM, Mehay S, Summons RE, Newman DK (2012) Identification and characterization of Rhodopseudomonas palustris TIE-1 hopanoid biosynthesis mutants. Geobiology 10:163–177. 12910 ∣ www.pnas.org/cgi/doi/10.1073/pnas.1208255109 22. Munoz R, et al. (2011) Release LTPs104 of the all-species living tree. Syst Appl Microbiol 34:169–170. 23. Ettwig KF, et al. (2010) Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464:543–548. 24. Summons RE, Jahnke LL, Roksandic Z (1994) Carbon isotopic fractionation in lipids from methanotrophic bacteria: Relevance for interpretation of the geochemical record of biomarkers. Geochim Cosmochim Acta 58:2853–2863. 25. Whittenbury R, Davies SL, Davey JF (1970) Exospores and cysts formed by methaneutilizing bacteria. J Gen Microbiol 61:219–226. 26. Scott D, Brannan J, Higgins IJ (1981) The effect of growth conditions on intracytoplasmic membranes and methane mono-oxygenase activities in Methylosinus trichosporium OB3b. J Gen Microbiol 125:63–72. 27. Birgel D, Peckmann J (2008) Aerobic methanotrophy at ancient marine methane seeps: A synthesis. Org Geochem 39:1659–1667. 28. Valentine DL (2011) Emerging topics in marine methane biogeochemistry. Ann Rev Mar Sci 3:147–171. 29. Kessler JD, et al. (2011) A persistent oxygen anomaly reveals the fate of spilled methane in the deep Gulf of Mexico. Science 331:312–315. 30. Navarro Llorens JM, Tormo A, Martinez-Garcia E (2010) Stationary phase in Gramnegative bacteria. FEMS Microbiol Rev 34:476–495. 31. Whittenbury R, Phillips KC, Wilkinson JF (1970) Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61:205–218. 32. Talbot HM, Squier AH, Keely BJ, Farrimond P (2003) Atmospheric pressure chemical ionization reversed-phase liquid chromatography/ion trap mass spectrometry of intact bacteriohopanepolyols. Rapid Commun Mass Spectrom 17:728–737. 33. Altschul SF, et al. (1997) Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. 34. Edgar RC (2004) MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. 35. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. 36. Letunic I, Bork P (2007) Interactive tree of life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 23:127–128. Welander and Summons