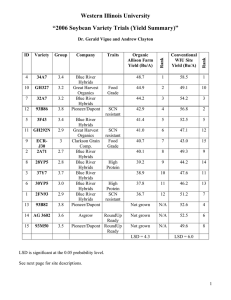
Characteristics of Mountain Mahogany (Cercocarpus) Species and Hybrids in Utah Hybrid Zone
advertisement

Characteristics of Mountain Mahogany (Cercocarpus) Species and Hybrids in Utah Hybrid Zone Scott C. Walker Deborah Turley Abstract—Species within the genus Cercocarpus, commonly called mountain mahogany, are valuable browse species for wildlife and livestock. Utah is the primary zone of overlap of the three species common to the Intermountain area. Hybridization occurs between curlleaf mountain mahogany (Cercocarpus ledifolius Nutt.), true mountain mahogany (C. montanus Raf.), and little-leaf mountain mahogany (C. intricatus Wats.) throughout central and northern Utah where the species come in contact. Hybrid frequency and characteristics are dependant upon parental varieties of each species. Cercocarpus species are vital components in native ecosystems. They increase diversity, maintain soil stability, and provide good quality habitat for many species of wildlife and domestic livestock. Three species of Cercocarpus are common in the Intermountain area of the Western United States: Cercocarpus montanus Raf. (true mountain mahogany), C. intricatus Wats. (little-leaf mountain mahogany), and C. ledifolius Nutt. (curlleaf mountain mahogany) represented by two distinct subspecies; var ledifolius and var intermontanus N. Holmgren. As shown in figure 1, Utah is the primary zone of overlap for these shrubs (Pyrah 1964; Davis 1990). The highest concentration of C. intricatus lies directly and almost entirely within the zone of overlap. Cercocarpus species are associated with desert shrub, sagebrush, pinyon-juniper, mountain brush, ponderosa pine, and mixed aspen-conifer zones (Davis 1990). Where the species of Cercocarpus come in contact with each other hybridization nearly always occurs. This report is not intended to be a comprehensive review of the hybridization of species within the genus Cercocarpus. It is more an overview of the occurrence of hybridization, a brief review of the literature, and a report of observations recorded by the authors. shrub is evergreen, intricately branched, and usually less than 1 m but can grow up to 2.5 m in height (Blauer and others 1975). Stutz (1974) suggests that C. intricatus is a dry, harsh site segregant of curlleaf. C. intricatus is distinguished from C. ledifolius solely on the basis of leaf size and plant stature. This morphology is not environmentally induced, but rather a genetic assimilation of adaptive characteristics from C. ledifolius var ledifolius. This becomes apparent when the two species are growing in a common garden and maintain their individuality, suggesting that these two taxa are genetically distinct (Pyrah 1964). C. ledifolius is an erect evergreen shrub that often grows into small trees, 2 to 8 m tall, with stiff sharp branches. C. ledifolius occurs on mountain slopes, often in pure stands as groves surrounded by open sagebrush slopes or mixed with mountain brush, pinyon-juniper, ponderosa pine, Douglasfir, or white fir. The best developed stands are routinely Discussion _____________________ Throughout their range of overlap each of the three species occupy rather distinct habitats. The more xeric C. intricatus occurs on harsh sites that are exposed to high temperature and drought (Blauer and others 1975). This In: McArthur, E. Durant; Ostler, W. Kent; Wambolt, Carl L., comps. 1999. Proceedings: shrubland ecotones; 1998 August 12–14; Ephraim, UT. Proc. RMRS-P-11. Ogden, UT: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. Scott C. Walker and Deborah Turley are Research Biologists, Utah Division of Wildlife Resources, Great Basin Research Center, 540 N. Main 32-7, Ephraim, UT 84627. 32 Figure 1—Distribution of curlleaf mountain mahogany, true mountain mahogany, and little-leaf mountain mahogany (from Davis 1990). USDA Forest Service Proceedings RMRS-P-11. 1999 found on all exposures of warm dry slopes, typically growing in shallow soils or rocky ridges on slopes averaging around 50 percent (Davis 1990; Blauer and others 1975; Holmgren 1987). The most distinguishing characteristic between C. ledifolius var ledifolius and C. ledifolius var intermontanus is leaf size and degree of pubescence on the leaf. C. ledifolius var intermontanus leaves are (ob)lanceolate or elliptic-lanceolate, 5 to 8 (10) mm wide, sparsely hairy, the midrib and lateral veins conspicuously visible. C. ledifolius var ledifolius leaves are narrowly lanceolate to linear, 1.5 to 4 (6.5) mm wide, densely white-hairy beneath, the pubescence sometimes obscuring the midrib and certainly the lateral veins (Holmgren 1987). C. montanus grows 1 to 2 m in height, less commonly, a small tree up to 4 m. C. montanus communities can usually be found at lower elevations than C. Ledifolius, occurring on similar slopes but usually with deeper soils (Davis 1990). Leaves are deciduous, short petiolate, the blade obovate to (ob)lanceolate or orbicular, 6 to 44 mm long, 5 to 23 mm wide, crenate-serrate (Welsh and others 1987; Blauer and others 1975). The flowering period for these species of Cercocarpus is from mid May to late June. Flowering periods for C. ledifolius and C. intricatus tend to overlap in areas of common occurrence. While C. montanus flowers nearly 2 weeks later than the evergreen types. The flowering period may overlap for plants on contrasting canyon slopes where aspect may affect plant distribution and flowering phenology. Hybrid plants generally begin flowering 2 weeks after the later flowering C. montanus (Pyrah 1964). Hybridization between the species is not a rare occurrence, suggesting genetic reproductive barriers between the taxa are weak. Though hybrids form upon contact between all of the taxa, frequency of F1 hybrids varies depending on parental species combinations. Only those that involve C. ledifolius var ledifolius as one of the parents produce hybrids with significant segregant progeny (table 1) (Stutz 1990). The duration of pollen viability is an important factor in species isolation in nature. As shown in table 2, a portion of the pollen of C. ledifolius and C. montanus remains viable for more than 10 days. Abnormal pollen tubes become increasingly abundant during this period of time (Stutz 1990; Pyrah 1964). This extended pollen viability allows for the cross pollination among these species. Hybridization of species within the genus Cercocarpus offers a unique perspective. At the ecotones where species of Cercocarpus meet, the distribution of each population is controlled by precipitation and soil characteristics. There is Table 1—Summary of natural hybridization in Cercocarpus (from Stutz 1990). Parentsa from F1 Opportunity for hybridization led led x led interm led led x intricatus led led x montanus led interm x intricatus led interm x montanus intricatus x montanus low high low moderate moderate moderate Abundance of F1 hybrids in contact zones high high high moderate high low a Progeny hybrids abundant abundant abundant moderate few none led led = C. ledifolius var ledifolius; led interm = C. ledifolius var intermontanus. USDA Forest Service Proceedings RMRS-P-11. 1999 often some overlap of the two populations at the ecotone. Where hybridization occurs, there is generally not a distinct band or zone that these hybrids occupy. For example, where C. ledifolius and C. montanus come in contact, C. ledifolius is generally growing upslope of C. montanus. When hybrids form between the two, they occur almost without exception within the bounds of the C. montanus population rather than where the two populations interface. This suggests that C. montanus is the maternal parent supported by what has been observed for flowering dates and pollen durability (Pyrah 1964). Since pollen from the earlier flowering C. ledifolius can remain viable for more than 10 days, crosspollination onto C. montanus is possible. However, before pollen is shed from C. montanus, the stigmas of C. ledifolius have withered, and as a result reciprocal pollination is usually not permitted. Generally it appears that pollination is most common from C. ledifolius to C. montanus. Noting that the flowering periods are similar for C. ledifolius and C. intricatus, one would expect similar trends for both species in hybridization with C. montanus. Observations were made that demonstrated exceptions to this pattern for hybrids between C. intricatus and C. montanus. One example occurs on the Chippean Rocks formation in the Abajo mountains of southeastern Utah (elevation 2,500 m). The spires of this weathered sandstone deposit extend up from a forest of ponderosa pine with a dense shrub understory. The formation is only a few hundred meters in height. C. montanus is scattered in the understory on lower slopes of the formation, while C. intricatus is distributed across upper slopes and on top. Hybrid plants are also located at the top of the formation within the C. intricatus stand. This representation of C. montanus not being the probable maternal parent suggests the possibility that reciprocal crossing can occur. Another site of interest is found near the Wind Caves (elevation 2,100 m) located in Logan Canyon in northern Utah. There is a variety of intermediate phenotypes between C. ledifolius var intermontanus and C. intricatus, all growing among a C. montanus population. The variety of intermediate phenotypes of the individuals within the populations may be a result of the hybridization of C. intricatus X C. ledifolius. Also present were hybrid products of C. ledifolius X C. montanus and plants suspected of being C. intricatus X C. montanus hybrids. Most of the dozen mahogany sites that were inspected for this project contained hybrid plants within the population. The Wind Cave site was unique in which hybrids occurred where all three species were growing within close proximity, and where there were hybrids derived from each combination of species. As this study was by no means a comprehensive inventory, other sites that may exhibit similar population dynamics are likely in the Intermountain area. When C. ledifolius is in contact with C. intricatus and C. montanus, hybridization nearly always occurs. However, each population, and progeny within populations, display a different pattern of hybrid products. Plants may show traits or characteristic of either parent. For example, a single hybrid plant might be more upright, have a tendency for evergreen leaves characteristics associated with C. ledifolius, and have multiple stems, a characteristic of C. montanus. One of the most telling 33 Table 2—Duration of pollen viabilitya for Cercocarpus montanus and C. Ledifolius var intermontanus (modified from Pyrah 1964). Age days Nb montanus germination #c % N var intermontanus germination # % 1 2 5 10 14 362 331 305 332 381 278 91 65 82 81 76.8 27.5 21.3 24.7 21.2 374 —— 222 308 228 152 —— 42 38 20 40.7 —— 18.9 12.3 8.8 a Pollen was germinated on sterile nutrient agar. Number in sample. c Number germinated. b and variable characteristics of hybrids is leaf morphology. Variability is high, both within species as well as among hybrids. Leaves taken from adjacent hybrid plants can demonstrate varying parental traits. For example, leaves may display strong C. ledifolius traits with more linear slightly toothed, and strongly enrolled margins, while leaves from other hybrids maintain stronger C. montanus traits with a more spatulate shape, stronger toothed and slightly enrolled margins. The seeds of hybrids are usually highly inviable, which suggests the presence of chromosomal or genetic sterility within the hybrid. Such sterility is probably due to differences in parental chromosomes or to incompatible gene interaction (Stutz 1990). For the three mahogany species, seed production always occurs on second-year branches or from second-year buds. The flowering phenology of a plant is synchronized and seed is produced in a single flush. However, C. montanus plants have been observed to demonstrate a unique partitioning of resources. One branch will put energy into copious seed production and will produce very little or no annual leader growth, while an adjacent branch, on that same plant, will produce very little or no seed, but instead will produce large amounts of annual growth. The trigger mechanisms for seed-versus-growth production within this genera are poorly understood. Among hybrid plants a unique flowering phenology also occurs. The triggering mechanism for bud and seed production in hybrids seems to be confused. Hybrid plants produce maturing seed, new flowers, and developing buds concurrently on the same branch of current-year’s growth, as compared to the synchronized flowering on second-year’s growth of nonhybrids. These branches may continue to produce buds and flowers well beyond the normal flowering period of both parent species. Hybridization or genetic assimilation is often the key to long-term survival, adaptation, or evolution of a species. The unique traits demonstrated by Cercocarpus hybrids include adaptation of parental characteristics. Hybrids exhibit characteristics that demonstrate a unique relationship to either parent. When hybrids become established in adaptive habitats, individuals tend to be larger than either parent. This is probably an expression of hybrid vigor and can be accounted for by the wide genetic diversity between these two species. 34 Hybridization among the three species of mountain mahogany is a common occurrence. This leaves one to surmise that the possibility that a stable hybrid could be produced; one that will produce viable seed, have desirable characteristics of both parents (such as being evergreen, and have the ability to resprout), and would be of benefit to the rangelands they occupied. Each parental species can produce unique products or perform different functions. Hybrids may have the ability to produce all products produced by both parents or perform both functions. This could explain the continual flowering process that occurs on hybrids and the ability to produce seed on current years’ growth. An interesting point is the idea that these hybrids may be able to synthesize new products not found in either parent. Gas chromatographic work done by Pyrah (1964) showed there are indeed unique compounds produced by the hybrids that are not present in either parent. Though not identified, these compounds may have additional value not yet understood. References _____________________ Blauer, A. C.; Plummer, A. P.; McArthur, E. D.; Stevens, R.; Guinta, B. C. 1975. Characteristics and hybridization of important Intermountain shrubs. I. Rose family. Res. Pap. INT-169. Ogden, UT: U.S. Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station. 35 p. Davis, J. N. 1990. General ecology, wildlife use, and management of the mountain mahoganies in the Intermountain West. In: Johnson, Kendall L., ed. Proceedings of the fifth Utah Shrub Ecology Workshop; 1988 July 13-14; Logan, UT. Logan, UT: Utah State University: 1-13. Holmgren, N. H. 1987. Cercocarpus ledifolius var intermontanus (Rosaceae), a new varietal name for the Intermountain curlleaf mountain-mahogany. Brittonia. 39: 423-427. Pyrah, G. L. 1964. Cytogenetic studies of Cercocarpus in Utah. Provo, UT: Brigham Young University. 44 p. Thesis. Stutz, H. C. 1974. Rapid evolution in Western shrubs. Utah Sci. 35: 16-20, 33. Stutz, H. C. 1990. Taxonomy and evolution of Cercocarpus in the Western United States. In: Johnson, Kendall L., ed. Proceedings of the fifth Utah Shrub Ecology Workshop; 1989 July 13-14; Logan, UT. Logan, UT: Utah State University: 15-25. Welsh, S. L.; Atwood, N. D.; Goodrich, S.; Higgins, L. C. 1987. A Utah flora. Great Basin Natur. Mem. 9. 894 p. USDA Forest Service Proceedings RMRS-P-11. 1999