CHM 111-02 College Chemistry I, Spring 2016
advertisement

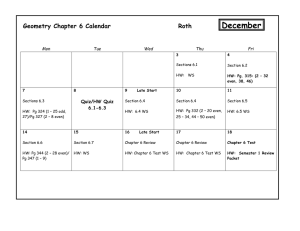
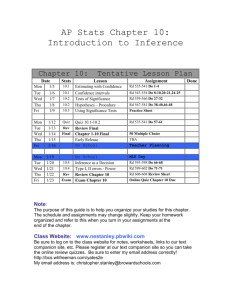
CHM 111-02 College Chemistry I, 4 Semester Hours Spring 2016 Course Information: Prerequisite(s) Course Description Student Learning Outcomes Class/Lab Time Class Location Required textbook and Course Materials Recommended Materials Last Date of Withdrawal Final Exam Recording Attendance and Excused Absences Tardiness Distractions Make-up policy Tutors Homework Videos MTH 112 or equivalent math placement score. Students are required to complete prerequisites for this course. Students who enroll without completing prerequisites for this course may be withdrawn by the College and may not qualify for a refund of tuition. It is the responsibility of the student to ensure that all course prerequisites are completed and documented at the College This is the first course in a two-semester sequence designed for the science or engineering major who is expected to have a strong background in mathematics. Topics in this course include measurement, nomenclature, stoichiometry, atomic structure, equations and reactions, basic concepts of thermochemistry, chemical and physical properties, bonding, molecular structure, gas laws, kinetic-molecular theory, condensed matter, solutions, colloids, and some descriptive chemistry topics. Laboratory is required. The student will demonstrate the ability to think critically and to use appropriate concepts to analyze qualitatively a problem or situation involving general chemistry. The student will demonstrate the ability to use appropriate mathematical techniques and concepts to obtain a quantitative solution to problems in general chemistry. The student will demonstrate the ability to collect and analyze data and to prepare a coherent report of his or her findings. Lecture – Tu/Th 8:30-9:45am, Room 2316 Lab – Th 11:30-2:30pm, Room 2316 Martin Campus Room 2316 Lecture – Chemistry, The Molecular Nature of Matter and Change, 7th edition by Silberberg. Scientific calculator. Lab – Lab Manual, splash protection goggles, shoes that cover the whole foot (heal and toes), and covered legs (pants down to shoes and without holes). Study Guide for Text; study sheets and videos available from my web page (http://www.sheltonstate.edu/faculty_staff/faculty_website_directory/saylor_rick.aspx) . Thursday, April 7, 2016 8:00-10:00am, Thursday, April 28, 2016 It is your responsibility to write your name on the attendance sheet yourself and to keep track of your own absences. If you miss a class or lab, please write a note on a 3x5 index card (available from me) providing your name, the date(s) missed, the reason you missed, the present date, and your signature. The note, along with any documentation, should be turned in within a week of your return to class. For an absence to be excused I must have something in writing. (Standard College Policies detail what is an excused absence.) Students are expected to be present for the entire class or lab. Lecture - Arriving late or leaving early is sometimes rude (if there is not a good reason) and always distracting. I will close the front door when I start. If you arrive late, please enter by the back (side) door. Don’t forget to sign the roll at the end of the class period. Test/Quiz - Arriving late does not extend the test/quiz period. You must arrive in the classroom before other test takers leave. Lab - As a safety matter, students arriving after the prelab presentation has begun are not permitted to do the lab. Please be on time. Actions or devices that distract your classmates or the instructor are inappropriate. People using computers or tablets should sit at the back of the classroom. Please turn off cell phones during class. The vibration of most cell phones is not silent. Daily Questions/In-class Problem – A missed question will be one of the dropped questions. Quiz – A missed quiz will be one of the dropped quizzes. (Except for safety quiz. See Lab below.) Lab – You may miss one lab without penalty. Only the safety lab may be made-up. (All students must complete the safety lab and the safety quiz before they work with reagent chemicals.) Test - The score for a missed test is determined from the respective portion of the cumulative final exam. Shelton State Community College is dedicated to the success of its students. To further that goal, free tutoring is available to all currently enrolled students. Check this webpage for additional tutoring info: www.sheltonstate.edu>Current Students> Tutoring (under Instructional Resources). If you need additional assistance to succeed, contact Annette Cook at acook@sheltonstate.edu. If you have a disability and need accommodations to help you be successful, contact Michele Minor at m.minor@sheltonstate.edu or visit her in the Office of Specialized Student Services. For chemistry the online Smarthinking site may be used. Textbook problems are not graded. Working these problems should help you: (a) to test your understanding of the material, and (b) to determine whether you should seek additional help. Several videos are available. Some videos will be optional tutorals on difficult topics covered in regular lecture. Other videos will be assigned minilectures, containing content that you will be tested and quizzed on. Assessments: Daily Questions Quizzes Labs Tests Lab “Final” Final Examination Most days there will be a 3 point question asked either at the beginning of class (related to material covered in the previous lecture session) or the end of class (related to material covered in class that day). Each 10 pt. quiz is given at the beginning of a lab period. Two quizzes are required at the beginning of the term (one covers the safety procedures and one covers the syllabus and standard policies). A quiz typically covers the material since the last test/quiz and does not include material from lecture the day of the quiz. For the satisfactory completion of each lab you will earn 10 points. For each lab to be satisfactory, you must: a) follow proper safety procedures (covered in the safety lab and each prelab); b) do all parts of the experiment correctly (follow the procedure carefully); c) clean the lab equipment and put it back where it belongs; d) clean up your bench and sink, and (if used) clean up the balance table and balances (if used); e) allow me to provide feedback about your lab report and bench; f) and turn in final corrected report before you leave. Deductions will be made for errors and omissions in lab report, not cleaning up properly, and not following safety procedures. The 100pt. midterm exams (tests) usually contain four types of questions: True/False; Fill in the Blank; Short Answer; and Calculation. Matching and/or Multiple Choice questions are possible but rare. The number of questions and their distribution will vary with the material. Each test will emphasize the current material but questions may be asked that relate to material covered on earlier tests. You may use a calculator but you may not share it. You may not use a cell phone, or “smart” device during the test. When the test begins, please put away and on the floor everything but a calculator, and a pen/pencil. Do not wear dark glasses or caps/hats during a test. This quiz will have approximately one question for each lab performed during the term. There will be True/False and Fill in the Blank questions that cover the processes (ie. weighing a sample)/principles (how solubility is affected by temperature)/observations (color of solid copper sulfate after heating) that were used during your labs. The cumulative final exam will have a section corresponding to each midterm test. There will be True/False, Fill in the Blank, and Calculations questions but no Short Answer questions. There may be a small number of multiple choice and/or matching questions. (continued on back) During the final exam, you may choose whether to replace the score of one 100pt. test with your score on the corresponding section of the final exam. (It will not be used to pull down your grade.) A missed test is automatically the one "replaced". Instructor Information: Instructor Student hours and location for Student Hours Instructor’s E-mail and phone number Web site Division Chair’s E-mail and phone number Dr. Richard Saylor Office: Room 2620 (enter Faculty Office on west side of atrium; short hall to large open area) Student Office Hours: (M/W 9:30-10:00am; M 1:45-2:15pm and 3:30-4:00pm; Tu/Th 10:00-10:30am and 5:30-6:00pm) My complete schedule is available on my office door and linked to on the web site below. e-mail: rsaylor@sheltonstate.edu Phone: 391-2269 Web site: http://www.sheltonstate.edu/faculty_staff/faculty_website_directory/saylor_rick.aspx Ms. Sharon Vincent Phone: 391-2208 e-mail: svincent@sheltonstate.edu Office: 2536 Safety Policy Information: Food and Drink NOTHING should be put into your mouth while you are in this room (not limited to "during" class or lab). While this obviously includes food (yes, candy too) and drink, you should also be wary of fingers, pencils, pens, calculators, . . . etc. Be cautious about rubbing your eyes as well. Residues from the benches get on your hands and objects that you handle. Always wash your hands after class or lab, especially before eating. The risk is small, but so easily avoidable. How much risk is an M&M, or a sip of coke worth? As soon as you reach your seat in the classroom/lab, any food or drink that you may have with you should be put away in a backpack or purse that is not on the lab bench. Lab Safety Students are expected to follow the general safety rules mentioned here, those on the Lab Safety Rules sheet (all students have a copy or are given a copy of when we cover safety), as well as the specific requirements covered before each lab. No credit will be given for work performed while violating these rules. For everyone’s safety, please be watchful for and helpful to anyone not following proper safety practices. As a matter of safety, students arriving after the prelab presentation has begun may not do the lab. College Policy Information: Emergency Preparedness and Sexual Misconduct Academic Misconduct Attendance Policy Standard College Policies Student Email (Bucs Mail) Quality Enhancement Plan Shelton State Community College continues to be committed to a safe teaching and learning environment for students and employees. In an effort to further strengthen efforts at keeping the College Community free from weapon related violence and to eradicate sexual misconduct crimes and infractions, SSCC has recently enacted the following policies that address these areas specifically. Sexual misconduct is an often underreported crime and victims should be aware that SSCC has a confidential process in place for reporting such actions and for helping victims identify resources for assistance. Links to these policies and other important emergency preparedness related topics may be found on the college website: http://www.sheltonstate.edu/discover_sscc/emergency_preparedness.aspx. Whether or not academic misconduct has occurred and what classroom sanctions, if any, are to be applied are matters to be determined by the respective instructor. A student who opposes the sanction imposed by an instructor may appeal the matter to the appropriate Associate Dean. (My Policy: Score on that assessment will be a zero and no make-up will be offered. The associate dean will be notified.) SSCC Attendance Policy: Students are expected to attend all classes for which they are registered, to be prompt and to remain in class/lab for the entire time. Attendance will be recorded at every class/lab meeting. On the final grade report, instructors are required to identify the last day of attendance for all students who receive a grade of “F” or “U.” Students who are unable to attend class regularly, regardless of the reason or circumstance, should withdraw from the class. Withdrawal from class can affect eligibility for federal financial aid. If a student is unable to attend at least 80 percent of class meetings, regardless of the reason or circumstance, it is recommended that the student withdraw from that class before excessive absences interfere with the student’s ability to successfully complete the course. The Standard College Policies apply to all classes at the college and are a part of every official course syllabus; each student receives a copy when he or she completes the vehicle registration/waiver procedure. It is also available from the College website, www.sheltonstate.edu. It is the responsibility of the student to have a copy of these policies and to abide by them. This class syllabus is intended to give further detail about the policies and expectations in this class. College policies are also published in the Schedule of Classes and the SSCC College Catalog/Student Handbook. Students are expected to be aware of and abide by College policies in every class. All students who are or have been registered for classes at Shelton State Community College are provided an email account. Students who are currently registered must have an email account. Electronic mail is the official method of communication for delivery of information. Shelton State designated communicators may use this email account to send official communications to the student body. Student email addresses will be recorded in the college’s electronic directories and records. To activate/sign in to your Bucs Mail account, visit the Bucs Mail icon at www.sheltonstate.edu. Shelton State’s Quality Enhancement Plan (QEP) Improving Student Success in Online Classes This table provides enough boxes for all your scores. See the Distribution of Points table for the actual number of assessments offered. Your Results Tests Quizzes Labs Daily Questions #1 #1 #6 #1 #7 #1 #7 #13 #19 #2 #2 #7 #2 #8 #2 #8 #14 #20 #3 #3 #8 #3 #9 #3 #9 #15 #21 #4 #4 #9 #4 #10 #4 #10 #16 #22 Final #5 #10 #5 #11 #5 #11 #17 #23 #6 #12 #6 #12 #18 #24 Grading: GRADE REPORT FORM: After the second and subsequent test; I will provide an update on how well you are doing relative to the grade you wish to earn. It will include the information and layout shown below. Your Name Test Scores 1 2 3 4 Assessment Tests Quizzes (drop 1) Daily (drop 4) Labs (drop 1) Lab "Final" Final Exam Totals Test Average Number Offered 3 6 20 12 1 1 Lab Total Daily Total Quiz Total Total Points Overall Average Distribution of Points Points Points Percentage of Each Total Grade (each) 100 pts 300 pts 15.8% 10 pts 50 pts 1.6% 3 pts 48 pts 0.5% 10 pts 110 pts 1.6% 24 pts 24 pts 3.8% 100 pts 100 pts 15.8% 632 pts Grade A,B,or C % of Points Remaining % of Pts Remaining (Replace Lowest Score) Letter grades will be assigned according to the following scale. Percentage Letter Grade 90-100 A 80-89 B 70-79 C 60-69 D 59 or less F Withdraw W Percentage of Grade (total) 47.5% 7.9% 7.6% 17.4% 3.8% 15.8% 100% Course Calendar: The dates shown on this page may be changed at the discretion of the instructor. Date Day 7-Jan Thu 12-Jan Tue 14-Jan Thu 19-Jan Tue 21-Jan Thu 26-Jan Tue 28-Jan Thu 2-Feb Tue 4-Feb Thu 9-Feb Tue 11-Feb Thu 16-Feb Tue 18-Feb Thu 23-Feb Tue 25-Feb Thu 1-Mar Tue 3-Mar Thu 8-Mar Tue 10-Mar Thu 15-Mar Tue 17-Mar Thu 22-Mar Tue 24-Mar Thu 29-Mar Tue 31-Mar Thu 5-Apr Tue 7-Apr Tue 7-Apr Thu 12-Apr Tue 14-Apr Thu 19-Apr Tue 21-Apr Thu 26-Apr Tue 28-Apr Thu No class Sections 1.3 1.1-1.4 1.5-2.4 2.5;2.6;2.9 7.1-7.2 7.3-7.4 8.1-8.2 8.3-8.4 9.1-9.4 2.7, 2.8 9.5-9.6 10.1 10.2-10.3 11.1-11.2 11.3 3.1-3.2 3.3-3.4 4.1 4.2-4.3 4.4-4.5 4.6-4.7 5.1-5.3 5.4-5.6 12.1-12.2 12.3-12.4 12.4-12.6 Lecture Topic Syllabus; Science Definitions; Measurement Measurement; Atomic Theory Elements, Compounds, Mixtures Light and the Bohr Model Matter/Energy Duality; Orbitals Electron Configuration Periodic Trends Ionic and Covalent Bonding Test 1 (Chap. 1, 7, 8, and some of 2) Naming Compounds Variations in Bonding Lewis Structure VSEPR and Polarity Valence Bond Theory Molecular Orbital Theory Moles and Chemical Formulas Test 2 (Chap. 9, 10, 11, and some of 2) Balancing Eq'ns and Stoichiometry Spring Break Spring Break Solution Stoichiometry Chemical Equations; Precipitation Rxn Acid-Base/Redox Reactions Redox/Reversible Reactions Gas Laws Last Day to Withdraw KMT - Explaining Gas Laws Real Gases; Phase Changes Intermolecular Forces; Liquids Water and Solids Test 3 (Chap. 3, 4, 5, and 12) Review/Lab Final Cumulative Final Exam (8:00-10:00am) Test dates shaded. Lab Topic Safety/Science Density Chemical and Physical Bright Line Emission Elem./Comp'ds/Bonding Lewis Structure/Naming (WS) Shape/Charge/Resonance (WS) Empirical Formula Balancing (WS) Precipitation Redox (WS) Molecular Mass Quiz dates shaded. The College does not discriminate on the basis of race, color, national origin, sex, disability, or age in its admissions, programs, and services in compliance with Title VI and VII of the Civil Rights Act of 1964, Section 504 of the Rehabilitation Act of 1973, the Age Discrimination Act of 1975, Title IX of the Educational Amendments of 1972, and the Americans with Disabilities Act of 1990. - See more at: http://www.sheltonstate.edu/discover_sscc/eeoc_statement.aspx#sthash.ZEfKOVpJ.dpuf