Phy 133 – assignment 12: A. 1.
advertisement

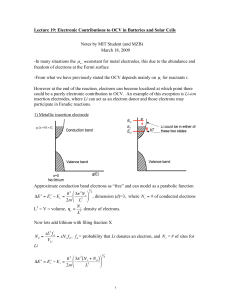
Phy 133 – assignment 12: A. 1. - In a(n) metal, the highest energy band containing electrons (the conduction band) is partly filled with many electrons. - A(n) insulator has an empty conduction band above a filled valence band. - A(n) semiconductor has an empty conduction band above a filled valence band at absolute zero, but the gap between bands is narrow enough for some electrons to cross it at room temperature. 2. Because Cooper pairs act collectively not individually. They are all in the same quantum state, so you can’t change the velocity of one pair without changing the velocity of them all. Imperfections and phonons aren’t capable of that. 3. a. Current develops on the superconductor’s surface, creating a magnetic field which cancels the permanent magnet’s inside the superconductor. b. Outside the superconductor, the surface current’s field repels the permanent magnet. 1/3 4. Nuclear radius: r = r0A where r0 = 1.2 fm. 1/3 (1.2 fm)(238 ) = 7.44 fm B. 1. All of them. Being “particles” with zero spin, the Pauli Exclusion Principle does not apply to Cooper pairs. So, there is nothing to keep them all from dropping into the lowest energy level there is. 2. C. 1. Connecting the battery one way sucks free charges out of the junction, making it like an insulator. (No current due to lack of anything free to flow.) The other way dumps lots of free charges in, for the opposite effect. 2. D. Because 5.4900 eV to 5.4904 eV is such a narrow range, we can treat E as a constant. (Otherwise we would have to integrate.) The center of the range, 5.4902 eV works best, although 5.49 is close enough. Total number = (number per cubic meter)(cubic meters) (An alternate approach, if you have a calculator which can integrate, is to integrate N(E)dE. That would work whether the range is very narrow or not.) E. 1. In diamond, when a photon tries to lift an electron into an empty state, the closest available states are 5.47 eV away. Visible light doesn’t have that much energy, so it can’t be absorbed and sails through the crystal. In silicon, the closest available states are only 1.14 eV away. Light can get electrons across that, so the light is absorbed and doesn’t go through the crystal. 2. Since the interval from 2.00 eV to 4.00 eV is not narrow, like the interval in quiz D was, we can’t say E ≈ constant and dE ≈ ΔE. Rather, 2.00 eV to 4.00 eV must be divided into many small intervals, and N(E) integrated: Number/volume = N(E)dE = f(E)g(E)dE In any material, states significantly below EF are almost all populated, so f(E) ≈ 1. (“Significantly” means a few times kT, which at room temperature would be something like a tenth of an eV.) (Unlike quiz D, this asked for the number per cubic meter rather than the total number. So, you don’t multiply by the volume this time.) F. 1. Charge gets stuck in the center layer. (Electrons get stuck in the p layer of an npn, for example.) This layer of charge, if allowed to accumulate, repels additional charge flowing toward it, shutting off the current. If it’s drained off, current flows. 2. Center (n) layer: donors. Donors donate electrons; with all those free electrons, conduction is dominated by negative particles. n is for negative. Outer (p) layers: acceptors. Acceptors take on electrons. Holes are left behind, so conduction is dominated by positive particles. p is for positive. 3. The Pauli Exclusion Principle does not apply to particles with integer spin. (Just particles with half-integer spin like electrons.) The pair’s total spin is zero, so there is nothing to keep them from all being in the same state. 4. The pairs break up, becoming individual electrons. (That’s why it stops being a superconductor.) 5. Alpha particles were scattered off the atoms in a gold foil.