Oxford Medical Genetics Laboratories, The Churchill, Old Road, Headington, Oxford,... 01865 225594
advertisement
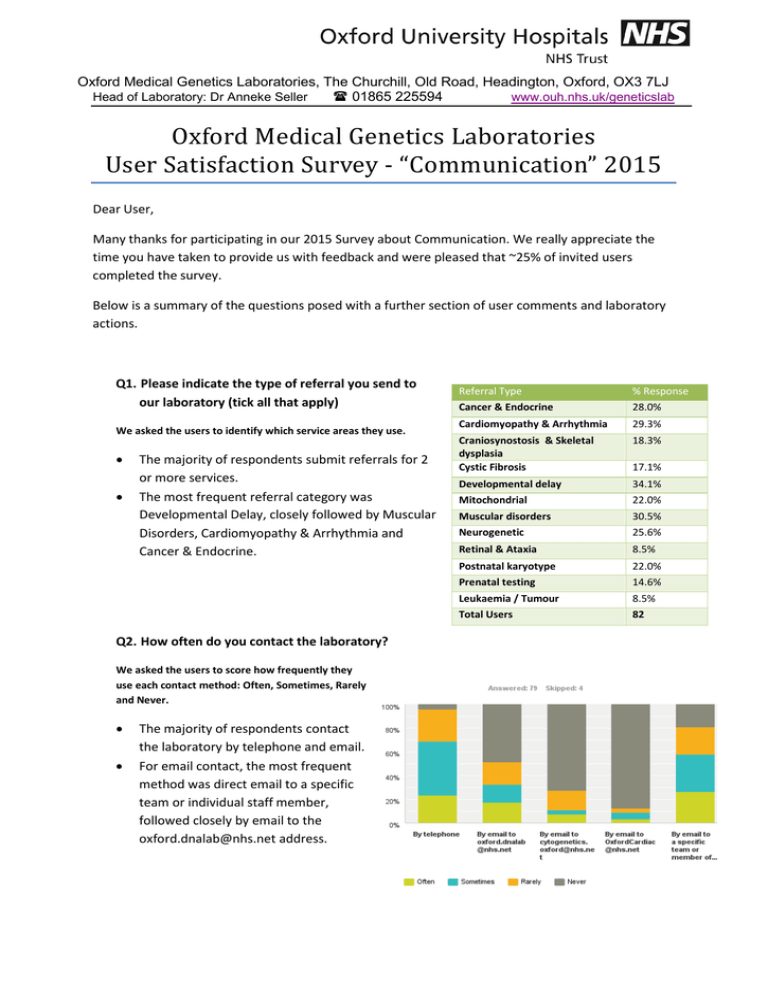
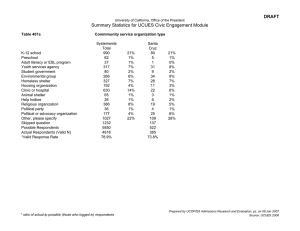
Oxford Medical Genetics Laboratories, The Churchill, Old Road, Headington, Oxford, OX3 7LJ Head of Laboratory: Dr Anneke Seller 01865 225594 www.ouh.nhs.uk/geneticslab Oxford Medical Genetics Laboratories User Satisfaction Survey - “Communication” 2015 Dear User, Many thanks for participating in our 2015 Survey about Communication. We really appreciate the time you have taken to provide us with feedback and were pleased that ~25% of invited users completed the survey. Below is a summary of the questions posed with a further section of user comments and laboratory actions. Q1. Please indicate the type of referral you send to our laboratory (tick all that apply) We asked the users to identify which service areas they use. • • The majority of respondents submit referrals for 2 or more services. The most frequent referral category was Developmental Delay, closely followed by Muscular Disorders, Cardiomyopathy & Arrhythmia and Cancer & Endocrine. Q2. How often do you contact the laboratory? We asked the users to score how frequently they use each contact method: Often, Sometimes, Rarely and Never. • • The majority of respondents contact the laboratory by telephone and email. For email contact, the most frequent method was direct email to a specific team or individual staff member, followed closely by email to the oxford.dnalab@nhs.net address. Referral Type Cancer & Endocrine % Response 28.0% Cardiomyopathy & Arrhythmia 29.3% Craniosynostosis & Skeletal dysplasia Cystic Fibrosis 18.3% Developmental delay Mitochondrial 34.1% 22.0% Muscular disorders Neurogenetic 30.5% 25.6% Retinal & Ataxia 8.5% Postnatal karyotype Prenatal testing 22.0% 14.6% Leukaemia / Tumour 8.5% Total Users 82 17.1% Q3. When you contact the laboratory ... We asked the users to provide feedback on the availability of relevant staff, whether the information provided was adequate and our speed of response. • • • 91% of respondents stated that they are able to reach an appropriate member of staff always or most of the time. 96% stated that they were provided with the information or advice required always or most of the time. 96% of respondents stated that we were able to deal with their enquiry promptly always or most of the time. Q4. Please rate the quality of information you receive on a scale of 1 to 5 (1 = poor, 5 = excellent). In this question we asked the users to rate the quality of information they received from each of the three main staffing groups. • • • • Overall the majority of respondents Q4. rated the quality of information 1 2 3 4 5 N/A Answer Options from all three staffing groups as 4 Office staff 2 0 4 22 36 13 Genetic and 5 (Very Good to Excellent). 2 0 2 13 43 17 Technologists The quality of information from Clinical Scientists 2 0 2 13 61 1 Clinical Scientists was rated highest among the three staffing groups. This reflects the fact that the majority of users contact the Clinical Scientists for advice. The genetic technologist group has significantly less contact with the users; 22.08% of respondents marked N/A against this group. Q5. Have you used our laboratory website? • The majority of respondents (>74%) have not used our laboratory web site or did not know it existed. The laboratory web site includes information on our: test repertoire, prices, facts about each disease / technical area, guides on how to send a sample, including sample referral cards and pre-referral forms. The web address is located in the header of all molecular genetics reports and in the footer of email signatures (http://www.ouh.nhs.uk/geneticslab) or Google us using ‘Genetics lab Oxford’. Total Response Count 77 77 79 Q6. If you have used the website did you find what you were looking for? We asked the users to categorise the availability of different types of information into 4 groups: “I found this information”, “I didn’t find this information”, “I didn’t look for this information” and “N/A”. • • • Most respondents who use our website could locate the information detailed in the 6 categories. Contact Information and Specific Disease/Test Information were the most commonly found. 15% of respondents, who use the website, said that they could not find our test prices Q7. Please grade different aspects of our clinical reports based on whether they meet your clinical needs (5 = completely meets your clinical needs, 1 = does not meet your clinical needs). We asked the users to rate six main aspects of our reports: turnaround times, presentation and clarity of results, detail of interpretive comments, scientific content, availability of additional information and overall quality. • • • Overall the majority of respondents rated all six aspects of our reports as 4 or above. The highest rated aspects of our clinical reports were scientific content (88%) and overall quality (87%). Some respondents felt that two aspects of our reports were not meeting their clinical needs, namely Turnaround times (ratings 1-3 33.7%) and Availability of additional information (ratings 1-3 26.3%). Q8. If you have suggestions on how we could improve our clinical reports, please provide them below. • A total of 19 comments were submitted for this question • 23.7% of these were complimentary towards our reports and service. • 76.3% of these were constructive/improvement suggestions Q9. If you have any further comments regarding our service or your experience of communicating with the laboratory, please provide them below. • A total of 25 comments were submitted for this question. • 88% of these were complimentary towards service delivery, specific teams and overall quality of service provided by the laboratory. • 12% of these were constructive criticisms or improvement suggestions. Q10. Contact Details • Just over 50% (47/83) of the total participants submitted their details • 27.66% of the 47 responses were internal (from the OUH Trust) and 72.34% were external. Overall Survey Participants 16% Internal (OUH) 43% External 41% Anonymous Summary of Comments and Actions We received some very complimentary comments about the services, the staff, the clinical reports (including the level of detail and interpretative comments) and the general availability of additional information via email contact or by speaking directly to members of staff. There were some comments about report turnaround times (TATs) and whilst the users generally feel that the TATs meet their clinical needs (as demonstrated in question 7) some did not feel that they meet the patient’s expectations. The reports issued from this department are currently meeting professional guidelines (Association for Clinical Genetic Science) for turnaround times. However, we constantly strive to improve the efficiency, effectiveness and quality of our tests by introducing new technology and improving laboratory workflow and processes. Additionally, in order to provide more information to patients and raise awareness of the complexities and multi-step processes involved in genetic testing, we have also been involved in a number of ‘patient engagement’ events over the last year. We will continue to increase our patient engagement over the coming year. Several constructive comments and suggestions were received regarding our departmental website and the availability of electronic or emailed reports. These are two areas that we will be targeting for improvement in the coming year: • We aim to have an updated set of disease information pages available on our web site by the end of August 2015. We will also improve links to other areas of the website, in particular accessibility of the price list. • We are investigating the technical and workload implications required to make our Clinical reports available in an electronic format. In the past this has not been possible due to technical constraints; mainly the configuration of our Laboratory Information Management System (LIMS) for encryption requirements. We anticipate that it may take several years before all of our reports are available electronically to all service users but we hope to make significant progress in this area over the coming year. A small number of comments were received about the availability of more interpretative information or general / basic level information about the tests, genes and results. Along with our colleagues in Clinical Genetics we are due to implement a new “Genetics Grand Round”, which will enable local Clinicians to join us each month for presentations of interesting cases. These sessions will focus on a specific disease area or technique each month. We are keen to invite external speakers/attendees to these sessions, so please let us know if you have any interesting cases to share with us or if you would like to be added to our mailing list to be informed about future sessions (oxford.dnalab@nhs.net). More information on the Genetics Grand Round will be available on our departmental website over the next few months. We hope you find this a useful summary and please do not hesitate to contact us with any further suggestions or comments you may have on the services we provide. Yours sincerely Karen McGuire Quality Manager for Genetics