CER MCO Principles and Natalizumab case study AcademyHealth, Boston MA
advertisement

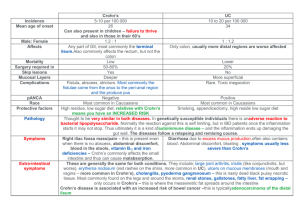
CER MCO Principles and Natalizumab case study AcademyHealth, Boston MA Tuesday June 29th, 2010 Fadia T. Shaya, PhD, MPH Center on Drugs and Public Policy University of Maryland School of Pharmacy fshaya@rx.umaryland.edu Objectives Payers’ considerations in CER CASE STUDY: Multiple Sclerosis / Crohn’s Disease clinical overview Health Related Quality of Life Cost-Effectiveness Analysis Formularyy Recommendations MCO survey Planning your OR study Adopt a perspective S Specify if the th disease di or condition diti Select relevant alternatives for comparison Define specific population Determine time horizon List all consequences related to the treatment alternatives for the patient populations of interest ECHO model Consequences: any effect related to the alternatives modeled Clinical intermediary: Measurements of a patient’s physical or biomedical status used d as a surrogate t for f or to t infer i f the th degree d off disease di (BP, (BP FEV) Clinical outcomes: Medical events that occur as a result of disease or treatment (stroke, disability, hospitalization) Humanistic intermediary: Factors that affect the formation of patients’ opinions about the effects of disease or treatment on their lives and wellbeing (perceptions) Humanistic outcomes: Patient self-assessment of the impact of disease or treatment on their lives and well-being g ((HRQoL,, PROs)) Costs: Direct medical, direct nonmedical, and indirect costs related to treatments Economic outcomes: Direct and indirect costs compared to consequences of medical treatment alternatives (CBA, CEA, CMA) Treatment modifiers: Factors that may alter intermediaries or outcomes associated with ith treatment t t t alternatives lt ti (side ( id effects, ff t compliance) li ) Factors that Affect Insurer D i i Decisions Consumer Co su e e expectations pectat o s DTC advertising Politics and public image Safety Efficacy Productivity, y satisfaction and QOL Effectiveness Acquisition cost Physician support FORMULARY DECISION Cost--effectiveness Cost HEDIS and NCQA Regulatory Issues Disease management programs Budget Impact PBM, physician and pharmacist contracts Discounts and Rebates BCBS: an Example Conduct ~20 to 25 new assessments of drugs, devices/ other technologies each year Generally y focus on clinical effectiveness rather than cost-effectiveness Have own criteria to evaluate drugs, devices, procedures and biological p g p products to improve p health outcomes Five Criteria by BCBS The technology must have final approval from the appropriate governmental regulatory bodies The scientific evidence must permit conclusions concerning the effect of the t h l technology on h health lth outcomes t The technology must improve net health The technology must be as beneficial as any established alternative The improvement must be attainable outside investigational settings Tysabri (natalizumab) Indication MS: A monotherapy treatment for patients with relapsing forms of multiple sclerosis who have had an inadequate response to or are unable to tolerate alternative therapies. Dosage: 300 mg/15 mL (20 mg/mL) IV every 4 weeks CD: CD: Inducing and maintaining clinical response and remission in adult patients with moderately to severely active Crohn’s disease with evidence of inflammation who have had an inadequate response to, or are unable to tolerate, conventional therapies and inhibitors of TNF-α. Safety Contraindications Tysabri is contraindicated in patients ti t allergic ll i to t natalizumab t li b or murine proteins. Precautions Tysabri® induces increases in circulating lymphocytes, monocytes, eosinophils, basophils, and nucleated red blood cells cells. These effects are reversible, usually within 16 weeks after the y induces mild last dose. Tysabri® decreases in hemoglobin levels that are frequently transient. Drug Interactions Concurrent use with i immunomodulators, d l t immunosuppressants, and antineoplastics may further increase the risk of PML and other opportunistic infections, and should not ordinarily be treated with Tysabri®. Adverse Events Headache Pain in extremity Fatigue Rash UTI Abdominal Abdominal Depression discomfort Arthralgia Gastorenteritis Lower Respiratory Tract Infection Vaginitis Diarrhea Di h TOUCH Prescribing Program Due to Tysabri’s® risk of PML, distribution is tightly regulated by Biogen Idec’s Tysabri® Outreach: Unified Commitment to Health (TOUCH™) Prescribing Program Program. The TOUCH™ program requires prescribers to: Educate patients on the risks and benefits before beginning treatment. treatment Report serious opportunistic and atypical infections. Evaluate the patient 3 months after the first infusion, 6 months after the first infusion infusion, and 6 months thereafter thereafter. Determine every 6 months whether treatment should be continued and if so reauthorize treatment. Submit it Biogen Idec the Reauthorization questionnaire and Tysabri Patient Status Report every 6 months. Multiple Sclerosis (MS) Multiple Sclerosis is an autoimmune disease affecting the autoimmune system. Prevalence: 2-150 p per 100,000 , Multiple Sclerosis is not a curable disease Only can delay disability progress, prevent new attacks T Tysabri b i iis only l iindicated di t d ffor R Relapsing/Remitting l i /R itti Multiple Sclerosis (RRMS) AFFIRM Drug Regimen n/Demographics Design End Points Results/Comments 1.NAT 300 mg IV every 4 weeks NAT 627 Placebo: 315 RCT, DB, PC, PG, MC Primary: -Cumulative probability of sustained disability progression (EDSS) _____ Placebo NAT 300 Cumulative probabilty 0.77 0.43 Secondary: -Annualized relapse rate at 2 years -New/growing lesions - Adverse events - Progression of disability after 2 years (MSFC) Adverse Events 2. Placebo Duration: 116 weeks Inclusion: -Age: 18-50 -RMS -EDSS ≤ 5 - 1 relapse previous 12 months Exclusion: -Progressive MS -Unstabilized relapse -Recent treatment with ith mostt other th therapies Probability of relapse 56% 28% MSFC -0.04 0.09 Lesions T2 Gd+ T1 Insignificant difference 6.1 1.2 - 1.2 0.1 76% reduction All results p<0 p<0.001 001 except MSFC MSFC, p≤0.005 NAT = natalizumab, IV = intravenous, RMS = relapsing multiple sclerosis, EDSS = expanded disability status scale, MS = multiple lti l sclerosis, l i MSFC = multiple lti l sclerosis l i functional f ti l composite, it RCT = randomized d i d controlled t ll d ttrial, i l DB = d double-blind, bl bli d PC = placebo-controlled, PG = parallel-group, MC = multicentered, p = probability value, Gd+ = gadolinium enhanced Polman CH, O’Conner PW, Hardova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899-910 Safety Evaluation Study Drug Regimen n/Demographics Design Results/Comments 1.NAT 300 mg IV n = 3116 Safety evaluation -No new cases of PML confirmed -44 cases referred to IAC -43 cases ruled out -1 case indeterminate due to lack off follow-up f -3 previous cases of PML (2 in MS), fulfilled diagnostic criteria for PML -Estimated incidence of PML with NAT: 1 case per 1 1,000 000 patients i (95% CI CI: 0 0.2, 2 2 2.8 8 per 1000) with a mean of 17.9 monthly doses 2. Placebo Duration: Mean of 17.9 months th Inclusion: -Patients who were previously in a NAT clinical trial (including placebo) Exclusion: - Patients who had a remote exposure to NAT. NAT = natalizumab, IV = intravenous, PML = progressive multifocal encephalopathy, IAC = Industry Advisory Council, MS = Multiple Sclerosis, Sclerosis CI = Confidence Interval Yousry TA, Habil M, Major EO, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med. 2006;354(9):924-933. Clinical Trials Summary - MS Probability of disease progression decreased with natalizumab Decreased relapse rate with natalizumab Decreased development and growth of lesions with natalizumab Comparable adverse events Risk of PML exists with natalizumab, but can be limited by TOUCH Prescribing Program Crohn’s Disease (CD) Crohn’s Disease is an inflammatory disease of the digestive system system. Autoimmune Affects 400,000-600,000 people in North America Tysabri is indicated for inducing/maintaining clinical response and remission in adult patients with moderately to severely active Crohn’s disease. Decision Tree – Crohn’s Disease ENABLE (CD1) Drug Regimen n/Demographics Design End Points Results/Comments 1.NAT 300 mg IV every 4 weeks (0,4,8, followed at 12) NAT 724 Placebo: 181 RCT, DB, PC Primary: - ≥70 point decrease in C CDAI score at week 10 _____ Primary* 2. Placebo Exclusion: -Various bowel conditions -TNF TNF iinhibitor hibi therapy h within 12 weeks Duration: 12 weeks Inclusion: -Age ≥ 18 -6 month history of CD -Moderate/severe CDAI (score ≥220 and ≤450) Placebo 49% S Secondary** ** 30% % NAT 300 56% 37% % Secondary: - <150 150 CDAI score (disease remission) *p = 0.05 **p = 0.12 NAT = natalizumab, IV = intravenous, CD = Crohn’s Disease, CDAI = Crohn’s Disease Activity Index, TNF = tumor necrosis f t RCT = randomized factor, d i d controlled t ll d ttrial, i l DB = d double-blind, bl bli d PC = placebo-controlled, l b t ll d p = probability b bilit value l Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353(18):1912-1925. ENABLE (CD3) Drug Regimen n/Demographics Design End Points Results/Comments 1.NAT 300 mg IV every 4 weeks (12-56, followed at 60) NAT: 168 Placebo: 171 RCT, DB, PC Primary: - Clinical response in study CD1 C sustained through week 36 _____ Primary* 2. Placebo Duration: 48 weeks Inclusion: - ≥70 point decrease in CDAI score at week 10 in CD1 Exclusion: - N/A Placebo 28% S Secondary** ** 26% % NAT 300 61% 44% % Secondary: S d - <150 CDAI score (disease remission) *p < 0.001 **p = 0.003 NAT = natalizumab, IV = intravenous, CDAI = Crohn’s Disease Activity Index, CD = Crohn’s Disease, RCT = randomized controlled trial, DB = double-blind, PC = placebo-controlled, p = probability value Sandborn WJ, Colombel JF, Enns R, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353(18):1912-1925. ENABLE Extension (CD2) Drug Regimen n/Demographics Design End Points Results/Comments 1.NAT 300 mg IV every 4 weeks (0,4,8,12) NAT: 259 Placebo: 250 RCT, DB, PC, PG, MC Primary: - ≥70 point decrease in C CDAI score at weeks 8 and 12 _____ Primary* 2. Placebo Duration: 12 weeks Inclusion: -Age ≥ 18 -6 month history of CD -Moderate/severe CDAI (score ≥220 and ≤450) -Elevated CRP (>2.87 mg/mL) Placebo 32% S Secondary** ** 16% % Secondary: -<150 150 CDAI score (disease remission) at weeks 8 and 12 NAT 300 48% 26% % Exclusion: - Various bowel *p < 0.001 conditions - TNF inhibitor therapy **p = 0.002 within 12 weeks NAT = natalizumab natalizumab, IV = intravenous intravenous, CD = Crohn’s Crohn s Disease Disease, CDAI = Crohn’s Crohn s Disease Activity Index Index, CRP = C C-reactive reactive protein, TNF = tumor necrosis factor, RCT = randomized controlled trial, DB = double-blind, PC = placebo-controlled, PG = parallel-group, MC = multicentered, p = probability value Targan SR, Feagan BG, Fedorak RN, et al. Natalizumab for the treatment of active Crohn's disease: results of the ENCORE Trial. Gastroenterology 2007;132(5):1672-1683. Clinical Trials Summary - CD Natalizumab not effective vs placebo d i fi during firstt 10 weeks k off ttreatment t t Effective beyond y 10 weeks Natalizumab effective during first 10 weeks of treatment when CRP is elevated Importance of HRQoL Patients with CD and MS often have lower QoL than the general population population. Available therapeutic options are costly. Evidence for humanistic outcomes is of interest because it shows additional value that clinical outcomes do not provide. Health H lth Quality Q lit off Life Lif for f C Crohn’s h ’ Disease Feagan et al. used disease-specific (IBDQ) and generic health (SF-36 (SF 36, EQ-5D EQ 5D, Subject Global Assessment) quality of life assessments to determine natalizumab's humanistic value in CD. The variety of assessments utilized offset the modest patient enrollment. Key finding: substantial improvements on HRQ L for HRQoL f patients ti t who h responded d d to t initial i iti l induction therapy and continued therapy for an additional 48 weeks of treatment treatment. Health H lth Quality Q lit off Life Lif for f M Multiple lti l Sclerosis Rudick et al. lacked variety in their assessmentt tools. t l High g p patient enrollment strengthened g the applicability of the results to clinical practice. Key finding: natalizumab treatment was shown h tto greatly tl iimprove th the HRQ HRQoL L ffor MS patients. COST-EFFECTIVENESS ANALYSIS HEALTH ECONOMIC MODEL(MS) from Managed Care Dossier: TYSABRI. New York, NY: Biogen Idec, 2008. COST-EFFECTIVENESS COST EFFECTIVENESS ANALYSIS(MS) Results in ICER Ratios $48,921 Managed Care and CER A Pilot Survey of MCOs How familiar are you with the concept i Eff i off C Comparative Effectiveness Research (CER)? 60.00 50.00 40.00 30.00 20.00 10.00 0.00 (1) Not at all (2) Minimal (3) Somewhat (4) Moderate (5) Very much How often do y you use CER to assess health outcomes? 60.00 50.00 40.00 30.00 20.00 10.00 0.00 (1) Not at all (2) Occasionally (3) Sometimes (4) Often (5) Always T what h extent d d CER for f To do you need your decision making? 60.00 50.00 40.00 30.00 20.00 10.00 0.00 (1) Not at all (2) Minimal (3) Somewhat (4) Moderate (5) Very much How does CER impact your decision on p y formulary lists? 60.00 50.00 40.00 30.00 20.00 10.00 0.00 (1) Not at all (2) Minimal (3) Somewhat (4) Moderate (5) Very much D you use CER iin your decisions d i i Do on formulary lists? 60.00 50.00 40.00 30.00 20.00 10.00 0.00 (1) Not at all (2) Minimal (3) Somewhat (4) Moderate (5) Very much In which of the following areas do you d CER most relevant l regard to your decision-making? 50.00 45 00 45.00 40.00 35.00 30.00 25.00 20 00 20.00 15.00 10.00 5.00 0.00 (1) Prescription drugs (2) Specialty drugs (3) Medical devices (4) Others Do you conduct your own CER? 60.00 50.00 40.00 30.00 20.00 10.00 0.00 (1) Not at all (2) Minimal (3) Somewhat (4) Moderate (5) Very much D you consider id using i i ValueV l Do CER in Based Benefit Design? 60.00 50.00 40.00 30.00 20.00 10.00 0.00 (1) Not at all (2) Minimal (3) Somewhat (4) Moderate (5) Very much H i i d How many participants are covered under a plan of your company? 45.00 40.00 35.00 30.00 25.00 20.00 15.00 10.00 5 00 5.00 0.00 (1) Less than 500,000 (2) 500,0001,000,000 (3) 1,000,0003,000,000 (4) 3,000,0005,000,000 (5) more than 5,000,000 References Tysabri® (natalizumab) [package insert]. Cambridge, MA: Biogen Idec, Inc; 2008. Managed Care Dossier: Tysabri® (Multiple Sclerosis) Sclerosis). Wellesley, MA: Biogen Idec, Inc., 2008. Managed Care Dossier: Tysabri® (Crohn’s Disease). Wellesley MA: Biogen Idec Wellesley, Idec, Inc Inc., 2008 2008. Kobelt G, Berg J, Lindgren et al. Modeling the costeffectiveness of a new treatment for MS ( natalizumab) compared p with current standard p practice in Sweden,, Multiple p Sclerosis: 2008; 14:679-690. Rudick RA, Miller D, Hass S, Hutchinson M, Calabresi PA, et al. Health-related Quality of Life in Multiple Sclerosis: Effects of N t li Natalizumab. b Ann A Neurol. N l 2007 O Oct;62(4):335-46. t 62(4) 335 46