Preparation parameters governing microstructure and grain
advertisement

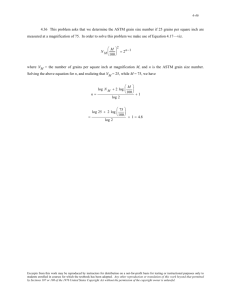
Physica C 295 Ž1998. 39–46 Preparation parameters governing microstructure and grain coupling of Bi 2 Sr2 CaCu 2 O xrAg tapes prepared by partial melting Ryoji Funahashi a b a,) , Ichiro Matsubara a , Hiroshi Ohashi b, Kazuo Ueno a , Hiroshi Ishikawa a Osaka National Research Institute, AIST, Midorigaoka, Ikeda, Osaka 563, Japan Osaka Electro-Communication UniÕersity, Hatcho, Neyagawa, Osaka 572, Japan Received 3 March 1997; revised 4 June 1997; accepted 4 July 1997 Abstract Bi 2 Sr2 CaCu 2 O x ŽBi-2212. tapes have been prepared using the isothermal partial melting ŽIPM. method, in which partial melting and solidification are carried out at the same temperature but in different atmospheres. Maximum critical current density Ž Jc . at 4.2 K under 0 T is 3.0 = 10 5 Arcm2 and 1.9 = 10 5 Arcm2 for the tapes prepared at 8658C and 8308C, respectively. This difference in Jc is attributed to Bi-2212 grain size, which is larger in the former tape than the latter. Large grains and a rough distribution of solid phases ŽBi-free and Cu-free phases. in a partially melted state lead to large Bi-2212 grains. Amount of impurities decreases and grain coupling becomes stronger with increasing solidification time Ž ts .. Jc is saturated in tapes solidified for more than a certain time, the length in which is dependent on melt processing temperature. More time is needed to achieve saturated Jc in tapes melted at higher temperature, because it takes longer for the large solid grains to react completely with a liquid phase in the partially melted state. It is clear that Bi-2212 grain size can be controlled by melt processing temperature, and that both amount of impurities and grain coupling strength can be controlled by solidification time. q 1998 Elsevier Science B.V. Keywords: Bi 2 Sr2 CaCu 2 O xrAg tapes; Isothermal partial melting method; Preparation parameter; Microstructure; Grain coupling 1. Introduction There have been many studies on the power applications of superconducting oxides, especially those of Bi–Sr–Ca–Cu–O and Y–Ba–Cu–O superconductors, some of which have reported the successful preparation of Bi 2 Sr 2 CaCu 2 O x ŽBi2212.rAg tapes w1–3x with critical current density ) Corresponding author. Tel.: q81 727 519541; Fax: q81 727 519622; E-mail: fune@onri.go.jp Ž Jc . of more than 10 4 Arcm2 at 77 K under zero magnetic field w1x. However, since the superconducting transition temperature of the Bi-2212 phase is close to 77 K and there are few effective pinning centers over 20 K, the power applications of Bi-2212 tapes under magnetic fields are restricted to low temperatures. On the other hand, the upper critical field Ž Hc2 . of such tapes is very high Ž) 20 T. at 4.2 K, which is favorable for application under high magnetic fields. Bi-2212 superconducting magnets which generate a magnetic field of up to about 3 T at 4.2 K have been produced w4,5x. 0921-4534r98r$19.00 q 1998 Elsevier Science B.V. All rights reserved. PII S 0 9 2 1 - 4 5 3 4 Ž 9 7 . 0 1 5 9 0 - 6 40 R. Funahashi et al.r Physica C 295 (1998) 39–46 Most Bi-2212 tapes with high Jc are prepared by partial melting. Since the Bi-2212 phase is an incongruent melting system, a liquid phase with a Bi-rich composition and solid phases with compositions of Sr–Ca–Cu–O ŽBi-free phase. and Bi–Sr–Ca–O ŽCu-free phase. are formed in the partially melted state w2,6,7x. The Bi-2212 phase is precipitated and grows by peritectic reaction between the liquid and the solid phases w8x. If the peritectic reaction is not completed, impurities such as the Bi-free phase, the Cu-free phase, and the Bi 2 Sr2 CuO x ŽBi-2201. phase originating in the liquid phase, remain in the tapes and depress Jc . Moreover, because Bi-2212 tapes are polycrystalline ceramics, Jc is also determined by grain size and grain coupling strength at grain boundaries. That is to say, for high Jc , control of microstructure and grain coupling strength are important. Under the conventional partial melting method, because the Bi-2212 grains are solidified by slow cooling, the optimal temperature range for the solidification reaction of Bi-2212 grains is maintained for only a short time. Bi-2201, Bi-free and Cu-free phases therefore remain as impurities. In this study, Bi-2212 tapes have been prepared using an isothermal partial melting ŽIPM. method to reduce the amount of impurities w9,10x. Since solidification is carried out by controlling atmospheres at a constant temperature, the optimal temperature range for solidification of Bi-2212 grains can be maintained indefinitely. At a given atmosphere, this method involves only two preparation parameters, melt pro- cessing temperature and solidification time. The effect of these parameters on microstructure and grain coupling of Bi-2212 tapes can therefore be observed independently in this method. This paper investigates that which of the preparation parameters of the IPM method governs Bi-2212 grain size, amount of impurities, and grain coupling strength in Bi-2212 tapes. 2. Experimental Bi-2212 superconducting tapes were prepared using the IPM method, in which partial melting and solidification were performed at the same temperature but in different atmospheres w10x. This method makes use of changes in the melting point of the Bi-2212 phase accompanying change in oxygen partial pressure Ž pO 2 .. The composition of the precursor Bi-2212 powder was Bi 2.0 Sr2.4 Ca 0.7 Cu 2.0 O x . The melting points of this powder measured using differential thermal analysis ŽDTA. were 8108C in an N2 atmosphere Ž pO 2 s 0%. and 8708C in pO 2 s 20%. The Bi-2212 powder was suspended in an organic binder and the suspension deposited on an Ag sheet Ž150 mm thick.. After evaporation of the binder, another Ag sheet was placed over the deposit. The specimens were pressed to densify under 240 MPa at room temperature. The green tapes were heated at 5008C in air in order to eliminate the organic binder completely, then partially melted at 8308C or 8658C for 5 min in an N2 gas flow Žpurity: 5 N, O 2 Fig. 1. SEM photographs of tapes solidified at 8308C Ža. and 8658C Žb. for 24 h. R. Funahashi et al.r Physica C 295 (1998) 39–46 41 mined by X-ray diffraction ŽXRD, Cu K a source. and electron probe micro-analysis ŽEPMA.. Microstructural observation was carried out using a scanning electron microscope ŽSEM.. For observation of the partially melted microstructure, tapes partially melted for 5 min in an N2 gas flow were quenched in water. In order to estimate grain coupling strength, AC susceptibility was employed using a SQUID magnetometer ŽQuantum Design.. 3. Results and discussion 3.1. Bi-2212 grain size Fig. 2. ts dependence of average Bi-2212 grain size for tapes solidified at 8308C Ž`. and 8658C ŽI.. contamination - 5 ppm.. Solidification was subsequently carried out at the same temperature in pO 2 s 20% Žbalanced with N2 gas. for 1–36 h Žsolidification time: ts .. After heat treatment, the tapes were allowed to cool down to 7508C in the furnace and then removed. Zero resistivity temperature ŽTc,zero . and Jc were measured using a standard four-probe method. Jc measurement was performed at 4.2 K under applied magnetic fields of 0–8 T. The criterion for the determination of Jc was 1.0 mVrcm. Composition and amount of impurities in the tapes were deter- SEM photographs of the tapes solidified at 8308C Ža. and 8658C Žb. for 24 h are shown in Fig. 1. Average Bi-2212 grain size is about 65 mm and 130 mm for the tapes prepared at 8308C and 8658C, respectively, and is independent of ts for the tapes treated at 8308C with ts G 3 h and at 8658C with ts G 12 h ŽFig. 2.. Holesinger et al. w9x have also reported that Bi-2212 grain size increases with melt processing temperature. The Bi-2212 phase is solidified by peritectic reaction between the solid Bi-free or Cu-free phases and the liquid Bi-rich phase formed by partial melting. Microstructural observation in the partially melted state is therefore indispensable to discuss the Bi-2212 grain size. Fig. 3 shows back scattering images for the tapes quenched from the partially melted state at 8308C Ža. Fig. 3. Back scattering images for tapes quenched from partially melted state at 8308C Ža. and 8658C Žb.. R. Funahashi et al.r Physica C 295 (1998) 39–46 42 and 8658C Žb.. The Bi-2212 phase melts incongruently so that the solid Bi-free and Cu-free phases and the liquid Bi-rich phase are formed. The dark spots in Fig. 3 correspond to the solid phases. ŽSr,Ca.CuO 2 and ŽSr,Ca. 2 CuO 3 as the Bi-free phases and Bi 2 ŽSr,Ca. 3 O6 as the Cu-free phase are observed in the tapes melted at both 8308C and 8658C. It is clear that the grains of the solid phases in the tape melted at 8658C are larger and more roughly dispersed than in the tape melted at 8308C. Since the grain size of the solid phases is smaller in the tape melted at 8308C, peritectic reaction is completed earlier. As the result, zero resistivity is not observed in the tape treated at 8658C with ts - 6 h, but is noted at 85 K in the tape solidified at 8308C even for 1 h. However, the large grains and the rough dispersion of the solid phases lead to larger Bi-2212 grains, because the grain growth of the Bi-2212 phase progresses peritectically at the edge of Bi-2212 grains through supply of cations from the solid Bi-free or Cu-free phases. It is clear from the above results that Bi-2212 grain size can be controlled by melt processing temperature, with which it increases. 3.2. Amount of impurities A diffraction peak of the Bi-free phase, ŽSr,Ca.CuO 2 , appears around 2 u s 35.68 in the tapes prepared at 8658C ŽFig. 4.. The intensity of this peak Fig. 4. XRD patterns for tapes solidified at 8658C. Fig. 5. Back scattering image for tape solidified at 8658C with ts s12. in the tapes with ts of 12 and 18 h is greater than in the tapes with ts of 24 and 36 h. This result indicates that the amount of Bi-free phase remaining in the former is larger than in the latter. Bi-free grains can be observed in back scattering images ŽFig. 5. in all tapes prepared at 8658C. Bi-free grain size decreases with increasing ts . Fig. 6 shows the ts dependence of average Bi-free grain size for the tapes prepared at 8658C. Average Bi-free grain size rapidly decreases in 18 - ts - 24 h. This indicates that the amount of impurities can be controlled by ts . Bi-free grains are surrounded by the solid Bi-2212 phase during the solidification stage ŽFig. 5.. Sr, Ca and Cu ions have to diffuse through the solid Bi-2212 phase to react with the liquid phase at the edge of the Bi-2212 grains. In consequence, it takes a longer time to obtain large Bi-2212 grains. No Bi-free grains are found in the tapes solidified at 8308C for 3 h, because the peritectic reaction is almost completed. The value of ts at which the peritectic reaction is completed depends on melt processing temperature and becomes longer with increasing grain size of the solid phases in the partially melted state. Because of the peritectic reaction, the amount of remaining Bi-2201 phase is proportional to the amount of Bi-free phase remaining in the tapes. Diffraction peaks due to the Bi-2201 phase are so weak in XRD patterns that the ts dependence of the amount of Bi-2201 phase is not clear. However, the amount of Bi-2201 phase seems to decrease with R. Funahashi et al.r Physica C 295 (1998) 39–46 Fig. 6. ts dependence of average Bi-free grain size for tapes solidified at 8658C. increasing ts . As mentioned above, Bi-2212 grains are surrounded by the liquid phase. The Bi-2201 phase originating in the liquid phase is present at the grain boundaries and makes the grain coupling weak. The relationship between the amount of impurities and the grain coupling strength at the grain boundaries is dealt with in Section 3.3. 3.3. Grain coupling strength Jc at 4.2 K under 0 T and transport superconducting transition width, DTc , depend on ts ŽFig. 7.. It is possible to classify tapes into two groups with borderlines at 18 - ts - 24 h and at 1 - ts - 3 h for the tapes treated at 8658C and 8308C, respectively. These 43 values of ts correspond with those beyond which impurities decrease rapidly. Transition width reflects grain coupling strength w11x. A wide transition indicates that grain coupling is weak at the grain boundaries. Because grain coupling is weak in the tapes with ts of 12 and 18 h at 8658C or with ts of 1 h at 8308C, which have large DTc , Jc for these tapes is lower than for tapes solidified for a longer time. This finding regarding weak coupling is supported by AC susceptibility measurement. Fig. 8 shows temperature dependence of AC susceptibility. A broad peak is observed in x Y for all tapes. This peak is due to AC field penetration into the grain boundaries w12–14x. With increasing ts peak temperature rises and magnetic superconducting transition in x X becomes sharper. Lower peak temperature is observed in tapes with weaker grain coupling. The x Y peak shifts downward in temperature with increasing AC amplitude, h ŽFig. 9.. Peak temperature decreases rapidly with increasing h in tapes affected seriously by weak grain coupling w12– 14x. This indicates conversely that grain coupling becomes strong with increasing ts . It is clear that grain coupling strength can be controlled by ts . As mentioned above, because the Bi-2212 phase grows by peritectic reaction, the amount of remaining Bifree and Cu-free phases is proportional to the amount of liquid phase Žpreceding Bi-2201 phase. remaining at the grain boundaries. Although more detailed experiments regarding location and amount of the Bi2201 phase are necessary, grain coupling is thought to be weakened by the Bi-2201 phase. That is to say, tapes including a large amount of Bi-free phase are affected seriously by weak coupling and their Jc is Fig. 7. ts dependence of Jc at 4.2 K under 0 T ŽI. and DTc Ž`. for tapes solidified at 8308C Ža. and 8658C Žb.. 44 R. Funahashi et al.r Physica C 295 (1998) 39–46 Fig. 8. Temperature dependence of AC susceptibility for tapes solidified at 8658C for 12 h Ž`., 18 h ŽI. and 36 h Ž^.. Temperature is normalized by magnetic transition onset temperaX ture ŽTc,mag . and susceptibility by absolute value of x at 5 K for each tape. limited to low values. It is reported that large tilt angle grain boundaries also make the grain coupling weak w15,16x. In this study, however, it is clear from XRD measurement that the c-axis alignment is comparable in the tapes treated at 8658C with ts ) 12 h. Therefore, the grain boundary angle seems to change little with ts . Because the h dependence of the peak temperature of x Y is almost the same in the tapes solidified for 24 h at 8658C and 8308C, grain coupling strength is comparable in such tapes. The difference in Bi2212 grain size causes a difference in Jc , which is 3.0 = 10 5 Arcm2 and 1.9 = 10 5 Arcm2 at 4.2 K under 0 T for the tapes solidified for 24 h at 8658C and 8308C, respectively. The increase in Jc is due to a decrease with increasing Bi-2212 grain size in the number of grain boundaries through which superconducting current flows. A schematic model of the solidification of the Bi-2212 phase is shown in Fig. 10. First, the solid Bi-free and Cu-free phases and the liquid phase are formed in the partially melted state by incongruent melting of the Bi-2212 phase. The composition of the Bi-free and Cu-free phases depends on melt processing temperature w17,18x. In this study, howev er, Ž S r,C a . C u O 2 , Ž S r,C a . 2 C u O 3 , an d Bi 2 ŽSr,Ca. 3 O6 are observed as solid phases in the tapes melted and quenched at both 8308C and 8658C. The grain size of the solid phases increases with melt processing temperature. After heat treatment, the larger solid grains lead to larger Bi-2212 grains. Solidification starts with increasing pO 2 , and the Bi-2212 phase is precipitated and grows by peritectic reaction. In the first stage of peritectic reaction, a product of the reaction is precipitated between the liquid and the solid phases w8,19x. Fig. 11 is a back scattering image for the tape solidified at 8658C for 6 h. The Bi-2212 phase is precipitated between the Bi-free solid phase and the Bi-2201 phase originating in the liquid phase. This photograph shows the first stage of peritectic reaction w8x. Starting time of the first stage of the peritectic reaction after increasing pO 2 is thought to depend on the composition of the solid phases. For the growth of the Bi-2212 grains, Sr, Ca and Cu ions diffuse through the solid Bi-2212 phase to react with the liquid phase at the edge of the Bi-2212 grains. Before the completion of peritectic reaction, the Bi-free, the Cu-free, and the liquid phases remain. In particular, the liquid phase which precedes the Bi-2201 phase is present at the grain boundaries and makes grain coupling weak. Even a small amount of Bi-2201 phase, which produces very weak diffraction peaks in XRD, affects Y Fig. 9. h dependence of peak temperature of x normalized by Tc,mag for tapes solidified at 8658C for 12 h Ž`., 18 h ŽI., 24 h Ž^. and 36 h Že.. R. Funahashi et al.r Physica C 295 (1998) 39–46 45 grain coupling. The completion time of peritectic reaction depends on the grain size of the Bi-free and the Cu-free phases in the partially melted state. A long time is needed for the large solid grains to react with the liquid phase completely. After the completion of peritectic reaction, impurities disappear and grain coupling becomes strong. In this study, the Bi-free grains are observed in the tape solidified at 8658C even for 36 h. It seems that the composition of the precursor powder has to be optimized to remove Bi-free grains completely. 4. Conclusion Fig. 10. Schematic model of solidification of Bi-2212 phase. Bi-2212 tapes have been prepared using the IPM method, in which partial melting and solidification are carried out at the same temperature but in different atmospheres. Maximum Jc is 3.0 = 10 5 Arcm2 and 1.9 = 10 5 Arcm2 for the tapes prepared at 8658C and 8308C, respectively. This difference in Jc is attributed to microstructural difference, i.e. Bi-2212 grains are larger in the former tape than the latter. It is clear that large grains and rough distribution of solid phases in the partially melted state lead to larger Bi-2212 grains. Bi-2212 grain size can be controlled by melt processing temperature while both amount of impurities and grain coupling strength can be controlled by ts . In order to obtain tapes with high Jc , melt processing temperature should be as high as possible and solidification time as long as possible. References Fig. 11. Back scattering image for tape solidified at 8658C with ts s6 h. w1x J. Kase, K. Togano, H. Kumakura, D.R. Dietderich, N. Irisawa, T. Morimoto, H. Maeda, Jpn. J. Appl. Phys. 29 Ž1990. L1096. w2x T. Kanai, N. Inoue, T. Kamo, J. Mater. Res. 9 Ž1994. 1363. w3x H. Kumakura, K. Togano, J. Kase, T. Morimoto, H. Maeda, Cryogenics 30 Ž1990. 919. w4x N. Tomita, M. Arai, E. Yanagisawa, T. Morimoto, H. Kitaguchi, H. Kumakura, K. Togano, T. Kiyoshi, K. Inoue, H. Maeda, K. Nomura, J.C. Vallier, IEEE Trans. Appl. Supercond. 5 Ž1995. 520. w5x M. Okada, K. Tanaka, N. Inoue, J. Sato, H. Kitaguchi, H. Kumakura, T. Kiyoshi, K. Inoue, K. Togano, Jpn. J. Appl. Phys. 34 Ž1995. L981. 46 R. Funahashi et al.r Physica C 295 (1998) 39–46 w6x W. Zhang, E.E. Hellstrom, Physica C 218 Ž1993. 141. w7x R.D. Ray II, P.A. Smith, E.A. Olsen, Physica C 251 Ž1995. 1. w8x M.J. Cima, X.P. Jiang, H.M. Chow, J.S. Haggerty, M.C. Flemings, H.D. Brody, R.A. Laudise, D.W. Johnson, J. Mater. Res. 5 Ž1990. 1834. w9x T.G. Holesinger, D.S. Phillips, J.Y. Coulter, J.O. Willis, D.E. Peterson, Physica C 243 Ž1995. 93. w10x R. Funahashi, I. Matsubara, T. Ogura, K. Ueno, H. Ishikawa, Physica C 273 Ž1997. 337. w11x D. Goldschmidt, Phys. Rev. B 39 Ž1989. 2372. w12x H. Kupfer, S.M. Green, C. Jiang, Y. Mei, H.L. Luo, R. Meier-Hirmer, C. Politis, Z. Phys. B-Condens. Matter 71 Ž1988. 63. w13x A.K. Sarkar, I. Maartense, T.L. Peterson, B. Kumar, J. Appl. Phys. 66 Ž1989. 3717. w14x S. Ravi, V. Seshu Bai, Phys. Rev. B 49 Ž1994. 13082. w15x M. Kawasaki, E. Sarnelli, P. Chaudhari, A. Gupta, A. Kussmaul, J. Lacey, W. Lee, Appl. Phys. Lett. 62 Ž1993. 417. w16x B. Soylu, N. Adamopoulos, D.M. Glowacka, J.E. Evetts, Appl. Phys. Lett. 60 Ž1992. 3183. w17x J. Polonka, M. Xu, Q. Li, A.I. Goldman, D.K. Finnemore, Appl. Phys. Lett. 59 Ž1991. 3640. w18x T. Hasegawa, H. Kobayashi, H. Kumakura, H. Kitaguchi, K. Togano, Physica C 222 Ž1994. 111. w19x H. Fredriksson, T. Nylen, Met. Sci. 16 Ž1982. 283.