CE 326 – Introduction to Environmental Engineering
advertisement

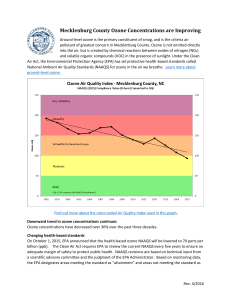
CE 326 – Introduction to Environmental Engineering Power Plant Field Trip and Air Pollution In-Class Assignment Alternative Homework As an alternative to either the power plant field trip or the in-class assignment, you may answer the following: 1) Convert 650 ppmv sulfur dioxide (SO2) to µg/m3. Assume standard temperature and pressure. 2) Determine the partial pressure of 500 µg/m3 carbon monoxide (CO) in a 10-L cylinder of helium gas at 20ºC and 2000 psi (1 psi = 6894.76 Pa) 3) A coal-fired power plant is emitting NO2 at a concentration of 300 ppmv and a flow rate of 50 m3/s from a 2-m diameter, 35-m tall stack. Assuming that the stack gas is at 200ºC, the atmospheric conditions are class C with a wind speed of 5 m/s to the east, temperature of 20ºC, and atmospheric pressure of 100 kPa, determine the ground level concentration of NO2 at 0.7-km directly east (x) and 0.1-km south (y) of the stack. 4) Go to the following US EPA websites and answer the questions below: http://www.epa.gov/oar/oaqps/greenbk/ http://www.epa.gov/airnow/index.html i) Which primary air pollutant exceeds NAAQS in more AQRs than any other? ii) Name eight states that have no AQRs that exceed NAAQS. iii) Select from the air now site ozone maps, Midwest, June 2002. Look at the June 22 map. Describe the ozone animation throughout the day, when do the concentrations start to increase? When do they decrease? Why do they change like this? iv) Look at the ozone maps for Los Angeles, June 16, 2002. Where would you rather be caught in a traffic jam at 5 pm in San Bernadino, Santa Clarita, or Hollywood? What does very unhealthy (the alert level) mean? Why do ozone concentrations on the coast stay lower than inland? v) What is today’s air quality forecast for Metropolitan Los Angeles? Give the date and forecast.