Infrared absorption cross section, radiative forcing, and GWP of four hydro#uoro(poly)ethers *
advertisement

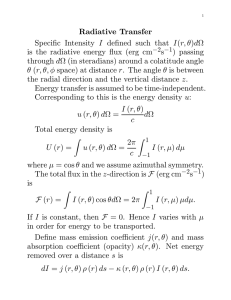
Atmospheric Environment 33 (1999) 4447}4458 Infrared absorption cross section, radiative forcing, and GWP of four hydro#uoro(poly)ethers G. Myhre *, C.J. Nielsen, D.L. Powell, F. Stordal Department of Geophysics, University of Oslo, P.O.Box 1022 Blindern, N-0315 Oslo, Norway Department of Chemistry, University of Oslo, P.O.Box 1033 Blindern, N-0315 Oslo, Norway Received 26 February 1998; received in revised form 30 March 1999; accepted 19 April 1999 Abstract Quantitative infrared cross-sections of the unbranched hydro#uoro(poly)ethers CHF OCHF , CHF OCF OCHF and CHF OCF CF OCHF have been obtained at 298 K in the region 25}4000 cm\. Radiative forcing calculations have been performed for these compounds and for CHF OCF OCF CF OCHF , and the values found per molecule are high compared to those of other CFCs and CFC replacements. Atmospheric lifetimes, calculated on the basis of experimental reaction rates with OH radicals, and global warming potentials are presented for all four compounds. 1999 Elsevier Science Ltd. All rights reserved. 1. Introduction Reduction of the ozone depleting chloro#uorocarbons (CFCs) in accordance with the Montreal Protocol has led to investigations of CFC replacements. Like most of the CFCs, the CFC replacements also absorb thermal infrared radiation and may contribute to an enhanced greenhouse e!ect. Several studies have investigated the climate e!ect of CFC replacements in use or with the potential for use in the future (Fisher et al., 1990; Shi and Fan, 1992; Clerbaux et al., 1993; Pinnock et al., 1995; Imasu et al., 1995; Christidis et al., 1997). The present study focuses on the four simplest linear hydro#uoropolyethers (HFPEs) with the general structure CF HO (CF O) (CF CF O) CF H, that is, CHF OCHF , CHF OCF OCHF , CHF OCF CF OCHF and CHF OCF OCF CF OCHF , later to be abbreviated HG-00, HG-10, HG-01, and HG-11 according to the values of (n, m) in the general structure formula. This class of hydro#uoropolyethers maintain several of the remarkable properties of per#uorinated compounds and can therefore be used in a variety of applications includ- * Corresponding author. Fax: #47-22855269. On leave from the College of Wooster, Wooster, Ohi, USA. E-mail address: gunnar.myhre@geofysikk.uio.no (G. Myhre) ing CFC substitution in the areas of solvents, highperformance #uids, "re suppressants, heat exchange, etc. Qualitative mid infrared spectra including ab initio calculations have been published for HG-10, HG-01 and HG-11 (Radice et al., 1998). Quantitative mid infrared vapour-phase spectra have previously been presented for HG-00 (Imasu et al., 1995; Heath"eld et al. 1998), HG-10 (Tuazon, 1997; Cavalli et al., 1998), HG-01 (Tuazon, 1997; Cavalli et al., 1998), and HG-11 (Tuazon, 1997; Christidis et al., 1997; Cavalli et al., 1998). Quantitative spectra have also been presented for an industrial mixture of HGs having n/m ratio of 1.03, an average molecular weight of 414 and a boiling point ranging from 40 to 1403C (BruK hl et al., 1993). No far infrared spectra of the compounds have so far been published. Reaction rate coe$cients for the OH reactions with the HG compounds have recently been reported (Zhang et al., 1992; Garland et al., 1993; Hsu and DeMore, 1995; DeMore, 1996; Tuazon, 1997; Cavalli et al., 1998). An UV-Visible vapour-phase spectrum at 298 K of an industrial mixture of HGs has also been presented (BruK hl et al., 1993). The radiative forcing concept is often used to compare radiative e!ects of di!erent atmospheric radiatively active components (IPCC 90; 95). Recently, Hansen et al. (1997) have shown that the relationship between radiative forcing and surface temperature changes is reasonably good for known radiatively active components. This 1352-2310/99/$ - see front matter 1999 Elsevier Science Ltd. All rights reserved. PII: S 1 3 5 2 - 2 3 1 0 ( 9 9 ) 0 0 2 0 8 - 3 4448 G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 is based on the assumption that radiative forcing is taken at the tropopause level and that the stratospheric temperature adjustment is taken into account. The experimental conditions and infrared cross-sections in the region 25}3250 cm\ for HG-00, HG-10, and HG-01 are presented in Sections 2 and 3, respectively. Calculations and results for lifetimes of HG-00, HG-10, HG-01, and HG-11 are described in Section 4. In Sections 5 and 6 we describe radiative transfer models used and radiative forcing due to the HGs. The potential climate e!ect of the four HGs is estimated using global warming potentials (GWP) which are presented in Section 7. Finally, a summary is given in Section 8. 2. Experimental The samples of CHF OCHF , CHF OCF OCHF and CHF OCF CF OCHF were obtained from Ausi mont S.p.A. and had stated purities better than 98%. They were used without further puri"cation after degassing and distillation in vacuo. Infrared spectra of the pure gases at room temperature were recorded in the region 25}4000 cm\ using a Bruker IFS 113v employing a nominal resolution of 1 cm\ and a 4P apodization (breakpoint 0.9) of the interferograms. KBr and Mylar威 beamsplitters of 3.5, 6, 12, 24, 50 and 75 lm thickness were used to cover the spectral region. To ensure optical linearity, only DTGS detectors were used. Cells of 10 and 19 cm length equipped with windows of CsI and high-density polyethylene were employed, respectively. The partial pressures of the gasses in the cells were from 0.5 to 90 mbar and were measured using MKS Baratron pressure transducers. The very strong absorption by the C}F stretching vibrations necessitated the use of low partial pressures and calibration procedures involving higher pressures and more reliable pressure measurements were based on the absorption in the well isolated C}H stretching region. On this basis the infrared cross sections are believed to be accurate within $10%. 3. Spectral results The IR spectra of HG-00, HG-10 and HG-01 are shown in Fig. 1. For completeness, Fig. 1 also includes the spectrum of HG-11 (courtesy of Cavalli et al., 1998). The integrated absorption cross sections (base e) are listed in Table 1 and compared to previous results (Imasu et al., 1995; Christidis et al., 1997; Heath"eld et al., 1998; Cavalli et al., 1998). Unfortunately, Tuazon (1997) only presents the spectra (base 10) and not the integrated cross sections, but a comparison between the peak heights of the HG-10 and HG-01 spectra in Fig. 1 with those shown by Tuazon reveals compatible spectral data with our values being slightly lower. Cavalli et al. (1998) used a long path system with a high sensitivity MCT detector. We suggest that some of the di!erences between our results and those of Cavalli et al. (1998) may be caused by non-linearity in their detection system. The bending and torsional modes situated in the far infrared region give rise only to very weak absorptions, Fig. 1. This agrees with results from ab initio calculations (Radice et al., 1998; Marstokk et al., 1999). Current investigations in our laboratory show that nearly all strong absorption bands of the di!erent conformers present in HG compounds overlap, and that only minor di!erences are observed in the region between 800 and 25 cm\ (e.g. Marstokk et al., 1999). Further, the minuscule absorption cross-sections of these modes imply that there will not be any substantial change with temperature relative to the room temperature mid infrared absorption cross section. 4. Lifetimes The UV-Visible absorption cross section of HGs was shown to have its maximum below 200 nm, where it is less than 10\ cm molecule\ dropping to less than 10\ cm molecule\ at wavelengths above 230 nm (BruK hl et al., 1993). Photolysis of the HGs will therefore only be an important removal mechanism in the upper stratosphere. The lifetimes of the four HGs are calculated on the basis of the reaction rates with OH only. Two methods have been used: one assuming the reaction rates with OH to be independent of temperature, the other assuming the same temperature dependence of the OH reaction rates for all four HGs. Table 2 collects the available kinetic data for OH reactions with the HGs. For HG-00 two investigations (Garland et al., 1993; Hsu and DeMore, 1995) report consistent reaction rate coe$cients and we have used an average of their results in our calculations, k (¹)"8.66;10\;exp(!1735/¹) and k " -& 2.6;10\ cm molecule\ s\. With this temperature dependence the reaction rate coe$cient is an order of magnitude smaller at 200 K than at 300 K. For the other three HGs we have used the experimental kinetic results of Cavalli et al. (1998) obtained at 298 K. Laboratory experiments have not been made to identify the temperature dependence of the reaction rates between the OH radical and HG-10, HG-01, and HG-11. However, the reactive parts of the four HG molecules considered are structurally similar, and we have therefore assumed that the OH reaction has the same temperature dependence for all the four HGs. With OH reaction rate coe$cients of these magnitudes, Table 2, the lifetimes are su$ciently long to ensure a relatively homogeneous distribution in the troposphere. Assuming the reaction rate to be spatially invariable (no temperature dependence) the lifetimes may then be G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 4449 Fig. 1. Quantitative vapour-phase infrared spectra 25}3250 cm\ of CHF OCHF (HG-00), CHF OCF OCHF (HG-10), CHF OCF CF OCHF (HG-01) and CHF OCF OCF CF OCHF (HG-11), the latter courtesy of Cavalli et al. (1998). The absorption cross sections are given in units of 10\ cm molecule\ (base e). estimated using averaged OH concentrations in the calculations. Monthly OH values are taken from a threedimensional (3D) chemistry transport model (CTM) (Berntsen and Isaksen, 1997) with a horizontal resolution of 103 in longitudinal and 83 in latitudinal direction and with 9 vertical layers. An average OH concentration of 1.1;10 molecules cm\ is calculated in the model leading to the lifetimes shown in Table 3. Data from the 3D chemistry transport model have also been used in combination with temperature-dependent reaction rates (see above) to calculate the lifetime of the HGs (Table 3). The calculated lifetimes are a factor of 2.5 4450 G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 Fig. 1. (continued) higher than the ones using temperature-independent reaction rates demonstrating that the loss of HGs to a large extent take place at temperatures which are signi"cantly lower than 298 K. We emphasise that the temperaturedependent reaction rate coe$cients used for HG-10, HG-10 and HG-11 are estimates and that the results obtained for these compounds must be used with caution. However, the temperature dependence of the reac- tion rates has to be greatly di!erent from the one used here to change the calculated lifetimes substantially. To investigate this further, we also performed a sensitivity study in which the activation energy was increased and decreased by 10 and 20%, respectively. In both cases the pre-exponential factors were adjusted to give the same values for the reaction rate coe$cients at 298 K as in the reference cases. Table 3 includes the results from this G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 4451 Table 1 Integrated absorption cross sections of hydro#uoro(poly)ethers Compound Integrated cross-section (10\ cm molecule\) Integration limits (cm\) Reference CHF OCHF (HG-00) 2.5$0.3 2.55 2.56$0.4 4.0$0.4 5.19$0.23 5.0$0.5 6.04$0.13 6.55$0.33 8.49$0.34 25}3250 cm\ Not stated 750}1480 cm\ 25}3250 cm\ 978}1584 cm\ 25}3250 cm\ 930}1501 cm\ 450}2000 cm\ 963}1587 cm\ This work Imasu et al. (1995) Heath"eld et al. (1998) This work Cavalli et al. (1998) This work Cavalli et al. (1998) Christidis et al. (1997) Cavalli et al. (1998) CHF OCF OCHF (HG-10) CHF OCF OCHF (HG-01) CHF OCF OCF OCF OCHF (HG-11) Table 2 Arrhenius parameters and reaction rate coe$cients at 298 K for the reaction of hydro#uoro(poly)ethers with the OH radical Compound A (10\ cm molecule\ s\) E/R CHF OCHF (HG-00) 5.4$3.5 19.0$1.9 1560$200 2006$200 CHF OCF OCHF (HG-10) CHF OCF CF OCHF (HG-01) CHF OCF OCF CF OCHF (HG-11) k (10\ cm molecule\ s\) Reference 25! 5.06$0.37 2.9 2.3 1.34 2.4$0.7 4.7$1.6 Zhang et al. (1992) Huie and Kurylu (1993) Garland et al. (1993) Hsu and DeMore (1995) DeMore (1996) Cavalli et al. (1998) Cavalli et al. (1998) 4.6$1.6 Cavalli et al. (1998) Table 3 Atmospheric lifetimes of hydro#uoro(poly)ethers Compound Lifetime (yr) k (¹)"k (298) -& -& CHF OCHF (HG-00) 11.3 CHF OCF OCHF (HG-10) 12.1 CHF OCF CF OCHF (HG-01) 6.2 CHF OCF OCF CF OCHF (HG-11) 6.3 k (¹)/A "exp(!1735/¹) -& -& #2.0 28.4 !3.7 #2.1 30.4 !4.0 #1.1 15.5 !2.0 #1.1 15.5 !2.1 Estimated uncertainty in the atmospheric lifetimes arising from an increase and decrease in the activation energy by 10 and 20%, respectively, see text. analysis showing that the lifetimes in these experiments increased and decreased by 7 and 13%, respectively. Cavalli et al. (1998) estimated lifetimes for HG-10, HG-01, and HG-11 using temperature-independent reac- tion rates. Christidis et al. (1997) assumed a lifetime for HG-11 of 48 y (based on unpublished data for HG-01). The Cavalli et al. (1998) values are about 1 yr higher than our lifetimes using temperature independent reaction 4452 G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 rates. Therefore our best estimates of lifetimes are about a factor of 2 higher than the Cavalli et al. (1998) values, and for the lifetime of HG-11 the Christidis et al. (1997) value is a factor of 3 higher than our value. 5. Radiative forcing: method 5.1. Radiative transfer models We used two thermal infrared radiative transfer models; one line-by-line (LBL) model and one broad-band model (BBM). The LBL model (Edwards, 1992) is used to calculate optical depths, whereas the discrete ordinate method of Stamnes et al. (1988) is used to calculate radiative #uxes (see also Myhre and Stordal, 1997). Absorption data from the Hitran-92 database (Rothman et al., 1992) are used for the greenhouse gases, except for the CFC replacements studied in this work. The BBM (Stordal, 1988; Myhre and Stordal, 1997) is shown to compare well with the LBL model, mostly within 5% (Myhre and Stordal, 1997; Myhre et al., 1998), and is used for most of the calculations in this work. The HG gases are represented by 7 bands for each of the components HG-00, HG-01, and HG-11, and 9 bands for HG-10 in the BBM. In both models the calculations are performed with 0.1 ppbv of the gases to ensure that the weak limit approximation is valid, which follows the procedure from Pinnock et al. (1995) and Myhre and Stordal (1997). 5.2. Radiative forcing In the de"nition of radiative forcing used in IPCC (1994) and WMO (1994) radiative forcing is calculated as the net change in irradiance at the tropopause level after allowing the temperature in the stratosphere to be adjusted to restore the radiative equilibrium in the stratosphere. The radiative forcing must represent global and annual mean atmospheric conditions. The "xed dynamical heating approximation (Ramanathan and Dickinson, 1979) is used. An iterative process is performed to restore the radiative equilibrium. We refer to radiative forcing calculations including stratospheric temperature adjustment as adjusted radiative forcing. The much less computer expensive calculations without stratospheric temperature adjustments are referred to as instantaneous radiative forcing. 5.3. Atmospheric proxles It has been shown by e.g. Myhre and Stordal (1997) that one global mean vertical pro"le of temperature, humidity, and ozone as used in radiative transfer calculations cannot represent globally averaged conditions with satisfactory accuracy. Freckleton et al. (1998) showed that using three carefully selected vertical pro"les representing the global atmosphere gives satisfactory results. We have performed instantaneous clear sky calculations to compare the BBM with the LBL model. In these calculations we use the three pro"les of Freckleton et al. (1998). In the adjusted radiative forcing calculations a resolution of 103 in latitudinal and longitudinal direction is used (Myhre and Stordal, 1997). Atmospheric distributions of temperature and water vapour are taken from European Centre for Medium-Range Weather Forecasts (ECMWF) whereas the ozone distribution is taken from Liang and Wang (1995). Cloud data have been taken from International Satellite Cloud Climatology Project (ISCCP) (Rossow and Schi!er, 1991). Overlap for the absorption bands of the HG molecules with bands of the well mixed greenhouse gases is taken into account, and mixing ratios of CO , CH , N O, CFC-11, and CFC-12 are taken from IPCC (1995) with values of 356 ppmv, 1.714 ppmv, 311 ppbv, 0.268 ppbv, and 0.503 ppbv, respectively. 6. Radiative forcing: results 6.1. Instantaneous radiative forcing The LBL model is only used to calculate global instantaneous radiative forcing using the three vertical pro"les described earlier. The spectral radiative forcings as calculated with the LBL model are shown in Fig. 2. In the results presented in Fig. 2 we have considered overlap with H O, CO , O , CH , N O, CFC-11, CFC-12. The results are shown for the spectral region 500}1500 cm\ as the contribution outside this spectral range is negligible. The thermal infrared energy decreases with increasing wavenumber for the spectral range shown in Fig. 2. Overlap with other greenhouse gases, especially water vapour, reduces the radiative forcing signi"cantly of bands outside the `atmospheric window regiona (800} 1200 cm\), but only weakly inside this spectral region. For instance, absorption bands near 1400 cm\ have very low forcing as a combination of low thermal infrared energy and high water vapour overlap. Table 4 shows instantaneous clear sky radiative forcing for the four molecules. The results are given for di!erent cases including overlap with various groups of greenhouse gases as well as a case where overlap with other gases has been omitted. Radiative forcing results are presented for the LBL model as well as the BBM. The results are for constant vertical pro"les for the three gases. In the LBL calculations, overlap with other gases reduces the forcing by about 35%, where water vapour is the dominating contributor to this reduction. Generally, the two radiative transfer models agree within 5%. The G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 4453 Fig. 2. Instantaneous clear sky radiative forcing due to HG-00, HG-10, HG-01, and HG-11. Absorption cross sections for HG-00, HG10, and HG-01 are from this work, whereas absorption cross-section from Cavalli et al. (1998) for HG-11 is used. The LBL model is used in the calculations and results are shown in the spectral region 500}1500 cm\ as the radiative forcing outside this spectral range is negligible. Overlap with other greenhouse gases is taken into account. 4454 G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 Fig. 2. (continued) overlap with water vapour in the BBM is in general agreement with the LBL model, whereas overlap with gases other than water vapour seems to be slightly underestimated in the BBM. HG-11 has the strongest forcing as the integrated band strength is largest for this gas. We have chosen to use the spectral data of Cavalli et al. (1998) for HG-11 although our results indicate that their cross sections are generally G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 4455 Table 4 Radiative forcing calculated with the LBL model and the BBM for overlap with all greenhouse gases (H O, CO , O , CH , N O, CFC-11, CFC-12), H O#CO #O , H O, and no overlap with other gases. Values are given in W m\ ppbv\ Compound HG-00 HG-10 HG-01 HG-11 LBL All gases H O#CO #O H O No overlap 0.51 0.83 1.07 1.65 0.53 0.89 1.12 1.77 0.55 0.93 1.16 1.83 0.75 1.35 1.61 2.54 0.55 0.89 1.11 1.83 0.73 1.29 1.58 2.52 BBM HG-00 HG-10 HG-01 HG-11 0.51 0.82 1.10 1.73 0.53 0.87 1.11 1.81 too large. This way we are likely to get an upper estimate of the radiative forcing for HG-11. The ratio between forcing including overlap with all gases and the integrated band strength di!ers by 10% among the four HG molecules. HG-01 has the higher ratio as more of the absorption is in the `atmospheric window regiona 800}1200 cm\ than for the three other HG gases, which absorb more strongly in the 1200}1300 cm\ region. 6.2. Ewect of decay of the mixing ratio with altitude The results shown in Table 4 are for a constant vertical pro"le of the HG molecules. This assumption is not quite realistic and the mixing ratio will most likely decrease with altitude in the stratosphere due to photochemical loss there. To study the sensitivity of the forcing to the vertical distribution of the gases in the stratosphere, we have assumed two further pro"les: one with a decay similar to CH , and one with zero concentration in the stratosphere. The forcings based on these two pro"les are compared to the forcings assuming constant vertical pro"les; the results are shown in Table 5. For instantaneous forcings the e!ect of including the gases in the stratosphere is up to about 10%. The vertical pro"le of the HG molecules can only be found through chemistry transport model calculations taking into account the photochemical destruction and the transport of the HGs. 6.3. Adjusted radiative forcing We have used the BBM and the atmospheric input data as described in Myhre and Stordal (1997) to perform globally averaged radiative forcing for the four HG species. The results are shown in Table 6. Clouds and stratospheric temperature adjustment are included. The decay Table 5 Relative di!erence in radiative forcing (%) compared to constant vertical pro"le Compound Decay as CH No concentration in the stratosphere HG-00 HG-10 HG-01 HG-11 !1.3 !1.5 !1.3 !1.2 !8.3 !9.5 !8.5 !8.0 of the mixing ratios of the HG molecules in the stratosphere is assumed to be similar to the decay of CH , as the main removal mechanism is the same. The calculated radiative forcing of HG-11 is 1.37 W m\ ppbv\. This is an extremely high radiative forcing per 1 ppbv and is obviously due to the many -CF - groups. Relative to CFC-11 the forcing of HG-11 is 5.5 times stronger per molecule. For the other three components the forcing is lower than for HG-11 but still very high compared to other CFCs or CFC replacements. The e!ects of clouds and stratospheric temperature adjustment on the forcing for the four species have very similar e!ects as most of the other halocarbons (Pinnock et al., 1995; Christidis et al., 1997; Myhre and Stordal, 1997). Omitting clouds increases the forcing by about 40% and omitting stratospheric temperature adjustment reduces the forcing by about 10%. The e!ect of changing the vertical pro"le in the stratosphere is larger in the adjusted case than in the instantaneous case for the HG molecules. Changing from a pro"le with decay like CH to no concentration in the stratosphere reduces the forcing by 20% in the adjusted 4456 G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 Table 6 Comparison of radiative forcing due to HGs. The value for HG-00 in Imasu et al. (1995) is given relative to CFC-11. We have used our best estimate of CFC-11 from Myhre et al. (1998) to scale the value of HG-00 in Imasu et al. (1995) Compound Radiative forcing (W m\ppv\) Absorption section Radiative transfer calculation HG-00 0.40 0.43 0.66 0.87 0.87 1.01 1.37 1.11 This work Imasu et al. (1995) This work Cavalli et al. (1998) This work Cavalli et al. (1998) Cavalli et al. (1998) Christidis et al. (1997) This work Imasu et al. (1995) This work This work This work This work This work Christidis et al. (1997) HG-10 HG-01 HG-11 case, but only 10% in the instantaneous case (see Table 5). The absorption of the gases in the stratosphere leads to a heating yielding a larger #ux from the stratosphere to the troposphere with a corresponding increase in the radiative forcing of about 10%, see above. Absorption by a halocarbon only in the troposphere leads to a small negative stratospheric temperature adjustment (Pinnock et al., 1995). Radiative forcing calculations due to HG-10 and HG01 using absorption cross sections from Cavalli et al. (1998) have also been performed. Table 6 shows radiative forcing due to HG-10 and HG-01 for absorption cross sections from this work as well as from Cavalli et al. (1998). The higher forcing using the Cavalli et al. (1998) data re#ects the higher absorption cross sections in their work than obtained in this work, as shown in Table 1. Christidis et al. (1997) have performed radiative forcing calculations of several CFC replacements including HG11, see also Table 6. They calculated 19% lower forcing using an integrated band strength which was 23% lower than in our work. They used a global and annual mean atmospheric pro"le and no decay of the gases in the stratosphere. They obtained a slightly lower e!ect from clouds than in this work. Imasu et al. (1995) have performed radiative forcing calculations for HG-00 among many others. However, their calculations are performed for a single vertical pro"le and radiative forcing is given relative to CFC-11. Clouds and stratospheric adjustment are not taken into account in the calculations by Imasu et al. (1995). Using our estimate from Myhre et al. (1998) for CFC-11 the radiative forcing due to HG-00 is about 5% lower in this work than in Imasu et al. (1995). For HG-10 and HG-01 no previous radiative forcing studies are known to the authors. 7. Global warming potential The GWP has been developed as a tool to rank the e!ectiveness of climate gases in terms of the relative contribution of their emissions to the greenhouse e!ect (IPCC, 1990,1995). It is de"ned to give the global and annually averaged radiative forcing, as a cumulative measure over a speci"ed time horizon, taking into account the decay of the atmospheric concentration following the emission of 1 kg of the compound. The GWP is given relative to another gas, usually CO or CFC-11. Table 7 shows GWP values relative to CFC-11. In the GWP calculations the radiative forcings presented in Table 6 from this work are used, and for HG-10 and HG-01 based on the cross sections from this work. The lifetimes based on temperature dependent reaction rates shown in Table 3 are used. The radiative forcing due to CFC-11 is taken from Myhre et al. (1998) using the BBM, with a forcing of 0.24 W m\. The lifetime of CFC-11 is 50 y (IPCC, 1995). The GWP values decrease with increasing time horizon as the HG lifetimes are shorter than for CFC-11. On a short-time horizon all HGs have higher GWP values than CFC-11, whereas for the longest time horizon when the e!ect decreases relative to CFC-11 the two HG-00 and HG-10 have higher GWP values than CFC-11, and HG01 and HG-11 have lower GWP values than CFC-11. HG-00 and HG-10 have higher GWP values than HG01 and HG-11, except in one case; nevertheless the radiative forcing due to HG-00 and HG-10 are lower on Table 7 GWP values of hydro#uoro(poly)ethers relative to CFC-11 for three time horizons Compound HG-00 HG-10 HG-01 HG-11 Time horizon (yr) 20 100 500 1.69 1.80 1.45 1.80 1.23 1.37 0.76 0.96 1.10 1.23 0.66 0.83 G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 Table 8 GWP values of hydro#uoro(poly)ethers relative to CO for three time horizons Compound HG-00 HG-10 HG-01 HG-11 Time horizon (yr) 20 100 500 10700 11400 9200 11400 6300 7000 3900 4900 2000 2300 1200 1500 a molecular basis. There are two reasons for this. First, GWP values are given relative to emitted mass and the molecular weight is lower for HG-00 and HG-10. Second, the lifetimes of HG-00 and HG-10 are higher compared to those of HG-01 and HG-11. GWP values relative to CO for the HGs are shown in Table 8. Radiative forcing due to CO is taken from Myhre et al. (1998) where 1990 is used as reference year for the CO concentration. We have used the same decay function for CO as IPCC (1995). The lifetimes are much shorter for the HG molecules compared to CO . The GWP values are therefore re duced substantially for long-time horizons. 8. Summary Absorption cross sections for HG-00, HG-10, and HG-01 in the spectral region 25}3250 cm\ have been measured. The contribution of bands in the thermal far-infrared region to the total integrated cross section is almost negligible. Our integrated cross sections are about 20% lower than those of Cavalli et al. (1998). Radiative forcing calculations are performed for HG00, HG-10, and HG-01 using absorption cross sections in this work and for HG-10, HG-01, and HG-11 using absorption cross sections from Cavalli et al. (1998). The radiative forcings per molecule are very high for the HGs compared to other CFCs and CFC replacements. The lifetime of the four HGs is found to be in the range 6}12 y assuming temperature-independent reaction rates with OH and using OH distribution from a 3D chemistry transport model. The lifetimes are found to increase by a factor of 2.5 when an estimate of the temperaturedependent reaction rates is used instead of the rates at 298 K. The resulting GWP values are comparable to those of CFC-11 on a 100 y time horizon, ranging from 0.76 to 1.37. Acknowledgements We thank Sigrun Karlsdottir for providing OH data from the Oslo 3D chemistry transport model. This 4457 work has received support from the Research Council of Norway (Programme for Supercomputing) through a grant of computing time, and from the Environment Programme of the University of Oslo. We thank one of the reviewers for drawing our attention to an error in the cross-section of HG-00 in the original manuscript. References Berntsen, T., Isaksen, I.S.A., 1997. A global three-dimensional chemical transport model for the troposphere;1. Model description and CO and ozone results. Journal of Geophysical Research 102, 21239}21280. BruK hl, C., Peter, T., Moortgat, G., Boglu, D., Meller, R., Brasseur, G., Isaksen, I., Crutzen, P., 1993. Modellstudie zu ozonstoK rung und Treibhause!ekt von halogenirten kohlenwassersto!en mit schwerpunkt bei FCKW-Ersazatzsto!en. Report 10402729, Umweltbundesamtes, Germany. Cavalli, F., Glasius, M., Hjorth, J., Rindone, B., Jensen, N.R., 1998. Atmospheric lifetimes, infrared spectra and degradation products of a series of hydro#uoropolyethers. Atmospheric Environment 32, 3767}3773. Christidis, N., Hurley, M.D., Pinnock, S., Shine, K.P., Wallington, T.J., 1997. Radiative forcing of climate change by CFC-11 and possible CFC replacements. Journal of Geophysical Research 102, 19597}19609. Clerbaux, C., Colin, R., Simon, P.C., Granier, C., 1993. Infrared cross sections and global warming potentials of 10 alternative hydrohalocarbons. Journal of Geophysical Research 98, 10491}10497. DeMore, W.B., 1996. unpublished results. Edwards, D.P., 1992. GENLN2: a general line-by-line atmospheric transmittance and radiance model. NCAR Technical Note, NCAR/TN-367#STR. National Center for Atmospheric Research, Boulder, CO. Fisher, D.A., Hales, C.H., Wang, W.-C., Ko, M.K.W., Sze, N.D., 1990. Model calculations of the relative e!ects of CFCs and their replacements on global warming. Nature 344, 513}516. Freckleton, R.S., Highwood, E.J., Shine, K.P., Wild, O., Law, K.S., Sanderson, M.G., 1998. Greenhouse gas radiative forcing: e!ects of averaging and inhomogeneities in trace gas distribution. Quarterly Journal of the Royal Meteorological Society 124, 2099}2127. Garland, N.L., Medhurst, L.J., Nelson, H.H., 1993. Potential chloro#uorocarbon replacements: OH reaction rate constants between 250 and 315 K and infrared absorption spectra. Journal of Geophysical Research 98, 23107}23111. Hansen, J., Sato, M., Ruedy, R., 1997. Radiative forcing and climate response. Journal of Geophysical Research 102, 6831}6864. Heath"eld, A.E., Anastasi, C., McCulloch, A., Nicolaisen, F.M., 1998. Integrated infrared absorption coe$cients of several partially #uorinated ether compounds: CF OCF H, CF HOCF H, CH OCF CF H, CH OCF CFClH, CH CH OCF CF H, CF CH OCF CF H and CH }CHCH OCF CF H. Atmospheric Environment 32, 23227}23238. Hsu, K.J., DeMore, W.B., 1995. Rate constants and temperature dependence for the reactions of hydroxyl radical with several 4458 G. Myhre et al. / Atmospheric Environment 33 (1999) 4447}4458 halogenated methanes, ethanes, and propanes by relative rate measurements. Journal of Physical Chemistry 99, 1235}1244. Imasu, R., Suga, A., Matsuno, T., 1995. Radiative e!ects and halocarbon global warming potentials of replacement compounds for chloro#uorocarbons. Journal of the Meterological Society Japan 73, 1123}1136. Intergovernmental Panel on Climate Change (IPCC), 1990. In: Houghton, J.T., Jenkins, G.J., Ephraums, J.J. (Eds.) Climate Change: The IPCC Scienti"c Assessment. Cambridge University Press, Cambridge, UK. Intergovernmental Panel on Climate Change (IPCC), 1994. In: Houghton, J.T., Meira Filho, L.G., Bruce, J. Hoesung Lee, Callander, B.A., Haites, E., Harris, N., Maskell, K. (Eds.), Radiative Forcing of Climate Change and an Evaluation of IPCC IS92 Emission Scenarios. Cambridge University Press, Cambridge, UK. Intergovernmental Panel on Climate Change (IPCC) 1995. In: Houghton, J.T., MeiraFilho, L.G., Callander, B.A., Harris, N., Kattenberg, A., Maskell, K. (Eds.), Climate Change 1995: The Science of Climate Change. Cambridge University Press, Cambridge. UK. Liang, X.-Z., Wang, W.-C., 1995. A GCM study of the climatic e!ect of 1979-1992 ozone trend. In: Wang, W.-C., and Isaksen, I.S.A.(Eds.), Atmospheric Ozone as a Climate Gas. NATO ASI series, Springer, Berlin. Marstokk, K.-M., M+llendal, H., Nielsen, C.J., Powell, D. L., 1999. Conformational Properties of Bis(di#uoromethyl) Ether as studied by Microwave, Infrared, Raman spectroscopy and by Ab Initio computations. Journal of Moleclar Structure (in press). Myhre, G., Stordal, F., 1997. Role of spatial and temporal variations in the computation of radiative forcing and GWP. Journal of Geophysical Research 102, 11181}11200. Myhre, G., Highwood, E.J., Shine, K.P., Stordal, F., 1998. New estimates of radiative forcing due to well mixed greenhouse gases. Geophysical Research Letters 25, 2715}2718. Pinnock, S., Hurley, M.D., Shine, K.P., Wallington, T.J., Smyth, T.J., 1995. Radiative forcing of climate by hydrochlorofluorocarbons and hydro#uorocarbons. Journal of Geophysical Research 100, 23277}23288. Radice, S., Causà, M., Marchionni, G., 1998. Vibrational spectra and ab initio calculation of Hydro#uoropolyethers (HFPE). Journal of Fluorine Chemistry (in press). Ramanathan, V., Dickinson, R.E., 1979. The role of stratospheric ozone in the zonal and seasonal radiative energy balance of the earth-troposphere system. Journal of Atmospheric Science 36, 1084}1104. Rossow, W.B., Schi!er, R.A., 1991. ISCCP cloud data products. Bulletin of American Meteorological Society 72, 2}20. Rothman, L.S., et al., 1992. The HITRAN molecular database: editions of 1991 and 1992. Journal of Quantitative Spectroscopy and Radiative Transfer 48, 469}507. Shi, G., Fan, X., 1992. Past, present and future climate forcing due to greenhouse gases. Advances in Atmospheric Science 9, 279}286. Stamnes, K., Tsay, S.-C., Wiscombe, W., Jayaweera, K., 1988. A numerically stable algorithm for discrete-ordinate-method radiative transfer in multiple scattering and emitting layered media. Applied Optics 27, 2502}2509. Stordal, F., 1988. A wide band model for transfer of infrared radiation: altitudinal, latitudinal and yearly variation of cooling rates in the troposphere and the stratosphere, Report 68, Institute for Geophysics, University of Oslo. Tuazon, E.C., 1997. Tropospheric degradation products of novel hydro#uoropolyethers. Environmental Science Technology 31, 1817}1821. World Meteorological Organization (WMO), 1994. Scienti"c Assessment of ozone depletion: 1994. Global Ozone Research and Monitoring Project, WMO, Report 37, Geneva. Zhang, Z., Saini, R.D., Kurylo, M.J., Huie, R.E., 1992. Rate constants for the reactions of the hydroxyl radical with several partially #uorinated ethers. Journal of Physical Chemistry 96, 9301}9304.