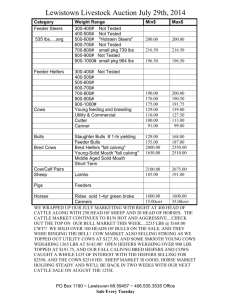
May 5,
advertisement

AN ABSTRACT OF THE THESIS OF Shelby Jean Filley for the degree of Doctor of Philosophy in Animal Science presented on May 5, 1998. Title: Utilization of Lipid by Primiparous Beef Heifers During the Postpartum Period. Abstract approved: Redacted for Privacy Harley A. urner Fredrick Stormshak Experiments were conducted to determine whether essential fatty acids administered to postpartum (PP) beef heifers would increase plasma concentrations of linoleic acid and prostaglandin Fat metabolite (PGFM), decrease days to first estrus, and increase subsequent pregnancy rate. In experiment 1, 20 heifers were assigned randomly to treatments of 1 L Intralipid (20% soybean oil; IL), 1 L 50% dextrose (DEXT; isocaloric to IL), .5 L Intralipid (.5M), and 1 L physiological saline (SAL) infused intravenously over 4 hours on days 7 through 11 PP. Capacity to produce prostaglandins, as reflected by systemic levels of PGFM, was evaluated after i.v. injection of 150 IU of oxytocin (OT) into IL and DEXT heifers on day 12 PP. Change in plasma PGFM 0 to 4 hour after injection was greater for IL-treated heifers compared with other treatments on day 7 (P = .04) and on day 11 (P = .01), but not different (P > .10) for day 9. Plasma linoleic acid on day 11, and OT-induced release of PGFM on day 12, were greater in IL compared with DEXT-treated heifers (P < .06 and P = .0001, respectively). In experiment 2, supplements of .23 kg-head-1d' calcium salts of fatty acids (CSFA; n = 20) or an isocaloric amount of barley (control; n = 19) were fed to heifers day 1 to 30 PP. Analysis of variance of contrast variables revealed an effect of treatment on change in PGFM from day 3 to 5 (P = .004). By day 7 and on day 9, plasma concentrations of PGFM were greater in heifers fed CSFA compared with those of controls (P = .02 and P = .06, respectively). On day 7, but not day 1, plasma from heifers fed CSFA had altered proportions of major FA (P < .01), including an increase in linoleic acid compared with that of controls (29.1 vs 25.6 weight %, SE = .75; P = .003). Days to first estrus with ovulation, pregnancy rate, and calving interval were not affected by treatments (P > .10). Although supplemental lipid administered to primiparous beef heifers increased plasma linoleic acid and PGFM in the early PP period, and increased the capacity of lipid-infused heifers to produce prostaglandin F2, it did not improve the fertility of these heifers in the subsequent breeding season. C Copyright by Shelby Jean Fil ley May 5, 1998 All Rights Reserved Utilization of Lipid by Primiparous Beef Heifers During the Postpartum Period by Shelby Jean Filley A THESIS submitted to Oregon State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Presented May 5, 1998 Commencement June 1998 Doctor of Philosophy thesis of Shelby Jean Filley presented on May 5, 1998 APPROVED: Redacted for Privacy Co-Major Professor, representing Animal Science Redacted for Privacy Co-Major Professor, representing Animal Science Redacted for Privacy Head of Department of Animal Sciences Redacted for Privacy Dean of Gradue School I understand that my thesis will become part of the permanent collection of Oregon State University libraries. My signature below authorizes release of my thesis to any reader upon request. Redacted for Privacy Shelby Jean Filley, A thor ACKNOWLEDGMENTS This dissertation summarizes only the technical information connected with my graduate studies. This section of acknowledgments personalizes the adventure never to be forgotten by me. There are many to whom I am moved to express my gratitude, the first and foremost is GOD for giving me strength, courage, and wisdom through a difficult time in both my professional career and personal life, and for a second chance at success. I want to thank my major professor, Harley Turner, who taught me much about feeding growing heifers, and allowed me to formulate my experimental plan and conduct my research without pushing or pulling me along. I am glad he took on one last graduate student, me, before his retirement from the Department of Animal Sciences. Next, I want to thank Dr. Stormshak, whom I still haven't gotten used to calling "Stormy." Dr. Stormshak began as a committee member, allowing me a place in his student offices and lab, and also helped with my experiment design. His contribution to my dissertation and professional career became so significant, I felt prompted to make him a Co-major professor on my committee. Also, I want to thank my other committee members, Frank Moore and Phil Whanger and my Graduate Council Member, Neil Christensen, for always being available to meet with me and review my proposals and dissertation, and for sharing their knowledge in the classroom or as I would pop in to visit them in their offices. A huge thank-you goes out to Dr. Rosemary Wander who allowed me to perform the fatty acid analysis in her laboratory, and for the interest she showed in my research (a fellow fatty acid fanatic). Another huge thank-you is extended to Dr. Dave Thomas for helping me with the statistical analyses of my data; I would still be trying to figure out how to program SAS if it weren't for him. I also want to thank Dr. Diane Carroll for the stimulating discussions on cattle nutrition and reproduction trials. Now, what would graduate student life be without fellow lab mates? Luckily, I didn't have to find out because I had some of the best: Jennifer E. Bertrand, thank-you for the friendship and standard of excellence in Reproductive Physiology I could look up to, and for sharing the Northern Blot experiences: "See June in July" and "Hey, there's that MARCKS mRNA." Joan Burke, thank-you for the discussions on prostaglandin, fatty acids, and statistics. Ursula Bechert, thank-you for the intellectual exchange in the discussions we had on our projects, and for all the baked German goodies. Timothy McLagan Hazzard, thank-you for the example of how a grad student can enjoy work too much, and still be successful. Kelly Pinckard, finishing within the same week as me, thank-you for the special kinship in the hard work and STRESS of completing our graduate degrees together. Ugur Salli, thanks for your encouragement and giving up the computer for me so often. Thank-you too, to Miss "Sarasota Sue" Supancic for always having a positive attitude and being so helpful and fun. For the great assistance, a special thank-you to the lab/office helpers Lisa, Christine, Jennifer, and Katharine. The donation of materials is gratefully acknowledged for lipid supplement from Church and Dwight, Inc., prostaglandin metabolite antibody from William Thatcher (University of Florida), and progesterone antibody from Gordon Niswender (Colorado State University). Also deserving of gratitude for monetary support are: Oregon Sports Lottery, Savery Award, and Fred. F. McKenzie Scholarship Foundations and Dr. Stormshak for additional support at the end of my work. Thank-you to the office staff and farm crew at Eastern Oregon Agricultural Research Center, Burns, Oregon. Thank-you to my parents, Robert F. Williams and Ruth P. Lucero, and my mother- in-law, Marilyn L. Fil ley, for their support. Thank-you to all my siblings for being proud of me and loving me. To Captain and Mrs. Hugh White, thank-you for your special help and friendship. The biggest THANK-YOU goes out to my husband Mr. Cameron L. Filley, and my children Miss Samantha Filley and Miss Tracy Filley, for their support, love, and faith in me over the years. Cameron was my most competent research assistant and most favorite cowhand, and once I got him chasing cows from horseback, I could barely get him to come down. Without the help of Cam, Sam, and T.C., my research assistants in halter breaking and feeding the heifers and technical help with the experiments, life would have been much harder and we might still be in Burns, an idea not half bad... TABLE OF CONTENTS Page 1. INTRODUCTION 1 2. REVIEW OF THE LITERATURE 3 The Estrous Cycle Folliculogenesis Corpus luteum formation and function Luteolysis Pregnancy Recognition of pregnancy Types of placentation. Ovarian and placental hormones Duration of pregnancy Parturition Role of the fetus Maternal role in parturition Postpartum Period Uterine involution and repair Role of prostaglandins in uterine involution Changes in ovarian function: hypothalamic control Influence of suckling on GnRH secretion Changes in ovarian function: local control Influence of prostaglandins on ovarian activity Short estrous cycles Impact of Nutrition on the Postpartum Period Supplemental energy Supplemental protein Supplemental lipid Energy status Communication of status 4 4 6 6 8 8 9 10 11 12 12 13 13 14 15 17 18 19 19 20 22 22 23 23 26 26 TABLE OF CONTENTS (Continued) Page Fatty Acids Nomenclature Dietary fatty acids Digestion of lipids in the rumen and abomasum Intestinal digestion and absorption of lipid Transportation of lipid Release of lipid into cells Cellular lipid processing Prostaglandin Synthesis in Animals Enzymatic reactions Site of synthesis and degradation Dietary influence on prostaglandin synthesis Characteristics of prostaglandin synthase Prostaglandin synthase in ovarian tissue Uterine prostaglandin synthase during the estrous cycle Uterine and placental prostaglandin synthase during pregnancy 27 27 29 30 31 32 33 33 34 34 36 37 38 40 40 41 3. STATEMENT OF THE PROBLEM 43 4. PROSTAGLANDIN F2, CONCENTRATIONS, FATTY ACID PROFILES, AND FERTILITY IN LIPID -INFUSED POSTPARTUM HEIFERS 44 Abstract 44 Introduction 45 Materials and Methods 46 Animals, treatments, and samples PGFM analysis Fatty acid analysis Progesterone analysis Statistical analysis 46 49 50 50 51 TABLE OF CONTENTS (Continued) Page Results 52 Discussion 62 5. PLASMA FATTYACIDS, PROSTAGLANDIN Fao AND REPRODUCTIVE RESPONSE IN POSTPARTUM HEIFERS FED RUMEN BY-PASS FAT 68 Abstract 68 Introduction 69 Materials and Methods 70 Animals, treatments, and samples Analysis of PGFM, fatty acids, and progesterone Statistical analysis 70 72 73 Results 73 Discussion 78 Implications 82 Acknowledgments 82 6. GENERAL CONCLUSIONS 83 BIBLIOGRAPHY 85 TABLE OF CONTENTS (Continued) Page APPENDICES 103 Appendix A: Metabolic Pathways for Fatty Acids and Eicosanoids 104 Elongation and desaturation of essential fatty acids Synthesis of eicosanoids from long chain fatty acids 104 105 Appendix B: Procedures for Androgen Treatment of Cows, PGFM Radioimmunoassay, and Fatty Acid Identification Androgen treatment of cows PGFM radioimmunoassay Identification of plasma fatty acids 106 106 107 109 Appendix C: Plasma Fatty Acids, Prostaglandin Concentrations, and Fertility in Postpartum Heifers Fed Rumen Protected Lipid and Biuret 111 Abstract 111 Introduction 112 Materials and Methods 113 Animals, treatments, and samples Analysis of PGFM, fatty acids, and progesterone Statistical analysis 113 115 115 Results 115 Discussion 118 References 119 LIST OF FIGURES Page Figure 2.1 Molecular formula and numbering system for linoleic acid 28 4.1 Experimental time line (0 to 150 days postpartum) 47 4.2 Mean (± SE) prostaglandin Fa, metabolite concentration days 1 through 15 postpartum 53 4.3. Mean (± SE) prostaglandin Fa, metabolite concentration hours 0, 4, and 8 on days 7, 9, and 11 postpartum 4.4 4.5 5.1 54 Mean (± SE) weight percentage of plasma linoleic (A) and linolenic (B) acids in response to infusion on day 7, hour 0, 4, and 8 57 Response in prostaglandin Fa, metabolite (PGFM) to oxytocin challenge (mean ± SE) 60 Mean (± SE) plasma concentration of prostaglandin Fa, metabolite (PGFM) on days 1 to 15 postpartum 74 LIST OF TABLES Page Table 2.1 Characteristic aspects of reproduction for selected species 4.1 Fatty acid profiles (mean ± SE) from whole plasma on days 7 and 11 postpartum 59 Mean number of days to first estrus, pregnancy rate, and calving interval 61 4.3 Mean body weights, body condition scores, and calf weights 62 5.1 Means (± SE) for weight percentage of total plasma fatty acids for days 1 and 7 postpartum 75 Mean number of days to first estrus, pregnancy rate, and calving interval 76 5.3 Mean body weights, body condition scores, and feed intake 77 5.4 Calving assistance, calf sex, and calf weights 78 4.2 5.2 3 LIST OF APPENDIX FIGURES Figure Page A.1. Conversion of linoleic acid to arachidonic acid 104 A.2. Conversion of a-linolenic acid to eicosapentaenoic and docosahexaenoic acids 104 A.3. Synthesis of one-series eicosanoids 105 A.4. Synthesis of two-series eicosanoids 105 A.S. Synthesis of three-series eicosanoids 106 A.6. Synthesis of four-series eicosanoids 106 C.1. Mean (± SE) plasma concentration of prostaglandin F2c, metabolite (PGFM) on days 1 through 13 postpartum 116 LIST OF APPENDIX TABLES Table Page C.1. Mean number of days to first estrus and pregnancy rate 117 C.2. Mean body weight, body condition score, and feed intake 117 C.3. Means of calving assistance, calf sex, and calf weight 118 UTILIZATION OF LIPID BY PRIMIPAROUS BEEF HEIFERS DURING THE POSTPARTUM PERIOD 1. INTRODUCTION Initiation of the estrous cycle in the postpartum cow is a complex phenomenon, and stress of parturition, uterine involution, escape from suckling-induced anestrus, physical presence of the calf, parity, increased energy demands for lactation, and nutritional status of the cow are a few of the factors that can influence the length of the interval from calving to first estrus (Malven, 1984). It is of economic importance to have a well defined breeding season in the commercial beef cattle industry, therefore it is imperative that cows return to estrus soon after parturition. Primiparous cows, i.e., first-calf heifers, have longer postpartum intervals than their multiparous herdmates, and failure of these heifers to rebreed is a major problem for beef producers. Diets differing in energy, protein, or fat content can influence reproduction parameters, and recent studies have investigated effects of fed or infused lipid on the metabolism of cholesterol, triglycerides and fatty acids, as well as plasma hormone profiles and the onset of estrous cycles in cattle and sheep. This dissertation addresses the topic of lipid utilization for increased fertility in heifers after their first parturition. Focus will be on the delivery of essential fatty acids as precursors of prostaglandin F20, a hormone important for reproduction. The major hypothesis under test was that supplemental lipid treatment of heifers would increase plasma concentration of prostaglandin and, hence, shorten the interval from parturition to first estrus, thus allowing a greater number of heifers to conceive earlier in the subsequent breeding season. The 2 benefits of feeding supplemental fats to this class of animals are further clarified and more information on the metabolism of linoleic acid in ruminants is contributed to the literature. 3 2. REVIEW OF LITERATURE Information pertaining to reproduction in the female bovine is summarized in this literature review, and the impact of nutrient utilization on reproduction is examined with particular emphasis on lipids. Studies with species other than cows are included for comparison and contrast. Species differences exist with respect to length of estrous cycle, placentation, hormonal support of pregnancy, gestational length, mechanism of parturition, and postpartum physiology. The diversity found in nature sometimes aids in the elucidation of function and control of these processes. Table 2.1 contains some characteristics of reproduction for selected domesticated animals. Table 2.1. Characteristic aspects of reproduction for selected species Animal Gestation length (days) Placenta Uterus Estrous cycle length (days) Cow 277-290 cotyledonary bipartite 19 - 21 Ewe 144-152 cotyledonary bipartite 16 - 17 Nanny 147-155 cotyledonary bipartite 21 Sow 112-116 diffuse bicornuate 19-20 Mare 330-345 diffuse bipartite 19-25 4 The Estrous Cycle Throughout the estrous cycle, characteristic hormone profiles are present at distinct times, and are controlled through the hypothalamic-pituitary-ovarian axis. The estrous cycle may be divided into 1) the follicular phase which is dominated by production of estradiol -1713 (E2) and the development, recruitment, and selection of follicles, and 2) the luteal phase which is characterized by the formation and growth of the corpus luteum (CL), a transient endocrine gland that is the source of progesterone (P4). Gonadotropin releasing hormone (GnRH) is released from the hypothalamus and causes the secretion of follicle stimulating hormone (FSH) and luteinizing hormone (LH) from the anterior pituitary. Negative feedback of these gonadotropins, and GnRH, on the hypothalamus helps regulate the release of GnRH (Hadley, 1992). Folliculogenesis Primordial or primary follicles are present within the ovary at or shortly after birth, and enter the pool of developing follicles by intraovarian mechanisms (Hafez, 1993a). Each follicle contains a fully grown oocyte surrounded by the zona pellucida and a single layer of flattened granulosa cells, all enveloped by a basement laminae. Secondary follicles are characterized by multiple layers of granulosa cells and gonadotropin responsiveness, whereas formation of an antrum or space within the follicle, and continued increase in number of granulosa cells describe early tertiary follicles. A mature tertiary or Graafian follicle is surrounded by thecal cells, and the expanding antrum accumulates estrogen rich follicular fluid (Hafez, 1993a). A two-cell theory exists for E2 production within the ovary (Falck, 5 1959). Granulosa cells have the capacity to produce P4, but are unable to convert it to androgens, the precursors of E2. However, theca cells found surrounding the basement membrane of the follicle convert P4 to androgens (androstenedione and testosterone) and supply these to the granulosa cells for aromatization to E2. Groups of follicles are developed together throughout the estrous cycle with atresia the usual end point (Fortune, 1994). However, if FSH increases just prior to time of atresia, some are recruited for further development in a wave-like manner, with one becoming dominant and others diminishing in size (Sirois and Fortune, 1988; Ginther et al., 1989). In the cow, there are two to three waves per cycle, but normally only one follicle is selected for recruitment and ovulation (Ginther et al., 1989). Pulsatile release of LH stimulates E2 secretion from the dominant follicle(s), which in turn increases the number of LH receptors (Fortune, 1994). The ewe is genetically more prolific, and normally two or three of the largest follicles are selected for ovulation. It is in dispute whether the development of these follicles is a continuous process or occurs in waves similar to the cow. In a study in which ovaries were examined daily by transrectal ultrasonography throughout the estrous cycle of 36 crossbred Suffolk ewes, Schrick et al. (1993) concluded that entry of follicles into the pool of gonadotropin-dependent follicles occurred as a continuum. Contrasting data were reported by Noel et al. (1993) who concluded there were 3 waves of follicular development in the ewe. In this latter study, only 5 Suffolk ewes were examined daily by laparoscopy. 6 Corpus luteum formation and function Increasing E2 relative to P4 causes a preovulatory surge of LH for luteinization of granulosa cells and also induces behavioral estrus (Louis et al., 1974). Increases in follicular prostaglandin (PG) F2, required for ovulation (Algire et al., 1992) and steroidogenesis are also stimulated by LH (Shemesh and Hansel, 1975). Ovarian PGF2a receptors in the cow are present throughout the estrous cycle, increasing from early to late luteal phase. However, they are significantly reduced in late pregnancy (Sakamoto et al., 1995). The ovulatory process has been likened to an inflammatory response, with PGE2 increasing blood flow and PGF2a activating proteolytic activity by fibroblasts which leads to the rupture of the ovarian surface and release of ova (Espey, 1980). After ovulation, the CL, a transient endocrine gland, is formed as a consequence of the luteinization of granulosa cells and invasion of theca cells (Warbritten, 1934). Under the influence of tonic secretion of LH, and with access to blood cholesterol, the CL produces massive amounts of P4. The bovine CL matures by 7 days and is sustained for 8 to 9 days more until it undergoes luteolysis at the end of the estrous cycle, unless fertilization of the ova occurred and the female is pregnant. Luteolysis Luteolysis is marked by a decrease in CL size and P4 production. In ruminants, uterine PGF2a is the luteolysin; however, in primates intraovarian mechanisms are responsible for demise of the CL (Young et al., 1997). The action of PGF2a on the CL was described in the early 1970's by McCracken and coworkers (McCracken et al., 1970). Uterine PGF2a enters the utero-ovarian vein, and via a counter current mechanism, diffuses into the closely 7 apposed ovarian artery to cause an increase in ovarian arterial PGF2a. Exposure of the CL to PGF2a initiates luteolysis and also causes release of oxytocin (OT) from the CL (as reviewed by McCracken et al., 1983). In a positive-positive feedback loop, this OT acts upon the uterine endometrium to cause further pulsatile release of PGF2a from the uterus, which eventually leads to the demise of the CL. Pulses of PGF2a have a requisite duration of 1 hour, with a 6-hour cycle (McCracken et al., 1983). The action of E2 acting on the P4 -primed uterus causes the production of PGF2a (Blatchley and Poyser, 1974). Hormonal control of uterine PGF2a secretion for luteolysis and its abrogation in early pregnancy has been shown to be orchestrated by steroid hormone receptor populations (McCracken et al., 1983; Silvia et al., 1991). During the mid-luteal phase of the cycle and pregnancy, P4 down-regulates the uterine estrogen receptor (McCracken et al., 1983). Without estrogen, the production of the OT receptor is inhibited and stimulation of PG synthesis and release is inhibited (Mirando et al., 1993). Oxytocin is synthesized not only in the CL, but also in the hypothalamus where it is packaged into secretory vesicles and transferred to the posterior pituitary for storage and release (Hafez, 1993b). Estrogen upregulates OT receptors and helps control release of OT from the posterior pituitary (Silvia et al., 1991). Neural stimulation from suckling and parturition trigger the release of neurohypophyseal OT, which causes milk letdown and uterine contractions (Nathanielsz, 1978). Uterine stimulation by OT from the neurohypophysis is capable of inducing the release of PGF2a by the same mechanism as OT of CL origin, and basal secretion of this OT may initiate the positive-positive feedback loop described above (McCracken et al., 1991). 8 The action of PGF2a on the bovine CL begins a phosphoinositide cascade and activation of protein kinase C which leads to release of luteal OT (as reviewed by Stormshak et al., 1995). Also, endothelin-1, an endothelial cell derived vasoconstrictor, increases in the bovine CL after treatment with PGF2, and may be a mediator in luteolysis (Ohtani et al., 1998). Oxytocin injections into cycling cows increases plasma concentrations of PGF2a (Oyedipe et al., 1984), with estrogen priming enhancing the response (Newcomb et al., 1977). Exogenous PGF2a is capable of causing luteolysis after day 7 (Louis et al., 1974), and is less effective if administered earlier in the estrous cycle (King et al., 1982b). Uterine refractoriness to OT is apparent, and intervals of 6 to 8 hours are needed between stimulation events, and similar refractoriness of the CL to uterine PGF2a exists (Silvia et al., 1991). A possible explanation of the refractoriness of uterine PGF2a is a delay in refilling available pools of arachidonic acid for PG release (Poyser, 1991). Progesterone has two effects on uterine PGF2a release. One effect is to stimulate the accumulation of substrates and enzymes for PGF2a production. Progesterone also inhibits the release of PGF2a which diminishes with prolonged exposure to P4 (Silvia et al., 1991). Ewes injected with OT on days 12, 13, 14, and 15 of the estrous cycle, failed to respond with increased secretion of PGF2a prior to day 14, but responded on days 14 and 15 (Silvia et al., 1992). Pregnancy Recognition of pregnancy If fertilization occurs, both production of uterine (Gross et al., 1988) and effect of exogenous PGF2a (Inskeep et al., 1975) for luteolysis is inhibited by the conceptus. Prior to 9 attachment, the ruminant placenta trophoblast, an extraembryonic membrane, produces proteins related to interferons, which were initially called ovine trophoblast-1 (OTP-1) and bovine trophoblast-1 (BTP-1) (Thatcher et al., 1994). These proteins down-regulate uterine estrogen receptors, and E2 is unable to support maintenance of myometrial OT receptor populations (Mirando et al., 1993). Hence, there is negligible response to PGF 2x, the CL persists, and pregnancy is maintained (as reviewed by Thatcher et al., 1994). Types of placentation The lumen of the uterus is enveloped by two major tissues, the endometrium (serosal) and the surrounding myometrium (smooth muscle layer). The lumen of the endometrium is lined with epithelial cells, resting on a basement membrane under which lies a bed of cells referred to as the stroma. Myometrium, which surrounds the stroma, consists of longitudinal and circular smooth muscle cells. Estrogens and P4 secreted during the estrous cycle prepare the uterine tissue for pregnancy. In domesticated animals attachment of the blastocyst to the uterine endometrium is superficial (King et al., 1982a); however, in primates and rodents the trophoblast invades the endometrium (Perry, 1981). Species differences are apparent in the type of placenta used to support the pregnancy, at least with respect to the number of tissue layers separating maternal from fetal blood. Common among the species is the arrangement of extraembryonic membranes. The inner most membrane of the placenta is the amnion, which immediately surrounds the fetus. The outer most layer is the chorion, which is connected to the uterine endometrium. The allantois separates the amnion from the chorion. For connection between maternal and fetal tissues, 10 villi protrude from the chorioallantoic membrane into the uterus. The mare and sow have a diffuse placenta with connections spanning the entire surface of the chorion, while cows, ewes and goats have a cotyledonary placenta characterized by localized areas of connections to the uterine endometrium. These areas are called placentomes, and are formed from maternal caruncles that exist in the endometrium and fuse via the villi with the fetal cotyledon. Cows have convex and ewes have concave cotyledons (Jainudeen and Hafez, 1993a). The placenta of the mare and the sow is termed epitheliochorial due to the fact that there are six layers of tissue separating maternal from fetal blood. Similarly, the placenta of the cow, ewe, and goat is also epitheliochorial, although the association is within the placentomes. Other types of placenta are endotheliochorial, hemochorial, and hemoendothelial and are found in the dog and cat, man, and rabbit, respectively. Ovarian and placental hormones Hormones that support pregnancy may be of placental and/or ovarian origin, again depending upon the species of animal. The CL is the primary source of P4 during early pregnancy, however, continued dependence on the CL differs among species. In the sow and goat the placenta does not produce enough P4 to sustain pregnancy until late gestation, and the CL is required throughout most of the gestation period (Cunningham, 1992a). Some cows ovariectomized at day 200 are capable of sustaining a viable fetus; however, in general, this results in a shortened gestation length, a high incidence of retained fetal membranes, and a high percentage of fetal and calf death loss (Estergreen et al., 1967). In the mare, equine chorionic gonadotropin stimulates luteinization of follicles for formation of accessory CL for 11 additional P4 to support pregnancy (Jainudeen and Hafez, 1993a). By day 70 of a 340 day gestation and day 50 of a 150 day gestation the horse and ewe, respectively, can do without CL because the placenta becomes the primary source of P4. Human placenta P4 production is sufficient to support pregnancy 2 to 3 weeks following implantation of the embryo (Cunningham, 1992a). In early pregnancy, neither fetus nor placenta can make steroids from cholesterol, and a maternal source is required (Cunningham, 1992b). Additionally, the production of estrogen from P4 requires a tandem effort between placenta and fetus until late gestation, when the placenta alone is capable of that task. Fetal P4 precursors are transferred to the placenta where conversion to androgens occurs. These androgens are then provided to the fetus and converted to estrogen (Cunningham, 1992b). Other hormones include placental lactogen, which is important for development of the mammary gland and coordinates the onset of lactation for the newborn calf (Thatcher et al., 1980), and relaxin, which in the cow, is produced by the CL and is required for relaxation of the pubic symphysis and dilation of the uterine cervix during parturition. Progesterone is also essential for uterine and mammary gland growth and maternal behavior (Hafez, 1993b). Duration of pregnancy Gestational length is genetically determined. It varies not only with species of animal, but also among breeds within species. It can also be altered by maternal, fetal, and environmental factors (Jainudeen and Hafez, 1993). A maternal factor is age of dam at conception, with young animals having a shorter gestation period than older animals; fetal 12 influences include sex of fetus, twinning, and fetal size; and environmental factors can include season of year and plane of nutrition (Jainudeen and Hafez, 1993). Parturition Role of the fetus Parturition may be divided into three phases; preparatory, expulsion of fetus and expulsion of placenta. Prior to parturition, plasma concentrations of hormones begin to change. In cows, P4 begins to decrease 60 days prepartum and declines rapidly 24 to 48 hours prior to parturition, while estrone increases 30 days prepartum and decreases after delivery of fetus (Edqvist et al., 1978). The fetus has an active role in the initiation of parturition; physical size of the conceptus is one possible explanation for signaling time for birth. The fetal hypothalamus, pituitary, and adrenal gland are involved (Nathanielsz, 1993), and the paraventricular nuclei of the fetal hypothalamus is the site of the signal that triggers the onset of parturition. In ruminants, fetal corticotropin releasing hormone is secreted, causing the release of adrenocorticotropic hormone from the fetal pituitary, and the production of cortisol from the fetal adrenal gland. As a result, the fetal lungs mature and maternal cytochrome P450 17ahydroxylase for conversion of P4 to estrogen is produced (Nathanielsz, 1993). Primates lack placental cytochrome P450 17a-hydroxylase and 17,20 lyase required for conversion of P4, however, fetal dehydroepiandrosterone is produced prior to parturition and is aromatized to estrogens (Nathanielsz, 1978). Estrogen is essential for 1) stimulation of the uterine endometrium (placentomes and fetal membranes ) to produce PG, 2) production of oxytocin 13 receptors in the myometrium, and 3) positive feedback to the hypothalamus to promote the release of OT from the posterior pituitary (Nathanielsz, 1993). Maternal role in parturition Oxytocin from the maternal neurohypophysis binds to its receptor in the endometrium (mucous membrane lining) and causes the release of PGF2a, which acts to promote contraction of the myometrium, i.e., intermediate smooth muscular layers of the uterus. Maternal cotyledons contain higher concentrations of PGF2, than the myometrium or fetal cotyledons, and appear to be the source of PGF2c, in the periparturient ewe (Mitchell and Flint, 1977). Oxytocin also binds to receptors in the myometrium and, through an increase in intracellular free calcium, causes uterine contractions (Carsten, 1972; Yu et al ., 1995). Estrogen, along with relaxin cause cervical softening (Nathanielsz, 1993), and the fetus is delivered. Soon after parturition, the placenta is also expelled. However, retention of the placenta may occur in some cases, which may cause metritis or inflammation of the endometrium. Prostaglandin Fa, has been used for treatment of bovine pyometra (Gustaffson, 1976) and metritis (Coulson, 1978). Immediately after injection, uterine cramping and expulsion of uterine fluid has been noted. This is consistent with a direct effect of PGF2c, on the uterine myometrium to cause vasoconstriction and contractions. Postpartum Period The period of time between parturition and resumption of estrous cycles is called the postpartum (PP) interval. During this time many changes take place and the animal is in a 14 state of anestrus. After parturition, and prior to another conception, the uterus must undergo the process of involution and repair, and ovulation must occur. Uterine involution and repair Uterine involution in the cow has been defined as that process which allows the uterus to attain its normal non-pregnant position in the pelvic cavity with two uterine horns of similar diameter having normal consistency and tone (Buch et al., 1955). In the cow, days for uterine involution as determined by manual palpation range from 16 to 53 (Lindell et al.,1982). According to Gier and Marion (1968), involution also has many histological as well as physical aspects which include reduction in size, loss of tissue and repair, and in the bovine takes between 40 and 60 days to complete. The process is delayed in cows with uterine infections, retained placentas, and poor physical condition. During the first week or two PP there is a uterine discharge, or lotia, which is mucous, blood, shreds of placental membranes, and caruncular tissue. In cows regeneration of caruncles begins 12 to 14 days PP, and the uterus is repaired and restored to condition by 40 to 60 days PP (Guilbault et al., 1988). Caruncles closest to the fetus are more numerous and take longest to repair (Guilbault et al., 1988). The uterine environment of cows and ewes is not conducive to conception during the first 3 weeks after parturition (Kiracofe, 1980). Immediately following this period, there are 2 or 3 weeks when fertility is less than optimal, but still may occur, and after 40 days PP conception rate improves. Unless delayed by inflammation or infection, uterine involution is not thought of as a barrier to fertility later in the PP period. Endometrial repair is faster in 15 animals with a diffuse placenta (mares and sows), and fertility often returns sooner after parturition than with those having localized placentomes (Jainudeen and Hafez, 1993 a). Also, uterine involution is completed a few days earlier in primiparous compared with multiparous cows, and also earlier in summer and autumn than in spring and winter (Buch et al., 1955). Guilbault et a1. (1985) reported that breed of service sire can decrease uterine involution time and is associated with higher prostaglandin metabolite (PGFM) concentrations in Holstein heifers. Lammoglia et al. (1995) confirmed sire effect and added that cow breed and calf sex also influences the systemic concentration of PGFM in cows. Role of prostaglandin in uterine involution Prostaglandin Fa, plays important roles in the PP cow. Injection of either PGF2a or OT causes uterine contractions in the PP cow (Eiler et al., 1981). Time for complete uterine involution is negatively correlated with the duration of elevated PGF2a, and cows with uterine infections have longer periods of PGF2a release and longer involution times (Lindell et al.,1982; Madej et al.,1984). Also, the duration of increased secretion of PGF2a is negatively correlated to the interval from parturition to the first normal length estrous cycle (Madej et al., 1984). The concentration of PGF2a from the utero-ovarian vein increases following parturition in the cow (Fairclough et al., 1975). Guilbault et al. (1984) hysterectomized cows within 8 hours after parturition, and demonstrated that the uterus was the primary source of PGF2a in the PP cow, and that caruncles produced more PGF2a than intercaruncular or myometrial tissue. In cattle, PP plasma concentration of PGF2a is positively correlated with 16 size of the previously gravid horn, and maximal systemic level of the eicosanoid occurs around day 3, and is diminished by day 15 (Eley et al., 1981). Relationship between uterine involution and plasma PGFM concentrations was studied in Brown Swiss cows given an inhibitor of prostaglandin synthesis every 12 hours (Guilbault et al., 1987a). Uterine involution (as determined by rectal palpation) proceeded with no difference among groups and it was concluded that PGF2c, is supportive, but not required for involution. However, concentrations of PGFM were only partially suppressed, and approached normal levels approximately 8 hours after injection of inhibitor. Exogenous PGF2c, given to cows (day 0 to 11 PP) with normal PG concentrations does not enhance uterine involution (Guilbault et al., 1988). Uterine manipulation on day 35, but not day 14 PP, increased PGFM (Tolleson and Randel, 1987). Cows (17 to 49 days PP) exposed to either uterine palpation plus exogenous PGF2c, (regardless of involution status) or uterine palpation alone (with incompletely involuted uteri) had an earlier return to estrus after parturition compared with controls (Tolleson and Randel, 1988). Further, uterine manipulation on day 35 PP results in increased systemic levels of PGFM in multiparous cows but not in primiparous cows (Wann and Randel, 1990). Cows suckling calves in the early PP period have a shorter time to uterine involution than unsuckled cows, however, calf removal at day 35 does not effect uterine involution (Guilbault et al., 1988). As discussed previously, suckling causes release of OT from the posterior pituitary. Exogenous OT causes the release of PGF20, in suckled Brahman cows from day 10 through 30 PP, with magnitude of response diminishing over time (Del Vecchio et al., 1990). Release of PGF2c, from the uterus of anestrous ewes was increased by injection 17 of estradiol alone but not with OT alone, while injection of OT after estradiol elicited the greatest response (Sharma and Fitzpatrick, 1974). Changes in ovarian function: hypothalamic control Some time after, or during the time of uterine involution and repair, resumption of estrous cycles occurs. This occurs as a consequence of the differential change in hypothalamo-hypophyseal-ovarian interrelationships. Higher centers of the central nervous system are connected to the hypothalamus through synaptic contacts, including those of neurons involved in the synthesis of neurotransmitters. Secretion of GnRH from the hypothalamus is controlled by catecholamines, probably norepinephrine and dopamine (Hadley, 1992). In this way, higher centers in the brain can evaluate positive and negative factors for resumption of estrous. During pregnancy the hypothalamic-hypophyseal axis is suppressed by progesterone and estrogen, therefore release of GnRH from the hypothalamus is inhibited, and as a consequence, systemic concentrations of LH and pituitary stores of this gonadotropin are low (Nett, 1987). Follicle stimulating hormone is not suppressed because no ovarian follistatin is present for negative feedback (Nett, 1987). It was postulated that GnRH stores were low and needed to be replenished, however, early administration of GnRH did not stimulate LH secretion, and it was concluded that there is insufficient storage of LH during the early PP period (Nett, 1987). After removal of the steroid inhibition, a period of GnRH stimulation of the pituitary gonadotrophs is required in order to replenish and release LH in sufficient quantity in a pulsatile manner (Nett, 1987). Besides steroid inhibition, there are other factors 18 such as presence of calf and energy balance (Short et al., 1972), responsible for controlling GnRH secretion. Influence of suckling on GnRII secretion Systemic levels of LH in the postpartum period are lower in suckled compared with nonsuckled cows (Short et al., 1972; Randel et al., 1976). Some studies have found that cows milked frequently (4x vs 2x daily; Wiltbank and Cook, 1958) and cows with high milk production (Morrow et al., 1966), have longer PP intervals than cows not suckling calves; however, energy balance may not have been adjusted for its effect on nutrient supply for reproduction (Short et al., 1972). Adjusting for energy balance, it was found that frequency of milking does not affect LH as does suckling (Carruthers and Hafs, 1980). Suckling- induced PP anestrous is due to a blocking of GnRH release. Neural stimulation at the mammary gland causes the maternal release of endogenous opioid peptides, blocking the release of neurotransmitters required for GnRH secretion (Nett, 1987). Without the release of GnRH from the hypothalamus, there is no stimulation of LH secretion from the pituitary. Administration of naloxone, an opioid antagonist, between day 24 to 35 PP increases LH secretion in suckled cows (Gregg et al., 1985), but not nonsuckled cows (Whisnant et al., 1985). Mastectomized cows removed from calves resumed estrus in comparable time to nonsuckled cows, which was earlier than for those suckling calves, demonstrating that residual milk within the mammary gland does not stimulate the inhibition of estrus in nonsuckled cows (Short et al., 1972). Mastectomized cows allowed to maintain contact with their calves remain anestrus similar to suckled cows, demonstrating that cow-calf interactions 19 other than suckling can suppress ovulation and estrus (Viker et al., 1989). In one study, restricted tactile stimuli failed to prolong postpartum interval in mastectomized cows (Viker et al., 1993), however, Griffith and Williams (1996) reported that either vision or olfaction interactions between cows and calves are sufficient to inhibit LH secretion if calves can be identified as their own. Changes in ovarian function: local control Frequency of first ovulation from the ovary contralateral to the previously gravid horn was found to be greater than from the ipsilateral ovary; however, it was unclear whether inhibition of ovulation from the ipsilateral ovary was due to a carry-over effect of the CL of the past pregnancy, or from uterine environment (Saiduddin et al., 1967). Morrow et al. (1968) suggested that the CL of pregnancy is non-functional after parturition because it does not produce P4 and is totally regressed by day 4 to 7 PP. The uterus plays an important role in regulating estrous cycles in cattle, and uterine infection, milk fever, metritis, ketosis, and retained placentas can all increase the PP interval (Morrow et al., 1968). Alternatively, the relative sensitivity of the ovaries to systemic hormones, FSH and LH, may differ and influence ovulation. Influence of prostaglandin on ovarian activity Postpartum cows with partially suppressed PGF2a had lower serum LH concentrations than controls (Guilbault et al., 1987b) and replacement with PGF2a increased ovarian activity ipsilateral to the previously gravid uterine horn (Guilbault et al., 1987a). Infusion of PGF2a 20 early in the postpartum period stimulates the development of large follicles, and in response to weaning, promotes development of those follicles toward ovulation or atresia (Villeneuve et al., 1988). Administration of 25 mg PGF2c, intramuscular (i.m.) to cows (14 to 28 days PP) increased first service conception rate; however, interval from calving to first service was not shortened (Young et al.,1984). This action of PGF2c, is apparently not a consequence of its effect on the ovary because cows with no systemic levels of P4 respond similarly to those with low levels of P4. Administration of PGF2a analog alfaprostol to beef cows increased pregnancy rate (Randel et al., 1988) and decreased PP interval regardless of uterine involution state (Tolleson and Randel, 1987) . Heat stress prepartum has been shown to reduce reproductive efficiency and milk production (Thatcher, 1974). In one study, prepartum Holstein cows exposed to heat stress had increased PP plasma concentrations of PGFM and uterine involution rate; however, compared with controls, subsequent fertility was not altered (Lewis et al., 1984). Heat stress attenuated negative effects of the previously gravid horn on ovarian volume and diameter, and percentage of ovaries with a CL, and reflects the control of the uterus over ovarian recrudescence. Short estrous cycles Transition of cows from PP anestrus to normal estrous cyclicity is often characterized by estrous cycles of less than normal length, i.e., short cycles, during which early regression of the CL occurs (Wright et al., 1988). Ewes also may exhibit short cycles if they lamb in the breeding season (Land, 1971). Occurrence of first ovulation without estrus is also frequent in cows, and the life span of the resultant CL is shorter than in subsequent ovulations 21 (Stevenson and Britt, 1979). However, short cycles are not a prerequisite for normal cyclicity (Peters and Lamming, 1984). In one study, short estrous cycles occurred in 198 out of 2,854 (7%) suckled fall calving Angus cows. Short cycles were between the first and second estrus PP and lasted 7 to 10 days, with 8 day cycles most frequent (Odde et al., 1980). Short cycles were more frequent in weaned compared with suckled (78.3 vs 25.0%) spring calving Simmental cows (Odde et al., 1980). In pregnant beef heifers induced to abort with exogenous PGF2c, the susequent occurrence of short estrous cycles appeared to be regulated by the previously gravid uterine horn (Wright et a., 1988). The untimely demise of the CL in short cycles is thought to be due to a premature release of PGF2c, and pre-exposure to P4 from a short cycle helps to counteract the problem in the subsequent cycle (Lishman and Inskeep, 1991). This may be why short cycles occur between the first and second estrus PP, and subsequent CL are maintained for a normal length of time. Beard and Hunter (1994) reported that ovariectomized ewes pre-treated with P4 had a decline in uterine OT receptors which are required for OT stimulation of PGF 2,, release and luteolysis. These researchers hypothesized that an appropriate P4 milieu in intact animals would prevent premature luteolysis in the estrous cycle. Immunization against PGF2c, was shown to extend the life span of the first CL PP (Copelin et al., 1989). Pre-estrus serum concentrations of FSH, but not LH, were lower for first estrous cycles of short duration than for second estrous cycles for five Hereford cows (Ramirez-Godinez et a., 1982). Also, systemic P4 was lower after first estrus as compared with levels of the steroid present after second estrus. First service conception rates at day 40 PP were higher in Holstein cows that had one or two previous estrous cycles (Stevenson and Call, 1983). 22 Impact of Nutrition on the Postpartum Period Numerous studies on the impact of nutrition on reproduction have been reported. Results most frequently reported indicate that feeding of diets low in energy or protein delays the onset of estrus after calving (Dziuk and Bellows, 1983). Effects of diets differing in energy and protein source and quantity at different times relative to breeding and calving were the focus of initial investigations, and results varied under the different experimental conditions. As more investigations on nutrition and reproduction were published, descriptions of hormones and nutritional metabolites in the circulation and specific tissues were included. Supplemental energy Feeding a low energy diet to cows prepartum increased time to first estrus, but did not affect conception rate, and feeding a low energy diet PP decreased conception rate but not days to first estrus (Wiltbank et al., 1962). Timing of supplemental energy (immediately PP or beginning 30 days PP) influenced follicular activity and altered days to first estrus (Wiltbank et al., 1964), with cows receiving high energy diets early PP having increased follicular activity and an earlier return to estrus. Pregnancy rates were greater for primiparous heifers fed high energy compared with those fed moderate and low energy diets PP. Low energy diets led to fewer heifers exhibiting estrus during the breeding season (Dunn et al., 1969). Subsequent studies revealed high energy level increased LH secretion and ovarian activity in PP cows (Perry et al., 1991). Response of the anterior pituitary to a GnRH 23 challenge differed among heifers fed high and low energy diets pre- and postbreeding, with secretion of LH being greatest in those fed high energy diets throughout the trial (LeersSucheta et al., 1994). Supplemental protein Inadequate protein intake prior to or after calving increases length of time from calving to first estrus in beef cows (Randel, 1990). In another trial using cows supplemented with high (1 kg/day) and low (.5 kg/day) protein, no effects on reproduction characteristics were found (Marston et al., 1995). Conception earlier in the breeding season was stimulated in PP primiparous heifers fed supplemental rumen undegradable protein compared with heifers fed control protein in one study (Appeddu et al., 1996) but not in other studies (Albertini et al., 1996; Renner et al., 1996). Amount and type of protein supplemented to PP cows can affect protein metabolism and composition of body weight loss, which can lead to differences in nutrient partitioning for reproduction and altered reproductive efficiency (Dhuyvetter et al., 1993). Supplemental lipid In order to increase dietary energy content and milk yield, supplemental fats have been added to dairy and beef cattle rations during the PP period, and changes in reproductive responses have been observed. As with energy and protein, results of fat supplementation varied depending on fat type, quantity, and time of delivery. Increased plasma cholesterol, nonesterified fatty acids (FA), triglycerides, and progesterone are the most frequent effects 24 detected in PP cows fed fat (Talavera et al., 1985; Williams, 1989; Carroll et al., 1990). Carroll et al. (1992) examined plasma lipoproteins from lactating dairy cows fed diets differing in added fat. Although plasma concentrations of progesterone were increased by dietary fat, they did not appear to be mediated by altered lipoprotein composition. Excess fat in the ruminant diet can cause nunen inefficiency, therefore rumen protected lipids have been designed to alleviate this problem (Chalupa et al., 1986). Protected fatty acids fed to PP dairy cattle have been shown to increase the number of medium and large follicles (Lucy et al., 1991a) and increase pregnancy rate (Ferguson et al., 1987; Sklan et al., 1991). In a similarly designed study Lucy et al. (1991b) found that FA fed to dairy cows increased medium sized follicles, but had no effect on changes in plasma concentrations of PGFM or LH. Protected fats fed to beef cows during the PP period enhanced follicular growth, and at least in one study, increased LH secretion (Hightshoe et al., 1991). Whole cottonseed, naturally high in fat, when fed to beef cows increased PP luteal activity compared with isocaloric control diets (Wehnnan et al., 1991). Increased life span of the CL was noted in a study with cows fed a high fat diet using whole cottonseed (Williams, 1989), and decreased PP intervals were reported in PP cows using pelleted supplements of tallow and yellow grease for 3 to 7% total dietary fat (Beam and Butler, 1997) Results of one study indicated an extended PP interval for well-conditioned heifers fed high fat diets. However, duration of fat supplementation was longer (from the third trimester of gestation through the third PP estrus) and amount of fat fed was greater than in other studies (Oss et al., 1993). The findings of this latter experiment are consistent with the report that reproductive response to fat supplement is dependent on body condition (Morgan and Williams, 1989; Spoon et al., 1990). 25 Acute effects of lipid on plasma lipids, hormones, follicular dynamics, and estrous cycle length have been studied by intravenous (i.v.) infusion of plant oil emulsions into cows and ewes during the estrous cycle. Holstein heifers infused with a soybean oil emulsion had increases in plasma PGFM, number and size of ovarian follicles, and estrous cycles of shorter duration (Lucy et al., 1990). Soybean and olive oil-infused ewes had increased systemic concentrations of cholesterol, P4, PGFM and PGE2, and shorter estrous cycles (Burke et al., 1996). The soybean oil emulsion was a commercially available i.v. fat emulsion (20% Intralipid; Kabi Pharmacia, Clayton, NC) designed to correct essential fatty acid deficiencies in human patients on total parenteral nutrition (TPN) or tube feeding. Content of neutral triglycerides is 50% linoleic, 26% oleic, 10% palmitic, 9% linolenic, and 3.5% stearic acids. The olive oil emulsion was prepared by Burke et al. (1996) and contained 72% oleic, 11% pahnitic, 8% linoleic, 2% stearic and 1% linolenic acids. Although reproductive responses can be altered and energy density increased significantly by supplementing fat, the diet of ruminants normally includes little fat. Other effects of increased fat in the ruminant diet are noteworthy and include increases in milk yield, percentage of milk fat, and decreased milk protein (Palmquist and Moser, 1981); decreased fiber digestibility and feed intake (Jerred et al., 1990); decreased acetate to propionate ratios (Jenkins 1993); and altered milk and plasma FA profile (Sklan et al., 1989; Jenkins, 1995). Feeding a rumen protected form of fat can help alleviate the negative effects of fat on fiber digestion and dry matter intake (Coppock and Wilks, 1991). Also, protected lipids do not cause alterations in ruminal acetate to propionate ratios because they do not decrease fiber digestion (Jenkins 1993), and can have higher energy diet while maintaining ration fiber concentration to sustain percentage of milk fat (Palmquist and Conrad, 1978). 26 Energy status Body condition scores, i.e., assessment of energy reserves, at various times pre- and postpartum also influences the reproductive efficiency in cows (Selk et al., 1988). Body condition prior to parturition had more influence on PP fertility than did level of supplemental energy during the PP period (Laflamme and Connor, 1992; DeRouen et al., 1994). Changes in body weight were also found to influence reproduction, with cows gaining weight during the breeding season having better conception rates than those losing weight (Wiltbank et al., 1964). The above trials in which fats were fed were well controlled by the feeding of isocaloric and isonitrogenous control diets; therefore the effects on reproduction, lactation, and digestion were not confined strictly to increasing energy in the diet. During the PP period cows are commonly in a state of negative energy balance, and systemic concentrations of growth hormone (GH) are increased, mobilization of body stores increases free FA, while insulin and insulin-like growth factor-1 (IGF-1) are suppressed (Rutter et al., 1989). However, investigations have shown cows fed high-fat diets have increased P4 in follicular fluid (Ryan et al., 1992), increased serum GH, and altered insulin secretion patterns, as well as increased in vitro production of IGF-1 from luteal tissue (Ryan et al., 1995). Cows fed soybean oil had increased follicular fluid IGF-1 and serum insulin (Thomas et al., 1994). Communication of status Although the above published results show that nutrition alters reproduction, exact mechanisms have not been identified. However, once the body has ample supplies to sustain 27 reproduction, there must be a way for the system to receive signals for action. It has been postulated that physiological stimuli signal higher brain centers to secrete GnRH for gonadotropin release (Randel, 1990). Progress is being made to elucidate the process(es). Leptin, a recently-discovered hormone produced in the adipocytes, serves as a metabolic signal to the reproductive system to signal that sufficient body stores of energy exist for reproduction. Adiposity increases and starvation decreases leptin concentrations (Barash et al., 1996; Ahima et al., 1996). The hypothalamus contains leptin receptors and the proposed mode of action of leptin is through the neuroendocrine system (Barash et al., 1996). In genetically leptin deficient mice, treatment with leptin increased LH and ovarian and uterine weights (Barash et al., 1996). Neuropeptide Y is the potential mediator to signal a starvation state, and leptin administration decreases neuropeptide Y in the rat (Ahima et al., 1996). There may also be direct effects of lipid on reproductive hormone concentrations that have the potential to enhance fertility. One explanation for increased fertility observed in fat feeding and infusion trials is an increased availability of a prostaglandin (PG) precursor, namely the essential fatty acid (EFA), linoleic. To better understand the processes associated with the use of EFA for PG production, the following sections on FA biochemistry, lipid metabolism in ruminants, and PG synthesis are included in this literature review. Fatty Acids Nomenclature The systematic nomenclature of fatty acids defines both the position and geometry of each double bond, counting from the terminal carboxylic acid function (alpha end of the 28 molecule). Linoleic acid is an 18-carbon FA with two double bonds, one between the ninth and tenth, and another between the 12th and 13th C atoms, and H atoms in the cis position, i.e., cis-9, cis-12-octadecadienoic acid (Figure 2.1). A shorthand nomenclature (Holman, 1966) indicates chain length, number of double bonds, and the position of the double bond nearest the terminal methyl group. This terminal methyl carbon is called the omega (w) carbon. For linoleic acid the designation is C18:26)6. Linolenic acid is designated as cis-9, cis-12, cis-15-octadecatrienoic acid, and C18:3(.i)3. Unlike plant enzyme systems, those of mammals cannot form these EFA from C16 or C18 unsaturated FA, nor can they interconvert the fatty acids from one class to another with respect to position of thew carbon double bond (Willis, 1987; Budowski, 1988). 18 11 1 CHACH2)4(CH=CH( CHYCH=CH)(012),COOH (06 Figure 2.1. Molecular formula and numbering system for linoleic acid (C18:2o)6) 29 Dietary fatty acids Inclusion of fat in the diet is required to eliminate deficiency symptoms such as skin disorders and slow rate of growth in the rat (Burr and Burr, 1929). Linoleic and arachidonic acids (AA) were found to be essential precursors of elongation products such as one and two series prostaglandins, whereas linolenic acid was found to be required for PG of the three series (Bergstrom et al., 1964; Van Dorp et al., 1964). The liver is an active site of FA metabolism, and rat liver microsomes can convert linoleic acid to AA (Pugh and Kates, 1977). Since these early studies, linoleic acid has been found to be required not only for prostaglandin synthesis, but also as a precursor of other eicosanoids such as prostacyclins, thromboxanes, and leukotrienes (Willis, 1987). Humans and rats require approximately 3% of dietary energy as linoleic acid, and have been diagnosed as EFA deficient at lower levels (Rivers and Frankel, 1981). Young growing animals have a higher EFA requirement than adult animals (Mattos and Palmquist, 1977). Information on required amounts of EFA in ruminants is sparse, and deficiencies in ruminants are rare. Calves appear to require only small amounts of EFA (Sklan et al., 1972), and lambs were found to have only one-half the EFA requirement of non-ruminants (Noble et al., 1971). Adipose and muscle lipids are lower and plasma is higher in EFA content for ruminants than in non-ruminant animals (Sklan et al., 1972). As with non-ruminants, tissue loading of EFA has been observed in ruminants, where the type of dietary fat ingested is reflected in milk and tissue fatty acid profiles as well as eicosanoid (EFA elongation) products (Tove and Mochrie, 1963; Sklan et al., 1972; Sinclair et al.,1994). Crawford and Gale (1969) found a consistent ratio of 0 )6:(03 FA elongation products in muscle tissue of ruminants, and 30 suggested a structural function for these acids, as well as precursors for PG. Triglycerides are the major lipid in plants and are generally confined to the seed for storage compounds (galactolipids and phospholipids; Van Soest, 1982). The so-called oil seeds, cottonseed, soybean, and canola that are sometimes fed to cattle and sheep, are high in fat, and are good sources of EFA, while forages contain negligible amounts of these acids (Van Soest, 1982). Digestion of lipids in the rumen and abomasum In the rumen, lipolysis and biohydrogenation of unprotected lipid occur so that the FA released to the hindgut are approximately 70% nonesterified (NEFA) and 10 to 20% are incorporated into phospholipids (Bauchart, 1993). Microbes biohydrogenate 70 to 90% of the dietary FA, i.e., saturate the C - C double bonds of FA with hydrogen, in order to detoxify lipids and stabilize the rumen environment (Bickerstaffe et al., 1972; Mattos and Palmquist, 1977; Fellner et al., 1995). Also, microbial synthesis of branched chained FA occurs (Van Soest, 1982). In spite of these alterations, linoleic acid at .5 to 1.5% of the total dietary energy escapes digestion in the rumen and is available for use by the ruminant (Bickerstaffe et al., 1972). It was found that if lipid is administered in ways that by-pass the rumen (i.v. or abomasal infusion), the FA deposited in milk fat and adipose tissue (Tove and Mochrie, 1963) and plasma (Moore et al., 1969) are similar to those provided. Feeding of rumen by-pass fats circumvents the alterations of FA that normally occur in the rumen because they are designed with the carboxyl end inaccessible to microbial action (Chalupa et al., 1986). Fatty acyl amides are one such protected FA product available for feeding to cattle (Fotouhi and 31 Jenkins, 1992). In a similar fashion, n-butylamine is used to protect linoleic acid from hydrogenation in the rumen, and plasma percentage of C18:2 can be increased with feeding this product relative to a control diet (Jenkins, 1995). Calcium salts of FA (CSFA) have also been designed for such purposes and are triglycerides (TG) from palm oil (Chalupa et al., 1986). These CSFA are insoluble at normal ruminal pH and are resistant to microbial fermentation. In the abomasum, pH decreases, dissociation of Ca' salts occurs, and TG are liberated (Sklan et al., 1989). However, digestion of NEFA and TG is inhibited by acid conditions in the abomasum (Bauchart, 1993). Intestinal digestion and absorption of lipid The small intestine is comprised of the duodenum, jejunum, and ileum, which in the ruminant, have a pH of 2 to 2.5, 2.8 to 4.2, and 4.2 to 7.6, respectively (Bauchart, 1993). Initially, NEFA and phospholipid (PL) are adsorbed to food particles, and as the digesta flows through the small intestine absorption into intestinal cells is facilitated by the dispersion action of bile and action of pancreatic phospholipase A2 to transfer PL to micelles (Bauchart, 1993). Dissociation of CSFA is favorable under these pH conditions, and TG are available for digestion and absorption (Sklan et al., 1989). However, pancreatic lipase and colipase function optimally at pH 7.5, thus release and absorption of free FA and monoglycerides from CSFA occur lower in the intestinal tract where the pH is higher (Bauchart, 1993). Digestibility of fat from CSFA is greater compared with that of other fat sources, possibly because of the different mode and site of digestion and absorption (Bauchart, 1993). Increases in plasma cholesterol in ruminants fed high fat diets appears to be due to increased 32 intestinal synthesis of cholesterol for FA absorption, and partially due to decreased fecal excretion of bile acids (Nestel et al., 1978; Park et al., 1983). Transportation of lipid Early studies with ruminants demonstrated that linoleic acid was transported in TG form to the liver and selectively incorporated into PL and cholesterol esters, but not used for resynthesis of TG (Moore et al., 1969). This selective incorporation retains EFA for essential functions and causes a sparing effect, a unique feature in ruminant animals which contributes to the rarity of EFA deficiencies (Van Soest, 1982). The EFA from dietary plant material are predominantly placed on the sn-2 or middle position of the triglyceride moiety, and this may help in the conservation process (Van Soest, 1982 ). Further investigations have shown that after absorption into the intestinal cells, micelles are disassembled, FA are released, and TG are reformed and transported by the lymphatic system through the thoracic duct to the vena cava and into the heart, then distributed throughout the body (Cunningham, 1992c). Transportation in lymph and plasma requires packaging into lipoproteins (LP), which by their chemical composition and rate of secretion, control lipid utilization by tissues (Bauchart, 1993). Major LP vary in TG, cholesterol, and phospholipid content and include (in increasing order of density) chylomicrons (CM), very low density LP (VLDL), intermediate density LP (IDL), low density LP (LDL), and high density LP (HDL) (Wendlandt and Davis, 1973; Ferreri and Gleockler, 1979). Lipoproteins circulate throughout the body to deliver the different lipid classes to the tissues for storage or utilization, and partitioning of lipid among tissues is accomplished with 33 the help of specialized attached proteins, apolipoproteins, recognized by receptors in the cell membrane or in the vascular wall (Bauchart, 1993). Release of lipid into cells Membrane lipoprotein lipase causes the release of FA, including linoleic acid, from chylomicrons and VLDL into the cell, with the remaining portion of the lipoprotein retained in the circulation as chylomicron remnants and DL, respectively (Bauchart, 1993). The LDL contain cholesterol esters, phospholipid, free cholesterol, and TG, and are taken into peripheral tissues by receptor mediated endocytosis for delivery of cholesterol (Goldstein et al., 1979). Cholesterol is often esterified with linoleic acid acquired from TG by intestinal and hepatic acyl-CoA:cholesterol acyltransferase (ACAT) and from plasma lecithin by lecithin:cholesterol acyltransferase (LCAT; Bauchart, 1993). The HDL, synthesized by liver and small intestine, pick up excess cholesterol from tissues and recycle it back to the liver for VLDL and bile synthesis (Bauchart, 1993). Cellular lipid processing Once inside the cell, LDL is metabolized so that linoleic acid is cleaved off cholesterol by lysosomal lipase, and free cholesterol is then used for membrane synthesis and steroid production, with excess providing negative feedback on de novo cholesterol synthesis (Goldstein et al., 1979). Linoleic acid from the cholesterol ester, along with VLDL linoleate, is used in membrane phospholipid synthesis and eicosanoid production (Bauchart, 1993). 34 The liver plays a central role in the metabolism of lipid. Some FA (e.g., C18:2co6 and C18:3033) are altered prior to leaving the liver by elongase and desaturase enzymes to form distinct series of FA (i.e., C20:4co6 and C20:50)3 and C22:6co3), and then transported in lipoproteins to various tissues of the body for use in essential functions (Moore et al., 1969; Budowski, 1988). Prostaglandin Synthesis in Animals Thus far, this review has covered the importance of prostaglandins in reproduction and the essentiallity and absorption of the carefully guarded linoleic acid as the dietary precursor of prostaglandins. Information that is lacking here concerns PG synthesis, which is controlled by cellular mechanisms for uptake, release, and oxygenation of the eicosanoid precursor FA (Weber, 1988). Understanding the process of PG synthesis, and factors affecting this, is essential to manipulating PG concentrations to favor fertility. The remainder of this review is therefore devoted to covering the regulation of these latter processes over time and location, with respect to substrates, products, competitors, inhibitors, and enzyme expression and activity. Enzymatic reactions Prostaglandin synthase (PGS) is an enzyme that is central to the formation of eicosanoids such as prostaglandins, thromboxanes, and leukotrienes from elongation products of the EFA, linoleic and linolenic acids. This section on enzymatic reactions was summarized from information found in the CRC Handbook of Eicosanoids: Prostaglandins and Related 35 Lipids (Willis, 1987). Eicosanoids are classified by family, e.g., E or F, and by series, e.g., 1,2, or 3, which differ in chemical properties and number of cis double bonds in the side chains, respectively. Appendix A contains a schematic summary of the reactions. In a series of steps, linoleic acid (C18:206) is elongated and desaturated to dihomo-ylinolenic acid (DHLA). From here, it can be acted upon by PGS, also called cyclooxygenase, to form the one-series PG or thromboxanes (TX). Elongation of DHLA forms arachidonic acid (AA), which upon oxygenation by PGS is converted to PGG2, which is an unstable endoperoxide. Further reduction via the peroxidation action of PGS yields a stable hydroxyperoxide, PGH2. The characteristics of PGS are unique and will be discussed in a following section. A reductase enzyme converts PGH2 to PGF2, while the action of an isomerase yields PGE2, i.e., the two-series PG. Prostacyclins (PC) and TX can also be formed from PGH2 but utilize PC synthase and TX synthase, respectively, to catalyze those reactions. Leukotrienes, a four-series eicosanoid product of AA, are formed using a lipoxygenase enzyme. The above products are formed from the 06 FA, and cannot be formed from co3 FA, i.e., no interconversion between FA series. Linolenic acid (C18:30) is also acted upon by elongase and desaturase enzymes; however, it yields eicosapentanoic (EPA; C20:503) and docosahexanoic (DHA; C22:603) acids. The three-series PG are another product of PGS action, but the substrate here is EPA, and contrary to above, is formed from co3 FA, but not o.)6 (Willis, 1987). It is evident that PG synthesis can only account for a small amount of the linoleic requirement in animals. Some of the important functions of PG in reproduction have been discussed in previous sections of this review. Thromboxanes play a role in platelet aggregation and vasoconstriction, while leukotrienes have a possible role in the leukocytes 36 of the immune system. Elongated lipids, AA, EPA, DHA, and adrenic acid (22:4(06, a product of AA) are all utilized as integral parts of plasma membranes, and help to maintain proper fluidity as well as provide substrate pools for the eicosanoids described above. There are many related FA and eicosanoid products, some share the same or similar metabolic pathways, and they may modulate each others biosynthesis (Willis, 1987). Site of synthesis and degradation Prostaglandins were first isolated in the prostate gland of the ram by Professors von Euler and Hammarstrom in 1937 (von Euler, 1982). Later, they were found in many other tissues, in which they can act locally to stimulate smooth muscle contraction by regulating intracellular Ca' transport (Carsten, 1972). Actual synthesis of PG, as well as elongation and desaturation of dietary precursors for substrates of PGS, occurs in the plasma membrane using membrane bound enzymes (Wong et al., 1989; Sugiura et al., 1995), and exogenous FA contributing to the substrate pool must first enter the membrane (Gan-Elepano et al., 1981). Liver microsomes are very active in elongation and desaturation of FA, and provide phospholipids and cholesterol esters as sources of linoleic, AA, linolenic, EPA, and DHA (Sprecher, 1988). Subsequent to the appropriate stimulus, phospholipase A2 (PLA2) directly cleaves off a molecule of AA from the membrane phospholipid. Alternatively, PLC can indirectly release AA via diacylglycerol from inositol glycerophospholipids. Ferreira and Vane (1967) found that PGE1 and PGE2 were inactivated in the pulmonary circulation of dogs, cats, and rabbits. Piper et al. (1970) found that 90% of 37 circulating PGF2a was inactivated by one pass through the lungs, presumably by 15hydroxyprostaglandin dehydrogenase to yield 13,14-dihydro-15-keto PGF2c, , i.e., PGFM. Dietary influence on prostaglandin synthesis Production of PGF2c, is limited by the availability of precursor and competitor substances in the diet (Mathias and DuPont, 1979; Sanders and Younger, 1981). Because little AA is derived from the diet, its conversion from linoleic acid is important and is modulated by linolenic acid concentrations (Budowski, 1988). Also, diets high in linolenic acid relative to linoleic acid have high amounts of EPA and DHA compared with AA, which compete for incorporation into membrane phospholipids and regulate availability of substrates for PG production (Sanders and Younger, 1981; Aukema and Holub, 1988). Prostaglandin biosynthesis is especially sensitive to alterations in diet if there is an EFA deficiency or if the linoleic acid to saturated FA ratio exceeds 5 (Mathias and DuPont, 1979). Substituting dietary 0)3 for co6 increases co3 elongation products in the PL fractions of membranes and eicosanoids produced (Mathias and Dupont, 1979). Dose responses with linoleic acid for PG production have been observed in vivo for humans (Adam et al., 1982 ), cows (Lucy et al., 1990), and ewes (Burke et al., 1996), but have also been compounded by altering linolenic concentrations. In vitro studies have been used to examine influences of different FA on PG production. It was determined that oleic, linoleic, and to a larger extent linolenic acid at concentrations of 1 to 4 mM inhibited PG synthesis in homogenates of sheep vesicular glands (Pace-Asciak and Wolfe, 1968). Endometrial cytosol was found to contain high levels of 38 linoleic acid and inhibited PGS in endometrial microsomal assays (Danet-Desnoyers et al., 1993). The potential of caruncular tissue to produce PG was determined to be stimulated by 13 hours, but inhibited after 20 hours of incubation with 25 and 200 pils,4 linoleic acid compared with 10 pM, and not affected by similar concentrations of AA (Oldick et al., 1994). Results of these studies demonstrate that FA can modulate PG synthesis, however, in vitro enzymatic reactions, particularly those involving FA metabolism are inaccurate with respect to conditions existing in the membranes (Gan-Elepano et al., 1981). The km for AA, i.e., the substrate concentration that provides one-half maximal PGS enzyme rate, is approximately .2 p.M in purified form (Harbon et al., 1979), whereas within membranes and in association with protein moieties the lcm may increase up to 500 p.M (Lands, 1988). No in vivo evidence for depressed PG levels with diets high in linoleic acid was found in the literature. In humans, it takes at least 24 hours to convert linoleic to arachidonic acid (Nichaman et al., 1967), and may take up to 2 to 3 days for dietary changes to significantly affect PG profiles (Adam et al., 1982). Some indication of immediate use of i.v. infused long chained fatty acids (linoleic) for PG synthesis is apparent in ewes (Burke et al., 1996) and cows (Lucy et al., 1990). In order to use a nutritional approach to altering PG for specific reproductive responses, appropriate timing and optimal quantities of linoleic acid supplementation, as well as accompanying dietary FA profile, remain to be determined. Characteristics of prostaglandin synthase Synthesis of PGF2a depends not only on substrate availability, but also on the presence of PGS, which varies with the tissue and the physiological state of the animal. There are two 39 isoforms of PGS, one with a MW of 69,000 (PGS-1) which is constitutively expressed and another with a MW of 72,000 (PGS-2) which is an inducible form (Wong and Richards, 1991; Lee et al., 1992; Sirois et al., 1992). The DNA of constitutively expressed genes, i.e., housekeeping genes, is transcribed to mRNA without the use of inducers, while the DNA of inducible genes is transcribed as a result of an inducer interacting with the promoter (Lewin, 1994). Although the transcription of PGS-1 is not inducible, the presence of protein may be regulated by affecting enzymes or cofactors at translational or post-translational levels (Sirois and Hawkins, 1992). The mRNA transcript is 2.8 and 4.0 kilobases for PGS-1 and PGS-2, respectively (Sirois and Hawkins, 1992). Isoforms also differ in their pharmacological response to glucocorticoids and non-steroidal anti-inflammatory drugs (Meade et al., 1993). Expression of PGS-2 is inhibited by glucocorticoid-induced transcription of mRNA (Bailey and Verma, 1991). Dexamethasone inhibited uterine PGS-2, but not PGS-1 expression in mice (Jacobs et al., 1994). Enzyme activity of PGS-2 is partially inhibited and PGS-1 is completely inhibited by non-steroidal anti-inflammatory drugs, e.g., indomethacin; while ibuprophen inhibits both isoforms (Meade et al., 1993). Early publications do not specify PGS isoform, however, most studies used antibodies raised against PGS from ram vesicular glands, which was later found to be anti- PGS-1 (Meade et al., 1993). This review will make assumptions of which isoform is being referred to in publications not specifying isoform based on the characteristics that are specified and will be so noted as "presumably PGS-1 or PGS-2" as appropriate. 40 Prostaglandin synthase in ovarian tissue Expression of PGS in ovarian tissue confirms local production of PG present within the ovary for luteinization and ovulation as was outlined earlier in this review. Production of PGS-2 is stimulated by FSH and LH in rat preovulatory follicles and is obligatory for ovulation (Wong and Richards, 1991). Protein kinase-A and protein kinase-C were found to induce PGS-2 protein required for luteinization of rat granulosa cells (Morris and Richards, 1995). Prostaglandins are also presumed to mediate GnRH and LH stimulation of ovulation through epidermal growth factor tyrosine kinases in a process distinct from luteinization (Wong and Richards, 1992). The predominant form of PGS in the rat CL of pregnancy is PGS-1 (Wong and Richards, 1991). Studies with bovine preovulatory follicles exposed to hCG demonstrated that PGS-2 induction by LH is conserved across mammalian species (Sirois, 1994). Because PGS-2 induction of ovulation is faster in rats than in cows, it may provide an explanation why ovulation time is decreased in rats as compared with cows (Sirois, 1994). Injection of PGF2c, into the ovarian vein stimulated PGS-2 mRNA, PGS protein, and endogenous PGF2c, in ovine large luteal cells, suggesting an autocrine/paracrine function of ovarian PGF2c, for ovulation (Tsai and Wiltbank, 1997). Uterine prostaglandin synthase during the estrous cycle Prostaglandin synthase (presumably PGS-1) in ovine uterine tissue increases late in the estrous cycle, and is most prevalent in lumina' epithelium, vascular endothelium, and stroma of the uterine caruncles (Huslig et al., 1979). Increased concentration of PGF2,,, days 41 12 to 15 of the guinea-pig estrous cycle, is stimulated by estradiol and blocked by intrauterine injection of actinomycin D (inhibitor of RNA synthesis) on day 10, indicating changes in level of PGS (presumably PGS-2; Poyser and Leaver, 1979). However, in another study, PGF2c, from guinea-pig endometrial tissue on days 7 to 15 of the estrous cycle, was not stimulated by estrogen (Riley and Poyser, 1987). Mouse preimplantation uterine stroma cell PGS-2 increases and is localized to implantation sites of the blastocyst, while the uterine lumina' epithelial cells have PGS-1 (Jacobs et al., 1994). Uterine and placental prostaglandin synthase during pregnancy The capacity for ovine cotyledonary (maternal) microsomes to produce PGF2c, and PGE2 increases during gestation (Rice et al., 1988). Increases in fetal membrane PGS in late pregnancy prior to stimulation of the hypothalamic-pituitary-adrenal axis and in the maternal epithelium 7 to 10 days prior to parturition have been observed in ewes (presumably PGS-1; Boshier et al., 1991). However, Wimsatt et al. (1993) reported ovine fetal tissue PGS-1 is minimally regulated throughout pregnancy, while PGS-2 gradually increases days 120 to 139 in the cotyledons, and PGS-1, but not PGS-2, is detected in amnion and allantochorion. In the rat, expression of uterine PGS-1 is highly regulated throughout gestation, is low in mid-gestation, and increases at parturition in myometrium and uterine epithelium (Sirois and Hawkins, 1992). It is important for labor, and decreases after delivery, possibly being regulated by E2/P4 ratio (Myatt et al., 1994). In humans, glucocorticoids stimulate PGS-2 and PGE2 in amnion by a newly discovered regulatory mechanism, while PGS-1 is unaffected (Zakar et al., 1995). The paradoxical effect of glucocorticoids on PGS-2, and differential 42 stimulation for inflammatory and pro-inflammatory cells has been discussed (Zakar et al., 1995). No publications on PG synthase in postpartum uteri were found for any species. 43 3. STATEMENT OF THE PROBLEM The primary problem in the beef industry, from the standpoint of reproduction, is failure to produce calves. In order to be economically efficient, each cow should produce one calf per year, which can only be realized if cows breed in a well defined breeding season. Pregnancy rates in range beef cattle are lower for primiparous heifers compared with multiparous cows. One reason for this reduced pregnancy rate is the duration from calving to first estrus, which is longer in younger than in older cows (Wiltbank, 1970; Dziuk and Bellows, 1983; Randel, 1990). Thus, many of these younger cows are not bred during the sometimes short breeding season. There is a definite need to improve efficiency of reproduction at this level, and attain a more profitable production system. Increased energy intake during the postpartum period has been shown to be beneficial in initiating comparatively early onset of estrous behavior. Effects of supplemental fat as a source of energy on reproductive characteristics has been studied in cycling dairy heifers and ewes, or postpartum multiparous dairy cows. More studies are needed to investigate the potential of short-term supplementation with FA for improving reproductive performances in beef heifers subsequent to their first parturition. Objectives of the present study reported herein were to provide linoleic acid to enhance plasma PGF2c, concentrations in anticipation of improving uterine environment, and to stimulate ovarian activity early in the postpartum period for a more prompt return to estrus. Overall, the desired effect was to shorten the postpartum interval and increase pregnancy rates of primiparous beef heifers. 44 4. PROSTAGLANDIN F2c, CONCENTRATIONS, FATTY ACID PROFILES, AND FERTILITY IN LIPID-INFUSED POSTPARTUM HEIFERS Abstract Effects of lipid infusion into postpartum (PP) beef heifers on plasma concentrations of linoleic acid and prostaglandin (PG) F2 metabolite (PGFM), days to first estrus, and pregnancy rate were examined. Treatments (n = 5/ group) of 1 L Intralipid (20% soybean oil; IL), 1 L 50% dextrose (DEXT; isocaloric to IL), 0.5 L Intralipid (0.5IL), and 1 L physiological saline (SAL) were infused i.v. over 4 hours on days 7 through 11. Capacity to produce PG was evaluated after i.v. injection of 150 IU of oxytocin (OT) to IL- and DEXT- treated heifers day 12 PP. Change in plasma PGFM 0 to 4 hours was greater for IL-treated heifers compared with other treatments on day 7 (P = 0.04) and on day 11 (P = 0.01), but not on day 9 (P > 0.10). Plasma linoleic acid on day 11, and OT-induced release of PGFM on day 12, were greater in IL-treated heifers compared with DEXT-treated heifers (P < 0.06 and P = 0.0001, respectively). There were no significant differences among treatments for mean days to first estrus or pregnancy rate. Infusion of lipid increased circulating linoleic acid and increased the capacity of PP heifers to produce prostaglandin F2, as indicated by plasma PGFM concentration after OT injection. 45 Introduction The interval between calving and resumption of estrous cycles is 45 to 60 days in mature beef cows, and 20 to 30 days longer in young heifers bred to calve as two-year olds (Wiltbank, 1970). This is partially due to the stress of calving and lactating for the first time, as well as higher nutrient requirements for growth. Long anestrous periods decrease pregnancy rates and profits if well defined breeding seasons are employed (Wiltbank, 1970), therefore, it is beneficial to shorten anestrous periods of primiparous beef heifers. In the postpartum cow (PP), fertility resumes after the uterus returns to its nonpregnant state through a process of involution and ovulation occurs. Prostaglandin F2c, is an eicosanoid that is important for uterine involution, and its primary site of synthesis in the postpartum cow is the uterine commies, where it acts locally on the myometrium to cause uterine contractions (Guibault et al., 1984). Duration of increased uterine synthesis of PGF2c, is negatively correlated with number of days to complete uterine involution and length of interval between parturition and resumption of normal ovarian activity (Madej et al., 1984). Prostaglandins are derived from the membrane phospholipid stores of arachidonic acid, which are synthesized from dietary linoleic acid, an essential fatty acid. There is some indication of immediate use of linoleate for prostaglandin production in lipid-infused estrous cycling heifers (Lucy et al., 1990) and ewes (Burke et al., 1996). It is not known whether administering linoleic acid to heifers in the early PP period increases PGF2. Objectives of this experiment were to determine whether infusion of PP heifers with essential fatty acids would increase plasma linoleic acid and PGFa, metabolite (PGFM), and promote increased PG production after oxytocin injection. In addition, the ability of 46 exogenous essential fatty acids to shorten the interval between parturition and onset of normal ovarian activity, and increase subsequent pregnancy rates was also examined. Materials and Methods Animals, treatments, and samples Thirty Hereford x Angus primiparous heifers were accustomed to handling, and body condition scored (BCS; scale 1 - 9; 1= emaciated, 9 = obese) prior to the start of the calving season. Twenty heifers were assigned randomly to one of four treatments (n = 5 per treatment) as they calved and as time and barn space allowed, and along with calves were weighed on day 1 postpartum (PP). All experimental procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Treatments consisted of i.v. infusions of 1 L fat emulsion (IL; Intralipid 20%, Pharmacia, Inc., Clayton, NJ; 20% soybean oil), 1 L 50% dextrose (DEXT; Phoenix Pharmaceutical, Inc., St. Joseph, MO; Isocaloric to IL), 0.5 L Intralipid (0.5IL), or 1 L physiological saline (SAL), into the heifers on days 7 through 11 PP (day 0 = parturition; Figure 4.1). One to 2 days prior to infusion, the jugular vein on each side of the neck was catheterized using a 14G x 5 1/4 " Angiocath catheter (Becton Dickinson Desert Medical, Sandy, UT) for convenient infusion of treatments and sampling of blood. Catheters were flushed with 1 ml of sodium heparin (40 U/ml) to keep them patent. Peristaltic pumps (Mmipuls 2, model HP-4, Gilson Medical Electronics, Inc., Middleton, Wisconsin) were used to ensure continuous infusion of treatment over a 4-hour period. Beginning day 7 PP, heifers 47 were led into individual stalls where they remained tethered and separated from their calves during infusion and blood sampling periods (0600 to1600 hours, days 7 through 11 PP). OXYTOCIN INJECTION DAY 12 OXYTOCIN CHALLENGE CALVING I I I 1 1 I 1 -60 -30 0 30 60 90 120 I 0 I 1 I I 3 5 1 1 1 7 9 11 13 15 1 I § 21 I 1 I 1 § MIN 1 I I I 150 DAYS DAY 21 TO 150 ESTRUS CHECK DAYS 7 TO 11 IV INFUSIONS 1 DAYS 1 TO 15 PGFM MEASURED -2 0 2 4 6 8 HOURS INFUSION Figure 4.1 Experimental time line (0 to 150 days postpartum). Day 0 was calving, prostaglandin Fat metabolite (PGFM) was measured from plasma on days 1 to 15 on alternate days, treatments were infused on days 7 through 11, and 150 IU of oxytocin (OT) was injected i.v. on day 12. Heifers were bled every 2 hours, -2 to 8 hours relative to initiation of infusion (time 0 days 7 through 11), and every 15 min, -60 to 120 min relative to OT injection (time 0 day 12). Return to estrus was determined by twice daily behavioral estrus checks using intact bulls and from plasma progesterone (P4) measured from blood collected twice weekly day 21 until 2 wk post-estrus (Standing for the bull and confirmed by plasma P4 > 1 ng/ml in two consecutive samples). 48 Blood samples were taken at time -2, -1, 0, 2, 4, 6 and 8 hours relative to initiation of infusion. All blood samples taken during this experiment (except where noted) were collected into 10 ml heparinized tubes, immediately placed on ice, and within 1 hour centrifuged at 2,500 x g for 15 min at 4 °C. Plasma was then removed and stored at -20 °C until analyzed for fatty acids (FA) and PGFM. After all infusions and blood samplings were completed for the day, heifers were moved to another stall and reunited with their calves. All heifers were then fed 2.3 kg alfalfa hay in individual feeders. After this hay was consumed, native flood meadow hay was fed ad libitum. Hay refusals were measured and 5-day feed intakes were calculated as a percentage of metabolic body weight (BW75). It was of interest whether lipid infusion would alter the ability of heifers to secrete PGF2c, independent of energy level, therefore only heifers from isocaloric treatments, IL (1 L Intralipid) and DEXT (1 L dextrose), were included in an oxytocin (OT) challenge as follows: On day 12 PP, heifers were separated from their calves, and 150 U of OT (Phoenix Pharmaceuticals) were administered i.v. via catheters. Blood samples were taken at time -30, -15, 0, 5, 15, 30, 45, 60, 75, 90, 105, and 120 min relative to OT injection. Behavioral activity and plasma concentrations of progesterone (P4) were used to determine number of days to first estrus. Sexual behavior of heifers (standing estrus) was observed twice daily from 21 to 150 days PP, initially by exposure of heifers to androgen- treated cows (Jerry Reeves, Washington State University, Pullman, WA, personal communication; Appendix B), and after the beginning of the breeding season (30 to 75 days PP), by use of intact bulls continually housed with heifers. Heifers were bled twice weekly (Mondays and Thursdays) for plasma concentrations of P4 beginning 3 wk PP until 12 days 49 after first behavioral estrus. First estrus with ovulation was defined as heifers standing for the bull with subsequent plasma P4 being greater than 1 ng/ml for two consecutive samples. Pre- estrus luteal activity was determined by evaluating plasma concentrations of P4 in samples taken during the 2 wk prior to first estrus. PGFM analysis Concentration of prostaglandin Fa, metabolite (PGFM; 13,14-dihydro-15-keto PGF2a) in plasma samples (100 1A1) from days 1 to 15 (every other day), from selected samples collected during the infusion period (time 0, 4, and 8 hours on days 7, 9, and 11), and from samples collected for the OT challenge was measured by radioimmunoassay (RIA) as described by Guilbault et al. (1984) and established in our laboratory by Burke et al. (1996; Appendix B). Intra- and interassay coefficients of variation (CV) were 7.7 and 11.7%, respectively, and the sensitivity of the assay was 50 pg/ml. The PGFM antibody (# J-53 antiPGFM) was a gift from William Thatcher (University of Florida), while PGFM standards and competitor (13,14-dihydro-15-keto [5,6,8,9,11,12,14(n)-311] PGFaL; 177 Ciimmol) were purchased from Cayman Chemical Company (Ann Arbor, MI) and Amersham (Arlington Heights, IL), respectively. Prostaglandin free plasma for use in standards was harvested from a cow treated twice (16 hours apart) with an intramuscular injection of a PG synthesis inhibitor, 20 ml of 50 mg/nil (1 g) flunixin meglumine (Banamine; Schering-Plough Animal Health Corp, Kenilworth, NJ;). Blood was collected into evacuated heparinized flasks 4 hours after the second injection, placed on ice, and centrifuged 2,500 x g for 15 min at 4 °C. Plasma was removed and stored at -20 °C. 50 Fatty acid analysis Fatty acids (FA) were extracted from plasma for day 7 (hour 0, 4, and 8) and day 11 (hour 0) with methanol:chloroform (2:1 vol/vol; Bligh and Dyer, 1959), methylated with boron trichloride and benzene, and then subjected to gas chromatography (Song and Wander, 1991) for identification (Appendix B). Samples selected for FA analysis were chosen in order to compare them to selected samples in which plasma concentrations of PGFM were found to be altered. Values for selected FA are reported as weight percentage of total (unfractionated) plasma FA. Progesterone analysis Plasma P4 was measured in duplicate (100 p.1) by RIA as described by Koligian and Stormshak (1976) using benzene:hexane (1:2 vol/vol) to extract plasma samples taken prior to and during the subsequent breeding season. Extraction efficiency was 80.3%, intra- and interassay CV were 9.3 and 14.8%, respectively, and sensitivity of the assay was 0.05 ng/ml. The antibody (#337 anti-progesterone-11-BSA) was a gift from Gordon Niswender (Colorado State University). Tritiated P4 ([1,2,6,7 -3H] 134; 12 x 103 dpm; 115 Ci/mmol) was purchased from New England Nuclear (Boston, MA) and P4 standards were purchased from Cayman Chemical Company (Ann Arbor, MI). 51 Statistical analysis Data on plasma concentrations of PGFM during the infusion period were subjected to a repeated measures analysis of variance (ANOVA) using Statistical Analysis Software (SAS, 1994). The factors included in the full model were treatment (TMT; IL, DEXT, 0.51L, and SAL), heifer within TMT, Hour, and Day. Also included were the interaction effects TMT x Hour, TMT x Day, and TMT x Hour x Day, and beginning PGFM (hour 0, day 7) was included as a covariate. To further define treatment effects, linear and quadratic orthogonal regression contrasts were used for analysis of Hour effects within TMT, and pairwise contrasts 0 to 4 and 4 to 8 hours relative to infusion were considered. Data for concentrations PGFM on days 1 to 15 and during the OT challenge (day 12, time -60 through 120 min) were also analyzed by repeated measures ANOVA, with hour 0 on day 12 used as a covariate for the OT data. Response in PGFM for the OT challenge was heterogeneous with respect to variance, therefore analysis was performed on log transformed data. Values for plasma weight percentage of FA during the infusion period on day 7 were analyzed by repeated measures ANOVA. The model was TMT, heifer within TMT, Hour, and TMT x Hour. Repeated measures ANOVA was also used to analyze for differences in plasma FA between days 7 and 11 PP time 0, with TMT, heifer within TMT, Day, and TMT x Day as factors in the model. Relationships between plasma weight percentage of fatty acid and PGFM concentration were evaluated by correlation analysis. Finally, ANOVA was used to analyze body weights (BW), BCS, 5-day feed intake, calf weights, number of days to first estrus with ovulation and calving interval, and chi square 52 analysis was used to analyze data for calving ease (no assistance or light pull), calf sex, and reproductive status at 150 days PP (estrus or anestrus) and pregnancy rate the subsequent fall (pregnant or nonpregnant). Results Plasma concentrations of PGFM decreased over days 1 to 15 PP (P < 0.05), the variability was high, and there was no significant treatment by day interaction (Figure 4.2). Changes in plasma PGFM in response to infusion of IL, DEXT, 0.5IL, and SAL on days 7, 9, and 11 are depicted in Figure 4.3, with concentrations decreasing significantly over days. There was no significant TMT x DAY x Hour interaction; however, response to infusion was characterized by a TMT x DAY interaction (P = 0.07), i.e., differences among treatments depended upon day of infusion. Variation in plasma concentrations of PGFM was also different (P < 0.0001) depending on day, therefore data for each day were analyzed separately. There were no differences (P > 0.10) among treatments for hour 0 plasma concentrations of PGFM on days 7, 9, or 11. Therefore, hour 0 on day 7 was used as a covariate in ANOVA for PGFM on hours 4 and 8 on days 7, 9, and 11 to account for beginning PGFM concentration. Upon examining data it was found that IL caused the greatest changes in prostaglandin concentrations over time. On a within day basis, plasma concentrations of PGFM on day 7, were significantly different among treatments for hour 4, with heifers that received IL having greater PGFM than DEXT-, 0.5IL- and SAL-treated heifers. No additional differences among treatments were found for day 7 hour 4 or 8. 4000 -9- Col 55 vs Col 56 0 Col 55 vs Col 59 9 Col 55 vs Col 63 3500 17 Col 55 vs Col 67 3000 i.v. Infusion Days 7 to 11 2500 2000 1500 1000 - 500 I I z 1 3 5 7 9 0 11 13 15 DAYS POSTPARTUM Figure 4.2. Mean (± SE) prostaglandin Fla metabolite concentration days 1 through 15 postpartum t 1600 -0- 1 L INTRALIPID DAV 7 1400 A --,- a 1200 1 L DEXTROSE .5 L INTRALIPID 1 L SALINE DAY1 1000 DAV 11 800 ..11111\ 411111111N!, 600 400 200 4ii hi C d 0 0 4 8 0 4 8 0 4 8 TIME (h) Figure 4.3. Mean (± SE) prostaglandin Fla metabolite concentration hours 0, 4, and 8 on days 7, 9, and 11 postpartum. ' On day 7, 1 L Intralipid differed (P = 0.04) from other treatments for 0 to 4 hours, 4 to 8 hours, and at 4 hours. b On day 11, 1 L Intralipid differed (P < 0.01) from other treatments for 0 to 4 hours. Day 11 hour 4, 1 L Intralipid differed (P = 0.06) from 1 L dextrose and 0.5 L Intralipid, but not from 1 L saline (P > 0.10). d Day 11 hour 8, 1 L saline differed (P = 0.07) from 1 L dextrose and 0.5 L Intralipid, but not from 1 L Intralipid (P > 0.10). 55 Because of the extreme variation in response, no significant treatment differences were found by ANOVA for PGFM on day 9 for any hour. On day 11, plasma PGFM was different among treatments for hour 4, with heifers receiving IL having greater concentrations of PGFM than DEXT- (P < 0.06) and 0.5IL-treated (P < 0.02), but not SAL-treated (P < 0.43) heifers; concentrations of PGFM from DEXT- and 0.5IL-treated heifers were not different from each other; and SAL-treated heifers had greater concentrations of PGFM than 0.5IL, but not different from IL- and DEXT-treated heifers. The only significant difference found on day 11 hour 8 was that concentrations of PGFM for SAL-treated heifers were greater than those of DEXT- and 0.5IL-treated heifers. The above results are supported by an overall regression analysis of the plasma concentrations of PGFM over hour 0, 4, and 8, again considering infusion day separately, but without the covariate of day 7 hour 0. This analysis and additional sums of squares F test, revealed a significant effect of treatment for day 7 (P = 0.05) and 11 (P = 0.05), but not on day 9 (P > 0.10). Subsequently, differences among means were tested for significance by orthogonal contrasts. On day 7 and 11 PP there was a quadratic response curve for heifers administered IL on the concentration of PGFM for 0, 4, and 8 hours which differed significantly from other treatments. This is reflected in the results of pairwise contrast analyses, which revealed that PGFM for heifers on IL significantly increased from 0 to 4 hours, then significantly decreased from 4 to 8 hours (days 7 and 11), and that these changes (0 to 4 and 4 to 8 hours) differed (P < 0.01) from those of the other treatments. Prostaglandin concentrations of heifers had no quadratic or linear responses to SAL or DEXT on any day examined (P > 0.10). For 0.5IL, only on day 9 was there a significant response over hour 0, 4, and 8 which was quadratic (P = 0.009) and differed from DEXT-treated (P 56 = 0.04), but not IL- or SAL-treated (P > 0.10) treated heifers. On day 9 a tendency (P = 0.11) for a quadratic response in PGFM was present for IL-treated heifers, but it was not different (13' > 0.20) from heifers on other treatments. The only significant change for DEXT- treated heifers was an increase in PGFM from 4 to 8 hours on day 7. Analysis of data for plasma FA on day 7 revealed that prior to infusion, the weight percentage of major FA in plasma were not different (13' > 0.10) among treatments; however, significant differences existed at 4 and 8 hours. Because linoleic acid is the dietary precursor of PG, and linolenic acid helps to modulate PG synthesis, profiles for these EFA were most pertinent to this experiment, and are shown in Figure 4.4. Only samples for day 7, when differences for 4-hour concentrations of PGFM were the greatest, were analyzed for FA profile; and differences within the same hour are specified in the figure. Differences in C18:2 and C18:3 between hour were also present and depended on treatment and hour (TMT x Hour interactions, P < 0.04). Regression analysis of these data revealed a significant quadratic response curve (i.e., increased 0 to 4 hours, then decreased 4 to 8 hours) for linoleic acid with all treatments except SAL. However, for linolenic acid, neither 0.5IL nor SAL significantly altered this FA, and IL-treated heifers had a linear decrease (P < 0.05) in C18:3 over time, while the changes in C18:3 in DEXT-treated heifers resulted in a quadratic response curve (P < 0.01). Other FA in plasma that increased or decreased in response to infusion of treatments are as follows: By 4 hours, plasma of heifers infused with IL had greater concentrations of C16:0 and C18:1 compared with those of DEXT-, 0.51L -, and SAL-treated heifers (P < 0.02 and P < 0.0001, respectively); while levels of C18:0 were greater in DEXT-treated (P < 0.06) but less than in SAL-infused heifers (P < 0.0001). By 8 hours, plasma C16:0 did not 57 30 F 25 0 20 E.") C-) EZ). 0 15 10 5 0 4 0 8 F.-1 1 L Intralipid MEM 1 L Dextrose 0.5 L Intralipid 1 itmoinal 1 L Saline B 30 25 20 15 a 10 0 4 8 TIME (HOUR) Figure 4.4. Mean (± SE) weight percentage of plasma linoleic (A) and linolenic (B) acids in response to infusion on day 7, hour 0, 4, and 8. ",Bars without a common letter within hour differ (P < 0.05) 58 differ (P > 0.10) among treatments; but IL-infused heifers had lower (P < 0.005) concentrations of C18:0 compared with those treated with SAL and greater (P < 0.04) C18:1 levels compared with heifers treated with DEXT, 0.511, and SAL. No correlation (P > 0.10) was present for percentage of linoleic acid and PGFM concentration on day 7 hour 4. On day 7, FA elongation products were as follows: Plasma AA (C20:4co6) was not different among treatments on day 7 for samples examined during the infusion period. By 4 hours, plasma levels of EPA (C20:5o3) were lower (P = 0.0007) for heifers receiving IL compared with that of heifers subjected to other treatments. Plasma levels of DHA (C22:6o3) were greater for 1L- treated heifers than for those treated with DEXT and SAL, but not 0.5IL. By 8 hours, EPA was lower (P = 0.03) for heifers given IL compared with those receiving DEXT and 0.5M. Results of FA plasma analyses revealed that initial plasma FA content did not differ significantly among treatments, with the exception of initial plasma content of stearic acid (C18:0) being greater in IL-treated heifers as compared with heifers from other treatments (Table 4.1; data for 0.511- and SAL-treated heifers not shown). Data from FA analysis for day 11 hour 0 plasma from IL- and DEXT-treated heifers were included in order to evaluate plasma FA content (specifically, linoleic acid) prior to OT challenge. Weight percentage of C18:2 in plasma was greater in heifers receiving IL (P = 0.001) than for DEXT-treated heifers. Additionally, day 12 OT-induced release of PGFM was greater (P < 0.06) for lipid- infused as compared with DEXT-infused heifers (Figure 4.5; untransformed data), but no significant correlation between linoleic acid on day 11 and PGFM response to OT injection on day 12 was detected. 59 Table 4.1. Fatty acid profiles (mean ± SE) from whole plasma on days 7 and 11 postpartum Treatment 1 L Intralipid Fatty Acid' Day 7 1 L Dextrose Day 11 Day 7 Day 11 Weight % C16:0 14.31.44 13.71.52 14.11.34 14.41.49 C18:0 14.91.24b 12.91.30 13.81.16' 12.31.35 C18:1 13.91.82 8.61.71d 12.81.54 12.31.35` C18:2 22.911.1 40.511.2d 22.71.57 22.91.71e C18:3 10.61.75 5.751.35d 11.31.37 11.71.29e C20:4 2.61.17f 2.91.14g 2.61.14f 3.021.28g C20:5 1.741.22 1.181.16d 2.041.14 2.341.07e C22:6 0.561.07 0.721.15d 0.521.05 0.441.02` 'Fatty acids C16:0, palmitic; C18:0, stearic; C18:1, oleic; C18:2, linoleic; C18:3, linolenic; C 20:4, arachidonic; C20:5, eicosapentaenoic; C22:6, docosahexaenoic. Day 7 means within the same row with different superscripts differ (P < 0.01). Day 11 means within the same row with different superscripts differ (P < 0.01). gl'ercentage of C20:4 differed (P < 0.01) between day 7 and 11, but not among treatments (P > 0.10). There were no differences (P > 0.10) among treatments for reproductive data (Table 4.2). According to the date of their second calving, all heifers diagnosed as pregnant conceived on their first estrus postpartum. Thirty-six percent had no detected progesterone rise prior to first estrus while in 64% of the heifers a transient (5.4 + 0.8 days) increase in plasma progesterone (0.65 + 0.25 ng/ml) was detected. 1200 ** * NM NM 1000 800 MO 0 400 200 --0 1 L Intralipid 0 -45 40 I I I I -15 0 15 30 T -0- 1 L Dextrose I 45 60 75 90 105 120 135 TIME (MIN) OT Figure 4.5. Response in prostaglandin F2c, metabolite (PGFM) to oxytocin challenge (mean ± SE). *13 < 0.10 and **P < 0.05. oo 61 Table 4.2. Mean number of days to first estrus, pregnancy rate, and calving interval Treatment' IL DEXT 0.51L SAL Common SEC Days to first estrus d 129.5 126.3 120.0 133.2 6.0 Pregnancy rate (%) 75 80 100 100 nd 409.7 406.3 397.7 410.4 6.3 Item b Calving Interval (days) 'IL, 1 L Intralipid, n = 4; DEXT, 1 L dextrose, n = 3; 0.5IL, 0.5 L Intralipid, n = 5; SAL, 1 L saline, n = 5. "No difference among treatments for any item (P > 0.10). Common estimate of the standard error. nd, not determined. d For two heifers from DEXT, days to first estrus was estimated from calving date and plasma progesterone. For one heifer each from IL and DEXT, days to first estrus was estimated from calving date and behavioral estrus. There were no differences (P > 0.10) among treatments for body weights and condition scores (Table 4.3). Heifers lost BW (P = 0.08) and BCS decreased (P = 0.0001) during the first 30 day PP. At 150 day PP, heifers body weight (P = 0.40) and body condition (P = 0.53) were not different from day 30 PP; however, BCS at day 150 was significantly lower (P < 0.0001) than was recorded for these heifers immediately prior to calving. No difference (P > 0.10) among treatments was found for calving assistance (75% unassisted), calf sex (50% male), and mean daily intake of meadow hay as a percentage of metabolic body weight (9.1% of BW"). 62 Table 4.3. Mean body weights, body condition scores, and calf weights Treatment' IL DEXT 0.5 IL BW 1d (kg) 363.6 336.6 368.6 BW 2 344.4 335.8 346.8 335.0 8.7 BW 3 344.6 332.1 348.8 345.6 8.2 BCS 1 °(1- 9) 5.4 5.3 5.3 5.1 1.0 BCS 2 4.3 4.0 3.9 3.4 0.96 BCS 3 3.9 3.7 3.5 3.8 0.71 Calf Wt lf (kg) 34.5 33.9 31.8 33.0 2.4 Calf Wt 2 129.7 136.2 131.4 132.1 7.8 Calf Wt 3 205.2 202.3 184.5 199.6 8.6 Item" SAL 341.8 Common SE' 8.9 'IL, 1 L Intralipid; DEXT, 1 L dextrose; 0.5EL, 0.5 L Intralipid; SAL, 1 L saline. "There were no differences among treatments for any item (P > 0.1). Common estimate of the standard error. BW, body weight 1 on day 1 postpartum (PP); BW2 at 30 days PP; BW3 at 150 days PP. Body condition score (1 = emaciated, 9 = obese); BCS 1 at day 1 PP; BCS 2 at 30 days PP; BCS 3 at 150 days PP. fCWT, Calf weight 1 at day 1 PP, CWT 2 at day 150 PP; CWT at weaning (day 240 PP). Discussion Infusion with 1 L Intralipid resulted in higher plasma linoleic acid and PGFM concentrations more consistently than other treatments. These data are compatible with the hypotheses that infusion of lipid changes plasma FA profile, and that increased plasma linoleic acid increases systemic PGF2C. However, administration of lipid only transiently increased 63 plasma PGFM; no carry-over effects on basal plasma concentrations of PGFM were detected on subsequent days. The decrease in PGFM over days 1 to 15 in this experiment is similar to that previously reported and reflects the naturally declining plasma concentrations of PGF2a in PP cows (Guilbault et al., 1984). The inability of short-term infusion of EFA (days 7 to 11) to alter overall PP profile of PGFM may be due to factors other than substrate availability controlling this process. Timing in the delivery of lipid is apparently an important factor in prostaglandin production as a response to lipid infusion. This is exemplified in previous experiments with heifers (Lucy et al., 1990) and ewes (Burke et al., 1996) in which response was dependent on day of the estrous cycle, and in this experiment with PP anestrous heifers, in which day of infusion affected PGFM response. Hence, to obtain a greater response a more appropriate regimen might have been to provide PP heifers with lipid earlier in the puerperium when PGF2a is normally present in a greater quantity. The time factor may be a reflection of synthesis capability, for example presence of enzymes, at various reproductive periods. In the guinea-pig, an increase in PG synthase (PGS) protein, the enzyme required for synthesis of PG, increases both uterine PG synthesis towards the end of the estrous cycle (Poyser and Leaver, 1979) and ovarian PG synthesis just prior to ovulation (Poyser, 1981). Similar patterns of PGS presence in these tissues exist for the ovine uterus (Huslig, et al., 1979) and bovine preovulatory follicles (Sirois, 1994). Studies on the presence and regulation of PGS in the PP uterus are lacking, but it is known that expression of PGS is highly regulated in the rat uterus during pregnancy and parturition (Sirois and Hawkins, 1992), and physiological stimulus for PG synthesis and release is regulated during luteolysis in the guinea-pig (Poyser, 1991). 64 Weight percentage of linoleic acid in plasma prior to treatment in this experiment is in agreement with values reported by Jenkins (1995). Changes in FA profiles during infusion were complex, but reflect the content of lipids administered. A dose response in PGFM with increasing quantity of lipid infused (no lipid [SAL], 0.5IL, and IL treatments) was observed at 4 hours after infusion. It remains a possibility that further supplementation with lipid could cause more PG to be released. Budowski (1988) demonstrated that production of PG in humans increased with increasing dietary linoleic acid up to a specific level, and then no further response was achieved. Infusion of lipid altered plasma FA profiles for at least 18 hours post-infusion (time between last infusion day 10 and pre-infusion samples obtained on day 11). These changes are pronounced and it is apparent that plasma and possibly tissue loading of EFA has occurred as reported with abomasal infusion of FA in sheep (Moore et al., 1969) and in cows fed or injected with fat (Tove and Mochrie, 1963). During infusions the change in weight percentage of FA was small and sometimes nonsignificant. The volume of infiisant and absolute quantity of individual FA infused was small relative to the volume of plasma in the animal and amount of FA in plasma. Some FA increased, and some decreased during the infusion period thus altering ratios of EFA to nonessential FA and ratio of saturated to polyunsaturated FA. Mathias and Dupont (1979) reported that PG production may be more sensitive to changes in ratios rather than absolute amount of FA. Burke et al. (1996) reported increases in plasma concentrations of PGFM during 4- hour lipid infusions into ewes during the midluteal phase of the estrous cycle and found a greater response with emulsions of olive oil (8% linoleic and 1% linolenic acids) as compared with soybean oil (50% linoleic and 9% linolenic acids). Relationships between lipid 65 administration and PG production must be scrutinized closely because linoleic, oleic, and to a greater degree linolenic acids are known to have inhibitory effects on production of PG in ram seminal vesicles and rat stomach homogenates (Pace-Asciak and Wolf, 1968). DanetDesnoyers et al. (1993) and Thatcher et al. (1994) also reported inhibitory effects on PGF2c, production by cytosolic components from endometrial explants of pregnant cows. These components were initially associated with serum albumin, and were later isolated and identified to included C16:0, C18:0, C18:1, C18:2 and C18:3 FA. Gan-Elepano et al. (1981) described the intricacy of PG production that occurs within microsomal membranes, and reported that membrane fluidity, and substrate, enzyme, and cytosolic protein concentrations are difficult to replicate. Leikin et al. (1979) also published information on a specific protein required for desaturation of C18:2co6, that is apoprotein-like in nature and is not substituted for by albumin. Albumin in that study bound the linoleic acid and inhibited its desaturation. No evidence of inhibition by lipid infusion was found in the present in vivo experiment. Infusion of lipid increased the capacity of PP heifers to synthesize and(or) secrete PGF2, as indicated by plasma concentration of PGFM after OT injection. Oxytocin causes the release ofPGF2c, from the endometrium by binding to OT receptors in that tissue (Roberts et al., 1976). The pattern of plasma PGFM increase after OT injection is in agreement with a previous report by Del Vecchio et al. (1990). Low baseline PGFM concentrations prior to OT injection demonstrates that the immediate increase in PGFM after OT is not merely decreased clearance of eicosanoid hormone caused by lipid infusion. Also, heterogeneity of variance apparent in the production of PGFM after OT in the current study exemplifies the difference in capacity of individual heifers to respond to supplemental lipid. Differences in ability to respond to supplemental lipid in a manner favoring fertility have also been reported 66 for cows with differing body condition score (Morgan and Williams, 1989; Spoon et al., 1990), different duration of supplement feeding (Oss et al., 1993; Lucy et al., 1991a), and type of supplemental lipid (Wehrman et al., 1991; Beam and Butler, 1997). Although PGFM concentrations were transiently altered early in the PP period by infusion of lipid into heifers, no change in fertility was found. The extended PP anestrous period for the heifers in the current experiment may have made uterine effects less influential on ovarian activity compared with cows that resume ovarian activity earlier in the PP period. This may provide an explanation for the lack of short cycles in these heifers, and facilitated pregnancy on first estrus after parturition. Cows returning to estrus after a period of PP anestrus frequently have estrous cycles of short duration (10 -12 days) with plasma concentrations of P4 greater than 1 ng/ml prior to resumption of estrous cycles of normal length (Odde et al., 1980); however, these short cycles are not a prerequisite for normal cyclicity (Peters and Lamming, 1984). This progesterone "priming" of the system is thought to be important for supporting a pregnancy from subsequent ovulations by controlling uterine OT receptor populations and premature release of PG responsible for early demise of CL frequently observed in early PP cows (Lishman and Inskeep, 1991; Beard and Hunter, 1994). Initial evidence that infused lipid alters PGFM in the PP period is provided in this study, and further investigations on how the FA are distributed among plasma lipid fractions of cholesterol esters (CE), phospholipids (PL), non-esterified FA (NEFA), or triglycerides (TG) are now underway (D. Palmquist, personal communication). According to the latter investigator, this information is important because in the ruminant, EFA are selectively used for storage CE and are incorporated into PL that are used for essential functions, e.g., PG production, rather than being incorporated into adipose tissue for energy stores as are TG and 67 NEFA which are incorporated into TG (Van Soest, 1982). Also, characterization of bovine uterine PGS in the PP period is needed, and further research to improve fertility in primiparous heifers using fatty acids or other specific nutrients to favorably alter hormone concentrations is warranted. In conclusion, infusion of Intralipid into PP heifers alters plasma FA profile to favor linoleic acid content, transiently increases systemic PGFM, and increases the capacity of PP heifers to produce PGF2c, as indicated by plasma PGFM after OT injection. Reproductive response was not improved by short-term infusion of lipid in the early PP period. 68 5. PLASMA FATTY ACIDS, PROSTAGLANDIN F2., AND REPRODUCTIVE RESPONSE IN POSTPARTUM HEIFERS FED RUMEN BY-PASS FAT Abstract An experiment was conducted to determine whether feeding rumen-protected fatty acids (FA) to postpartum (PP) heifers would increase plasma concentrations of linoleic acid and PGF2a, shorten the interval from calving to first rise in plasma progesterone (P4), and increase pregnancy rate relative to controls. Thirty-nine Hereford x Angus heifers (346 kg) were assigned randomly to supplement treatments of .23 kg-head-1d' calcium salts of FA (CSFA; n = 20) or an isocaloric amount of barley (control; n = 19) for the first 30 days PP. Supplements, with .23 kg barley as a vehicle, and a basal diet of meadow and alfalfa hays were pen fed to heifers (5/pen). Heifers were bled on alternate day (days 1 to 30) and 2x weekly (d 21 to 2 wk after first estrus) for RIA of plasma PGF2c, metabolite (PGFM) and P4, respectively. Weight percentage of major FA in plasma on days 1 and 7 was determined by gas chromatography. First behavioral estrus was detected by use of intact bulls, and confirmed by an increase in plasma P4. Data were analyzed using x2 and ANOVA (repeated measures and one-way). On day 7, but not day 1, plasma from heifers fed CSFA had altered proportions of major FA (P < .01), including an increase in linoleic acid compared with that of controls (29.1 vs 25.6 weight %, SE = .75; P = .003). Analysis of variance of contrast variables revealed an effect of treatment on change in PGFM from days 3 to 5 (P = .004). By day 7 and on day 9, plasma concentrations of PGFM were greater in heifers fed CSFA compared to those of controls (P = .02 and P = .06, respectively). There was no significant 69 difference in PGFM between treatments on days 1, 3, 5, 11, 13 and 15 PP. Days to first estrus with ovulation, pregnancy rate, and calving interval were not affected by treatments (P > .10). Although supplemental lipid fed to primiparous beef heifers increased plasma levels of linoleic acid and production of PGF2a in the early PP period, it did not improve the fertility of these heifers in the subsequent breeding season. Key Words: Prostaglandin, Fatty Acids, Cattle Introduction It is of economic importance for commercial beef cattle producers to have a well defined breeding season whereby each cow produces one calf per year. Because the postpartum (PP) interval to first behavioral estrus with ovulation is longer in primiparous than in multiparous cows, pregnancy rates in first calf heifers may be improved by shortening this PP period (Wiltbank, 1970). Fertility resumes after uterine involution and repair and the ovulations that occur result in cycles of normal duration (Kiracofe, 1980). The hormone prostaglandin (PG) F, is important for uterine involution and ovarian function. Duration of increased production of PGF2c, in the PP period is negatively correlated with the number of days to complete uterine involution and the length of interval between parturition and resumption of normal ovarian activity (Madej et al., 1984). Nutritional approaches, including altered dietary lipid content, have been used to improve reproductive and lactational performance in cattle. In particular, plasma cholesterol levels, lipid profiles, and hormone concentrations can be altered by including fat in livestock diets (Dunn et al., 1969; Schneider 70 et al., 1988). Calcium salts of fatty acid (CSFA) fed to PP dairy cattle have been reported to positively affect ovarian follicular dynamics (Lucy et al., 1991a). Linoleic acid, an essential fatty acid (EFA), is converted to arachidonic acid, the immediate precursor of prostaglandins, and the infusion of lipid into ruminants (Lucy et al., 1990; Burke et al., 1996; Filley et al., 1997) and feeding EFA to non-ruminants (Mathias and DuPont, 1979; Adam et al., 1982) increased plasma PG. Because 70 to 90% of unsaturated FA entering the rumen are biohydrogenated (Bickerstaffe et al., 1972), protected lipids increase the amount of unsaturated FA absorbed by the ruminant (Tove and Mochrie, 1963). It is not known whether PP beef heifers fed lipids containing EFA have increased plasma concentrations of linoleic acid and PGFM and improved reproductive performance. The objectives of this experiment were to determine whether feeding rumen-protected fatty acids to PP heifers would increase plasma concentrations of linoleic acid and PGF2a, shorten the interval from calving to first estrus, and increase pregnancy rate. Characteristics of the calf that may affect production of PGF2c, and heifer performance, such as dystocia, calf sex, and calf weight, were also examined. Materials and Methods Animals, treatments, and samples Thirty-nine Hereford x Angus primiparous beef heifers (346 kg) in the immediate PP period were assigned to treatments randomly as they calved, and fed native flood meadow hay and trace mineral salts ad libitum, with alfalfa hay (2.3 kghead-l.d4) as a source of additional protein. Treatments consisted of either fat (n=20) or control (n=19) supplement fed days 1 71 through 30 PP. Fat supplement, at approximately 3% of estimated dry matter intake, was .23 kghead-1 -d-1 of rumen inert fat as calcium salts of fatty acids [CSFA; Church and Dwight, Inc., Ca salts of palm oil; 44% palmitic (C16:0), 40% oleic (C18:1), 9.5% linoleic (C18:2) and 5% stearic (C18:0) acids] mixed with .23 kg ground barley. Control supplement was an isocaloric amount of ground barley (.72 kg). Animals were fed in groups of 5 (8 pens; 4 replicate pens per treatment), and calves remained with dams. Heifers were body condition scored (BCS; scale 1 - 9; 1 = emaciated, 9 = obese) approximately 2 wk prior to calving, at 30 day PP, and at the end of the breeding season (150 day PP). Heifers were weighed at 1, 30, and 150 days PP. Calving ease was scored either as unassisted, light pull, or hard pull. Only heifers scored as unassisted or light pull were included in this experiment. Calf weight at 1 and 150 days PP (CWT 1 and CWT 2, respectively), and calf sex were recorded. Bay was fed twice daily, with alfalfa fed in the morning and meadow hay in the afternoon. Unconsumed meadow hay was removed every morning, and daily intake was calculated as kilogram of meadow hay consumed per kilogram of metabolic body weight. Experimental supplements were fed once daily at noon. Feed bunks were designed to accommodate up to 20 heifers, and each of the five heifers in the pen had adequate room to receive the supplement as an individual. Heifers were eager to consume the supplement and care was taken that each heifer ate only her portion. In order to assess plasma fatty acid profiles on days 1 and 7 and PGF production for days 1 to 15, heifers were bled by venepuncture every other morning using heparinized vacutainer tubes. The blood was immediately placed on ice, and within 1 hour centrifuged at 2,500 x g for 15 min at 4°C and the plasma stored at -20°C. After 30 days on the supplements, heifers were released to pastures where they continued to receive meadow and alfalfa hays. At approximately 60 days PP, heifers were moved to 72 summer ranges to graze high desert grasses and forbes. Behavioral activity and plasma P4 concentration were used to determine number of days to first estrus. Heifers were observed twice daily for sexual behavior from 30 to 150 days PP, and were bled twice weekly for plasma P4 concentrations beginning 30 days PP until 12 days after first behavioral estrus. First estrus with ovulation was defined as heifers standing for the bull with subsequent plasma concentrations of P4 being greater than 1 ng/ml for two consecutive samples. Pre-estrus luteal activity was determined by evaluating plasma P4 concentrations in samples taken during the 2 wk prior to first estrus. Analysis of PGFM, fatty acids, and progesterone A subset of heifers (12 per treatment) was selected randomly for plasma PGFM (13,14-dihydro-15-keto PGF) and FA analyses, performed as in experiment 1 and described by Guilbault et al. (1984) and Song and Wander (1991), respectively. Plasma PGFM intra- and interassay CV were 7.7 and 11.7%, respectively, and sensitivity was 50 pg/ml. Values for selected fatty acids are reported as weight percentage of total (unfractionated) plasma fatty acids. Plasma progesterone was measured in duplicate (100 ill) for all heifers by R1A as in experiment 1 and as described by Koligian and Stormshak (1976) using benzene:hexane (1:2 vol/vol) to extract plasma samples taken prior to and during the subsequent breeding season. Extraction efficiency was 58.5%, intra- and interassay CV were 6.6 and 7.8%, respectively, and sensitivity of the assay was 50 pg/ml. 73 Statistical analysis All data were analyzed using SAS (1994). Analysis of variance (ANOVA) was used to analyze body weights, body condition scores, feed intake, calf weights, and number of days to first estrus with ovulation. Data for PGFM concentration and fatty acid profiles were analyzed by repeated measures (ANOVA). Chi square was used to analyze data for calving ease (no assistance or light pull), calf sex, and reproductive status at 150 days PP (estrus or anestrus) and pregnancy rate the subsequent fall (pregnant or nonpregnant). Simple correlation analysis was used to evaluate relationships between weight percentage of FA and plasma PGFM concentrations. Results Plasma concentrations of PGFM decreased (P = .0001) from day 1 to basal levels on day 15 PP reflecting the natural decline of PGFM during the PP period (Figure 5.1). Also, there was a significant treatment by day interaction (P < .0001), which is explained by the increase in plasma PGFM for CSFA-fed heifers as compared with controls on days 5, 7, and 9. Analysis of variance of contrast variables revealed a significant effect of treatment on change in PGFM from days 3 to 5 PP (P = .004). By day 7 PP and on day 9 PP, plasma concentrations of PGFM were greater for heifers fed CSFA as compared to controls (P = .02 and P = .06, respectively). There was no significant difference in PGFM between treatments on days 1, 3, 5, 11, 13 and 15 PP. 3000 - II- CONTROL 0- CSFA 2500 2000 1500 1000 500 1 3 5 7 9 11 13 15 DAY POSTPARTUM Figure 5.1. Mean (± SE) plasma concentration of prostaglandin Fa, metabolite (PGFM) on days 1 to 15 postpartum. CSFA (calcium salts of FA; n = 12) and control (barley; n = 12) supplements. *P = .02 on day 7 and *P = .06 on day 9 postpartum. 75 On day 1 PP, the plasma fatty acid profiles of heifers were not different between treatments; however, by day 7, proportions of major FA in plasma were different (P < .01) in heifers fed CSFA compared with that of controls, with some FA decreasing and some increasing (Table 5.1). Weight percentage of C18:2 on day 7 was correlated with plasma concentration of PGFM on day 5 (r = .46; P = .02) and day 9 (r = .39; P = .06), but not correlated on day 7 (r = .31; P = .14). Table 5.1. Means (± SE) for weight percentage of total plasma fatty acids for days 1 and 7 postpartum Fatty acid Palmitic (C16:0) Stearic (C18:0) Oleic (C18:1) Linoleic (C18:2) Linolenic (C18:3) Arachidonic (C20:4) Day CSFAa Control' 1 15.7 ± .27 15.2 ± .27 7 16.4 ± .55b 14.1 ± .55' 1 13.1 ± .23 12.6 ± .23 7 12.7 ± .36b 14.1 1 14.1 ± .53 12.8 ± .53 7 14.1 ± .66b 11.6 1 .66' 1 24.7 ± .70 25.0 ± .70 7 29.1 ±:75 25.6 1 .75' 1 10.8 1 .38 11.5 ± .38 7 7.4 ± .35b 10.8 ± .35' 1 2.6 ± .07 2.6 ± .07 7 2.1 ± .09b 2.5 ± .09' .36' aCSFA (calcium salts of fatty acid; n = 20) and control (barley; n = 19). b''Means with different superscripts within the same row differ (P < .01). 76 Reproductive performance was not different (P > .10) between treatments (Table 5.2). The experiment was terminated at the end of the breeding season, at which time heifers averaged 130 days PP and 73.3% had exhibited estrus with no differences (P > .10) between treatments. Days to first estrus with ovulation, pregnancy rate, and calving interval were not affected by treatments (P > .10), and 92% of the pregnancies were from first PP ovulation with estrus. No typical short cycles occurred in these heifers, with 26% having no detected pre-estrus rise in plasma P4, and only transient (5.7 + .18 d) increases in plasma P4 (.92 ± .1 ng/ml) detected in 73.9% of the heifers that exhibited estrus. Table 5.2. Mean number of days to first estrus, pregnancy rate, and calving interval Treatment' Item b CSFA Control Common SEC Days to first estrus 110.6 114.8 3.3 Pregnancy rate (%) 72.2 68.4 nd 389.9 401.2 6.3 Calving Interval (days) CSFA, calcium salts of fatty acid; Control, barley. b No difference among treatments for any item (P >.10). Common estimate of the standard error. nd, not determined. Heifer body weights (BW) and BCS were not different (P > .10) between treatments on days 1 and 30 PP. Kilograms of meadow hay consumed per day (as a percentage of metabolic body weight) and BW at 150 days PP were not different (P >.10) between treatments. However, BCS at 150 days PP was greater (P = .05) for heifers fed CSFA than for heifers fed control diets their first 30 days PP (Table 5.3). 77 Table 5.3. Mean body weights, body condition scores, and feed intake Item' CSFAb Control' Common SE' BW 1 (kg) 347.5 344.9 4.5 BW 2 (kg) 328.7 335.3 4.4 BW 3 (kg) 341.9 333.1 4.6 BCS 1 (1-9) 5.13 5.09 .52 BCS 2 (1-9) 4.10 4.15 .49 BCS 3 (1-9) 3.88d 3.65' .41 FI (%BW '75) 9.6 10.3 .88 a Body weight (BW) and condition score (BCS; scale 1 to 9) 1 at day 1 postpartum (PP), BW 2 and BCS 2 at day 30 PP, BW 3 and BCS 3 at day 150 PP. Daily feed intake (F1) was kg of meadow hay consumed as a percentage of metabolic BW. b Calcium salts of fatty acid (CSFA; n = 20) and control (barley; n = 19) supplement. ' Common estimate of the standard error. d'e Means with different superscripts within the same row differ (P = .05). Calving ease and calf sex were not different (P = .12 and P = .90, respectively) between treatments (Table 5.4). Although heifers were assigned to treatment randomly, initial calf weight (CWT 1) was greater (P = .06) for heifers fed CSFA than controls. However, when only the subset of heifers with PGFM measured (n = 12/ treatment) were included in statistical analysis, there was no difference (P = .19) for CWT 1, and no data were adjusted for CWT 1. This subset of heifers was chosen randomly, however, four out of 12 heifers fed CSFA had assistance in calving and all 12 heifers from the control group calved unassisted. Using chi square analysis, no statistical difference between treatments could be found (quasicomplete separation) for the occurrence of calving assistance. The assistance given was 78 recorded as easy hand pulls, and the biological significance of this event on PGFM concentration is uncertain. Using calving assistance as a factor in ANOVA, PGFM concentrations for heifers fed CSFA were not different between heifers that calved assisted and unassisted. There was no difference (P > .10) between treatments for CWT 2 (day 150 PP). Table 5.4. Calving assistance, calf sex, and calf weights CSFAb Control' Common SEC Calving assistance (%) 30.0 15.8 nd Calf sex (% male) 55.0 52.9 nd CWT 1 (kg) 32.8d 35.0' 1.2 CWT 2 (kg) 122.7 125.2 3.4 Item a a Calf weight (CWT) 1 at day 1 postpartum (PP), CWT 2 at day 150 PP. Calcium salts of fatty acid (CSFA; n = 20) and control (n = 19) supplements. Common estimate of the standard error. nd, not determined. d'e Means with different superscripts within the same row differ (P = .05). Discussion Feeding CSFA to PP heifers resulted in higher plasma linoleic acid and PGFM concentrations. Feeding rumen protected FA changes plasma FA profile, and increases systemic PGF2c, probably as a consequence of the increased systemic levels of linoleic acid. To my knowledge, this is the first study to report increased PGFM by feeding of lipid to ruminants. Moore et al. (1969) demonstrated that intra-abomasal infusion of seed oils 79 increased EFA in plasma lipids of sheep, and Lucy et al. (1990) and Burke et al. (1996) reported increases in PGFM with intravenous infusion of lipid into ruminants. It has also been reported that in vitro treatment of bovine blastocysts, endometrium (Lewis, et al., 1982), and ovarian tissue (Shemesh and Hansel, 1975) with arachidonic acid (an elongation product of linoleic acid) increases PGFM production by these tissues. The greater plasma PGFM on days 7 and 9 for heifers fed CSFA is consistent with data demonstrating that consumption of EFA by humans and rats (Mathias and Dupont, 1979) alters PG concentrations. In humans, plasma PGF2c, increases after 4 to 5 days of increased dietary linoleic acid (Adam et al., 1982). Data from this study suggest that this lag time apparently applies to ruminants as well, and is supported by the observed change in PGFM concentration from days 3 to 5 of the feeding trial for the heifers fed CSFA. The decrease in plasma PGFM over days 1 through 15 PP is consistent with published data, and reflects the natural decline in PGF2c, during this period (Madej et al., 1984). Greater day 7 plasma C16:0 and C18:1 in heifers fed CSFA compared with controls is not surprising given that CSFA is 44% C16:0 and 40% C18:1. However, although CSFA is only 9.5% C18:2, a 17.8% increase in plasma linoleate from days 1 to 7 is remarkable when compared to a 4.4% increase for C16:0. It is known that ruminants conserve EFA for essential functions (Moore et al., 1969), and increased plasma retention may be a part of that process. Lower plasma percentages of C18:0 and C18:3 in lipid-fed heifers were not surprising due to the low and negligible levels of these FA in CSFA. In sheep, abomasal infusion with linoleic acid increased plasma C18:2 and decreased concentrations of C16:0 and C18:0 (Moore et al., 1969). From plasma FA profiles determined here, it can be seen that some changes do not directly reflect FA profile of the diet. As exemplified above by the 80 increase in plasma C18:2, EFA are conserved in the ruminant, while other FA tend to be in equilibrium with tissue FA (Moore et al., 1969). The latter is evident in plasma C18:1, where the high content in CSFA did not lead to a large increase in plasma C18:1. Proper fluidity of membranes is maintained by controlling lipid content, with melting point of FA increasing with increasing chain length and degree of saturation (Singer and Nicolson, 1972). Although both C16:0 and C18:1 were major FA in the lipid supplement, the disproportionate change in plasma may have been due to adjustments for maintaining plasma membrane integrity and other cellular functions. Lower plasma AA (C20:4) was unexpected for CSFA fed heifers, because in non-ruminants, supplemental C18:2 induces increased C20:4 in plasma phospholipids (Sanders and Younger, 1981). More information should be gathered by examining changes in specific lipid fractions, e.g., phospholipid or cholesterol esters of plasma in ruminants fed lipids. Although plasma PGFM for CSFA fed heifers was significantly greater than for controls, reproductive performance was not improved. Duration of elevated PGFM is negatively correlated with days to complete uterine involution and days to first estrus (Lindell et al., 1982; Madej et al., 1984); however, many other factors also influence the length of interval from calving to resumption of estrus (Malven, 1984). Uterine involution was :not determined in this experiment because the manipulation shortens involution time, increases plasma PGFM (Tolleson and Randel, 1987), and could have led to confounding of dietary influence on PGFM concentrations. Mean days to first estrus (112.7 + 8.7 days) was greater than published observations (60 to 80 days) for primiparous beef heifers (Kiracofe, 1980; Randel, 1990), and uterine environment is not considered to influence fertility after the early PP period unless infection or trauma to the uterus is present (Kiracofe, 1980). Because of 81 the extended anestrous period, it is concluded that in this instance, return to estrus was not dependent on or influenced by early PP uterine environmental conditions imposed by pregnancy. Prior to estrous cycles of normal length, the first estrus PP is frequently associated with a cycle of short duration, generally 8 to 10 days in length, with P4 less > 1 ng/ml (Odde et al., 1980; Peters and Lamming, 1984; Wright et al., 1988). An improperly steroidconditioned uterus is thought to be the cause of premature release of PGF2c, and an early demise of the corpus luteum. Pre-exposure of the uterus to P4 improves fertility (Lishman and Inskeep, 1991); however, short cycles are not a prerequisite for fertility. Because no typical short cycles occurred in these heifers, it is theoretically possible that the uterus had ample time to recover through long-term low ovarian or adrenal gland steroid production. Feeding of CSFA in this experiment may have been too early PP to positively affect ovarian follicular dynamics as reported for the feeding of CSFA to PP dairy cattle (Lucy et al., 1991a). A longer period or altered delivery scheme of lipid supplementation may be required to maximally affect reproduction, particularly with respect to ovarian function. Reproductive response to lipid supplement has been shown to be dependent on BCS (Ryan et al., 1994). Although heifers in this study were at an acceptable body condition score at calving, their body weight was less than optimal, which can also delay return to estrus (Wiltbank et al., 1964). Heifers fed CSFA did perform better than controls with respect to terminal heifer BCS and calf weight gain. This may have been due to positive effects of CSFA feeding such as increased milk yield and increased efficiency of energy utilization with respect to milk fat synthesis (Schneider et al., 1988). 82 It is known that male calves suckle more vigorously than female calves and that OT is released upon stimulation by suckling (Hafez, 1993b). The binding of OT to its endometrial receptor causes secretion of PGF2a from the uterus (Roberts et al., 1976). Also, severe dystocia, or calving difficulty, causes trauma to the uterus and requires repair to the uterine lining and delays return to normal uterine environment (Kiracofe, 1980). Although these factors may influence PGF2a secretion during the PP period, neither percentage of calves born male nor calving assistance as recorded in this experiment were different between treatments. Implications Although supplemental lipid fed to primiparous beef heifers altered plasma fatty acid profile to favor increased linoleic acid, and increased plasma PGFM in the early PP period, it did not improve the fertility of these heifers in the subsequent breeding season. Continued studies in nutrition and reproduction using specific nutrients to affect specific hormones and initiation factors are warranted. Acknowledgments Calcium salts of fatty acids were donated by Church and Dwight Co., Inc., Princeton, N.J. The PGFM antibody (# J-53 anti-PGFM) was a gift from William Thatcher (University of Florida). The P4 antibody (#337 anti-progesterone-11-BSA) was a gift from Gordon Niswender (Colorado State University). 83 6. GENERAL CONCLUSIONS The two experiments conducted provided differing perspectives in administration of EFA to primiparous beef heifers for PG production early in the PP period, both with the goal of decreasing days to first estrus and increasing pregnancy rate. Experiment 1 was a basic study, and examined effects of short-term infusion of lipid on immediate response of PGF2a in order to establish whether providing EFA early during the PP could increase the production of this eicosanoid, and improve fertility. Experiment 2 was an applied study, and addressed the effects of feeding supplemental lipid, at a time convenient for most producers, to improve reproductive efficiency of primiparous beef heifers under range conditions through enhanced early PP increases in uterine PGF2c, synthesis. Both trials were successful in increasing PGI'M early PP, however, no improvement in reproduction was detected by either method employed. Because of the delay in time between parturition and return to estrus, some researchers discount the influence of the PP uterine environment in affecting length of PP anestrous period. Kiracofe (1980) discussed fertility in the early PP period, citing reports of ovulations prior to uterine capacity to support pregnancy in dairy cattle, and Randel (1990) emphasized body weight and condition score and absolute amounts of energy or protein as primary determinants influencing return to estrus. One important aspect I discovered in the literature is that primiparous heifers have shorter uterine involution times than multiparous cows (Kiracofe, 1980), which raises some questions about the importance of uterine involution for return to estrus in primiparous heifers. However, evidence of uterine environment affecting ovarian activity is also evident in the literature. Saiduddin et al. ( 1967) reported the frequency of first ovulation from the 84 ovary contralateral to the previously gravid horn was greater than from the ipsilateral ovary. Also, an improperly steroid conditioned uterus is thought to be the cause of premature release of PGF2a, hence, the occurrence of short cycles. It has been demonstrated that pre-exposure of the uterus to endogenous P4 improves fertility (Lishman and Inskeep, 1991). The advantage of PGF2c, treatments of PP anestrous cattle in stimulating shorter PP intervals has also been studied (Randel et al., 1988). Better understanding of uterine and ovarian control of eicosanoid production, particularly with respect to PG synthase in the PP uterus is needed. It would be interesting to study the differences in PG production between uterine caruncules found in the previously gravid and the contralateral horns and determined the effect of these differences in PG levels on the function of the adjacent ovaries. It is uncertain if positive effects of lipid are the result of direct stimulation of ovarian function or indirectly through improved uterine environment, through second messengers, or other mechanisms such as physiological stimulus to the hypothalamus. It is encouraging that plasma concentrations of PGF2c, were altered by diet, and the potential remains for successful administration of nutritional regimens to improve uterine conditions that favor earlier return to estrus. Further study of the effects of exogenous lipids on utero-ovarian interrelationships is needed. 85 BIBLIOGRAPHY Adam, 0., G. Wolfram, and M. Zollner. 1982. Prostaglandin formation in man during intake of different amounts of linoleic acid in formula diets. Ann. Nutr. Metab. 26:315-323. Ahima, RS., D. Prabakaran, C. Mantzoros, D. Qu, B. Lowell, E. Maratos-Flier, and J. Flier. 1996. Role of leptin in the neuroendocrine response to fasting. Nature 382:250-252. Albertini, H.L., D.E. Hawkins, M.K. Petersen, D.M. Hallford, T.T. Ross, R.A. Renner, K..K. Kane, and L.A. Appeddu. 1996. Undegradable intake protein (UIP) and fat on reproductive and endocrine function of young beef cows. Proc. West. Sect. Amer. Soc. Anim. Sci. 47:197-200. Algire, J. E., A. Srikandarkumar, L.A. Guilbault, and B.R. Downey. 1992. Preovulatory changes in follicular prostaglandins and their role in ovulation in cattle. Can. J. Vet. Res. 56:67-69. Appeddu, L.A., M.K. Petersen, J.S. Serrato-Corona, L.F. Gulino, I. Tovar-Luna, G.B. Donart, D.E. Hawkins, J.R. Strickland, E.E. Parker, and S. Cox. 1996. Postpartum reproductive responses of two year old range cows supplemented with protein, fat, or energy. Proc. West. Sec. Amer. Soc. Anim. Sci. 47:2-6. Aukema, H.M., and B.J. Holub. 1988. The effect of dietary fish oil supplementation and in vitro collagen stimulation on human platelet phospholipid fatty acid composition. In: C. Galli and A.P. Simopoulos (Eds.) Dietary co3 and o.)6 Fatty Acids. Biological Effects and Nutritional Essentiality. NATO ASI Series A Life Sci. 171:81-90. Bailey, J.M., and M. Verma. 1991. Analytical procedures for a cryptic messenger RNA that mediates translational control of prostaglandin synthase by glucocorticoids. Anal. Biochem. 196:11-18. Barash, IA, C.C. Cheung, D.S. Weigle, H. Ren, E.B. Kabigting, J.L. Kuijper, D.K. Clifton, and R.A. Steiner. 1996. Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144-3147. Bauchart, D. 1993. Lipid absorption and transport in ruminants. J. Dairy Sci. 76:3864-3881. Beam, S.W., and W.R. Butler. 1997. Energy balance and ovarian follicle development prior to the first ovulation postpartum in dairy cows receiving three levels of dietary fat. Biol. Reprod. 56:133-142. 86 Beard, A.P., and M.G. Hunter. 1994. Effects of progesterone pretreatment on the oxytocin receptor concentration and the response to oxytocin during the simulated early luteal phase in the ovariectomized ewe. J. Reprod. Fertil. 102:57-63. Bergstrom, S., H. Danielsson, D. Klenbert, and B. Samuelsson. 1964. The enzymatic conversion of essential fatty acids into prostaglandins. J. Biol. Chem. 239:4006-4009. Bickerstaffe, R., D.E. Noakes, and E.F. Annison. 1972. Quantitative aspects of fatty acid biohydrogenation, absorption, and transfer into milk fat in the lactating goat, with special reference to the cis- and trans- isomers of octadecenoate and linoleate. Biochem. J. 130:607-617. Blatchley, F.R., and N.L. Poyser. 1974. The effect of estrogen and progesterone on the release of prostaglandins from the uterus of the ovariectomized guinea-pig. J. Reprod. Fertil. 40:205-209. Bligh, E.G., and W.J. Dyer. 1959. A Rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. Boshier, D.P., R.A. Jacobs, V.K.M. Han, W. Smith, S.C. Riley, and J.R.G. Challis. 1991. Immunohistochemical localization of prostaglandin H synthase in the sheep placenta from early pregnancy to term. Biol. Reprod. 45:322-327. Buch, N.C., W.J. Tyler, and L.E. Casida. 1955. Postpartum estrus and involution of the uterus in an experimental herd of Holstein-Friesian cows. J. Dairy Sci. 38:73-79. Budowski, P. 1988. Alpha-linolenic acid and the metabolism of arachidonic acid. In: C. Grath and A.P. Simopoulos (Eds.) Dietary co3 and 0)6 Fatty Acids. Biological Effects and Nutritional Essentiality. NATO ASI Series A Life Sci. 171:97-110. Burke, J.M., D.J. Carroll, K.E. Rowe, W.W. Thatcher, and F. Stormshak. 1996. Intravascular infusion of lipid into ewes stimulates production of progesterone and prostaglandin. Biol. Reprod. 55:169-175. Burr, G.O., and M.M. Burr. 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 82:345-367. Carroll, D.J., R.R. Grummer, and M.K. Clayton. 1992. Stimulation of luteal cell progesterone production by lipoproteins from cows fed control or fat-supplemented diets. J. Dairy Sci. 75:2205-2214. Carroll, D.J., M.J. Jerred, R.R. Grummer, D.K. Combs, R.A. Pierson and E.R. Hauser. 1990. Effects of fat supplementation and immature alfalfa to concentrate ratio on plasma progesterone, energy balance, and reproductive traits of dairy cattle. J. Dairy Sci. 73:2855-2863. 87 Carruthers, T.D., and H.D. Hafs. 1980. Sucking and four-times daily milking: influence on ovulation, estrus and serum luteinizing hormone, glucocorticoids and prolactin in postpartum Holsteins. J. Anim. Sci. 50:919-925. Carsten, M.E. 1972. Prostaglandin's part in regulating uterine contraction by transport of calcium. In: E.M. Southern (Ed.) The Prostaglandins. Clinical Application in Human Reproduction. Futura Publishing Company, Inc. p 59-66. Mount Kisco, New York. Chalupa, W., B. Vecchiarelli, A.E. Elser, D.S. Kronfeld, D. Skian, and D.L. Palmquist. 1986. Ruminal fermentation in vivo as influenced by long-chain fatty acids. J. Dairy Sci. 69:1293-1301. Copelin, J.P., M.F. Smith, D.H. Keisler, and H.A. Garverick. 1989. Active immunization of prepartum and postpartum cows against prostaglandin F2c, (PGF2a): effect of lifespan and progesterone secretion of short-lived corpora lutea. J. Anim. Sci. 67(Suppl. 2): 140(Abstr.). Coppock, C.E., and D.L. Wilks. 1991. Supplemental fat in high-producing rations for lactating cows: effects on intake, digestion, milk yield, and composition. J. Anim. Sci. 69:3826-3837. Coulson, A. 1978. Treatment of metritis in cattle with PGF2a. Vet. Rec. 103:359. Crawford, MA, and M.M. Gale. 1969. Linoleic acid and linolenic acid elongation products in muscle tissue of Syncerus caffer and other ruminant species. Biochem. J. 115::2527. Cunningham, J.G. 1992a. Control of ovulation and the corpus luteum. In: L. Mills (Ed.) Textbook of Veterinary Physiology. p 437-445. W.B. Sanders Co., Philadelphia, PA. Cunningham, J.G. 1992b. Pregnancy and parturition. In: L. Mills (Ed.) Textbook of Veterinary Physiology. p 460. W.B. Sanders Co., Philadelphia, PA. Cunningham, J.G. 1992c. Digestion and absorption: the nonfermentative processes. In: L. Mills (Ed.) Textbook of Veterinary Physiology. p 310. Philadelphia, PA. W.B. Sanders Co., Danet-Desnoyers, G., J.W. Johnson, S.F. O'Keefe, and W.W. Thatcher. 1993. Characterization of a bovine endometrial prostaglandin synthesis inhibitor (EPSI). Biol. Reprod. 48(Suppl. 1):115 (Abstr.). Del Vecchio, R.P., C.C. Chase Jr., P. Bastidas, and R.D. Randel. 1990. Oxytocin-induced changes in plasma 13,14 dihydro-15 keto prostaglandin F2c, concentrations on days 10, 20 and 30 postpartum in the bovine. J. Anim. Sci.68:4261-4266. 88 De Rouen, S.M., D.E. Franke, D.G. Morrison, W.E. Wyatt, D.F. Coombs, T.W. White, P.E. Humes, and B.B. Greene. 1994. Prepartum body condition and weight influences on reproductive performance of first-calf beef cows. J. Anim. Sci. 72:1119-1125. Dunn, T.G., J.E. Ingalls, D.R. Zimmerman and J.N. Wiltbank. 1969. Reproductive performance of 2-year-old Hereford and Angus heifers as influenced by pre- and post-calving energy intake. J. Anim. Sci.29:719-726. Dhuyvetter, D.V., M.K. Peterson, R.P. Ansotegui, R.A. Bellows, B. Nisley, R. Brownson, and M.W. Tess. 1993. Reproductive efficiency of range beef cows fed different quantities of rumen undegradable protein before breeding. J. Anim. Sci. 71:25862593. Dziuk, P.J., and R.A. Bellows. 1983. Management of reproduction of beef cattle, sheep and pigs. J. Anim. Sci. 57(Suppl. 2):355-379. Edqvist, L.E., H. Kindahl, and G. Stabenfeldt. 1978. Release of prostaglandin Fa, during the bovine peripartal period. Prostaglandins 16:111-119. Eiler, H., J. Oden, R. Schuab, and M. Sims. 1981. Refractoriness of both uterus and mammary gland of the cow to prostaglandin F2c, administration: clinical implications. Amer. J. Vet. Res. 42:314-317. Estergreen Jr., V.L., O.L. Frost, W.R. Gomes, RE. Erb, and J.F. Bullard. 1967. Effect, of ovariectomy on pregnancy maintenance and parturition in dairy cows. J. Dairy Sci. 50:1293- 1295. Eley, D.S., W.W. Thatcher, H.H. Head, R.J. Collier, C.J. Wilcox, and E.P. Call. 1981. Periparturient and postpartum endocrine changes of conceptus and maternal units in Jersey cows bred for milk yield. J. Dairy Sci. 64:312-320. Espey, L.L. 1980. Ovulation as an inflammatory reaction - A hypothesis. Biol. Reprod. 22:73-106. Fairclough, R.J., J.T. Hunter, and R.A.S. Welch. 1975. Peripheral plasma progesterone and utero-ovarian prostaglandin F concentrations in the cow around parturition. Prostaglandins 9:901-914. Falck, B. 1959. Site of production of oestrogen in rat ovary as studied in micro-transplants. Acta Physiol. Scand. 47(Suppl. 163):1-101. Fellner, V., F.D. Sauer, and J.K.G. Kramer. 1995. Steady-state rates of linoleic acid biohydrogenation by ruminal bacteria in continuous culture. J. Dairy Sci. 78:18 I51823. 89 Ferguson, J.D., S. Shotzberger, W. Chalupa, D. Sklan, and D.S. Kronfeld. 1987. Reproductive responses in lactating cows fed diets supplemented with long chain fatty acids. J. Dairy Sci. 70(Suppl.1):207 (Abstr.). Ferreira, S.H., and J.R. Vane. 1967. Prostaglandins: their disappearance from and release into the circulation. Nature 216:868-873. Ferreri, L.F., and D.H. Gleockler. 1979. Electrophoretic characterization of bovine lipoprotein subfractions isolated by agarose gel chromatography. 62:1577-1582. Filley, J. Dairy Sci. H.A. Turner, and F. Stormshak. 1997. Prostaglandin concentrations in lipidinfused postpartum heifers. Biol. Reprod. 56(Suppl. 1):207 (Abstr.). Fortune, J.E. 1994. Ovarian follicular growth and development in mammals. Biol. Reprod. 50:225-232. Fotouhi, N., and T.C. Jenkins. 1992. Resistance of fatty acyl amides to degradation and hydrogenation by ruminal microorganisms. J. Dairy Sci. 75:1527-1532. Gan-Elepano, M., E. Aeberhard, and J. F. Mead. 1981. On the mechanisms of fatty acid transformations in membranes. Lipids 16:790-795. Gier, H.T., and G.B. Marion. 1968. Uterus of cow after parturition: involutional changes. Amer. J. Vet. Res. 29:83-96. Ginther, 0.J., L. Knopf and J.P. Kastelic. 1989. Temporal associations among ovarian events in cattle during oestrous cycles with two and three follicular waves. J. Reprod. Fertil. 87:223-230. Goldstein, J.L., R.G.W. Anderson, and M.S. Brown. 1979. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 279:679-685. Griffith, M.K., and G.L. Williams. 1996. Roles of maternal vision and olfaction in suckling-mediated inhibition of luteinizing hormone secretion, expression of maternal selectivity, and lactational performance of beef cows. Biol. Reprod. 54:761-768. Gross, T.S., W.W. Thatcher, P.J. Hansen, J.W. Johnson, and S.D. Helmer. 1988. Presence of an intracellular endometrial inhibitor of prostaglandin synthesis during early pregnancy in the cow. Prostaglandins 35:359-378. Guilbault, L.A., W.W. Thatcher, R.J. Collier, C.J. Wilcox and M. Drost. 1985. Carry-over effects of periparturient endocrine changes on postpartum reproductive function of Holstein heifers bred to genetically different service sires. J. Anim. Sci. 61:15161525. 90 Guilbault, L.A., W.W. Thatcher, M. Drost and S.M. Hopkins. 1984. Source of F series prostaglandins during the early postpartum period in cattle. Biol. Reprod. 31:879887. Guilbault, L.A., W.W. Thatcher, and C.J. Wilcox. 1987a. Influence of a physiological infusion of prostaglandin F2, into postpartum cows with partially suppressed endogenous production of prostaglandins. Theriogenology 27:947-957. Guilbault, L.A., W.W. Thatcher, and C.J. Wilcox. 1987b. Interrelationships of hormonal, ovarian and uterine responses. Theriogenology 27:931-946. Guilbault, L.A., P. Villeneuve, and J.J. Dufour. 1988. Failure of exogenous prostaglandin F2c, to enhance uterine involution in beef cows. Can. J. Anim. Sci. 68:669-676. Gustaffson, B., 1976. Treatment of bovine pyometra with PGF2a : an evaluation of field study. Theriogenology 6:45-50. Hadley, M.E. 1992. Endocrinology (3rd Ed.). pp 127-134. Prentice Hall. Englewood Cliffs, NJ. Hafez, E.S.E. 1993a. Folliculogenesis, egg maturation, and ovulation. E.S.E. Hafez (ed.) In: Reproduction of Farm Animals (6th Ed.). pp 114-142. Lea and Febiger, Philadelphia. Hafez, E.S.E. 1993b. Hormones, growth factors, and reproduction. E.S.E. Hafez (ed.) In: Reproduction of Farm Animals (6th Ed.). pp 59-93. Lea and Febiger, Philadelphia. Harbon, S., L. Do Khac, M.F. Vesin, D. Leiber, and Z. Tougui. 1979. Prostaglandins and cyclic nucleotides in the regulation of uterine contractility. The role of endogenous prostacyclin. Prostaglandins Reprod. Physiol. 91:205-220. lilghtshoe, R.B., RC. Cochran, L.R. Corah, G.H. Kiracofe, D.L. Harmon, and R.C. Perry. 1991. Effects of calcium soaps of fatty acids on postpartum reproductive function in beef cows. J. Anim. Sci. 69:4097-4103. Holman, R.T. 1966. General introduction to polyunsaturated acids. Prog. Chem. Fats and other Lipids. 9:3-12. Huslig, R.L., R.L. Fogwell, and W. L. Smith. 1979. The prostaglandin forming cyclooxygenase of ovine uterus: relationship to luteal function. Biol. Reprod. :21: 589-600. Inskeep, E.K., W.J. Smutny, R.L. Butcher, and J.E. Pexton. 1975. Effects of intrafollicular injections of prostaglandins in non-pregnant and pregnant ewes. J. Anim. Sci. 41:1098-1104. 91 Jacobs, A.L., D. Hwang, J. Julian, and D.D. Carson. 1994. Regulated expression of prostaglandin endoperoxide synthase-2 by uterine stroma. Endocrinology 135:18071815. Jainudeen, MR., and E.S.E. Hafez. 1993a. Gestation, Prenatal Physiology, and Parturition. In: E. S.E. Hafez (Ed.) Reproduction of Farm Animals (6th Ed.). pp 213-236. Lea and Febiger, Philadelphia. Jainudeen, M.R., and E.S.E. Hafez. 1993b. Reproductive cycles; cattle and buffalo. In: E.S.E. Hafez (Ed.) Reproduction of Farm Animals (6th Ed.). pp 315-329. Lea and Febiger, Philadelphia. Jenkins, T.C. 1993. Lipid metabolism in the rumen. J. Dairy Sci. 76:3851-3863. Jenkins, T.C. 1995. Butylsoyamide protects soybean oil from ruminal biohydrogenation: effects of butylsoyamide on plasma fatty acids and nutrient digestion in sheep. J. Anim. Sci. 73:818-823. Jerred, M.J., D.J. Carroll, D.K. Combs, and R. R. Grummer. 1990. Effects of fat supplementation and immature alfalfa to concentrate ratio on lactation performance of dairy cattle. J. Dairy Sci. 73:2842-2854. King, G.J., B.A. Atkinson, and H.A. Robertson. 1982a. Implantation and early placentation in domestic ungulates. J. Reprod. Feral. 31(Suppl. 1):17-30. King, M.E., G.H. Kiracofe, J.S. Stevenson, and R.R. Schalles. 1982b. Effect of stage of the estrous cycle on interval to estrus after PGF2c, in beef cattle. Theriogenology 18:191200. Kiracofe, G.H. 1980. Uterine involution: its role in regulating postpartum intervals. J. Arian. Sci. 51 (Suppl. 2):16-28. Koligian, K.B., and F. Stormshak. 1976. Progesterone synthesis by ovine fetal cotyledons in vitro. J. Anim. Sci. 42: 439-443. Laflamme, L.F., and M.L. Connor. 1992. Effect of postpartum nutrition and cow body condition at parturition on subsequent performance of beef cattle. Can. J. Anim. Sci. 72:843-851. Lammoglia, MA., J.W. Holloway, A. W. Lewis, D.A. Neuendorff, and R.D. Randel. 1995. Influence of maternal and service-sire breed on serum progesterone and estrogen before calving and plasma 13,14-dihydro-15-keto-prostaglandin Fla after calving. J. Anim. Sci. 73:1167-1173. 92 Land, R.B. 1971. The incidence of oestrus during lactation in Finnish Landrace, Dorset Horn, and Finn-Dorset sheep. J. Reprod. Fertil. 24:345-352. Lands, W.E.M. 1988. Differences in n-3 and n-6 eicosanoid precursors. In: B. Samuelsson, P. Y.-K. Wong, and F.F. Sun (Eds). Advances in Prostaglandin, Thromboxane, and Leukotriene Research. 19:602-609. Raven Press, Ltd., New York. Lee, S.H., E. Soyoola, P. Chanmugam, S. Hart, W. Sun, H. Zhong, S. Liou, D. Simmons, and D. Hwang. 1992. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J. Biol. Chem. 267:25934-25938. Leers-Sucheta, S., P.K. Chakraborty, K.E. Rowe, H.A. Turner, and F. Stormshak. 1994. Gonadotropin-releasing hormone-induced secretion of luteinizing hormone in postpartum beef heifers maintained on two planes of nutrition before and after breeding. J. Anim. Sci. 72:998-1003. Leikin, A.I., A.M. Nervi, and R.R. Brenner. 1979. Lipid binding properties of a factor necessary for linoleic acid desaturation. Lipids 14:1021-1026. Lewin, J. 1994. Genes V. p. 1239. Oxford University Press Inc., New York. Lewis, G.S., W.W. Thatcher, F.W. Bazer, and J.S. Curl. 1982. Metabolism of arachidonic acid in vitro by bovine blastocysts and endometrium. Biol. Reprod. 27:431-439. Lewis, G.S., W.W. Thatcher, E.L. Bliss, M. Drost, and R.J. Collier. 1984. Effects of heat stress during pregnancy on postpartum reproductive changes in Holstein cows. J. Anim. Sci. 58: 174-186. Lindell, J-0., H. Kindahl, L. Jansson, and L. Edqvist. 1982. Post-partum release of prostaglandin F2a and uterine involution in the cow. Theriogenology 17:237-245. Lishman, A.W., and E.K. Inskeep. 1991. Deficiencies in luteal function during re-initiation of cyclic breeding activity in beef cows and in ewes. S. Afr. J. Anim. Sci. 21:59-75. Louis, T.M., H.D. Hafs, and D.A. Morrow. 1974. Intrauterine administration of prostaglandin Fa, in cows: progesterone, estrogen, LH, estrus and ovulation. J. Anim. Sci. 38:347-353. Lucy, M.C., T.S. Gross, and W.W. Thatcher. 1990. Effects of intravenous infusion of a soybean oil emulsion on plasma concentration of 15-keto-13,14-dihydroprostaglandin F2c, and ovarian function in cycling Holstein heifers. In: Livestock Reprod. in Latin Amer. Int. Atomic Energy Agency. Vienna, Austria. 119-132. 93 Lucy, M.C., C.R. Staples, F.M. Michel, W.W. Thatcher, and D.J. Bolt. 1991a. Effect of feeding calcium soaps to early postpartum dairy cows on plasma prostaglandin F2a luteinizing hormone, and follicular growth. J. Dairy Sci. 74:483-489. Lucy, M.C., C.R. Staples, F. M. Michel, and W.W. Thatcher. 199 lb. Energy balance and size and number of ovarian follicles detected by ultrasonography in early postpartum dairy cows. J. Dairy Sci. 74:473-482. Madej, A., H. Kindahl, W. Woyno, L.E. Edqvist, and R. Stupnicki. 1984. Blood levels of 15keto-13,14-dihydro-prostaglandin F2a during the postpartum period in primiparous cows. Theriogenology 21:279-287. Malven, P.V. 1984. Pathophysiology of the puerperium: definition of the problem. Proc. 10th Int. Congr. Anim. Reprod. Artificial Insemination. IV:III-1. Marston, T.T., K.S. Lusby, R.P. Wettemann, and H.T. Purvis. 1995. Effects of feeding energy or protein supplements before or after calving on performance of springcalving cows grazing native range. J. Anim. Sci. 73:657-664. Mathias, M.M., and J. Dupont. 1979. The relationship of dietary fats to prostaglandin biosynthesis. Lipids 14: 247-252. Mattos, W., and D.L. Palmquist. 1977. Biohydrogenation and availability of linoleic acid in lactating cows. J. Nutr. 107:1755-1761. McCracken, J.A., W. Schramm, and W.C. Okulicz. 1983. Hormone receptor control of pulsatile secretion of PGF2afrom the ovine uterus during luteolysis and its abrogation in early pregnancy. Anim. Reprod. Sci. 10:31-55. McCracken, J.A., T.T. Smith, J.C. Lamsa, and A.G. Robinson. 1991. The effect of estradiol17 beta (E) and progesterone (P) on the oxytocin (OT) pulse generator in the ovexed sheep. Biol. of Reprod. 44(Suppl.):87 (Abstr.). Meade, E.A., W.L. Smith, and D.L. Dewitt. 1993. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 268:6610-6614. Mirando, MA., J.P. Harney, Y. Zhou, T.F. Ogle, T.L. Ott, J.R. Moffat, and F.W. Bazer. 1993. Changes in progesterone and oestrogen receptor mRNA and protein and oxytocin receptors in endometrium of ewes after intrauterine injection of ovine trophoblast interferon. J. Mol. Endocrinol. 10:185-192. Mitchell, M.D., and A.P. Flint. 1977. Prostaglandin concentrations in intra-uterine tissues from late-pregnant sheep before and after labour. Prostaglandins 14:563-569. 94 Moore, J.H., R.C. Noble, and W. Steele. 1969. The incorporation of linolenic and linoleic acids into plasma lipids of sheep given intra-abomasal infusions of linseed oil, maize oil or linoleic acid. Br. J. Nutr. 23:141-152. Morgan, A.R. , and G.L. Williams. 1989. Effect of body condition and postpartum dietary lipid intake on lipid metabolism and GnRH- induced luteal function in postpartum beef cows. J. Anim. Sci. 67 (Suppl. 2):60 (Abstr.). Morris, J.K., and J.S. Richards. 1995. Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology 136:1549-1558. Morrow, D.A., S.J. Roberts, and K. McEntee. 1968. Postpartum ovarian activity and involution of the uterus and cervix in dairy cattle. 1. Ovarian activity. Cornell Vet. 59:173-189. Morrow, D.A., S.J. Roberts, K. McEntee, and H.G. Gray. 1966. Postpartum ovarian activity and uterine involution in dairy cattle. J. Amer. Vet. Med. Assoc. 149:1596-1609. Myatt, L., G. Langdon, and D.E. Brockman. 1994. Identification and changes in concentrations of prostaglandin H synthase (PGHS) isoforms in rat myometrium at parturition. Prostaglandins 48:285-296. Nathanielsz, P.W. 1978. Endocrine mechanisms of parturition. Ann. Rev. Physiol. 40:411445. Nathanielsz, P.W. 1993. A time to be born: how the fetus signals to the mother that it is time to leave the uterus. Cornell Vet. 83:181-187. Nestel, P.J., A. Poyser, R.L. Hood, S.C. Mills, M.R. Willis, L.J. Cooke, and T.W. Scott. 1978. The effect of dietary fat supplements on cholesterol metabolism in ruminants. J. Lipid. Res. 19:899-909. Nett, T.M. 1987. Function of the hypothalamic-hypophysial axis during the post-partum period in ewes and cows. J. Reprod. Fertil. 34(Suppl. 1):201-213. Newcomb, R., W.D. Booth, and L.E.A. Rowson. 1977. The effect of oxytocin treatment on the levels of prostaglandin F in the blood of heifers. J. Reprod. Fertil. 49:17-24. Nichaman, M.Z., R.E. Olson and C.C. Sweeley. 1967. Metabolism of linoleic acid-1-'4C in normolipemic and hyperlipemic humans fed linoleate diets. Amer. J. Clin. Nut. 20:1070-1083. Noble, R.C., W. Steele, and J.H. Moore. 1971. Diet and the fatty acids in the plasma of lambs during the first eight days after birth. Lipids 6:26-34. 95 Noel, B., J.L. Bister, and R. Paquay. 1993. Ovarian follicular dynamics in Suffolk ewes at different periods of the year. J. Reprod. Fertil. 99:695-700. Odde, K.G., H.S. Ward, G.H. Kiracofe, R.M. McKee, and R.J. Kittok. 1980. Short estrous cycles and associated serum progesterone levels in beef cows. Theriogenology 14:105-112. Ohtani, M., S. Kobayashi, A. Miyamoto, K. Hayashi, and Y. Fukui. 1998. Real-time relationships between intraluteal and plasma concentrations of endothelin, oxytocin, and progesterone during prostaglandin Flx-induced luteolysis in the cow. Biol. Reprod. 58:103-108. Oldick, B.S., C.R. Staples, M.J. Thatcher, and W.W. Thatcher. 1994. In vitro production of prostaglandin Fa, and 13, 14 dihydro-15 keto PGF2c, (PGFM) by bovine caruncular tissue explants incubated with linoleic and arachidonic acid. 72(Supp1.1):352 (Abstr.). J. Anim. Sci. Oss, G.M., D.N. Schutz, and K.G. Odde. 1993. Effects of a high fat diet on reproductive performance in pre- and postpartum beef heifers. Proc. West. Sec. Amer. Soc. Anim. Sci. 44:44-47. Oyedipe, E.O., B. Gustafsson, and H. Kindahl. 1984. Blood levels of progesterone and 15keto -13, 14-dihydroprostaglandin Fa, during the estrous cycle of oxytocin-treated cows. Theriogenology 22:329-339. Pace-Asciak, C. and L.S. Wolfe. 1968. Inhibition of prostaglandin synthesis by oleic, linoleic and linolenic acids. Biochim. Biophys. Acta 152:784-787. Palmquist, D.L., and H.R. Conrad. 1978. High fat rations for dairy cows, effects on feed intake, milk and fat production, and plasma metabolites. J. Dairy Sci. 61:890-901. Palmquist, D.L., and E.A. Moser. 1981. Dietary fat effects on blood insulin, glucose utilization, and milk protein content of lactating cows. J. Dairy Sci. 64:1664-1670. Park, C.S., W. Rafalowski, and G.D. Marx. 1983. Effect of dietary fat supplement on lipid metabolism of Holstein heifers. J. Dairy Sci. 66:528-534. Perry, J.S. 1981. The mammalian fetal membranes. J. Reprod. Fertil. 62:321-335. Perry, RC., L.R. Corah, RC. Cochran, W.E. Beal, J.S. Stevenson, J.E. Minton, D.D. Simms, and J. R. Brethour. 1991. Influence of dietary energy on follicular development, serum gonadotropins, and first postpartum ovulation in suckled beef cows. J. Anim. Sci. 69:3762-3773. 96 Peters, A.R., and G.E. Lamming. 1984. Reproductive activity of the cow in the post-partum period. II. Endocrine patterns and induction of ovulation. Br. Vet. J. 140:269-279. Piper, P.J., J.R. Vane, and J.H. Wyllie. 1970. Inactivation of prostaglandins by the lungs. Nature 225:600-604. Poyser, N.L. 1991. A possible explanation for the refractoriness of uterine prostaglandin production. J. Reprod. Fertil. 91:371-384. Poyser, N.L. 1981. Hormonal control of prostaglandin in reproductive tissues. In: Y.S. Bakhle (Series Ed.) Prostaglandins in Reproduction. p. 213-229. Research Studies Press. Chichester, United Kingdom. Poyser, N.L., and H.A. Leaver. 1979. Prostaglandin production by the uterus. Prostaglandins Reprod. Physiol. 91:195-204. In: Pugh, E.L., and M. Kates. 1977. Direct desaturation of eicosatrienoyl lecithin to arachidonoyl lecithin by rat liver microsomes. J. Biol. Chem. 252:68-73. Ramirez-Godinez, J.A., G.H. Kiracofe, RR. Schalles, and G.D. Niswender. 1982. Endocrine patterns in the postpartum beef cow associated with weaning: a comparison of the short and subsequent normal cycles. J. Anim. Sci. 55:153-158. Randel, RD. 1990. Nutrition and postpartum rebreeding in cattle. J. Anim. Sci. 68:853-862. Randel, RD., RE. Short, and RA. Bellows. 1976. Suckling effect on LH and progesterone in beef cows. J. Anim. Sci. 42:267 (Abstr.). Randel, R.D., R.P. Del Vecchio, D.A. Neuendorff, and L.A. Peterson. 1988. Effect of alfaprostol on postpartum reproductive efficiency in Brahman cows and heifers. Theriogenology 29:657-670. Renner, RA., D.E. Hawkins, M.K. Petersen, E.E. Parker, S. Cox, M. Dumensil, E.L. McFadin, J.E. Bruemmer, H. Albertini, K.K. Kane, and T.T. Ross. 1996. The effects of protein and (or) fat supplementation on growth and reproductive parameters in yearling beef heifers. Proc. West. Sec. Amer. Soc. Anim. Sci. 47:250-252. Rice, G.E., M.H. Wong, and G.D. Thorburn. 1988. Gestational changes in prostaglandin synthase activity of ovine cotyledonary microsomes. J. Endocrinol. 118:265-270. Riley, S.C., and N.L. Poyser. 1987. Effects of oestradiol, progesterone, hydrocortisone and oxytocin on prostaglandin output from the guinea-pig endometrium maintained in tissue culture. Prostaglandins 34:535-551. 97 Rivers, J.P.W., and T.L. Frankel. 1981. Essential fatty acid deficiency. Br. Med. Bulletin. 37:59-64. Roberts, J.S., J.A. McCracken, J.E. Gavagan, and M.S. So loff. 1976. Oxytocin-stimulated release of prostaglandin Fla from ovine endometrium in vitro: correlation with estrous cycle and oxytocin-receptor binding. Endocrinology 99:1107-1114. Rutter, L.M., R. Snopek, and J.G. Maims. 1989. Serum concentrations of IGF-1 in postpartum beef cows. J. Anim. Sci. 67:2060-2066. Ryan, D.P., B. Bao, M.K. Griffith, and G.L. Williams. 1995. Metabolic and luteal sequelae to heightened dietary fat intake in undernourished, anestrous beef cows induced to ovulate. J. Anim. Sci. 73:2086-2093. Ryan, D.P., R.A. Spoon, M.K. Griffith, and G.L. Williams. 1994. Ovarian follicular recruitment, granulosa cell steroidogenic potential and growth hormoneimsulin-like growth factor-I relationships in suckled beef cows consuming high lipid diets: effects of graded differences in body condition maintained during the puerperium. Domest. Anim. Endocrinol. 11:161-174. Ryan, D.P., R.A. Spoon, and G.L. Williams. 1992. Ovarian follicular characteristics, embryo recovery, and embryo viability in heifers fed high-fat diets and treated with follicle stimulating hormone. J. Anim. Sci. 70:3505-3513. SAS. Version 6.10. 1994. Cary, NC: Statistical Analysis Systems Institute, Inc. Saiduddin, S., J.W. Riesen, W.J. Tyler, and L.E. Casida. 1967. Some carry-over effects of pregnancy on post-partum ovarian function in the cow. J. Dairy Sci. 50:1846-1847. Sakamoto, K., K Miwa, T. Ezashi, E. Okuda-Ashitaka, K. Okuda, T. Houtani, T. Sugimoto, S. Ito, and 0. Hayaishi. 1995. Expression of mRNA encoding the prostaglandin F, receptor in bovine corpora lutea throughout the oestrous cycle and pregnancy. J. Reprod. Fertil. 103:99-105. Sanders, T.A.B., and K. Younger. 1981. The effect of dietary supplements of co3 polyunsaturated fatty acids on the fatty acid composition of platelets and plasma choline phosphoglycerides. Br. J. Nutr. 45:613-616. Schneider, P., D. Sklan, W. Chalupa, and D.S. Kronfeld. 1988. Feeding calcium salts of fatty acids to lactating cows. J. Dairy Sci. 71:2143-2150. Schrick, F.N., RA. Surface, J.Y. Pritchard, R.A. Dailey, E.C. Townsend, and E.K. Inskeep. 1993. Ovarian structures during the estrous cycle and early pregnancy in ewes. Biol. Reprod. 49:1133-1140. 98 Se lk, G.E., R.P. Wettemann, K.S. Lusby, J.W. Oltjen, S.L. Mobley, R.J. Rasby, and J.C. Garmendia. 1988. Relationships among weight change, body condition, and reproductive performance of range beef cows. J. Anim. Sci. 66:3153-3159. Sharma, S.C., and R.J. Fitzpatrick. 1974. Effect of oestradiol -1713 and oxytocin treatment on prostaglandin Fac release in the anoestrous ewe. Prostaglandins 6:97-105. Shemesh, M, and W. Hansel. 1975. Stimulation of prostaglandin synthesis in bovine ovarian tissues by arachidonic acid and luteinizing hormone. Biol. Reprod. 13:448-452. Short, RE., R.A. Bellows, E.L. Moody, and B.E. Howland. 1972. Effects of suckling and mastectomy on bovine postpartum reproduction. J. Anim. Sci. 34:70-74. Silvia, W.J., G.S. Lewis, J.A. McCracken, W.W. Thatcher, and L. Wilson, Jr. 1991. Hormonal regulation of uterine secretion of prostaglandin F2, during luteolysis in ruminants. Biol. Reprod. 45:655-663. Silvia, W.J., R.E. Raw, S.L. Aldrich, and S.H. Hayes. 1992. Uterine secretion of prostaglandin F2c, in response to oxytocin in ewes: changes during the estrous cycle and early pregnancy. Biol. Reprod. 46:1007-1015. Sinclair, A.J., L. Johnson, K. ODea, and R.T. Holman. 1994. Diets rich in lean beef increase arachidonic acid and long-chain co3 polyunsaturated fatty acids levels in plasma phospholipids. Lipids 29:337-343. Singer, S.J., and G.L. Nicolson. 1972. The fluid mosaic model of the structure of cell membranes. Science 175:720-731. Sirois, J. 1994. Induction of prostaglandin endoperoxide synthase-2 by human chorionic gonadotropin in bovine preovulatory follicles in vitro. Endocrinology 135:841-848. Sirois, J., and J.E. Fortune. 1988. Ovarian follicular dynamics during the estrous cycle in heifers monitored by real-time ultrasonography. Biol. Reprod. 39:308-317. Sirois, J., and H.K. Hawkins. 1992. Upregulation of rat uterine prostaglandin endoperoxide synthase (isoform 1) at parturition. Biol. Reprod. (Suppl. 1) 46:178 (Abstr.). Sirois, J., D.L. Simmons, and J.S. Richards. 1992. Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. J. Biol. Chem. 267:11586-11592. Sklan, D., E. Bogin, Y. Avidar, and S. Gur-Arie. 1989. Feeding calcium soaps of fatty acids to lactating cows: effect on production, body condition, and blood lipids. J. Dairy Res. 56:675-681. 99 Sk lan, D., U. Moallem, and Y. Folman. 1991. Effect of feeding calcium soaps of fatty acids on production and reproductive responses in high producing lactating cows. J. Dairy Sci. 74:510-517. Sklan, D., R. Volcani, and P. Budowski. 1972. Effects of diets low in fat or essential fatty acids on the fatty acid composition of blood lipids of calves. Br. J. Nutr. 27:365-374. Song, J., and R.C. Wander. 1991. Effects of dietary selenium and fish oil (MaxEPA) on arachidonic acid metabolism and hemostatic function in rats. J. Nutr. 121:284-292. Spoon, RA, A.R. Morgan, T.H. Welsh, and G.L. Williams. 1990. Interactions of postpartum dietary lipid intake and body condition on serum nonesterified fatty acid and insulin concentrations in multiparous beef cows. J. Anim. Sci. 68(Suppl. 1):421 (Abstr ). Sprecher, H. 1988. (n-3) and (n-6) fatty acid metabolism. In: C. Galli and A.P. Simopoulos (Eds.) Dietary co3 and co6 Fatty Acids. Biological Effects and Nutritional Essentiality. NATO ASI Series A Life Sci. 171:69-78. Stevenson, J.S., and J.H. Britt. 1979. Relationships among luteinizing hormone, estradiol, progesterone, glucocorticoids, milk yield, body weight, and postpartum ovarian activity in Holstein cows. J. Anim. Sci. 48:570-577. Stevenson, J.S., and E.P. Call. 1983. Influence of early estrus, ovulation, and insemination on fertility in postpartum Holstein cows. Theriogenology 19:367-375. Stormshak, F., K.E. Orwig, and J.E. Bertrand. 1995. Dynamics of molecular mechanisms underlying ovarian oxytocin secretion. J. Reprod. Fertil. Suppl. 49:379-390. Sugiura, T., N. Kudo, T. Ojima, S. Kondo, A. Yamashita, and K. Waku. 1995. Coenzyme A- dependent modification of fatty acyl chains of rat liver membrane phospholipids: possible involvement of ATP-independent acyl-CoA synthesis. J. Lipid. Res. 36:410450. Talavera, F., C.S. Park, and G. L. Williams. 1985. Relationships among dietary lipid intake, serum cholesterol and ovarian function in Holstein heifers. J. Anim. Sci. 60:10451051. Thatcher, W.W. 1974. Effects of season, climate, and temperature on reproduction and lactation. J. Dairy Sci. 57:360-368. Thatcher, W.W., C.R. Staples, G. Danet-Desnoyers, B. Oldick, and E.P. Schmitt. 1994. Embryo health and mortality in sheep and cattle. J. Anim. Sci. 72(Suppl. 3):16-30. 100 Thatcher, W.W., C. J. Wilcox, R.J. Collier, D.E. Eley, and H.H. Head. 1980. Bovine conceptus-maternal interactions during the pre-and postpartum periods. J. Dairy Sci. 63:1530-1540. Thomas, M.G., B. Bao, and G.L. Williams. 1994. Dietary fatty acids may alter folliculogenesis and luteal function through multiple physiological mechanisms in the bovine. J. Anim. Sci. 72(Suppl. 1):16 (Abstr.). Tolleson, D.R., and R. D. Randel. 1987. Physical manipulation of the postpartum bovine uterus and the subsequent release of prostaglandin Fla. J. Anim. Sci. 65(Suppl. 1) :414 (Abstr). Tolleson, D.R., and R. A. Randel. 1988. Effects of alfaprostol and uterine palpation on postpartum interval and pregnancy rate to embryo transfer in Brahman influenced beef cows. Theriogenology 29:555-564. Tove, S.B., and R.D. Mochrie. 1963. Effect of dietary and injected fat on the fatty acid composition of bovine depot fat and milk fat. J. Dairy Sci. 46:686-689. Tsai, S.J., and M.C. Wiltbank. 1997. Prostaglandin F2, induces expression of prostaglandin G/H synthase-2 in the ovine corpus luteum: a potential positive feedback loop during the luteolysis. Biol. Reprod. 57:1016-1022. Van Dorp, D.A., R.K. Beerthuis, D.H. Nugteren, and H. Vonkeman. 1964. Enzymatic conversion of all-cis-polyunsaturated fatty acids into prostaglandin. Nature 23:839841. Van Soest, P.J. 1982. Lipids. In: Nutritional Ecology of the Ruminant. pp 260-268. 08&B Books, Inc., Corvallis, OR. Viker, S.D., R.L. Larson, G.H. Kiracofe, R.E. Stewart, and J. S. Stevenson. 1993. Prolonged postpartum anovulation in mastectomized cows requires tactile stimulation by the calf. J. Anim. Sci. 71:999-1003. Viker, S.D., W.J. McGuire, J.M. Wright, K.B. Beeman, and G.H. Kiracofe. 1989. Cow-calf association delays postpartum ovulation in mastectomized cows. Theriogenology 32:467-474. Villeneuve, P., J.J. Dufour, and L.A. Guilbault. 1988. Influence of infusion of prostaglandin Fla (PGF2,) and weaning on surface and histologic populations of ovarian follicles in early postpartum beef cows. J. Anim. Sci. 66:3174-3184. von Euler, U.S. 1982. Prostaglandin, historical remarks. Prog. Lipid Res. 20:31-35. :101 Warm, RA, and R.D. Randel. 1990. Effect of uterine manipulation 35 days after parturition on plasma concentrations of 13,14-dihydro-15-keto prostaglandin F2, in multiparous and primiparous Brahman cows. J. Anim. Sci. 68:1389-1394. Warbritten, V. 1934. The cytology of the corpora lutea of the ewe. J. Morphol. 56:181-202. Weber, P.C. 1988. Modification of the arachidonic acid cascade by long-chain co-3 fatty acids. In: C. Galli and A.P. Simopoulos (Eds.) Dietary 0)3 and co6 Fatty Acids. Biological Effects and Nutritional Essentiality. NATO ASI Series A Life Sci. 171:201-209. Wehrman, M.E., T.H. Welsh, Jr., and G.L. Williams. 1991. Diet-induced hyperlipidemia in cattle modifies the intrafollicular cholesterol environment, modulates ovarian follicular dynamics, and hastens the onset of postpartum luteal activity. Biol. Reprod. 45:514522. Wendlandt, RM., and C.L. Davis. 1973. Characterization of bovine serum lipoproteins. J. Dairy Sci. 56:337-339. Whisnant, C.S., T.E. Kiser, F.N. Thompson, and C.R. Barb. 1985. Influence of calf removal on the serum luteinizing hormone response to naloxone. J. Anim. Sci. 61(Suppl.):42 (Abstr.). Williams, G.L. 1989. Modulation of luteal activity in postpartum beef cows through changes in dietary lipid. J. Anim. Sci. 67:785-793. Willis, AL. 1987. The eicosanoids: an introduction and overview. In: Willis AL. (Ed.). CRC Handbook of Eicosanoids: Prostaglandins and Related Lipids. Vol 1 Part A. pp 3-46. CRC Press, Inc., Boca Raton, FL. Wiltbank, J.N. 1970. Research needs in beef cattle reproduction. J. Anim. Sci. 31:755-762. Wiltbank, J.N., and AC. Cook. 1958. The comparative reproductive performance of nursed and milked cows. J. Anim. Sci. 17:640-648. Wiltbank, J.N., W.W. Rowden, J.E. Ingalls, K.E. Gregory, and R.M. Koch. 1962. Effect of energy level on reproductive phenomena of mature Hereford cows. J. Anim. Sci. 21:219-225. Wiltbank, J.N., W.W. Rowden, J.E. Ingalls, and D.R. Zimmerman. 1964. Influence of post- partum energy level on reproductive performance of Hereford cows restricted in energy intake prior to calving. J. Anim. Sci. 23:1049-1053. Wimsatt, J., P.W. Nathanielsz, and J. Sirois. 1993. Induction of prostaglandin endoperoxide synthase isoform-2 in ovine cotyledonary tissues during late gestation. Endocrinology 133:1068-1073. 102 Wong, W.Y.L., D.L. DeWitt, W.L. Smith, and J.S. Richards. 1989. Rapid induction of prostaglandin endoperoxide synthase in rat preovulatory follicles by luteinizing hormone and cAMP is blocked by inhibitors in transcription and translation. Mol. Endocrinol. 3:1714-1723. Wong, W.Y.L., and J.S. Richards. 1991. Evidence for two antigenically distinct molecular weight variants of prostaglandin H synthase in the rat ovary. Mol. Endocrinol. 5:1269-1279. Wong, W.Y. L., and J. S. Richards. 1992. Induction of prostaglandin H synthase in rat preovulatory follicles by gonadotropin-releasing hormone. Endocrinology 130:35123521. Wright, J.M., G.H. Kiracofe, and K.B. Beeman. 1988. Factors associated with shortened estrous cycles after abortion in beef heifers. J. Anim. Sci. 66:3185-3189. Young, I.M., D.B. Anderson, and R.W.J. Plenderleith. 1984. Increased conception rate in dairy cows after early postpartum administration of prostaglandin F2c, THAM. Vet. Rec. 115:429-431. Young, F.M., P.J. Illingworth, S.F. Lunn, D.J. Harrison, and H.M. Fraser. 1997. Cell death during luteal regression in the marmoset monkey (Callithrix jacchus). J. Reprod. Fertil. 111:109-119. Yu, H., R. Zhuge, and W.H. Hsu. 1995. Lysine vasopressin-induced increases in porcine myometrial contractility and intracellular Ca2+ concentrations of myometrial cells: involvement of oxytocin receptors. Biol. Reprod. 52:584-590. Zakar, T., J.J. Hirst, J.E. Mijovic, and D. M. Olson. 1995. Glucocorticoids stimulate the expression of prostaglandin endoperoxide H synthase-2 in amnion cells. Endocrinology 136:1610-1619. 103 APPENDICES 104 Appendix A: Metabolic Pathways for Fatty Acids and Eicosanoids Elongation and desaturation of essential fatty acids linoleic acid (6)6) or C18:26)6 (9,12 octadecadienoic acid) ItA6 desaturase y-linolenic acid or C18:36)6 (6,9,12 octadecatrienoic acid) elongase dihomo-y-linolenic acid or C20:36)6 (8,11,14 eicosatrienoic acid) 05 desaturase arachidonic acid (AA) or C20:46)6 (5,8,11,14 eicosatetraenoic acid) 11 Figure A.1. Conversion of linoleic acid to arachidonic acid a-linolenic acid (o3) or C18:3 6)3 (9,12,15 octadecatrienoic acid) A6 desaturase C18:46)3 (6, 9, 12, 15 octadecatetraenoic acid) elongase C20:46)3 (8, 11, 14, 17 eicosatetraenoic acid) A5 desaturase eicosapentaenoic acid (EPA) or C20:56)3 (5, 8, 11, 14, 17-EPA) elongase and A4 desaturase docosahexaenoic acid (DHA)or C22:66)3 (4, 7, 10, 13, 16, 19-DHA) Figure A.2. Conversion of a-linolenic acid to eicosapentaenoic and docosahexaenoic acids 105 Synthesis of eicosanoids from long chain fatty acids dihomo-y-linolenic acid (DHLA, C20:3(46) prostaglandin synthase (PGS) PGG1 PGS PGHI (PG endoperoxide) Three possible products: 1) Reductase to yield PGF1. 2) PG isomerase to yield PGEI 3) Thromboxane synthase to yield TXAI & TXB1 Figure A.3. Synthesis of one-series eicosanoids Arachidonic acid (AA, 20:4(46) 11 prostaglandin synthase (PGS) PGG2 PGS PGH2 (PG endoperoxide) Four possible products: 1) Reductase to yield PGF2c, 2) PG isomerase to yield PGE2 (-- >PGF2.) 3) Thromboxane synthase to yield TXA2 & M32 4) Prostacyclin synthase to yield PGI2 Figure A.4. Synthesis of two-series eicosanoids 106 eicosapentaenoic acid (EPA) or C20:5603 (5, 8, 11, 14, 17-EPA) prostaglandin synthase (PGS) PGG3 11 PGS PGH3 Four possible products: 1) Reductase to yield PGF3(X 2) PG isomerase to yield PGE3 (--- >PGF3c,) 3) Thromboxane synthase to yield TXA3 andTXB3 4) Prostacyclin synthase to yield PGI3 Figure A.5. Synthesis of three-series eicosanoids arachidonic acid (AA; 20:46)6) lipoxygenase leukotrienes A4 through F4 .11 Figure A.6. Synthesis of four-series eicosanoids Appendix B: Procedures for Androgen Treatment of Cows, PGFM Radioimmunoassay and Fatty Acid Identification Androgen treatment of cows In experiments 1 and 2, mature Hereford x Angus cows were treated with androgens for use in monitoring heifers for return to estrus prior to the breeding season when bulls were available. Testosterone propionate (Sigma Chemical Co., St. Louis, Missouri) was dissolved 107 in benzyl benzoate (1 g/10 ml), then diluted to 40 mg/ml with vegetable oil (safflower oil). The preparation is stable at room temperature, therefore 1 L was prepared. Eight mature non-pregnant Hereford X Angus cows were injected with 200 mg (5 ml) every other day for 20 days, then boosted every 10th day for 40 days (Jerry Reeves, Washington State University, Pullman, WA, personal communication). After 1 wk, six of the androgen-treated cows began to exhibit sexual interest in each other, however, two cows showed little response to treatment and were removed from the group. PGFM radioimmunoassay Radioimmunoassay was used to determine concentration of prostaglandin (PG) Fes, metabolite, 13,14-dihydro, 15-keto prostaglandin Fla (PGFM) as developed by Guilbault et al. (1984) and validated for use in our laboratory (Burke et al., 1996). There was no indication that infusions of intralipid interfered with the assay of PGFM. Prostaglandin free plasma collected as described in experiment 1, was used to construct a standard curve using serial dilutions (5 to 500 pg/tube) of PGFM (Cayman Chemical Company, Ann Arbor, /41) in .5 M Tris-HC1, pH 7.5, and along with samples and reference plasma (200 ill), assayed with .08% bovine serum albumin (BSA) used as a carrier protein, and approximately 13,000 cpm of radiolabeled competitor (177 Ci/mmol; 13,14-dihydro-15-keto [5,6,8,9,11,12,14(n)-11] PGF2; Amersham, Arlington Heights, IL) and PGFM antibody (# J-53 anti-PGFM), a gift from William Thatcher (University of Florida), as described below. 108 Assay procedure Day 1 A. Standards (triplicates) 1. Pipette 2001i1 PG free plasma into all 12 x 75 standards tubes. 2. Place 100 p.1 of standards into appropriate tubes. 3. Pipette 200 gl of Tris-HC1 buffer into non-significant binding (NSB) tubes and 100 pi of Tris -HCI buffer into zero binding (Bo) tube. B. Samples and reference plasma (duplicates) 1. Pipette 200 µl of samples or reference plasma into appropriate tubes. 2. Pipette 100 111 of Tris-HC1 buffer into all tubes. C. Samples and standards 1. Add 100 pl of .5% BSA to all tubes, vortex. 2. Incubate for 15 min at room temperature. 3. Add 100 pl of rabbit J53 anti-PGFM (1/15,000) to all tubes except NSB, vortex. 4. Incubate 30 min at room temperature. 5. Add 100 pi of 3H -PGFM to all tubes and to three scintillation vials (total count tubes), vortex. 6. Incubate for 1 h at room temperature and then overnight at 4 °C. Day 2 1. Add 750 pl of 40% Polyethylene glycol (PEG) to all tubes and vortex for 1 minute. 2. Centrifuge for 30 min at 1,620 x g at 4 °C. 3. Put tubes in foam racks, invert to drip dry (10 min). 4. Redissolve pellets in 750 pl of Tris -HCI buffer and vortex for 1 min. 5. Add 750 pl of PEG to all tubes and vortex 1 minute. 6. Centrifuge for 30 min at 1,620 x g at 4 °C. 7. Put tubes in foam racks, invert to drip dry (10 min). 8. Dissolve pellets in 1 ml Tris -HCI buffer and vortex for 2 min. 9. Transfer entire solution to scintillation vials, add 6 ml of counting fluid (Ecolume, ICN), vortex and let sit overnight at room temperature. Day 3 1. Determine counts per minute in beta counter. 2. Determine concentration of PGFM in samples and reference plasma using RIA-Aid or hand calculation. 109 Identification of plasma fatty acids Fatty acids were extracted from plasma using methanol:chloroform (2:1 vol/vol; Bligh and Dyer, 1959), methylated with boron trichloride and benzene, and then subjected to gas chromatography (Song and Wander, 1991) for identification as follows. Fifteen microliters of an internal standard, C17:0 methyl ester at approximately 10 mg/ml in iso-octane, was added to 100 x 10 ml, dried under nitrogen gas, and .2 ml of plasma was added. Lipid was then extracted with 3.75 ml methanol:chloroform (2:1 vol/vol) with 50 mg/L butylated hydroxytoluene (BHT). Samples were placed on a shaker for 1 hour and then centrifuged 3,000 g for 10 min. The supernatant was transferred to a 120 x 10 mm test tube (with Teflon cap) by Pasteur pipette, and the residue was extracted with 1 ml distilled water and 3.75 ml methanol-chloroform mixture, centrifuged again for 10 min, and supernatant transferred to the 120 x 10 mm test tube as the first extraction. Distilled water and chloroform (2.5 ml each) were added, the contents mixed, and then centrifuged. The upper layer was removed by aspiration, and the lower was transferred to a clean test tube and dried under nitrogen gas (N). Fatty acids remaining in the sample were then methylated as follows: .2 ml benzene and 1 ml boron trichloride were added, the top purged with N2 and capped tightly. The tube was heated in 95°C heating block for 90 min and then cooled to room temperature. Five milliliters of distilled water and 5 ml hexane were added, the tube vortexed 2 min, centrifuged 1,000 x g for 10 min, and the top hexane layer transferred to a 125 x 16 mm test tube. Five milliliters of hexane were added to the first tube and again vortexed 2 min, centrifuged 1,000 x g for 10 min, and the top hexane layer transferred to the 125 x 16 mm test tube. Sodium 110 sulfate (.3 g) was added to the combined extracts and vortexed 45 sec. The extract was transferred to a 100 x 10 mm test tube and the hexane evaporated under N2. The sample was then dissolved in .5 ml iso-octane in preparation for gas chromatography. Two microliters of the fatty acid methyl esters were injected onto a 30 m x .25 mm i.d., .25 p.m film thickness SP 2330 column (Supelco, Bellefonte, PA) connected to a Hewlett-Packard 5890 gas chromatograph interfaced with a Hewlett-Packard Chem Work Station (Avondale, PA). Helium was the carrier gas and was used at a flow rate of 1 ml/min with a split ratio of 100:1. Hydrogen and air flow rates were 30 and 300 ml/min, respectively. Both the injector and detector were maintained at 170°C. The column was programmed for 4 min at 170°C and then a 3° C/min rise to 225 °C. Fatty acid methyl esters in the samples were identified by comparing to authentic standard mixtures (Sigma; NuChek Prep, Elysian, NM; Supelco). The chromatogram graphed elution time and peak areas for each fatty acid. Weight percentage of individual fatty acids were calculated by dividing the area of a specific fatty acid's peak by the total area of all fatty acids injected onto the column. When internal standard (C17:0) was applied or preservative (BHT) registered on the chromatogram, these values were subtracted from the total prior to calculating weight percentage of individual fatty acids. 111 Appendix C: Plasma Fatty Acids, Prostaglandin Concentrations, and Fertility in Postpartum Heifers Fed Rumen Protected Lipid and Biuret Abstract An experiment was conducted to determine whether feeding of rumen protected fatty acids (FA), along with biuret as a non-protein nitrogen source, to postpartum (PP) heifers would increase plasma concentrations of PGF2a, metabolite (PGFM), shorten the interval from calving to first estrus with ovulation, and increase pregnancy rate. Forty crossbred beef heifers (354 kg) were stratified by breed and assigned randomly as they calved to supplement treatments of .23 kg-head-1d' calcium salts of FA (CSFA; n = 20) or an isocaloric amount of barley (control; n = 20) for the first 30 days PP. Supplements, with .23 kg barley as a vehicle and .2 kg biuret, and chopped meadow hay (ad libitum) were pen fed to heifers (5/pen). Blood samples were collected from heifers on alternate days (days 1 to 30) for assay of PGFM. Twenty-four additional heifers were also pen fed experimental diets, however, they were not bled as above. For all heifers, first behavioral estrus was detected by use of intact bulls, and confirmed by an increase in plasma concentrations of progesterone in blood collected twice weekly (day 30 to 2 wk after first estrus). Data were analyzed using x2 and ANOVA (repeated measures and one-way). There were no differences between treatments in plasma concentrations of PGFM for days 1 through 13 PP, days to first estrus with ovulation, or pregnancy rate(P > .10). Supplemental lipid plus non-protein nitrogen fed to primiparous beef heifers did not alter plasma concentrations of PGF2c, in the early PP period, and did not improve the fertility. 112 Introduction Economic impact and physiological characteristics of long postpartum anestrous periods in primiparous beef heifers (Wiltbank, 1970; Randel, 1990), and the role of PGF2c, for uterine involution (Madej et al., 1984) and ovarian function (Guilbault, et al. 1987) were discussed in experiments 1 and 2. Recall that duration of increased production of PGF2c, in the PP period is negatively correlated with the number of days to complete uterine involution and the with length of interval between parturition and resumption of normal ovarian activity (Madej et al., 1984). Nutritional approaches, including altered dietary lipid content, have been used to improve reproductive and lactational performance in cattle (Dunn et al., 1969; Schneider et al., 1988). It has been demonstrated that plasma cholesterol levels, lipid profiles, and hormone concentrations can be altered by including fat in livestock diets (Talavera et al., 1985; Williams, 1989; Carroll et al., 1990). Linoleic acid, an EFA, is converted to arachidonic acid, the immediate precursor of prostaglandins, and the infusion of lipid into ruminants (Lucy et al., 1990; Burke et al., 1996; Filley et al., 1997) and feeding EFA to non- ruminants (Adam et al., 1982) increases plasma PG. Because 70 to 90% of unsaturated FA entering the rumen are biohydrogenated (Bickerstaffe et al., 1972), protected lipids increase the amount of unsaturated FA absorbed by the ruminant (Tove and Mochrie, 1963). The present experiment was conducted not only to repeat results of experiment 2, with respect to increasing PGFM in the PP period, but also to improve reproductive performance of postpartum (PP) heifers fed calcium salts of fatty acids (CSFA). Increased numbers of heifers (64 total) compared to experiment 1 (20) and 2 (39), and therefore more observations and increased statistical power of the design to test the reproductive data were 113 also available for use in this experiment. Barton and Carroll (1992) reviewed research protocols for nutrition and reproduction and reported that large numbers of animals are needed to find statistical differences in reproductive traits. One example given was that the number of animals needed to attain a 5% level of significance in conception rates was 150. The objectives of this experiment were to determine whether feeding rumen-protected fatty acids to PP heifers would increase plasma concentrations of PGF2, shorten the interval from calving to first estrus, and increase pregnancy rate. Differences between experiment 2 and the present protocol are protein source, hay presentation form, year conducted, and heifer crop and heifer breed. Materials and Methods Animals, treatments, and samples Forty Hereford x Angus (H x A) and HxAx Longhorn primiparous heifers (353 kg) were stratified by breed and otherwise assigned to treatments randomly as they calved. Days 1 through 30 PP, heifers were fed chopped meadow hay and trace mineral salts ad libitum, with .2 kg biuret included as a source of non-protein nitrogen that was mixed into treatments consisting of either fat (n = 20) or control (n = 20) supplement. Fat supplement, representing 3% of estimated dry matter intake, was .23 kghead-ld-1 of rumen inert fat as CSFA (Church and Dwight, Inc.; Ca salts of palm oil; 44% palmitic, 40% oleic, 9.5% linoleic and 5% stearic acids) mixed with .23 kg ground barley. Control supplement was an isocaloric amount of ground barley (.72 kg). Animals were fed in groups of 5 (8 pens; 4 replicate pens per treatment), and calves remained with dams. Heifers were body condition scored (BCS; scale 114 1 to 9; 1= emaciated, 9 = obese) approximately 2 wk prior to calving, at 30 days PP, and at the end of the breeding season (150 days PP). Calf sex was recorded, and heifers and calves were weighed at 1, 30, and 150 days PP. Calving ease was scored either as unassisted, light pull, or hard pull. Two heifers scored as hard pull, and were excluded from the experiment. Orts were removed weekly for calculation of daily intake expressed as kilogram of meadow hay consumed per kilogram of metabolic body weight. Experimental supplements were fed once daily at noon using removable supplement feeders designed to accommodate up to 20 heifers. Heifers had adequate room to receive the supplement as individuals, were eager to consume the supplement, and care was taken that each heifer ate only her portion. In order to assess PGF2a, production after parturition, blood samples were collected from heifers by venepuncture every other morning days 1 through 30 PP. The blood was immediately placed on ice, and within 1 hour centrifuged at 2,500 x g for 15 min at 4°C and the plasma stored at -20°C. Twenty-four additional heifers were also pen-fed experimental diets, and weighed and BCS on 1, 30, and 150 days PP; however, they were not bled as above, nor was their hay intake measured. Calf weights were also collected on 1, 30, and 150 days of the experiment. After 30 days on the supplements, all heifers were released to pastures where they received meadow and alfalfa hays. At approximately 60 days PP, heifers were moved to summer ranges to graze high desert grasses and forbes. In order to determine number of days to first estrus, heifers were observed twice daily for sexual behavior using intact bulls continuously housed with heifers from 60 to 150 days PP, and were bled twice weekly for plasma progesterone (P4) concentrations for 14 days after first behavioral estrus. First estrus with ovulation was defined as heifers standing for the bull with subsequent plasma P4 being greater than 1 ng/ml for two consecutive samples. 115 Analysis of PGFM, fatty acids, and progesterone A subset of heifers (12 per treatment) was selected randomly for plasma PGFM (13,14-dihydro-15-keto PGF2a) analyses, performed as in experiment 1 and described by Guilbault et al. (1984). Plasma PGFM intra- and interassay CV were 7.5 and 7.9%, respectively, and sensitivity was 50 pg/ml. Plasma progesterone was measured in duplicate for all heifers by RIA as in experiment 1 using the method described by Koligian and Stormshak (1976). Plasma samples collected during the breeding season were extracted using benzene:hexane (1:2 vol/vol). Extraction efficiency was 68.8%, intra- and interassay CV were 7.7 and 9.9%, respectively, and sensitivity of the assay was 50 pg/ml. Statistical analysis All data were analyzed using SAS (1994). Analysis of variance (ANOVA) was used to analyze body weights, body condition scores, feed intake, calf weights, and number of days to first estrus with ovulation. Data for plasma PGFM concentration were analyzed by repeated measures ANOVA. Chi square was used to analyze data for calving ease (no assistance or light pull), calf sex, reproductive status at 150 days PP (estrus or anestrus), and pregnancy rate the subsequent fall (pregnant or nonpregnant). Results Plasma concentrations of PGFM from days 1 through 13 PP were not affected by treatments (P > .10; Figure C.1). Power curve analysis revealed that including the remaining 116 samples in the PGFM analysis would not result in statistical differences between treatments, therefore no further samples were included. Also no difference between treatments (P > .10) was found for days to first estrus, pregnancy rate (Table C.1), body weights, BCS, hay intake (Table C.2.), or calving assistance and calf sex (Table C.3). Calf weight, however, was lower (P < .04) for heifers fed CSFA compared with controls on days 1, 30, and 150 days PP. When only calf weights from heifers included in PGFM analysis were analyzed, no difference (P > .10) was found for day 1 and day 30 calf weight. 3000 --0-- CONTROL -0-- CSFA 2500 2000 12.4 1500 1000 500 1 3 5 7 9 11 13 DAY POSTPARTUM Figure C.1. Mean (± SE) plasma concentration of prostaglandin F2c, metabolite (PGFM) on days 1 through 13 postpartum 117 Table C.1. Mean number of days to first estrus and pregnancy rate Item a CSFA b Control b Common SEC Days to first estrus 101.7 103.6 2.5 Pregnancy rate (%) 86.2 92.6 nd 'No difference among treatments for either item (P >.10). bCSFA (calcium salts of fatty acid) and control (barley) supplements. 'Common estimate of the standard error. nd, not done. Table C.2. Mean body weight, body condition score, and feed intake Itema CSFAb Control" Common SEC BW 1 (kg) 350.0 356.4 3.5 BW 2 (kg) 324.1 337.1 3.4 BW 3 (kg) 353.7 360.1 3.4 BCS 1 (1 to 9) 5.03 5.10 .4 BCS 2 (1 to 9) 4.28 4.36 .4 BCS 3 (1 to 9) 3.66 3.63 .4 FI (%BW .") 13.8 14.2 .7 aBW, body weight and BCS, body condition score (scale 1 to 9) 1 at day 1 postpartum (PP), BW 2 and BCS 2 at day 30 PP, BW 3 and BCS 3 at day 150 PP. Feed intake (FI) was kg of meadow hay consumed (per day) as a percentage of metabolic BW. No difference between treatments for any item (P > .10). b CSFA (calcium salts of fatty acid) and control (barley) supplements. c Common estimate of the standard error. 118 Table C.3. Means of calving assistance, calf sex, and calf weight Item a CSFAb Controlb Calving assistance (%) 12.9 13.3 nd Calf sex (% male) 46.9 59.4 nd CWT 1 (kg) 34.7d 37.3` 1.2 CWT 2 (kg) 53.0d 57.2' 1.7 CWT 3 (kg) 118.7d 128.4' 2.4 Common SE 'Calf weight (CWT) 1 on day 1, CWT 2 on day 30, and CWT 3 at day 150 postpartum. b CSFA (calcium salts of fatty acid) and control (barley) supplements. Common estimate of the standard error. d'e Means with different superscripts within the same row differ (P = .05). Discussion The lack of change in plasma concentrations of PGFM in response to supplemental lipid containing dietary PG precursors ( i.e., linoleic acid) was disappointing considering the increase in PGFM in PP heifers infused and fed lipid (experiment 1 and 2, respectively). Differences between the current experiment and experiment 2 include heifer crop and year (1996 vs 1997 calving dates), protein supplement (protein vs non-protein nitrogen), and presentation form of hay (long hay vs chopped). Cow breed can influence plasma PGFM concentrations (Lammoglia et al., 1995); however, heifer breeds differed only slightly between experiments in that only 6 H x Ax Longhorn heifers were included along with 58 H x A heifers, whereas experiment 1 and 2 used only H x A heifers. Because primiparous heifers are the animal of choice, the same animals could not be used twice. Postpartum body 119 weights and condition scores were similar between experiments, although the range was greater in experiment 2. Lipid metabolism (Serrato-Corona et al., 1996) and reproductive performance (Randel, 1990) have been shown to be altered by form and amount of protein in the diet. Presentation form of hay, i.e., long or coarse chopped, does not affect feed intake, digestibility, or rate of passage through the rumen (Van Soest, 1982). The inability to repeat results of supplemental lipid on PP hormone concentrations, and to affect reproductive performance, serves to emphasize the complexity of the physiological balance that exists in the PP cow. References Adam, 0., G. Wolfram, and M. Zollner. 1982. Prostaglandin formation in man during intake of different amounts of linoleic acid in formula diets. Ann. Nutr. Metab. 26:315-323. Barton, B., and D.J. Carroll. 1992. Dietary crude protein and reproductive efficiency in dairy cows: considerations when designing or evaluating research protocols. Pacific NW Anim. Nutr. Conf. 1-20. Bickerstaffe, R., D.E. Noakes, and E.F. Annison. 1972. Quantitative aspects of fatty acid biohydrogenation, absorption, and transfer into milk fat in the lactating goat, with special reference' to the cis- and trans- isomers of octadecenoate and linoleate. Biochem. J. 130:607-617. Burke, J.M., D.J. Carroll, K.E. Rowe, W.W. Thatcher, and F. Stormshak. 1996. Intravascular infusion of lipid into ewes stimulates production of progesterone and prostaglandin. Biol. Reprod. 55:169-175. Carroll, D.J., M.J. Jerred, RR. Grummer, D.K. Combs, R.A. Pierson and E.R. Hauser. 1990. Effects of fat supplementation and immature alfalfa to concentrate ratio on plasma progesterone, energy balance, and reproductive traits of dairy cattle. J. Dairy Sci. 73:2855-2863. 120 Dunn, T.G., J.E. Ingalls, D.R. Zimmerman and J.N. Wiltbank. 1969. Reproductive performance of 2-year-old Hereford and Angus heifers as influenced by pre- and post-calving energy intake. J. Anim. Sci.29:719-726. Filley, S.J., H.A. Turner, and F. Stormshak. 1997. Prostaglandin concentrations in lipidinfused postpartum heifers. Biol. Reprod. 56(Suppl. 1):207 (Abstr.). Guilbault, L.A., W.W. Thatcher, M. Drost and S.M. Hopkins. 1984. Source of F series prostaglandins during the early postpartum period in cattle. Biol. Reprod. 31:879887. Guilbault, L.A., W.W. Thatcher, and C.J. Wilcox. 1987. Influence of a physiological infusion of prostaglandin Fla, into postpartum cows with partially suppressed endogenous production of prostaglandin. Theriogenology 27:947-957. Guilbault, L.A., P. Villeneuve, and J.J. Dufour. 1988. Failure of exogenous prostaglandin F2c, to enhance uterine involution in beef cows. Can. J. Anim. Sci. 68:669-676. Koligian, K.B., and F. Stormshak. 1976. Progesterone synthesis by ovine fetal cotyledons in vitro. J. Anim. Sci. 42: 439-443. Lammoglia, M.A., J.W. Holloway, A. W. Lewis, D.A. Neuendorff, and RD. Randel. 1995. Influence of maternal and service-sire breed on serum progesterone and estrogen before calving and plasma 13,14-dihydro-15-keto-prostaglandin F2 calving. J. Anim. Sci. 73:1167-1173. Lucy, M.C., T.S. Gross, and W.W. Thatcher. 1990. Effects of intravenous infusion of a soybean oil emulsion on plasma concentration of 15-keto-13,14-dihydropro staglandin Fa, and ovarian function in cycling Holstein heifers. In: Livestock Reprod. in Latin Amer. Int. Atomic Energy Agency. Vienna, Austria. 119-132. Madej, A., H. ICindahl, W. Woyno, L.E. Edqvist, and R. Stupnicki. 1984. Blood levels of 15keto-13,14-dihydro-prostaglandin Fla, during the postpartum period in primiparous cows. Theriogenology 21:279-287. Randel, RD. 1990. Nutrition and postpartum rebreeding in cattle. J. Anim. Sci. 8:853-862. SAS. Version 6.10. 1994. Cary, NC: Statistical Analysis Systems Institute, Inc. Schneider, P., D. Sklan, W. Chalupa, and D.S. Kronfeld. 1988. Feeding calcium salts of fatty acids to lactating cows. J. Dairy Sci. 71:2143-2150. 121 Serrato-Corona, J.S., I. Tovar-Luna, L.A. Appeddu, M.K. Petersen, H. Albertini, J.E. Sawyer, and A. Gomez. 1996. Plasma glucose and nonesterified fatty acids responses to glucose and epinephrine in protein supplemented beef heifers. Proc. West. Sec. Amer. Soc. Anim. Sci. 47:310-313. Talavera, F., C.S. Park, and G. L. Williams. 1985. Relationships among dietary lipid intake, serum cholesterol and ovarian function in Holstein heifers. J. Anim. Sci. 60:10451051. Tove, S.B., and R.D. Mochrie. 1963. Effect of dietary and injected fat on the fatty acid composition of bovine depot fat and milk fat. J. Dairy Sci. 46:686-689. Van Soest, P.J. 1982. Lipids. In: Nutritional Ecology of the Ruminant. p. 260-268. O&B Books, Inc., Corvallis, OR. Williams, G.L. 1989. Modulation of luteal activity in postpartum beef cows through changes in dietary lipid. J. Anim. Sci. 67:785-793. Wiltbank, J.N. 1970. Research needs in beef cattle reproduction. J. Anim. Sci. 31:755-762.