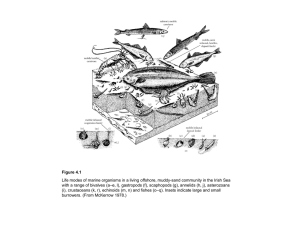
ficance of foraminiferal assemblages dominated by small-sized Environmental signi fluenced deposits
advertisement

Earth-Science Reviews 99 (2010) 31–49 Contents lists available at ScienceDirect Earth-Science Reviews j o u r n a l h o m e p a g e : w w w. e l s ev i e r. c o m / l o c a t e / e a r s c i r ev Environmental significance of foraminiferal assemblages dominated by small-sized Ammodiscus and Trochammina in Triassic and Jurassic delta-influenced deposits Jenö Nagy ⁎, Silvia Hess, Elisabeth Alve Department of Geosciences, University of Oslo, P.O. Box 1047, Blinden, N-0316 Oslo, Norway a r t i c l e i n f o Article history: Received 13 March 2009 Accepted 8 February 2010 Available online 16 February 2010 Keywords: foraminifera low diversity agglutinated small-sized low salinity reduced oxygen Triassic and Jurassic a b s t r a c t The sediment packages analyzed for benthic foraminifera consist of mudstones with interbedded sandstones deposited in shallow delta-influenced shelf to deltaic environments. The sections are located in Spitsbergen, the Barents Sea, northern North Sea and Yorkshire, and range in age from Late Triassic to Middle Jurassic. Salient features of the foraminiferal successions are: (1) The assemblages consist entirely or dominantly of agglutinated taxa. (2) The faunal diversities are extremely low. (3) The dominant genera are Ammodiscus and Trochammina. (4) The species are generally of small size compared to usual dimensions within the genera. The features listed above suggest that the assemblages were adapted to restricted conditions (clearly divergent from those of a normal marine shelf), where the main limiting factors were low salinity and reduced amount of dissolved oxygen in unstable, storm-influenced environments. Evidence for environmental conditions is obtained from modern analogues, although the large evolutionary changes in foraminifera during post-Jurassic time make it difficult to find such analogues. Additional information is derived from functional morphology, sedimentary features and paleogeography. The analyzed sediment packages show close faunal similarities suggesting opening of a marine pathway, which connected the paleo-Arctic Ocean with the western European shelf seas in Early Jurassic. A depositional biofacies model of the small-sized Ammodiscus–Trochammina assemblages envisages a deltainfluenced shelf environment, where high freshwater influx would have created a density-stratified water column with a tendency to develop hypoxic conditions in its deeper parts. The depth interval between fairweather and storm wave base (the offshore-transition zone) is indicated as the habitat of the small-sized Ammodiscus–Trochammina assemblages. In this zone, benthic biota would have been stressed by intermittent periods with moderate hypoxia combined with lowered salinity and storm impacts. © 2010 Elsevier B.V. All rights reserved. Contents 1. 2. Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.1. Background and purpose of study . . . . . . . . . . . . . 1.2. Main features of Jurassic foraminiferal facies . . . . . . . . 1.2.1. Normal marine shelf assemblages . . . . . . . . . 1.2.2. Delta-influenced assemblages . . . . . . . . . . . 1.2.3. Hypoxic shelf assemblages . . . . . . . . . . . . Distribution of small-sized Ammodiscus–Trochammina assemblages . . . 2.1. Knorringfjellet Formation at Festningen, western Spitsbergen 2.2. Knorringfjellet Formation, central Spitsbergen . . . . . . . . 2.3. Knorringfjellet Formation, Wilhelmøya . . . . . . . . . . . 2.4. Ragnarok Formation of the Mjølnir structure, Barents Sea . . 2.5. Rannoch Formation, Gullfaks Field, North Sea . . . . . . . . 2.6. The Yons Nab Beds, Yorkshire Coast . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ⁎ Corresponding author. Tel.: + 47 22 85 66 48; fax: +47 22 85 42 15. E-mail addresses: jeno.nagy@geo.uio.no (J. Nagy), silvia.hess@geo.uio.no (S. Hess), elisabeth.alve@geo.uio.no (E. Alve). 0012-8252/$ – see front matter © 2010 Elsevier B.V. All rights reserved. doi:10.1016/j.earscirev.2010.02.002 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32 32 32 32 32 33 34 34 34 34 35 35 36 32 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 3. Environmental significance of assemblage features . . 3.1. Main aspects of biofacies . . . . . . . . . . . 3.2. The agglutinated faunal component . . . . . . 3.3. The calcareous faunal component . . . . . . . 3.4. Species diversities . . . . . . . . . . . . . . 3.5. Dominant foraminiferal taxa . . . . . . . . . 3.6. Occurrence of ostracods . . . . . . . . . . . 3.7. Taphonomy of assemblages . . . . . . . . . 4. Test size and shape of foraminiferal taxa . . . . . . 4.1. Size distribution in the studied sections . . . 4.2. Literature-based size distributions . . . . . . 4.3. Size comparisons . . . . . . . . . . . . . . 4.4. Test morphology and habitats . . . . . . . . 5. Modern analogues . . . . . . . . . . . . . . . . . 5.1. Drammensfjord . . . . . . . . . . . . . . . 5.2. Aso-kai Lagoon . . . . . . . . . . . . . . . 6. Triassic and Jurassic environments of small-sized taxa 6.1. Delta-influenced shelf embayment . . . . . . 6.2. Prodelta–delta front transition . . . . . . . . 6.3. Interdistributary bay . . . . . . . . . . . . . 6.4. A depositional biofacies model . . . . . . . . 7. Conclusions . . . . . . . . . . . . . . . . . . . . Acknowledgments . . . . . . . . . . . . . . . . . . . . References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1. Introduction 1.1. Background and purpose of study Benthic foraminiferal successions heavily dominated by Ammodiscus and Trochammina are known from several Late Triassic to Middle Jurassic sediment packages along the Atlantic margin of northwestern Europe, from the North Sea up to the Arctic (Nagy et al., 1990; Bremer et al., 2003). Several of the successions are typified by small dimensions of these and other taxa, extremely low species diversities and, with a few exceptions, by consisting entirely of agglutinated forms. The present study examines geographically widely spaced assemblages of this type, recognized in several sediment packages as follows (Figs. 1 and 2): The Knorringfjellet Formation of Spitsbergen studied at Festningen, in central Spitsbergen and on Wilhelmøya; the Ragnarok Formation of the Mjølnir impact crater in the western Barents Sea; the lower Rannoch Formation of the Gullfaks Field in the northern North Sea; the Yons Nab Beds on the Yorkshire coast of northeast England. The above-listed sedimentary successions are composed mainly of mudstones and sandstones interbedded in varying proportions. In the literature, these successions are attributed to shallow shelf, deltaic and coastal marine environments of restricted nature, based on sedimentary features combined with foraminiferal biofacies. Until recently, low salinity shallow water conditions were regarded as the main restricting factor in these environments (Nagy et al., 1990), although tendency to hypoxic conditions in a stratified water column was suggested as an additional factor affecting depositional conditions of the Knorringfellet Formation (Nagy and Berge, 2008). The objectives of the present study are: (1) Outlining the regional and stratigraphic occurrence of small-sized Ammodiscus and Trochammina assemblages. (2) Comparison of these assemblages in order to delineate their common features. (3) An assessment of the environmental significance of small-sized agglutinated assemblages. (4) To contribute to the knowledge of delta-influenced and marginal marine foraminiferal assemblages, which have received little attention in spite of the high geological importance of shallow shelf to coastal marine deposits. 1.2. Main features of Jurassic foraminiferal facies 1.2.1. Normal marine shelf assemblages Assemblages of this type consist entirely or dominantly of calcareous foraminifera, belonging mainly to Nodosariacea, although . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36 36 37 37 38 38 39 39 39 39 41 41 42 43 43 44 45 45 46 46 46 47 48 48 agglutinated taxa can also form a significant component. High species diversities are typical. There are numerous studies dealing with assemblages of this type from various sedimentary successions e.g.: Exton (1979), Pliensbachian and Toarcian of Portugal; Pietrzenuk (1961), Sinemurian and Toarcian of northeastern Germany; Copestake and Johnson (1989), Hettangian to Toarcian of North Wales; Barnard et al. (1981), Callovian and Oxfordian (Oxford Clay), England; Norling (1972), Pliensbachian of Western Scania, Sweden. The main faunal proxies for normal marine shelf assemblages of the Northern North Sea Basin, were calculated by Nagy et al. (1990) in two sediment packages. (1) The Ladys Walk Shale Member in the Moray Firth Basin is of Late Sinemurian to Early Pliensbachian age. It comprises 3 foraminiferal assemblage units with average alpha diversities from 4.9 to 7.3 and proportion of calcareous taxa from 73 to 99%. (2) The Amundsen Formation of the Statfjord area includes 6 assemblage units with average alpha values ranging from 4.0 to 8.4 and frequency of calcareous taxa varying from 0.3 to 38%. In both successions the dominant genera are Marginulina, Mesodentalina, Lenticulina and Dentalina. The alpha diversities are generally well above 5, corresponding to the values of modern normal marine shelves. 1.2.2. Delta-influenced assemblages Paralic foraminiferal assemblages dominated by large-sized Ammodiscus were reported by Løfaldli and Nagy (1980) from the Toarcian to Bathonian Kongsøya Formation sampled on Kong Karls Land (easternmost part of the Svalbard Archipelago). Dominant species are the robust Ammodiscus asper (Terquem, 1862) and the medium-sized A. limitatus (Terquem, 1864). The diversities are extremely low, with number of species per sample varying from 1 to 3. Several samples are barren and thin coal seams are present in the section. The assemblages are attributed to shallow, strongly hyposaline, well-oxygenated waters in lagoonal or estuarine settings. The varied foraminiferal succession of the Middle Jurassic Safa Formation of Sinai has been analyzed by Ghandour and Maejima (2007) who distinguished two agglutinated biofacies. (1) The medium diversity Ammobaculites biofacies is referred to brackish prodelta environments dominated by Trochammina and Verneuilinoides in addition to the nominate genus. (2) The low diversity Ammodiscus–Glomospira biofacies is ascribed to brackish delta plain and estuarine conditions dominated by Miliammina in addition to the nominate taxa. J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 33 Fig. 1. Paleogeographic map for Toarcian to Aalenian time showing position of sections containing low diversity agglutinated foraminiferal assemblages characterized by small-sized taxa dominated by Ammodiscus and Trochammina discussed in this paper. Map based on own interpretations and information from Ziegler (1982), Nøttvedt et al. (1992), van Veen et al. (1992), Torsvik et al. (2002), Riis et al. (2008). Other low diversity, agglutinated, delta-influenced assemblages are recognized in the Jurassic of the western and northwestern European margin, in the 6 sections that form the basis of the present study. As shown in the following chapters, hyposaline conditions are here combined with varying degrees of hypoxia leading to small test dimensions. 1.2.3. Hypoxic shelf assemblages The western and northwestern European margin comprises several sediment packages composed of organic-rich shales. The foraminiferal assemblages of three of these are referred to hypoxic depositional conditions and are outlined here. (1) The Black Stone Band, a subunit of the Kimmeridge Clay Formation of Southern England, is strongly dominated by agglutinated taxa with an extremely small calcareous component (Jenkins, 2000). The alpha diversity is typically around 3 and the dominant genera are Reophax, Ammobaculites, Textularia and Trochammina. (2) The Brora Shale Member (Callovian) on the northwestern margin of the Moray Firth Basin is heavily dominated by agglutinated forms with a strongly subordinate calcareous component (Nagy et al., 2001). The average alpha value is 2.5 and 34 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 Fig. 2. Stratigraphic positions of sections containing small-sized foraminiferal assemblages dominated by Ammodiscus and Trochammina. Intervals with poorly defined stratigraphic age are stippled. the dominant genera include Haplophragmoides, Gaudryina and Ammobaculites. (3) The Lardyfjellet Member (Oxfordian) of the Agardhfjellet Formation contains exclusively agglutinated assemblages (Nagy and Basov, 1998). The species diversity is extremely low and the dominant genera are Trochammina, Recurvoides and Thuramminoides. The examples cited above reveal that the hypoxic black shale assemblages are entirely agglutinated or contain only a very small additional calcareous component. The species diversities in these deposits are extremely low. The generic composition of the assemblage is variable, although Trochammina seems to be the most typical genus. while the Teistberget comprises its Early Jurassic part. The intervening hiatus is believed to represent the Late Norian to Early Pliensbachian time interval. The lower part of the Tverrbekken member consists of highly bioturbated sandstones, while its middle and upper parts are composed of mudstones with siderite-cemented sandstone interbeds. The mudstones are generally dark grey in color, and their TOC content ranges from 0.9 to 1.6%, while their calcium carbonate content varies from 0.02 to 2.00%. The presence of indistinct lamination suggests a very low degree of bioturbation. The sandstone interbeds are heavily bioturbated. The lower and middle parts of the Teistberget member consist mainly of mudstones, while its upper part is dominated by bioturbated sandstones (Fig. 3). The mudstones are light grey in color, silty and weather to pieces of blocky appearance suggesting the presence of diffuse bioturbation. The TOC content of these mudstones ranges from 0.2 to 1.0%, while their calcium carbonate content varies from 0.03 to 1.68%, except for the disconformity level at base of the member where 33% carbonate was measured. The foraminiferal assemblages of the Knorringfjellet Formation are entirely agglutinated except for the occurrence of Eoguttulina liassica Strickland 1846 in two samples and Astacolus sp. in a single sample; in each case they account for less than 1%. The most common species throughout the formation is Ammodiscus aff. yonsnabensis Nagy, Løfaldli and Bomstad 1983, increasing in frequency upwards (Fig. 3). Trochammina aff. eoparva Nagy and Johansen, 1991 is abundant in the Tverrbekken member and occurs in reduced amounts in the Teistberget member. Less common but characteristic species include Reophax metensis Franke 1936, Bulbobaculites aff. vermiculus Nagy and Seidenkrantz 2003 and Verneuilinoides subvitreus Nagy and Johansen, 1991. Three other species occur in more than 30% of the samples but with frequencies less than 10%: Evolutinella sp.1, Thurammina sp.1 and Trochammina sp.1. The faunal diversity is low. This is shown by the number of species per sample, with a mean value of 6.2 and a range from 1 to 10. There is a general diversity decrease upwards through the Teistberget member, leading to a monospecific assemblage at the top of the member (Fig. 3). More details about the composition of these assemblages are given by Nagy and Berge (2008). 2.2. Knorringfjellet Formation, central Spitsbergen 2. Distribution of small-sized Ammodiscus–Trochammina assemblages 2.1. Knorringfjellet Formation at Festningen, western Spitsbergen Festningen is located close to the western coast of Spitsbergen (the main island of the Svalbard Archipelago). Here, the low diversity agglutinated assemblages dominated by small-diameter Ammodiscus and Trochammina are typical of the 22.8 m thick Knorringfjellet Formation of Late Norian to Toarcian age (Fig. 3). The formation represents the upper part of the Kapp Toscana Group. The group consists of shallow marine shelf to deltaic mudstones, siltstones and sandstones, having a wide regional distribution in Spitsbergen and on the western Barents Shelf (Mørk et al., 1982). The Knorringfjellet Formation consists mainly of mudstones with sandstone interbeds (Fig. 3). The occurrence of phosphatic horizons and siderite-cemented levels indicate a condensed succession, and in accordance with this, the presence of at least two major and several minor hiatuses has been suggested (Mørk et al., 1982; Nagy and Berge, 2008). Both the base and top of the formation are disconformities, marked by transgressive phosphatic conglomerates. In the Festningen section the formation is informally subdivided into a lower Tverrbekken member and an upper Teistberget member, separated by a major disconformity (Pčelina, 1980; Mørk et al., 1999). The Tverrbekken constitutes the Late Triassic part of the formation, In addition to the Festningen section, similar low diversity agglutinated assemblages dominated by small-sized Ammodiscus and Trochammina are observed in three sites exposing the Knorringfjellet Formation in central Spitsbergen: (1) Detailed analyzes of a section at Marhøgda showed that also here the dominant species are T. aff. eoparva and A. aff. yonsnabensis, while the species diversity varies from 4 to 11 around a mean of 8.8 (Nagy and Berge, 2008). (2) Two analyzed samples from a section at Juvdalen revealed a high dominance of A. aff. yonsnabensis and extremely low species diversity. (3) A pilot study of a section located close to the front of Drønbreen demonstrated a dominance of T. aff. eoparva and A. aff. yonsnabensis once more associated with low species diversities. 2.3. Knorringfjellet Formation, Wilhelmøya This island is located on the northeastern coast of Spitsbergen where the Knorringfjellet Formation is thicker, more sandy, and apparently more complete than in western and central areas. Two samples were analyzed from the Wilhelmøya exposures; both contain low diversity agglutinated assemblages of small-sized taxa, among which T. aff. eoparva and A. aff. yonsnabensis are the most common. J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 35 Fig. 3. The Knorringfjellet Formation at Festningen, western Spitsbergen, showing lithology, number of species and distribution of the three dominant taxa. 2.4. Ragnarok Formation of the Mjølnir structure, Barents Sea The Mjølnir structure is a crater-like depression with a diameter of 40 km seen in pre Cretaceous deposits located in the central Barents Sea (Fig. 1). Interpretation of seismic data and drill-hole sediment cores reveal that the structure was formed by the impact of a major meteorite (Tsikalas et al., 1998; Bremer et al., 2003; Dypvik and Jansa, 2003). The impact has taken place in latest Tithonian time, when the target area formed a deeper part of the paleo-Barents Sea shelf. A cored drill-hole, 7329/03-U-01, located within the Mjølnir crater, penetrated a 24 m thick succession of Ryazanian to Valanginian post impact deposits. These rest on a 97 m thick impact-affected sediment package, the Ragnarok Formation, of Late Triassic to Early Jurassic age (Fig. 4). The formation is severely deformed by the impact, but its lithology indicates that it originally formed a succession of mudstones interbedded with sandstones. Thus, both the age and lithology of the Ragnarok indicate that it is correlative with the Kapp Toscana Group, including the Knorringfjellet Formation of Spitsbergen. The foraminiferal and palynomorph content of the Mjølnir drill core 7329/03-U-01 are presented by Bremer et al. (2003) and the following foraminiferal data are derived from that paper. The foraminifera occurring in the Ragnarok Formation are entirely agglutinated, small in size and form low diversity assemblages. The dominant species are A. aff. yonsnabensis, Trochammina minutissima Dain, 1972 and Evolutinella sp. 1 (Fig. 4). The number of specimens per sample ranges from 3 to 12, with a mean of 6.1. 2.5. Rannoch Formation, Gullfaks Field, North Sea The Rannoch Formation of Aalenian age belongs to the sanddominated deltaic Brent Group deposited in the northern North Sea Basin in Middle Jurassic time (Nagy et al., 1990). The small-sized Ammodiscus–Trochammina assemblages were recognized in the lower 10 m of the Rannoch in well 34/10-1 drilled on the Gullfaks Oil Field. The lower Rannoch is a succession of silty mudstones containing thin interbeds of sandstone (Fig. 5). A thin coal seam is present at the top. 36 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 Fig. 4. The Ragnarok Formation of the Mjølnir Structure, western Barents Sea, showing lithology, number of species and distribution of the three dominant taxa. The TOC content of the lower Rannoch mudstones ranges from 0.1 to 2.6%. The calcium carbonate content varies from 0.0 to 3.8% in the lower part of the interval, while in the upper part it attains 37% although the values are strongly variable. The mudstone-dominated succession of the lower Rannoch grades upwards into a thick package of sandstones which comprise the main body of the formation, interpreted to have been deposited as an advancing wave-dominated delta front (Graute et al., 1987) including distributary mouth bar and prograding shoreface sands. The foraminiferal assemblages in the lower Rannoch deposits consist almost entirely of agglutinated taxa (Nagy and Johansen, 1991). The dominant species are A. yonsnabensis, T. eoparva and Reophax metemsis (Fig. 5). Other species that are locally common include Trochammina aff. sablei Tappan, 1955, Trochammina sp. 1 and Lagenammina aff. inanis (Gerke and Sosipatrova 1961). Calcareous taxa are observed only in three samples, and then at frequencies b1%. The calcareous species are Spirillina numismalis Terquem and Bertehlin 1875 and Astacolus sp. The number of species per sample ranges from 1 to 8 with a mean of 4.9. lower and upper parts, and bivalve shell horizons in the middle part of the succession (Fig. 6). Good exposures are present on the foreshore at Yons Nab where the thickness of the unit is around 8 m. At this site, the foraminiferal assemblages were analyzed in a south-eastern section and a north-western section by Nagy et al. (1983). The faunal composition in the two sections proved to be closely similar, although the south-eastern section is generally richer both in species and individuals. The data presented in this paper are therefore those derived from the latter. The foraminiferal assemblages of the Yons Nab Beds show low diversities with an overall dominance of agglutinated taxa, although calcareous species are also represented in significant amounts, particularly within the middle part of the section and at the base (Fig. 6). Spirillinids dominate the calcareous component but nodosariids are also significant. A range chart of all the species observed in the section is presented in Nagy et al. (1983). The dominant species (in decreasing abundance) are: A. yonsnabensis, Turrispirillina punktulata (Trequem 1870), Conicospirillina pictonica (Berthelin 1879) and Trochammina sp. The number of species per sample ranges from 1 to 8 with a mean of 4.1. Ostracod carapaces occur in most samples. 2.6. The Yons Nab Beds, Yorkshire Coast 3. Environmental significance of assemblage features The Yons Nab Beds together with the underlying Millepore Beds comprise the Cayton Bay Formation of Aalenian age (Cope et al., 1980). This formation represents a major marine intercalation in the Middle Jurassic deltaic succession of Yorkshire. The lithology of the Yons Nab Beds is dominated by mudstones with interbedded sandstones. Accessory lithologies include thin siderite beds in the 3.1. Main aspects of biofacies As shown on the preceding pages, the foraminiferal facies of the analyzed sections are characterized by the following main features: (1) The assemblages are virtually entirely agglutinated, except some J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 37 Fig. 5. The lower part of the Rannoch Formation in the Gullfaks Field, northern North Sea, showing lithology, number of species and distribution of the three dominant taxa. samples in the Yons Nab Beds where calcareous taxa occur in varying amounts; (2) the species diversities are low, showing values below those typical for normal marine shelves; (3) the species are generally of small dimensions; and (4) the dominant species belong to Ammodiscus and Trochammina. The following is a closer consideration of the environmental significance of these biofacies features. 3.2. The agglutinated faunal component The foraminiferal assemblages of the central Spitsbergen sections and the Mjølnir core (Fig. 4) are entirely agglutinated. The assemblages of the Festningen and Gullfaks sections (Figs. 3 and 5) are agglutinated except for a very few scattered samples containing calcareous taxa that account for less than 1% of the assemblages. The Yons Nab Beds are heavily dominated by agglutinated taxa, although in the lower and particularly in the middle part of the section calcareous forms also occur in significant amounts (Fig. 6). In an overview of Jurassic foraminiferal facies in the northern North Sea Basin, Nagy et al. (1990) demonstrated the occurrence of agglutinant-dominated assemblages in Toarcian, Aalenin and Callovian strata, which they referred to brackish conditions in proximal to distal deltaic settings, and to hypoxic conditions in organic-rich shelf environments. These interpretations imply that Jurassic environments divergent from those of normal marine shelves developed low diversity agglutinated assemblages. The Rannoch Formation and the Yons Nab Beds were included in that Jurassic study. 3.3. The calcareous faunal component As shown above, the foraminiferal assemblages are composed exclusively of the agglutinated suborder Textulariina in three of the analyzed sites (central Spitsbergen, Wilhelmøya and Mjølnir). Two other sites (Festningen and Gullfaks) are virtually agglutinated, and provided only a few samples containing very small (b1%) calcareous components belonging to nodosariids. These features are consistent with the restricted nature of the assemblages typifying the five sites. Nagy et al. (1990) have demonstrated the environmental significance of calcareous assemblages in Early to Middle Jurassic deposits of the North Sea Basin, where normal marine shelf strata were found to be typified by high diversity faunas dominated by nodosariids. Significant divergences from normal shelf conditions result in low species diversity coupled with high dominance of agglutinated forms or entirely agglutinated assemblages. The dominant calcareous group of the Mesozoic, the nodosariids, was apparently not adapted to restricted conditions during that era, as is also the case with the Cenozoic descendents of this superfamily, although strongly reduced both in abundance and diversity in most Cenozoic and modern environments. 38 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 Fig. 6. The Yons Nab Beds of the Cayton Bay Formation, Yorkshire Coast, showing lithology, number of species, distribution of the four dominant foraminiferal taxa and ostracod occurrences. Among the studied sections, only the basal and middle parts of the Yons Nab Beds contain significant amounts of calcareous foraminifera. The calcareous component here is dominated by spirillinids, while nodosariids are subordinate. In modern faunas, spirillinids are common in lagoons, living principally on seaweeds (Davies, 1970; Brasier, 1975) while nodosariids are generally regarded to occur in a much wider range of normal marine habitats. Significant amounts of these calcareous components in the lower and middle part of the Yons Nab Beds indicate marine ingressions, which resulted in temporary increased salinity in an overall brackish development. 3.4. Species diversities In all the studied sections, the number of species per sample is generally low (Figs. 3–6). In accordance with this, both the alpha diversity and H(S) values are strongly reduced (Fig. 7). The alpha diversities can be summarized as follows: Festningen section, mean 1.05 (range 0.3–1.9); Mjølnir structure, mean 1.26 (range 0.6–2.5); Gullfaks Field, mean 1.09 (range 0.4–1.7); Yons Nab section, mean 1.01 (range 0.2–2.0). Comparisons with the alpha values of modern faunas suggest that these low diversity assemblages were formed under restricted conditions. In modern faunas, alpha values below 5 are typical of environments where the restricting factor is low salinity, as in numerous estuaries and hyposaline lagoons (Murray, 1973). However, similar low alpha values are also found in assemblages from modern hypoxic estuarine waters, as demonstrated by fjord environments in southeastern Norway (Alve and Nagy, 1986; Nagy and Alve, 1987; Alve, 1990). In accordance with this, strongly reduced diversities are also typical for faunas occurring in organic-rich shales, as shown in the Middle to Late Jurassic of Spitsbergen and Scotland (Nagy et al., 1988, 1990). Diversity diagrams where alpha is plotted against H(S) sow typically low values in modern hyposaline environments as summarized by Murray (2006). Cross plots of these two indices reveal comparable low values in the four sections analyzed in detail in the present study (Fig. 7). 3.5. Dominant foraminiferal taxa The most common genus in the analyzed sample material is Ammodiscus (Figs. 3–6). It is represented by the small-sized A. aff. yonsnabensis in the Knorringfjellet Formation of the Festningen, central Spitsbergen, and Wilhelmøya sections, as well as in the Ragnarok Formation of the Mjølnir structure (Plate 1, figs. 1, 2, 3). The closely related small form, A. yonsnabensis, is dominant in the North Sea Basin where it occurs in the Rannoch Formation of the Gullfaks Field and in the Yons Nab Beds of the Yorkshire coast (Plate 1, figs. 16, 22, 23, 24). The next most common genus is Trochammina, represented by several small-sized species: T. aff. eoparva in the five Spitsbergen sections, T. minutissima in the Mjølnir structure and T. eoparva in the Gullfaks Field (Plate 1, figs. 6, 7, 18, 19, 20). In the Yons Nab section the second most abundant species is the calcareous C. pictonica. Other dominant species, all of comparatively small size, include: Reophax metensis at Festningen and Gullfaks, Evolutinella sp.1 in the Mjølnir Structure, and Turrispirillina punctulata and Trochammina sp.2 at Yons Nab (Plate 1, figs. 5, 9, 25). In the Festningen section, A. aff. yonsnabensis is associated with low to extremely low species diversities. The percentage frequency of this species reveals a gradual upward increase, which is inversely related to the species diversity (Fig. 3). This development is portrayed in more detail in Fig. 8, showing that the percentage of A. aff. yonsnabensis is variable at lower diversities but increases strongly J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 39 Fig. 7. Species diversities in four analyzed sections expressed by plots of the alpha index against H(S). with further diversity reduction. At extremely low diversities the frequency of the species is above 90%, with a single sample containing a monospecific fauna. This relationship suggests that among the species occurring in the Festningen section, A. aff. yonsnabensis is the taxon most tolerant of restricting conditions that controlled the distribution of foraminiferal species. 3.6. Occurrence of ostracods A significant feature of the Yons Nab section is its content of ostracods (Fig. 6). They are smooth-walled and occur in 11 of the 17 analyzed samples where they range in number from 3 to 179 carapaces per 100 gram sediment. These microscopic crustaceans have a highly mobile mode of life (compared to foraminifera), which implies a higher level of metabolism that requires increased oxygenation of the water column. Consequently, ostracods are totally absent or extremely rare in organic-rich shales. This is demonstrated by their total absence from 50 samples of shales with high organic content that were analyzed for microfossils from the Spekk Formation (Late Jurassic) of the Mid Norwegian Shelf. Among several hundred samples disintegrated form organic-rich shales of the Agardhfjellet Formation (Middle to Late Jurassic) of Spitsbergen, only a single one contained a few carapaces, while almost all samples produced agglutinated foraminifera. conditions may potentially dissolve calcareous tests if originally present. Nevertheless, even if all calcareous species are lost through dissolution, the remaining agglutinated assemblages may still hold useful ecolocical information (Murray and Alve, 2000). In this context it must be noted, however, that environments with extremely low content of dissolved calcium carbonate might physiologically exclude calcareous taxa. Among the analyzed sections, only the Yons Nab Beds contain significant amounts of calcareous fossils, including foraminifera, ostracods, bivalves and crinoids (Fig. 6). No traces of dissolution are observed on the foraminiferal and ostracod shells (the two groups studied in detail), which thus excludes carbonate dissolution. Two of the studied sections, Festningen and Gullfaks, contain a very small calcareous foraminiferal component of b1% in a few scattered samples, but no partially dissolved calcareous tests were observed. The assemblages studied from central Spitsbergen, Wilhelmøya and Mjølnir are entirely agglutinated. Regarding carbonate dissolution in the present material, of particular note is the presence of pyrite internal molds in many of the non-compressed calcareous and agglutinated tests. Pyrite precipitation in chamber voids is a pre-compressional process, which usually takes place immediately after burial. In the samples analyzed in this study, pyrite internal molds without a covering shell have not been observed, which suggests that no significant post-compressional dissolution of carbonate has taken place. 3.7. Taphonomy of assemblages 4. Test size and shape of foraminiferal taxa When interpreting fossil foraminiferal assemblages of agglutinated or mixed (calcareous-agglutinated) type, taphonomic dissolution of calcareous tests is a relevant issue. Dissolution of calcium carbonate can take place before burial (e. g. Murray and Alve, 1999), during diagenesis and through modern weathering processes. Of particular interest are stagnant environments, where low pH bottom water 4.1. Size distribution in the studied sections The foraminiferal taxa occurring in the studied sections are characterized by generally small test dimensions (Plate 1, figs 1–25). This feature is exemplified by the common to dominant species: (1) 40 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 Plate 1. Common and characteristic foraminifera occurring in the small-sized Ammodiscus and Trochammina assemblages. 1, 2, 3: Ammodiscus aff. yonsnabensis, Knorringfjellet Fm Festningen, sample 20, X180. 4: Trochammina sp. 1, Knorringfjellet Fm Festningen, sample 7, X180. 5: Evolutinella sp. 1, Knorringfjellet Fm Festningen, sample 10, X180. 6, 7: Trochammina aff. eoparva, spiral and umbilical view (respectively), Knorringfjellet Fm Marhøgda, sample 7, X180. 8: Trochammina aff. eoparva, peripheral view, Knorringfjellet Fm Festningen, sample 8, X180; 9: Reophax metensis, Knorringfjellet Fm Marhøgda, sample 2, X180. 10: Reophax sp. 1, Knorringfjellet Fm Festningen, sample 10, X180. 11: Bulbobaculites aff. vermiculus, Knorringfjellet Fm Marhøgda, sample 9, X180. 12: Verneuilinoides aff. kirillae, Knorringfjellet Fm Marhøgda, sample 10, X110. 13: Verneuilinoides subvitreus, Knorringfjellet Fm Marhøgda, sample 4, X180. 14: Lagenammina aff. inanis, Knorringfjellet Fm Festningen, sample 2, X180. 15: Thurammina sp. 1, Knorringfjellet Fm Marhøgda, sample 9, X180. 16: Ammodiscus yonsnabensis, Rannoch Fm Gullfaks Field, sample 10, X180. 17: Bulbobaculites oviloculus, Rannoch Fm Gullfaks Field, sample 1, X180. 18, 19, 20: Trochammina eoparva, spiral, umbilical and peripheral view (respectively), Rannoch Fm Gullfaks Field, sample 10, X180. 21: Glomospira aff. perplexa, Rannoch Fm Gullfaks Field, sample 1, X200. 22, 23, 24: Ammodiscus yonsnabensis, Yons Nab Beds Yorkshire, sample 10, X190. 25: Turrispirillina punctulata, Yons Nab Beds Yorkshire, sample 7, X172. J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 41 Fig. 8. The percentage frequency of Ammodiscus aff. yonsnabensis plotted against H(S) diversity. Diagram based on specimens from the Knorringfjellet Formation, Festningen section. Number of overlapping plots given. Ammodiscus aff. yonsnabensis, highly dominant in the Knorringfjellet Formation of Spitsbergen and in the Ragnarok Formation of the Barents Sea; (2) the closely related A. yonsnabensis, dominant in the Rannoch Formation of the Gullfaks Field and in the Yons Nab Beds of the Yorkshire Coast; (3) T. aff. eoparva, locally dominant in the Knorringfjellet Formation; (4) the closely related T. eoparva, dominant in the Rannoch Formation; (5) T. minutissima, dominant in the Ragnarok Formation; (6) Trochammina sp. 2, common in the Yons Nab Beds; and (7) Evolutinella sp. 1, common in the Ragnarok Formation. In addition, the dimensions of most of the rare species also appear to be reduced. To quantify the size distribution of the two most common species, the diameters of A. aff. yonsnabensis and T. aff. eoparva were measured in assemblages from the Knorringfjellet Formation of the Festningen section. Graphic presentation of the measurements reveals (Fig. 9) that the diameter of A. aff. yonsnabensis has a mean value of 0.16 mm within a range of 0.09 to 0.23 mm. Trochammina aff. eoparva attains a mean diameter of 0.17 mm and varies from 0.12 to 0.24 mm. The low diversity agglutinated assemblages typical for the other sections are dominated by similarly small species, as shown in the following by their mean values with ranges in parentheses. Ragnarok Formation of the Mjølnir structure: diameter of A. aff. yonsnabensis, 0.14 mm (0.08–0.18 mm); diameter of T. minutissima, 0.15 mm (0.11– 0.17 mm). Rannoch Formation of the Gullfaks Field: diameter of A. yonsnabensis 0.22 mm (0.16–0.32 mm); diameter of T. eoparva, 0.22 mm (0.20–0.26 mm). Yons Nab Beds of the Yorkshire coast: diameter of A. yonsnabensis 0.19 mm (0.15–0.29 mm). 4.2. Literature-based size distributions The size distributions of Ammodiscus and Trochammina, observed in the studied sections, are placed in a wider regional and stratigraphic context by comparison with the dimensions of the same two genera recorded in the literature, covering an extensive range of sedimentary successions located outside the area of the present study. To obtain the most relevant data set, this literature study is restricted to the Triassic and Jurassic time interval. It must be noted, however, that from the Triassic only a moderate number of relevant size measurements could be obtained, owing to the generally small number of papers dealing with foraminifera of this period. The database is then used for constructing the regional size distribution diagrams displayed in Fig. 10, which are based on measurement data extracted from 27 papers for Ammodiscus and from 28 papers for Trochammina. Most of the information used in the diagrams originates from the papers referred to in Tables 1 and 2. Fig. 9. Diameter categories of Ammodiscus aff. yonsnabensis and Trochammina aff. eoparva calculated as percentages from the total number of measured specimens (n). Data from the Knorringfjellet Formation, Festningen section. 4.3. Size comparisons The literature-based regional data set demonstrate that the genus Ammodiscus is extremely variable in size, with reported diameter measurements ranging from 0.09 to 4.69 mm (Fig. 10). The smallsized A. aff. yonsnabensis recorded in the present study, with diameter of 0.09 to 0.23 mm, is restricted to the lowermost part of this regional size range (Fig. 11). Similarly, the mean diameter of Ammodiscus in the literature-based dataset is 0.35 mm, while that of A. aff. yonsnabensis in the present samples is 0.16 mm. The diameter range of the genus Trochammina found in the literature search is from 0.07 to 1.26 mm (Fig. 10), while that of the generally very small T. aff. eoparva in our material varies from 0.12 to 0.23 mm. In accordance with this, the literature-based main diameter of Trochammina is 0.25 mm, while that of T. aff. eoparva in our material is 0.17 mm (Fig. 11). These comparisons demonstrate that the diameters of both A. aff. yonsnabensis and T. aff. eoparva are significantly smaller than is typically found for Ammodiscus and Trochammina in a wide range of Triassic and Jurassic sedimentary successions (Fig. 11). We propose that the reduced size is an adaptive response to restricted environmental conditions. It seems highly probable that the main restricting factors were low salinity and low oxygen content, apparently in varying combinations. In addition, rapid fluctuations in environmental 42 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 Fig. 10. Diameter categories of various Triassic and Jurassic species of Ammodiscus and Trochammina calculated as percentages from the total number of measured specimens (n). Data derived from published sources (see Tables 1 and 2). conditions may have contributed to the overall stress. In more extreme cases, destruction of the life faunas might have occurred, which was followed by subsequent recolonization. A special feature of the interdistributary bay Yons Nab Beds (on the Yorkshire Coast) is their content of ostracods, which indicates increased oxygenation of benthic habitats. Thus, the main restricting factor during deposition of these strata was apparently low salinity, particularly at stratigraphic levels where ostracods are abundant in association with significant amounts of calcareous foraminifera. Low diversity agglutinated assemblages dominated by small-sized foraminifera are recognized in several Jurassic formations of organicrich shales ascribed to stagnant bottom conditions. Two examples observed by the present authors are the Spekk Formation of the Mid Norwegian Shelf, heavily dominated by the tiny Trochammina annae Levina 1972, and the Brora Shale Member of northeast Scotland dominated by the small Haplophragmoides aff. pygmaeus (Haeusler 1882). Low diversity agglutinated assemblages composed of smallsized taxa are also recorded from several localities as recolonization faunas appearing immediately after the Cenomanian–Turonian boundary anoxic event (Kuhnt, 1992; Coccioni et al., 1995). 4.4. Test morphology and habitats Two of the sections included in the present study, the lower Rannoch Formation and the Yons Nab Beds, have been analyzed for foraminiferal morphogroups by Nagy (1992). The analysis applied a system of 4 morphogroups and 7 subgroups based on test shape combined with life position and microhabitat. Both of the sections are heavily dominated by the flattened subgroup 4a. In the Rannoch Formation this subgroup consists mainly of the planispiral A. yonsnabensis and the low trochospiral T. eoparva. In the Yons Nab Beds A. yonsnabensis is strongly dominant, and flattened trochospiral spirillinids are common. The foraminiferal succession of the Ragnarok and Knorringfjellet formations appears to have a morphological composition closely similar to those of the two North Sea Basin sections cited above. The Ragnarok assemblages are dominated by A. aff. yonsnabensis, T. minutissima and Evolutinella sp. 1, all belonging to morphosubgroup 4a. The same is the case with taxa dominant in the Knorringfjellet Formation, A. aff. yonsnabensis and T. aff. eoparva. Based on comparisons with modern analogues, the species composing J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 Table 1 List of selected papers used to delineate the regional size distribution of Triassic and Jurassic Ammodiscus. Paper Age Region Table 2 List of selected papers used to delineate the regional size distribution of Triassic and Jurassic Trochammina. Paper Triassic Jurassic Late Ten Dam (1945) Kristan-Tollmann (1964) Copestake (1989) Wicher and Bartenstein (1962) Azbel and Grigyalis (1991) Kaptarenko-Chernousova (1959) Gerke (1961) Nagy and Johansen (1991) Tappan (1955) Souaya (1976) Early Middle Late x x x Caucasus, Siberia, Pechora Ukraine x x North-Central Siberia North Sea Basin x x x x x x Wall (1983) x x x Løfaldli and Nagy (1980) x Wall (1960) Bartenstein (1972) Dain (Ed.) (1972) Hedinger (1993) Late Netherlands Austria England Germany x Age Region Triassic Jurassic x x x x x 43 x x x x x Arctic slope, Alaska Canadian Arctic Archipelago Canadian Arctic Archipelago Kong Karls Land, Svalbard Saskatchewan, Canada Eastern Indian Ocean Western Siberia Northern Territories, Canada subgroup 4a are regarded as mobile epifaunal forms, living on the seabed surface or on marine vegetation in shallow brackish waters. 5. Modern analogues This study demonstrates that low diversity agglutinated assemblages dominated by Ammodiscus and Trochammina appear to have been widespread in Late Triassic to Middle Jurassic restricted environments along the northwestern and western European margin, from Spitsbergen to the North Sea Basin. In spite of their extensive regional and temporal ancient distribution, it seems difficult to find modern analogues to these assemblages. The main reason of this circumstance should be sought in the large time gap between Jurassic and Recent, which includes an extensive evolutionary expansion of calcareous foraminifera that lasted from Late Cretaceous to Recent time. During this expansion, calcareous taxa became adapted to a wide range of environments, replacing the originally agglutinated faunas, which were typical and dominant in many habitats during the Jurassic. A number of modern oxygen-depleted environments are dominated by small-sized mainly calcareous foraminiferal species, according to Bernhard and Sen Gupta (1999). They cite several small-sized examples, but also mention that there are exceptions from this development. The present study demonstrates that reduced dimensions are a feature of low diversity stressed assemblages, and the authors support the widely-held view that this feature has a double function. (1) Small size means an increase of the surface area relative to body volume, enhancing oxygen uptake. (2) Small size reflects rapid reproduction rate, which is a feature of opportunistic taxa adapted to rapidly changing conditions typical for several paralic environments where fast recolonization is an advantage. Two examples of modern analogue assemblages are recorded below. Kristan-Tollmann (1964) x Copestake (1989) x Kaptarenko-Chernousova (1959) Gerke (1961) Brouwer (1969) Souaya (1976) Said and Barakat (1958) Ziegler (1959) Lutova (1981) Yakovleva (1984) Nagy and Basov (1998) Løfaldli and Nagy (1980) Sharovskaya (1961) Wall (1960) Oesterle (1968) Kuznetsova (1972) Dain (Ed.) (1972) Hedinger (1993) Early Middle Late Austria England Ukraine x x x x x x x x x x x x x x x x x x x North-Central Siberia Northwestern Europe Canadian Arctic Archipelago Sinai, Egypt Bavaria, Germany Middle Siberia Pechora Basin Spitsbergen Kong Karls Land, Svalbard Northern Siberia Saskatchewan, Canada Switzerland Eastern Indian Ocean Western Siberia Northern Territories, Canada estuarine stratification owing to the high freshwater discharge into the head of the fjord by the river Drammenselva (Fig. 12). The freshwater influx shows large yearly fluctuations changing the composition of the upper water layers in particular. The salinity of these layers drops to minimum values in the spring months and attains peak values during the winter. The presence of a sill 20 km down from the head of the fjord reduces water exchange with the more open Oslofjord, contributes significantly to the development of stagnant deeper water. The water depth is mainly around 10 m at the river mouth, but increases to a maximum of 124 m close to the sill. The depth of the sill itself is 10 m. The upper water layer in Drammensfjord is nearly fresh to brackish, with a salinity that varies between 1 and 20‰ with the seasons. A pronounced halocline located around 15 m separates this highly brackish and unstable upper layer from the underlying 5.1. Drammensfjord A modern low diversity Ammodiscus-dominated foraminiferal fauna has been recorded by Alve (1995) from Drammensfjord in south-eastern Norway. The water column in this fjord reveals a typical Fig. 11. The size ranges (diameters) of Triassic and Jurassic Ammodiscus and Trochammina based on published data, compared with the size ranges of Ammodiscus aff. yonsnabensis and Trochammina aff. eoparva measured in the Knorringfjellet Formation, Festningen section. Mean values of dimensions are also indicated. Based on datasets used in Figs. 9 and 10. 44 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 Fig. 12. Depth section from Drammensfjord, south-eastern Norway, showing distribution of salinity, dissolved oxygen, alpha diversity index and percentage of the small-sized Ammodiscus gullmarensis. Based on data from Alve, 1995. moderately brackish transitional layer, which extends down to about 38 m and shows a salinity increase from 24 to 30‰. Below this depth, the salinity distribution is fairly constant at 30.0 to 31.5‰. The amount of dissolved oxygen in the water column decreases from 8.5 ml/l at the surface to 4.4 ml/l at the halocline (Fig. 12). Through the transitional layer the oxygen content decreases further to 1.7 ml/l at 38 m. Below this water depth, anoxic conditions might develop. The TOC content of the sediments in the transitional zone is on average 0.8%. According to Alve (1995), the shallowest and highly brackish waters of the fjord are dominated by the agglutinated form Miliammina fusca (Brady 1870). The depth interval between this and the top of the transitional layer (at 15 m) is dominated by the calcareous species Elphidium excavatum (Terquem 1870) and E. albiumbilicatum (Weiss 1957). The strongly hypoxic stable deeper water from 38 to 50 m is dominated by the agglutinated taxon Spiroplectammina biformis (Parker and Jones 1865) and the opportunistic calcareous form Stainforthia fusiformis (Williamson 1858). In the moderately brackish transitional zone (15–38 m) the foraminifeal assemblages are highly dominated by agglutinated taxa that account for 60 to 100% (mostly 90%) of the assemblages, while the alpha diversity ranges from 1.9 to 3.9 (average 2.9). The species most commonly dominant is the small-sized Ammodiscus gullmarensis Höglund 1948, comprising 21 to 58% (average 37%) of the fauna. Other dominant species in the transitional zone include the agglutinated forms Eggerelloides scabrus (Williamson 1858) and Adercotryma glomerata (Brady 1878), as well as the calcareous form E. excavatum. Below the halocline, the salinity and oxygen distribution of the fjord waters shows only minor seasonal fluctuations. The environment is nevertheless subjected to major long term changes, as demonstrated by the recorded occurrence of a long period (N5 years) with increased anoxia, when the redox boundary moved upward to a water depth of 30–35 m (Alve, 1995). This event was followed by increased oxygenation leading to recolonization by S. fusiformis and S. biformis, followed by A. gullmarensis. As shown above, the foraminiferal distribution in Drammensfjord is controlled by two main restricting factors, low salinity in shallow waters and hypoxia combined with reduced salinity in the transitional interval (Fig. 12). In addition, changing conditions create a stressed habitat. At greater water depths the restricting effect of hypoxia increases strongly. The foraminiferal fauna occurring in the transi- tional zone in Drammensfjord shows considerable similarities to the Triassic–Jurassic assemblages presented here, by virtue of its low diversities coupled with high dominance of small-sized Ammodiscus. Calcareous species occurring in this depth zone of the fjord belong to the abundant genera Elphidium and Stainforthia, and to the less common Ammonia and Haynesina. However, all these genera have evolved since the Eocene, according to stratigraphical ranges given by Loeblich and Tappan (1988). 5.2. Aso-kai Lagoon This lagoon is located on the western coast of central Japan, and its benthic foraminiferal assemblages were recorded by Takata et al. (2005). The lagoon is partially separated from the Sea of Japan by a sand barrier, and communicates with offshore waters through a narrow channel. The lagoonal water column is maximum 15 m deep, brackish (mostly in the shallower layers) and oxygen-poor (particularly at deeper levels) owing to limited water exchange with the open sea. The hydrography of the water masses reveals large seasonal changes, with highest temperatures and lowest oxygen values during the summer. In winter time, the salinity of the surface waters is around 23‰ and increases to 29‰ at a halocline located between 3 and 4 m depth (Fig. 13). From this level and downwards (to the deepest measurements at 12 m) there is a smooth salinity increase to 30‰. The oxygen content of the lagoon water is highest in February, when the upper 3.5 m of the water column contains 11.0 to 12.6 mg/l oxygen. From here, there is a rapid drop in oxygen content to 3 mg/l at a depth of 5 m. The underlying depth interval down to 12 m is characterized by low values, varying from 2 to 4 mg/l. The foraminiferal diversities of the Aso-kai Lagoon are generally low but spatially highly variable, with alpha values ranging from 1.0 to 7.9 (average 3.1). The lowest values are found in the deeper parts of the lagoon (Fig. 13), although also here with large local changes. Most of the samples from shallow waters were collected along the seaward margin of the lagoon. In these samples, the assemblages are strongly dominated by the small-sized Trochammina cf. japonica Ishiwada 1950, attaining 60%, followed by species of Rosalina, Ammonia, Elphidium, Haynesina and Miliolina. In the deepest, central parts of the lagoon, the frequency of Trochammina cf. japonica is strongly reduced. The assemblages here J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 45 Fig. 13. Depth section from the Aso-kai Lagoon, central Japan, showing distribution of salinity, dissolved oxygen, alpha diversity index and percentage of the small-sized Trochammina cf. japonica. Based on data from Takata et al., 2005. are highly dominated by Stainforthia fragilis (Grindell and Collen, 1976), an opportunistic species particularly adapted to low oxygen conditions. It is of interest to note that high proportions of T. cf. japonica are positively correlated with slightly increased alpha diversities. This is in contrast to several other brackish Japanese lagoons with a tendency to hypoxia, where T. cf. japonica is among the most dominant species, as also exemplified by Lake Saroma (Takata et al., 2005). The foraminiferal distribution of the Aso-kai Lagoon is controlled by two main restricting factors, low salinity and reduced oxygen content, as is the case in Drammensfjord. The common features of the foraminiferal faunas of the lagoon, and of the analyzed Triassic–Jurassic assemblages, are the low diversities and the dominance of Trochammina. Also in this case the most obvious difference is that the modern lagoon contains comparatively large amounts of calcareous taxa, belonging mostly to post-Jurassic genera, particularly: Virgulina evolved in the Early Cretaceous, while Rosalina, Ammonia, Elphidium and Haynesina developed during the Tertiary (Loeblich and Tappan, 1988). 6. Triassic and Jurassic environments of small-sized taxa The application of modern faunal features to the interpretation of Triassic–Jurassic foraminiferal facies is complicated by the fact that modern hyposaline and hypoxic environments can be heavily dominated by either agglutinated or calcareous taxa, or contain a balanced mixture composed of both groups, features demonstrated in several papers (e.g. Alve and Nagy, 1986; Alve, 1990) and by the two examples presented here. Low diversity, however is, a common feature of modern and ancient (e.g. Mesozoic) faunas. As mentioned previously, the large differences in taxonomic composition are explained by major evolutionary changes that have taken place in the order Foraminiferida, mainly during Cenozoic times. When interpreting Mesozoic foraminiferal facies, it is therefore important to integrate faunal data with sedimentary features, paleogeography and transgressive–regressive relationships. 6.1. Delta-influenced shelf embayment The Knorringfjellet Formation of Spitsbergen (Fig. 3) and its facies equivalents in the Barents Sea, the Ragnarok (Fig. 4) and Stø formations, were deposited in an extensive, shallow marine shelf embayment covering the western Barents Sea platform. It is assumed that this embayment received a large freshwater supply from surrounding land areas, with a particularly massive discharge from the southeast, as shown by extensive delta outbuilding from this direction (Van Veen et al., 1992; Riis et al., 2008). Delta outbuilding is also assumed from the northeast, connected with the ancient Lomonosov Ridge terrain (Fig. 1). This paleogeography, involving a large fluvial influx, would explain the brackish conditions in the shallow Barents Shelf embayment. The high freshwater input implies the formation of density-stratified water masses, which potentially would have led to hypoxia in the lower part of the water column below fair-weather wave base to storm wave base. An example of modern shallow water continental shelf hypoxia was presented by Rabalais et al. (1991) from the inner and middle shelf of the Gulf of Mexico, off the Louisiana coast. In this area, development of a stratified water column is mainly controlled by the freshwater discharge (from the Mississippi and Atchafalaya rivers), adequate marine current regime and regional wind fields. The hypoxia develops seasonally, during midsummer, within the 5 to 60 m depth interval. The sandstone interbeds of the Knorringfjellet Formation are thin (less than 50 cm), and show sharp base and top contacts (Fig. 3). They are believed to have been deposited in the offshore-transition zone by episodic major storm events punctuating the background offshore mud sedimentation of quite periods. Storm-generated sedimentary structures have not been observed in these sandstones, obviously because they have been erased by the intensive bioturbation. This development of bioturbation is in accordance with increased bottom water oxygenation during storm events. The calcium carbonate contents of the Knorringfjellet Formation are extremely low in the Festningen section (averaging 0.76% in the Tverrbekken and 0.61% in the Teistberget member), and similar values were also obtained in central Spitsbergen. Such low values, close to the detection limit, are expected with deposition in hyposaline waters. The absence of unequivocal normal marine salinity indicators (such as ammonites, belemnites and echinoderms) points in the same direction. The TOC content of the sediments forming the Festningen section is intermediate (averaging 1.3% in the Tverrbekken and 0.8% in the Teistberget member), and the same is the case in central Spitsbergen (average 1.0%). These rather modest values are lower than those of typical hypoxic deposits. This does not, however, exclude seasonal hypoxia since an originally high organic influx might have been diluted by massive input of silt and clay from active deltaic sources. 46 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 6.2. Prodelta–delta front transition In the northern North Sea (Gullfaks Field), small-sized Ammodiscus– Trochammina assemblages occur in the lower part of the Rannoch Formation (Fig. 5) deposited in the East Shetland Basin, a marine paleoembayment between the East Shetland Platform and Norway. During the Middle Jurassic this embayment was the site of extensive delta progradation resulting in the deposition of the Brent Group delta system, the lower part of which is made up of the Rannoch Formation (Fig. 1). As shown by its coarsening upward lithology, the lower Rannoch succession of sandy mudstone with subordinate sandstone interbeds represents a transition from prodelta to delta front conditions in a distal delta front setting (Parry et al., 1981; Nagy and Johansen, 1991). The lower Rannoch sandstone interbeds are thin, bioturbated and are typified by abrupt basal and top contacts. They have been interpreted as intermittent storm deposits by Brown et al. (1987). Consequently, the depositional setting of the lower Rannoch was the shelf-transition zone, above storm wave base, with fair-weather mud deposition. During the outbuilding of the Rannoch delta, extensive fluvial discharge from the south created hyposaline conditions in the prodelta–delta front area. The carbonate content in the lower part of the analyzed interval is extremely low (0.0 to 3.8%) and accords well with hyposaline conditions. In the upper part of the interval, the carbonate values are strongly variable (0 to 37%) but it is possible that the carbonate here is siderite or ankerite. The TOC content of the sediments is intermediate (average 1.8%), and accords with extensive influx of organic matter to a delta frontprodelta area where the siliciclastic depositional rate was high. Intensive freshwater supply to this area might have created a salinitystratified water column with hypoxic conditions in near-bottom waters. The strongly variable foraminiferal assemblage composition suggests changing hydrographic conditions (Fig. 5). 6.3. Interdistributary bay The Yons Nab Beds (Fig. 6) together with the underlying Millepore Bed comprise a transgressive–regressive cycle that has inundated a delta plain of the Middle Jurassic deltaic series of Yorkshire (Fig. 1). The main transgressive phase is represented by the Millepore Bed of calcareous sandstones, which were deposited in shallow water marine sandwave environments. The regressive phase is formed of the Yons Nab Beds of mudstones and sandstones, deposited in shallow water interdistributary bay environments (Hancock and Fischer, 1980). This regressive stage is succeeded by a shale-sandstone package, including freshwater swamp deposits. The relatively coarse lithologies of the Yons Nab Beds with distinct vertical changes are in accordance with the shallow water marginal marine nature of the environment. Thinner sand beds with sharp base and top contacts probably represent storm deposits, while thicker ones with upwards-coarsening base are regarded as minor mouth bar progradations (Fig. 6). The interdistributary bay depositional setting of these strata implies hyposaline depositional conditions, although major changes in sediment influx and salinity are suggested by the lithology and foraminiferal fauna. The calcium carbonate content is generally low (mainly below 2%, maximum 12%). The foraminiferal succession of the Yons Nab Beds is dominated by agglutinated taxa (mainly Ammodiscus), but the lower and particularly the middle parts of the section contain rather large amounts of calcareous forms. The calcareous group is mainly composed of species belonging to spirillinids (Fig. 6) but species of nodosariids are also significant. Levels with increased frequencies of calcareous taxa represent intermittent ingressions of marine salinities into an overall brackish environment with low species diversities. The marine impact culminates in the middle part of the section, signaled by peak values of calcareous foraminifera and associated shell beds and crinoid stems. As mentioned previously, the Yons Nab Beds contain ostracod shells in 65% of the samples although in strongly varying abundance. The presence of these highly active biota in significant amounts can be taken as indicative of well-oxygenated bottom waters at certain horizons in the shallow water interdistributary bay succession. Likewise, intervals with an absence of ostracods but high frequencies of Ammodiscus imply hypoxic bottom water conditions in a salinitystratified water column. 6.4. A depositional biofacies model The scenario for the Late Triassic to Early Jurassic delta progradations in the North Sea–Mid Norway–Barents Sea region is an extensive, north–south trending seaway (Fig. 1). It was widely open in the north towards the paleo-Arctic Ocean, and continued southwards between Greenland and Norway as a narrow inlet. In the Late Triassic, the southern termination of the inlet was located outside Mid Norway (Nagy and Berge, 2008). During Early Jurassic time, this seaway extended further southward, and connected the paleo-Arctic Ocean with the western European shelf seas. This southwards extension provided a marine pathway for migration of foraminiferal faunas between deltas of the Barents Shelf, northern North Sea and Yorkshire. The main factors controlling the habitats of the small-sized Ammodiscus–Trochammina assemblages were low salinity combined with reduced access to oxygen in a density-stratified water column, as suggested by the main faunal features and analogies with modern assemblages recorded above. A depositional biofacies model delineating the environments of these assemblages envisages extensive but shallow prodelta shelf to delta front areas (Fig. 14), or an interdistributary bay in the case of the Yons Nab Beds. The salient feature of the model is the extensive influx of river water from deltaic sources. In such settings fluvial discharge creates salinity-stratified water masses, with a tendency to hypoxia below fair-weather wave base and particularly below storm wave base. This development implies rapidly changing bottom water properties creating stressful conditions for benthic biota. According to the prodelta–delta front model (Fig. 14), the Ammodiscus–Trochammina assemblages occupied the offshore-transition zone (located between fair-weather and storm wave base) where a moderate degree of hypoxia is combined with reduced salinity. As demonstrated by the modern Drammensfjord, a combination of these factors favored the development of low diversity Ammodiscus-dominated faunas in the depth interval between the fresh to highly brackish surface water layer and the strongly oxygendepleted deeper water (Fig. 12). The depositional setting of the Ammodiscus–Trochammina assemblages in the offshore-transition zone is supported by the sedimentary succession of the analyzed sections, consisting mainly of mudstones with interbeds of thin sandstones typically developed in the Knorringfjellet and Rannoch formations (Figs. 3 and 5). The sandstone beds are interpreted as storm sands interrupting the background sedimentation of offshore muds (containing the foraminifera). This bathymetric setting implies periodic mixing of the upper water layers, leading to intermittent increase of oxygenation during storm events. Emplacement of storm sands would wipe out the foraminiferal faunas of the area, which would be recolonized in subsequent calm periods by low diversity small-sized assemblages. Another but principally related environmental setting for these assemblages is a deltaic interdistributary bay, which is suggested by the stratigraphic and paleogeogtaphic position of the Yons Nab Beds of Yorkshire (Fig. 6). Presence of upwards-coarsening sandstone beds of supposed distributary mouth bar origin is consistent with such a J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 47 Fig. 14. Depositional biofacies model of the small-sized Ammodiscus–Trochammina assemblages, developed particularly for the Knorringfjellet Formation of Spitsbergen with its correlative units in the Barents Sea, and for the lower Rannoch Formation of the North Sea. depositional environment. Storm impacts ate signaled by thinner, sharp-bounded sandstones. 7. Conclusions The analyzed sections from Spitsbergen, the Barents Sea, North Sea and Yorkshire span the time range from Late Triassic to Middle Jurassic and display sedimentary successions consisting of mudstones and sandstone deposited in shallow shelf to deltaic environments. The sections contain mainly agglutinated (benthic) foraminifera, commonly in high abundance. Distinctive compositional features of the foraminiferal successions are explained by adaptation to restricted environmental conditions, which means conditions divergent from those of a normal marine shelf. Salient features of the assemblages are outlined below: 1) The assemblages consist exclusively or almost entirely of agglutinated taxa, except for segments of one of the studied sections (Jons Nab). These agglutinated assemblages are attributed to restricted environments where the controlling factors were low salinity combined with low amounts of dissolved oxygen in storminfluenced temporarily unstable habitats. Absence of calcareous foraminifera from such settings is explained by the presumption that Triassic and Jurassic calcareous taxa (mainly nodosariids) were not adapted to restricted conditions, as is still the case with their modern descendants. 2) The faunal diversities are expressed by the number of species, alpha index and the Shannon–Weaver index H(S). The measured values are low and accord with restricted conditions; the alpha indices are clearly below 5 which is the lower diversity limit displayed by modern normal marine shelf assemblages. 3) The test dimensions of foraminifera are generally smaller than usual. This has been quantified by measuring the diameter of the two dominant species of the Knorringfjellet Formatuin, A. aff. yonsnabensis and T. aff. eoparva, and comparing these data sets with dimensions of Triassic and Jurassic Ammodiscus and Trochammina extracted from the literature. The comparison demonstrates that the average diameter of A. aff. yonsnabensis is 46% of that usual for the genus Ammodiscus, while the average diameter T. aff. eoparva is 68% of that usual for Trochammina. 4) Ostracods are absent from the analyzed sections, except in the Yons Nab Beds where they are most common at horizons containing significant amounts of calcareous foraminifera. The presence of these highly active crustaceans indicates better-oxygenated bottom waters, at least if they occur in adequate abundance. Modern analogues to the small-sized Ammodiscus–Trochammina assemblages are difficult to find owing to the large time gap, which in addition includes a major evolutionary expansion of calcareous foraminifera that radically changed assemblage compositions through time. A close analogue occurs in the modern Drammensfjord (Norway) where the small-sized A. gullmarensis is dominant in a low salinity oxygen-depleted interval of the density-stratified water column. Another cited analogue is the Aso-kai Lagoon (Japan) where Trochammina cf. japonica dominates in low salinity oxygen-poor waters with density stratification. The facies features and depositional setting of the studied sections reveal useful criteria for recognizing the habitat of the small-sized Ammodiscus–Trochammina assemblages: (1) The Knorringfjellet Formation of Spitsbergen and correlative strata of the Barents Sea (the Ragnarok and Stø formations) were deposited in the same extensive shallow shelf embayment with a high deltaic influx creating a brackish, salinity-stratified water column with a tendency to develop bottom water hypoxia. (2) The lower Rannoch Formation of the northern North Sea was deposited at the prodelta to delta front transition in a wide shelf embayment receiving high deltaic discharge leading to a brackish, stratified water column potentially oxygen-depleted in the lower parts. (3) The Yons Nab Beds of Yorkshire are referred to a delta plain interdistributary bay setting with hyposaline and hypoxic conditions intermittently modified by open marine influence. 48 J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 The foraminiferal assemblage occurring in the studied sediment packages reveal close similarities, which indicate marine faunal communications between the various sedimentary basins. The pathway of these communications were establishes in the Early Jurassic along Greenland and Norway, and connected the paleo-Arctic Ocean and the Barents Shelf with the western European shelf seas. A depositional model envisaging the main environment of the low diversity small-sized Ammodiscus–Trochammina assemblages applies a combination of hyposaline and hypoxic conditions formed in a gravity-stratified water column created by high deltaic discharge (Fig. 14). The occurrence of these assemblages in mudstone packages with thin sandstone interbeds, suggests deposition in the offshoretransition zone, between fair-weather and storm wave base. This setting implies large temporal variations of ecological factors, such as salinity and dissolved oxygen, increasing the stress level of the habitats of benthic organisms. Storm impacts contributed to the instability of these environments. Acknowledgments The project was supported by the StatoilHydro-VISTA program, which covered general research expenses. The University Centre of Svalbard is gratefully acknowledged for making field work at Festningen possible. We are also indebted to Henning Dypvik for giving access to samples from the Mjølnir structure. Special thanks are extended to Merethe G. A. Bremer and Sigrid H. Berge for inspiring discussions. We much appreciate the comments of Sorin Filipescu and Adrian Read improving the manuscript. References Alve, E., 1990. Variations in estuarine foraminiferal biofacies with diminishing oxygen conditions in Drammensfjord, SE Norway. In: Hemleben, C., Kaminski, M.A., Kuhnt, W., Scott, D.B. (Eds.), Paleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera. Kluwer Academic Press, Dordrecht, the Netherlands, pp. 621–657. Alve, E., 1995. Benthic foraminiferal distribution and recolonization of formerly anoxic environments in Drammensfjord, southern Norway. Marine Micropaleontology 25, 169–186. Alve, E., Nagy, J., 1986. Estuarine foraminiferal distribution in Sandebukta, a branch of the Oslo Fjord. Journal of Foraminiferal Research 16, 261–284. Azbel, A.J., Grigyalis, A.A. (Eds.), 1991. Practical Advisory in Micropaleontology of the SSSR. : Vol. 5, Mesozoic Foraminifera. Vsesoyuznyy Nauchno-issledovatelskiy Geologo-razvedotchnyy Institut (VNIGRI). 375 pp. (in Russian). Barnard, T., Cordey, W.G., Shipp, D.J., 1981. Foraminifera from the Oxford Clay (Callovian–Oxfordian of England). Revista Espanola de Micropaleontologia 13 (3), 383–462. Bartenstein, H., 1972. Upper Jurassic–Lower Cretaceous primitive arenaceous foraminifera from DSDP sites 259 and 261, eastern Indian Ocean. Initial Reports of the Ocean Drilling Project 27, 683–695. Bernhard, J.M., Sen Gupta, B.K., 1999. Foraminifera of oxygen-depleted environments. In: Sen Gupta, B.K. (Ed.), Modern Foraminifera. Kluver Academic Publisheres, Great Britain, pp. 201–216. Brasier, M.D., 1975. Ecology of modern sediment-dwelling and phytal foraminifera from the lagoons of Barbuda, West Indies. Journal of Foraminiferal Research 5, 42–62. Bremer, G.M., Smelror, M., Nagy, J., Vigran, J.O., 2003. Biotic response to the Mjølnir meteorite impact, Barents Sea: evidence from a core drilled within the crater. In: Dypvik, H., Burchell, M., Claeys, P. (Eds.), Cratering in Marine Environments and on Ice. Springer Verlag, pp. 21–38. Brouwer, J., 1969. Foraminiferal assemblages of the Lias of North-Western Europe. Verhandlungen der Koningklijke Nederlandse Akademie van Wetenschappen, Afd. Natuurkunde 1 (25), 1–48. Brown, S., Richards, P.C., Thomas, A.R., 1987. Patterns in the deposition of the Brent Group (Middle Jurassic) UK North Sea. In: Brooks, J., Glennie, K. (Eds.), Petroleum Geology of North West Europe. Graham and Trotman, London, pp. 399–413. Coccioni, R., Galeotti, S., Gravili, M., 1995. Latest Albian–earliest Turonian deepwater agglutinated foraminifera in the Bottaccione section (Gubio, Italy) — biostratigraphic and paleoecologic implications. Revista Espanola de Paleontologia, No. Homenaje al Dr. Guillermo Colom, pp. 135–152. Cope, J.C.W., Duff, K.L., Parsons, C.F., Torrens, H.S., Wimbledon, W.A., Wright, J.K., 1980. A correlation of Jurassic rocks in the British Isles. Part two: Middle and Upper Jurassic. Geological Society of London, Special Report 15, 1–109. Copestake, P., 1989. Triassic. In: Jenkins, D.G., Murray, J.W. (Eds.), Stratigraphic Atlas of Fossil Foraminifera. Ellis Horwood Limited, Chichester, p. 593. Copestake, P., Johnson, B., 1989. Jurassic. The Hettangian to Toarcian. In: Jenkins, D.G., Murray, J.W. (Eds.), Stratigraphic Atlas of Fossil Foraminifera. Ellis Horwood Limited, Chichester, pp. 81–105. Dain, L.G. (Ed.), 1972. Foraminifera of Upper Jurassic Deposits of Western Siberia: Trudy Vsesoyuznogo Neftyanogo Nauchno-issledovatelskogo Geologo-razvedochnogo Instituta (VNIGRI), 371, 1–272 (in Russian). Davies, G.R., 1970. Carbonate bank sedimentation, East Shark Bay, Western Australia. American Association of Petroleum Geologists, Memoir 13, 85–168. Dypvik, H., Jansa, L.F., 2003. Sedimentary signatures and processes during marine bolide impacts: a review. Sedimentary Geology 116, 309–337. Exton, J., 1979. Pliensbachian and Toarcian microfauna of Zambujal, Portugal: systematic paleontology. Carleton University, Ottawa, Canada, Geological Paper 79-1, 1–102. Gerke, A.A., 1961. Foraminifera of the Permian, Triassic and Liassic strata of the petroliferous region of north central Siberia. Trudy Nauchno-Issledovatelskogo Instituta Geologii Arktiki (NIIGA) 120, 1–518 (in Russian). Ghandour, I.M., Maejima, W., 2007. Benthic foraminiferal biofacies distribution in the Middle Jurassic Safa Formation, Gebel Al-Maghara, Northern Sinai, Egypt: paleoenvironmental implications. Neues Jahrbuch für Geologie und Paläontologie – Abhandlungen 245 (3), 273–294. Graute, E., Helland-Hansen, W., Johansen, J., Lømo, L., Nøttvedt, A., Rønning, K., Ryseth, A., Steel, R., 1987. Advance and retreat of the Brent Delta system, Norwegian North Sea. In: Brooks, J., Glennie, K. (Eds.), Petroleum Geology of North West Europe. Graham and Trotman, London, pp. 915–938. Hancock, N.J., Fischer, M.J., 1980. Middle Jurassic North Sea Deltas with Particular Reference to Yorkshire. Proc. Conf. Petroleum Geology of the Continental Shelf of North West Europe. Heydon & Son Limited, London, pp. 186–195. Hedinger, A.S., 1993. Upper Jurassic (Oxfordian–Volgian) foraminifera from the Husky Formation, Aklavik Range, District of Mackenzie, Northwest Territories. Geological Survey of Canada Bulletin 439, 1–172. Jenkins, C.D., 2000. The Ecological Significance of Foraminifera in the Kimmeridgian of Southern England. In: Hart, M.B., Kaminski, M.A., Smart, C.W. (Eds.), Proceedings of the Fifth International Workshop on Agglutinated Foraminifera: Grzybowski Foundation Special Publication 7, pp. 167–178. Kaptarenko-Chernousova, O.K., 1959. Foraminifera of Jurassic strata of the DnieperDonets Basin. Trudy Instituta Geologicheskikh Nauk, Akademiya Nayk Ukraynskoy SSR. Seriya Stratigrafii i Paleontologii 15, 1–121 (in Russian). Kristan-Tollmann, E., 1964. Die Foraminiferen aus den Rhaetischen Zalmbachmergeln der Fischerwiese bei Aussee im Salzkammergut. Jahrbuch der Geologischen Bundesanstalt, Sonderband 10, 1–189. Kuhnt, W., 1992. Abyssal recolonization by benthic foraminifera after the Cenomanian– Turonian boundary anoxic event in the North Atlantic. Marine Micropaleontology 19, 257–274. Kuznetsova, K.I., 1972. A new genus Marginulinita K. Kuznetsova, and some new species of it in the Late Jurassic of the Russian Platform. Voprosy Micropaleontologii 15, 91–102 (in Russian). Loeblich Jr., A.R., Tappan, H., 1988. Foraminiferal Genera and Their Classification. Van Nostrand Reinhold, New York. 970 pp., 874 pl. Løfaldli, M., Nagy, J., 1980. Foraminiferal stratigraphy of Jurassic deposits on Kongsøya, Svalbard. Norsk Polarinstitutt Skrifter 172, 63–96. Lutova, Z.A., 1981. Callovian stratigraphy and foraminifera from the northern part of Middle Siberia. Trudy Institut Geologii i Geofiziki Sibirskoe Otdelenie, Akademia Nuk SSSR 427, 1–134 (in Russian). Mørk, A., Knarud, R., Worsley, D., 1982. Depositional and diagenetic environments of the Triassic and Lower Jurassic of Svalbard. In: Embry, A.F., Balkwill, H.R. (Eds.), Arctic Geology and Geophysics: Canadian Society of Petroleum Geologists Memoir, 8, pp. 371–398. Mørk, A., Dallmann, W.K., Dypvik, H., Johannessen, P., Larssen, G.B., Nagy, J., Nøttvedt, A., Olaussen, S., Pĉelina, T.M., Worsley, D., 1999. Mesozoic lithostratigraphy. In: Dallmann, W.K. (Ed.), Lithostratigraphic Atlas of Svalbard. Norwegian Polar Institute, Tromsø, pp. 127–214. Murray, J.W., 1973. Distribution and Ecology of Living Benthic Foraminiferids. Heinemann Education Books, London. 247 pp. Murray, J.W., 2006. Ecology and Applications of Benthic Foraminifera. Cambridge University Press, New York. 426 pp. Murray, J.W., Alve, E., 1999. Natural dissolution of modern shallow water benthic foraminifera: taphonomic effects on the palaeoecological record. Palaeogeography, Palaeoclimatology, Palaeoecology 146, 195–209. Murray, J.W., Alve, E., 2000. Do Calcareous Dominated Shelf Foraminferal Assemblages Leave Worthwhile Ecological Information after Their Dissolution? In: Hart, M.B., Kaminski, M.A., Smart, C.W. (Eds.), Proceedings of the Fifth International Workshop on Agglutinated Foraminifera: Grzybowski Foundation Special Publication 7, pp. 311–331. Nagy, J., 1992. Environmental significance of foraminiferal morphogroups in Jurassic North Sea deltas. Palaeogeography, Palaeoclimatology, Palaeoecology 95, 111–134. Nagy, J., Alve, E., 1987. Temporal changes in foraminiferal faunas and impact of pollution in Sandebukta, Oslofjord. Marine Micropaleontology 12, 109–128. Nagy, J., Basov, V.A., 1998. Revised foraminiferal taxa and biostratigraphy of Bathonian to Ryazanian deposits of Spitsbergen. Micropaleontology 44 (3), 217–255. Nagy, J., Berge, S.H., 2008. Micropalaeontological evidence of brackish water conditions during deposition of the Knorringfjellet Formation, Late Triassic–Early Jurassic, Spitsbergen. Polar Research 27, 413–427. Nagy, J., Johansen, H.O., 1991. Delta-influenced foraminiferal assemblages from the Jurassic (Toarcian–Bajocian) of the northern North Sea. Micropaleontology 37 (1), 1–40. Nagy, J., Løfaldli, M., Bomstad, K., 1983. Marginal Marine Microfaunas of the Jurassic (Bajocian) Yons Nab Beds of the Yorkshire Coast. In: Verdenius, J.G., van Hinte, J.E., J. Nagy et al. / Earth-Science Reviews 99 (2010) 31–49 Fortuin, A.R. (Eds.), Proceedings of First Workshop Arenaceous Foraminifera. Continental Self Institute, Norway, pp. 111–127. Nagy, J., Løfaldi, M., Bäckström, S.A., 1988. Aspects of foraminiferal distribution and depositional conditions in Middle Jurassic to Early Cretaceous shales in eastern Spitsbergen. Abhandlungen der Geologischen Bundesanstalt 30, 297–300. Nagy, J., Pilskog, B., Wilhelmsen, R., 1990. Facies controlled distribution of foraminifera in the Jurassic North Sea Basin. In: Hemleben, C., Kaminski, M.A., Kuhnt, W., Scott, D. B. (Eds.), Papleoecology, Biostratigraphy, Paleoceanography and Taxonomy of Agglutinated Foraminifera. Kluwer Academic Press, Dordrecht, the Netherlands, pp. 621–657. Nagy, J., Finstad, E.K., Dypvik, H., Bremer, M.G.D., 2001. Response of foraminiferal facies to transgressive–regressive cycles in the Callovian of Northeast Scotland. Journal of Foraminiferal Research 31 (4), 324–349. Norling, E., 1972. Jurassic stratigraphy and foraminifera of Western Scania, southern Sweden. Sveriges Geologiska Undersökning, Avhandlingar, Serie Ca 47, 1–120. Nøttvedt, A., Cecchi, M., Gjelberg, G.J., Kristensen, S.E., Lønøy, A., Rasmussen, A., Rasmussen, E., Skott, P.H., van Veen, P.M., 1992. Svalbard–Barents Sea correlation: a short review. In: Vorren, T.O., Bergsager, E., Dahl-Stamnes, Ø.A., Holter, E., Johansen, B., Lie, E., Lund, T.B. (Eds.), Arctic Geology and Petroleum Potential: Norwegian Petroleum Society Special Publication 2, pp. 363–375. Oesterle, M., 1968. Foraminiferen der Typlokalität der Birmenstorfer-Schichten, unterer Malm. Ecloge Geologicae Helvetica 61 (12), 695–712. Parry, C.C., Whitley, P.K.J., Simpson, R.D.H., 1981. Integration of paleontological and sedimentological methods in facies analysis of the Brent Formation. In: Illing, L.W., Hobson, G.D. (Eds.), Petroleum Geology of the Continental Shelf of Northwest Europe. Institute of Petroleum, London, pp. 205–215. Pčelina, T.M., 1980. New evidence on the Triassic/Jurassic boundary beds on Svalbard. In: Semevskij, D.V. (Ed.), Geologija Osadnochnogo Chesla Archipelaga Svalbard. NIGA, Leningrad, pp. 363–375 (in Russian). Pietrzenuk, E., 1961. Zur Mikrofauna einiger Liasvorkommen in der Deutschen Demokratischen Republik. Freiberger Forschungshefte C 113, 1–129. Rabalais, N.N., Turner, R.E., Wiseman Jr., W.J., Boesch, D.F., 1991. A brief summary of hypoxia on the northern Gulf of Mexico continental shelf: 1985–1988. In: Tyson, R. V., Pearson, T.H. (Eds.), Modern and Ancient Continental Shelf Anoxia: Geological Society Special Publication 58, pp. 35–47. Riis, F., Lundschien, B.A., Høy, T., Mørk, A., 2008. Evolution of the Triassic shelf in the northern Barents Sea region. Polar Reseach 27, 318–338. Said, R., Barakat, M.G., 1958. Jurassic microfossils from Gebel Maghara, Sinai, Egypt. Micropaleontology 4 (3), 231–272. 49 Sharovskaya, N.V., 1961. Some foraminiferal species from Upper Jurassic deposits of the Nordvik area. Sbornik statei, Nauchno- issledovatelskiy Institut Geologii Atktiki (NIIGA). Paleontologiya i Biostratigrafiya 27, 17–77 (in Russian). Souaya, F.J., 1976. Foraminifera of Sun-Gulf-Global Linckens Island Well P-46, Arctic Archipelago, Canada. Micropaleontology 22 (3), 249–306. Takata, H., Seto, K., Sakai, S., Tanaka, S., Takayasu, K., 2005. Correlation of Virgulinella fragilis Grindell & Collen (benthic foraminiferid) with near-anoxia in Aso-kai Lagoon, central Japan. Journal of Micropaleontology 24, 159–167. Tappan, H., 1955. Foraminifera of the Arctic slope of Alaska; part 2, Jurassic foraminifera. U.S. Geological Survey, Professional Paper 236-B, 21–90. Ten Dam, A., 1945. Een nieuwe soort int het geslacht Ammodiscus Reuss in het Rhaet bij Winterswijk. Geologie en Mijnbow 7, 3. Torsvik, T.H., Carlos, D., Mosar, M., Cocks, L.M.R., Malme, T., 2002. Global reconstructions and North Atlantic paleogeography 440 Ma to recent. In: Eide, E.A. (Ed.), BATLAS — Mid Norway Plate Reconstruction Atlas with Global and Atlantic Perspectives. Geological Survey of Norway, pp. 18–39. Tsikalas, F., Gudlaugsson, S.T., Faleide, J.I., 1998. The anatomy of a buried complex impact structure: the Mjølnir structure, Barents Sea. Journal of Geophysical Research 103, 30,469–30,484. Van Veen, P.M., Skjold, L.J., Kristensen, S.E., Rasmussen, A., Gjelberg, J., Stølen, T., 1992. Triassic sequence stratigraphy in the Barents Sea. In: Vorren, T.O., Bergsager, E., DahlStamnes, Ø.A., Holter, E., Johansen, B., Lie, E., Lund, T.B. (Eds.), Arctic Geology and Petroleum Potential: Norwegian Petroleum Society Special Publication 2, pp. 363–375. Wall, J.H., 1960. Jurassic microfaunas from Saskatchewan. Department of Mineral Resources Saskatchewan, Report 53, 1–229. Wall, J.H., 1983. Jurassic and Cretaceous foraminiferal biostratigraphy in the eastern Sverdrup Basin, Canadian Arctic Archipelago. Bulletin of Canadian Petroleum Geology 31 (4), 246–281. Wicher, C.A., Bartenstein, H., 1962. Trias. Ausgewählte Beispiele aus dem norddeutschen Keuper. Arbeitskreis deutscher Mikropaleontologen. Leitfossilien der Mikropaläontologi. Gebrüder Borntraeger Berlin-Nikolassee, pp. 67–72. Yakovleva, S.P., 1984. Bathonian–Kallovian foraminifera of the Pechora Basin. Microfauna of oil-bearing areas of the SSSR. Vsesoyuzniy ordena Krasnogo Znameni Neftyanoy Nauchno-issledovatelskiy Geologo-razvedochniy Instituta (VNIGRI) 563, 50–58 (in Russian). Ziegler, J.H., 1959. Mikropaläontologische Untersuchungen zur Stratigraphy des Braunjura in Nordbayern. Geologia Bavarica 40, 9–128. Ziegler, P.A., 1982. Geological Atlas of Western and Central Europe. Elsevier Scientific Publishing Company, Amsterdam, the Netherlands. 130 pp., 40 enclosures.