Analysis of Volatile Organic Compounds (VOCs) in Soil via
Passive Sampling: Measuring Partition and Diffusion
Coefficients
by
Hanqing Liu
B.S Chemistry
Renmin University of China, 2014
SUBMITTED TO THE DEPARTMENT OF CIVIL AND ENVIRONMENTAL ENGINEERING IN PARTIAL
FULLFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF
MASTER OF ENGINEERING IN CIVIL AND ENVIRONMENTAL ENGINEERING
AT THE
-MASSACHUSETTS INSTMTUTE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
ARCHNME
OF TECHNOLOLGY
JUNE 2015
JUL 02 2015
@2015 Hanqing Liu. All rights reserved.
LIBRARIES
The author hereby grants to MIT permission to reproduce and to distribute publicly paper and
electronic copies of this thesis document in whole or in part
in any medium now known or hereafter created.
Signature of Author:
Signature redacted
Department of Civil an&Environmental Engineering
Certified by:
21, 2015
Signature redacted
Philip M. Gschwend
Professor of Cix'il and Environmental Engineering
Signature red acted
5,s 2 ,
-
Accepted by:
Thesis Supervisor
Heidi Nepf
Donald and Martha Harleman Professor of Civil and Environmental Engineering
Chair, Departmental Committee for Graduate Students
1
Analysis of Volatile Organic Compounds (VOCs) in Soil via Passive Sampling: Measuring
Partition and Diffusion Coefficients
by
Hanqing Liu
Submitted to the Department of Civil and Environmental Engineering on May 21, 2015
in Partial fulfillment of the requirements for the Degree of Master of Engineering in Civil
and Environmental Engineering
ABSTRACT
Passive sampling has been used as a qualitative and semi-quantitative method in
detecting volatile organic compound (VOCs) concentrations in soil vapors or water.
Passive sampling for soil vapor takes an absorptive material and places it underground for
a period of time to allow the VOCs to diffuse into the absorptive materials. In this report,
I use low density polyethylene (PE) as the absorptive material and determine two key
parameters for passive sampling: the PE-water partition coefficient (Kpew) and diffusion
coefficient in PE (Dpe). These two parameters help passive sampling to transition from a
qualitative method to a quantitative method. The report describes the steps used to carry
out the experiments, gives the results for several specific VOCs, and makes an attempt to
draw more general conclusions on how to estimate these two parameters according to
some other well-known properties.
Thesis Supervisor: Philip M. Gschwend
Title: Professor of Civil and Environmental Engineering
2
Table of Contents
C hap ter 1 Introduction ...........................................................................................................................
C hapter 2 Partition Coefficient T est .........................................................................................
Chapter 3 D iffu sion Coefficient T est.......................................................................................
C hapter 4 C onclusion ...........................................................................................................................
R eferences ..................................................................................................................................................
A p p en dix .....................................................................................................................................................
3
4
. 14
. 34
54
55
57
Chapter 1 Introduction
Background.
This chapter had been jointly written along with Yu Xiang Jaren Soo and David G. Jensen,
both of whom were working on separate aspects of the project. Soo focused on bench where
controlled settings can be achieved so that he can change different variables of the
experiment. Jensen focused on developing the mass transfer model for soil gas and also
"
developing the probe prototype to be used for the eventual field testing.2
Every year, there is a large amount of chemicals released into the environment either
intentionally or by accident. In 1986 the United States issued a program trying to regulate
the underground storage tanks (USTs).
There are approximately 571,000 underground
storage tanks (USTs) nationwide that store petroleum or hazardous substances. The
greatest potential threat from leaking USTs, which mostly contain petroleum products for
service stations, is contamination of groundwater, the source of drinking water for nearly
half of all Americans.1 In fact, the EPA has reported between 6000 and 7000 confirmed
releases of contaminants by registered USTs every year since 2009.2 Statistics shows that
just the state of Massachusetts has about 10,000 active USTs, which is almost equivalent to
1 potential release site every square mile.3 These UST tanks are widely spread and may
cause a great threat for public health via different exposure path ways,
intrusion.
4
such as vapor
Vapor intrusion is an important exposure pathway in risk assessment and often related to
leaking petroleum situations. This is because when these volatile organic compounds are
leaking into the ground, they could volatilize from the contaminant source and be inhaled
by people living in the area. People spend a large amount of time inside buildings and they
breathe a large volume of air, so this can result in a significant risk of chronic health effects. 4
Because of the large number of USTs and the possible threat that they might bring, EPA has
promulgated 40 CFR Part 280. One EPA requirement of 40 CFR Part 280 is "the UST must
have a leak detection method that provides monitoring for leaks at least once every 30
days... and that ... leak detection can consist of monitoring vapors in the soil provided that
the device is protected from moisture such that the results will not be rendered useless."5
And USTs are just an example of all kinds of chemical release today. Since we cannot stop
factories
and manufacturers
from using chemicals in their production, we cannot
completely avoid chemicals leaking into the environmental. Based on this, it is important to
find a way to detect and evaluate a leak as soon as it occurs.
Active and Passive Sampling.
Soil vapor sampling and analysis is a valuable tool for
assessing the nature and extent of contamination. Soil gas samples are typically collected by
applying a vacuum to a probe inserted below ground in order to collect a whole-gas sample,
or by drawing the gas through a tube filled with an adsorbent. These approaches are called
active sampling. But there are challenges associated with flow and vacuum levels in low
permeability materials, and leak prevention and detection during active sample collection
can be cumbersome.
5
Passive sampling has been available as an alternative to such conventional gas sample
collection. Passive sampling involves of several steps. First, one must choose an absorptive
material, for example polyethylene (PE).
When PE is cleaned with dichloromethane,
methanol and pure water, the PE does not have organic contaminants in it before placing
underground. Then, the absorptive material is attached to a frame made of a non-absorptive
material such as aluminum. The frame, usually a pipe shape, has many small holes on its
surface, which enables the absorptive material to contact soil vapors and at the same time
stay away from soil particles. The passive sampling is commonly performed by drilling a
hole into the ground, removing the soil from the hole, and putting the passive sampler frame
into the hole. A commonly seen frame is shown in figure 1-1 below 6. After that, the
excavated soil is back filled to the mouth of the hole and the absorptive material is left there
for a period of time to achieve equilibrium. Finally, the frame is retrieved from underground
and the equilibrated absorptive material is passed to laboratory for concentration analysis
using methods like gas chromatography.
6
Holder
Prnmovable
Sorbent Tube
Sample Chamber
0-Ring
O-Ring
Buna-n Washer
Gas Entry Holes
Diftuslonal Tube
Membrane
Shield
Stainless Steel
Point
6
Figure 1-1. Schematic representation of sampling chamber and sorbent tube
Much of the historic use of passive sampling has been indoor and outdoor air quality
7
monitoring and industrial hygiene applications. -11
Previous passive soil-gas sampling
techniques include a method that uses a thin ferromagnetic wire coated with activated
charcoal to collect organic compounds. The sample is analyzed in the laboratory using
methods
like
gas
spectrometry.
chromatography-mass
However,
the
accumulated
contaminant masses are not simply related to soil-gas concentrations. Several passive soil
7
gas sampling methods have been developed over the past quarter century since the earliest
efforts1 2, including
Petrex
tubes,13 1' 4
EMFLUX~cartridges,1
5
Beacon
B-Sure
Sample
Collection KitsTM16 and GoreTMModules (formerly known as the Gore-Sorber@).1 7 Each of
these methods provides results in units of the mass adsorbed over the duration of the
sample; however,
the correlation between
the mass adsorbed and the soil vapor
concentration has not been quantitatively established.15,16,1 7 Concentration values are
needed for comparison to risk-based screening levels when assessing human health risks
via vapor intrusion, so many regulatory guidance documents caution that passive soil gas
8
sampling should only be used as a qualitative or semi-quantitative screening tool.1 '
19
Absorptive Material. In the passive sampling design, we need to choose an absorptive
material in order to sorb the contaminant in soil gases. Some of the commonly seen
materials are activated carbon or charcoal. However, these have some disadvantages like
they are hard to handle and it is not straight forward to relate their sorbed loads to vapor
concentrations.
Hence, I chose polyethylene as my absorbent for the following reasons. Several polymers
have had success in the application as a passive sampling material for uptake of organic
environmental pollutants. Materials tested in passive sampling devices from earlier studies,
including semi-permeable membrane devices (SPMDs), solid-phase microextraction (SPME)
fibers,
had
simple
polymeric
materials
such
as
polyoxymethylene
polydimethylsiloxane (PDMS) and low-density polyethylene (PE).
20
(POM),
Each of these polymers
exhibits different properties, such as the free volume within the polymer and the segmental
mobility of the polymer chains. Transport of contaminants inside polymers depends on
these factors. The glass transition temperature (Tg) of a polymer defines these properties,
8
21
and polymers with lower Tg have higher chemical diffusivities within the polymer. While
in search of the most suitable material for the setup, we wanted the polymer to have a low
Tg as it facilitates the diffusion process. Amongst these materials, PE has several advantages
over the rest. It is readily available, inexpensive, robust, has a modest Tg, and is easy to
deploy. Therefore, PE was chosen to be the passive sampler material for our passive
samplers.
Key Parameters. Based on what we had discussed above, we need to know some key
parameters in order to change passive sampling from a qualitative tool to a quantitative one.
Two key parameters for a contaminant of interest are its polyethylene-water partition
coefficient
(Kpew)
and its diffusion coefficient in polyethylene (Dpe).
In order to translate the concentration of contaminant in PE into the concentration in soil
vapor, we need the compound's polyethylene-air partition coefficient, Kpea, which is defined
by:
Kpea =
Cpe
a.
Cair
where Cpe is the concentration of contaminant in the PE and Cair is the concentration in soil
air.
However, it is somewhat difficult to directly measure
Kpea
values due to the difficulty of
controlling the concentration in vapor phase. As a result, we measured a related parameter,
the polyethylene-water partition coefficient, Kpew, defined by:
9
-Cpe
Cw
Kpew
because the Kpew can be linked to Kpea by the following equation:
Kpea
_Kpew
K w
-
Kaw
where Kaw is the air-water partition coefficient of the same chemical.
number of reported Kaw values, so as long as we can measure the
There is a large
values, we will be able
Kpew
to estimate the Kpea accordingly. Through a literature review, we can find some reported
data on Kpew for some large compounds like polycyclic aromatic hydrocarbons (PAHs) and
polychlorinated biphenyls (PCBs), however, the contaminants that we are more interested
in are fuels and include chemicals like benzene-toluene-ethyl benzene-and xylenes (BTEX)
and alkanes. The Kpew values for these chemicals has not been reported, although values for
many larger chemicals have been measured (Figure 1-2).
a)
b)
7
A
Met
lA.2
o Adarns @
*
I
a
*
A
x
7
.2007
22
A 8-1 at al., 2003 (70 -)m(28)
o Adams t al. 2007 (51 un)(10)
7
urn) (1)
ConmeIase eta*I.2008(100un)(29(
2009( 25 um)(21)
F...anL.
Smnedebl a.. 2009(70 urn) (30
Perronat al.,2009(lIOGun) (31)
Hale etal. 2010 (26 uM) (32)
Haleta. 2010 (51 um) (32)
6
A
A
5
0
4
(70
8
8
5
t al..
um) (10)
2007
(29)
* COrneissen at al. 2006 (100 urn)
Q Fernandeaa..2009 (25 urn)(11)
a S dttal, 20 (7 um) (30
Peron etal 2009(100Li)(31)
AHAle t 1A2010 (26 urn) (32)
x HMO.t al. 2010 (5Lurn) (32)
-Pmalcted
line
0 Adam
(7 umt0
4
4
A
30
4
-5
-4
-2
-3
0
Iog C."(L)
log K.
Figure 1-2. Log Kpew for PAHs versus (a) log Kow or (b) log Cwsat (L).
22
Notice that the way we obtain the concentration in gas from the concentration in PE is
10
based on the condition that the contaminant has enough time to diffuse into the PE and get
equilibrium. However, it is not easy to determine whether phase equilibrium has been
reached or not. One key factor influencing the rate of approach to equilibrium is a
compound's
Dpe
value, and so knowing such values will be useful for supporting
development of passive sampling.
Choose Performance Reference Standards. In the design for our passive sampling, we use
performance reference standards (PRCs) impregnated into PE before deployment to allow
evaluation of a given sampler's approach to equilibrium.
23
In its simplest form, the PRCs
have similar chemical and physical properties to the target compounds diffusing into the
sampler. After deployment, a measurement of the remaining PRC mass permits the
calculation of the extent to equilibrium reached. Using this information and the deployment
time, one can correct measured concentrations of the target compound in the PE to be what
.
they would have been at equilibrium with the environment 23
Using a PRC for every target compound would be expensive, so Fernandez et al. proposed
using a method to extrapolate PRC properties to different compounds.
23
Using a 1D
diffusion mass transport model, they were able to generate a linear regression from a small
number of PRCs with which to infer necessary mass transfer properties for other target
compounds. A major assumption for this model is that the PRCs experience the same factors
limiting their diffusion rates as affect the target compounds.
In a later study, Apell and Gschwend validated the PRC diffusion assumption of the
quantitative passive sampling method for sediment samping.24 They showed that they could
11
accurately determine equilibrium concentrations in sediment porewater using quantitative
passive samplers removed at different times prior to equilibrium (figure 1-3).
Deduced quilibrium
Concentration
Target Accumulation
U
l I
PRC Loss
t
i
i
t
i
i
I
I
Time
Figure 1-3. Relationship between target accumulation and PRC loss 2 4
Furthermore, they confirmed the ability to use a linear regression generated from the mass
transfer model to infer transport properties. Using these inferred properties they could
calculate PE-deduced concentrations that reasonably matched measured equilibrium
concentrations (Figure 1-4).
12
I
a
.1
CL 0. 11
10
0
0.0
0.0
0.
Measured Porewater (ng/L)
Figure 1-4. Porewater concentrations of PCB congeners 52 (black), 101 (red), 153 (blue), and
180 (green) in seven different sediments from Lake Cochituate measured in laboratorytumbled polyethylene pieces and in extracted porewater. 2 4
It is important that we could use PRCs that are similar to the target chemicals so that we can
use the calculation method mentioned above.
However, the reported data for
focused at large compounds like PAHs and PCBs (Figure
1-5).22
are
As a result, we need to do
the experiments to measure the Dpe values for the compounds we are interested in.
13
Dpe
Ibweilo0 taL, 1985 (66)
Siko t al., 1999 (65)
x
Rsina et al., 2007 (64)
Hab et al.. 2010 (51 um) (32)
1
*
R ina at al. 2010 (63)
Rsina et al, 2010- best fit
--- best f ft
100
A
-12
2X
-13
-14
0
-15
x
-16.
_
50
0
100
200
150
Vm (SPARC)
250
_
300
350
Figure 1-5. Measured log Dpe for selected organic compounds versus their molar volume, Vm
(estimated from SPARC).
22
Besides this, Dpe values also play an important role in determining how long do we need to
deploy the passive sampler. If the Dpe value is quite large, the diffusion process might be
accomplished within one day or a few hours. On the other hand, if the value is quite small, a
different deployment time may be needed to reach equilibrium.
In chapter 2, I will report the detail process of measuring PE-water partition coefficients for
several volative organic compounds or VOCs (Kpew),
results and calculation details.
including the experiment steps and
And in chapter 2, I will report how did I measured the
diffusion coefficient for VOCs in PE (Dpe) and the results I obtained.
14
Chapter 2 PE-Water Partition Coefficients
Measures for Volatile Organic Compounds
(VOCs)
Introduction
The goal of this work was to find polyethylene-water partition coefficients for several VOCs
expected to be important contaminants in soils from leaking fuels.
Test Materials. Additive-free low-density polyethylene (PE) with 102 um (4mil) and 2Sum
(1mil) thicknesses (density: 0.92g/cm3) was obtained from Ambicat" The PE was put into
dichloromethane (J.T.Baker) for 1 day, and then taken out for soaking in another container
with clean dichloromethane for 1 more day. After that, the PE was taken out from
dichloromethane and put into methanol (J.T.Baker) for 1 day and changed into a clean
methanol solution for another day. After cleaning with these solvents, the PE was put into
pure water (Vaponics, model: Aries, 11OV) to leach out any residual organic solvent and
then it was ready for use.
The chemicals
used in the experiment
included:
toluene (J.K.Baker),
ethylbenzene
(J.K.Baker), o-xylene (Aldrich Chemical Company), pentane (J.K.Baker), hexane (J.K.Baker,
95% n-hexane), and hexadecane (Aldrich Chemical Company, 99%).
The instruments used for analysis included a Carlo Erba gas chromatograph (HRGC 5300)
-
and Tekmar purge and trap connected to a Perkin Elmer gas chromatograph. GC Column
J&W Scientific, DB-624 capillary column, 60m, 1.40mm film thickness.
15
Toluene Test. I chose toluene as the chemical with which to start partition coefficient
measurements for the following reasons. First, there are some results from some other's
previous work 22, so I can support my findings by comparing with those results. Second,
toluene has the Henry's Law constant of 6.61 X 1O-3 atm m3 /mol (US Air Force 1989) which
is not too big for a volatile organic compound. This means that conducting the experiment
with toluene would be a little easier compared with chemicals that more easily to escape
into the air, such as pentane and hexane. As a result, the data for toluene would not be
affected significantly by any little air bubbles that may appear in the experiment.
When I began to do the experiment for toluene partition coefficient measurement, I first
prepared
several
biological
A1,A2,A3,B1,B2,B3,C1,C2,C3.
oxidation
demand
(BOD)
bottles,
numbered
with
Then, I filled sets of bottles, A1-A3, B1-B3, C1-C3, with 0.4%,
1.2% and 2% of saturated toluene solution, respectively.
The saturated toluene solution
was made by mixing toluene with pure water in a separatory funnel and held without
mixing to keep the aqueous solution clear of toluene droplets in the lower layer. After filling
the BOD bottles with known toluene concentration solutions, I then cut 6 pieces of precleaned PE (each about 120 mg) and put them into Al, A2, B1, B2, C1, C2 and sealed each
bottle. After 72 hours absorption time, I used gas chromatography with flame ionization
detection to measure the toluene concentrations in the water of these 9 bottles. In a given
series, for example A1,A2,A3, all bottles has the same initial toluene added, the difference in
the concentration between Al and A3, A2 and A3 should represent the toluene that
absorbed into the PE. Hence, these results allowed us to calculate the toluene concentration
in the PE. And the reason that I made two comparisons is that I want to make sure that the
results are replicable.
16
After the impregnation period, the PE was taken out from BOD bottles and the aqueous
samples were sent to gas chromatography (GC) for analysis. I injected 1 uL of each solution
into the GC to measure toluene in the water. The temperature for GC oven was initially set
at 103 'C, then it was increased at 10 degrees per minute to 200*C. After that, the
temperature increased at 25 degrees per minute to 225'C and stayed at 225 C for 1 minute.
The peak height of GC analysis was recorded and translated to concentrations by fitting
with a series of toluene standards (figure 2-1).
120
100
y =15.96
(0.48) x +0.67
(0.006)
80
.
60
40
20
0.000
1.000
2.000
3.000
4.000
5.000
6.000
concentration (ng/uL)
Figure 2-1. Concentration of toluene standards versus peak height in GC. Black thinner line
shows the linear regression line of standard points with regression equation on left side.
And the peak height was translated into concentrations of samples Al to C3 (Table
2-1).
17
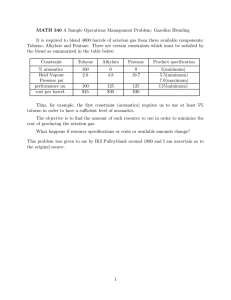
Table 2-1. Tare weight, water filled weight, and volume of BOD bottles of Al to C3. PE dry
weight before the test. Peak height from GC analysis and calculated concentrations.
Sample
Number
Jar Tare
wgt.
(g)
PE dry
Jar H20
filled
Jar Volume
(g)
(mL)
Al
A2
89.78
117.17
A3
116.29
BI
118.07
B2
114.72
B3
118.94
Cl
116.46
C2
118. 12
154.
176.
176.
177.
175.
177.
175.
177.
18
01
73
67
35
72
C3
118.42
178. 52
75
49
64.4
58.84
60.44
59.6
60.63
58.78
59.29
59. 37
60. 1
wgt.
Peak
Height
Concentrat
ion
(mg)
(mm)
(ng/uL)
330.8
329.68
n. d.
340.5
20.5
22
338.56
n. d.
67
352.22
330.8
n. d.
1. 243
1. 337
2.
4.
4.
6.
34
72
110
089
470
156
851
120
7. 352
7. 477
184
11. 488
118
Take Al and A3 as an example to calculate the Kpew for toluene, the calculation is shown
here:
Concentrationdifference = Concentration(A3)- Concentration(A1)
Jar Volume =
Cpe =
JarH20 filled weight - JarTare weight
density of H20
concentrationdifference * Jar Volume
PE dry weight
Cpe =
Cpe
Cw
Concentration(A1)
Using this approach, we found the partition coefficient (Kpew) of toluene is about 105
g(PE)/ml(water), which gives log (Kpew) of about 2.02
18
0.06 (Table 2-2)
14.1
Table 2-2. Kpew and log (Kpew) values of toluene calculated from 6 data sets.
Sample Set
Kpew
log (Kpew)
(ml (H20) /g (PE))
(ml/g)
Al, A3
A2,A3
Bl,B3
B2,B3
C1,C3
C2,C3
Mean
Standard Deviation
132
100
93.2
116
94.7
2.12
2.00
1.97
2.06
1.98
96.3
1.98
105
2.02
14. 1
0.06
If we take the equation from Lohmann (log Kpew= 0.99 log Kow - 0.07) to estimate the Kpew
values for toluene, we estimate the number to be about 2.59. Given that the equation was
drawn from data of PCBs, perhaps it is not surprising that the extrapolated result was lower
than my measurements. So I decided to move to the next compound.
o-xylene and ethylbenzene Tests. Having the experience with toluene, I then decided to
test o-xylene and ethylbenzene together. For one reason, these two chemicals have some
similar properties with toluene (Table 2-3)
Table 2-3. Comparison of log (Cwsat (H20)) and log (Kow) of toluene, o-xylene and ethylbenzene (all values are taken from textbook Environmental Organic Chemistry,
Schwarzenbach et al. 2003).
Toluene
o-xylene
ethylbenzene
Formula
C7H8
C8H10
C8H10
-log (Cosat(H20)) (L)
2.22
2.75
2.8
-log (Kow)
2.69
3.16
3.2
Chemical
property
19
So it is reasonable to assume that the Kpew values for these compounds should not differ too
much from each other.
The method for testing o-xylene and ethylbenzene was the same as toluene. I first prepared
biological
several
oxidation
A1,A2,A3,B1,B2,B3,C1,C2,C3.
demand
(BOD)
bottles,
numbered
with
Then, I filled sets of bottles, A1-A3, B1-B3, C1-C3, with 0.4%,
1.2% and 2% of saturated o-xylene and ethyl-benzene mixed solution, respectively. After
filling the BOD bottles with known o-xylene, ethylbenzene mixed concentration solutions, I
then cut 6 pieces of pre-cleaned PE (each about 320 mg) and put them into Al, A2, B1, B2,
C1, C2 and sealed each bottle. After 72 hours absorption time, I used gas chromatography
with flame ionization detection to measure the o-xylene and ethyl-benzene concentration in
these 9 bottles. The temperature program of GC was same too.
The peak height of GC
analysis was recorded and translated to concentrations by fitting with a series of o-xylene
and ethylbenzene standards (figure 2-2).
(a)
20
-
160
y = 24.8
140
(1.1)x + 3.0
R2 = 0.987
(3.7)
120
100
80
Cu
60
40
20
0
0.000
1.000
2.000
3.000
4.000
5.000
6.000
5.000
6.000
concentration (ng/uL)
(b)
180
160
y=29.8
140
(0.61) x+4.6
R2 = 0.98515
(2.3)
3.000
4.000
120 -i
100 -i
80
Cu
60
40
20
0
0.00 0
1.000
2.000
concentration(ng/uL)
Figure 2-2. Concentration of (a) o-xylene (b) ethylbenzene standards versus peak height in GC.
Black thinner line shows the linear regression line of standard points with regression
equation on left side.
And the peak height was translated into concentrations of samples Al to C3 (Table
21
2-4).
Table 2-4. Tare weight, water filled weight, and volume of BOD bottles of Al to C3. PE dry
weight before the test. Peak height from GC analysis and calculated concentrations for (a) oxylene (b) ethylbenzene
(a)
H20
Tare
filled
Jar
PE
sample
weight
weight
Volume
dry weight
number
(g)
(g)
(mL)
(mg)
Al
A2
89. 78
117.17
154. 18
64.4
176.01
58.84
A3
Bl
B2
B3
C1
C2
C3
116.29
118.07
114.72
118.94
176.73
177.67
116.46
118.12
175.75
118.42
178.52
175. 35
177.72
177.49
Peak Height
Concentration
o-xylene
o-xylene
(mm)
(ng/uL)
60.44
59.6
60.63
58.78
330.8
329.68
n. d.
340.5
338.56
n. d.
5. 5
5. 5
15. 5
21
59.29
59.37
60. 1
352.22
330.8
n. d.
34
19.5
51.5
34.5
90
0.102
0.102
0.506
0.728
0.667
1.959
1.253
1.273
3. 514
(b)
Tare
H20
filled
sample
weight
weight
number
(g)
(g)
Jar
PE
Volume
dry weight
Peak Height
ethylbenzene
(mg)
(mm)
Al
89.78
154. 18
(mL)
64.4
A2
A3
BI
B2
B3
117. 17
116.29
176.01
176.73
58.84
60.44
329.68
n.d.
118.07
177.67
175.35
59.6
60.63
C1
116.46
177.72
175. 75
58.78
59.29
C2
C3
118. 12
177.49
178.52
59.37
60.1
340.5
338.56
n. d.
352.22
330.8
n. d.
114.72
118.94
118.42
330.8
22
5
5
13.5
Concentration
ethylbenzene
(ng/uL)
0.014
0.014
0.300
18
0.451
0.451
45
33
1.358
0.955
32.5
0.938
2.768
18
87
Using the same approach as toluene, we found the partition coefficient (Kpew) of o-xylene is
about 480
185 g(PE)/ml(water), which gives log
(Kpew)
0.17; and the
1735 g(PE)/ml(water), which
partition coefficient (Kpew) of ethylbenzene is about 1453
gives log (Kpew) of about 2.87
of about 2.65
0.54 (Table 2-5). However, as we can see from table 2-4, the
A serie samples for ethylbenzene is higher than the other two series. The might due to the
regression error when fitting into a small concentration. If we ignore the A series in
ethylbenzene test, the partition coefficient (Kpew) of ethylbenzene is about 335
25
0.02.
g(PE)/ml(water), which gives log (Kpew) of about 2.52
Table 2-5. Kpew and log(Kpew) for o-xylene and ethyl-benzene calculated from 6 sets.
Sample Set
A1,A3
A2,A3
B1,B3
B2,B3
C1,C3
C2,C3
o-xylene
log (Kpew)
Kpew
(ml (H20) /g(PE))
769
705
296
347
381
381
Mean
Standard Deviation
2.89
2.85
2.47
2.54
2.58
2.58
2.65
0.17
480
185
ethyl-benzene
log (Kpew)
Kpew
(ml(H20)/g(PE))
3.59
3850
3.55
3529
2.55
351
2.56
360
2.48
305
2.51
325
1453
1735
2.87
0.54
Pentane and Hexane Test. After obtaining the values for these three aromatic compounds
above, I then assessed another two aliphatic chemicals, pentane and hexane. These two
compounds are more difficult to test because they have large Henry's Law constants, which
means pentane and hexane tend to partition into any bubbles in the test system rather just
stay in the PE and the water. Since my testing relied on the analysis of aqueous samples, a
few air bubbles existing in the BOD bottles may cause a big error in the measurement of
concentration in aqueous phase.
23
We can take pentane as an example, the log (Kaw) value for pentane is 1.69 (Schwarzenbach
et al. 2003), which gives the Kaw to be 48.9. If we have a 60 mL BOD bottle full of 1%
saturated pentane solution and a 1 mL air bubble appears in it, the fraction of pentane that
go into the air bubble will be:
fw
ai
0.54
1
mass in water
total mass
1 + Kaw
*
.
Vair
Vwater
This means just a 1 mL air bubble can cause more than half mass loss in aqueous phase and
gives an error in our analysis.
First, I tried to follow the same procedure as the other three compounds. However, there
were two problems appeared in the experiment. One is that some air went into the BOD
bottles and created air bubbles during the 72 absorption time. The other is that the
concentrations of the aqueous samples were about the same as the minimum detection limit
of GC. As a result the analysis was not very reproducible (Table 2-6).
Table 2-6. Kpewl and log (Kpew)J for pentane and hexane calculated from 6 sets.
Sample Set
Al,A3
A2,A3
B1,B3
B2,B3
pentane
log (Kpew)
Kpew
(ml (H20) /g (PE))
2.83
676
2.53
336
2.65
2.70
442
496
Kpew
hexane
log (Kpew)
(ml (H20) /g(PE))
498
189
3.55
1.LE+03
1.8E+03
2.55
2.56
2.7E+03
2.92
825
C1,C3
3.2E+03
2.97
924
C2,C3
1.6E+03
2.76
617
Mean
1.2E+03
0.17
230
Standard Deviation
samples
are around the
for
the
the
concentration
Kpew
means
subscript
J
for
(the
24
3.59
2.48
2.51
2.87
0.54
detection
limit of the instrument)
Based on the problems stated above, I made two changes in the experiment. One is that I
placed the BOD bottles filled with PE and aqueous solutions into a big plastic jar that was
filled with clean water. I hope that in this way, the water that surrounded BOD bottles
would keep air from going into the samples during the desorption time. Another change
was that I switched to a purge and trap instrument for sample analysis. The purge and trap
method captures the interested chemicals from a solution by blowing air through the
sample. And the good part is that we can inject the VOC content of up to 5 mL of sample
instead of 1 uL into GC, which can give us a much more lower detection limit.
To optimize the purge and trap method, I made some changes to the temperature program.
The temperature first stayed at 35-C, then climbed to 165'C with a speed of 10 degrees per
minute. And then temperature stayed at 165 *C for 1 minute. The reason that the upper
temperature of GC program was decreased from 225 "C to 165*C is that both pentane and
hexane are rather volatile and elute at around 100 degree. As a result, we don't need to wait
the temperature to reach 225 *C. The purge and trap program was: purge time was set to 4
minutes, followed by desorption heating of 2 minutes, and then bake for 8 minutes.
The method for preparing testing samples are the same with above. I first prepared several
biological oxidation demand (BOD) bottles, numbered with A1,A2,A3,B1,B2,B3,C1,C2,C3.
Then, I filled sets of bottles, A1-A3, B1-B3, C1-C3, with 0.4%, 1.2% and 2% of saturated
hexane and pentane mixed solution, respectively. After filling the BOD bottles with known
hexane, pentane mixed concentration solutions, I then cut 6 pieces of pre-cleaned PE (each
about 80 mg) and put them into Al, A2, B1, B2, C1, C2 and sealed each bottle. After 72
25
hours absorption time, I used gas chromatography purge and trap method to measure the
hexane and pentane concentration in these 9 bottles. The peak area of GC analysis was
recorded and translated to concentrations by fitting with a series of hexane and pentane
standards (figure 2-3).
(a)
0.45
0.4
y = 0.2881x
R 2 = 0.99093
0.35
f0-
0.3
0.25
0.2
0.15
0.1
0.05
0
1.5
1
0.5
0
2
Area(uV*sec)
(b)
0.14
y = 0.0445x
0.12
R
=
0.99703
0.10
0.08
S0.06
8 0.04
0.02
0.00
0
0.5
1.5
1
2
Peak Area(uV*sec)
26
2.5
3
Figure 2-3. Concentration of (a) pentane (b) hexane standards versus peak height in GC. Black
thinner line shows the linear regression line of standard points with regression equation on
left side.
And the peak area was translated into concentrations of samples Al to C3.(Table 2-7)
Table 2-7. Tare weight, water filled weight, and volume of BOD bottles of Al to C3. PE dry
weight before the test. Peak area from GC analysis and calculated concentrations for (a)
pentane (b) hexane
(a)
Tare
H20 filled
Jar
PE
sample
weight
weight
Volume
dry weight
hexane
number
Al
A2
A3
BI
B2
(g)
(g)
(mg)
(uV*sec)
B3
C1
89. 78
117. 17
116. 29
118. 07
114. 72
118. 94
116. 46
C2
C3
118. 12
118. 42
154.35
176.04
176.93
177.7
175.29
177. 76
175.48
177.8
178.44
(mL)
64.57
Peak Area
92.3
81.6
n. d.
77.5
81.9
58.87
60.64
59.63
60. 57
58.82
59.02
n. d.
86.5
81.9
n. d.
59.68
60.02
1. 48E+04
2. 43E+04
3. 35E+05
3.
3.
2.
1.
75E+05
40E+05
92E+06
02E+06
1. 56E+06
7. 12E+05
Concentration
hexane
(ng/uL)
4. 27E-03
7.
9.
1.
9.
8.
2.
00E-03
66E-02
08E-01
79E-02
42E-01
94E-01
4. 50E-01
1. 03E+00
(b)
Tare
sample
number
Al
A2
A3
BI
B2
B3
C1
C2
weight
H20 filled
weight
Jar
Volume
Peak Area
PE
(g)
89.78
(g)
154.35
(mL)
64.57
117. 17
116.29
118.07
114.72
118.94
116.46
118. 12
176.04
176.93
Concentration
dry weight
hexane
hexane
(mg)
(uV*sec)
(ng/uL)
92.3
4.94E+04
2.20E-06
58.87
60.64
81.6
4.35E+04
8.32E+05
1.94E-06
3.70E-05
177.7
175.29
177. 76
175.48
59.63
60. 57
58.82
59. 02
77.5
81.9
5.68E+05
2.23E+05
2.53E-05
9.92E-06
n. d.
2.01E+06
8.93E-05
86.5
1.41E+06
6.27E-05
177.8
59.68
81.9
1.39E+06
6.20E-05
27
n. d.
C3
n.d.
60.02
178.44
118.42
1.15E+06
2.56E-04
Using the same calculation steps as toluene, we found the partition coefficient (Kpew) for
pentane is about 6.3 * 103
5.4 * 103 g(PE)/ml(water), which gives log (Kpew) of about 3.64
0.45; and the partition coefficient (Kpew) of hexane is about 6.06 * 103
g(PE)/ml(water), which gives log (Kpew) of about 3.65
4.9 * 103
0.38 (Table 2-8)
Table 2-8. Kpew and log(Kpew) for pentane and hexane calculated from 6 sets.
hexane
pentane
Sample Set
log (Kpew)
Kpew
log (Kpew)
Kpew
(ml (H20) /g (PE))
(ml(H20)/g(PE))
A1,A3
1.51E+04
4.18
1.11E+04
4.04
A2,A3
B1,B3
9.23E+03
5.23E+03
3.97
3.72
1.31E+04
1.95E+03
4.12
3.29
B2,B3
C1,C3
5.62E+03
1.70E+03
C2,C3
Mean
Standard Deviation
9.33E+02
6.30E+03
5.44E+03
3.75
3.23
2.97
3.64
5.92E+03
2.10E+03
2.28E+03
6.06E+03
4.92E+03
3.77
3.32
3.36
3.65
0.45
0.38
We can see that there is still a relatively large deviation between different experiment sets,
which may decrease our confidence in the consistency of the data. The possible reason for
the deviation may be: 1. There were air bubbles in the BOD bottles during desorption that
cause a large fraction of chemicals going into the air. Although I didn't see air bubbles when
did the analysis, there might be numbers of tiny little bubbles in there. 2. Because we put
BOD bottles in a large jar filled with water, it is possible that the cap for BOD bottles were
not tight enough so that chemicals can go outside the samples. 3. There might be some
pentane and hexane droplet sticking onto the volume pipettes, thus causing a larger
concentration than what I would expect. If this is true, I would suspect the high number in
Table 2-8 because the droplet effect.
28
Also notice that the calculated results for A series are obviously higher than the other. This
might due to 1. a little droplet in low concentration solutions will cause a larger impact on
results than in a higher concentration solution. 2. When we are fitting a linear regression
line for the standards of each chemical, there might be a larger error for the low
concentration fittings.
Discussion
Desorption Time Optimization. Before discussing the partition coefficients, I would like to
first explain why I choose 72 hours as the desorption time in the experiment.
I did an
experiment that set the desorption time as independent variable and tested how the
concentration of samples changed as the desorption time changed. I chose chlorobenzene
and toluene as my test compounds. First, I prepared a bottle with a mixed solution of 1%
toluene and 2% of chlorobenzene in hexadecane. Then I sealed the bottle and let it sit on the
bench for half an hour in order to make the chemicals vaporize into the air space. Then I put
a large piece (18 cm * 25 cm) of pre-cleaned PE into the bottle without directly contacting
the liquid phase solution. After 24 hours absorption, toluene and chlorobenzene in the
vapor phase has reached equilibrium with the PE According to John K MacFarlane's test
results (shown in Appendix A), 8 hours is long enough to reach the air-PE equilibrium; here
I choose 24 hours just because it is convenient to continue the experiment after one day.
Then, I cut the large piece of PE into 9 small pieces, each with a size about 2cm*4cm, and
placed these pieces in BOD bottles numbered from D1 to D9 for desorption. If toluene and
chlorobenzene diffused into the PE evenly, the concentration in these small pieces should be
identical. However, considering there are some errors in the real practice, we assume that
the original concentrations in these pieces of PE are about the same. These small pieces of
PE were put into BOD bottles at the same time, and the PE was taken out of the bottle with
29
an increasing time.
For example, the PE in bottle D1 was immediately taken out of the
bottle after putting in, and the PE in D2 was taken out after 1 hour and so on.
The result showed toluene in the water increased with time (Figure 2-4).
4.OOE+07
y = $E+061n(x) + 2E+07
R 2 = 0.91708
3.50E+07
3.OOE+07
2.50E+07
--
1.50E+07...
1.OOE+07
-y
=
5.OOE+06
- -
-
Z 2.OOE+07
2E+061n(x) + 9E+06
R2 = 0.80593
O.OOE+00
40
20
-5.OOE+06
60
80
desorption time(hour)
*Toluene
*Cholorobenzene
Figure 2-4. Peak Area (uV *sec) measured by Perkin Elmer gas chromatograph versus
desorption time (hours). The blue points represent toluene and the red points represent
chlorobenzene. The solid and dashed black curves are exponential fitting of the toluene and
chlorobenzene respectively, with the equation of each line on its side.
For the reason that the data in desorption test was somewhat similar to first order reaction
curve, so I also try to fit the data into a first order like formula:
Ct = Ceq ( 1 -
e-kt)
assuming that the desorption has achieved equilibrium after 72 hours. And the fitted k for
toluene, chlorobenzene is 0.0968[uV*sec/hour], 0.0914[uV*sec/hour], respectively. (figure
2-5)
30
0
40
20
60
80
-1
-2
0--%
-3
-4
-5
-6
-7
y = -0.0968x
=
-8
0.86346
y = -0.0914x
R2
=
0.79377
desorption time (hour)
4 toluene
U chlorobenzene
Figure 2-5. Desorption data exponential fitting. The x-axis is desorption time. The red square
points are chlorobenzene and the blue diamond points are toluene. The solid line is the linear
fitting line for chlorobenzene while the dashed one is for toluene.
As we can see from Figure 2-4, desorption of toluene and chlorobenzene from PE into water
almost got equilibrium after the first 24 hours. The reason I chose 72 hours as a desorption
time was simply to guarantee that compounds with larger volume than toluene were able to
achieve equilibrium. However, it appears reasonable to reduce the time to 48 hours instead
of 72 to save time.
Kpew results discussion. We can now proceed to the discussion of partition coefficients for
these five VOCs. Polyethylene is a thermoplastic polymer consisting of long hydrocarbon
31
chains. 25 So compared with water, which has polar structure, these organic compounds
should be more likely to go into a nonpolar structure of PE. So it is reasonable to guess that
(Kow) values or Csat values because these two
Kpew values may have a relationship with
parameters both represent some property relating to compatibility with water. Also, some
previous work, Lohmann found a correlation between log(K0 w) and log(Kpew) values
22
. So
here I also made a graph trying to figure out the potential relationship between log (Kpew)
and log (Kow)(figure 2-6).
6.00
y = 1.30x - 1.32
R2 = 0.6136
5.00
4.00
3.00
2.00
1.00
0.00
0
-1.00
1
3
2
4
5
6
log(Kow)
*toluene U o-xylene A ethyl-benzene X pentane )Khexane
Figure 2-6.
log(Kpew) values versus log(K 0 .) values for toluene, o-xylene, ethyl-benzene,
pentane and hexane. Red line is the linear regression line with its equation on left side.
for the linear regression and the equation for the linear regression is log(Kpew) = 1.30(
0.19)
log(K 0 w) -1.31 ( 0.65) ( r2 = 0.61, se = 0.45, n= 30 ). Notice that the points of toluene and oxylene are all within the 95% confidential area. However the results for other 3 compounds
32
all have some outliers, especially for hexane and pentane.
As stated above, pentane and
hexane tend to escape into the air, so a tiny air bubble in the sample may contribute to a
large loss of concentration. So it seems likely that some error in conducting the experiment
may contribute to the outliers in the results.
If we compare the results we got with those reported in Lohmann's review 22, the new data
reported here appear consistent, albeit variable, with the rest (Figure 2-7).
dm
C) Adaf:~maa
Z0 -W
o
27:
Mi.30717')wiw 261,
1 101
a)
wna*hdgzuia
7
2O9l26,.ni'1i
0AO*@ 5die1.6 'CI7I"U UMJC.
6
PW1Sqt M 2009 1OCw'K"
4
a
kdwiIM.1~ O77 ' 10IM09
0
II
46
3
7
44
4
7
Figure 2-6. Comparison of data from Lohnman's review and our results. Red points are the
results obtained by our experiment.
The equation from Lohmann's review in Figure 2-7 is log (Kpew) = 1.22
1.22 (
0.24)
(
0.046)log (KOW)-
(r 2= 0.94, SE= 0.27, n= 65). We can see that the equation of our result is very
close to the equation from the summary of previous work and given that the summary
equation contains large compounds like PCBs and PAHs, we can have confidence that our
33
results give some of the facts about partition coefficients of these chemicals.
Chapter 3
Diffusion
Coefficients
in
Polyethylene
Introduction. In the previous chapter, we focus on the measurement of partition
coefficients
(Kpew)
for several VOCs. However, knowing the Kpew values alone is not enough
to guide passive sampling work. Here are the reasons. First, we need to choose a PRC that is
similar in property with a target compound so that we can use the method mentioned in
Chapter 1 to calculate equilibrium status. Second, the diffusion coefficient (Dpe) can help us
to decide how long to put the passive samplers in the testing field. It is easy to understand
that chemicals with larger Dpe, which means they move faster, will need less deployment
time. On the other hand, chemicals with smaller Dpe values will need longer diffusion time in
order to achieve equilibrium.
Test Materials. Additive-free low-density polyethylene (PE) with 102 um (4 mil) thickness
and density of 0.92g/cm 3 was obtained from Ambicat" Here I used 4 mil PE instead of 1 mil
PE in the partition experiment because I am testing relatively small compounds compared
to PCBs and PAHs. By pre-calculation, we can estimate the time for toluene to diffuse
through 1 mm thickness of PE.
34
diffusion time =
distance2
Dpe
given that Dpe of toluene is around 10-11 (m 2 /s) according to Lohnman 22, the time for toluene
to diffuse through 1 mm thickness of PE should be around:
time =
(1 * 10-3)2
10-11
=10)10s
s ~ 28 hour
In other words, if we choose 4 mil (about 100 um) PE to do the diffusion test, it will take
about 1000s to diffuse through the PE, which is less than 1 hour. This means if we take 1 mil
PE like we did in partition experiment, the diffusion time will decrease 16 times.
For the experiment, I impregnated a piece of PE with a target chemical, and then inserted
the impregnated piece between sheets of other pre-cleaned PE. In this way, the chemicals in
the middle layer will diffuse out because of the concentration gradient and finally all diffuse
out through all the sheets of PE. For the convenience of the experiment, I collected samples
for a time span of hour scale. It is not hard to image that if the diffusion time decreased to a
few minutes, it would be difficult to do the experiment.
The PE was cleaned using the same method as it was for partition coefficient experiments.
And the chemicals used in the experiment included: toluene (J.K.Baker), ethylbenzene
(J.K.Baker), o-xylene (Aldrich Chemical Company), pentane (J.K.Baker), hexane (J.K.Baker,
95% n-hexane) and hexadecane (Aldrich Chemical Company, 99%).
The instruments used for analysis includes Tekmar purge and trap system, connected to a
35
Perkin Elmer gas chromatograph.
Toluene Test. Again, I chose toluene as my first compound to do the diffusion test. In order
to calculate diffusion coefficient (Dpe), we need to know the diffusion velocity of toluene
within the PE. So I designed the experiment as follows. First, I cut 7 pieces of PE (about 30
cm * 20 cm) and chose one of them to impregnate with toluene. Here I prepared jar, which
at the bottom of it is 10 mL mix solution of 1% toluene in hexadecane. The air in the jar was
expected to build up to about 1% of toluene's vapor pressure (about 1 mg/Lair). Then I
placed the PE inside the jar without contacting the liquid and capped it. In this way, the PE
equilibrated with toluene in gas phase. Since the PE-air partition coefficient for toluene can
be estimated from the ratio of this compound's
Kpew/Kaw =
105/0.25 = 420, then we can
expect the toluene will build up to about 400 mg/kgpe at equilibrium
After the impregnation, I put two lead bricks on a flat bench, and then put a rectangle of
aluminum frame above the lead bricks (Figure 3-1). I used a vacuum sealer to seal the edge
of the toluene-loaded PE together with other 6 pieces, placing the PE with toluene at the
middle of all 7 layers. The sealed layers of PE are then place on the aluminum frame and
placed under another rectangle of Al frame. Finally, another two lead bricks are put onto
these so that two layers of aluminum frames will be pushed tightly together.
The reason for doing this is to keep air out of the space between each layer of PE. We are
trying to measure the Dpe for PE, so air existing between the PE would cause error in the
measurement for the reason that toluene need to diffuse through air between the PE layers.
36
Step 2
Step 1
Step 3
Figure 3-1 Photos showing experimental setup.
One hour after the experiment began, a small area of PE (1.5 cm * 3 cm) was cut at the same
location from each of 7 PE sheets and put into a 60 mL BOD bottle completely filled with
clean water. Another small area was also cut out after 2 hours. In this way, we got 14 BOD
bottles numbered from D1-D7, E1-E7 containing a small piece of PE from each of 7 layers.
And after
72 hours of desorption,
the samples were taken for analysis by gas
chromatography.
The temperature program for GC first stayed at 35'C, then climbed to 225'C with a speed of
10 degrees per minute. And then temperature stayed at 225 'C for 1 minute. The purge and
trap program was: purge time was set to 4 minutes, followed by desorption heating of 2
37
minutes, and then bake for 8 minutes.
Because the PE originally impregnated with toluene was put at the middle of 7 layers and
the time for diffusion was not long enough for all the toluene in middle to diffuse out, we
would expect the concentration in the middle one should be the largest and decrease as the
diffusion distance increases. Moreover, we expected the peak concentration in the middle
PE sheet would be greater at 1 hour than what was seen at 2 hour.
This pattern is
consistent with what we found (Table 3-1).
Table 3-1. Toluene diffusion test results from GC analysis. Time (hour) represents the
diffusion time, and Distance (m) represents the distances from the middle layer (diffusion
distance). Creal is the real concentration measured by GC, and Cest is the estimate
concentration by fitting an optimized Dpe values into the concentration equation.
Time
Distance
Creal in H20
Cest in H20
(hour)
(m)
(ng/uL)
(ng/uL)
1
1
0
0.0003048
0.0002032
0.0001016
0
0.0001016
1
1
0.0002032
0.0003048
2
2
0.0003048
0.0002032
2
2
0.0001016
0
2
2
2
0.0001016
0.0002032
0.0003048
0
1
1
1
2.98
0.17
0.35
0.58
0.78
0.57
0.33
0.15
0.10
0.27
0.39
0.43
0.39
0.24
0.12
n.d.
0.16
0.35
0.58
0.68
0.58
0.35
0.16
0.24
0.30
0.34
0.36
0.34
0.30
0.24
Here I will give an example of how the Cest in H20 in table 3-1 is calculated. We know from
38
Schwarzenbach et al. (2003) that when the boundary condition for diffusion process is set
to 0, the concentration at a specific location can be calculated by:
M *
C(x, t) = 2e(xDt)1/2
2 ep(-
2(w~)'/
x2
-)
4Dt
This is a normal distribution with standard deviation
ox = (2Dt)1/ 2
26
M* is the total mass per unit area which can be calculated by :
*
C(x)dx = M
We know the concentration of toluene in PE from t=0 at the beginning of the test by
measuring the aqueous sample equilibrated with PE, which can be denoted as Cw(0,0). Then,
in order to calculated the mass per unit area M*, we assume the concentrations in the
impregnated PE at t=0 are the same for every tiny part. In this way, M* can be calculated by:
M * = Cw(0,0) * Kpew * density of PE * thickness of PE
And given a values for M* and Dpe, we can calculate an estimated concentration of toluene in
a piece of PE (Cpe) by:
M2
Cpe(x,
x2
t ) = 2 (7D pet)1/2 exp~ 4D pet
39
Then using the Kpew value that we obtained from previous chapter, we can calculate the
toluene concentration in H20 (Conc in H20) by:
Cest in H20(x, t) = fW
*
Cpe(X, t) *
Mpe
Vw
and the fraction of toluene in the water at equilibrium could be calculated by:
1
fw
1 + Mpe
Kpe
w
Cest in H20 is the theoretical number given a particular
Dpe,
and we would like to find a Dpe
that can best fit our real concentration profile into the theoretical distribution. So that we
first calculate the concentration difference between measurement and theoretical values by:
ConcDiff = Creal in H20 - Cest in H20
Then, we add the ConcDiff for the whole 7 sets of data to give the sum square errors.
SSE
=
~ConcDiff
Conc in H20
In the excel spreadsheet, I tried several Dp, values in order to minimize SSE value, and the
Dpe
that gives the minimum SSE values is considered to be the optimized Dpe. By doing the
40
(
optimization for 1 and 2 hour test separately, I found the Dpe of the toluene to be: 2.23
0.01)
* 10-12
[m 2 /s] and log(Dpe) = - 11.65 (
0.004) [m 2 /s]. And by plotting the theoretical
curve with the optimized Dpe value, we can see how well is the real data fit into theory.
(figure 3-2).
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
-0.0006
-0.0004
-0.0002
0
0.0002
0.0004
0.0006
Diffusion Distance(m)
4
Creal_1
Creal_2
Cest_1
Cest_2
Figure 3-2. Toluene concentration profile of diffusion test for 1 and 2 hour tests. The diamond
the
points represent the data collected from the 1 hour test, and the triangle points represent
data collected from the 2 hour test. The x-axis is diffusion distance in unit of m, and the y-axis
is the concentration in H20 with PE equilibrated.
The blue solid line is the theoretical
distribution of toluene concentration given the Dpe value of 3.51 *
10-12,
and the dashed green
line is the theoretical distribution of toluene concentration given the Dpe value of 2.22
41
*
10-12.
Notice that all the diamond points are above the triangle points, which indicates that the
concentration in PE after the first hour is higher than that of the second hour. This is
reasonable because the pieces of PE at the end of both sides were directly contacting the air,
which means toluene will eventually diffuse into the air and cause concentration in PE to
decrease. Also, the distribution of the concentration is similar to what we would expect: the
middle layer has the highest concentration and concentration decrease as the diffusion
distance increases.
O-xylene Test. I then moved forward to do the diffusion test on another compound: oxylene. The experiment steps were very similar to the experiment with toluene. First, I cut
two pieces of 4 mil pre-cleaned PE (25cm * 30cm) and impregnated them by equilibrium
with the gas phase of a mix solution. The solution at the bottom of the jar was prepared with
mixing 1% o-xylene into hexadecane. Since the vapor pressure for hexadecane is much
lower than the other two volatile organic compounds, again we assume that the vapor phase
had about 1% of the vapor pressures of o-xylene . After the impregnation, I then stacked
the PE with another 6 pieces of clean PE and wrap them as flat as possible in order to keep
away the air between different layers. The stack of PE were then put between two layers of
aluminum frame and lead bricks (Figure 3-1). I cut a small area from the PE for each of 7
pieces after 1 and 2 hours and put them into BOD bottles filled with clean airless water.
After 72 hours of desorption time, I then tested the sample on gas chromatography using
the same temperature program with toluene. The results of concentration distribution are
shown as below (Table 3-2).
Table 3-2. o-xylene diffusion test results from GC analysis. Time (hour) represents the
42
diffusion time, and Distance(m) represents the distances from the middle layer(diffusion
distance). Creal is the real concentration measured by GC, and Cest is the estimate
concentration by fitting an optimized Dpe values into the concentration equation.
Time
Distance
Creal in H20
Cest in H20
(hour)
(i)
(ng/uL)
(ng/uL)
0
0.0003048
0.0002032
0.0001016
0
0.0001016
0.0002032
0.0003048
0.0003048
0.0002032
3.22
0.04
n. d.
0.03
0.26
0.60
0.97
0.64
0.24
0. 77
1.13
0.77
0.24
0.03
0.13
0. 36
0.19
0.03
0.09
0. 37
0.47
0.54
0.46
0. 33
0.13
0.0001016
0
0.0001016
0.0002032
0.0003048
0.68
0.84
0.68
0.36
0.13
Using the same approach with toluene, by doing the optimization for 1 and 2 hour test
11.7 (
0.08) * 10-12 [m 2/s] and log(Dpe) =
-
separately, I found the Dpe of the o-xylene to be: 1.78 (
0.01) [m2 /s]. And by plotting the theoretical curve with the optimized Dpe value, we
can see how well is the real data fit into theory (figure 3-3).
43
1.2
1
0
-0.0006
*
0.0002
0
-0.0004 -0.0002
Diffusion Distance(m)
Creal_1
Creal_2
"
0.0004
Cest_1
0.0006
Cest_2
Figure 3-3. o-xylene concentration profile of diffusion test for 1 and 2 hour tests. The diamond
points represent the data collected from the 1 hour test, and the triangle points represent the
data collected from the 2 hour test. The x-axis is diffusion distance in unit of m, and the y-axis
is the concentration in H20 with PE equilibrated.
The blue solid line is the theoretical
distribution of o-xylene concentration given the Dpe value of 1.85 *
10-12,
and the dashed green
line is the theoretical distribution of toluene concentration given the Dpe value of 1.70 *
10-12.
Notice that if we separately investigate the data for each hour's test, they both apply to
normal distribution. However, the concentration of the 2 hour test is higher than the 1 hour
in the position away from the middle layer, which includes diffusion distance more than 0.2
mm. It is interesting to guess what happened here. My hypothesis is that the diffusion
coefficient (Dpe) of o-xylene is lower than that of toluene, so the chemicals moving velocity
in the PE will be slower. This means some o-xylene could accumulate in the outer layers and
gave the results like this. Knowing that the o-xylene has one more methyl group than
toluene so it has a larger molar volume, it is reasonable to guess the Dpe of o-xylene is lower
44
than toluene. And the theoretical curve has confirmed my guess (figure 3-3).
When the
diffusion distance is larger than 0.2 mm, the analytical concentration of the second hour test
is bigger than that of the first hour, which is indicted by a higher position in the figure.
Chlorobenzene Test. For the chlorobenzene diffusion coefficient measurement, I first used
*
the same method as toluene and o-xylene. I cut two pieces of 4 mil pre-cleaned PE (25cm
30cm) and impregnated them by equilibrium with the gas phase of a mix solution of 1%
chlorobenzene in hexadecane. After the impregnation, I then stacked the PE with another 6
pieces of clean PE and wrap them as flat as possible in order to keep away the air between
different layers. The stack of PE were then put between two layers of aluminum frame and
lead bricks (Figure 3-1). I cut a small area from the PE for each of 7 pieces after 1 and 2
hours and put them into BOD bottles filled with clean airless water.
After 72 hours of
desorption time, I then tested the sample on gas chromatography using the same
temperature program with toluene and o-xylene. The results of concentration distribution
are shown as below (Table 3-3).
Table 3-3. Chlorobenzene diffusion test results of 1 hour and 2 hour tests. Time (hour)
represents the diffusion time, and Distance(m) represents the distances from the middle
layer(diffusion distance). Creal is the real concentration measured by GC, and Cest is the
estimate concentration by fitting an optimized Dpe values into the concentration equation.
Time
Distance
Creal in H20
Cest in H20
(hour)
(M)
(ng/uL)
(ng/uL)
0
1
1
0
3.22
-0.0003048
-0.0002032
0.0006
0.0026
0.000558
0.003874
1
1
-0.0001016
0
0.0060
0.0116
0.012388
0.018251
45
1
1
0.0001016
0. 0002032
1
2
2
2
2
2
2
2
0.0003048
-0. 0003048
-0. 0002032
-0.0001016
0.0053
0. 0014
0.0006
0. 0004'
0. 0008'
0. 0016'
0. 0023'
0. 0017'
0. 0007'
0. 0005'
0
0. 0001016
0. 0002032
0. 0003048
Notice that all the results from the second hour test has
0.012388
0. 003874
0.000558
0. 003458
0. 006284
0.008993
0.010134
0. 008993
0. 006284
0. 003458
J label. This is because the peak
height was about as large as background noise and does not show a clear peak of a
reasonable scale. As a result, although the data still have a normal distribution, I didn't
include them for fitting Dpe.
Using the same approach with toluene, by doing the optimization for 1 hour test, I found the
Dpe of the o-xylene to be: 2.46 * 10-12 [m 2 /s] and log(Dpe) = - 11.6 [m 2 /s]. And by plotting
the theoretical curve with the optimized Dpe value, we can see how well is the real data fit
into theory. (figure 3-4).
46
(a)
0.02
0.018
0.016
1.014
4.012
00.01
1.008
).006
).004
).002
0
-0.0006 -0.0004 -0.0002
0
0.0002 0.0004 0.0006
Diffusion Distance(m)
"nCest
1
4 Creal_1
(b)
0.02
0.018
0.016
)O.014
7).012
S0.01
)..008
.2.006
bO.004
r0.002
0
-0.0006 -0.0004 -0.0002
0
0.0002 0.0004 0.0006
Diffusion Distance(m)
"
+ Creal_1
Cest_1
47
(c)
0.02
0.018
0.016
ZO.014
o.012
0.01
EC.008
).006
-b.004
'0.002
0
-0.0006 -0.0004 -0.0002
0
0.0002 0.0004 0.0006
Diffusion Distance(m)
-Cest_1
*
Creal_1
Figure 3-4. Chlorobenzene concentration profile of diffusion test for 1 hour test. The x-axis is
diffusion distance in unit of m, and the y-axis is the concentration in H20 with PE equilibrated.
The blue solid line is the theoretical distribution of chlorobenzene concentration given the Dpe
value of (a) 2.46 * 10- 12[mZ/s] (b) 3 * 10- 12 [m2 /s] (c) 2
*
10-1 2 [m2 /s]
For the reason that the data for the second hour test was not reliable enough and the fitted
Dpe for chlorobenzene seems smaller than what I would expect, I decided to do another test
for chlorobenzene.
For the second diffusion test with chlorobenzene, I increased the number of PE layers to 19
instead of 7 in order to make a better fit of normal distribution. The reason that this could
work is that by increasing the number of layers, we can investigate in a narrow area of the
48
concentration profile, which can fit a more sooth curve than before.
The steps I took were not a big difference from previous tests. First, I cut 19 pieces of PE
(about 30 cm * 20 cm) and chose one of them to impregnate with chlorobenzene. Here I
prepared a jar with 10 mL of 2% chlorobenzene in hexadecane. Then I placed the PE inside
the jar without contacting the liquid and capped it. In this way, the PE equilibrated with
chlorobenzene in vapor phase. After the impregnation, I put two lead bricks on a flat bench,
and then put a circle of aluminum frame above the lead bricks. I used the vacuum sealer to
seal the edge of the chlorobenzene loaded PE together with other 18 pieces, placing the PE
with toluene at the middle of all 19 layers. The sealed layers of PE are then place on the
aluminum frame and placed another circle of frame on them. Finally, another two lead
bricks are put onto these so that two layers of aluminum frames were pushed tightly
together.
After 6 hours diffusion time, I cut a small piece ( 3 cm * 6 cm) from the same location of all
19 pieces of PE and put them into BOD bottles filled with clean water. Because I increased
the number of layers of PE, which is diffusion distance, I increased the diffusion time
correspondingly in order to make sure that chlorobenzene have enough time to diffuse out a
certain distance. Otherwise we may only be able to detect chlorobenzene in a few layers
near the middle one.
Because I only have one chemical (chlorobenzene) in my testing samples, I did some
changes in the GC temperature program. I set the temperature to be isothermal at 140 'C
and recorded peak area every time I injected a sample. The purge and trap program was set
to purge for 5 minutes, then followed by 2 minutes heating for desorption, and baked for 8
49
minutes to clean up the residuals. The concentration measured by GC is below.(table 3-4).
Table 3-4. Chlorobenzene diffusion test results of 6 hour test. Time (hour) represents the
diffusion time, and Distance(m) represents the distances from the middle layer(diffusion
distance). Creal is the real concentration measured by GC, and Cest is the estimate
concentration by fitting an optimized Dpe values into the concentration equation.
Time
Distance
Creal in H20
Cest in H20
(hour)
()
(ng/uL)
(ng/uL)
0
6
6
6
6
6
6
6
0
-0.0009144
-0.0006096
-0.0003048
27.97420734
0.08
0.62
1.67
2.58
1.51
0.76
0.03
0
0.0003048
0.0006096
0.0009144
n. d.
0.057032863
0.567480281
2.25240319
3.566245637
2.25240319
0.567480281
0.057032863
Using the same approach with toluene, by doing the optimization for 6 hour test, I found the
Dpe of the o-xylene to be: 2.34 *
10-12
[m 2 /s] and log(Dpe) = - 11.63 [m 2 /s]. And by plotting
the theoretical curve with the optimized Dpe value, we can see how well is the real data fit
into theory (figure 3-5).
50
(a)
4
3.5
3
0
1.5
1
10.5
-0
-0.0012
-0.0007
-0.0002
0.0003
0.0008
Diffusion Distance(m)
Creal_6
Cest_6
(b)
4
3.5
3
25
1.5
0.5
-0.0012
-0.0007
-0.0002
0.0003
Diffusion Distance(m)
Cest_6
Creal_6
51
0.0008
Figure 3-5. Chlorobenzene concentration profile of diffusion test for 6 hours' test. The x-axis is
diffusion distance in unit of m, and the y-axis is the concentration in H20 with PE equilibrated.
The blue solid line is the theoretical distribution of chlorobenzene concentration given the Dpe
value of (a) 2.34 *
10-12
[m 2 /s] (b) 2.0 *
10-12
Results Visualization and Discussion.
[m 2 /s]
Diffusion coefficients (Dpe) quantify how fast a
compound can move in a particular medium. When a molecule moves through PE, it needs
to pass through the spaces between all kinds of straight and branched structures, so it is
reasonable to guess that increasing molecular weight/ volume would decrease the diffusion
coefficient. And results from previous work have confirmed this conclusion already. So I
decided to plot Dpe we got in my experiments with their molar volume, Vm, which is
calculated by a chemical calculation online software called SPARC27. As expected, increasing
Vm for the three VOCs tested correlated (figure 3-6).
-11.6
1 0
-11.62
I
105
110
115
120
125
-11.64
-11.66
-11.68
i
-11.7
l
-11.72
y = -0.007x - 10.903
R 2 = 0.95111
I
-11.74
-11.76
U
-11.78
Vm(SPARC)[cmA3/mol]
*toluene
Uo-xylene
52
chlorobenzene
Figure 3-6. Measured log (Dpe) for toluene, o-xylene and chlorobenzene versus their molar
volume, Vm (predicted from SPARC). Fit the data with linear regression and the equation is
shown on left side.
Notice that we had a clear trend that the Dpe decreases as the Vm increases, which is
-
exactly what we would expect. The equation of the linear regression line is log (Dpe) =
0.007( 0.09) Vm
-
10.903( 0.08) (r 2
=
0.95, se = 0.17, n= 6).
To assess our measures, we incorporated our results into previous work summarized by
Lohmann 22 (figure 3-7). The results from our experiment fit pretty well on the regression
line from previous work, so that we can have some confidence that our results are within
the benchmark.
so
100
0s
o
35 o
Z ast sa 90 661ZOD
A
x
*
SMetsa 1999(65)
ofee at 200 (641
m
P4M
aet.h
2012i3
*tat, 2010 bwst fit
..-...--------E
I
Q
I
434
-
A6
x
0
50
100
150
20
V. SPAP
250
X0
350
Figure 3-7. Incorporate measured Dpe data into Lohnman's review data 22. The equation for
2
Lohnman's regression line is: log (Dpe) = -0.00145( 0.001) Vm - 10.1( 0.2) ( r = 0.76, se =
0.24, n= 74).
53
Chapter 4 Conclusion
The fundamental motivation for carrying out my part of the project is to support the design
and implement of passive sampling in a gasoline leaking site. The parameters that I have
been measuring are critical to the sampler design and concentration interpretation.
For the partition coefficient part, I have measured the Kpew values for toluene, o-xylene,
ethylbenzene, pentane and hexane. The results for aromatic compounds seem to be
consistent and reasonable. However, there was a quite large variation between different
concentration sets for pentane and hexane experiments. Since these alkanes are harder to
test in aqueous samples, future work future work may consider to get more data on alkanes.
For the diffusion coefficient part, the measured Dpe values have a clear trend with molar
volume. However, more data will be helpful to draw a more general relationship with
relatively small volatile organic compounds.
So that we can estimate a new compound's
diffusion coefficient given its molar volume data, which will be save a significant time to
measure each compound separately.
54
References
1.
US.EPA:
http://www.epa.gov/oust/
2.
USEPA, Semiannual Report Of UST PerformanceMeasures. End Of Fiscal Year 2014, 2014
3.
USEPA, Conceptual Model Scenarios for the Vapor Intrusion Pathway,2012
4.
McAlary, T., Wang, X., Unger, A., Groenevelt, H., & G6recki, T. (2014). Quantitative
&
passive soil vapor sampling for VOCs-part 1: theory. EnvironmentalScience: Processes
Impacts, 16(3), 482-490.
5.
USEPA, 40 CFR Part 280
6.
Karp, Kenneth E. "A diffusive sampler for passive monitoring of underground storage
tanks." Groundwater Monitoring & Remediation 13.1 (1993): 101-106.
J. Environ.
7.
R. H. Brown,
8.
J. Namie'snik,
Monit., 2000, 2, 1-9.
B. Zabiegala, A. Kot-Wasik, M. Partyka and A. Wasik, Anal. Bioanal. Chem.,
2005, 381, 279-301.
9.
S. Seethapathy, T. G'orecki and X. Li,
10. T. G'orecki and
J.
J. Chromatogr.
A, 2008, 1184, 234-253.
Namie'snik, TrAC, Trends Anal. Chem., 2002, 21(4), 276-291.
11. D. P. Adley and D. W. Underhill, Anal. Chem., 1989, 61(8), 843-847.
12. Kerfoot, Henry B., and C. L. Mayer. Use of industrial-hygiene samplers for soil-gas
measurement. No. PB-89-166359/XAB. Lockheed Engineering and Management Services
Co., Inc., Las Vegas, NV (USA), 1989.
13. Gomes, D. C., Alarsa, M., Salvador, M. C., & Kupferschmid, C. (1994). Environmental Soil
and Ground Water Assessment Using High Resolution Passive Soil-Gas Samplers-Petrex
&
Method: Methodology and Results of a Case Study Performed in Brazil. Water Science
Technology, 29(8), 16 1-172.
55
14. M. Anderson and G. Church,
J. Environ. Eng., 1998, 124, 555.
15. Environmental Technology Veriocation Report, Soil Gas Sampling Technology, Quadrel
Services, Inc., EMFLUX Soil Gas System, U.S. EPA Office of Research and Development.
EPA Report no. 600/R-98/096,1998.
16. Beacon, 2013. http:://www.beacon-usa.com/services/passive-soil-gas-surveys/
17. Environmental Technology Veriocation Report, Soil Gas Sampling Technology, W. L.
Gore & Associates, Inc. GORE-SORBER Screening Survey, U.S. EPA Office of Research and
Development. EPA Report no. 600/R-98/095, 1998.
18. ASTM Standard D7758, New Practice for Passive Soil Gas Sampling in the Vadose Zone
for Source Identication,
Intrusion
Evaluations,
Spatial Variability Assessment,
ASTM
International,
West
Monitoring
Conshohocken,
and Vapor
PA,
2011,
http://www.astm.org.
19. Final Guidance for the Evaluation and Mitigation of Subsurface Vapor Intrusion to
Indoor
Air
(Vapor
Intrusion
Guidance),
California
Environmental
Protection
Agency/Department of Toxic Substances Control (EPA/DTSC), October 2011. http://
www.dtsc.ca.gov/AssessingRisk/upload/Final VIG Oct 2011.
20. T.P.Rusina,F.Smedes,and.Klanova,J.Appl.Polym.Sci.,2010,1803-1810
21. M.Saleem,A.DF.A.Asfour,D.DeKee,andB.Harrison,J.Appl.Polym.Sci.1989,37,617-625
22. Lohmann, Rainer. "Critical review of low-density polyethylene's partitioning and
diffusion coefficients for trace organic contaminants and implications for its use as a
passive sampler." Environmentalscience & technology 46.2 (2011): 606-618.
23. L.A.Fernandez, C.F.Harvey, and P.M.Gschwend, Environ. Sc. Technol.,2009, 43, 8888-94.
24. J.N.Apell and P.M.Gschwend, Environ. Sci. Technol., 2014, 48, 10301-7.
25. Wikipedia: http://en.wikipedia.org/wiki/Polyethylene
26. Schwarzenbach, Rene P., Philip M. Gschwend, and Dieter M. Imboden. Environmental
56
OrganicChemistry.John Wiley & Sons, 2003.
27. SPARC: ARChem's physicochemical calculator
57
Appendix
Appendix A. Toluene Desorption Time course
10.0
9.0
o
8.0
7.0
6.0
o _
5.0
E 4.0
3.0
2.0
0~1
1.0
0
0.00
10
20
30
40
50
60
Vapor Phase Sorption Time (hours)
Figure A-1. Desorbed water concentration versus vapor phase sorption time testing on
toluene.
Method: 1 mL of 100:1 hexadecane:toluene was added to a tared, -64 mL volume, glassstoppered jar. The 4 mil PE was 'ribboned' onto aluminum wire supports for suspension
above the hexadecane:toluene solution. At t=0, 24 and 50h PE was removed and placed into
a water-filled, glass-stoppered jar. The toluene-exposed, 4 mil PE (removed from the
aluminum wire support) was added to a tared, glass-stoppered jar filled with Aries H 2 0 and
after 72 hours test the concentration in GC.
58