Protein Engineering for Cancer Therapy
MSSACHUSETTS INSTITUTE
by
OF TECiNOLOY
Al4 2012
David Victor Liu
B.S., M.S. in Chemical Engineering,
Stanford University, 2005
LIBRARIES
ARCHIVES
Submitted to the Department of Chemical Engineering in
Partial Fulfillment of the Requirements for the Degree of
DOCTOR OF PHILOSOPHY IN CHEMICAL ENGINEERING
at the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
February 2012
0 2011 Massachusetts Institute of Technology
All Rights Reserved
Signature of Author:
Department of Chemical Engineering
October 24, 2011
Certified by:
K Dane Wittrup
C.P. Dubbs Professor of Chemical Engineering and Biological Engineering
Thesis Supervisor
Accepted by:
William M. Deen
Engineering
Chemical
of
Professor
Chairman, Committee for Graduate Students
1
Protein Engineering for Cancer Therapy
by
David Victor Liu
Submitted to the Department of Chemical Engineering on October 24, 2011 in
partial fulfillment of the requirements for the degree of Doctor of Philosophy in
Chemical Engineering
Abstract
The immunosuppressive effects of CD4*CD25* regulatory T cells (Tregs) interfere with
anti-tumor immune responses in cancer patients. In the first part of this work, we present a novel
class of engineered Interleukin-2 (IL-2) analogues that antagonize the IL-2 receptor, for
inhibiting Treg suppression. These antagonists are engineered for high affinity to the IL-2
receptor a subunit and low affinity to either the P or y subunit, resulting in a signaling-deficient
IL-2 analogue that sequesters the IL-2 receptor a subunit from wild type IL-2. Using this design,
human and mouse IL-2 antagonists were generated with inhibition constants ranging from
200 pM to 5 nM in vitro. Genetic fusions with IgG2a Fc enhanced serum half-life up to 30
hours. In order to study the effects of IL-2 antagonism, Fc fragments with disrupted effector
functions were used. Fc-antagonist fusions bound to but could not deplete peripheral Tregs.
They downregulated CD25 on Tregs, but could not perturb Treg function in a syngenic tumor
model, presumably due to the high sensitivity of the IL-2 receptor and a high threshold for
antagonism in vivo.
In the second part of this work, we present a novel multi-agent protein-based system for
targeted siRNA delivery that provides potential advantages over other nanoparticle- and proteinbased delivery vehicles. In the first agent, the double stranded RNA binding domain (dsRBD) of
human protein kinase R is used as an siRNA carrier, in fusion proteins that target epidermal
growth factor receptor (EGFR). Targeted dsRBD proteins deliver large amounts of siRNA to
endosomal compartments in an EGFR expressing cell line, but efficient gene silencing is limited
by endosomal escape. The use of a second agent that contains the cholesterol dependent
cytolysin, perfringolysin 0, enhances endosomal escape of siRNA. Targeted delivery of
perfringolysin 0 induces gene silencing in a dose-dependent and EGFR-dependent manner.
However, cytotoxicity of the cytolysin creates a narrow therapeutic window. Multiepitopic
EGFR binders that induce EGFR clustering are explored as tools for enhancing gene silencing
efficiency. Interestingly, they not only enhance gene silencing potency but also protect against
toxicity from EGFR-targeted cytolysins, thus significantly widening the therapeutic window of
this method.
Thesis Supervisor: K. Dane Wittrup
Title: C.P. Dubbs Professor of Chemical Engineering and Biological Engineering.
2
Acknowledgements
I would like to first thank my advisor, Dane Wittrup, for giving me the opportunity to
join his lab. He has been an amazing mentor and I admire his ability to see the big picture while
also staying aware of the small details when discussing an experiment. His guidance, patience,
and advice over the years have been invaluable. I appreciate the independence he has given me
as a researcher while also providing direction in my work. I would also like to thank my thesis
committee members, Harald von Boehmer and Arup Chakraborty, for their time, ideas, and
discussions.
I would like to thank all of the members of the Wittrup lab, past and present, who have
helped me in the lab. Annie Gai, Ben Hackel, and Mike Schmidt were very helpful and patiently
answered my many questions when I was getting started in the lab. Ben Hackel and Margie
Ackerman were especially helpful in teaching me yeast display. Chris Pirie and Nicole Yang
provided excellent discussions on the siRNA delivery work. Stefan Zajic, Andy Rakestraw,
Ginger Chao, Pankaj Karande, Shanshan Howland, Greg Thurber, Wai Lau, Steve Sazinsky,
Shaun Lippow, Eileen Higham, Kelly Davis, Jordi Mata-Fink, Jamie Spangler, John Rhoden,
Tiffany Chen, Xiaosai Yao, Seymour de Picciotto, Peter Bak, Cary Opel, James van Deventer,
Byron Kwon, Alice Tzeng, and Michael Santos all provided useful reagents, discussion, and
protocols. Undergraduate researchers, Diana Wieser, Jarrett Remsberg and Abigail van Hook
worked hard and contributed to work presented here.
I would also like to acknowledge the help of collaborators. Lisa Maier in the Hafler Lab
at Brigham and Women's Hospital helped with human primary Treg assays. Jens Nolting and
Carolin Daniel in the von Boehmer lab at the Dana Farber Cancer Institute, and Ed Kim in the
Mempel lab at Mass General Hospital helped with mouse Treg work.
On a personal note, I would like to thank my friends in the Boston area for all of their
support. The friends I have made through the Living Root Dragon Boat Club have always been
there when I wanted to take my mind off of work.
I especially would like to thank my family, who has always encouraged me to do my
best. My parents taught me the importance of hard work, and without them, I would not be here
today. My brother always made me feel proud of what I accomplished. Last, and certainly not
least, I would like to thank my partner, Kali Drake, who has always been a source of
unconditional support throughout graduate school. She helped me keep things in perspective
after a tough day in lab. I certainly could not have done this without her support and
encouragement. Thank you.
3
Table of Contents
Chapter 1: Introduction
Part 1: Engineered Interleukin-2 antagonists for the inhibition of regulatory T cells....... 8
Immunotherapy as a therapeutic modality for cancer...................................8
.10
Regulatory T cells in cancer..........................................................
12
...
CD25 for Treg targeting...........................................................
Part 2: A novel protein based method for targeted delivery of short interfering RNA.....15
15
RNA interference as a powerful therapeutic modality.......................................
Nanoparticle-based methods for targeted delivery of siRNA.............................17
18
Protein-based methods for targeted siRNA delivery...................................
Double stranded RNA binding domains as an alternative siRNA carrier.......... 20
Endosomal Escape as a barrier to effective siRNA delivery....................... 22
.. 25
Thesis Overview................................................................
26
References......................................................
Chapter 2: Engineered human Interleukin-2 antagonists for the inhibition of regulatory
T cells
Abstract........................................................32
Introduction.....................................................33
Materials and Methods.............................................36
40
Results........................................................
Design of human IL-2 mutant antagonists............................................40
IL-2 analogue binding affinity to the IL-2 receptor a subunit........................41
Disruption of binding to the IL-2 receptor p and y subunits.......................44
46
Antagonism by the IL-2 analogues......................................................
48
Discussion.........................................................................
52
Acknowledgements....................................................
References......................................................53
Chapter 3: Engineered mouse Interleukin-2 antagonists for the inhibition of regulatory
T cells
Abstract........................................................56
Introduction........................................................57
...... 59
Materials and Methods.............................................
Results...............................................................................................64
64
Design of mouse IL-2 antagonists....................................................
67
In vitro characterization of FEc fusion proteins......................................
Pharmacokinetic characterization of Fc fusion proteins............................69
69
Characterization of Fc fusion proteins in vivo......................................
Discussion...........................................................73
Acknowledgements................................................76
77
References.........................................................................
4
Chapter 4: A two-agent protein-based system for targeted siRNA delivery
. 80
Ab stract............................................................................................
. 81
Introduction .......................................................................................
M aterials and M ethods..............................................................................85
R esults.........................................................................................
. . .. 92
92
Preparation of E6N2.....................................................................
Analysis of siRNA / dsRBD interactions.............................................93
pH sensitivity of binding of siRNA and dsRBD.....................................93
siRNA uptake by E6N2................................................................
95
PFO fusion proteins for endosomal escape..........................................97
D iscu ssion .......................................................................................
. .. 99
A cknow ledgem ents................................................................................103
R eferen ces..........................................................................................104
Chapter 5: EGFR clustering for the enhancement of targeted siRNA delivery
Ab stract.............................................................................................
10 6
Introdu ction .........................................................................................
107
M aterials and M ethods............................................................................109
Results.........................................................111
Enhancement of siRNA Uptake by multispecific constructs........................Ill
Enhancement of GFP knockdown by multispecific constructs..................... 112
Cytotoxicity profiles of Fn3-PFO in the presence of HNB-LCD.................. 113
115
D iscu ssion ..........................................................................................
A cknow ledgem ents................................................................................118
119
Referen ces..........................................................................................
Appendix A: Delivery of siRNA using targeted dsRBD fusions to CD25 and CEA
exp ressin g cells..........................................................................
120
Appendix B: pH sensitive binders to perfringolysin 0............................................125
Appendix C: DNA Sequences...........................................................................
5
131
List of Figures and Tables
Figure 1.1 Immune activation and suppression in an antitumor immune response..................11
16
Figure 1.2 RNAi pathways in mammalian cells.......................................................
Figure 1.3 Crystal structure of a dsRBD bound to double stranded RNA............................21
Figure 1.4 Crystal structure of two cholesterol dependent cytolysins.................................24
Table 2.1 Mutations for disrupting IL-2 receptor subunit binding.................................
42
Figure 2.1 Interleukin-2 antagonist design................................................................
42
Figure 2.2 Kit225 cell surface titrations to measure IL-2Ra binding affinity.....................
43
Figure 2.3 Lack of agonism by Q126T and V91R....................................................
45
Figure 2.4 Antagonism by Q126T and V91R.........................................................47
Figure 3.1 Mouse IL-2 antagonist design..............................................................65
66
Figure 3.2 Characterization of two lead mouse IL-2 antagonists...................................
Figure 3.3 Characterization of Fc-IL-2 antagonist fusion proteins.................................68
Figure 3.4 Characterization of Fc-IL2 antagonists in GFP-Foxp3 reporter mice.................71
72
Figure 3.5 Characterization of Fc-IL2 antagonists in an EG7 tumor model.......................
Figure 4.1 Schematic of the two-agent system for siRNA delivery.................................83
92
Table 4.1 EGFR binding affinity for dsRBD and PFO fusion constructs............................
Figure 4.2 Characterization of the interaction between E6N2 and siRNA...........................94
Figure 4.3 siRNA uptake limitations and requirements.............................................
96
Figure 4.4 Characterization of Fn3-PFO fusion proteins.............................................
98
Figure 5.1 Enhancement of siRNA uptake by multispecific constructs.............................
111
Table 5.1 Summary of multispecific constructs evaluated............................................
112
Figure 5.2 Effects of HNB-LCD on the therapeutic window of E6N2/Fn3-PFO based
siRN A delivery .................................................................................
114
6
List of Abbreviations
ADCC
Ago2
BSA
CDC
CDC
CEA
dsRBD
dsRBM
DTX
d2EGFP
EC50
FACS
FBS
Fc
Fn3
GFP
HBSS
HEK
IC 50
IgG
IL-2
IL-2R
IL-2Ru
IL-2Rp
IL-2Ry
KD
KD, app
Ki
LLO
MBP
miRNA
PBMC
PBS
PBSA
PFO
RISC
RNAi
siRNA
STAT5
TGFp
Treg
antibody dependent cell-mediated cytotoxicity
argonaute 2
bovine serum albumin
cholesterol dependent cytolysin (used in Chapters 4 and 5)
complement dependent cytotoxicity (used in Chapter 3)
carcinoembryonic antigen
double stranded RNA binding domain
double stranded RNA binding motif
diphtheria toxin
destabilized GFP
half-maximal effective concentration
fuorescence activated cell sorting
fetal bovine serum
fragment crystallizable region
10th type 3 fibronectin domain
green fluorescent protein
Hank's balanced salt solution
human embryonic kidney
half-maximal inhibitory concentration
immunoglobulin G
Interleukin-2
IL-2 receptor
IL-2 receptor a subunit
IL-2 receptor P subunit
IL-2 receptor y subunit
dissociation constant
apparent dissociation constant
inhibition constant
listeriolysin 0
maltose binding protein
micro RNA
peripheral blood mononuclear cells
phosphate buffered saline
phosphate buffered saline with 1 mg/mL bovine serum albumin
perfringolysin 0
RNA Induced Silencing Complex
RNA interference
small interfering RNA
signal transducer and activator of transcription 5
transforming growth factor P
regulatory T cell
7
Chapter 1: Introduction
The work presented here consists of two main parts. The first part describes work to
inhibit immunosuppression by regulatory T cells using Interleukin-2 antagonists. The second
part describes the development of a novel protein based method for the targeted delivery of small
interfering RNA.
Part 1: Engineered Interleukin-2 antagonists for the inhibition of Regulatory T cells
Immunotherapy as a therapeutic modality for cancer
The idea that the body's immune system can play a protective role in cancer was first
proposed over 100 years ago (1). Since then, there has been plenty of evidence that the immune
system can recognize tumor cells as malignant and in some cases, develop adaptive and innate
immune responses against a tumor (2). In the field of cancer immunotherapy, approaches are
sought to activate or strengthen this immune response against cancer.
Current strategies for cancer immunotherapy generally rely on the fact that there are
antigens expressed differentially on tumor cells versus normal cells that can be utilized for tumor
targeting.
The tumor antigen can be targeted directly by the therapeutic, such as with
monoclonal antibodies that bind tumor antigens.
In this case, the Fc region of the
immunoglobulin G (IgG) antibody isotype can activate antibody-dependent cellular cytotoxicity,
in which natural killer cells are recruited to lyse the target cell. Fe regions can also activate
complement dependent cytotoxicity, which leads to target cell lysis by the membrane attack
complex. A number of such antibodies have been successful in the clinic and have received
8
FDA approval for marketing, such as cetuximab and panitimumab, which target EGFR in
colorectal cancer; trastuzumab, which targets Her2 in breast cancer; rituximab, which targets
CD20 in non-Hodgkin's lymphoma and alemtuzumab which targets CD52 in chronic
lymphocytic leukemia. Alternatively, immune responses against tumor antigens can be induced
without direct binding of the therapeutic to the antigen. For example, fusion proteins of tumor
antigens with immunological adjuvants such as GM-CSF are infused into a patient, where they
activate antigen presenting cells.
This in turn induces a T cell responses against the tumor
antigen (3).
Cell-based therapy, such as adoptive cell transfer, is another strategy that is being
investigated for cancer immunotherapy. Specific cell populations are isolated from a patient,
modified or expanded ex vivo and then infused into the patient to activate antitumor immune
responses. This is the strategy behind sipuleucel-T, the first FDA-approved autologous cell
therapy for prostate cancer, in which autologous dendritic cells (DC's) are pulsed ex vivo with
the fusion protein containing GM-CSF and the prostate cancer marker, prostatic acid
phosphatase (PAP). The DC's are then infused back into the patient, where they activate T cell
responses against PAP and the malignancy (4).
In addition to autologous DC's, cell-based
immunotherapies can also employ tumor infiltrating lymphocytes, which contain tumor antigen
specific T cells found at the tumor site. These T cells can be isolated and expanded ex vivo,
during which they can be subjected to various conditions to boost their antitumor effector
functions prior to infusion back into the patient (5). For example, they can be activated with
soluble cytokines, or genetically engineered to express tumor antigen specific T cell receptors or
to enhance survival (6-8). Although no T cell based immunotherapies have been approved by
the FDA to this date, adoptive T cell transfer has been shown to be highly effective in the clinic.
9
In metastatic melanoma patients who have undergone lymphodepleting treatments prior to
adoptive T cell transfer, objective response rates of up to 72% have been reported (9).
In these adjuvant and cell based approaches, the end goal is to induce the effector
functions of tumor antigen specific cytotoxic T lymphocytes (CTL's) to attack and eliminate
tumor cells (Figure 1.1).
However, in many cancer patients and in mouse models of cancer,
there already exist tumor specific DC's and T cells, but they are unable to carry out an immune
response and eradicate the tumor (10-12). Based on this observation, there seem to be inhibitory
factors that prevent the antigen-specific DC's and T cells from carrying out the immune response
against the tumor. Indeed it has been shown that the tumor environment is immunosuppressive.
Tumors can tolerize CTL responses using immunosuppressive cytokines, such as transforming
growth factor
P
(TGF-p), Interleukin-10, and vascular endothelial growth factor (13-15).
Additionally, there are a number of cell types that can suppress antitumor immune responses,
such as myeloid derived suppressor cells (16), type II natural killer T cells (17), and regulatory T
cells (18). The inhibition or elimination of these suppressive factors presents an opportunity to
significantly potentiate antitumor immune responses. Of the suppressive cell types, regulatory T
cells are the best characterized in the context of cancer immunotherapy and is the focus of this
work.
Regulatory T cells in cancer
A subset of thymus derived cells that exhibited immunosuppressive function was initially
discovered in 1970 (19).
However, with the lack of proper tools for cellular and molecular
analysis of these suppressor cells, it was not until 25 years later when Sakaguchi and coworkers
10
Ant ge
uptake
i
I
MHC TCR
class I
Tumor
cells
Tumo-specific
Prostaglandins, IL-10,
TGF-0, VEGF,
other factors
Antigen
uptake
Dysfunctiona
APO
87-HI and
other signals
H
class i
Figure 1.1 Immune activation and suppression in an antitumor immune response.
Tumor-specific antigens are taken up by antigen presenting cells (APC), which
activate tumor antigen specific CTL's. However, tumors secrete suppressive factors,
which activate Tregs that inhibit immune responses by tumor antigen specific CTL's.
Figure reproduced with permission from reference (18).
identified these cells as CD4+ CD25+ Tregs (20). The immunosuppressive functions of Tregs
have been shown to be important in preventing over-reaching immune responses against selfantigens and protecting against autoimmune disease.
Given that the majority of cell surface
markers of cancerous cells can be quite similar to untransformed cells with the exception of a
small subset of surface antigens, it is perhaps not surprising that Tregs can inadvertently inhibit
antitumor immune responses.
11
Regulatory T cells have been shown to inhibit the effector functions of tumor antigen
specific CTL's.
When these tumor specific CTL's are isolated and cultured ex vivo in the
absence of Tregs, their effector functions are active and the cells are capable of eradicating tumor
cells (10). Furthermore, Tregs are found in the circulation of patients of various cancers (21-26)
as well as near tumor sites (23, 25), and they also correlate with reduced survival (25), further
underscoring the importance of this subset of cells in the context of cancer therapy. Clearly,
suppression by Tregs is a significant hurdle to any antitumor immune response, whether it is
naturally occurring, or induced by cancer immunotherapy (Figure 1.1). Therefore, the inhibition
or elimination of Treg suppression could significantly enhance the efficacy of cancer
immunotherapeutic strategies. Recent adoptive T cell transfer clinical trials have shown this to
be the case: lymphodepletion of the patient by radiation prior to infusion of activated T cells can
significantly enhance the potency of adoptive T cell transfer.
This is presumably due to a
number of effects, one of which is the removal of immunosuppressive Tregs in the lymphocyte
compartment.
CD25 for Treg targeting
While the expression of the nuclear transcription factor Foxp3 appears to be the most
specific marker for Tregs (27-30), surface receptors for targeting Tregs include glucocorticoidinduced tumor necrosis factor receptor, cytotoxic T-lymphocyte antigen 4, and folate receptor 4
(31-33). We chose to target CD25, the Interleukin (IL)-2 receptor a subunit (IL-2Ra), which is
constitutively expressed at high levels on CD4*CD25* Tregs.
utilized successfully for Treg depletion.
12
The CD25 marker has been
For example, a fusion protein comprising IL-2 and a diphtheria toxin, DAB389-IL2 (also
known as denileukin difitox, or ONTAK), has been shown to have beneficial effects on cancer
immunotherapy in both mouse models and human patients. In a breast cancer model in mouse,
depletion of Tregs by DAB389-IL2 administration inhibited tumor progression in a dose-specific
manner (34). DAB389-IL2 shows early promise in clinical trials as well, both as a therapy alone
for Treg depletion (35-36), but also in conjunction with a dendritic cell cancer vaccine (37).
Anti-CD25 monoclonal antibodies can also effectively inhibit the suppressive functions of Tregs.
Inhibition of Treg in mice by anti-CD25 antibodies have been shown to be beneficial in mouse
cancer models both by inhibition alone (38) or in conjunction with a dendritic cell cancer vaccine
(39), or prior to adoptive immunotherapy (40).
However, these methods remain sub-optimal for several reasons.
First, the diphtheria
toxin portion of DAB 389 -IL-2 exhibits both toxicity and immunogenicity. In clinical trials for
DAB389-IL2 for cutaneous T cell lymphoma, all patients experienced some adverse effect, 60%
of the patients exhibited acute hypersensitivity type reactions within 24 hours of administration
and 98% of patients developed neutralizing antibodies to DAB389-IL2 by the second round of
treatment (41). Anti-CD25 antibodies can cause elimination of Tregs by complement or by
antibody dependent cell-mediated cytotoxicity, thereby disturbing T cell homeostasis (42).
While the inhibition of Treg function is desirable, their outright elimination would potentially be
more disruptive to normal immune regulation and prevention of autoimmunity. Treg functional
inhibition may be preferable to depletion as a therapeutic strategy, because Treg inhibition
maintains normal T cell numbers, whereas Treg depletion simply leads to replenishment with
new, uninhibited Tregs (43):
13
In this work, we propose a novel approach to CD25-mediated Treg inhibition, with the
use of engineered IL-2 analogues that antagonize the IL-2 receptor. Instead of using CD25
merely as a surface marker to identify and target Tregs, the goal is to inhibit the downstream
effects of IL-2 receptor signaling. IL-2 is essential to Treg biology, and is required for the
expansion, maintenance and the suppressive function of Tregs in mice (44-47). In humans,
CD25 has been shown not to be essential for Treg maintenance, even though Treg function can
be modulated by CD25 blockade (48). While Treg function was not explored in the context of
cancer therapy in this study, this nevertheless provides supporting evidence that it may be
possible to inhibit Tregs without eliminating them through CD25.
14
Part 2: A novel protein based method for targeted delivery of short interfering RNA
RNA interference as a powerful therapeutic modality
The use of double stranded RNA for RNA interference (RNAi) was first discovered in
1998 by Fire and Mello in Caenorhabditis elegans (49).
In 2001, Tuschl and coworkers
demonstrated that sequence-specific gene silencing can be induced in mammalian cells by the
exogenous introduction of short interfering RNA (siRNA), double stranded RNA 21-23
nucleotides in length (50). Since then, RNAi has been demonstrated in a wide range of species,
including humans, mice, yeast, worms and insects (51-53). The prospect of targeting any gene
for silencing simply by adjusting the sequence of the siRNA molecule makes RNAi an extremely
powerful therapeutic modality that can address virtually any target.
RNAi occurs endogenously as a method for translational regulation through the
transcription of non-coding regions of the genome to generate long pieces of hairpin RNA
(Figure 1.2). This hairpin RNA is processed by the enzymes Drosha and Dicer to generate 21-23
nucleotide micro RNA (miRNA) which generally have imperfect sequence complementarity.
miRNA molecules are then loaded into Argonaute 2 (Ago2) and the RNA-induced silencing
complex (RISC), where the passenger or sense strand is unwound, leaving the guide or antisense
strand bound to the RISC/Ago2 complex. The sense strand recognizes the target messenger
RNA (mRNA) and the imperfect complementarity results in translational repression (54).
RNAi can alternatively be mediated by the introduction of synthetic siRNA, which is
differentiated from miRNA in that siRNA are generally exogenously introduced, and have
perfect sequence complementarity to the gene target (Figure 1.2). siRNA molecules are loaded
15
Pre-tA
dsRNA-~~
Cytoplasm
=MnOO Pre m RNA
Dtcer processing
and RiSC loading
AiNA
RISC passenger-strand
cleavage (siRNA) or
-c
E
unwinding (miRNA)
RISC actvaton
I
AA
1
RNA
Nature Reviews I Drug Discover y
Figure 1.2 RNAi pathways in mammalian cells. In mammalian cells, RNAi can
occur through either the endogenous micro RNA pathway (right) or the siRNA
pathway from exogenously delivered siRNA (left).
Figure reproduced with
permission from reference (54).
into the RISC/Ago2 complex, and the sense strand is cleaved.
Based on the perfect
complementarity, the target mRNA transcript is degraded, resulting in gene silencing (54).
The ability to target virtually any gene target for gene silencing, simply by altering the
siRNA nucleotide sequence makes RNAi an extremely powerful and attractive tool for
therapeutic intervention. In 2003, Song et al first utilized siRNA as a novel therapeutic modality
in an animal model for disease (53). Since then, siRNA has been shown to have applications in a
number of diseases, including (53, 55-59). In cancer, a large number of molecular markers and
16
cellular processes have been identified as activated in cancerous cells but are otherwise
"undruggable" by small molecule drugs or biologics. The ability to eliminate these markers or
key proteins in these pathways makes cancer a particularly interesting disease target for siRNAbased therapeutics (60, 61).
Nanoparticle-based methods for targeted delivery of siRNA
While the delivery of "naked" siRNA is technically possible, only a limited number of
organs such as the liver and brain, are capable of taking up siRNA in this type of formulation and
only under extreme conditions (53, 62). Generally, siRNA molecules are too large (-14kDa) and
too negatively charged to readily pass through the cell plasma membrane of the cell. In the cases
mentioned above, successful delivery of naked siRNA was achieved through non-specific fluid
phase uptake, induced through either significant hydrostatic perturbation (63) or injection of very
high concentrations of siRNA (10pM) (62).
Because of the limited bioavailability of naked siRNA, a delivery vehicle is generally
required for efficient delivery to the cell cytoplasm, where it is therapeutically active. It is also
important that the delivery vehicle is able to specifically target the cell of interest, in order to
minimize off-target effects. Viral vectors are effective delivery vehicles that were used in initial
RNAi studies, but they suffer from problems of immunogenicity and toxicity (64). This has led
to great interest and effort in developing nonviral methods for targeted siRNA delivery.
The majority of nonviral delivery systems are nanoparticulate in nature. They include
cationic polymers, cationic lipids, liposomes and dendrimers, and are typically 10-300nm in
diameter. Although nanoparticle-based delivery vehicles can carry large siRNA payloads, they
suffer from several disadvantages. First, nanoparticles are taken up rapidly by phagocytes which
17
leads to accumulation in the liver and spleen through the reticuloendothelial system (65),
resulting in poor pharmacokinetics and biodistribution.
The large size of nanoparticles also
limits their ability to extravasate from the tumor vasculature and penetrate efficiently into solid
tumors (66).
Some nanoparticle formulations are aggregate prone (67), and nanoparticles are
generally complex to synthesize and purify in a reproducible and monodisperse manner (68).
Although there have been only preliminary studies on the consequences of polydisperse polymer
preparations, polydispersity has been linked to some cases of tissue toxicity (68). Dendrimers
are capable of being synthesized in a monodisperse fashion (69), but they still suffer from
increased uptake by the liver and spleen (70).
Protein-based methods for targeted siRNA delivery
The use of proteins for targeted siRNA delivery may solve some of the pharmacokinetic,
biodistribution and polydispersity issues that nanoparticle-based vehicles face.
The use of
recombinant DNA technology and standard protein purification techniques allows proteins to be
prepared in a reproducible and monodisperse manner. Also, proteins can be engineered to fall
within the window of 60 kDa and 500 kDa, which would be large enough to avoid rapid renal
clearance, yet small enough for efficient extravasation, tumor penetration, and avoiding
phagocytic clearance (66). In addition to molecular weight, the antigen binding affinity can be
optimized for tumor uptake as well (66).
While limited in number, there do exist protein-based delivery vehicles for siRNA that
combine a targeting agent (e.g. an antibody fragment or DARPin), and an siRNA complexation
agent in the form of a polycationic peptide, such as polyarginine or protamine. It is important to
note that polycationic peptides such as polyarginine, MPG or Pep1 have been used for siRNA
18
delivery in peptide formulations that do not contain macromolecular targeting agents as
described above.
These formulations are prepared with peptide:siRNA ratios of 10-50:1 and
form aggregates 100-300 nm in size (71, 72), which gives them very similar biophysical
properties to the nanoparticle formulations described above, along with the associated limitations
in pharmacokinetics, biodistribution, reproducibility and monodisperesity. On the other hand,
when conjugated to macromolecular targeting agents, polycationic peptides like polyarginine and
protamine instead behave like monomeric, non-aggregated siRNA carriers, with typical
protein:siRNA ratios of 1:1-5.
Nevertheless, the use of polycationic peptides as siRNA carriers has not lived up to the
potential that protein-based delivery vehicles can offer. For example, the conjugation of the tat
peptide to an antibody (73) or to streptavidin (74) significantly increased the plasma clearance
rate when compared to the unconjugated protein. This was shown to be caused by the highly
charged nature of the lysine- and arginine-rich tat peptide, which led to significant liver uptake
for the antibody-tat fusion and global organ uptake for the streptavidin conjugate. Next, Kumar
et al showed successful siRNA delivery and gene silencing using a fusion protein containing an
anti-CD7 single chain variable fragment (scFv) and polyarginine. However, this method was
inefficient and required very large amounts of protein and siRNA, typically 1gM or more (75).
The fusion protein was also made through chemical conjugation by a disulfide linkage, which
adds an extra layer in the synthesis and purification. It also makes the fusion protein labile due
to the high reducing power on the surfaces of a wide range of cell types (76, 77). This limits the
efficiency of the method, as well as its adaptability to a wide range of cell targets.
Another protein-based delivery technique employes the use of fusion proteins of a
targeting moiety (eg. antibody fragments or DARPin) and protamine, another arginine-rich
19
peptide (78-80).
Protamine binds to nucleic acids through electrostatic interactions with the
phosphate backbone. As a result, they are not specific to siRNA and can bind to other sources of
nucleic acid present during the preparation, such as genomic DNA, mRNA, or plasmid DNA. In
our experience, it is not trivial to remove these contaminating nucleic acids. In purification
protocols reported in the literature, the protein is generally denatured and refolded, which is
required to remove nucleic acid contamination (80). Protein refolding is a poorly understood
process that introduces variability into the preparation step and is generally undesirable. In some
cases, proteins cannot always be refolded properly from a denatured state.
Double stranded RNA binding domains as an alternative siRNA carrier
Double stranded RNA binding domains (dsRBD) may provide an attractive alternative to
the polycationic peptides used as an siRNA carrier. dsRBD's are a class of protein domains
found in a number of RNA-binding proteins across different species (81). Although the common
function of all dsRBD's are to bind to double stranded RNA, the proteins that contain the dsRBD
can have a number of different functions, from RNA editing, viral RNA detection, RNA editing,
and even RNAi. dsRBD's contain 1-3 double stranded RNA binding motifs (dsRBM), which
exhibit a high degree of structural homology across different proteins and different species.
dsRBD's are particularly attractive as siRNA carriers for targeted siRNA delivery for
several reasons.
First, dsRBM's complex with the backbone of double-stranded RNA in a
sequence-independent fashion (82) (Figure 1.3). Each dsRBM has been measured to interact
with 11-12 base pairs of double-stranded RNA (83). This makes the dsRBD particularly suited
as an siRNA carrier because an siRNA designed to any gene target can be loaded onto dsRBD
20
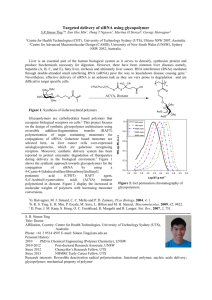
IV
Figure 1.3 Crystal structure of a dsRBD bound to double stranded RNA. The dsRBD
of human protein kinase R (purple ribbon model) contains two dsRBM's that bind to
opposite sides of the double stranded RNA (spherical model). Figure reproduced
with permission from reference (82).
for delivery. The dsRBD/RNA interaction is non-covalent and reversible, which is important for
the siRNA molecule to be loaded into the RISC/Ago2 complex in the cytoplasm. dsRBM's also
exhibit a low charge density, with most dsRBM's containing an average net charge per amino
acid residue between -0.05 and +0.1 per residue, compared to +1.0 for polyarginine, +0.67 for
tat, and +0.54 for protamine. Since many of the problems associated with polycationic peptides
can be traced to its highly charged nature, the relatively low charge density is likely to prevent or
lessen any issues with poor pharmacokinetics, biodistribution, and complexity of synthesis and
purification.
21
Endosomal Escape as a barrier to effective siRNA delivery
The use of ligands or antigen binding moieties such as antibody fragments for targeted
delivery can enhance the internalization of nanoparticles or siRNA cargo that is loaded onto an
siRNA carriers.
However, internalization through a receptor or cell-surface antigen target
generally results in endocytosis and entry into endosomal compartments. If the siRNA cargo
cannot escape from the endosome into the cytoplasm where it is loaded into the RISC/Ago2
complex for RNAi, it will undergo degradation in lysosomes.
Endosomal escape has been shown to be a limiting factor for effective targeted siRNA
delivery and RNAi, and a number of approaches to enhance endosomal escape have been
attempted. For example, the addition of positive charge on polycationic vehicles takes advantage
of the proton sponge effect (84). Here, the protonable moieties of the cationic vehicles buffer
against the acidification of endosomal compartments.
This results in further ATP-mediated
influx of protons as well as counter ions, leading to osmotic swelling and eventually endosomal
disruption.
Endosomal escape can also be enhanced through the inclusion of membrane-interacting
agents.
These are generally delivered in cis with the therapeutic cargo, but they could also
potentially be delivered in trans as a separate, second agent (85). For example, when mixed in
liposomal preparations, fusogenic lipids such as dioleoylphosphatidyl-ethanolamine (86) can
enhance the ability of liposomes to associate and fuse with endosomal membranes, thus releasing
the encapsulated cargo into the cytoplasm. Short fusogenic peptides, both synthetic (eg. GALA)
and virus-derived (eg. HA2, INF7, TAT), have also been used to enhance endosomal escape (87,
88). pH sensitive peptides such as GALA, HA2, and INF7 have an additional advantage of
adopting an active conformation for membrane penetration only at endosomal pH (88).
22
Proteins can also mediate endosomal escape. Type II exotoxins such as diphtheria toxin
from Corynebacterium diphthteria and exotoxin A from Pseudomonas aeruginosa contain
translocation domains for endosomal escape into the cytoplasm, where the catalytic domain is
active. Fragments of these proteins containing the translocation domain have been employed to
improve the cytosolic delivery of macromolecular cargo such as plasmid DNA and therapeutic
proteins (89-91).
For siRNA that is delivered by a non-polycationic carrier, the proton sponge effect is less
useful.
Proteins, peptides and lipids that interact with endosomal membranes can mediate
cytosolic delivery through one of two mechanisms: 1) direct fusion with endosomal membranes,
or 2) the formation of pores, through which the therapeutic payload can diffuse to reach the
cytoplasm. For some endosomal escape agents, there is evidence that both mechanisms may
play a role (92-94). Direct fusion is particularly suited for cargo that is encapsulated, such as in a
nanoparticle formulation, because the cargo can be emptied into the cytoplasm after endosomal
membrane fusion. If direct fusion leads to translocation across the endosomal membrane, it is
unclear whether siRNA will also be translocated if it is non-covalently bound to an siRNA
carrier on the same molecule as the endosomal escape agent. Also, a direct fusion translocation
mechanism would not be useful if the siRNA has already dissociated from the siRNA carrier, or
if it is delivered as a separate, second agent in trans. Therefore, the most reliable method of
endosomal escape is likely through the formation of pores.
However, the pores formed in
endosomal membranes must be large enough for efficient diffusion of siRNA, which is 7-8 nm in
length and 2.6 nm in diameter (90, 91).
This is not the case for some of the pore-forming
endosomal escape agents described above, such as GALA and exotoxin A (93, 97).
23
Cholesterol dependent cytolysins (CDC) are a class of pore-forming proteins found
across different species with a high degree of sequence and structural homology. The crystal
structures of several CDC's have been solved (Figure 1.4). CDC's contain four domains, with a
highly conserved undecapeptide in domain 4 which mediates binding to cholesterol-containing
membranes (98). The CDC's then oligomerize to form a pre-pore complex, which then forms a
pore in the membrane 5-50 nm in diameter (99).
CDC's evolved in bacteria to mediate the
escape of bacteria cells from phagosomes (100), and as a result, are generally retain activity at
acidic pH. This property, along with the large pore size formed by CDC's, make this class of
protein an attractive candidate for the use as an endosomal escape agent for macromolecular
delivery.
ILY
PFO
D1
D2
F318
D3
wllsA
D4
wioA
Undcapepd
Loop1-3
Figure 1.4 Crystal structure of two cholesterol dependent cytolysins. The crystal
structures of two CDC's, perfringolysin 0 and intermedilysin Y, from the
bacterial species Clostridium perfringens and Streptococcus intermedius,
respectively are shown here. CDC's contain a high degree of structural homology,
with four distinct domains, and a highly conserved undecapeptide in domain 4
believed to be responsible for cholesterol binding. Figure reproduced with
permission from reference (98).
24
Thesis Overview
The development of IL-2 antagonists for the inhibition of regulatory T cells is covered in
Chapters 2 and 3. In Chapter 2, the development and characterization of human IL-2 are
described. Here, data on a human T cell line and human primary Tregs ex vivo are presented. In
Chapter 3 the development and characterization of mouse IL-2 antagonists are described. Here,
mouse IL-2 antagonists in both monovalent form as well as in Fc fusions are characterized in
vitro and in vivo.
The development of a multi-agent protein-based vehicle for the targeted delivery of
siRNA is described in Chapters 4 and 5. In Chapter 4, the use of dsRBD fusion proteins for
targeted siRNA delivery and CDC fusions for targeted endosomal escape is described.
In
Chapter 5, the induction of EGFR clustering is shown to expand the therapeutic window of
siRNA delivery using dsRBD and CDC fusion proteins targeted to EGFR. This is shown as a
proof of principle with a third agent that clusters EGFR by binding simultaneously to multiple
epitopes on EGFR.
25
References
1. Ehrlich P. Ueber den jetzigen Stand der Karzinomforschung. Ned Tijdschr. Geneeskd.
5:273 (1909).
2. Disis ML. Immune regulation of cancer. J Clin Oncol. 28:4531-8 (2010).
3. Disis ML, Grabstein KH, Sleath PR, et al. Generation of immunity to the HER-2/neu
oncogenic protein with breast and ovarian cancer using a peptide-based vaccine. Clin
Cancer Res. 5:1289-97 (1999).
4. Cheever MA and Higano CS. PROVENGE (sipuleucel-T) in prostate cancer: the first
FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 17:3520-3526 (2011).
5. Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: a clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299-308 (2008).
6. Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after
transfer of genetically engineered lymphocytes. Science. 314:126-129 (2006).
7. Hooijberg E, Ruizendaal JJ, Snijders PJ, et al. Immortalization of human CD8+ T cell
clones by ectopic expression of telomerase reverse transcriptase. J Immunol. 165:42394245 (2000).
8. Charo J, Finkelstein SE, Grewal N, et al. Bcl-2 overexpression enhances tumor-specific
T-cell survival. Cancer Res. 65:2001-2008 (2005).
9. Rosenberg SA and Dudley ME. Adoptive cell therapy for the treatment of patients with
metastatic melanoma. Curr Opin Immunol. 21:233-240 (2009).
10. Dhodapkar, MV, Krasovsky J and Olson K. T cells from the tumor microenvironment of
patients with progressive myeloma can generate strong tumor specific cytolytic responses
to autologous tumor loaded dendritic cells. Proc Natl Acad Sci USA. 99:13009-13013
(2002).
11. Mempel TR, Pittet MJ, Khazaie K, et al. Regulatory T cells reversibly suppress cytotoxic
T cell function independent of effector differentiation. Immunity. 25:129-141 (2006).
12. Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T
cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 102:419424 (2005).
13. Salazar-Onfray F. Interleukin-10: a cytokine used by tumors to escape
immunosurveillance. Med Oncol. 16:86-94 (1999).
14. Li MO, Wan YY, Sanjabi S, et al. Transforming growth factor-beta regulation of immune
responses. Annu Rev Immunol. 24:99-146 (2006).
15. Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth
factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med.
2:11096-1103 (1996).
16. Bronte V, Serafini P, Apolloni E, et al. Tumor-induced immune dysfunctions caused by
myeloid suppressor cells. J Immunother. 24:431-446 (2001).
17. Terabe M and Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new
immunoregulatory axis. Trends Immunol. 28: 491-496 (2007).
18. Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 117:1167-1174
(2007).
19. Gershon RK and Kondo K. Cell interactions in the induction of tolerance: the role of
thymic lymphocytes. Immunology. 18:723-737 (1970).
26
20. Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by
activated T cells expressing IL-2 receptor alpha chain (CD25). Breakdown of a single
mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 155:11511164 (1995).
21. Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are
abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 103:1755-1762
(2004).
22. Woo EY, Chu S, Goletz TJ, et al. Regulatory CD4+CD25+ T cells in tumors from
patients with early stage non-small lun cancer and late-stage ovarian cancer. Cancer Res.
61:4766-4772 (2001).
23. Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in
peripheral blood and tumor microenvironment of patients with pancreas or breast
adenocarcinoma. J Immunol. 169:2756-2761 (2002).
24. Javia LR and Rosenberg SA. CD4+CD25* suppressor lymphocytes in the circulation of
patients immunized against melanoma antigens. J Immunother. 26: 85-93 (2003).
25. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian
carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 10: 942-949
(2004).
26. Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4* regulatory T cells and
their ligands: implications for immunotherapy. Immunity. 20(1): 107-118 (2004).
27. Fontenot JD and Rudenski AY. A well adapted regulatory contrivance: regulatory T cell
development and the forkhead family transcription factor Foxp3. Nat Immunol. 6:331-7
(2005).
28. Fontenot JD, Gavin MA and Rudensky AY. Foxp3 programs the development and
function of CD4+CD25+ regulatory T cells. Nat Immunol. 4:330-6 (2003).
29. Hori S, Nomura T and Sakaguchi S. Control of regulatory T cell development by the
transcription factor Foxp3. Science. 299:1057-61 (2003).
30. Khattri R, Cox T, Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ T
regulatory cells. Nat Immunol. 4:337-42 (2003).
31. McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4+CD25+ immunoregulatory T cells
gene expression analysis reveals a functional role for the glucocorticoid-induced TNF
receptor. Immunity. 16:311-323 (2002).
32. Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by
CD25+ CD4+ regulatroy T cells constitutively expressing cytotoxic T lymphocyteassociated antigen 4. J Exp Med. 192:303-9 (2000).
33. Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigenspecific regulatory T cells expressing the folate receptor. Immunity.;27:145-59 (2007).
34. Knutson KL, Dang Y, Lu H, et al. IL-2 immunotoxin therapy modulates tumorassociated regulatory T cells and leads to lasting immune-mediated rejection of breast
cancers in neu-transgenic mice. J Immunol. 177:84-91 (2006).
35. Salazar L, Higgins D, Childs J. Phase I-II study of denileukin difitox (ONTAK) in
patients with advanced refractory breast cancer. Cancer Res. 69(Supplement 3): 4130
(2009).
36. Barnett B, Kryczek I, Cheng P, et al. Regulatory T cells in ovarian cancer: biology and
therapeutic potential. Am J Reprod Immunol. 54: 369-377 (2005).
27
37. Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor imunity
in cancer patients after depletion of regulatory T cells. J Clin Invest. 115:3623-3633
(2005).
38. Fecci PE, Sweeney AE, Grossi GM, et al. Systemic anti-CD25 monoclonal antibody
administration safely enhances immunity in murine glioma without eliminating
regulatory T cells. Clin Canc Res. 12:4294-4305 (2006).
39. Van Meirvenne S, Dullaers M, Heirman C, et al. Thielemans K. In vivo depletion of
CD4*CD25* regulatory T cells enhances the antigen-specific primary and memory CTL
response elicited by mature mRNA-electroporated dendritic cells. Mol Ther. 12:922-932
(2005).
40. Prasad SJ, Farrand KJ, Matthews SA, et al. Dendritic cells loaded with stressed tumor
cells elicit long-lasting protective tumor immunity in mice depleted of CD4* CD25*
regulatory T cells. J Immunol. 174:90-98 (2005).
41. Olsen E, Duvic M, Frankel A, et al. Pivotal Phase III Trial of Two Dose Levels of
Denileukin Diftitox for the Treatment of Cutaneous T-Cell Lymphoma. J Clin Oncol.
19:376-88 (2001).
42. Stephens LA, Gray D and Anderton ST. CD4*CD25* regulatory T cells limit the risk of
autoimmune disease arising from T cell receptor crossreactivity. Procl Natl Acad USA.
102:17418-23 (2005).
43. Colombo MP and Piconese S. Regulatory T-cell inhibition versus depletion: the right
choice in cancer immunotherapy. Nat Rev Canc. 7:880-7 (2007).
44. Setoguchi R, Hori S, Takahashi T, et al. Homeostatic maintenance of natural
Foxp3*CD25*CD4* regulatory T cells by interleukin (IL)-2 and induction of autoimmune
disease by IL-2 neutralization. J Exp Med. 201:723-35 (2005).
45. Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin 2 in Foxp3expressing regulatory T cells. Nat Immunol. 6:1142-51 (2005).
46. Thornton AM, Donovan EE, Piccirillo CA, et al. Cutting Edge: IL-2 is critically required
for the in vitro activation of CD4*CD25* T cell suppressor function. J Immunol.
172:6519-23 (2004).
47. Yu A and Malek TR. Selective availability of IL-2 is a major determinant controlling the
production of CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 177:5115-21 (2006).
48. de Goer de Herve MG, Gonzales E, Hendel-Chavez H, et al. CD25 appears non essential
for human peripheral Treg maintenance in vivo. PLoS ONE. 5:e 11784 (2010).
49. Fire A, Xu S, Montgomery M, et al. Potent and specific genetic interference by double
stranded RNA in Caenorhabditis elegans. Nature. 391:806-811 (1998).
50. Elbashir SM, Harborth J, Lendeckel W, et al. Duplexes of 21-nucleotide RNAs mediate
RNA interference in cultured mammalian cells. Nature. 411:494-498 (2001).
51. Drinnenberg IA, Weinberg DE, Xie KT, et al. RNAi in budding yeast. Science.
326:544-550 (2009).
52. Huvenne H and Smagghe G. Mechanisms of dsRNA uptake in insects and potential of
RNAi for pest control: a review. J Insect Physiol. 56:227-235 (2010).
53. Song E, Lee SK, Wang J, et al. RNA interference targeting Fas protects mice from
fulminant hepatitis. Nat Med. 9:347-351 (2003).
54. de Fougerolles A, Vornlocher HP, Maraganore J, et al. Interfering with disease: a
progress report on siRNA-based therapeutics. Nat Rev Drug Disc. 6:443-453 (2007).
28
55. Morrissey DV, Lockridge JA, Shaw L, et al. Potent and persistent in vivo anti-HBV
activity of chemically modified siRNAs. Nat Biotechnol. 23: 1002-1007 (2005).
56. Okumura A, Pitha PM and Harty RN. ISG15 inhibits Ebola VP40 VLP budding in an Ldomain-dependent manner by blocking Nedd4 ligase activity. Proc Nati Acad Sci
USA. 105: 3974-3979 (2008).
57. Ptasznik A, Nakata Y, Kalota A, et al. Short interfering RNA (siRNA) targeting the Lyn
kinase induces apoptosis in primary, and drug-resistant, BCR-ABL1 leukemia cells. Nat
Med. 10: 1187-1189 (2004).
58. Kim SH, Jeong JH, Lee SH, et al. Local and systemic delivery of VEGF siRNA using
polyelectrolyte complex micelles for effective treatment of cancer. J Control Release.
129: 107-116 (2008).
59. Xia CF, Zhang Y, Zhang Y, et al. Intravenous siRNA of brain cancer with receptor
targeting and avidin-biotin technology. Pharm Res. 24: 2309-2316 (2007).
60. Bild AH, Yao G, Chang JT et al. Oncogenic pathway signatures in human cancers as a
guide to targeted therapies. Nature. 439: 353-357 (2006).
61. Abdelrahim M, Safe S, Baker C et al. RNAi and cancer:implications and applications. J
RNAi Gene Sil. 2:136-145 (2006).
62. DeFiglia , Sena-Esteves M, Chase K, et al. Therapeutic silencing of mutant huntington
with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc
Natl Acad Sci USA. 104:17204-17209 (2007).
63. Crespo A, Peydro A, Dasi F, et al. Hydrodynamic liver gene transfer mechanism involves
transient sinusoidal blood sasis and massive hepatocyte endocytic vesicles. Gene Ther.
12:927-935 (2005).
64. Barquinero J, Eixarch H and Perez-Melgosa M. Retroviral vectors: new applications for
an old tool. Gene Ther. 11:S3-S9 (2004).
65. Alexis F, Pridgen E, Molnar LK, et al. Factors affecting the clearance and biodistribution
of polymeric nanoparticles. Mol Pharm. 5:505-515 (2008).
66. Schmidt MM and Wittrup KD. A modeling analysis of the effects of molecular size and
binding affinity on tumor targeting. Mol Cancer Ther. 8:2861-2871 (2009).
67. Tokatlian T, and Segura T. siRNA applications in nanomedicine.
Nanomed
Nanobiotechnol. 2:305-315 (2010).
68. Moghimi SM, Hunter AC, Murray JC.
Long-circulating and target-specific
nanoparticles: theory to practice. Pharmacol Rev. 53:283-318 (2001).
69. Kukowska-Latallo JF, Bielinska AU, Johnson J, et al. Efficient transfer of genetic
material into mammalian cells using starburst polyamidoamine dendrimers. Proc Natl
Acad Sci USA. 93:4897-4902 (1996).
70. Malik N, Wiwattanapatapee R, Klopsch R, et al. Dendrimers relationship between
structure and biocompatibility in vitro, and preliminary studies on the biodistribution of
1251-labelled polyamidoamine dendrimers in vivo. J Control Release. 65:133-148
(2000).
71. Simeoni F, Morris MC, Hetiz F, et al. Insight into the mechanism of the peptide-based
gene delivery system MGP: implications for delivery of siRNA into mammalian cells.
Nucl Acids Res. 31:2717-2724 (2003).
72. Deshayes S, Morris M, Hetiz F, et al. Delivery of proteins and nucleic acids using a noncovalent peptide-based strategy. Adv Drug Deliv Rev. 60:537-547 (2008).
29
73. Niesner U, Halin C, Lozzi L, et al. Quantitation of the tumor-targeting properties of
antibody fragments conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug
Chem. 13: 729-36 (2002).
74. Lee HJ and Pardridge WM. Pharmacokinetics and delivery of tat and tat-protein
conjugates to tissues in vivo. Bioconjug Chem12: 995-9 (2001).
75. Kumar P, Ban HS, Kim SS, et al. T cell-specific siRNA delivery suppresses HIV-1
infection in humanized mice. Cell. 134:577-586 (2008).
76. Sahaf B, Heydari K, Herzenberg LA, et al. Lymphocyte surface thiol levels. Proc Natl
Acad Sci USA. 100:4001-4005 (2003).
77. Jiang XM, Fitzgerald M, Grant CM, et al. Redox control of exofacial protein
thiols/disulfides by protein disulfide isomerase. J Biol Chem. 275:2416-2423 (1999).
78. Song E, Zhu P, Lee SK, et al. Antibody mediated in vivo delivery of small interfering
RNAs via cell-surface receptors. Nat Biotechnol. 23:709-717 (2005).
79. Peer D, Zhu P, Carman CV, et al. Selective gene silencing in activated leukocytesby
targeting siRNAs to the integrin lymphocyte function-associated antigen-1. PNAS.
104:4095-4100 (2007).
80. Winkler J, Martin-Killias P, Pluckthun A, et al. EpCAM-targeted delivery of
nanocomplexed siRNA to tumor cells with designed ankyrin repeat proteins. Mol Canc
Ther. 8:2674-83 (2009).
81. Chang KY and Ramos A. The double-stranded RNA-binding motif, a versatile
macromolecular docking platform. FEBS J. 279: 2109-2117 (2005).
82. Nanduri S, Carpick BW, Yang Y, et al. Structure of the double-stranded RNA-binding
domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated
activation. EMBO J. 117:5458-5465 (1998).
83. Bevilacqua PC and Cech TR. Minor-groove recognition of double-stranded RNA by the
double stranded RNA-binding domain from the RNA-activated protein kinase PKR.
Biochemistry. 35:9983-9994 (1996).
84. Dominska M and Dykxhoom DM. Breaking down the barriers: siRNA delivery and
endosomal escape. J Cell Sci. 123:1183-1189 (2010).
85. Pirie CM, Liu DV and Wittrup KD. Independently targeted cytolysin and gelonin
immunotoxins combine to exhibit mechanistic synergy in vitro and in vivo, submitted.
86. Farhood H, Serbina N and Huang L. The role of dioleoyl phosphatidylethanolamine in
cationic liposome mediated gene transfer. Biochim Biophys Acta. 1235:289-295 (1995).
87. Hatakeyama H, Ito E, Akita H, et al. A pH-sensitive fusogenic peptide facilitates
endosomal escape and greatly enhances the gene silencing of siRNA-containing
nanoparticles in vitro and in vivo. J Control Release 139:127-132 (2009).
88. Plank C, Oberhauser B, Mechtler K, et al. The influence of endosome-disruptive peptides
on gene transfer using synthetic virus-like gene transfer systems. J Biol Chem. 269:
12918-12924 (1994).
89. Kakimoto S, Hamada T, Komatsu Y, et al. The conjugation of diphtheria toxin T domain
to poly(ethylenimine) based vectors for enhanced endosomal escape during gene
transfection. Biomaterials. 30: 402-408 (2009).
90. Prior TI, Fitz Gerald DJ and Pastan I. Translocation mediated by domain II of
Pseudomonas exotoxin A: transport of barnase into the cytosol. Biochemisry. 31: 35553559 (1992).
30
91. Bruell D, Stocker M, Huhn M, et al. The recombinant anti-EGF receptor immunotoxin
425(scFv)-ETA' suppresses growth of a highly metastatic pancreatic carcinoma cell line.
Int J Oncol. 23: 1179-1186 (2003).
92. Varkouhi AK, Scholte M, Storm G, et al. Endosomal escape pathways for delivery of
biological. J Control Release. 151:220-228 (2011).
93. Kuehne J and Murphy RM. Synthesis and characterization of membrane-active GALAOKT9 conjugates. Bioconjug Chem. 12:742-749 (2001).
94. von Rossenberg SMW, Sliedregt-Bol KM, Meeuwenoord NJ. Targeted lysosome
disruptive elements for improvement of parenchymal liver cell-specific gene delivery. J
Biol Chem. 277:45803-45810 (2002).
95. van den Hout M, Vilfan ID, Hage S, et al. Direct force measurements on double-stranded
RNA in solid-state nanopores. Nano Lett. 10:701-707 (2010).
96. Sinden RR. DNA structure and function. Academic Press (1994).
97. Zalman LS and Wisnieski BJ. Characterization of the insertion of Pseudomonas exotoxin
A into membranes. Infect Immun. 50:630-635 (1985).
98. Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins.
Infect Immun. 73:6199-6209 (2005).
99. Portnoy DA, Tweten RK, Kehoe M, et al. Capacity of listeriolysin 0, streptolysin 0, and
perfringolysin 0 to mediate growth of Bacillus subtilis within mammalian cells. Infect
Immun. 60:2710-2717 (1992).
100.Rosado CJ, Kondos S, Bull TE, et al. The MACPF/CDC family of pore-forming toxins.
Cell Microbiol. 10:1765-1774 (2008).
31
Chapter 2: Engineered human Interleukin-2 antagonists for
the inhibition of regulatory T cells
Abstract
The immunosuppressive effects of CD4* CD25 high regulatory T cells interfere with antitumor immune responses in cancer patients. Here, we present a novel class of engineered human
Interleukin (IL)-2 analogues that antagonize the IL-2 receptor, for inhibiting regulatory T cell
suppression. These antagonists have been engineered for high affinity to the a subunit of the IL2 receptor and very low affinity to either the
P or y
subunit, resulting in a signaling-deficient IL-2
analogue that sequesters the IL-2 receptor a subunit from wild type IL-2. Two variants, "V91R"
and "Q126T" with residue substitutions that disrupt the
P and
y subunit binding interfaces,
respectively, have been characterized in both a T cell line and in human primary regulatory T
cells. These mutants retain their high affinity binding to IL-2 receptor a subunit, but do not
activate STAT5 phosphorylation or stimulate T cell growth. The two mutants competitively
antagonize wild-type IL-2 signaling through the IL-2 receptor with similar efficacy, with
inhibition constants of 183 pM for V91R and 216 pM for Q126T. Here, we present a novel
approach to CD25-mediated Treg inhibition, with the use of an engineered human IL-2 analogue
that antagonizes the IL-2 receptor.
Major portions of this chapter were previously published in:
Liu DV, Maier LM, Hafler DA and Wittrup KD. Engineered interleukin-2 antagonists for the
inhibition of regulatory T cells. J Immunother. 32: 887-94 (2009).
32
Introduction
CD4*CD25 high regulatory T cells (Tregs) suppress immune responses (1, 2) and have
been found in the circulation of patients with various cancers (3-6) as well as near tumor
sites (5-6), correlating with reduced survival in some cancers (6-8). Tregs dampen anti-tumor
immune responses by inhibiting the effector functions of tumor-specific CD4* and CD8* T
cells (6, 9-11). As a result, the inhibition of Treg suppression of an anti-tumor immune response
is one approach to cancer therapy currently being studied.
While the expression of the nuclear transcription factor Foxp3 appears to be the most
specific marker for Tregs (12-15), surface receptors for targeting Tregs include glucocorticoidinduced tumor necrosis factor receptor, cytotoxic T-lymphocyte antigen 4, and folate
receptor 4 (16-18). We chose to target CD25, the Interleukin (IL)-2 receptor a subunit (IL-2Ra),
which is constitutively expressed at high levels on CD4*CD25high Tregs. The CD25 marker has
been utilized successfully for Treg depletion. For example, anti-CD25 monoclonal antibodies
and the diphtheria toxin / Interleukin -2 fusion protein, DAB 389-IL-2 (also known as denileukin
difitox, or ONTAK) have been used to deplete Tregs alone or in conjunction with a cancer
vaccine to induce effective anti-tumor immune responses in mice and humans (19-23). However,
these remain sub-optimal for several reasons.
For example, the diphtheria toxin portion of
DAB 389-IL-2 exhibits both toxicity and immunogenicity (24). Anti-CD25 antibodies can cause
elimination of Tregs by complement or by antibody dependent cell-mediated cytotoxicity,
thereby disturbing T cell homeostasis (25). While the inhibition of Treg function is desirable,
their outright elimination would potentially be more disruptive to normal immune regulation and
prevention of autoimmunity.
Treg functional inhibition may be preferable to depletion as a
33
therapeutic strategy, because Treg inhibition maintains normal T cell numbers, whereas Treg
depletion simply leads to replenishment with new, uninhibited Tregs (26).
Here, we present a novel approach to CD25-mediated Treg inhibition, with the use of an
engineered human IL-2 analogue that antagonizes the IL-2 receptor. Instead of using CD25
merely as a surface marker to identify and target Tregs, our approach is to inhibit the
downstream effects of IL-2 receptor signaling. IL-2 is essential to Treg biology; it is required for
the expansion, maintenance and the suppressive function of Tregs (27-30). An IL-2 antagonist
that is also an IL-2 analogue could potentially minimize the chances for systemic toxicity and
immunogenicity, providing inhibition of Treg function without their elimination.
To construct competitive IL-2 antagonists, we started with 2-4 IL-2, an IL-2 analogue
that was originally designed for more potent T cell signaling responses and a better therapeutic
index with selective targeting for CD25* T cells over CD25~ natural killer cells. 2-4 IL-2 was
engineered for increased binding affinity to the IL-2 receptor a subunit using directed evolution
and yeast surface display (31). For antagonists, we constructed and characterized two variants of
2-4 IL-2, one with a valine to arginine substitution at position 91, and the second with a
glutamine to threonine at position 126, both on the 2-4 IL-2 background. These mutants, termed
V91R and Q126T, are designed to disrupt binding to the IL-2 receptor P (IL-2Rs) and IL-2
receptor y (IL-2Ry) subunits, respectively.
They exhibit antagonistic properties without
stimulating IL-2 receptor signaling in both the human T cell line Kit225 and human primary
CD4*Foxp3* Tregs ex vivo.
These analogues could prove useful as pharmacological Treg
inhibitors in the context of cancer immunotherapy. In addition, to the best of our knowledge,
this is the first report of engineering a cytokine analogue with high affinity to a non-signaling
receptor subunit, and low affinity to the signaling receptor subunits to create effective cytokine
34
antagonists. Therefore, this approach of engineering signaling deficient cytokine analogues that
bind tightly and sequester the receptor from the wild type cytokine represents a novel paradigm
that can be more broadly applied to antagonist engineering, such as for other y common receptor
cytokines, or for inflammatory cytokines such as IL- 1, IL-6, or IL- 12.
35
Materials and Methods
Preparation of IL-2 mutant proteins
Single point mutations were introduced to the 2-4 IL-2 coding sequence using the
Quikchange kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. IL-2
mutants were expressed in yeast with an N-terminal FLAG tag and a C-terminal c-myc tag as
previously described (31). The supernatant was concentrated and buffer exchanged with PBS
using a lOkDa MWCO ultrafiltration unit (Millipore, Billerica, MA). The retentate was purified
using an anti-FLAG M2 agarose affinity gel (Sigma-Aldrich, St. Louis, MO), followed by size
exclusion chromatography with a Superdex 75 column (GE Healthcare, Piscataway, NJ).
Elution fractions that contained only monomeric protein were pooled and the protein
concentration was determined using the Micro BCA Protein Assay kit (Pierce, Rockford, IL).
Tissue culture
To characterize the IL-2 mutants, the human IL-2 dependent T cell line Kit225, which
constitutively expresses all three subunits of the IL-2 receptor, was used. The cells were cultured
in a humidified atmosphere in 5% CO 2 in RPMI 1640 supplemented with 10% heat-inactivated
fetal bovine serum, 40 pM IL-2, 2 mM L-glutamine, 2 mg/mL sodium bicarbonate, 50 U/mL
penicillin, 50 pg/mL streptomycin and 50 jig/mL gentamicin.
Determination of IL-2Ra binding affinities of IL-2 mutants
The equilibrium binding affinities of V91R and Q126T binding to IL-2Ra were evaluated
using a modification of a previously described protocol (31). Kit225 cells were incubated in
36
phosphate buffered saline (PBS) + 0.1% bovine serum albumin (BSA) at 37'C for 30 minutes, at
8x10 5 cells per sample with varying IL-2 mutant concentrations.
At low IL-2 mutant
concentrations, the total volume was increased to maintain an excess number of IL-2 mutant
molecules over the number of IL-2Ra. After incubation, cells were kept on ice and stained with
mouse anti-FLAG monoclonal antibody M2 (Sigma Aldrich, St. Louis, MO), followed by an
Alexa Fluor 488 conjugated goat anti-mouse antibody (Invitrogen, Carlsbad, CA), to detect cellsurface bound protein. The mean single-cell fluorescence was measured, and the dissociation
constant (KD) and the 95% confidence interval were determined as previously described (31),
using the following equation: Fb, = cLO / (KD + L0 ),where Fos is the background-corrected mean
fluorescence, Lo is the initial concentration of the protein being measured, and c is the
proportionality constant. The KD of IL-2 C125S, which is equivalent to aldesleukin (Proleukin,
Novartis, Basel, Switzerland) and is referred to as wild type IL-2, was also measured as a control.
Analysis of STAT5 phosphorylation
For all STAT5 phosphorylation assays, Kit225 cells were starved of IL-2 for 36 hours.
Kit225 cells were incubated per at 106cells/mL culture medium at 37'C with IL-2 mutants for 30
minutes (for agonism studies), or with IL-2 mutants and 3 pM wild type IL-2 for 15 minutes (for
antagonism studies).
The cells were fixed and permeabilized using the method optimized by
Krutzik et al. 32 The cells were stained with anti-pSTAT5 antibody clone 47 conjugated with
Alexa Fluor 488 (BD Biosciences, San Jose, CA) and the mean single-cell fluorescence was
measured. For antagonism studies, the half-maximal inhibitory concentration (IC 50 ) and the 95%
confidence interval were determined using the following equation Fb, = C/
-- LO /(IC50 + LO )),
where all variables are defined above. A global fit nonlinear regression was performed for each
37
protein, using a global IC 50 value and the proportionality constant from each of two separate
experiments.
For analysis of human whole blood, phosphorylation state analysis was performed using
BD Phosflow technology according to the manufacturer's instructions (BD Biosciences, San Jose,
CA), and as previously described (33). All human blood samples were obtained with informed
consent and according to the Institutional Ethics Review Board Protocols. All blood samples
were collected in sterile 10 mL lithium heparin Monoject tubes. For each condition and time
point, 4 mL fresh, ex vivo blood from healthy control donors were used. Blood samples were
incubated with IL-2 or with a cocktail of IL-2 and IL-2 antagonists in 50 mL polypropylene
Falcon conical tubes for 30 min in a 370 C water bath. Fixation of cells and preservation of
phosphorylation status was obtained by adding pre-warmed BD Lyse/Fix buffer and incubation
in a 370 C water bath. Permeabilization of cells was performed by incubation of cells in BD Perm
Buffer III on ice for 30 min. Cells were subsequently washed twice with 2% FBS/PBS and
stained using BD Staining Buffer (all reagents from BD Bioscience, San Jose, CA). Cells were
stained using APC mouse anti-human CD4 (clone RPA-T4) (BD Bioscience, San Jose, CA), PE
anti-human Foxp3 (clone 206D) (Biolegend, US) and Alexa Fluor-488 mouse anti-human
pSTAT5 (pY694; clone 47) (BD Bioscience, San Jose, CA).
Kit225 cell proliferation assays
Kit225 cells were starved of IL-2 for 36 hours. Then, 4x10 5 cells were incubated in 3 mL
culture medium at 370 C with IL-2 mutants, either in the absence (for agonism studies), or
presence (for antagonism studies) of 25 pM wild type IL-2. At each time point, the live cell
number in 100pL culture medium was determined in triplicate using the CellTiter-Glo assay
38
(Promega, Madison, WI) and a Cary Eclipse luminometer (Varian, Palo Alto, CA) according to
the manufacturer's instructions.
39
Results
Design of human IL-2 mutant antagonists
IL-2 analogue antagonists were designed using the following criteria: 1) high binding
affinity to IL-2Ra, the IL-2 specific capture subunit, and 2) low predicted binding affinity to IL2Rp or IL-2Ry, the two subunits responsible for receptor signaling. The high binding affinity to
IL-2Ra leads to preferential IL-2Ra binding of the IL-2 analogue over wild type IL-2, while the
low binding affinity to IL-2Rp or IL-2Ry would prevent the IL-2 analogue from activating the
IL-2 receptor signal itself.
We achieved the first design criterion by using a previously
engineered mutant of human IL-2, 2-4 IL-2, as a starting point for our IL-2 analogue. 2-4 IL-2 is
an IL-2 analogue previously developed in our lab using directed evolution and yeast surface
display to have high binding affinity to IL-2Ra (31). The KD of 2-4 IL-2 binding to IL-2Ra is
-200 pM whereas the KD of wild type IL-2 binding to IL-2Ra is -30 nM. 2-4 IL-2 persists on
the surface of cells expressing IL-2Ra for days, significantly longer than the cell surface
persistence of wild type IL-2 (31).
For the second design criterion, we used recently published crystal structures of wild type IL-2
bound to the three IL-2 receptor subunits (34, 35) to identify candidate residues likely to make
energetically important interactions with the IL-2R3 or IL-2Ry subunits. Assuming that these
interactions are preserved in 2-4 IL-2 binding to IL-2Rp and IL-2Ry, we disrupted binding of 2-4
IL-2 to IL-2Rp or IL-2Ry by introducing amino acid substitutions at these locations.
Five
mutants, each with a single residue substitution on the 2-4 IL-2 background were generated in
small scale pilot studies (Table 2.1). Several of these point mutations have been reported in the
literature on a wild type IL-2 background to disrupt biological activity (36) or more explicitly, to
40
disrupt IL-2 receptor subunit binding affinity (37-40). Of the five mutants generated, V91R and
Q126T, which contain single residue substitutions at the binding interfaces with IL-2Rp and IL2Ry, respectively, were secreted in yeast most efficiently and were characterized further
(Figure 2.1). On wild type IL-2, a valine at position 91 is in the center of the IL-2 / IL-2R
interface and makes van der Waals interactions with IL-2Rp (35). Therefore, a charged amino
acid substitution, such as arginine, at position 91 (V91R) was hypothesized to disrupt binding to
IL-2Rs.
As for IL-2Ry binding, previous reports have shown the importance of Q126 for
biological activity (40, 41); the crystal structures used also identified Q126 as the most important
IL-2 residue that interacts with IL-2R 7 (34, 35). Cassell and coworkers performed an extensive
study of the activity of wild type IL-2 mutants on T cells with each of the 20 amino acids in the
126 position, and showed that threonine yielded the lowest activity (36). We assumed that this
was due to abrogated IL-2Ry binding and was the basis for introducing a threonine substitution
at position 126 (Q126T) on the 2-4 IL-2 background.
IL-2 analogue binding affinity to the IL-2 receptor a subunit
The first design criterion for the IL-2 antagonists was to maintain high binding affinity to
IL-2Ra in order for IL-2Ra to preferentially bind the antagonist over wild type IL-2. Therefore,
the first step in characterizing the IL-2 analogues was to measure their IL-2Ra binding affinity
using Kit225, a human T cell line that is dependent on IL-2 for growth.
Kit225 constitutively
expresses all three subunits of the IL-2 receptor, with IL-2Ra in -10 fold excess. The surface
labeling of Kit225 is thus dominated by binding to IL-2Ra, and the IL-2Ra binding affinity can
be measured using cell surface titrations on Kit225.
41
Table 2.1 Mutations for disrupting IL-2 receptor subunit binding
Subunit Binding Disrupted
IL-2Rp3
IL-2Ry
IL-2Rp and IL-2Ry
Mutation
D88R*, V91R
Q126T, Q1261
E15W
* Wild type IL-2 has an asparagine at position 88, but 2-4
IL-2 has an asparagine to an aspartic acid substitution
C
Figure 2.1 Interleukin-2 antagonist design. The crystal structure of IL-2 (orange)
complexed with the full IL-2 receptor complex, IL-2Ra (yellow), IL-2Rp (blue), and
IL-2Ry (magenta), is shown with the valine 91 and glutamine 126 residues
highlighted (A). Close-ups are shown of the IL-2 / IL-2Rp interface with V91 (B),
and the IL-2 / IL-2Ry interface with Q126 (C). The crystal structure was calculated
by Wang et al (32).
42
The binding domains to each of the three IL-2 receptor subunits are on distinct areas of
the surface of IL-2 (34, 35). Therefore, single residue substitutions at the IL-2Rp or IL-2Ry
interfaces on the 2-4 IL-2background were estimated to have little or no effect on the binding
affinity to IL-2Ra. Indeed, the measured IL-2Ra binding affinities of V91R and Q126T are
similar to that of 2-4 IL-2, indicating that the introduction of each of the two point mutations did
not disrupt high affinity binding to IL-2Ra (Figure 2.2). On the other hand, a Q126T/V91R
double mutant that we sought to explore had significantly lower IL-2Ra binding affinity
(KD= 2 nM, data not shown), presumably due to some synergistic destabilization by the two
mutations that reduces the IL-2Ra binding affinity by disrupting the protein's conformation.
Although this was an unexpected result, this example underscores the importance of ensuring
that the protein's conformation and its IL-2Ra affinity is preserved when introducing other
residue substitutions for other antagonists designed in this manner.
1.4
-_
1.
Q126T
._A V91R
0.8 -
E 0.2
E 0
0.001
O
2-4IL-2
0
0.1
10
1000
Concentration(nM)
Figure 2.2 Kit225 cell surface titrations to measure IL-2Ra binding affinity. The
binding isotherms are shown for: Q126T (KD = 109±19 pM), V91R (KD = 119±45
pM), 2-4 IL-2 (KD = 199±56 pM), and wild type IL-2 (KD = 46±36 nM). The
fluorescence is normalized to the maximum fluorescence of 2-4 IL-2 as determined
by least squares regression, and KD values are reported with 95% confidence
intervals.
43
Disruption of binding to the IL-2 receptor P and y subunits
The second design criterion for the IL-2 antagonists was the disruption of binding affinity
to the IL-2Rp and IL-2Ry subunits, so that the IL-2 mutants themselves would not agonize the
IL-2 receptor.
The binding affinities of wild type IL-2 to IL-2Rp or to IL-2Ry alone are
relatively low, with KD values of approximately 500 nM and 700 tM, respectively (42). The
affinities of the IL-2 analogues with disrupted binding interactions to IL-2RP or IL-2Ry would
likely be too low to be measured reliably. Therefore, instead of directly measuring those binding
affinities, the inability of the IL-2 analogues to agonize the IL-2 receptor at both an early and late
signaling event was measured.
The Jak/STAT pathway is activated by the IL-2 receptor in both non-regulatory T cells
and regulatory T cell (43), and thus phosphorylated STAT5 (pSTAT5) was used as an early
marker of IL-2 receptor activation. During initial testing, STAT5 phosphorylation in Kit225 was
found to be extremely sensitive to wild type IL-2, with a measured half-maximal effective
concentration (EC5 o) of approximately 2 pM wild type IL-2
(Figure 2.3A).
However, the
pSTAT5 profiles of cells treated with 100 nM V91R or Q126T are indistinguishable from those
of untreated cells (Figure 2.3B), thus indicating that the V91R and Q126T mutations severely
inhibit the IL-2 mutants' ability to activate the IL-2 receptor.
Because the Kit225 cell line is dependent on exogenous IL-2 for growth, Kit225 cell
proliferation was used as a late signaling event in measuring IL-2 receptor activation. The half
maximal effective concentration for wild type IL-2 induced cell growth has been reported to
be -10 pM (44), consistent with our results (data not shown). Similar to the pSTAT5 analysis,
there was minimal to no Kit225 proliferation stimulated by V91R and Q126T at concentrations
44
as high as 100 nM (Figure 2.3C). At 100 nM, V91R did induce a very slight residual amount of
cell growth, but this was still significantly less than the growth induced by 25 pM IL-2,
signifying an over 4000-fold reduction in cell proliferative activity on a molar basis.
A
B
0
-.
1.2
1
0.6
U
-
0
0.8
o
0.4
an 0.2
0.
0. 1
CL
1
10
100
1000
[wild type IL-2] (pM)
10O
101
102
10a
104
Phosphorylated STAT5
C
1.2
1
0.8
E 0.6
0.4
0
N
0.2
0
0
1
2
3
4
Time (Days)
Figure 2.3 Lack of agonism by Q126T and V91R. STAT5 phosphorylation in
Kit225 cells is highly sensitive to IL-2. In an IL-2 dose response curve (A) in the
absence of antagonist, the measured EC 50 is 2.1 1.2pM, and these data are
representative of two independently repeated experiments. In both a STAT5
phosphorylation assay (B) and a cell proliferation assay (C) in Kit225, Q126T and
V9 1R were significantly inhibited in their ability to activate the IL-2 receptor. Error
bars represent the standard deviation of the live cell number at each data point
measured in triplicate. Cell number is normalized to the mean cell number of the 25
pM wild type IL-2 group on day 4. These data are representative of three
independently repeated experiments.
45
Antagonism by the IL-2 analogues
Next, the ability of the IL-2 mutants to antagonize the IL-2 receptor was studied using the
same early and late signaling events measured in the agonism studies. In pSTAT5 assays in
Kit225, both V91R and Q126T antagonized the IL-2 receptor with equal efficacy and IC50 values
of ~500 pM in the presence of 3 pM wild type IL-2, and -2 nM in the presence of 25 pM wild
type IL-2 (Figure 2.4A, 2.4B). In Kit225 cell proliferation assays, V91R and Q126T effectively
antagonized IL-2 receptor as well (Figure 2.4C).
V91R and Q126T were also tested for
antagonism of STAT5 phosphorylation in primary human Tregs ex vivo.
As shown in
Figure 2.4D, these antagonists potently interfere with wild-type IL-2 signaling.
For a competitive antagonist, the Cheng-Prusoff relationship (45) describes the
relationship between the half-maximal inhibitory concentration of antagonist, IC5 0 , and the
inhibition constant, K.
It is given by: IC50 = KI(1+ [A]/EC 50 ), where [A]=wild type IL-2
agonist concentration, and the ECso is the half-maximal effective concentration of wild type IL-2
agonist in the absence of antagonist. Based on the measured IC 50 values and the wild type IL-2
concentration used in Figure 2.4A, the corresponding K1 values are 183 pM for V91R and 216
pM for Q126T. These values are consistent with the binding affinity measured (Figure 2.2).
They are also consistent when repeating the pSTAT5 antagonism assay with a different wild type
IL-2 agonist concentration (Figure 2.4B).
Because IC50 values are dependent on assay
conditions, such as wild type IL-2 agonist concentration, the measured IC 50 values are not
directly applicable to other in vitro or in vivo assays.
Instead, a better indicator of the
antagonists' effectiveness and potency is the assay-independent Ki which are sub-nanomolar for
both antagonists and indicate relatively potent antagonists.
V91R and Q126T also potently
antagonize wild type IL-2 in human primary Tregs. However, a similar analysis based on the
46
Cheng-Prusoff equation for a consistency check cannot be performed, due to a lack of an EC5 o
value for the assay. The donor variability in IL-2 sensitivity as well as limitations in the amount
of blood taken from a single donor make it difficult to measure both an IL-2 agonist dose
response (for EC5 o determination) and antagonist inhibition curves (for IC5 o determination) for
use in calculating K1 values in primary Tregs.
A
B__
B
1.2
.
_
_
_
_
---
_
_
_
Q126T
R1
e--V91R
--
E 0.6
Z0
-- O4
E 0.6
A
0.4-
_
. 0.8
i0
V91R
---
"
_
V
-- 0-- Q126T
.N 0.8
_
0
1L
19
to
< 0.2
0.2
U-
a.
_
1.2
CL)
0 0.01
C
0.1
.Antagonist
1.2 -
1
10
0
0.01
100
D
Concentration (nM)
0.8
1
10
100
Antagonist Concentration (nM)
Ah-
-0-- wtIL-2 + Q126T
-&- wtIL-2 + V91R
-0- Media only
El
0.1
0.8
-o--
V91R
Q126T -
0.6
0. 6
S0.4
=0.4
0.2
E~
0.2
CL
0
0100
0.1
0
2
4
1
10
100
1000
10000
Antagonist:Agonist Molar Ratio
Time (Days)
Figure 2.4 Antagonism by Q126T and V91R. The two mutants, Q126T and V91R, were assayed
for antagonism in the presence of 3 pM (A) or 25 pM (B) wild type IL-2 in a phosphorylated
STAT5 assay, where the IC50 values and 95% confidence intervals were determined to be
525±252 pM Q126T, and 445±90pM V91R at 3 pM wild type IL-2 and 1.86±0.46 nM Q126T
and 2.35±0.63 nM V91R at 25 pM wild type IL-2. Data for each antagonist are combined from
two independent experiments. Fluorescence is normalized to the maximum fluorescence of each
antagonist as determined by least squares regression. In the Kit225 cell proliferation assay (C),
100 nM of Q126T or V91R was able to antagonize 25pM wild type IL-2. These data are
representative of three independently repeated experiments. Cell number is normalized to the
mean cell number of the 25 pM wild type IL-2 group on day 4. Error bars represent the standard
deviation of the cell number at each data point measured in triplicate. Antagonism of STAT5
phosphorylation in primary human Treg cells ex vivo in the presence of 40 pM wild type IL-2
was also measured (D). Fluorescence was normalized to a value of 1.0 for 40 pM IL-2 in the
absence of antagonist, and 0.0 in the absence of either antagonist or agonist.
47
Discussion
Protein engineering techniques were used to customize the binding affinities of IL-2 to
each of its receptor subunits, resulting in a novel class of IL-2 analogues with high affinity to IL2Ra and low affinity to either IL-2Rp or IL-2Ry. These mutants antagonize wild type IL-2 in
both a T cell line and in primary Tregs ex vivo. By targeting the downstream effects of the IL-2
receptor rather than simply using CD25 as a cellular marker to deliver cytotoxic payloads, these
IL-2 antagonists provide a novel mode of pharmacological Treg inhibition potentially of use for
cancer immunotherapy.
The IL-2 variants created by substituting on 2-4 IL-2 either a valine to arginine at
position 91 or a glutamine to threonine at position 126 retain high affinity IL-2Ra binding,
minimally activate the IL-2 receptor, and antagonize the IL-2 receptor. The assumption that the
wild type IL-2 / IL-2 receptor crystal structure could be used to rationally design mutants of 2-4
IL-2 appears to hold up well. In fact, it was surprising that single residue substitutions at V91R
or Q126T were so effective at disrupting binding to IL-2Rp and IL-2Ry, respectively, and
created such signaling deficient analogues of 2-4 IL-2. Of course, effective antagonists are not
limited to these substitutions on the 2-4 IL-2 background. Residue substitutions, such as those
listed in Table I, or other unidentified substitutions at the IL-2Rp or IL-2Ry binding interfaces, or
combinations of these, could also potentially yield potent antagonists on the 2-4 IL-2 background.
The potency of antagonism of both mutants tested was nearly identical in this study.
However, if additional residue substitutions are explored, a 2-4 IL-2 mutant with disrupted IL2Ry affinity may be preferable to one with disrupted IL-2Rp affinity. Unbound IL-2Ra and IL2Rp have been shown to pre-associate on the cell surface 4 6-4 8 and an IL-2 mutant with high IL-
48
2Ra affinity may still bind preformed IL-2Ra/IL-2Rp complexes, even with lowered IL2-Rp
affinity (49). Once bound to IL-2Ra/IL-2Rp complexes, the mutant might bind IL-2Ry due to
undisrupted IL-2R 7 binding affinity, and undesirably create a signaling complex. We speculate
this may be the cause of the low levels of agonism by V9 1R. Second, if a mutant with disrupted
IL-2Rp binding affinity only binds IL-2Ra, this leaves IL-2Rs and IL-2R7 available for
signaling by wild type IL-2, although signaling in the absence of IL-2Ra is much less efficient.
A 2-4 IL-2 analogue with disrupted binding to both IL-2Rp and IL-2Ry is another potential
design to be explored, but such a double mutant may not sequester preformed IL-2Ra/IL-2Rp
complexes from wild type IL-2 as efficiently as a mutant with disrupted IL-2Ry affinity only.
Unfortunately, our attempts to express a Q126T/V91R double mutant unexpectedly yielded a
presumably misfolded protein with significantly lower IL-2Ra binding affinity.
The requirement of IL-2 signaling for many biological functions of Tregs, including
activation of their suppressive functions, has been well documented in the literature (27-30). Our
antagonists inhibit IL-2 mediated proliferation in a human T cell line, and STAT5
phosphorylation in both a human T cell line and human primary Tregs. Since IL-2 does not
activate the PI3K/Akt and MAPK signaling pathways in Tregs (43), the inhibition of STAT5
phosphorylation in primary Tregs is significant, because this represents blockage of all known
signaling pathways downstream of the IL-2 receptor. Therefore, the documented requirement of
IL-2 signaling for Treg suppressive function, coupled with data that IL-2 signaling is blocked in
Tregs by our antagonists, provide strong evidence that these antagonists are capable of inhibiting
Treg suppressive function.
To measure Treg suppression in vitro, a coculture assay is traditionally performed, where
the proliferation of effector T cells is measured in the presence of Tregs, CD3 activating
49
antibodies and costimulation (1).
However, for testing the effects of the antagonists on Tregs,
there exist many confounding and competing effects that make the results of such an assay
ambiguous and not definitive of the antagonists' effects in vivo. First of all, the anti CD25
antibody used for Treg purification is detectably bound to the cell's surface for 48 hours or more
after purification, and could inhibit binding of the antagonists to CD25 (data not shown). This
was part of the reason that whole blood, instead of purified T cell subsets, was used to analyze
the effects of the antagonists on gated CD4*Foxp3* cells, without staining for CD25. Next, the
usefulness of the standard in vitro Treg coculture suppression assay is limited, because IL-2
antagonism would also have mixed effects on activated effector T cells that are upregulated for
CD25. Antagonism of wild type IL-2 may inhibit proliferation and other effector functions in
activated T cells.
However, there is also the possibility that the antagonists may inhibit
activation induced cell death, thereby enhancing effector functions by prolonging the lifespan of
the effector T cells. Given these competing effects, the results from an in vitro co-culture Treg
suppression assay would be difficult to interpret and ambiguous at best. Furthermore, the results
would likely be dependent on assay conditions (1) such as absolute and relative Treg and effector
T cell numbers used, the type and amount of costimulation, the cell isolation method, and other
culture conditions; the in vitro assay would thus inaccurately reflect what occurs in vivo.
Therefore, our next steps focused on testing these antagonists in vivo. Unfortunately, the 2-4
human IL-2 mutant does not bind with enhanced affinity to the mouse IL-2 receptor a subunit, so
the antagonists developed in this study cannot be tested in a mouse model. We are currently
recapitulating the approach used here for engineering human IL-2 antagonists with mouse IL-2.
It will be interesting to contrast the effects of these specific IL-2 signaling antagonists to those of
50
ablative CD25-targeted therapies such as DAB 389 -IL-2 and anti-CD25 antibodies in an in vivo
model system.
It will also be important to test the selectivity of the IL-2 antagonists for Tregs over
activated effector T cells. The significant variability and dependence on assay conditions, as
described above and previously reviewed (1), all make an in vitro assay unsuitable for testing
this selectivity. Nevertheless, there are several factors that would potentially favor a selective
effect on Tregs in vivo. First, effector T cells are activated by IL-2 in an autocrine manner,
whereas Tregs do not secrete IL-2 and instead require IL-2 that is secreted by neighboring
effector T cells in a juxtacrine manner. Therefore, the local IL-2 concentration surrounding
effector T cells would be higher than for Tregs, making it easier to antagonize IL-2 effects on
Tregs.
Second, activated T cells express CD25 in a transient manner, and therefore have a
shorter window of time for inhibition by IL-2 antagonists than Tregs, which constitutively
express CD25.
Lastly, from an empirical standpoint, Tregs are selectively depleted by the
DAB 389-IL-2 fusion protein in several studies (19-21). Though the mechanism of this selectivity
has not been elucidated, the analogy can be tentatively applied to IL-2 antagonists because both
rely on the interactions between IL-2 and the high affinity trimeric IL-2 receptor.
While these antagonists were originally designed as a novel class of Treg inhibitors, their
use is not necessarily limited to biological inhibitors of regulatory T cell function. For example,
these antagonists may be of use for inhibiting the effects of soluble CD25 in the body. Another
potential use may be for targeting CD25 expressing cells for the delivery of drug payloads or for
gene delivery, where IL-2 receptor activation, antibody dependent cell cytotoxicity or
complement-mediated elimination may be undesirable.
51
Acknowledgements
We thank Dr. Lisa Maier (Brigham and Women's Hospital) and Dr. David Hafler (Yale
University) for their assistance with performing the human primary regulatory T cell assays.
52
References
1. Baecher-Allan C, Viglietta V and Hafler DA. Human CD4+CD25+ regulatory T cells.
Semin Immunol. 16:89-98 (2004).
2. Fehervari Z and Sakaguchi S. Development and function of CD25+CD4+ regulatory T
cells. Curr Opin Immunol. 16:203-208 (2004).
3. Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are
abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 103:1755-1762
(2004).
4. Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4*CD25* T cells in tumors from
patients with early-stage non-small cell lung cancer and late-stage ovarian cancer.
Cancer Res. 61:4766-4772 (2001).
5. Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in
peripheral blood and tumor microenvironment of patients with pancreas or breast
adenocarcinoma. J Immunol. 169:2756-61 (2002).
6. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian
carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 10:942-9
(2004).
7. Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the
identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin
Oncol. 24:5373-80 (2006).
8. Kobayashi N, Hiraoka N, Yamagumi W, et al. FOXP3+ regulatory T cells affect the
development and progression of hepatocarcinogenesis. Clin Cancer Res. 13:902-11 (2007).
9. Mempel TR, Khazaie PK, Weninger W, et al. Regulatory T cells reversibly suppress
cytotoxic T cell function independent of effector differentiation. Immunity. 25:129-41
(2006).
10. Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T
cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 102:419-24
(2005).
11. Nishikawa H, Jager E, Ritter G, et al. CD4+ CD25+ regulatory T cells control the
induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood.
106:1008-11 (2005).
12. Fontenot JD and Rudenski AY. A well adapted regulatory contrivance: regulatory T cell
development and the forkhead family transcription factor Foxp3. Nat Immunol. 6:331-7
(2005).
13. Fontenot JD, Gavin MA and Rudensky AY. Foxp3 programs the development and
function of CD4+CD25+ regulatory T cells. Nat Immunol. 4:330-6 (2003).
14. Hori S, Nomura T and Sakaguchi S. Control of regulatory T cell development by the
transcription factor Foxp3. Science. 299:1057-61 (2003).
15. Khattri R, Cox T, Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ T
regulatory cells. Nat Immunol. 4:337-42 (2003).
16. McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4+CD25+ immunoregulatory T cells
gene expression analysis reveals a functional role for the glucocorticoid-induced TNF
receptor. Immunity. 16:311-323 (2002).
53
17. Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by
CD25+ CD4+ regulatroy T cells constitutively expressing cytotoxic T lymphocyteassociated antigen 4. J Exp Med. 192:303-9 (2000).
18. Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigenspecific regulatory T cells expressing the folate receptor. Immunity.;27:145-59 (2007).
19. Knutson KL, Dang Y, Lu H, et al. IL-2 immunotoxin therapy modulates tumor-associated
regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in
neu-transgenic mice. J Immunol. 177:84-91 (2006).
20. Dannull J, Su, Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor imunity
in cancer patients after depletion of regulatory T cells. J Clin Invest. 115:3623-33 (2005).
21. Litzinger, MT, Fernando R, Curiel TJ, et al. IL-2 immunotoxin denileukin difitox reduces
regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood. 110:3192-201
(2007).
22. Curiel TJ, Barnett B, Kryczek I, et al. Regulatory T cells in ovarian cancer: biology and
therapeutic potential. Am J Reprod Immunol. 54:369-77 (2005).
23. Shimizu J, Yamazaki S and Sakaguchi S. Induction of tumor immunity by removing
CD25+CD4+ T cells: a common bais between tumor immunity and autoimmunity. J
Immunol. 163:5211-18 (1999).
24. Olsen E, Duvic M, Frankel A, et al. Pivotal Phase III Trial of Two Dose Levels of
Denileukin Diftitox for the Treatment of Cutaneous T-Cell Lymphoma. J Clin Oncol.
19:376-88 (2001).
25. Stephens LA, Gray D and Anderton ST. CD4*CD25* regulatory T cells limit the risk of
autoimmune disease arising from T cell receptor crossreactivity. Procl Natl Acad USA.
102:17418-23 (2005).
26. Colombo MP and Piconese S. Regulatory T-cell inhibition versus depletion: the right
choice in cancer immunotherapy. Nat Rev Canc. 7:880-7 (2007).
27. Setoguchi R, Hori S, Takahashi T, et al. Homeostatic maintenance of natural
Foxp3*CD25*CD4* regulatory T cells by interleukin (IL)-2 and induction of autoimmune
disease by IL-2 neutralization. J Exp Med. 201:723-35 (2005).
28. Fontenot JD, Rasmussen JP, Gavin MA, et al. A function for interleukin 2 in Foxp3expressing regulatory T cells. Nat Immunol. 6:1142-51 (2005).
29. Thornton AM, Donovan EE, Piccirillo CA, et al. Cutting Edge: IL-2 is critically required
for the in vitro activation of CD4*CD25* T cell suppressor function. J Immunol.
172:6519-23 (2004).
30. Yu A and Malek TR. Selective availability of IL-2 is a major determinant controlling the
production of CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 177:5115-21 (2006).
31. Rao BM, Driver I, Lauffenburger, DA, et al. High-affinity CD25-binding IL-2 mutants
potently stimulate persistent T cell growth. Biochemistry. 44:10696-701 (2005).
32. Krutzik PO, Nolan QP. Intracellular phospho-protein staining techniques for flow
cytometry: monitoring single cell signaling events. Cytometry A. 55:61-70 (2003).
33. Maier LM, Anderson DE, De Jager PL, et al. Allelic variant in CTLA4 alters T cell
phosphorylation patterns. Proc Natl Acad Sci USA. 104:18607-12 (2007).
34. Wang X, Rickert M and Garcia KC. Structure of the quaternary complex of Interleukin-2
with its a, P, and ye receptors. Science. 310:1159-63 (2005).
54
35. Stauber DJ, Debler EW, Horton PA, et al. Crystal structure of the IL-2 signaling
complex: paradigm for a heterotrimeric cytokine receptor. Proc Natl Acad Sci USA.
103:2788-93 (2006).
36. Cassell DJ, Choudhri S, Humphrey R, et al. Therapeutic enhancement of IL-2 through
molecular design. Curr Pharm Des. 8:2171-83 (2002).
37. Shanafelt AB, Lin Y, Shanafelt MC, et al. A T-cell-selective interleukin 2 mutein
exhibits potent antitumor activity and is well tolerated in vivo. Nat Biotechnol. 18:1197202 (2000).
38. Collins L, Tsien WH, Seals, J, et al. Identification of specific residues of human
interleukin 2 that affect binding to the 70-kDa subunit (p70) of the interleukin 2 receptor.
Proc Nati Acad Sci USA. 85:7709-13 (1988).
39. Eckenberg R, Xu D, Moreau JL, et al. Analysis of human IL-2/IL-2 receptor P chain
interactions: monoclonal antibody H2-8 and new IL-2 mutants define the critical role of a
helix-A of IL-2. Cytokine. 9:488-98 (1997).
40. Buchli P and Ciardelli T. Structural and biologic properties of a human aspartic acid-126
Interleukin-2 analog. Arch Biochem Biophys. 307:411-15 (1993).
41. Liang SM, Thatcher DR, Liang CM, et al. Studies of structure-activity relationships of
human interleukin-2. J Biol Chem. 261:334-37 (1986).
42. Liparoto SF, Myszka DG, Wu Z, et al. Analysis of the role of the Interleukin-2 receptor
y chain in ligand binding. Biochemistry. 41:2541-51 (2002).
43. Bensinger SJ, Walsh PT, Zhang J, et al. Distinct IL-2 receptor signaling pattern in
CD4+CD25+ regulatory T cells. J Immunol. 172:5287-96 (2004).
44. Smith KA. The Interleukin 2 receptor. Annu Rev Cell Biol. 1989;5:397-425 (1989).
45. Cheng Y and Prusoff WH. Relationship between the inhibition constant (KI) and the
concentration of inhibitor which causes 50 per cent inhibition (150) of an enzymatic
reaction. Biochem Pharmacol. 22:3099-108 (1973).
46. Saragovi H and Malek TR. Direct identification of the murine IL-2 receptor p55-p75
heterodimer in the absence of IL-2. J Immunol. 141:476-82 (1988).
47. Goldstein B, Jones D, Kevrekidis IG, et al. Evidence for p55-p75 heterodimers in the
absence of IL-2 from Scatchard plot analysis. Int Immunol. 4:23-32 (1992).
48. Roessler E, Grant A, Ju G, et al. Cooperative interactions between the interleukin 2
receptor a and P chains alter the interleukin 2-binding affinity of the receptor subunits.
Proc Natl Acad Sci USA. 91:3344-7 (1994).
49. Fallon EM, Liparoto SF, Lee KJ, et al. Increased endosomal sorting of ligand to recycling
enhances potency of an interleukin-2 analog. J Biol Chem. 275:6790-7 (2000).
55
Chapter 3: Engineered mouse Interleukin-2 antagonists for
the inhibition of regulatory T cells
Abstract
Human IL-2 antagonists that are engineered for high affinity to non-signaling IL-2Ra and
low affinity to either IL-2Rp or IL-2Ry, the signaling subunits, show potential for inhibiting the
immunosuppressive functions of regulatory T cells (Tregs).
However these human IL-2
antagonists are not cross reactive with mouse IL-2 receptor. Here, we present the development
of murine versions of these IL-2 antagonists and their characterization in vitro and in vivo. Two
mouse IL-2 variants, D34W and Q141D, were generated by incorporating single point
substitutions on either the IL-2Rp or IL-2Ry binding interface, respectively, of a high IL-2Ra
affinity mutant.
D34W and Ql41D antagonize IL-2 dependent proliferation and STAT5
phosphorylation in a T cell line with K1 values of 3.7 nM and 510 pM, respectively.
When
reformatted as Fc fusion proteins, Fc-D34W and Fc-Q141D retain their antagonistic potency in
vitro. However, IL-2 antagonism by Fc-Q141D with deactivated Fc effector functions are not
capable of depleting peripheral Tregs, or disrupting Treg function in a syngenic tumor model,
despite inducing CD25 downregulation in vivo. We believe that this is due to the high sensitivity
of the IL-2 receptor system and the high threshold required for successful antagonism in vivo.
56
Introduction
CD4*CD25high Tregs suppress immune responses (1,2) and have been found in the
circulation of patients with various cancers (3-6) as well as near tumor sites (5,6), correlating
with reduced survival in some cancers (6-8). Tregs dampen anti-tumor immune responses by
inhibiting the effector functions of tumor-specific CD4* and CD8* T cells (6, 9-11). As a result,
the inhibition of Treg suppression of an anti-tumor immune response is one approach to cancer
therapy currently being studied.
While the expression of the nuclear transcription factor Foxp3 appears to be the most
specific marker for Tregs (12-15), surface receptors for targeting Tregs include glucocorticoidinduced tumor necrosis factor receptor, cytotoxic T-lymphocyte antigen 4, and folate receptor 4
(16-18). We chose to target CD25, the Interleukin (IL)-2 receptor a subunit (IL-2Ra), which is
constitutively expressed at high levels on CD4*CD25high Tregs. IL-2 signaling has been shown
to be critical for the homeostasis and suppressive functions of Tregs (19, 20), and therefore, we
sought to inhibit Treg suppression by antagonizing the IL-2 receptor.
Previously, we developed human IL-2 analog antagonists using the following design
criteria: 1) high binding affinity to the a subunit of the IL-2 receptor (IL-2Ra), the IL-2 specific
capture subunit, and 2) low binding affinity to the
subunits responsible for receptor signaling.
P or y subunits
(IL-2Rp or IL-2Ry), the two
The high binding affinity to IL-2Ra leads to
preferential IL-2Ra binding of the IL-2 analog over wild type IL-2, while the low binding
affinity to IL-2Rp or IL-2Ry would prevent the IL-2 analog from activating the IL-2 receptor
signal itself. Briefly, the residue substitutions V91R and Q126T were individually introduced
onto a high IL-2Ra affinity background, 2-4 IL-2 (KD-200 pM). This resulted in IL-2 variants
that retained high affinity binding to IL-2Ra but disrupted binding to IL-2Rp or IL-2Ry,
57
respectively.
These variants, termed "V91R" and "Q126T," inhibited IL-2 mediated STAT5
phosphorylation with K1 ~ 200 pM, and also antagonized the IL-2 dependent proliferation in a
human T cell line.
These antagonists also antagonized STAT5 phosphorylation in human
primary Tregs ex vivo (21).
However, 2-4 IL-2 and in turn, V91R and Q126T, do not bind to mouse IL-2Ra with high
affinity, and therefore, this design strategy was recapitulated to develop mouse IL-2 antagonists
for in vivo characterization. A mouse IL-2 variant, QQ6.2-10, was recently engineered using
directed evolution and yeast surface display for increased affinity to mouse IL-Ra, with KD~100
pM. Mouse IL-2 antagonists were engineered by incorporating single mutations that disrupted
binding to either IL-2Rp or IL-2Ry onto the QQ6.2-10 background. These constructs were
characterized both in monovalent form as well as in fusion proteins with the mouse IgG2a Fc
fragment for improved serum half-life in vivo.
These constructs were able to inhibit
phosphorylated STAT5 and cell proliferation in an IL-2 dependent mouse T cell line in vitro.
When characterized in vivo, these constructs were able to bind to Tregs and downregulate IL2Ra expression levels, but were unable to potently antagonize Treg suppressive functions in a
syngenic mouse tumor model. We believe that this is due to the high sensitivity of the IL-2
receptor system to wild type IL-2 (22) and a high threshold required for receptor antagonism.
Nevertheless, this paradigm of high affinity binding to non-signaling subunits and disrupted
affinity to signaling subunits may be useful for antagonist designs in multimeric receptor systems
with a lower threshold for receptor antagonism.
58
Materials and Methods
Preparation of IL-2 Mutant Proteins
Single point mutations were introduced to the QQ6.2-10 coding sequence using the
Quikchange kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. IL-2
mutants were expressed in yeast with an N-terminal FLAG tag and a C-terminal c-myc tag as
previously described. For pilot studies, IL-2 mutants were expressed in 5mL pilot cultures, and
buffer exchanged with PBS using a 1OkDa MWCO Amicon spin column, followed by
purification using an anti-FLAG M2 agarose affinity chromatography gel according to the
manufacturer's instructions (Sigma Aldrich, St. Louis, MO). The concentration of IL-2 mutant
monomer was determined using a quantitative SDS-PAGE, with BSA as a standard, and Simply
Blue (Invitrogen, Carlsbad, CA) for visualization of protein. For further characterization of lead
IL-2 mutants, yeast secretions were performed at the 1 L scale as described (21).
Fc Fusions
Fusion genes between Mouse IgG2a and IL-2 variants were made using a PCR based
method (23). Fusion PCR products were cloned into the gWiz vector (Genlantis, San Diego,
CA) using the method of Geiser et al (24). The D265A mutation for disrupted effector functions
was inserted by the Quikchange kit according to the manufacturer's instructions. Fc-IL-2 variant
fusions were expressed in stable transfectants of HEK293F cells (Invitrogen, Carlsbad, CA) for 8
days. The supernatant was then pH -adjusted to 7.4 and the protein was purified using affinity
chromatography with Protein A agarose resin (Pierce, Rockford, IL), followed by size exclusion
chromatography with a Superdex 200 column (GE Healthcare, Piscataway, NJ).
59
Elution
fractions that contained only monomeric protein were pooled and the protein concentration was
determined using the Micro BCA Protein Assay kit (Pierce, Rockford, IL).
Tissue Culture
To characterize the IL-2 mutants in vitro, the mouse IL-2 dependent T cell line CTLL-2,
which constitutively expresses all three subunits of the IL-2 receptor, was used. The cells were
cultured in a humidified atmosphere in 5% CO 2 in RPMI 1640 supplemented with 10% heatinactivated fetal bovine serum, 200 pM IL-2 (R&D Systems, Minneapolis, MN), 2 mM Lglutamine, 2 mg/mL sodium bicarbonate, 50 U/mL penicillin, 50 tg/mL streptomycin and 50
gg/mL gentamicin.
Determination of IL-2Ra binding affinities of IL-2 mutants
The equilibrium binding affinities to IL-2Ra were evaluated using a modification of a
previously described previously, except that CTLL-2 cells are used instead (21). CTLL-2 cells
were incubated in phosphate buffered saline (PBS) + 0.1% bovine serum albumin (BSA) at 37'C
for 30 minutes, at 8x10 5 cells/sample with varying IL-2 mutant concentrations. At low IL-2
mutant concentrations, the total volume was increased to maintain an excess number of IL-2
mutant molecules over the number of IL-2Ra. After incubation, cells were kept on ice and
stained with mouse anti-FLAG monoclonal antibody M2 (Sigma Aldrich, St. Louis, MO),
followed by an Alexa Fluor 488 conjugated goat anti-mouse antibody (Invitrogen, Carlsbad,
CA), to detect cell-surface bound protein. The mean single-cell fluorescence was measured, and
the dissociation constant (KD) and the 95% confidence interval were determined as previously
described, 3 1 using the following equation: Fh, = cLO / (KD + L0
60
),where
Fos
is the background-
corrected mean fluorescence, Lo is the initial concentration of the protein being measured, and c
is the proportionality constant.
Analysis of Stat5 phosphorylation
For all STAT5 phosphorylation assays, CTLL-2 cells were starved of IL-2 for 4 hours.
CTLL-2 cells were incubated per at 106cells/mL culture medium at 370 C with IL-2 mutants for
30 minutes (for agonism studies), or with IL-2 mutants and 25 pM wild type IL-2 for 15 minutes
(for antagonism studies). The cells were fixed and permeabilized using the method optimized by
Krutzik et al (25). The cells were stained with anti-pSTAT5 antibody clone 47 conjugated with
Alexa Fluor 488 (BD Biosciences, San Jose, CA) and the mean single-cell fluorescence was
measured. For antagonism studies, the half-maximal inhibitory concentration (IC 5 o) and the 95%
confidence interval were determined using the following equation Fs = c / (I
-
LO / (IC 50
+ LO
)),
where all variables are defined above. A global fit nonlinear regression was performed for each
protein, using a global IC 50 value and the proportionality constant from each of two separate
experiments.
CTLL-2 Cell Proliferation Assays
CTLL-2 cells were starved of IL-2 for 4 hours. Then, 4x 105 cells were incubated in 3 mL
culture medium at 370 C with IL-2 mutants, either in the absence (for agonism studies), or
presence (for antagonism studies) of 25 pM wild type IL-2. At each time point, the live cell
number in 10pL culture medium was determined in triplicate using the CellTiter-Glo assay
(Promega, Madison, WI) and a Cary Eclipse luminometer (Varian, Palo Alto, CA) according to
the manufacturer's instructions.
61
Serum half-life determination
Wild type and D265A Fc-D34W were labeled using a near-infrared dye, Li-Cor
IR800CW NHS ester (Li-Cor, Lincoln, NE) at a dye:protein ratio of 2-3:1. 50tg of either wt FeD34W or D265A Fc-D34W was injected retroorbitally into duplicate Balb/c mice, and at each
time point a blood sample was collected from the tail in a heparin coated capillary tube. The
blood was ejected and the red blood cells were removed with a 12 minute centrifugation at
1500g. The supernatant was recollected into a fresh capillary tube and analyzed on a Li-Cor
Odyssey Imaging system (Li-Cor, Lincoln, NE).
The decay in IR800CW serum signal was
measured and fit to a bi-exponential decay model.
Treg depletion assay
Foxp3-GFP reporter mice were injected retroorbitally in duplicate with either PBS, wt
Fc-Ql41D or D265A Fc-Q141D.
At each time point, 3-4 drops of blood were obtained
retroorbitally and mixed with a 10% heparin solution to prevent coagulation.
PBMC's were
isolated using Ficoll-Paque density centrifugation (GE Healthcare, Piscataway, NJ), and washed
twice with HBSS + 10% FBS. PBMC's were stained with anti CD4-Pacific Blue at 1:400 and
anti CD25-PerCP Cy5.5 at 1:200 (BD Biosciences, San Jose, CA) for 20 minutes on ice, and
washed twice with HBSS + 10% FBS, before analysis by flow cytometry.
Syngenic tumor model
DEREG mice (26) and Foxp3-GFP reporter mice were crossed on a Thyl.2 background.
Tumor challenged was initiated using 106 EG7 cells.
62
On Days 1 and 2 after EG7 tumor
challenge, PBS, Ip g diphtheria toxin, or 500pg D265A was injected retroorbitally. On Day 3
after tumor challenge, 106 CFSE-stained OT-1 CD8 T cells obtained from a Thyl.1 mouse were
administered by tail vein injection. On Day 7, the mice were sacrificed, and the spleen, tumor
draining lymph node and non-draining lymph node was harvested. Cells from each organ were
washed, and split into two. One set was stained with with one set stained with the following
antibodies: CD4-PE-PerCP, Thyl.1-APC, and CD25-biotin, followed by a Streptavidin-PE-Cy7
secondary stain. The second set was restimulated with OVA peptide for 4 hours, and Golgi-Plug
(BD Biosciences, San Jose, CA), then fixed and permeabilized, and stained with IFNy-PE and
Thyl.1-APC. All antibodies and streptavidin conjugates were purchased from BD Biosciences
(San Jose, CA).
63
Results
Design of mouse IL-2 antagonists
Since the crystal structure of the mouse IL-2 / IL-2 receptor complex has not yet been
solved, we constructed homology models based on the human IL-2 / IL-2R crystal structure from
Wang et al (27) using SWISS-Model (28).
From this homology model, as well as from
previously reported substitution mutagenesis analysis (29-31), we identified 5 candidate residues
for substitution on the QQ6.2-10 background, shown in Figure 3. lA-C.
In pilot scale studies,
antagonism by mouse IL-2 mutants with single point mutations on the QQ6.2-10 background
was compared (Figure 3. 1D). From this initial screening, two mutants were chosen for further
characterization in the CTLL-2 mouse T cell line, D34W and Q141D, which have disrupted
binding to IL-2Rp and IL-2Ry, respectively.
Using cell surface titrations on CTLL-2 cells, the KD of IL-2Ra binding was measured.
As with the human IL-2 antagonists, the addition of these residue substitutions did not alter the
mouse IL-2Ra binding affinity of the QQ6.2-10 background (Figure 3.2A). Based on STAT5
phosphorylation, their ability to agonize the IL-2 receptor is significantly inhibited (Figure 3.2B).
They are also capable of antagonizing the IL-2 receptor, with Q141D the more potent antagonist
(Figure 3.2C).
64
C
A
A
B
D34
N103
Q30
1.2 -
Q4
10 nM IL-2 mutant
+25pM wtIL-2
1
0.8
0.6U.
0.4
1!
0.2
0*
CDP
Figure 3.1 Mouse IL-2 antagonist design. A homology model was created for the
mouse IL-2 / IL-2 receptor complex homology model (A). IL-2 is green, IL-2Ra is
yellow, IL-2R is blue IL-2Ry is magenta. The locations of candidate mutation are
V106, N103, D34 and Q30 at the IL-2 / IL-2Rp interface (B), and Q141 at the IL-2 /
IL-2Ry interface (C). Substitutions on the QQ6.2-10 background were tested in pilot
pSTAT5 studies (D) with 10 nM variant in the presence of 25 pM wild type mouse
IL-2.
65
£1.4 -B
A
14
112 -
L- 1.2
0.6
m 0.4
0.2
|2
-
<
D34W
Q141D
*1nM
__
__
EOnM
S0.
6
1OOnM
4
2
0
0.001
-
0.1
10
1000
Media
only
Concentration (nM)
C
25pM 1L2
Q141D
D3,
18
. 16
--
U. 14 --
Q141D
I4D
1*
10
8 -N
+25pM wtIL-2
6
C. 4 -
-D34W
2
0
0.01
0.1
1
10
100
1000
Antagonist Concentration (nM)
Figure 3.2 Characterization of two lead mouse IL-2 antagonists. The KD of binding
to the CTLL-2 cell line was measured to be 145 pM for Q141D and 160 pM for
D34W (A). Agonism was measured by a STAT5 phosphorylation assay (B), and
antagonism was measured in the presence of 25 pM wild type mouse IL-2 (C). The
measured IC50 values were 5.2±2.6 nM for Q141D and 38±18 nM for D34W.
66
In vitro characterization of Fc fusion proteins
Initial testing of these antagonists in vivo was limited by the short serum half-life of the
antagonists. The IL-2 antagonists are l8kDa in size, and its estimated serum half-life is 20-30
minutes, due to rapid renal clearance. In order to address this issue, the IL-2 antagonists were reformatted as C-terminal fusions with the mouse IgG2a Fc domain. Due to interactions with the
neonatal Fc receptor (FcRn), IgG antibodies and fusion proteins containing an Fc fragment
benefit from extended serum half-lives (32). Also, the substitution of an aspartic acid in position
265 of the mouse IgG2a Fc to an alanine (D265A) has been shown to disrupt Fc effector
functions, including antibody dependent cellular cytotoxicity (ADCC), and complement directed
cytotoxicity (CDC) (33). This D265A modification thus enabled us to study the effects IL-2
antagonism alone, in the absence of additional Fc effector functions.
Both D34W and Q141D were expressed as fusion proteins with mouse IgG2a Fc, both
wild type Fc and D265A Fc. To confirm IL-2 antagonist potency in the Fc fusion protein format,
the Fc fusion proteins were characterized in vitro on CTLL-2 cells. They retained high binding
affinity to IL-2Ra (Figure 3.3A). STAT5 phosphorylation antagonism profiles were similar to
monovalent IL-2 antagonists as well (Figure 3.3C). In CTLL-2 proliferation studies, D265A FcD34W weakly agonized IL-2 mediated proliferation (Figure 3.3D). Also, proliferation was fully
antagonized by Fc-Q141D fusions and partially antagonized by Fc-D34W fusions (Figure 3.3E),
consistent with their respective potencies in STAT5 phosphorylation assays.
In all CTLL-2
assays, there was no discemable effect of wild type versus the D265A versions of the Fc on the
activities of each IL-2 antagonist.
67
A
B
1.2
p
1
IL
,//
/'/
0.4
''
-V
0.2
C
'U
10
0)
,,e
0.6
0
00
~
0
0.8
C
p
0
0 .001
----o-----a---
0.1
wt Fc-Q141D
D265A Fc-Q141D
wt Fc-D34W
D265A Fc-D34W
0.1
_ 0.01
0
L 0.001
10
1000
0
Concentration (nM)
C
50
100
150
Time post injection (hr)
20
16
IL
+25pM wtIL-2
12
I-
8
0.
---
4
---.....
-
wt Fc-Q141D
D265A Fc-Q141D
wt Fc-D34W
D265A Fc-D34W
Media only
wtIL2 only
0
1
100
10
1000
[Antagonist] (nM)
D
E
3000
-
3000
2500
2000
E 1500
-
1000
'10/
500
-0-
Media only
2500
-O-
wtL2
2000
1
-- O- wtlL2
E 1500
-0-D265A FcZ 1000
Q141D
-- o- D265A Fc- ) 500
Z
D34W
2
-0-wtL-2 + D265A
Fc-Q141D
-4-wtL-2 + D265A
Fc-D34W
0
0
0
-4--Media only
0
3
1
2
3
Time (Days)
Time (Days)
Figure 3.3 Characterization of Fc-IL-2 antagonist fusion proteins. The KD of IL-2Ra binding
was measured on CTLL-2 cells (A) and was 206±40 pM for wt Fc-Q141D, 193±25 pM for
D265A Fc-Q141D, 270±35 pM for wt Fc-D34W and 230±70 pM for D265A Fc-D34W.
Pharmacokinetic decay curves are shown in (B).
Based on a biexponential decay
pharmacokinetic model, the fitted parameters are: tl/2,a =22±6 min and tii 2 ,p=37±6 hr for wt FcD34W and tl/2 ,a =19±6 min and tv/2,s=29±4 hr for D265A Fc-D34W. Fluorescence intensities
are normalized to the intensity of serum taken at 3 minutes post injection. Error bars represent
the standard deviation of measurements taken from mice tested in duplicate. Antagonism of
STAT5 phosphorylation in the presence of 25pM wild type IL-2 is shown in (C). CTLL-2
proliferation is shown for 100 nM protein in the absence (D) or presence (E) of 200 pM wild
type IL-2. Error bars represent the standard deviation of wells tested in triplicate.
68
Pharmacokinetic characterization of Fc fusion proteins
The serum half-lives were measured for FEc fusion proteins administered retroorbitally.
This was important for D265A Fc, because it was hypothesized that D265A disrupted ADCC
and CDC functions through a conformational change in the Fc fragment (33), which could
potentially disrupt FcRn interactions and thus its serum half-life. Although this conformational
change did not affect effector functions or serum half-life when the homologous substitution was
introduced on mouse IgG1, the effect of D265A on mouse IgG2a had not been directly tested.
After fitting the data to a bi-exponential decay, the
p phase half-lives of the wild type and D265A
Fc fusions were found to be similar to each other, approximately 30 hours (Figure 3.3B). This
indicated that there is a significant improvement in serum half-life of the Fc fusions compared to
monovalent IL-2 antagonists and the D265A substitution did not affect the extended half-life
benefit of the Fc fusion.
Characterization of Fc fusion proteins in vivo
Since IL-2 signaling has been shown to be required for Treg maintenance in the periphery
in mice (19, 20), Fc-Q141D was assayed in an in vivo model for their effects on peripheral Tregs.
Wild type Fc as well as D265A Fc were used, in order to isolate the effects of IL-2 antagonism.
For this assay, reporter mice expressing Foxp3 as a fusion protein with GFP were used for
convenient quantification of peripheral Tregs. Daily injections of 20 g wt Fc-Ql41D were able
to deplete the CD4'Foxp3' T cells in the peripheral blood by Day 4, the first time point
measured. In contrast, daily injections of 685 g D265A Fc-Ql41D were unable to affect the
frequency of peripheral CD4*Foxp3* T cells. This indicated that the Fc-Q141D constructs were
capable of binding Tregs, because wt Fc-Ql4lD was able to deplete Tregs, most likely through
69
its intact effector functions. However, IL-2 antagonism alone had no effect on the peripheral
Treg frequency in the case of D265A Fc-Ql41D. (Figure 3.4A)
The robust Treg depletion observed in vivo with daily administration of 20pg wt FcQ141D makes this construct analogous to an anti CD25 monoclonal antibody, where the Q141D
portion binds to CD25, and the Fc fragment induces effector functions. Despite this observed
effect on peripheral Tregs, we decided not to pursue and further characterize this construct
because of the limited novelty of this molecule compared to an anti CD25 monoclonal antibody.
Since our original hypothesis was that IL-2 antagonism alone would be able to antagonize Treg
fnction, only the D265A Fc variant was further examined in vivo.
Although IL-2 antagonism had no effect of the frequency of peripheral CD4*Foxp3*
Tregs, it caused a marked downregulation of CD25 expression levels in Tregs (Figure 3.4B). To
further investigate the possibility that CD25 downregulation may indicate a perturbation in Treg
biological function, D265A Fc-Q141D was characterized in a syngenic mouse tumor model
using OVA-expressing EG7 tumors.
These experiments were performed on a DEREG
background, in which Tregs express the diphtheria toxin receptor and can be selectively depleted
by administration of diphtheria toxin (DTX) (26). As shown in Figures 3.5A and 3.5B, DTX is
capable of inducing significant proliferation and IFN-y secretion in adoptively transferred OT-1
cells, consistent with Treg depletion. However, D265A Fc-Q141D did not differ from the PBS
negative control with respect to inducing proliferation or IFN- y secretion in adoptively
transferred OT-1 cells (Figure 3.5A, 3.5B). This indicates that IL-2 antagonism by D265A FcQ141D under the dosing scheme tested was not able to perturb Treg function, despite the fact
that it could still bind to Tregs and induce IL-2Ra downregulation (Figure 3.5C).
70
7
A
6
5
X
a
II.
3
2
1
0
D265A FcQ1 41 D
(680ug/inj)
wt Fc-Q141D
(20uglinj)
PBS
D265A
Fc-Q141D
B
01
PBS
02
0its
DayO0
to 1
1.
it
04 'a
02
0
10
se.
92
444
Oil
toI
00
0)
":
061
10"
Day 4
to
to
CD25
f
4,
to
t0
14
0
10
o
Foxp3
*04
It
to
120
Day 7
i4
lot
to)
to
10
'o'
Figure 3.4 Characterization of Fc-IL2 antagonists in GFP-Foxp3 reporter mice. Mice were
injected daily for 4 days with either wt Fc-Q141D, D265A Fc-Q141D, or PBS, and at each time
point, PBMC's were analyzed by flow cytometry. The number of CD4+Foxp3* Tregs are shown
in (A), while CD25 expression levels in the Treg subset are shown in (B). Error bars represent
the standard deviation of measurements from mice tested in duplicate.
71
A
B
80
CD4+ subset
70
-
TDLN
-NDLN
+ 60
a Spleen
50
40
+
30
20
10
0
PBS
DEREG
D265AFc-0141D
C 30000
STDLN
25000
*NDLN
20000
MSpleen
~~
15000
10000
Q
5000
0
PBS
CFSE-FLI
DEREG
D265AFc-Q141D
Figure 3.5 Characterization of Fc-IL2 antagonists in an EG7 tumor model. PBS,
DTX, or 500ug D265A Fc-Q141D was injected on Days 1 and 2 after EG7 tumor
implantation into DEREG Foxp3-GFP mice. On Day 3, CFSE labeled Thy 1.1 OT-1
cells were transferred, and on Day 7, the tumor draining lymph node (TDLN), nondraining lymph node (NDLN), and spleen were isolated. Lymphocytes and
splenocytes were analyzed for CFSE dilution (A), IFN-y secretion (B) and CD25
expression (C).
72
Discussion
Previously, human IL-2 antaognists, V91R and Q126T, were engineered with nearly
identical antagonistic potencies (21). While the two mouse IL-2 antagonists developed in this
study potently antagonized the IL-2 receptor with Ki values of 0.5 nM and 3.7 nM, Q141D was
significantly more potent than D34W as an antagonist. Both D34W and Q141D had equivalent
binding affinity to IL-2Ra, indicating that the difference in antagonist potencies are due to
differences in their binding dynamics to IL-2Rp and IL-2Ry. Specifically, an IL-2 antagonist
with disrupted IL-2Ry affinity would theoretically be preferable to one with disrupted IL-2Rp
affinity. Unbound IL-2Ra and IL-2Rp have been shown to pre-associate on the cell surface (3436) and an IL-2 mutant with high IL-2Ra affinity may still bind preformed IL-2Ra/IL-2Rp
complexes, even with lowered IL2-Rp affinity (37). Once bound to IL-2Ra / IL-2Rp complexes,
the mutant is able to bind IL-2Ry due to undisrupted IL-2Ry binding affinity, and undesirably
create a signaling complex. This would explain the improved antagonistic potency of Q141D,
with disrupted IL-2Ry binding, over D34W, with disrupted IL-2Rp binding.
It would also
explain the higher levels of agonism observed by D34W (Figure 4B). It was hypothesized that
the relative lack of agonism and the relatively improved antagonistic potency observed by the
human homolog V91R is due to more effective disruption of IL-2Rp binding by V91R than by
D34W.
Based on this hypothesis, the optimization of disruption at these receptor binding
interfaces was attempted, using combination mutants with multiple residue substitutions at the
IL-2Rp interface, or at both the IL-2Rp interface and IL-2Ry interface. However, these residue
substitutions on the QQ-6.2-10 background did not exhibit increased antagonism potency relative
to the single mutants D34W and Q141D on the QQ6.2-10 background (data not shown).
73
It was disappointing that D265A Fc-Q141D was unable to antagonize Treg function in
vivo, despite exhibiting potent antagonism in vitro, with K1 ~ 0.5 nM. We believe that this is due
to incomplete antagonism of IL-2R in vivo. Past studies that show the dependence of Treg
homeostasis on IL-2 have done so with a complete loss of IL-2 signaling through gene knockouts
of IL-2 or IL-2R subunits (19, 20).
In contrast, competitive antagonism is by definition never 100% complete. The relative
concentration required for competitive antagonism can be estimated using the Cheng-Prussof
equation, which describes the relationship between K1, and the half maximal inhibitory
concentration in an assay (IC 50 ). It is given by IC50 = K1( 1+[A]/ EC5 o), where [A] is the wild
type IL-2 agonist concentration and EC50 is the half maximal effective concentration of wild type
IL-2 in the absence of antagonist. For in vitro assays with CTLL-2 cells, the wild type IL-2
concentration is controlled and chosen for convenient quantification of the IC 50 for the assay.
However, the endogenous wild type IL-2 concentration is not known in the tumor local
microenvironment.
Thus, it is possible that the wild type IL-2 concentration is too high for
potent antagonism at the antagonist concentrations delivered, despite the sub-nanomolar K1 .
Additionally, there is a precipitous drop in serum Fc-Q141D concentration during the a phase of
tissue distribution, and the IL-2 receptor system is extremely sensitive to wild type IL-2, with an
EC5 0-10
pM (22).
Imperfect extravasation and tumor penetration can further reduce the
antagonist concentration in a solid tumor. All of these factors work against the ability of D265A
Fc-Q141D to meet the threshold for potent antagonism of Treg function.
It is also possible that signal activation may have occurred in Tregs in the presence of the
antagonists, through either IL-2/IL-2Rp/IL-2Ry interactions or other gamma-common cytokines.
Although other groups have observed upregulation of receptor subunits of other gamma-common
74
cytokines, such as IL-7Ra and IL-15Ra (37), this was not observed in our system (data not
shown). However, IL-2 signaling through IL-2R$/IL-2Ry is consistent with a recent study where
IL-2Rp was upregulated in CD4*Foxp3* T cells relative to CD4*Foxp3- T cells in patients
treated with the anti CD25 monoclonal antibody, basiliximab (38). The endogenous wild type
IL-2 concentration in the tumor local microenvironment would have to be relatively high, since
signaling through IL-2Rp/IL-2Ry is relatively inefficient (EC50
1InM) compared to IL-2Ra/IL-
2Rp/IL-2Ry (EC5o0-O pM). Nevertheless, this possibility cannot be ruled out since the local IL2 concentration is unknown.
In conclusion, although the IL-2 antagonists met our design criteria and were able to
potently antagonize the IL-2 receptor in vitro, they were unable to antagonize Treg suppressive
function in vivo, which we believe is due to the high sensitivity of the IL-2 receptor system.
Nevertheless, similar antagonist designs have been successfully implemented with disrupted
binding to signaling subunits with unperturbed binding affinity to other receptor subunits.
Examples include antagonists against IL-4 and IL-13 (Aerovant, developed by Aerovance), and
IL-15 (40). It is possible that the potency of these antagonists could be improved by increasing
binding affinity to the receptor subunit whose affinity is currently unperturbed, using the
techniques established in this study.
75
Acknowledgements
Annie Gai engineered and provided the genetic constructs for the high IL-2Ra affinity
mutant QQ6.2-10.
Diana Wieser assisted with optimization of the IL-2 antagonists. Jarrett
Remsberg assisted with optimizing expression of the D265A Fc-Q141D construct. Dr. Jens
Nolting and Dr. Carolin Daniel (Dana Farber Cancer Institute) assisted with the Foxp3-GFP
reporter mouse assays. Dr. Ed Kim (Massachusetts General Hospital) assisted with the EG7
tumor model assays.
76
References
1. Baecher-Allan C, Viglietta V and Hafler DA. Human CD4+CD25+ regulatory T cells.
Semin Immunol. 16:89-98 (2004).
2. Fehervari Z and Sakaguchi S. Development and function of CD25+CD4+ regulatory T
cells. Curr Opin Immunol. 16:203-208 (2004).
3. Marshall NA, Christie LE, Munro LR, et al. Immunosuppressive regulatory T cells are
abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood. 103:1755-1762
(2004).
4. Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4*CD25* T cells in tumors from
patients with early-stage non-small cell lung cancer and late-stage ovarian cancer.
Cancer Res. 61:4766-4772 (2001).
5. Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in
peripheral blood and tumor microenvironment of patients with pancreas or breast
adenocarcinoma. J Immunol. 169:2756-61 (2002).
6. Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian
carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 10:942-9
(2004).
7. Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the
identification of high-risk breast cancer patients and those at risk of late relapse. J. Clin
Oncol. 24:5373-80 (2006).
8. Kobayashi N, Hiraoka N, Yamagumi W, et al. FOXP3+ regulatory T cells affect the
development and progression of hepatocarcinogenesis. Clin Cancer Res.13:902-11
(2007).
9. Mempel TR, Khazaie PK, Weninger W, et al. Regulatory T cells reversibly suppress
cytotoxic T cell function independent of effector differentiation. Immunity. 25:129-41
(2006).
10. Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T
cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 102:419-24
(2005).
11. Nishikawa H, Jager E, Ritter G, et al. CD4+ CD25+ regulatory T cells control the
induction of antigen-specific CD4+ helper T cell responses in cancer patients. Blood.
106:1008-11 (2005).
12. Fontenot JD and Rudenski AY. A well adapted regulatory contrivance: regulatory T cell
development and the forkhead family transcription factor Foxp3. Nat Immunol. 6:331-7
(2005).
13. Fontenot JD, Gavin MA and Rudensky AY. Foxp3 programs the development and
function of CD4+CD25+ regulatory T cells. Nat Immunol. 4:330-6 (2003).
14. Hori S, Nomura T and Sakaguchi S. Control of regulatory T cell development by the
transcription factor Foxp3. Science. 299:1057-61 (2003).
15. Khattri R, Cox T, Yasayko SA, et al. An essential role for Scurfin in CD4+CD25+ T
regulatory cells. Nat Immunol. 4:337-42 (2003).
16. McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4+CD25+ immunoregulatory T cells
gene expression analysis reveals a functional role for the glucocorticoid-induced TNF
receptor. Immunity. 16:311-323 (2002).
77
17. Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by
CD25+ CD4+ regulatroy T cells constitutively expressing cytotoxic T lymphocyteassociated antigen 4. J Exp Med. 192:303-9 (2000).
18. Yamaguchi T, Hirota K, Nagahama K, et al. Control of immune responses by antigenspecific regulatory T cells expressing the folate receptor. Immunity.;27:145-59 (2007).
19. Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in
Foxp3-expressing regulatory T cells. Nat Immunol. 6:1142-1151 (2005).
20. D'Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+
regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol 6: 1152-1159
(2005).
21. Liu DV, Maier LM, Hafler DA, et al. Engineered interleukin-2 antagonists for the
inhibition of regulatory T cells. J Immunother. 32: 887-94 (2009).
22. Smith KA. The Interleukin 2 receptor. Annu Rev Cell Biol. 5:397-425 (1989).
23. Warrens AN, Jones MD, Lechler RI. Splicing by overlap extension by PCR using
asymmetric amplification: an improved technique for the generation of hybrid proteins of
immunological interest. Gene. 186: 29035 (1997).
24. Geiser M, Cebe Regis, Drewello D, et al. Integration of PCR fragments at any specific
site within cloning vectors without the use of restriction enzymes and DNA ligase.
Biotechniques. 1: 88-92 (2001).
25. Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow
cytometry: monitoring single cell signaling events. Cytometry A. 55:61-70 (2003)
26. Lahl K, Loddenkemper C, Drouin C, et al. Selective depletion of Foxp3+ regulatory T
cells induces a scurfy-like disease. J Exp Med. 204: 57-63 (2007).
27. Wang X, Rickert M, and Garcia KC. Structure of the quaternary complex of Interleukin-2
with its a, P, and Yc receptors. Science. 310:1159-63 (2005)
28. Schwede T, Kopp J, Guex N, et al. SWISS-MODEL: an automated protein homologymodeling server. Nucl Acids Res. 31:3381-5 (2003)
29. Zurawski SM, Imler J and Zurawski G. Partial agonist/antagonist mouse interleukin-2
proteins indicate that a third component of the receptor complex functions in signal
transduction. EMBO J. 9:3899-905 (1990).
30. Zurawski SM and Zurawski G. Receptor antagonist and selective agonist derivatives of
mouse interleukin-2. EMBO J. 11:3905-10 (1992).
31. Zurawski SM, Vega F, Doyl EL et al. Definition and spatial location of mouse
interleukin-2 residues that interact with its heterotrimeric receptor. EMBO J. 12:5113-19
(1993).
32. Roopenian DC and Akilesh S. FcRn: the nenonatal Fc receptor comes of age. Nat Rev
Immunol. 7:715-25 (2007).
33. Baudino L, Shinohara Y, Nimmerjahn F, et al. Crucial role of aspartic acid at position
265 in the CH2 domain for murine IgG2a and IgG2b Fc-Associated Effector Functions. J
Immunol. 181:6664-9 (2008).
34. Saragovi H, Malek TR. Direct identification of the murine IL-2 receptor p55-p75
heterodimer in the absence of IL-2. J Immunol. 141:476-82 (1988).
35. Goldstein B, Jones D, Kevrekidis IG, Perelson AS. Evidence for p55-p75 heterodimers in
the absence of IL-2 from Scatchard plot analysis. Int Immunol. 4:23-32 (1992).
78
36. Roessler E, Grant A, Ju G, Tsudo M, Sugamura K, Waldmann TA. Cooperative
interactions between the interleukin 2 receptor a and P chains alter the interleukin 2binding affinity of the receptor subunits. Proc Natl Acad Sci USA. 91:3344-7 (1994).
37. Fallon EM, Liparoto SF, Lee KJ, Ciardelli TL, Lauffenburger DA. Increased endosomal
sorting of ligand to recycling enhances potency of an interleukin-2 analog. J Biol Chem.
275:6790-7 (2000).
38. Vang KB, Yang J, Mahmud SA, et al. IL-2, -7, and -15, but not thymic stromal
lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J
Immunol. 181: 3285-90 (2008).
39. de Goer de Herve MG, Gonzales E, Hendel-Chavez H, et al. CD25 appears non essential
for human peripheral Treg maintenance in vivo. PLoS ONE. 5:e 11784 (2010).
40. Pettit DK, Bonnert TP, Eisenman J, et al. Structure-function studies of interleukin 15
using site specific mutagenesis, polyethylene glycol conjugation and homology
modeling. J Biol Chem. 272: 2312-8 (1997).
79
Chapter 4: A two-agent protein-based system for targeted siRNA delivery
Abstract
Protein-based methods for targeted siRNA delivery have the potential to solve some of
the problems faced by nanoparticle-based methods, including poor pharmacokinetics and
biodistribution, low tumor penetration, and polydispersity. Protein-based methods employed so
far for targeted delivery have been limited to polycationic peptide carrier fusion proteins with a
macromolecular targeting moiety. However, polycationic peptides are not ideal due to their high
charge density, which results in poor pharmacokinetics and biodistribution, and requires complex
purification schemes.
Here we present a two-agent protein-based approach for targeted siRNA delivery to
EGFR, using a non-polycationic carrier for siRNA.
The first agent contains a 10th type 3
fibronectin (Fn3) engineered for EGFR binding and a double stranded RNA binding domain
(dsRBD). dsRBD provides several advantages over other peptide-based carriers including low
charge density, as well as reversible and pH-dependent non covalent interactions with siRNA. In
the high EGFR expressing cell line, A431, targeted dsRBD fusions can internalize up to 1.3x 106
molecules of siRNA per cell in 6 hours, but successful RNA interference is inhibited by poor
endosomal escape of siRNA. The use of a second agent that contains the cholesterol dependent
cytolysin, perfringolysin 0 enhances endosomal escape of siRNA.
Targeted delivery of
perfringolysin 0 induces gene silencing in A431 cells in a dose-dependent and EGFR-dependent
manner. However, the cytotoxicity of the cytolysin provides a narrow therapeutic window that
must be addressed for this technique to become a viable option for RNAi in vivo.
80
Introduction
The use of short interfering RNA (siRNA) for RNA interference (RNAi) is a powerful
tool that can silence gene expression at the messenger RNA (mRNA) level. siRNA molecules
are double-stranded RNA typically 21-23 base pairs in length, and can be designed for sequence
specificity to potentially any gene target. However, the major barrier to its use in the clinic is the
efficient and specific delivery into the target cell's cytoplasm, where it is loaded into the
RISC/Ago2 complex for sequence-specific mRNA degradation.
The large majority of targeted siRNA delivery efforts employ the use of nanoparticlebased delivery vehicles (1),
with small molecule or macromolecular ligands tethered to the
nanoparticle surface for targeting and internalization.
Although nanoparticle-based delivery
vehicles can carry large siRNA payloads, they suffer from several problems that limit their
efficacy, and that protein-based delivery can potentially solve. Nanoparticle delivery vehicles
suffer from poor pharmacokinetics and biodistribution.
They are rapidly phagocytosed by the
reticuloendothelial system and accumulate in the liver and spleen. They also exhibit poor
extravasation and penetration into solid tumors due to their large size (2). On the other hand,
protein-based systems can be engineered to fall within the window of 60 kDa and 500 kDa,
which would be large enough to avoid rapid renal clearance, yet small enough for efficient
extravasation and avoiding phagocytic clearance. Also, nanoparticle formulations are difficult to
prepare in a reproducible and monodisperse manner.
In contrast, proteins are relatively
straightforward to synthesize using recombinant DNA technology, and can generally be purified
in a straightforward, reproducible manner to monodispersity.
While limited in number, there do exist protein-based delivery vehicles for siRNA that
combine a targeting agent, such as an antibody fragment, with an siRNA complexation agent,
81
usually a short polycationic peptide (3-6). However, these methods have limitations that have
prevented them from reaching the potential of protein-based delivery methods. For example,
they suffer from poor pharmacokinetics and biodistribution, due to high global organ uptake as a
result of their high positive charge (7, 8). They also tend to be inefficient and require large
amounts of siRNA, up to 1IM or more (3).
These agents require complex preparation or
purification schemes, such as protein refolding (6), or chemical conjugation (3). Also, in our
experience, these polycationic peptides are prone to aggregation and are generally poorly
behaved and difficult to work with.
In this work, we propose the use of a two agent protein based siRNA delivery system for
targeted siRNA delivery agent (Figure 4.1). The first agent is a fusion protein containing 3
components: E6, an EGFR-binding variant of the 10th type 3 fibronectin (Fn3) for targeting (9),
mouse IgG2a Fc, and the double stranded RNA binding domain (dsRBD) of human protein
kinase R for siRNA complexation (10). The dsRBD moiety has a low charge density, which
would help avoid the pharmacokinetic limitations of polycationic peptides. Additionally, dsRBD
interacts with the phosphate backbone of siRNA molecules in a double-stranded RNA specific
and a nucleotide sequence independent fashion, thus allowing siRNA molecules for any gene
targeted to be loaded onto the dsRBD moiety. Previous reports describe a weak siRNA binding
affinity of dsRBD, with KD~ 200 nM (11). Therefore, dsRBD was expressed as a fusion with
mouse IgG2a Fc and E6, in order to allow bivalent dsRBD to improve the avidity of siRNA
binding. This construct was termed E6N2. E6N2 was found to complex with siRNA in a pH
dependent manner, and internalize large amounts of siRNA into the endosomes of A431 cells, a
high EGFR expressing cell line.
Although dsRBD has also been used previously for
82
dsRBD
EGFR-binding Fn3
Mouse
IgG2a Fc
C-
PFO
EGFR-binding
Fn3, E6
E6N2
Fn3-PFO
Figure 4.1 Schematic of the two-agent system for siRNA delivery. E6N2 is on the left and binds
siRNA and delivers it to endosomes through EGFR. It contains an N-terminal dsRBD, mouse
IgG2a Fc, and a C-terminal EGFR-binding Fn3 clone, E6. Fn3-PFO is on the right and contains
an EGFR-binding Fn3 clone, and the cholesterol dependent cytolysin PFO for mediating the
endosomal escape of siRNA delivered by E6N2. Fn3-PFO fusions with multiple EGFR-binding
Fn3 clones were constructed and evaluated.
non-targeted siRNA delivery (12, 13), this is the first report of dsRBD as an siRNA carrier for
targeted siRNA delivery.
The second agent is designed to co-internalize with E6N2/siRNA complexes and enhance
the endosomal escape of endocytosed siRNA. It consists of a fusion of one of several EGFRbinding Fn3's and the cholesterol dependent cytolysin (CDC), perfringolysin 0 (PFO). CDC's
bind to cholesterol-containing membranes, oligmerize into pre-pore complexes containing
approximately 50 monomers, and then create pores in plasma membranes up to 50 nm in
diameter (10). Previous work with CDC fusion proteins for endosomal disruption employed an
83
alternative CDC, Listeriolysin 0 (LLO) (14). LLO exhibits a high degree of pH sensitivity, with
over 10 fold difference in lytic activity in hemolysis assays (15), which makes it appear to be an
attractive candidate for endosome- and lysosome-specific membrane disruption activity.
However, it has been shown that this pH dependence is the result of an irreversible degradation
and aggregation of LLO at neutral pH (16), which is consistent with our experience working with
Fn3-LLO fusions as well (data not shown). Native LLO is expressed by Listeria monocytogenes
in acidic endosomal compartments, whereas CDC fusions must maintain endosomolytic function
while at neutral pH prior to endocytosis in order to be useful as drug delivery agents. Due to the
irreversible nature of the degradation and deactivation of membrane disruptive activity at neutral
pH, this makes LLO a less desirable candidate for endosomal disruption. For this reason, a CDC
that is stable at neutral pH such as PFO was chosen for use as an endosomal disruption agent.
When combined with E6N2/siRNA complexes, Fn3-PFO fusions are able to enhance the
endosomal escape of endocytosed siRNA. Gene silencing is dependent on both EGFR-specific
delivery of siRNA by E6N2/siRNA complexes as well as endosomal disruption by Fn3-PFO
fusion proteins. The endosomolytic effects of PFO fusion proteins are dependent on binding of
the Fn3 to EGFR. PFO fusions are potent, with efficient gene silencing at sub-nanomolar
concentrations of PFO and 100 nM E6N2/siRNA.
84
Materials and Methods
Construction and preparation of dsRBD fusions
The dsRBD gene was a kind gift from Dr. James Cole (University of Connecticut).
Genetic fusions containing E6-mouse IgG2a Fc-dsRBD with an N-terminal His tag were
constructed using a modified Quikchange reaction as described by Geiser et al (17) and inserted
into the gWiz vector (Genlantis, San Diego, CA). The Cl21V and C135V mutations, which
were shown not to be important for binding (18), were incorporated into dsRBD using the
Quikchange mutagenesis kit according to the manufacturer's instructions (Agilent, Santa Clara,
CA). E6N2 was expressed in transiently transfected HEK293F cells (Invitrogen, Carlsbad, CA)
for 8 days. E6N2 was purified from the supernatant using a Talon column according to the
manufacturer's instructions (Clontech, Mountain View, CA).
Construction and preparation of Fn3-PFO fusions
Fn3-PFO genetic fusions with a C-terminal His tag and a C215A mutation were
constructed using a modified Quikchange reaction as described by Geiser et al (17) and inserted
into the pmal-c2x vector with a TEV cleavage site immediately downstream of the Factor Xa
site. In total, 4 fusions with EGFR-binding Fn3's (E6-PFO, C-PFO, D-PFO, E-PFO) and one
fusion with a CEA-binding Fn3 (C7-PFO) were constructed.
Fn3-PFO fusion proteins were
transformed into Rosetta 2 (DE3) E. coli (Novagen, San Diego, CA).
Cells were grown to
OD600=0.5-1.0 and induced with 0.5 mM IPTG for 6 hours at 30"C. Resuspended cell pellets
were sonicated and the lysates stored were subjected to purification on an amylose column
according to the manufacturer's instructions (New England Biolabs, Ipswich, MA).
85
The eluate was dialyzed overnight at 4"C into 50 mM Tris pH 8.0, 50 mM NaCl, 1 mM
EDTA, 0.5 mM DTT and TEV protease at a 50:1 (w/w) ratio in a Slide-a-lyzer dialysis cassette
with 3.5 kDa MWCO (Thermo Fisher Scientific, Rockford, IL). The resulting digestion mixture
was clarified with a 0.2 pm filter, and loaded onto a HiTrap
Q ion
exchange column (GE Life
Sciences, Piscataway, NJ) equilibrated with 20 mM phosphate pH 7.0. TEV protease eluted with
the flow through, and with the exception of C-PFO, the Fn3-PFO's eluted as the first peak in a
0-0.5M NaCl gradient over 30 mL. C-PFO co-eluted with MBP and was purified using a Talon
column according to the manufacturer's instructions (Clontech, Mountain View, CA). Fractions
containing Fn3-PFO were pooled and stored at -80"C in single-use aliquots.
Tissue culture
The human epidermoid carcinoma cell line, A43 1, was cultured in a humidified
atmosphere in 5% CO 2 in DMEM supplemented with 10% heat-inactivated fetal bovine serum
and 1% Pen/Strep.
A431 cells stably expressing d2EGFP under the CMV promoter were
generated by transfection of pd2EGFP-N1 (Clontech, Mountain View, CA) using the Amaxa
Nucleofector 2b (Lonza, Germany) according to the manufacturer's instructions. 48 hours after
transfection, 0.75 mg/mL G418 (Invitrogen, Carlsbad, CA) was added to the culture medium.
G418-resistant cells were propagated and the GFP-expressing fraction was sorted twice by FACS
using a Mo-Flo sorter (Cytomation, Carpinteria,
CA).
The resulting cells, termed
A43 1-d2EGFP, were >99% GFP-positive and were cultured using a maintenance G418
concentration of 0.1 mg/mL.
86
Agarose gel shift assay
50 pmol siRNA was mixed with varying amounts of E6N2 for 30 minutes at room
temperature.
The resulting complexes were run on a 2% agarose gel and visualized using
SYBR-Gold (Invitrogen, Carlsbad, CA).
Measurement of KD of dsRBD and siRNA binding
In order to quantify the siRNA binding affinity of the dsRBD portion of E6N2, E6N2 was
loaded onto Protein A Dynal Beads according to the manufacturer's instructions (Invitrogen,
Carlsbad, CA). Typically, 2 pL Protein A beads were used per tube. The Protein A beads were
washed in PBSA and resuspended in DMEM + 10% FBS adjusted to the specified pH with
Alexa 488 labeled AllStars Negative Control siRNA (Qiagen, Valencia, CA) at the specified
concentration at 37"C for 1 hour. The beads were washed twice in ice cold PBSA and analyzed
by flow cytometry on an Accuri C6 flow cytometer (Accuri, Ann Arbor, MI). The KD,app
of
binding was determined as described previously (19).
Measurement of KD of Fn3 and EGFR binding
In order to quantify the EGFR binding affinity of E6N2, A431 cells were mixed with
varying concentrations of E6N2 at 4"C for 6 hours. The cells were washed twice with PBSA,
and stained with Alexa 488 labeled Goat anti Mouse IgG (Invitrogen, Carlsbad, CA) for 20
minutes at 4 0C. The cells were washed twice with PBSA and analyzed by flow cytometry with
the KD of binding determined as described previously (19).
In order to quantify the EGFR binding affinity of E6-PFO, C-PFO, D-PFO and E-PFO,
A431 cells could not be used due to PFO binding to cellular membranes, even at 4'C. Therefore,
87
yeast displaying EGFR ectodomain 404SG were employed (19). The yeast cells were incubated
with varying amounts of Fn3-PFO for 6 hours at 4"C, washed twice with PBSA, then stained
with rabbit anti His antibody (Abcam, Cambridge, MA) labeled with the Alexa 647 Microscale
Labeling Kit (Invitrogen, Carlsbad, CA). The cells were washed twice with PBSA and analyzed
by flow cytometry with the KD of binding determined as described previously (20).
siRNA cell uptake assay
A431 cells were plated in 96 well flat bottom plates and serum starved overnight. Alexa
488 labeled Negative AllStars siRNA was complexed with E6N2 at a 1:1 ratio for 30 minutes at
room temperature. Complexes or free siRNA in the absence of E6N2 were added to the cells at a
100 nM final concentration, in complete media (10% FBS), in the presence or absence of
0.3 mM monensin, or vehicle, 0.3% ethanol. Cells incubated with monensin were additionally
pre-treated with monensin for 20 minutes prior to incubation with E6N2/siRNA complexes. At
each time point, the cells were washed twice with PBS and trypsinized for 20 minutes. Cells
were washed twice with complete media and resuspended in PBS + 2% FBS for analysis by flow
cytometry.
In order to correlate the fluorescence signal with the number of siRNA molecules, a
calibration curve was determined using the Quantum Simply Cellular anti-Mouse beads
according to the manufacturer's instructions (Bangs Laboratories, Fishers, IN), using E6N2
labeled with Alexa 488 at a 6 dye : 1 protein ratio.
88
Fluorescence microscopy
Cells were plated on MatTek chambers with a 0.13 mm glass coverslip bottom (Ashland,
MA), and serum starved overnight. Alexa 488 labeled siRNA was complexed with E6N2 at a
1:1 ratio for 30 minutes at room temperature and added to the cells at a 100 nM final
concentration in complete media. After 6 hours, the cells were washed with complete media, and
then stained with LysoTracker Red (Invitrogen, Carlsbad, CA) and DAPI (Roche Applied
Sciences, Indianapolis, IN) according to the manufacturer's instructions (Invitrogen, Carlsbad,
CA).
Cells were imaged using a Delta Vision fluorescence microscope (Applied Precision,
Issaquah, WA).
Amaxa calibration curve
In order to determine the number of siRNA molecules required for knockdown, Amaxa
electroporation was used as a positive control for cytoplasmic delivery. A43 1-d2EGFP cells or
A431 cells were electroporated according to the manufacturer's instructions with varying
amounts of either GFP-targeting siRNA, or Alexa 488 labeled control siRNA, respectively. To
measure the amount of siRNA delivered, A431 cells with Alexa 488 labeled siRNA were
measured by flow cytometry within 1 hour of electroporation by flow cytometry. The number of
siRNA delivered was determined using a calibration curve generated by the Quantum Simply
Cellular beads, as described above.
The resulting GFP expression levels were measured in
A43 1-d2EGFP cells 24 hours after transfection.
89
GFP knockdown assay
A43 1-d2EGFP cells were plated in 96 well flat bottom plates and serum starved
overnight. siRNA was complexed with E6N2 at a 1:1 ratio for 30 minutes at room temperature.
Complexes or free siRNA in the absence of E6N2 were added to the cells at a 100 nM final
concentration in complete media with varying amounts of Fn3-PFO. At 6 hours, cells were
washed and incubated for 24 hours in complete media. For GFP expression measurements, the
cells were trypsinized, washed twice with PBS + 2% FBS and analyzed by flow cytometry.
Hemolysis assay
Fresh red blood cells were obtained from Fitzgerald Industries (Acton, MA). Red blood
cells were washed twice in PBSA pH 7.5, and once in PBSA adjusted to the pH for the assay,
either 7.5 or 5.5. 50 ptL of a 10% suspension of red blood cells were used per sample. The cells
were then incubated for 45 minutes at 370 C with varying amounts of PFO, or 10% Triton-X 100
as a positive control for lysis. The cells were centrifuged for 4 minutes at 14000g and the
supernatants were measured for hemoglobin release by absorbance at 541 nm.
Cytotoxicity of PFO fusion proteins
Cytotoxicity measurements were performed using the Wst-1 reagent with a 1 hour
incubation according to the manufacturer's instructions (Roche Applied Science, Indianapolis,
IN). They were performed either prior to trypsinization for flow cytometry analysis of GFP
expression in GFP knockdown assays or in separate cytotoxicity assays. When performed prior
to GFP analysis, the cells were washed twice with PBS prior to trypsinization. Alternatively,
cytotoxicity assays were performed in A431 cells using the same treatment as in the GFP
90
knockdown assay up to trypsinization, as described above. The presence of d2EGFP did not
affect Wst-1 reagent performance.
The Wst-1 reagent also did not affect GFP expression
measurements in knockdown assays.
91
Results
Preparation of E6N2
E6N2 was expressed in transient transfections of HEK293F cells, with a single affinity
chromatography purification step. Purification by Protein A or cobalt based resins typically
yielded 1-3 mg protein per liter of cell culture, and the resulting protein ran as a single band on
SDS-PAGE analysis and as a single monomer peak by size exclusion chromatography (data not
shown). Compared to the refolding or chemical conjugation steps of protamine or oligoarginine
fusion constructs (3, 6), dsRBD fusions are well behaved and are relatively straightforward to
purify. The EGFR binding Fn3 moiety also retains high affinity binding to EGFR in the E6N2
construct, with KD~ 2.1 nM (Table 4.1).
Table 4.1 EGFR binding affinity for dsRBD and PFO fusion constructs
Construct
E6N2
E6-PFO
C-PFO
D-PFO
E-PFO
Source of EGFR
A431
YSD 404SG*
YSD 404SG
YSD 404SG
YSD 404SG
KD (nM)
2.1 0.91
4.1 0.5
0.40 0.23
6.0 0.8
0.96 0.25
Error bars indicate a 68% confidence interval
Since E6N2 contains bivalent E6, the reported value is an apparent
EGFR 404SG ectodomain displayed on the surface of yeast
92
KD
Analysis of siRNA / dsRBD interactions
In order to visualize the complexation between the siRNA and E6N2, an agarose gel shift
assay was performed. As shown in Figure 4.2A, a shift in the siRNA band is visible in the
presence of E6N2, with partial complexation at a dsRBD: siRNA ratio of 1.0, based on the
partial disappearance of the free siRNA band.
Complete complexation is observed at a
dsRBD:siRNA ratio of 2, indicating potent complexation between the siRNA and dsRBD.
For a more quantitative measurement of the dsRBD/siRNA binding affinity, titrations of
fluorescently labeled siRNA were performed on Protein A magnetic beads pre-loaded with
E6N2.
The titrations were performed in complete media at 37"C to simulate physiological
conditions.
In our measurements,
KD,app
of siRNA binding to E6N2 was measured to be
3.5+0.2 nM (Figure 4.2B). This represents a significant avidity enhancement from the bivalency
of dsRBD in the E6N2 construct.
pH sensitivity of binding of siRNA and dsRBD
In order for the siRNA cargo to be loaded into the RISC/Ago2 complex in the cytoplasm
for RNAi, it must be able to dissociate from dsRBD. Therefore, we measured the
KD,app Of
binding at pH 7.4, 6.5, and 5.5 to determine if siRNA could dissociate at endosomal or lysosomal
pH once it is internalized. As shown in Figure 4.2B, the
KDapp of
siRNA/E6N2 binding is pH
dependent, with a difference of over 2 orders of magnitude between pH 7.4 and pH 5.5. This
indicates that siRNA will be able to dissociate from dsRBD within the acidic conditions of the
endosome or lysosome.
Secondary staining using (Fab') 2 fragments against mouse Fc was used to detect the
levels of bound E6N2 to Protein A beads after incubation at each pH . There was no detectable
93
difference between the amount of loaded E6N2 after exposure to all pH conditions tested (data
not shown), indicating that the differences in measured
KD,app
is not due to dissociation of E6N2
from the Protein A beads.
A
Free siRNA
E6N2:siRNA
Molar ratio
0
1.2
N
1
0.8
0
0
.0
z
0.25 0.5
-pH
7.4
-pH
6.5
-pH
1
2
4
8
5.5
0.6
0.4
0.2
0
0.1
1
10
100
[siRNA] (nM)
1000
10000
Figure 4.2 Characterization of the interaction between E6N2 and siRNA.
Complexation between E6N2 and siRNA were qualitatively measured in a gel
shift assay at varying molar ratios of E6N2 and siRNA (A). More quantitatively,
the binding affinity was also measured on E6N2-loaded Protein A beads and
fluorescently labeled siRNA (B). The KD,app values of binding and 68%
confidence intervals were measured to be 3.5 ± 0.2 nM at pH 7.4, 11.4 ± 0.9 nM
at pH 6.5, and 925 ± 88 nM at pH 5.5.
94
siRNA uptake by E6N2
Using a modification of the methods of Austin and coworkers (21), the amount of siRNA
taken up by E6N2/siRNA complexes was measured A431 cells.
Initial experiments were
performed using an Alexa 488 binding antibody to quench surface fluorescence. However, both
quenched and unquenched samples exhibited equivalent fluorescence intensities (data not
shown), suggesting that the trypsin treatment used to detach the cells from the well plates was
sufficient to cleave either EGFR or surface-bound E6N2, thus releasing surface-bound siRNA.
Therefore, all subsequent siRNA uptake assays were performed without the Alexa 488
quenching antibody, under the assumption that trypsinization of cells is sufficient to eliminate
surface signal of fluorescent siRNA.
Upon ligand binding to EGFR, the EGFR internalization rate is increased 5-10 fold and
internalized EGFR is degraded (22, 23). However, in the absence of ligand binding, EGFR still
exhibits a high level of constitutive internalization and recycling (22). Recent work suggests that
E6 does not activate EGFR (24), and therefore, internalized E6N2/siRNA complexes may be
recycled back to the cell surface. In order to quantify siRNA uptake for RNA interference, the
total number of siRNA that has been internalized independent of recycling is more relevant than
the net uptake of siRNA. Therefore, to better estimate this number, an inhibitor of EGFR
recycling, monensin, was used to minimize loss of siRNA through recycling.
Using a calibration of fluorescence signal to number of siRNA molecules, it was
determined that 1.3x106 molecules of siRNA is internalized into monensin-treated A431 cells
after 6 hours of treatment with E6N2/siRNA complexes. Approximately 70% of these molecules
are retained in cells not treated with monensin (Figure 4.3A). There is negligible internalization
of siRNA by fluid-phase pinocytosis in the absence of E6N2.
95
A
1.6E+06
CL
4.
Z
1.4E+06
1.2E+06
-+-E6N2/si
1.OE+06
-
8.OE+05
+0.2mM monensin
6.OE+05
--
+0.3%EtOH
4.OE+05
-+-
siRNA only
2.OE+05
0.0E+00
0
2
4
6
8
Time (hours)
B
C
1.2
LL
C
1
0.8
0.6
.
U.
0.4
*ctrl
0.
0.2
*gfp
0
0
1000
10000
100000 1000000
# siRNA internalized per cell
LysoTracker Red
Merge
Figure 4.3 siRNA uptake limitations and requirements. E6N2-mediated uptake of
siRNA is measured in A431 cells in the presence or absence of 0.2 mM monensin or
0.3% ethanol solvent-only control. Uptake of free siRNA not complexed with E6N2
is negligible (A). Error bars represent the standard deviation of triplicate wells. As a
positive control for cytoplasmic delivery of siRNA, gfp or control siRNA is
delievered by Amaxa electroporation in A43 1-d2EGFP cells, in order to determine
the cytoplasmic delivery requirements for GFP knockdown (B). E6N2-mediated
uptake of fluorescently-labeled siRNA is visualized in A431 cells (C). Nuclei are
stained in blue, siRNA is shown in green, late endosomes and lysosomes are stained
red. The large amount of co-localization of siRNA and endosomal and lysosomal
staining indicates that E6N2-delivered siRNA are largely trapped in endosomal and
lysosomal compartments. Scale bar represents 15 pm.
96
Using Amaxa electroporation as a positive control for delivery directly to the cytoplasm,
fewer than 104 molecules of siRNA are required in the cytoplasm for observable knockdown of
GFP protein expression in A431 cells stably transfected with d2EGFP, a destabilized form of
GFP with a 2 hour half-life (Figure 4.3B). However, no GFP knockdown is observed in these
cells with over 106 molecules of gfp siRNA delivered by E6N2 (data not shown). Fluorescence
microscopy reveals that virtually all of the detectable internalized siRNA are trapped within
endosomal and lysosomal compartments (Figure 4.3C). This implies that endosomal escape is
the critical barrier for effective RNAi by siRNA delivered by dsRBD fusion proteins.
PFO fusion proteins for endosomal escape
Four EGFR-binding Fn3 clones were chosen for expression as Fn3-PFO fusion proteins.
Clones C and E bind competitively to EGFR, whereas clone D and E6 (also known as clone A)
do not compete for EGFR binding with each other or with clones C or E. All EGFR-binding Fn3
moieties retain high binding affinity as PFO fusions (Table 4.1). The PFO domain was also
active, as evidenced by hemolysis assays and A431 cytotoxicity assays (Figures 4.4A and 4.4B).
When added to A431-d2EGFP cells along with 100 nM E6N2/siRNA complexes, all Fn3-PFO
fusion proteins induce GFP knockdown in a dose dependent manner, presumably due to
enhancement of endosomal escape of endocytosed siRNA (Figure 4.4C). Reductions in GFP
expression are not due to a global downregulation of cell proteins, or an artifact of PFO
cytotoxicity, because the GFP expression levels are unchanged when negative control siRNA is
delivered (Figure 4.4C). GFP knockdown is also dependent on delivery by E6N2, as free siRNA
cannot induce GFP knockdown in the presence of E6-PFO.
97
Of the various EGFR-binding Fn3-PFO fusions, E6-PFO was the least potent, while CPFO, D-PFO and E-PFO were roughly equivalent in GFP knockdown potency (Figure 4.4D).
The PFO fusion protein with C7, an Fn3 that binds an irrelevant antigen, carcinoembryonic
antigen (CEA), does not result in GFP knockdown (Figure 4.4D). This reveals that the reduction
in GFP protein expression is dependent on Fn3-PFO binding to EGFR.
A
1.2
B
-
1.2-
1
0.8
0.8
L-
0.6
-
Z 0.6
E 0.4
-
.0.4
0.2
-
O
0
U-
00.001
0.01
0.1
1
10
-
0.2
-
00.001
100
0.01
[E6-PFO] (nM)
C
I-
LL
0
0
0.
1
10
100
D
1.6
M
0.1
[E6-PFO] (nM)
1.4
1.4
1.2
1
M
T
'I
4
0.8
0.6
0.4
0.2
-
L-
-4-+-4*
E6N2/siGFP
E6N2/siCtrl
siGFP only
E6N2 only
0
0.1
1
[E6-PFO] (nM)
.2
0
1.2
1
1~
4
-T -*-
E6-PFO
C-PFO
-+
D-PFO
- E-PFO
-+C7-PFO
+
0.8
0.6
x 0.4
o
0.
0.2
Iii
0
U-
U-
a
10
0
0.01
0.1
1
[Fn3-PFO] (nM)
Figure 4.4 Characterization of Fn3-PFO fusion proteins. The membrane disruptive
efficiency of the PFO moiety in E6-PFO was tested in hemolysis assays (A), where
hemoglobin release in mouse red blood cells is used as a measurement of membrane
disruption. Hemolysis was normalized to a value of 0 in untreated cells and 1 in 10%
Triton-X treated cells. Cytotoxicity of a 6 hour pulse of E6-PFO and 100 nM E6N2 was
measured at 24 hours post treatment in A431 cells (B). Here, viability is normalized to a
value of 0 in blank wells without cells and 1 in untreated cells. In GFP knockdown
assays, GFP expression is measured in A43 1-d2EGFP cells 24 hours after a 6 hour pulse
of E6-PFO and either gfp or control siRNA delivered by 100 nM E6N2 or gfp siRNA
alone, or 100 nM E6N2 alone (C). GFP expression is also measured in A431-d2EGFP
cells 24 hours after a 6 hour pulse with 100 nM E6N2/gfp siRNA complexes and varying
amounts of different Fn3-PFO clones (D). In (C) and (D), GFP expression is normalized
to a value of 0 for non-GFP expressing A431 cells and 1 for untreated A431-d2EGFP
cells. Error bars represent the standard deviation of triplicate wells.
98
Discussion
The dsRBD moiety is expressed and purified in a straightforward manner, without any
chemical conjugation required. In our experience, it was not prone to aggregation, presumably
due to its relative low charge density, unlike the highly-charged polycationic peptides used
previously for siRNA complexation (3-6).
The dsRBD moiety also binds reversibly and
specifically to double stranded RNA, and provides protection against siRNA degradation by
RNases (13).
In combination with PFO-fusions, dsRBD fusion proteins deliver enough siRNA
to the cytoplasm for potent gene silencing; 1OOnM siRNA is typically used in this system, as
opposed to concentrations up to 1p M or more using polyarginine as an siRNA carrier (3). These
properties make dsRBD fusion proteins an attractive option to other peptide based methods for
complexing siRNA.
PFO fusion proteins are effective at enhancing endosomal escape of siRNA delivered by
dsRBD fusions. They also induce GFP knockdown in a dose dependent manner. The decreased
potency of E6-PFO relative to C-PFO, D-PFO and E-PFO is consistent with the fact that E6-PFO
must compete with E6N2 for EGFR binding, whereas Fn3 clones C, D and E do not compete
with E6 (24). Despite the fact that E6-PFO is the least potent of the EGFR-binding Fn3-PFO
constructs tested, the fact that it is still active at sub-nanomolar concentrations in the presence of
1OOnM E6N2 is intriguing. Given that the measured KD of EGFR binding is 4.1 +0.5 nM, this
raises the question as to whether E6-PFO is co-localizing to siRNA-containing endosomes by a
non-specific fluid phase pinocytic uptake.
At sub-nanomolar concentrations of E6-PFO, any
fluid phase uptake is likely negligible; based on a conservative estimate of a typical endosome
radius of 100 nm and a perfect mixing assumption, there is less than one molecule present on
average within the volume of a spherical endosome, based on first principle calculations. The
99
negligible role of fluid phase pinocytic uptake is confirmed experimentally as well. C7-PFO,
which binds to the irrelevant antigen CEA, does not induce GFP knockdown in A43 1-d2EGFP
cells despite an active PFO moiety, indicating that PFO-mediated endosomal escape is dependent
on binding to EGFR.
When comparing the efficacy of GFP knockdown to the cytotoxicity of PFO fusion
proteins, a narrow therapeutic window is revealed for all EGFR-binding Fn3 clones tested.
Although there is added complexity arising from the use of two agents, the large difference in
effective concentrations for each agent requires the use of both agents separately, as opposed to a
single agent containing both functions in the form of a fusion protein. Nevertheless, in order for
this method to be useful in a therapeutic setting, it will be important to expand this therapeutic
window. This can be achieved either by increasing the potency of RNAi, or by reducing the
cytotoxicity of the PFO fusions.
For a given target for RNAi, any improvement in RNAi potency would be the result of
increasing the number of siRNA molecules that are delivered to the cytoplasm. This would
require an increase in either the number of siRNA molecules that are internalized per endosome,
or the proportion of endosomal compartments that are disrupted per cell. This can be achieved
by a number of different methods. First, EGFR clustering induced by multi-specific binders can
create a concentrating effect of EGFR when endocytosed (25).
This would allow for more
E6N2/siRNA complexes or Fn3-PFO fusions to be endocytosed, allowing for a higher
concentration of siRNA within each endosome, as well as more potent endosomal disruption.
Second, the cytotoxicity of PFO requires the use of Fn3-PFO concentrations that do not saturate
EGFR. Therefore, affinity improvements in the EGFR binding Fn3 would allow for more Fn3PFO to be endocytosed, thus improving the efficiency of endosomal escape.
100
Third, the
interaction of dsRBD and siRNA is non-covalent, with a measured
KD,app~
3.5 nM. Assuming a
typical association rate for a protein binder, the dissociation half-life is estimated to be on the
order of several hours, and this is consistent with the 3.7 hour dissociation half-life measured for
another Fc-dsRBD fusion protein (Appendix A). As a result, over the course of treatment with
E6N2/siRNA complexes, a significant amount of siRNA can dissociate from E6N2, thus
reducing the efficiency of siRNA internalization. Therefore, this loss in siRNA internalization
can be reduced through the affinity maturation of the dsRBD/siRNA interaction by directed
evolution of dsRBD.
The second general approach to expanding the therapeutic window involves decreasing
the cytotoxicity of the PFO fusions. This approach is based on the assumption that PFO-based
cytotoxicity is caused by disruption of the plasma membrane of the cell, as opposed to disruption
of endosomal compartments. Several lines of evidence support this assumption. First, when
comparing the therapeutic windows of PFO fusions with different Fn3 clones, the variation arises
primarily in the potency of GFP knockdown, while cytotoxicity profiles are nearly equivalent.
Thus, the lack of correlation between cytotoxicity and degree of endosomal disruption as
determined by GFP knockdown potency indicates that endosomal disruption is likely not the
primary cause of cytotoxicity. When comparing the cytotoxicity potency of PFO fusions on
A431 cells to the potency of membrane disruption of mouse red blood cells which do not express
EGFR, the cytotoxicity profiles are very similar.
The potency is also consistent with the
cytotoxicity seen in CEA-binding C7-PFO, indicating that cytotoxicity is not dependent on
EGFR. These observations suggest that plasma membrane disruption is likely the cause of Fn3PFO mediated cytotoxicity.
101
Hemolysis assays indicate that Fn3-PFO exhibits a low degree of pH-sensitive cytolytic
activity, but this pH dependence can be enhanced for reduced cytotoxicity. Specifically, in order
to reduce the cytotoxicity of PFO, while maintaining GFP potency, the cytolytic activity of PFO
must be decreased at physiological pH, while maintained at endosomal pH.
This may be
possible through the use of protein engineering, for example, through directed evolution of PFO.
Since cholesterol-dependent membrane binding is the first step to creating the pre-pore complex
in membrane disruption (10), it is possible that cholesterol binding could be used as a proxy for
cytolytic activity, using yeast surface display (26).
Another approach that is currently being
explored is through the use of engineered pH-sensitive Fn3 that bind PFO such that the epitope
of membrane binding is obscured by the bound Fn3 at neutral pH, but unobscured when the Fn3
dissociates at endosomal pH.
Of the approaches described above to expand the therapeutic window of PFO-mediated
RNAi, two are currently being explored. First is the use of EGFR clustering using multiepitopic
antibody-Fn3 fusion proteins. The second is the engineering of pH sensitive Fn3 binders to PFO.
These efforts are described in more detail in the following chapter and in Appendix B.
102
Acknowledgements
We thank Dr. James Cole (University of Connecticut) for providing the gene encoding
dsRBD, and Dr. Jacqueline Lees (Massachusetts Institute of Technology) for providing the
pd2EGFP-N 1 plasmid. We thank Dr. Frank Gertler for our use of the Amaxa electroporator, and
the Koch Institute Flow Cytometry Core at MIT for technical assistance with FACS. We thank
Chris Pirie and Nicole Yang for useful discussions.
103
References
1. Jarvis L. Delivering the promise. Chem Eng News. 87: 18-27 (2009).
2. Schmidt MM and Wittrup KD. A modeling analysis of the effects of molecular size and
binding affinity on tumor targeting. Mol Cancer Ther. 8: 2861-71 (2009).
3. Kumar P et al. T cell-specific siRNA delivery suppresses HIV-1 infection in humanized
mice. Cell. 134: 577-86 (2008).
4. Song E et al. Antibody mediated in vivo delivery of small interfering RNAs via cellsurface receptors. Nat Biotechnol. 23: 709-17 (2005).
5. Peer D, Zhu P, Carman CV et al. Selective gene silencing in activated leukocytesby
targeting siRNAs to the integrin lymphocyte function-associated antigen-1. PNAS.
104:4095-4100 (2007).
6. Winkler J, Martin-Killias P, Pluckthun A, et al. EpCAM-targeted delivery of
nanocomplexed siRNA to tumor cells with designed ankyrin repeat proteins. Mol Canc
Ther. 8:2674-83 (2009).
7. Niesner U et al. Quantitation of the tumor-targeting properties of antibody fragments
conjugated to cell-permeating HIV-1 TAT peptides. Bioconjug Chem. 13: 729-36 (2002).
8. Lee HJ and Pardridge WM. Pharmacokinetics and delivery of tat and tat-protein
conjugates to tissues in vivo. Bioconjug Cheml2: 995-9 (2001).
9. Hackel BJ, Ackerman ME, Howland SW, et al. Stability and CDR composition biases
enrich binder functionality landscape. J Mol Bio. 401: 84-96 (2010).
10. Rosado CJ, Kondos S, Bull TE, et al. The MACPF/CDC family of pore-forming toxins.
Cell Microbiol. 10: 1765-1774 (2008).
11. Bevilacqua PC and Cech TR. Minor-Groove Recognition of Double-Stranded RNA by
the Double-Stranded RNA-Binding Domain from the RNA-Activated Protein Kinase
PKR. Biochemistry. 35: 9983-9994 (1996).
12. Eguchi A, Meade BR, Chang YC, et al. Efficient siRNA delivery into primary cells by a
protein transduction domain-dsRNA binding domain fusion protein. Nat Biotechnol. 27:
567-571 (2009).
13. Kim J, Lee SH, Choe J, et al. Intracellular small interfering RNA delivery using
genetically engineered double-stranded RNA binding protein domain. J Gene Med. 11:
804-12 (2009).
14. Pirie CM, Liu DV and Wittrup KD. Independently targeted cytolysin and gelonin
immunotoxins combine to exhibit mechanistic synergy in vitro and in vivo, submitted.
15. Decatur AL, Portnoy DA. A PEST-like sequence in listeriolysin 0 essential for Listeria
monocytogenes pathogenicity. Science. 290: 992-995 (2000).
16. Schuerch DW, Wilson-Kubalek EM and Tweten RK. Molecular basis of listeriolysin 0
pH dependence. Proc Natl Acad Sci USA. 102: 12537-12542 (2005).
17. Geiser M, Cebe Regis, Drewello D, et al. Integration of PCR fragments at any specific
site within cloning vectors without the use of restriction enzymes and DNA ligase.
Biotechniques. 1: 88-92 (2001).
18. Spanggord RJ and Beal PA. Site specific modification and RNA crosslinking of the
RNA-binding domain of PKR. Nucl Acid Res. 28: 1899-1905 (2000).
19. Chao G, Cochran JR and Wittrup KD. Fine epitope mapping of anti-epidermal growth
factor receptor antibodies through random mutagenesis and yeast surface display. J Mol
Biol. 342: 539-50 (2004).
104
20. Liu DV, Maier LM, Hafler DA, et al. Engineered interleukin-2 antagonists for the
inhibition of regulatory T cells. J Immunother. 32: 887-94 (2009).
21. Austin CD, de Maziere AM, Pisacane PI, et al. Endocytosis and sorting of ErbB2 and the
site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell. 15:
5268-82 (2004).
22. Wiley HS. Traffickign of the ErbB receptors and its influence on signaling. Exp Cell
Res. 284: 78-88 (2003).
ErbB-2 amplification inhibits
23. Worthylake R, Opresko LK and Wiley HS.
downregulation and induces constitutive activation of both ErbB-2 and epidermal growth
factor receptors. J Biol Chem. 274: 8865-8874 (1999).
24. Hackel BJ. Fibronectin Domain Engineering. MIT PhD Thesis (2009).
25. Spangler JB, Manzari MT, Rosalia EK, et al. Triepitopic antibodies inhibit cetuximabresistant BRAF- and KRAS-mutant tumors via EGFR signal repression, submitted
(2011).
26. Chao G, Lau WL, Hackel BJ, et al. Isolating and engineering human antibodies using
yeast surface display. Nat Protoc. 1:755-768 (2006).
105
Chapter 5: EGFR clustering for the enhancement
of targeted siRNA delivery
Abstract
The double stranded RNA binding domain (dsRBD) can be used as an siRNA carrier in
targeted fusion proteins for the delivery of siRNA to endosomal compartments. A second agent
that contains the cholesterol dependent cytolysin, perfringolysin 0 (PFO) can be delivered in a
targeted manner to enhance endosomal disruption and allow internalized siRNA to access the
cytoplasm. However, this method is limited by the cytotoxicity of the PFO, which results in a
relatively narrow therapeutic window.
Here we present the proof of principle that EGFR
crosslinking with the use of multiepitopic EGFR binders can widen the therapeutic window of
dsRBD- and PFO-based gene silencing. EGFR clustering increases the EGFR internalization
rate and creates a concentrating effect due to multiple clustered EGFR molecules simultaneously
internalizing into the same endosome.
This leads to an enhancement in siRNA uptake of
approximately 2.4 fold and a 3-4 fold improvement in gene silencing potency. Interestingly,
multiepitopic binders also protect against the cytotoxicity of targeted PFO fusions.
This
significantly widens the therapeutic window, with approximately a 100 fold difference in the half
maximal lethal concentration and the half maximal effective concentration of targeted PFO
fusions for gene silencing.
106
Introduction
The use of protein based delivery systems for targeted siRNA delivery has the potential
to solve some of the problems faced by nanoparticle-based delivery systems that are more
commonly used, such as poor pharmacokinetics, biodistribution, tumor penetration, and
monodispersity (1). The use of proteins for targeted siRNA delivery have primarily focused on
polycationic peptides as siRNA carriers conjugated to macromolecular targeting agents.
However, these methods have not lived up to the potential of protein-based vehicles, because of
their complex purification and preparation schemes, poor biodistribution and pharmacokinetics
(2-4), which are largely due to the high positive charge density of the peptide carriers.
Previously, we showed that the use of E6N2, a fusion protein containing the double
stranded RNA binding domain (dsRBD) of human protein kinase R, can be used to noncovalently complex siRNA with high affinity
(KD,app=
3.5 nM) and mediate siRNA uptake into
endosomes through targeted EGFR binding. The protein-based nature of this approach, and the
low charge density of dsRBD have the potential of solving some of the problems with
nanoparticle and polycationic peptide vehicles. Endosomal escape of siRNA was mediated with
a second agent delivered in trans, a fusion protein containing EGFR-binding Fn3 and the
cholesterol dependent cytolysin, perfringolysin 0 (PFO) (5).
When comparing the cytotoxicity of Fn3-PFO fusions and the potency of GFP
knockdown, the therapeutic window is relatively narrow. In this work, we investigate the use of
EGFR clustering to improve the potency of PFO-mediated GFP knockdown. Fusion proteins
containing binders to unique epitopes of EGFR can cross link and cluster multiple EGFR
molecules, which enhances the EGFR internalization rate due to multiple clustered EGFR
entering the cell simultaneously (6, 7). This allows for more siRNA to be internalized per
107
endosome, and therefore, more siRNA released into the cytoplasm per endosome that is
disrupted.
The multispecific constructs used in this work have been described in detail previously
(7), but briefly, multiple EGFR-binding Fn3 clones that bind to distinct epitopes of EGFR are
expressed as fusions with the anti EGFR IgG C225, or cetuximab. Two tri-specifics, with Fn3
clones on the heavy chain N terminus and the light chain C terminus of C225, and one tetra
specific, with Fn3 clones on the heavy chain N and C termini, and the light chain C terminus of
C225, were evaluated for enhancement of E6N2-mediated siRNA uptake.
Of the three multispecifics tested, the tri-specific HNB-LCD most effectively enhanced
siRNA uptake, by approximately 2.4 fold.
Similar enhancements were observed in GFP
knockdown potency of siRNA delivered by E6N2, when combined with either D-PFO or E6PFO. Interestingly, HNB-LCD also reduces the cytotoxicity of EGFR-binding Fn3-PFO fusion
proteins by approximately 3-4 fold. The exact mechanism for this protective effect is unknown
but was shown to be dependent on EGFR binding of the Fn3-PFO fusion protein. Eventually,
EGFR clustering capability will be incorporated into the dsRBD fusion construct or the PFO
construct in order to eliminate the need of a third agent for siRNA delivery. This work provides
an interesting proof of principle for the use of EGFR clustering to expand the therapeutic
window by simultaneously enhancing GFP knockdown potency and reducing the cytotoxicity of
Fn3-PFO.
108
Materials and Methods
Protein expression and purification
The expression and purification for the following proteins have been described
previously. E6N2 was expressed in transient transfections of HEK293F cells, while E6-PFO, CPFO, D-PFO and E-PFO was expressed in E. coli. Expression and purification protocols are
described in Chapter 4. HNB-LCD, HND-LCA, HNB-HCA-LCD was expressed in transient
transfections of HEK293F cells, and is described by Spangler et al (7).
siRNA uptake assay
A431 cells were plated in 96 well flat bottom plates and serum starved overnight. Alexa
488 labeled Negative AllStars siRNA was complexed with E6N2 at a 1:1 ratio for 30 minutes at
room temperature.
Complexes were added to the cells at a 100 nM final concentration, in
complete media (10% FBS), in the presence or absence of varying amounts of HNB-LCD, HNDLCA, or HNB-HCA-LCD for 6 hours. Cells were washed twice with PBS and trypsinized for 20
minutes. Cells were washed twice with complete media and resuspended in PBS + 2% FBS for
analysis by flow cytometry using an Accuri C6 flow cytometer (Accuri, Ann Arbor, MI).
GFP knockdown assay
A43 1-d2EGFP cells were plated in 96 well flat bottom plates and serum starved
overnight. siRNA was complexed with E6N2 at a 1:1 ratio for 30 minutes at room temperature.
siRNA complexes and HNB-LCD were added to the cells at a final concentration of 100 nM and
7.5 nM, respectively, with varying amounts of Fn3-PFO. At 6 hours, cells were washed and
109
incubated for 24 hours in complete media. For GFP expression measurements, the cells were
trypsinized, washed twice with PBS + 2% FBS and analyzed by flow cytometry.
Cytotoxicity measurements
Cytotoxicity measurements were performed using the Wst-1 reagent with a 1 hour
incubation according to the manufacturer's instructions (Roche Applied Science, Indianapolis,
IN).
They were performed either prior to trypsinization for flow cytometry analysis of GFP
expression in GFP knockdown assays or in separate cytotoxicity assays. When performed prior
to GFP analysis, the cells were washed twice with PBS prior to trypsinization. Alternatively,
cytotoxicity assays were performed in A431 cells using the same treatment as in the GFP
knockdown assay up to trypsinization, as described above. The presence of d2EGFP did not
affect Wst-1 reagent performance.
The Wst-1 reagent also did not affect GFP expression
measurements in knockdown assays
110
Results
Enhancement of siRNA uptake by multispecific constructs
Three different multispecific antibody-Fn3 fusion proteins were evaluated for their ability
to enhance the uptake of Alexa 488 labeled siRNA complexed with E6N2. The potency of the
multispecific constructs for EGFR clustering and downregulation are summarized in Table 5.1.
Compared to the tri-specific HND-LCA, and the tetra-specific HNB-HCA-LCD, the tri-specific
HNB-LCD provided the greatest enhancement in E6N2-mediated siRNA, despite the fact that
HNB-LCD induces the least amount of EGFR clustering and downregulation of the three in
A431 cells (7).
HNB-LCD provides a -2.4 fold improvement in uptake with a broad
concentration optimum, whereas HND-LCA and HNB-HCA-LCD provide less than a 2 fold
improvement, with a narrower concentration optimum (Figure .5.1, Table 5.1). Due to the wide
concentration optimum and the improved enhancement of siRNA uptake, HNB-LCD was used
for siRNA uptake enhancement for all following experiments.
3.5E+06
2.0E+06
1.5E+06
!
U
---
HND-LCA
HNB-LCD
HNB-HCA-LCD
-+------- E6N2/si only
1.OE+06
--
5.OE+05
1
10
100
[Multispecific] (nM)
Figure 5.1 Enhancement of siRNA uptake by multispecific constructs. Three
multispecific constructs are evaluated for their ability to enhance siRNA uptake by
100 nM E6N2. Baseline uptake by 100 nM E6N2/siRNA complexes in the absence
of any multispecific construct in A431 cells after 6 hours is shown as a dotted line.
Error bars represent the standard deviation of triplicate wells.
I1
Table 5.1 Summary of multispecific constructs evaluated
Construct
EGFR downregulation
induced in A431 cellst
Peak fold enhancement of
siRNA uptake
HND-LCA
HNB-LCD
HNB-HCA-LCD
50%
30%
80%
1.8
1.9
2.4
Degree of induced EGFR downregulation was determined in reference (7).
Enhancement of GFP knockdown by multispecific constructs
Next, HNB-LCD was tested to see if the enhancement in siRNA uptake could result in
Of the EGFR-binding clones identified and
enhanced knockdown of GFP expression.
characterized, all bind to an epitope on EGFR that competes with either E6N2 or HNB-LCD.
Therefore, PFO fusion proteins with clones E6, C, D and E were all tested for their ability to
enhance endosomal escape and induce GFP knockdown in the presence of E6N2 and HNB-LCD.
As shown in Figure 5.2, E6-PFO and D-PFO showed enhancements in GFP knockdown
in the presence of 7.5 nM HNB-LCD, whereas E-PFO showed no change, and C-PFO showed a
worsening in GFP knockdown. Between E6-PFO and D-PFO, GFP knockdown by D-PFO was
enhanced to a larger degree by HNB-LCD.
This makes the combination of HNB-LCD and D-
PFO the best combination of constructs for the delivery of siRNA by E6N2 to the cytoplasm of
A431 cells.
In the presence of 7.5 nM and 100 nM E6N2/siRNA complexes, D-PFO has an
EC 50 < 15 pM for GFP knockdown (Figure 5.2).
112
Cytotoxicity profiles of Fn3-PFO in the presence of HNB-LCD
It was originally hypothesized that the addition of a multi-epitopic EGFR binder would
only enhance GFP knockdown through clustering, without any effect on PFO-related
cytotoxicity. However, when the cytotoxicity profiles were measured for the various Fn3-PFO
fusion proteins, it was found that the presence of HNB-LCD had a protective effect on A431
cells. This effect was consistent across all EGFR-binding Fn3-PFO constructs, and provided an
effective 3-4 fold reduction in Fn3-PFO cytotoxicity (Figure 5.2).
When the cytotoxicity of
CEA-binding C7-PFO was measured in A431 cells, there was no difference in the presence or
absence of HNB-LCD. This indicates that the reduction in Fn3-PFO cytotoxicity by HNB-LCD
requires EGFR binding by the Fn3-PFO construct.
113
1.2
1.2
1 ---
1
-
0.8
0.6
0.8
U.
0.4
0.2
-
00.01
LI".
0.1
1
[E6-PFO] (nM)
-
0.4 0.2 -
-
10
0.01
1.2
1.2
1
1
0.8
*
0
0.6
U-
~0.6
-
-
0
0.1
1
[C-PFO] (nM)
0.8
0.6
I-
U.
0.4
0.2
0
0.01
,
0.1
1
[D-PFO] (nM)
1.4
10
0.4
-
0.2
-
00.01
0.1
1
[E-PFO] (nM)
10
-
1.6
--
1.2
0
10
*
1
- -+
Viability (No HNB-LCD)
- Viability (+HNB-LCD)
0.8
U.
0.6
-+-
0.4
--
GFP Expression (No HNB-LCD)
- GFP Expression (+HNB-LCD)
0.2
0
0.01
0.1
1
10
[C7-PFO] (nM)
Figure 5.2 Effects of HNB-LCD on the therapeutic window of E6N2/Fn3-PFO based
siRNA delivery. In order to visualize the therapeutic windows of E6N2-mediated siRNA
delivery in the presence of various Fn3-PFO clones, GFP expression in A43 1-d2EGFP cells
is overlayed with viability curves. In all experiments, 100 nM E6N2 / gfp siRNA
complexes, and Fn3-PFO are pulsed for 6 hours with or without 7.5 nM HNB-LCD. GFP
expression or viability was measured 24 hours after treatment. Five Fn3-PFO clones were
evaluated, four that bind EGFR (E6-PFO, C-PFO, D-PFO and E-PFO) and one that binds
the irrelevant antigen CEA (C7-FO). GFP expression was normalized to a value of 0 for
non-GFP expressing A431 cells and 1 for untreated A431-d2EGFP cell fluorescence.
Viability was normalized to a value of 0 for blank wells and 1 for untreated cells. Error bars
represent the standard deviation of triplicate wells.
114
Discussion
The degree of EGFR downregulation correlates to the degree of EGFR clustering induced
by antibodies or antibody-Fn3 fusion proteins (6-8).
EGFR clustering has been shown to
enhance the rate of EGFR internalization through the internalization of multiple clustered EGFR
molecules, which we hypothesized would in turn enhance EGFR-mediated siRNA uptake (7).
Therefore, based on downregulation efficiency alone, the tetraspecific IINB-HCA-LCD should
be most potent, while HNB-LCD should be the least potent. However, in siRNA uptake assays,
HNB-LCD provided the greatest enhancement of siRNA uptake. This is most likely due to the
fact that HNB-LCD is the only multispecific construct tested that does not compete with E6N2,
since clone A and E6 refer to the same EGFR-binding Fn3 clone (9).
The slight decrease in
enhancement at 40-100 nM HNB-LCD is likely due to monovalent binding. Monovalent binding
cannot be ruled out as a mechanism for decreases in uptake enhancement by 40-100 nM HNDLCA and HNB-HCA-LCD.
However, the fact that the decrease is more severe compared to
HNB-LCD suggests that competition is likely an important factor at the concentrations tested.
The use of multispecific antibody-Fn3 fusion constructs was motivated by the prospect of
enhancing RNAi potency by inducing EGFR clustering and increasing the number of EGFR
internalized per endosomal compartment.
This use of multispecific constructs was not
hypothesized to have any effect on PFO-mediated cytotoxicity. The observation that HNB-LCD
can reduce the cytotoxicity of EGFR-binding Fn3-PFO constructs was indeed surprising,
especially since EGFR downregulation induced by clustering has been shown to reduce viability
(7).
The exact mechanism for this protective effect has yet to be elucidated. Initially, it was
hypothesized that the downregulation induced by HNB-LCD resulted from decreased exposure
to epidermal growth factor from FBS in the media, which has been shown to inhibit growth in
115
A431 cells (10). If this were the case, the protective effect by HNB-LCD would be independent
of the Fn3-PFO construct used. However, when using the CEA-binding C7-PFO, no difference
in cytotoxicity is observed in the presence or absence of HNB-LCD. This indicates that HNBLCD can only reduce the cytotoxicity of EGFR-binding Fn3-PFO constructs.
When inducing EGFR downregulation with HNB-LCD, EGFR-bound Fn3-PFO will
likely be co-internalized. At the Fn3-PFO concentrations used, the number of EGFR molecules
and Fn3-PFO molecules is the same order of magnitude, and thus, it is possible that increasing
the EGFR internalization rate by receptor clustering would more rapidly deplete extracellular
Fn3-PFO. Combining this with the assumption that Fn3-PFO cytotoxicity arises from plasma
membrane disruption, it is possible that the reduction in cytotoxicity from EGFR clustering and
downregulation induced by HNB-LCD is due to decreased extracellular Fn3-PFO available for
plasma membrane disruption.
The addition of 7.5 nM HNB-LCD to 100 nM E6N2/siRNA and D-PFO significantly
expands the therapeutic window, with approximately 2 orders of magnitude difference in the half
maximal lethal dose of D-PFO and the half maximal effective dose of D-PFO for GFP
knockdown. This arises from both the enhancement of GFP knockdown and the decrease in DPFO toxicity by HNB-LCD.
As a therapeutic modality, a third agent would significantly
increases the complexity of treatment, and therefore the final goal is to combine multispecific
binding with one or both of the siRNA delivery agent and endosomal disruption agent. Efforts
are currently underway to make these constructs.
As this system is characterized in vivo, it will be interesting to see how the
pharmacokinetic, biodistribution and tumor penetration properties compare to those of
nanoparticle based delivery systems.
Another important hurdle will be immunogenicity,
116
especially from PFO, a bacterial protein.
Bacterial CDC's with homologous structures have
been shown to induce immunogenicity (11, 12).
However, perforin, a human protein which
mediates the cytosolic delivery of granzyme B, has high structural homology to bacterial CDC's.
Perforin can mediate the endosomal escape of granzyme B (13), and has the potential to be used
as a non-immunogenic alternative to PFO (13).
117
Acknowledgements
Michael Santos and Dr. Jamie Spangler provided HNB-LCD, HND-LCA and HNBHCA-LCD proteins. We thank Nicole Yang and Chris Pirie for useful discussions.
118
References
1. Schmidt MM and Wittrup KD. A modeling analysis of the effects of molecular size and
binding affinity on tumor targeting. Mol Cancer Ther. 8: 2861-71 (2009).
2. Niesner U et al. Quantitation of the tumor-targeting properties of antibody fragments
conjugated to cell-permeating HIV- 1 TAT peptides. Bioconjug Chem. 13: 729-36 (2002).
3. Lee HJ and Pardridge WM. Pharmacokinetics and delivery of tat and tat-protein
conjugates to tissues in vivo. Bioconjug Cheml2: 995-9 (2001).
4. Winkler J, Martin-Killias P, Pluckthun A, et al. EpCAM-targeted delivery of
nanocomplexed siRNA to tumor cells with designed ankyrin repeat proteins. Mol Canc
Ther. 8:2674-83 (2009).
5. Rosado CJ, Kondos S, Bull TE, et al. The MACPF/CDC family of pore-forming toxins.
Cell Microbiol. 10: 1765-1774 (2008).
6. Friedman LM, Rinon A, Schechter B, et al. Synergistic down-regulation of receptor
tyrosine kinases by combinations of mAbs: Implications for cancer immunotherapy. Proc
Natl Acad USA. 102: 1915-1920 (2005).
7. Spangler JB, Manzari MT, Rosalia EK, et al. Triepitopic antibodies inhibit cetuximabresistant BRAF- and KRAS-mutant tumors via EGFR signal repression, submitted
(2011).
8. Spangler JB, Neil JR, Abramovitch S, et al. Combination antibody treatment downregulates epidermal growth factor receptor by inhibiting endosomal recycling. Proc Natl
Acad USA. 107: 13253-7 (2010).
9. Hackel BJ. Fibronectin domain engineering. MIT PhD Thesis (2009).
10. Barnes DW. Epidermal growth factor inhibits growth of A431 human epidermoid
carcinoma in serum-free cell culture. J Cell Biol. 93:1-4 (1982).
11. Barry RA, Bouwer A, Portnoy DA, et al. Pathogenicity and immunogenicity of Listeria
monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell
spread. Infect Immun. 60:1625-1632 (1992).
12. Umeda M, Tomita T, Shibata H, et al. Homogeneous liposome lysis assay for
determination of anti-streptolysin 0 antibody titer in serum. J Clin Microbiol. 26:804807 (1998).
13. Thiery J, Keefe D, Boulant S, et al. Perforin pores in the endosomal membrane trigger the
release of endocytosed granzyme B into the cytosol of target cells. Nat Immunol. 12:
770-777 (2011).
119
Appendix A: Delivery of siRNA using targeted dsRBD fusions
to CD25 and CEA expressing cells
In addition to E6N2 for EGFR-targeted siRNA delivery, constructs for targeted delivery
to CD25 and CEA expressing cells were also made (Figure A. 1). N2-IL2 contains an N-terminal
dsRBD, a mouse IgG2a Fe fragment, and a C-terminal weak IL-2 agonist, D34W, which is
described in Chapter 3. sm3e-dsRBD consists of the CEA-binding human IgG1 sm3e with
dsRBD on the C terminus of the heavy chain. Both constructs were expressed in transient
transfections of HEK293F cells and purified using a Talon column.
dsRBD
*-
S
#.
Anti-CEA human
IgG 1, sm3e
Mouse
IgG2a Fc
-
Mouse IL2
dsRBD
variant, D34W
N2-1L2
sm3e-dsRBD
Figure A.1 Protein construct topologies explored for dsRBD based targeted delivery
of siRNA. Left: N2-IL2 is a fusion protein containing dsRBD, mouse IgG2a Fc, and
the mouse IL-2 variant D34W for targeted siRNA delivery to CD25 expressing cells.
Right: sm3e-dSRBD is a fusion protein containing antiCEA IgG sm3e and dsRBD
for targeted delivery to CEA expressing cells.
120
The siRNA complexation properties of N2-IL2 and sm3e-dsRBD were determined to be
similar to those of E6N2.
N2-IL2 and sm3e-dsRBD complex efficiently with siRNA, as
determined by an agarose gel shift assay, with partial complexation at a 1:1 stoichiometric ratio
and nearly complete complexation at 2:1 (Figure A.2A). The KD,app
of
binding for both N2-IL2
and sm3e-dsRBD are comparable to that of E6N2, in the single-digit nanomolar range (Figure
A.2B, A.2C).
Like E6N2, the siRNA binding affinity of N2-IL2 is also pH dependent, with
significant loss of affinity at pH 5.5 (Figure A.2B).
A
N2-IL2
sm3e-dsRBD
Complexed
siRNA
Free siRNA
Protein:siRNA
0 0.25 0.5
1
2
4
8
8
0 0.25 0.5 1 2
4
8
8
molar ratio
B
1.2
Cl
-pH
E 1 - -pH
-
7.4
1.4
1.2 -
6.5
.
1
pH 5.5
/ 4.88
00.8
-
0.6
<
00.6
0 .4
0.4
0.2
L0.2 -
0
0
0.1
10
0.1
1000
10
1000
[siRNA] (nM)
[siRNA] (nM)
Figure A.2 Characterization of N2-IL2 and sm3e-dsRBD interactions with siRNA.
The interaction between siRNA and either N2-IL2 or sm3e-dsRBD is shown
qualitatively in gel shift assays (A).
The KD,app values were also determined in
Protein A beads loaded with either N2-IL2 or sm3e-dsRBD.
For N2-IL2 (B), the
KD,app values were determined to be 8.5 ± 1.5 nM at pH 7.4, 31.5 ± 5.1 nM at pH 6.5
and > 1 pM at pH 5.5. For sm3e-dsRBD, the KD,app at pH 7.4 was determined to be
2.8 ± 0.6 nM. Error bars represent a 68% confidence interval.
121
For siRNA uptake assays, we used the CD25 expressing CTLL-2 cell line for N2-IL2
mediated delivery, and the CEA expressing HT-CEA cell line for sm3e-dsRBD mediated
delivery of Alexa-488 siRNA. N2-IL2 and sm3e-dsRBD delivered approximately 5x10 5 and
2x106 siRNA molecules after a 6-8 hour incubation with lOOnM N2-IL2/siRNA or sm3edsRBD/siRNA complexes, respectively (Figure A.3A, A.3B). For the adherent HT-CEA cell
line, trypsinization was found to sufficiently eliminate surface signal, as determined by
comparing samples that were treated with an Alexa 488 quenching antibody (Invitrogen,
Carlsbad, CA), and those that were not treated. For uptake assays with the CTLL-2 cells, the
surface and internalized siRNA signal was determined by splitting cells from each measurement
and quenching surface Alexa-488 signal. Surface and internalized contributions to the total
Alexa 488 fluorescence signal were determined using the calculations described previously (1).
The surface signal on CTLL-2 cells decays over time (Figure A.3C). CTLL-2 uptake
assays are typically performed with over 10 fold excess of N2-IL2 molecules over CD25
molecules. Based on the net siRNA uptake rate measured in CTLL-2 cells (A.3A), extracellular
N2-IL2 is not depleted, indicating that CD25 is saturated with bound N2-IL2 during the entire
time-course. Therefore, the decay in surface signal can be explained by dissociation of siRNA
from N2-IL2. Using an exponential decay model, the dissociation half-life was calculated to be
3.7±0.3 hr, which translates to a dissociation rate of koff= 5.2x10- 5 s-1.
For knockdown assays, we used HT-CEA cells that stably express d2EGFP on a CMV
promoter (HT-CEA-d2EGFP) and CTLL-2 cells that stably expressed firefly luciferase on a
EF 1-htLV promoter (Cffluc, a kind gift from Christina Smolke, Caltech).
For endosomal
disruption of sm3e-dsRBD-delivered siRNA, the CEA-binding C7-PFO was used. No CD25-
122
A
B
5.OE+05
2.5E+06
z4.OE+05
Z 2.OE+06
3.OE+05
1.5E+06
2.OE+05
1.OE+06
5S 1.OE+05
5 .OE+05
0.OE+OO
p
O.OE+0
0
C
*- sm3e-dsRBD / siRNA
-s+ siRNA only
2
4
Time (hours)
6
8
0
2
4
6
Time (hr)
2.OE+06
z 1.5E+06
8
1.OE+06
c 5.OE+05
n n+nn
0
2
4
6
Time (Hours)
8
10
Figure A.3 siRNA uptake by N2-IL2 and sm3e-dsRBD. The number of internalized siRNA
is measured in CTLL-2 cells and HT-CEA cells delivered by N2-IL2 (A) and sm3e-dsRBD
(B), respectively. Uptake of free siRNA in the absence of N2-IL2 or sm3e-dsRBD is also
measured. The decay of surface siRNA from cell surface-bound N2-IL2 is shown in (C).
From these data, the dissociation half-life of siRNA from N2-IL2 can be determined by
performing a exponential decay curve fit. Here, the half-life was determined to be 3.7 ± 0.3
hours. Error bars represent the standard deviation of duplicate wells.
1.4
1.2
M
-+-
1
*
0.8
0
z
*
CL 0.4
IL
0
sm3e-dsRBD/siCtrl
siGFP only
--
0.6
sm3e-dsRBD/siGFP
sm3e-dsRBD only
0.2
0
0
10
100
[C7-PFO] (pM)
1000
Figure A.4 Gene silencing in HT-CEA-d2EGFP cells. In the presence of C7-PFO, delivery
of gfp siRNA by sm3e-dsRBD can mediate GFP knockdown compared to control siRNA.
The degree of knockdown is modest, but is statistically significant by a paired Student's t-test
(** p<0.05). Error bars represent the standard deviation of triplicate wells.
123
binding Fn3 was available, and the primary sequence of commonly used mouse CD25 binding
antibodies were not readily available.
Therefore, CD25-targeted delivery of PFO was not
attempted. Untargeted PFO was unable to induce knockdown of luciferase siRNA delivered by
N2-IL2 in Cffluc cells (data not shown). This is consistent with results in Chapter 4, where the
CEA-binding C7-PFO was unable to induce gene silencing from siRNA delivered by E6N2 to
CEA-negative A431-d2EGFP
cells.
The combination of 1OOnM sm3e-dsRBD/siRNA
complexes with C7-PFO was able to reduce GFP expression in HT-CEA-d2EGFP cells at
modest but statistically significant levels (Figure A.4).
References
1. Austin CD, De Maziere AM, Pisacane PI, et al. Endocytosis and sorting of ErbB2 and the
site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Bio Cell. 15:526882 (2004).
124
Appendix B: pH sensitive binders to perfringolysin 0
The use of Fn3-PFO fusion proteins used for enhancing endosomal escape of siRNA is
limited by the cytotoxicity of the PFO moiety at the cell surface plasma membrane. PFO was
chosen due to its stability at physiological pH, but it exhibits limited pH sensitivity
(Figure 4.4A). Listeriolysin 0 (LLO) is another cholesterol dependent cytolysin (CDC) that was
used in earlier work for endosomal disruption due to its pH sensitivity.
However, this pH
sensitivity arises from an irreversible degradation at physiological pH prior to internalization,
which reduces its endosomolytic activity and makes it unsuitable for effective cytoplasmic
siRNA delivery (1).
The ideal CDC would have low membrane disruptive activity but also be
stable and retain endosomolytic potential while at physiological pH prior to internalization.
Endosomolytic activity would only be activated at endosomal or lysosomal pH. No naturally
occurring CDC has these properties to the best of our knowledge.
In this work, we propose the use of engineered Fn3 binders to incorporate reversible and
pH sensitive membrane disruptive activity into PFO. These pH sensitive Fn3's would bind and
sterically hinder the membrane-binding epitope of PFO at physiological pH, blocking its ability
to bind to cholesterol-containing membranes and disrupt the cell surface membrane.
Once
internalized into endosomal or lysosomal compartments, the resulting reduction in pH would
cause the pH sensitive Fn3 to dissociate, allowing the PFO to regain its membrane disruptive
activity.
To isolate pH-sensitive PFO binders, we used a pooled combination of the YS, G2 and
G4 Fn3 libraries previously developed (2).
PFO containing an N-terminal Avi tag was co-
expressed with BirA biotin ligase and purified in E. coli, with approximately 40% of purified
125
PFO biotinylated. The libraries were initially screened with magnetic Dynalbeads as described
(3) using bare Streptavidin Dynal beads for negative selection and biotinylated PFO coated
Dynal beads at pH 7.5 for positive selection.
Bead selections were performed until robust
enrichment was observed and binders were visible by flow cytometry, after which the libraries
were selected by fluorescence activated cell sorting (FACS). FACS sorted libraries alternated
between a positive sort at pH 7.5 for binders and a negative sort at pH 5.5 for non-binders to
generate pH sensitive binders. For pH 5.5 staining, only the initial incubation step with PFO was
performed at pH 5.5.
All subsequent wash steps and secondary staining incubations were
performed at pH 7.5. This selection scheme is shown in Figure B. 1.
Negative selection with bare
Positive selection of binders
streptavidin Dynal beads
of PFO at pH 7.5 by FACS
Grow and
re-induce
Grow and
re-induce
Positive Selection with
PFO coated Dynal beads
Negative selection of binders
of PFO at pH 5.5 by FACS
Figure B.1 Selection scheme to isolate pH-sensitive Fn3 binders. Libraries were
initially sorted using magnetic Dynal beads until binders were visible by flow
cytometry. Negative selection using bare magnetic beads was performed to prevent
the isolation of binders to streptavidin. Once visible by flow cytometry, FACS-based
sorting was used. Typically, random mutagenesis was performed after every 2-3
selections to maintain high library diversity.
126
After 2 rounds of mutagenesis with 2-4 successive selections for each round, the 2.3 and
2.4 libraries were generated and exhibited moderate pH sensitivity in analysis of pooled clones.
Individual clones were selected for further analysis, and are summarized in Table B.1.
All
clones exhibited pH sensitive binding to PFO with up to ~7 fold difference between the KD's
of
binding at pH 7.5 and 5.5. Sequence analysis of these individual clones revealed two distinct
families of clones with similar loop sequences.
This provided two classes of binders with
potentially distinct epitopes.
Table B.1 Summary of Fn3 clones isolated from pH-denpendent PFO affinity maturation
Clone
BC Loop
DE Loop
FG Loop
Framework
Kd (pH7.5)
Kd (pH5.5)
(nM)
(nM)
Kd(5.5)/Kd(7.5)
2.3-5
2.3-6
2.4-3
YSRYDSV
YSRYDSV
YSRHDSV
RSASK
RSASK
RSASK
YNGYSYRYSF
YNGYSYRYSF
S2G, N94D
55.2±10. 1T
YNGYSYRYSF
T50A, 199T
108.1±27.1
94.8±13.1
193.7±34.5
448.1±51.1
462.7±47.1
3.51
4.15
4.88
2.3-3
2.3-7
2.4-8
2.4-10
FNPHSCS
PNLHPSCS
PNLHPSCS
HSHSLCH
DSFSI
ASTAHPQ
ASTAHPQ
ATARSSI
WNCFSE
WSCYSG
WSCYSG
WKCYSP
T16A
199V,
K91E
E48G, 191V,
Q113P
45.0±4.5
59.4±15.0
50.2±7.9
28.1±3.1
84.7±34.9
398.2±68.5
82.1±29.7
171.0±18.0
1.88
6.70
1.64
6.09
2.3-8
PNLHPSCS
ASTAHPQ
RNCYSA
2.4-5
QPSDSANS
SSVSS
DCYLCGSFSN
2.4-9
2.4-13
2.4-2
2.3h
YSRYDSV
YSCYDSV
RNSASCS
YSRYDSV
RSASK
RSASK
GCGT
RSASK
YDGYPYRYPF
-INGYSYRYSF
WRCYSR
YNGYSYRYSF
T161, E48G,
193V, N94S
D5G, V6A,
G64C,
Q104R
S23N, 1791
A15T
D100G,
S103P
N.B.
N.B.
N.B.
N.D.*
N.D.
N.D.
N.D.
68% confidence intervals are reported
N.B. denotes that no binding detected
* N.D. denotes that binding was determined but the KD of binding was not determined
127
In order to test the ability for the Fn3 clones to inhibit the hemolytic activity of E6-PFO,
two clones were chosen for further analysis, one from each family of binders, 2.3-6 and 2.4-10.
These, along with wild type Fn3 as a negative control, were expressed as soluble Fn3's or as
MBP fusions. Hemolysis assays were performed as described in Chapter 4, with the addition of
300 nM Fn3 or MBP-Fn3, which is a saturating concentration based on the measured KD's.
Fn3's were preincubated with E6-PFO for 20 minutes at room temperature prior to addition of
red blood cells to allow pre-binding to the PFO moiety. Wild type Fn3 was used as a negative
control. As shown in Figure B.2, no change was observed in the hemolytic activity of PFO in
the presence of either 2.3-6 or 2.4-10, indicating that these Fn3's do not bind to an epitope on
PFO that interferes with hemolytic activity.
1.2
1.2
1
1
0.8
~++--
L
--
a
0.6
i -
6
----
E 0.4
E
.-
0.2 -'"
0 -
0.001
E6PFO pH7
E6PFO pH5
u.
+300nM Fn3 wt pH7
.L
WI'
I
0.1
-r
1
+300nM MBP-2.4-10 pH7
0.6
+300nM Fn3 wt pH5
0__
E 0'4
+300nM 2.3-6 pH7
-- +--- +300nM 2.3-6 pH5
0.01
4---E6PFO pH7
-
02
0
10
100
0.01
[E6PFO] (nM)
1
[E6PFO] (nM)
Figure B.2 Analysis of the effect of two PFO binding clones on hemolytic activity.
The effects of two PFO binding clones on the hemolytic activity of E6-PFO are
shown here. Saturating amounts of PFO binding clones in soluble form or as MBP
fusion proteins were pre-bound to E6-PFO. Wild type Fn3 was used as a negative
control. Mouse red blood cells were added and the mixtures were incubated for 1
hour at 37"C. The supernatants were measured for absorbance at 541 nm.
Hemolysis was normalized to a value of 0 for untreated red blood cells, and 1 for red
blood cells treated with 10% Triton-X detergent.
128
100
Although this brief study did not result in an Fn3 clone that could inhibit membrane
disruption by PFO in hemolysis assays, it nevertheless reveals that Fn3 libraries can be
engineered for pH sensitive binding using FACS. It is possible that pH sensitive selection prior
to clonal analysis for hemolytic inhibition may have resulted in the loss of clones that bind to the
correct epitope but do not initially exhibit pH sensitivity. pH sensitivity could then potentially be
introduced into those clones without significant epitope drift, using techniques such as histidine
scanning (4). Therefore, future efforts should focus on identifying Fn3 clones in earlier libraries
that can inhibit PFO hemolytic activity before performing pH-based positive and negative
selections.
Ultimately, it is envisioned that pH sensitive Fn3's will be expressed as fusion proteins
with PFO. This creates a high local concentration of the Fn3, although this parameter can be
altered by adjusting the linker length. Nevertheless, the high local concentration allows for
selection of weak binders (eg. KD ~ 100 nM) at physiological pH, which allows for a wide range
of potential clones to screen for binding against the proper epitope.
Although the Fn3 clones described here are not suitable for use as an inhibitor of PFO
membrane disruption, they may be useful for other applications. For example, when screening
further for other PFO binders for membrane disruptive activity, these clones can be used to
obscure inactive epitopes to prevent the selection of other Fn3 clones that bind to similar
epitopes. Also, if a single protein with both PFO and dsRBD functionality were desired, these
Fn3 clones could be used to create non-covalent complexes without affecting PFO activity. This
would bypass potential problems when attempting to express PFO fusions in a cellular host with
cholesterol-containing membranes, such as mammalian cells.
129
Acknowledgements
Abigail van Hook assisted with selecting for PFO binders.
References
1. Schuerch DW, Wilson-Kubalek EM and Tweten RK. Molecular basis of listeriolysin 0
pH dependence. Proc Natl Acad Sci USA. 102: 12537-12542 (2005).
2. Hackel BJ. Fibronectin Domain Engineering. MIT PhD Thesis (2009).
3. Ackerman M, Levary D, Tobon G, et al. Highly avid magnetic bead capture: an efficient
selection method for de novo protein engineering utilizing yeast surface display.
Biotechnol Prog. 25:774-783 (2009).
4. Murtaugh ML, Fanning SW, Sharma TM, et al. A combinatorial histidine scanning
library approach to engineer highly pH-dependent protein switches. Protein Sci.
20:1619-31 (2011).
130
Appendix C: DNA Sequences
V91R
(pRS316 vector)
Q126T (pRS316 vector)
CTCTTT
D34W
(pRS316 vector)
CTCTTT
131
Q141D
(pRS316 vector)
CTCTTT
Wild type Fc-D34W
(gWiz vector)
132
Wild type Fc-Q141D (gWiz vector)
133
D265A Fc-D34W
(gWiz vector)
134
D265A Fc-Q141D
(gWiz vector)
135
E6N2
(gWiz vector)
-
-
dsRBD
-
TGCTGGTGATCTTTCAGCAGGTTTCTTCATGGAGGAACTTAATACATA
CCGTCAGAAGCAGGGAGTAGTACTTAAATATCAAGAACTGCCTAATTCAGGACCTCCACATGAT
AGGAGGTTTACATTTCAAGTTATAATAGATGGAAGAGAATTTCCAGAAGGTGAAGGTAGATCAA
AGAAGGAAGCAAAAAATGCCGCAGCCAAATTAGCTGTTGAGATACTTAATAAGGAAAAGAAGGC
AGTTAGTCCTTTATTATTGACAACAACGAATTCTTCAGAAGGATTATCCATGGGGAATTACATA
GGCCTTATCAATAGAATTGCCCAGAAGAAAAGACTAACTGTAAATTATGAACAGGTTGCATCGG
GGGTGCATGGGCCAGAAGGATTTCATTATAAAGTCAAAATGGGACAGAAAGAATATAGTATTGG
TACAGGTTCTACTAAACAGGAAGCAAAACAATTGGCCGCTAAACTTGCATATCTTCAGATATTA
TCAGAAGAAACCTCAGTGAAATCTGACGGTGGAGGTGGATCA
136
E6-PFO
(pMal-c2x vector with upstream TEV site)
-M---
137
C-PFO
(pMal-c2x vector with upstream TEV site)
-- n-r
138
D-PFO
(pMal-c2x vector with upstream TEV site)
-- n-r
139
E-PFO
(pMal-c2x vector with upstream TEV site)
-- n-r
140
C7-PFO
(pMal-c2x vector with upstream TEV site)
-M---
141
N2-IL2
(gWiz vector)
-
-
-
-
dsRBD
-
TGGCTGGTGATCTTTCAGCAGGTTTCTTCATGGAGGAACTTAATACATA
CCGTCAGAAGCAGGGAGTAGTACTTAAATATCAAGAACTGCCTAATTCAGGACCTCCACATGAT
AGGAGGTTTACATTTCAAGTTATAATAGATGGAAGAGAATTTCCAGAAGGTGAAGGTAGATCAA
AGAAGGAAGCAAAAAATGCCGCAGCCAAATTAGCTGTTGAGATACTTAATAAGGAAAAGAAGGC
AGTTAGTCCTTTATTATTGACAACAACGAATTCTTCAGAAGGATTATCCATGGGGAATTACATA
GGCCTTATCAATAGAATTGCCCAGAAGAAAAGACTAACTGTAAATTATGAACAGGTTGCATCGG
GGGTGCATGGGCCAGAAGGATTTCATTATAAAGTCAAAATGGGACAGAAAGAATATAGTATTGG
TACAGGTTCTACTAAACAGGAAGCAAAACAATTGGCCGCTAAACTTGCATATCTTCAGATATTA
TCAGAAGAAACCTCAGTGAAATCTGACGGTGGAGGTGGATCA
142
sm3e Heavy Chain -
dsRBD
(gWiz vector)
Variable Heavy
-
-
dsRBD -
-
GTCGAGA
TGGCTGGTGATCTTTCAGCAGGTTTCTTCATGGAGGAACTTAATACATACCGTCAGAAGCAGGG
AGTAGTACTTAAATATCAAGAACTGCCTAATTCAGGACCTCCACATGATAGGAGGTTTACATTT
CAAGTTATAATAGATGGAAGAGAATTTCCAGAAGGTGAAGGTAGATCAAAGAAGGAAGCAAAAA
ATGCCGCAGCCAAATTAGCTGTTGAGATACTTAATAAGGAAAAGAAGGCAGTTAGTCCTTTATT
ATTGACAACAACGAATTCTTCAGAAGGATTATCCATGGGGAATTACATAGGCCTTATCAATAGA
ATTGCCCAGAAGAAAAGACTAACTGTAAATTATGAACAGGTTGCATCGGGGGTGCATGGGCCAG
AAGGATTTCATTATAAAGTCAAAATGGGACAGAAAGAATATAGTATTGGTACAGGTTCTACTAA
ACAGGAAGCAAAACAATTGGCCGCTAAACTTGCATATCTTCAGATATTATCAGAAGAAACCTCA
TGATAA
GTGAAATCTGACGGTAGT
143
sm3e Light Chain
(gWiz Vector)
- Variable Light -
144