Math 1321 Week 7 Lab Worksheet Due Thursday 02/28
advertisement
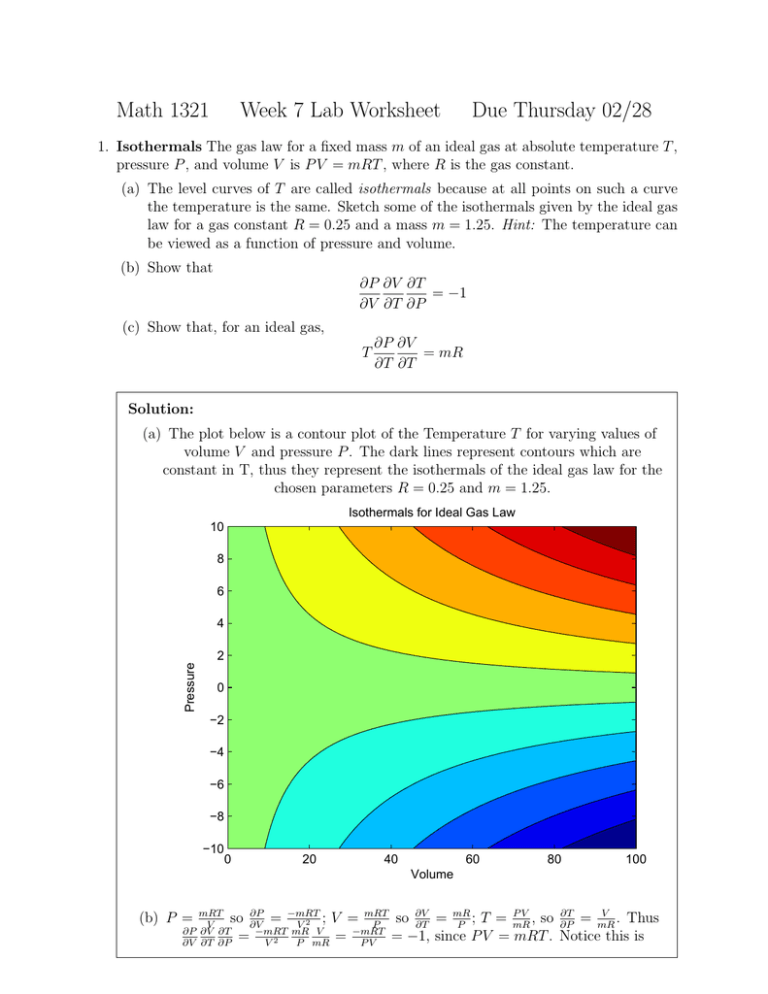
Math 1321 Week 7 Lab Worksheet Due Thursday 02/28 1. Isothermals The gas law for a fixed mass m of an ideal gas at absolute temperature T , pressure P , and volume V is P V = mRT , where R is the gas constant. (a) The level curves of T are called isothermals because at all points on such a curve the temperature is the same. Sketch some of the isothermals given by the ideal gas law for a gas constant R = 0.25 and a mass m = 1.25. Hint: The temperature can be viewed as a function of pressure and volume. (b) Show that ∂P ∂V ∂T = −1 ∂V ∂T ∂P (c) Show that, for an ideal gas, T ∂P ∂V = mR ∂T ∂T Solution: (a) The plot below is a contour plot of the Temperature T for varying values of volume V and pressure P . The dark lines represent contours which are constant in T, thus they represent the isothermals of the ideal gas law for the chosen parameters R = 0.25 and m = 1.25. Isothermals for Ideal Gas Law 10 8 6 4 Pressure 2 0 −2 −4 −6 −8 −10 0 20 40 60 80 100 Volume mRT ∂P so ∂V = −mRT ; V = mRT V V2 P ∂P ∂V ∂T −mRT mR V −mRT = = 2 ∂V ∂T ∂P V P mR PV (b) P = PV ∂T V so ∂V = mR ; T = mR , so ∂P = mR . Thus ∂T P = −1, since P V = mRT . Notice this is exactly negative of the quantity we would arrive at if we treated all of the terms ∂P , ∂V , and ∂T as fractions and canceled “like” terms. Thus we see derivatives are “almost” like fractions but still something a little different. , so ∂P = mR . Also, (c) By part (a), P V = mRT =⇒ P = mRT V ∂T V ∂V mR PV mRT , we have P V = mRT =⇒ V = P and ∂T = P . Since T = mR ∂P ∂V P V mR mR T ∂T ∂T = mR V P = mR. 2. Frost Penetration In a study of frost penetration it was found that the temperature T at time t (measured in days) at a depth x (measured in feet) can be modeled by the function T (x, t) = T0 + T1 e−λx sin(ωt − λx) where ω = 2π/365 and λ is a positive constant. (a) Find (b) Find ∂T . ∂x ∂T . ∂t What is its physical significance? What is its physical significance? (c) Show that T satisfies the heat equation Tt = kTxx for a certain constant k. (d) If λ = 0.2, T0 = 0, and T1 = 10, graph T (x, t). (e) What is the physical significance of the term −λx in the expression sin(ωt − λx)? Solution: (a) ∂T = T1 e−λx [cos(ωt − λx)(−λ)] + T1 (−λe−λx sin(ωt − λx) ∂x = −λT1 e−λx [sin(ωt − λx) + cos(ωt − λx)] This quantity represents the rate of change of temperature with respect to the depth below the surface at a given time t. (b) ∂T = T1 e−λx [cos(ωt − λx)(ω)] = ωT1 e−λx cos(ωt − λx) ∂t This quantity represents the rate of change of temperature with respect to time at a fixed depth x. (c) Txx ∂ ∂T = ∂x ∂x = −λT1 e−λx [cos(ωt − λx)(−λ) − sin(ωt − λx)(−λ)] + · · · +e−λx (−λ) [sin(ωt − λx) + cos(ωt − λx)] = 2λ2 T1 e−λx cos(ωt − λx) From part(b) we saw, Tt = ωT1 e−λx cos(ωt − λx) = the function T satisfies the heat equation. ω T . 2λ2 xx So with k = ω , 2λ2 (d) (e) The term −λx is a phase shift: it represents the fact that since heat diffuses slowly through soil, it takes time for changes in the surface temperature to affect the temperature at deeper points. As x increases, the phase shift also increases. For example, when λ = 0.2, the highest temperature at the surface is reached when t ≈ 91, whereas at a depth of 5 feet, the peak temperature is attained at t ≈ 149, and at a depth of 10 feet, at t ≈ 207.








