Interaction of multispecies ecosystems and climate *Manuscript Ivan Sudakov
advertisement

*Manuscript
Click here to view linked References
Interaction of multispecies ecosystems and climate
Ivan Sudakova,∗, Sergey A. Vakulenkob,c , Dubrava Kirievskayad,e , Kenneth M.
Goldenf
a University of
Dayton, Department of Physics, 300 College Park, SC 111, Dayton, OH 45469-2314 USA
of Problems in Mechanical Engineering, Russian Academy of Sciences, Bolshoy pr., 61, V.O.,
St. Petersburg 199178, Russia
c University ITMO, Kronverkskiy pr., 49, St. Petersburg 197101, Russia
d
University of Dayton, Department of Geology, 300 College Park, SC 179, Dayton, OH 45469-2364 USA
e Research Center, Novgorod State University, Bolshaya St. Petersburgskaya ul., 41, Veliky Novgorod
173003, Russia
f University of Utah, Department of Mathematics, 155 S 1400 E, RM 233, Salt Lake City, UT 84112-0090,
USA
b Institute
Abstract
We propose a conceptual model of an ecosystem with many species which depends
on climate and exhibits complex behavior. We prove a general assertion on attractor
existence for this model. One of the sufficient conditions for attractor existence is that
species self-regulation is stronger than species competition. The development of the
proposed model allows for the investigation of climate-ecosystem feedback loops. In
the case of positive feedback in climate-ecosystem interactions, we demonstrate the
possibility of catastrophic bifurcations, where all species go extinct under the impact
of climate warming.
Keywords: ecosystem, climate feedback, Lotka-Volterra model, plankton, dynamical
systems.
1. Introduction
Models of ecosystems form an important class of dynamical systems generating
complicated patterns and strange (chaotic) attractors (Ulanowicz and Kemp, 1979).
However, modeling these large systems is made difficult by rapid, small scale biological evolution and gaps in observations to use for comparison. Also, there is uncertainty
in how to set up reliable experiments on such ecosystems.
Large multispecies marine ecosystems such as phytoplankton play an important
role in the climate system, and are sensitive indicators of climate change (Doney et
al., 2012). As a key part of the global ecosystem, they influence climate feedback processes and possible tipping points (Selkoe et al., 2015). Complex ecosystems have been
studied in terms of internal processes within the ecosystem such as the competition for
∗ Corresponding author
Email address: isudakov1@udayton.edu (Ivan Sudakov)
Preprint submitted to Elsevier
March 10, 2016
resources, conditions for chemical reactions, etc. However, recent observations have
shown that climate change may be be a leading factor influencing ecosystem behavior
(Walther, 2010).
A well studied example is the ocean ecosystem, where the main resource for many
species is phytoplankton. Phytoplankton plays an important role in the dynamics of the
climate system through the oceanic carbon cycle by removing about half of all carbon
dioxide from the atmosphere during photosynthesis (Field et al., 1998). Previous studies (Arhonditsis and Brett, 2004; Travers et al., 2007) have shown that phytoplankton
communities respond to climate warming through changes in diversity and productivity. However, it was determined (Toselandet al., 2013) that changing the climate
temperature directly impacts the chemical cycles in plankton, affecting the system as
much as nutrients and light.
Another example where environmental temperature may be related to the dynamics
of the plankton ecosystem is the very recent appearance of a fall phytoplankton bloom
in the Arctic Ocean (Ardyna et al., 2014). In contrast to previous observations, the
new plankton dynamics may be approaching a possible bistable regime, where plankton bloom not only during the spring time, but in early fall as well. This in turn could
affect surface ocean temperature and delay sea ice re-freezing. Also, an unusual massive phytoplankton bloom which has been found underneath the Arctic ice pack in the
Chukchi Sea (Arrigo et al., 2012) may correlate with planetary warming. Thus, theoretical investigation of the dynamical properties of large ecosystems under external
forcing makes a significant contribution to understanding properties of the ecosystems
as well as the climate system, and their interactions.
We consider here a model of a large ecosystem where many species share few resources. It extends the model in (Huisman and Weissing, 1999), taking into account environmental dependence of resources, in particular climate factors, and self-limitation
and competition effects. Our aim is to study the connection between complexity of
time behavior, biodiversity, and the structure of the climate-ecosystem interaction.
Note that competition may occur as a result of allelopathy (Legrand et al., 2003),
while self-limitation is critically important to support the coexistence of many species
in the system (Roy and Chattopadhyay, 2007). There are a number of species of phytoplankton which have the ability to produce some toxic or inhibitory compounds. The
toxic materials compensate for the competitive disadvantages among phytoplankton
species that leads to self-limitation effects. The resources may depend on the environment via temperature or greenhouse gas concentration.
If the resource turnover rate is large enough, the model reduces to a Lotka-Volterra
system (Vakulenko, 2013; Kozlov and Vakulenko, 2013). If we remove self-limitation
effects for the Lotka-Volterra system, one finds (Hofbauer and Sigmund, 1988; Takeuchi,
1996) that a single species can survive only in an ecosystem for certain fixed climate
parameters. Biologically, it is the competitive exclusion principle. In the framework
of the phytoplankton model, it is the so-called plankton paradox studied in many interesting works (Hutchinson, 1961; Tilman, 1977; Huisman and Weissing, 1999; Irigoien
et al., 2004). In fact, in contrast to the exclusion principle, we observe the coexistence
of many plankton species sharing the same niche. Numerical simulations (Hutchinson,
1961; Tilman, 1977; Huisman and Weissing, 1999) have shown that in such systems
chaos and unpredictable behavior occur. In (Hsu et al., 1977; Smith, 1981) it was shown
2
that a temporal variability of the nutrient supply can lead to coexistence of species.
The paper is organized as follows. In the next section we formulate the standard
model of species coexistence and the extended model, which takes into account climatic factors. Further, in section 3 we prove a general assertion on attractor existence
for this model. In section 4 it is shown that for large turnovers D the system admits an
asymptotic solution and, under additional assumptions, can be reduced to the LotkaVolterra model. This model is well studied and the known results allow us to describe
the influence of climate and climate warming in large ecosystems (see section 5). In
section 6 for the case of a single resource we show that the global attractor consists of
equilibria and derive an equation for species abundances. This investigation is aimed
at describing the influence of climate on biodiversity.
2. Models of large ecosystems
2.1. Standard model
Consider the following model of an ecosystem, which extends the model in (Huisman and Weissing, 1999):
where
φ j (v) =
N
dxi
= xi (−ri + φi (v) − ∑ γi j x j ),
dt
j=1
(1)
N
dv
= D(S − v) − ∑ c j x j φ j (v),
dt
j=1
(2)
a j v+
,
Kj + v
(3)
a j , K j > 0,
v+ = max{v, 0},
are Michaelis-Menten’s functions, xi are species abundances, ri are the species mortalities, D is the resource turnover rate, S is the supply of resource v, and ci is the content
of the resource in the i-th species. These constants define how different species share
resources. Note that if all ci = 0 then the equation for v becomes trivial and v(t) → S
for large times t, i.e., the resource equals the resource supply.
The terms γii xi define self-regulation of species populations that restrict the species
abundances, and γi j x j with i 6= j define a possible competition between species for
resources. These effects can appear as a result of species allelopathy (Legrand et al.,
2003), where phytoplankton allelopathy is considered, and an ability to produce some
toxic or inhibitory compounds (Roy and Chattopadhyay, 2007).
The coefficients ai are specific growth rates and the Ki are self-saturation constants.
The coefficients ci determine how the species share the resource (nutrient supply). It is
natural thus to assume that ∑Ni=1 ci = 1, ci > 0.
For the case of M resources we have more complicated equations
N
dxi
= xi (−ri + φi (v) − ∑ γi j x j ),
dt
j=1
3
(4)
N
dv j
= D j (S j − v j ) − ∑ c jk xk φk (v),
dt
k=1
(5)
where v = (v1 , v2 , ..., vM), and
φ j (v) = min{
a j v1+
a j vM +
, ...,
},
K1 j + v1
KM j + vM
(6)
where a j and Ki j > 0. This model is widely used for primary producers like phytoplankton and it can also be applied to describe competition for terrestrial plants
(Tilman, 1977). Relation (6) corresponds to the von Liebig minimum law, but we
can consider even more general φ j satisfying the conditions
φ j (v) ∈ C 1 ,
0 ≤ φ j (v) ≤ C+,
(7)
where C+ > 0 is a positive constant, and
φk (v) = 0,
∀k,
v ∈ ∂ RN>
(8)
where ∂ RN> denotes the boundary of the positive cone RN> = {v : v j ≥ 0, ∀ j}. Note
that condition (8) holds if φ j are defined by (6). Similarly as above, we assume that
∑Nk=1 cik = 1, cik > 0.
When γi j = 0 for all i, j this system is equivalent to those in works where the plankton paradox (Huisman and Weissing, 1999) is studied. The choice γii = γi > 0 and
γi j = 0 for i 6= j allows us to take into account the self-limitation effects, important for
these systems. It was shown in Roy and Chattopadhyay (2007).
2.2. Extended standard model with climate influence
We extend system (4) and (5) to describe effects connected with a possible climate
influence. For one and two species (N = 1, 2) a model of climate influence was proposed in (Sekerci and Petrovskii, 2015). We consider the case of arbitrary N but, in
certain aspects, our model is simpler than in (Sekerci and Petrovskii, 2015), in particular, we do not take into account zooplankton and, therefore, possible predator-prey
interactions in an explicit form.
Temperature has a significant effect on the maximum growth rate of phytoplankton
(Richardson et al., 2000), and can be considered as a crucial growth factor.
Let us assume that the resource supplies Sk can depend on the environmental parameters, for example, temperature T : Sk = Sk (T ). In turn, T may depend on species
abundances, for example, via albedo (Chapin et al., 2002). We assume, for simplicity,
that this effect is linear:
N
T = T̄ + ∆ T,
∆T =
∑ µk j x j ,
(9)
k=1
where µik are coefficients and T̄ is a reference temperature corresponding to the earth
albedo without the ecosystem influence. If the temperature variations ∆ T induced by
the species are small, we have
Sk = S̄k + ∆ Sk + O(∆ T 2 ),
N
∆ Sk =
∑ bk j x j ,
k=1
4
k = 1, ..., M
(10)
k (T̄ )
where bk j = dSdT
µk j . If all bk j > 0 we are dealing with a purely positive feedback
(then the species abundance increase resources) and if bk j < 0 one has a purely negative feedback. There is possible, however, an interesting case where one part of the
coefficients bk j are positive numbers and another part consists of negative bk j (mixed
feedback). For the mixed feedback a cumulative effect of the climate-ecosystem feedback on the resource supplies may be small since the different terms in ∆ Sk may cancel
each other.
There also are possible alternative physical mechanisms leading to relations like
(10). An important resource for phytoplankton is oxygen (Sekerci and Petrovskii,
2015). The production of oxygen is proportional to the phytoplankton concentration
and depends on temperature T .
Finally, the extended model takes the form
N
dxi
= xi (−ri + φi (v) − ∑ γi j x j ),
dt
j=1
(11)
N
dv j
= D j (S j (x) − v j ) − ∑ c jk xk φk (v),
dt
k=1
(12)
where
N
Sk (x) = S̄k + ∑ bk j x j ,
k = 1, ..., M.
(13)
k=1
To describe consequences of climate warming with this model, we can assume that
some coefficients depend on T (for example, bik or ai in (6)). In fact, as it was mentioned in the Introduction, temperature is an important factor that determines oxygen
and phytoplankton production.
In the next section we show that under some assumptions this model is well posed.
3. General properties of model
Let us first describe some sufficient conditions, which guarantee that systems (1),
(2), (4), (5) and (11), (12) are dissipative and have a global attractor. Define the matrix
Γ with the entries γi j to satisfy one of the following conditions:
Assumption 1A. The matrix Γ with the entries γi j has a positive dominant diagonal:
γii − ∑ |γi j | = κi > 0.
(14)
j6=i
Assumption 1B The matrix Γ has non-negative entries
γi j ≥ 0,
γii > 0 ∀i, j.
(15)
The assumption 1A means that species self-regulation is stronger than competition
between species while assumption 1B implies that all species compete in our ecosystem.
5
Let us show that the solutions to (11), (12) exist, and that they are non-negative and
bounded.
Lemma 1. Assume the functions φ j satisfy (7). Let us consider for (11), (12) the
Cauchy problem with positive initial data for x and positive initial resources
xi (0) > 0,
v j (0) > 0.
(16)
Then, if either assumption 1A or 1B hold, solutions of this Cauchy problem are positive
and bounded for large times t, that is,
0 < xi (t) < X(t) = X0 + |X0 − max xi (0)| exp(−κ t),
i
t > 0,
(17)
where X0 is a positive constant, κ = γ X0 and
0 < v j (t) < v j (0) exp(−D j t) + max V j (s),
s∈[0,t]
where
(18)
N
V j (t) = S̄ j + b̄ j X(t),
b̄ j = ∑ (b ji )+ ,
i=1
and f + = max{ f , 0}.
Proof. The proof proceeds in the following steps.
Step 1. Positivity of xi follows from the fact that the i-th right hand side of system
(4) is proportional to xi , thus, xi (t) = xi (0) exp(ξi (t)), where ξi is a function.
Step 2. Let us prove that v j (t) > 0. Assume that this fact is violated. Then there
exists an index j0 and a time t0 > 0 such that
v j0 (t0 ) = 0,
dv j0
≤ 0,
dt
v j (t0 ) ≥ 0,
f or all j 6= i.
(19)
Condition (8) entails that the term ∑Nk=1 c jk xk φk (v) equals zero. Then we substitute
these inequalities into the j0 -th equation (12) and obtain a contradiction.
Step 3. Let us prove estimate (17). Suppose that assumption 1B is satisfied (for assumption 1A the proof is analogous). Let E(t) = max{x1 (t), ..., xN (t)}. Let us estimate
dE/dt for large E. Let i0 (t) be an index such that E(t) = xi0 (t). According to (7) the
φi are uniformly bounded by C+ . Therefore within any open interval, where i0 is fixed,
one has
dxi0
≤ xi0 Ri0 , Ri0 ≤ C+ − γ xi0 (t),
dt
where γ = mini γii > 0 due to assumption (14) on Γ . This inequality implies that E(t) ≤
X(t), where X(t) the solution of the Cauchy problem
dX
= X(C+ − γ X),
dt
X(0) = max xi (0).
i
Let X0 = V+ /γ and κ = γ X0 . If X(0) < X0 , then the last equation shows that X(t) ≤ X0
for all t and (17) follows. If X(0) > X0 , then X(t) > X0 for all t. By the change
X̃ = X − X0 we obtain that X̃ > 0 and thus
d X̃
= −γ (X0 + X̃ )X̃ ≤ −γ X0 X̃
dt
6
that entails X̃(t) ≤ X̃ (0) exp(−γ X0 t), and we obtain (17).
Step 4. Having (17), we can prove (18). Indeed, using the non-negativity of the c jk
and φk , one obtains
dv j
≤ D j (S j (x(t)) − v j ).
dt
Therefore,
Z
t
v j (t) = exp(−D j t)(v j (0) +
0
S j (x(s)) exp(D j s)ds)
that gives
v j (t) ≤ exp(−D j t)v j (0) + maxs∈[0,t] S j (x(s)).
Here S j (x(t)) ≤ S̄ j + b̄ j X(t). These two last inequalities imply v j (t) ≤ V j (t) that completes the proof.
Due to boundness of solutions for large t we then obtain the following corollary.
Theorem. Under the conditions of the previous lemma, system (11), (12) is dissipative and has a compact global attractor.
4. Asymptotic approach
Our next step is to find asymptotic solutions of system (11) and (12), where Sk are
defined by (10). We suppose as above that D j >> 1 and that γi j = 0 for all i, j, i.e., we
have the standard plankton model without competition and self-limitation. Note that
a reduction to a Lotka-Volterra system described below also holds for bounded D and
large resource supplies Sk >> 1. To fix ideas we consider the case of large D j . For
simplicity, we assume that D j = D >> 1. Let us make the change
vk = Sk (x) − ṽk ,
τ = Dt.
(20)
System (11) and (12) takes then the form
N
dxi
= ε xi (−ri + φi (S(x) − ṽ) − ∑ γi j x j ),
dτ
j=1
(21)
d ṽ j
= −ṽ j − εU j (x, ṽ),
dτ
where ṽ = (ṽ1 , ..., ṽM),ε = D−1 << 1 and
N
U j (x, v) =
(22)
N
∑ c jkφk (S(x) − ṽ) + ∑ b jk(φk (S(x) − ṽ) − rk − ∑ γkl xl ).
k=1
k=1
(23)
kl
For small ε equations (21), (22) form a typical system with slow variables x j and fast
variables ṽ. We can find an asymptotic solution of (22), which has the form
ṽ j = εU j (x, 0) + O(ε 2 ).
(24)
Finally, for the species abundances xi one obtains
N
dxi
= xi (φi (S(x)) − ri − ∑ γi j x j ) + O(ε ).
dt
j=1
7
(25)
5. Qualitative analysis of large time behaviour
If the coefficients bl j are small, i.e., the feedback between the resource supply and
the climate is weak, then the system (25) can be simplified by the Taylor expansion
φi (S(x)) = φi (S̄) +
∂ φi
(S̄)bl j x j + ... .
l=1,...,M j=1,...,N ∂ Sl
∑
∑
Removing terms quadratic in xi we reduce (25) to the Lotka -Volterra system
N
dxi
= xi (Ri − ∑ Ki j x j ).
dt
j=1
where
(26)
M
Ri = φi (S̄) − ri ,
Ki j = γi j − ∑ ail bl j ,
(27)
l=1
and
∂ φi
(S̄).
(28)
∂ Sl
The Lotka-Volterra systems are very well studied (see, for example, (Hofbauer and
Sigmund, 1988; Takeuchi, 1996)) and we can use these results in order to understand
how climate warming can affect ecosystems. We assume that assumption 1B holds
and consider the two limiting cases, the so-called “weak climate” (WC) regime and
the “strong climate” (SC) regime. The WC case corresponds to a weak climate influence, where the ecosystem-climate interaction via coefficients bik is much weaker with
respect to competition effects associated with the coefficients γi j . This means that all
|bik | << γ , where γ = ||Γ || is a characteristic magnitude of the entries γi j .
In the SC case (regime of a strong climate influence; coefficients determining climate feedback are stronger than coefficients that define competition and self-limitation),
we assume that inversely |bik | >> γ .
In the case of WC systems, (26) is close to so-called competitive systems which
are well studied (Hirsch, 1985). These systems exhibit no stable periodic or chaotic
regimes: almost all trajectories converge to equilibria, which will be investigated in
section 6.
Consider the case SC. We set γi j = 0 for all i, j. Then equations (26) represent
a Lotka-Volterra system with special structure. An analysis (Hofbauer and Sigmund,
1988) shows that, for general Ri , no more than M species can coexist (the competitive
exclusion principle). Mathematically this means that if N > M then for some i either
the corresponding xi (t) → 0 or xi (t) → +∞ as t → +∞, i.e., the system is not permanent
(Hofbauer and Sigmund, 1988). However, if the condition
ail =
M
Ri =
∑ aikθk ,
∀i = 1, ..., N
(29)
k=1
for some θk is fulfilled, then it is possible that all N species can coexist. In this case
system (26) can be reduced to a reduced system involving M variables q j only (Kozlov
8
and Vakulenko, 2013):
dq j
= G j (q),
dt
N
(30)
M
G j (q) = −θi + ∑ b jiCi exp(− ∑ ai j q j ),
i=1
(31)
j=1
where Ci are arbitrary positive constants. The dynamics in (30) completely determines
the x-dynamics by the relations
M
xi = Ci exp(− ∑ ai j q j ).
j=1
Note that Ci = xi (0) and therefore the vector field G(q) depends on initial data and the
species number N.
The main results on system (30) can be outlined as follows (see (Kozlov and Vakulenko, 2013) for more details). Let Ω be a compact connected domain in RM with a
smooth boundary, F(q) be a compact C 1 smooth field on Ω and ε > 0 be a number.
Then there exist a number N and coefficients ai j > 0,Ci > 0 and bil that the corresponding field G approximates F in the domain Ω in C 1 -norm with accuracy ε . This
approximation result implies that system (30) with M variables q j can generate all
structurally stable dynamics in dimension M. In particular, due to the Theorem on Persistence of hyperbolic sets (Ruelle, 1989), system (30) can induce all (up to topological
orbital equivalences) hyperbolic dynamics including periodic and chaotic, for example,
Smale horseshoe, Anosov flows etc.
Under condition (29) we find that the time behavior of solutions of system (26)
sharply depends on M. Assume that aik > 0 (it is natural since this means φi increases
as a resource supply Sl increases).
If M = 1 it is possible that all N species survive in an equilibrium state, and N may
be large. Although the periodic and chaotic trajectories are impossible, we can observe
multistability (coexistence of many equilibria).
For M = 2 and bik of different signs system (26) can have time periodic solutions
and for M > 2 this system can produce time chaotic solutions (we can obtain then all
possible hyperbolic invariant sets of dimension ≤ M). If all bik < 0 or all bik > 0 we
have no complex behavior for trajectories and they are convergent. Therefore, the most
interesting situation arises in the biodiversity case when bik have different signs. In the
next subsection we will study some bifurcations for the case M = 2 and we will see
that in this case Andronov-Hopf bifurcations are possible.
5.1. Bifurcations, complexity and biodiversity
If there exists a positive climate-ecosystem feedback, and bik > 0, then a time periodic (for M > 1) or even chaotic (for M > 2) behaviour and complicated bifurcations
can occur.
We consider two cases: M = 1 ( a single resource) and M = 2. We are going to
investigate existence of different bifurcations, in particular, the Andronov-Hopf bifurcations. If M = 1 there are possible saddle-node, pitchfork and transcritical bifurcations
9
but the Andronov-Hopf does not occur. The main climate effect in the case M = 1 is
a destruction of the ecosystem under the climate influence that can be described as
follows. Let us consider a population consisting of N species with random parameters
and denote q = q1 , G = G1 . We can assume, for example, that parameters ai , Ki in (3)
and b1i in (10) are normally distributed random numbers. The equilibria are defined by
roots of equation θ = G(q).
Let us consider system (30) for M = 2. Let (Q1 , Q2 ) be a steady state for this
system. Let us define a matrix M of size 2 × 2 with the entries
Ml j =
∂ Gl
(Q1 , Q2).
∂qj
We introduce vectors b(l) = col(bl1 , bl2, ...., blN) and
Ea (Q)( j) = col(a1 j exp(−a11 Q1 − a12 Q2 ), ..., aN j exp(−aN1 Q1 − aN2 Q2 ).
We define the scalar product together with the corresponding norm by
N
h f , giC =
∑ C j f j g j,
|| f ||C2 = h f , f iC
j=1
This scalar product is defined for N-component vectors and depends on non-negative
coefficients C j > 0, j = 1, ..., N. Then we obtain
Mkl = hb(k), Ea (Q)(l)iC ,
l, k ∈ {1, 2}.
An Andronov-Hopf bifurcation occurs if the trace TrM of the matrix M changes its sign
as the bifurcation parameter b goes through a critical value bc and if the determinant
detM of M is positive at this critical value. By notation introduced above we obtain
DetM = M11 M22 − M12 M21 ,
(32)
TrM = hb(1) , Ea (Q)(1) iC + hb2 , Ea (Q)(2) iC .
(33)
These relations allow us to see connections between bifurcations, feedbacks and diversity. First let us observe that components of the vectors Ea (Q) j are always positive.
Note that if the climate influence is absent, then all the components of bl are negative.
Then it is clear that TrM does not change its sign thus in this case the Andronov-Hopf
bifurcations are absent. The same fact holds if all the climate-ecosystem feedbacks are
negative. For purely positive or mixed feedbacks these bifurcations are possible under
additional conditions. In order to find a biological meaning of these conditions, we
define φl j (C) as angles between the vectors bl and Ea (Q)(2) . One has
φl j (C) = hb(l), Ea (Q)( j)iC ||b(l)||C−1||Ea (Q)( j)||C−1.
Then the condition DetM > 0 reduces to
φ11 (C)φ22 (C) > φ12 (C)φ21 (C).
10
(34)
The condition TrM = 0 implies that φ11 (C) and φ22 (C) have opposite signs. Then (34)
means that φ12 (C) and φ21 (C) also have opposite signs. If all the species affect the
climate in a similar manner (for example, we have a purely positive feedback) then one
can expect that all φl j have the same sign, and, therefore, Andronov-Hopf bifurcations
are impossible. We conclude then that not only a feedback positivity but also biodiversity and a complex ecosystem structure support a complicated time periodic behavior.
Moreover, all bifurcation conditions depend on the initial data C. From a biological
point of view, this means that all bifurcation effects have a ”memory”, i.e., depend on
the choice of initial data.
6. Equilibria
6.1. Equation for equilibrium resource value
On the attractor structure, one can say more for the particular case of system (11),
(12), where we have a single resource: M = 1. Numerical simulations for this case
show that all trajectories tend to equilibria. To understand this fact, let us recall the fundamental concept of cooperative systems (Hirsch, 1985; Smith and Thieme, 1991). The
condition, which determines a cooperative system (Hirsch, 1985; Smith and Thieme,
1991) does not hold for (1), (2) but if we change variables to yi = −xi , then in the new
variables this system becomes cooperative. This fact implies that all local attractors are
stable equilibria. The stable rest points (x̄, v̄) of systems (1) and (2) can be found as
follows. Let us set, for simplicity, that
γi j = γi δi j ,
γi > 0.
(35)
Setting dxi /dt = 0 in (1), we obtain x̄i = γi−1 (φi (v̄)−ri )+ , where f + denotes max{ f , 0}.
This gives the following nonlinear equation for v̄:
D(R(v̄) − v̄) = ∑ ci γi−1 (φi (v̄) − ri )+ φi (v̄),
(36)
i=1
where
N
R(v) = S̄ + ∑ b j x̄ j (v).
j=1
We have obtained a complicated equation with a non-smooth nonlinearities. One
can show that a solution v̄ always exists. Below we present results on a numerical
solution of this equation.
6.2. Numerical results
In the general case the equations for equilibria can be resolved numerically for
N = 50. We choose the coefficients in eq. (36) as follows. The positive coefficients ai
and ri are random numbers subject to log-normal distributions. This means that ln(ai )
are distributed normally, lnai ∈ N(Ea , sa), where Ea is the mean and sa is the deviation.
Similarly, ln ri ∈ N(Er , sr ). We set
bi (T ) = b̄i α (T ),
α = exp(k0 (T − 300)/300),
11
4.85
Biomass of Ecosystem
4.8
4.75
4.7
4.65
4.6
300
300.1
300.2
300.3
300.4
300.5
300.6
300.7
300.8
300.9
301
Temperature
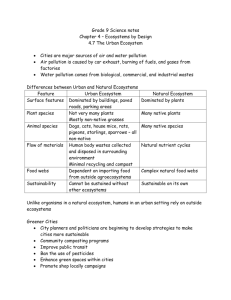
Figure 1: The graph shows the influence of the temperature on species abundances for
negative feedback case, p = 0.8. The curve shows the total biomass of ecosystems as
a function of temperature.
where |b̄i | ∈ N(Eb , sb ) and the absolute temperature T ∈ (300, 301). This relation describes a dependence of the feedback loop on temperature. Moreover, we choose the
sign of b̄i randomly. With a probability p ∈ (0, 1) we set b̄i = |b̄i | and with probability
1 − p we set b̄i = −|b̄i |. Therefore, the parameter p determines the sign of ecosystemclimate feedback loop. If p < 0.5 we have, on average, a positive feedback, for p > 0.5
we have a negative feedback and p ≈ 0.5 corresponds a neutral case.
The parameters were taken as follows: D = 10, K = 3, S̄ = 10, Ea = 1, sa = 0.1, Eb =
1, sb = 0.2, k0 = 4 and γi = 1.
The results are shown by Fig.2, Fig.2 and Fig.3 . These plots correspond to the
cases p = 0.8, p = 0.5 and p = 0.2, respectively.
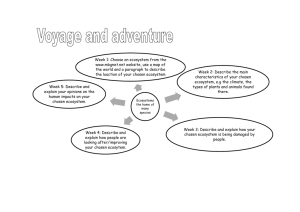
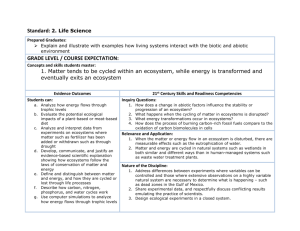
These graphs show the following effects. For negative and neutral feedback cases
the biomass and the number of coexisting species (such that xi (v̄) > 0) is more than for
positive feedback. Indeed, for p = 0.8 and p = 0.2 less than Ne = 20 species coexist.
For p = 0.8 we have Ne = 34 for T < Tc but Ne = 0 for T > Tc , i.e., we observe a total
ecological catastrophe. This catastrophic effect depends on the ecosystem parameters:
it is possible if S̄/(S̄ + K) ≈ P̄, where P̄ is the average of ri /ai .
7. Conclusions
In this paper, a resource model for phytoplankton species is developed, which generalizes the well known model in (Huisman and Weissing, 1999) and takes into account
12
15.35
Biomass of Ecosystem
15.3
15.25
15.2
15.15
15.1
15.05
15
14.95
300
300.1
300.2
300.3
300.4
300.5
300.6
300.7
300.8
300.9
301
Temperature
Figure 2: The graph shows influence of the climate on species abundances for p = 0.5,
a neutral case with no climate effects.
species self-regulation, extinction, and a dependence on the environment. Such conceptual models describe a simple and easily understandable mechanism for resource
competition. For the case of fixed parameters, a general assertion on attractor existence for this model is proved. One of the sufficient conditions of the attractor existence is that species self-regulation is stronger than the species competition. For the
case of a single resource the large time behavior of solutions can be described since
then the system is cooperative. We find that all local attractors are stable steady points.
These points can be found from a nonlinear equation for the equilibrium resource level.
Climate-ecosystem feedbacks are an important problem in terms of uncertainty in predictions and modeling future climate change. The proposed model enables investigation of climate-ecosystem feedbacks for large ecosystems. For the case of positive
feedback in the ecosystem-climate interaction, the numerical results show a possibility
of catastrophic bifurcations when all species become extinct under the impact of climate warming. For negative feedback the biomass and biodiversity are less but we do
not observe catastrophes. Note that in the contemporary world the human impact on
the climate can lead to a positive feedback.
To investigate more complicated situations, when complex dynamics may be possible, we have considered the case of a few resources. We find asymptotic solutions
for the case of a large resource turnover. This allows us to reduce this system to the
Lotka-Volterra model, which is very well studied. We propose a multispecies ecosystem where ecological interactions reduce to competition and self-limitation as well as a
parameter that determines the intensity of ecosystem-climate interactions. We find that
13
20
18
Biomass of Ecosystem
16
14
12
10
8
6
4
2
0
300
300.1
300.2
300.3
300.4
300.5
300.6
300.7
300.8
300.9
301
Temperature
Figure 3: The curve shows the total biomass of the ecosystems as a function of temperature, in the case of p = 0.2 with a positive feedback loop.
all local attractors are equilibria and we do not observe complicated dynamical effects
when the system is under the negative feedback loop. If the ecosystem-climate interaction involves both positive and negative feedback loops, there are possible AndronovHopf bifurcations and time periodic behavior for the case of two resources and chaotic
behavior for three resources.
Acknowledgments
This study was funded by RFBR, according to the research project No.16-31-60070
mol a dk. We gratefully acknowledge support from the Government of the Russian
Federation through mega-grant 074-U01 as well as from the Division of Mathematical
Sciences and the Division of Polar Programs at the U.S. National Science Foundation
(NSF) through Grants DMS-0940249 and DMS-1413454. We are also grateful for
support from the Office of Naval Research (ONR) through Grant N00014-13-10291.
Finally, we would like to thank the NSF Math Climate Research Network (MCRN) as
well for their support of this work.
References
Ardyna, M., Babin, M., Gosselin, M., Devred, E., Rainville, L., Tremblay, J.-E., 2014.
Recent Arctic Ocean sea ice loss triggers novel fall phytoplankton blooms. Geophys.
14
Res. Lett. 41, 62076212.
Arhonditsis, G.B., Brett, M.T., 2004. Mar. Ecol. Prog. Ser. 271, 13-26.
Arrigo, K.R., Perovich, D.K., Pickart, R.S., Brown, Z.W., van Dijken, G.L., Lowry,
K.E., Mills, M.M., Palmer, M.A., Balch, W.M., Bahr, F. et al., 2012. Massive phytoplankton blooms under Arctic sea ice. Science 336(6087), 1408.
Chapin, F.S., Matson, P.A., Mooney H.A., 2002. Principles of Terrestrial Ecosystem
Ecology. Springer Science and Business Media.
Doney, S.C., Ruckelshaus, M., Duffy, J.E., Barry, J.P., Chan, F., English, C.A.,
Galindo, H.M., Grebmeier, J.M., Hollowed, A.B., Knowlton, N., Polovina, J., Rabalais, N.N., Sydeman, W.J., Talley, L.D., 2012. Climate change impacts on marine
ecosystems. Ann. Rev. Mar. Science 4, 11-37.
Field, C.B., Behrenfeld, M.J., Randerson, J.T., Falkowski, P., 1998. Science 281,
237240.
Hirsch, M., 1985. Systems of differential equations that are competitive or cooperative.
II: Convergence almost everywhere, SIAM J. Math. Anal., 16. 423-439.
Hofbauer, J., Sigmund, K., 1988. Evolutionary Games and Population Dynamics. Cambridge University Press, Cambridge.
Hsu, S.B., Hubbell, S., Waltman, P., 1977. A mathematical theory for single-nutrient
competition in continuous cultures of microorganisms. SIAM J. Appl. Math. 32,
366-383.
Huisman, J., Weissing, F.J., 1999. Biodiversity of plankton by species oscillations and
chaos, Nature 402, 407-410.
Hutchinson. G.E., 1961. The paradox of the plankton. Am. Nat., 95, 137145.
Irigoien, X., Huisman, J., Harris, R.P., 2004. Global biodiversity patterns of marine
phytoplankton and zooplankton. Nature 429: 863867.
Kozlov, V., Vakulenko, S., 2013. On chaos in Lotka-Volterra systems: an analytical
approach. Nonlinearity 26 , 2299-2314.
Legrand, C., Rengefors, K., Fistarol, G.O., Granli, E., 2003. Allelopathy in phytoplankton Biochemical, ecological and evolutionary aspects. Phycologia 42, 406-419.
Richardson, T.L., Gibson, C.E., Heaney, S.I., 2000. Temperature, growth and seasonal
succession of phytoplankton in Lake Baikal, Siberia. Freshw. Biol., 44, 431440.
Roy, S., Chattopadhyay, J., 2007. Towards a resolution of ’the paradox of the plankton’:
A brief overview of the proposed mechanisms. Ecol. Complex. 4, 26-33
Ruelle, D., 1989. Elements of differential dynamics and bifurcation theory. Academic
Press, Boston.
15
Sekerci, Y., Petrovskii, S., 2015. Mathematical modelling of plankton-oxygen dynamics under the climate change. Bull. Math. Biol. 77, 2325-2353.
Selkoe, K.A., Blenckner, T., Caldwell, M.R., Crowder, L., Erickson, A., Essington,T.E.
et al. 2015. Principles for managing marine ecosystems prone to tipping points.
Ecosystem Health and Sustainability 1, art17.
Smith, H.L., 1981. Competitive Coexistence in an Oscillating Chemostat. SIAM J.
Appl. Math. 40, 498-522.
Smith, H.L., Thieme, H.R., 1991. Convergence for strongly order-preserving semiflows, SIAM J. Math. Anal. 22, 10811101.
Takeuchi, Y., 1996. Global Dynamical Properties of Lotka-Volterra Systems. World
Scientific, Singapore.
Tilman, D., 1977. Resource competition between platonic algae: an experimental and
theoretical approach. Ecology 58, 338-348.
Toseland, A., S.J., Daines, Clark, J.R., Kirkham, A., Strauss, J., Uhlig, C., Lenton,
T.M., Valentin, K., Pearson, G.A., Moulton, V., Mock, T., 2013. The impact of temperature on marine phytoplankton resource allocation and metabolism. Nat. Clim.
Chang. 3, 979-984.
Travers, M., Shin, Y.J., Jennings, S., Cury, P., 2007. Towards end-to-end models for investigating the effects of climate and fishing in marine ecosystems. Prog. Oceanogr.
75, 751770.
Ulanowicz, R.E. Kemp, W.M.,1979. Prediction, Chaos and ecological perspective. In:
Efraim Halfon, (ed.), Theoretical Systems Ecology. Academic Press, NY.
Vakulenko, S., 2013. Complexity and Evolution of Dissipative Systems. An Analytical
Approach. Berlin, Boston: De Gruyter.
Walther, G.R., 2010. Community and ecosystem responses to recent climate change.
Phil. Trans. R. Soc. B 365, 20192024.
16