Potential for Pathogen Growth, Fecal Indicator Growth
advertisement

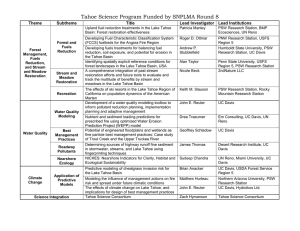
Title: Subtheme this proposal is responding to Principal Investigator and Receiving Institution Co-Principal Investigator Agency Collaborators Potential for Pathogen Growth, Fecal Indicator Growth and Phosphorus Release under Clam Removal Barriers in the Lake Tahoe Basin 2b: Special status species and communities and priority invasive species Dr. Stefan Wuertz Dept of Civil and Environmental Engineering University of California-Davis Davis, California 95617 Phone: (530) 754 6407 FAX: (530) 530 752 7872 Email: swuertz@ucdavis.edu Dr. Geoffrey Schladow Tahoe Environmental Research Center University of California- Davis Davis, California 95617 Phone: 530-752-3942 FAX: 530 754 9364 Email: gschladow@ucdavis.edu Doug Smith Chief, Lake Tahoe TMDL Unit Regional Water Quality Control Board 2501 Lake Tahoe Blvd. South Lake Tahoe, CA 96151 Phone number: 530 542-5453 DFSmith@waterboards.ca.gov Jack Landy US EPA Lake Tahoe Basin Coordinator c/o TRPA PO Box 5310, Stateline, NV 89449 Phone number: 775 589-5248 FAX: 775 588 4527 landy.jacques@epa.gov Grants Contact Person Funding requested: Tim Hagan Tahoe Regional Planning Agency Water Quality Program PO Box 5310, Stateline, NV 89449 Phone number: (775) 589-5314 FAX: (775) 588-4527 thagan@trpa.org George Malyj John Muir Institute of the Environment (JMIE) Watershed Sci Bldg, RM 1105G Davis, CA 95616 Phone: 530 752 3938 FAX: 530 754 9364 gjmalyj@ucdavis.edu $99,395 1 II Proposal Narrative a. Project Abstract The project seeks to measure the impact of clam barriers –rubber sheets that are spread on the bottom of Lake Tahoe to create anaerobic conditions to kill Asian clams – on the survival and re-growth of fecal indicator bacteria (FIB) and potential bacterial pathogens, and the release of soluble reactive phosphorus (SRP) from the anaerobic sediments that are produced through the treatment. The project is motivated by recent in-lake pilot experiments that demonstrated that barriers, when properly designed and installed, are effective at killing Asian clams in near-shore areas but that the anaerobic, relatively warm, and nutrientrich conditions that are produced under the barriers may result in undesirable water quality impacts. Experiments testing the hypothesis that elevated FIB levels observed in preliminary experiments at Marla Bay and Lakeside Marina are due to bacterial re-growth and not actual contamination with fecal waste of human or non-human origin will be conducted in laboratory-based microcosms designed to mimic environmental conditions at the bottom of the lake. The simultaneous SRP measurements are intended to quantify release rates of phosphorus under anaerobic conditions (internal nutrient loading). The goals of the project are to (1) establish if FIB can re-grow under low oxygen conditions underneath clam barriers positioned in the lake, (2) perform spiking experiments with fecal material to track the fate of FIB and two relevant bacterial pathogens, Campylobacter jejuni and Salmonella enterica, and (3) quantify the release rates of phosphorus from the sediments associated with Asian clam growth in Lake Tahoe. This information is critical to helping agencies make an informed decision about both the benefits and risks of using bottom barriers to contain the spread of priority invasive species, and will be required as part of permitting associated with large-scale deployments of this technology b. Justification Statement Asian clams (Corbicula fluminea) are considered a major threat to the ecosystem in Lake Tahoe; since their discovery in April 2008 they have increased in density to visibly impair the near-shore areas both through the production of shells and through facilitating the growth of nuisance periphyton (attached algae) and metaphyton (filamentous, non-attached algae). As part of ongoing efforts to control the spread of this invasive species, UC Davis and University of Nevada (UNR) researchers have partnered with a number of agencies including Tahoe Regional Planning Agency (TRPA), Tahoe Resource Conservation District TRCD, U.S. Fish and Wildlife Service (USFWS), Nevada Department of Wildlife (NDOW), Nevada Division of State Lands (NDSL) and the Lahontan Regional Water Quality Control Board (Lahontan) to create an Asian Clam Workgroup (ACWG) and develop a science-based management plan. As part of the approach, several mechanical management operations have been tested in pilot-scale studies: 1) physical removal of clams from lake sediments by suction dredging; 2) installation of bottom barriers to cover resident Asian clam populations and disrupt their access to oxygen and food, eventually leading to anaerobic conditions; and 3) some combination of the two treatments. The studies in Lake Tahoe led to the complete die off of clams under the barriers; concurrently, elevated levels (1-3 orders of magnitude) of fecal indicator bacteria (FIB) were observed where previously counts were very low or below the limit of detection. It is unclear whether the high FIB counts are due to input of fecal material or whether they are linked to the perishing of clams and the associated algal biomass. An alternative possibility is that FIB find refuge in the bottom-associated algae that occur in the vicinity of Asian clam beds. In addition, when lake sediments become anaerobic, phosphorus that is previously bound to the sediment resolubilizes and becomes bioavailable. In some systems this internal nutrient loading is the largest source of bioavailable phosphorus (see for example Robertson et al. 2008). Preliminary field measurements indicated that soluble reactive phosphorus (SRP) concentrations were significantly higher under bottom barriers. Therefore, the proposal addresses the subtheme 2B in the RFP to “evaluate the likelihood or effectiveness of current or potential approaches to prevent establishment, constrain spread, or reduce populations of priority invasive species.” This project will quantify the water quality impacts when bottom barriers are applied to Lake Tahoe. The 2 project has been designed to isolate and quantify the key factors that are believed to contribute to sharply elevated FIB levels below bottom barriers in the lake and apply state-of-the-art molecular tools to detect indicator bacteria. These are the same methods that stand to be adopted by EPA as the new federal recreational criteria. Consequently, the study results will have validity well past 2012 when new recreational water quality criteria are expected to be established by EPA (http://www.epa.gov/waterscience/standards/academy/basic_course/24-bacteria-7-10-09.pdf). The study will also look at the release of SRP under bottom barriers, to determine whether large scale deployment of barriers will have a significant impact on the lake clarity TMDL and a future nearshore TMDL. By studying the response of Asian clam under laboratory conditions, it will be possible to evaluate the potential that bottom barriers may have (1) on the effective in-lake control of Asian clam as well as the control of future quagga and zebra mussel introduction and establishment, (2) on the fate of fecal indicator bacteria that have been filtered by clams and may become established in near-bottom algal mats, (3) on the survival of two important bacterial pathogens and corresponding human health risks to recreational users of the lake and drinking water supplies, and (4) on the release of phosphorus from the bottom sediments. c. Concise Background and Problem Statement Asian clams demonstrate great fecundity and are believed to be responsible for the benthic blooms of the green filamentous algae Zygnema sp. and Spirogyra sp. of summer 2008 (Wittmann, pers. comm.). Their deposition of feces concentrate nutrients in the sediments and hence provide an environmental niche conducive to bacterial growth and oxygen depletion. By increasing local concentrations of + ammonium (NH4 ) and soluble reactive phosphorus (SRP) clams stimulate algal growth and elevated concentrations of these chemical species have been reported in clam beds in Lake Tahoe (Wittmann et al. 2008). Further, Asian clams bioconcentrate calcium and hence create conditions favoring the invasion by other bivalves such as the zebra or quagga mussel. None of these ecological changes are conducive with the use of Lake Tahoe as a drinking water source for the surrounding communities and management strategies that contain these invasive species are urgently needed. Bottom barriers constitute a promising and simple technology to control the spread of Asian clams in the Lake Tahoe basin. Preliminary work in Marla Bay and Lakeside Marina showed that anoxia developed under the barriers within 24 hours. 100% clam mortality was achieved within 2 months. An unexpected observation was the concurrent increase in fecal indicator bacteria levels associated with the use of bottom barriers. Both total coliforms and E. coli were detected at concentrations exceeding water quality criteria when the barriers were removed at the completion of the test. The key question is whether elevated FIB concentrations in the vicinity of bottom barriers represent a public health risk and a reason for concern for the implementation of this technology to control Asian clams in the basin. We will address this concern by conducting controlled laboratory experiments involving microcosms where clams will be exposed to bottom barriers. The decay of traditional indicators, spiked bacterial pathogens and human-specific microbial source identifiers under anoxic/anaerobic conditions will be followed using molecular (DNA-based) methods and traditional indicator tests that are growth-based. The opportunity provided by the experiment will also be used to quantify the release of phosphorus under the same conditions. While not representing a human health risk, phosphorus is the limiting nutrient in Lake Tahoe and large release rates from the sediments may significantly counter the gains made through the TMDL program. The results of this project will significantly contribute to the evaluation of the bottom barrier approach as a management tool. d. Goals, objectives and hypotheses to be tested The goals of this project are to: (1) Assess the potential of bottom barriers to create environmental conditions favoring the survival and 3 re-growth of fecal indicator bacteria (2) Determine the fate of two relevant bacterial pathogens under bottom barriers (3) Quantify the release rate of soluble reactive phosphorus under bottom barriers The objectives are to: (1) Construct flow-through microcosms in the laboratory that mimic the environmental conditions of clam beds in the lake (2) Place intact box cores from Marla Bay and Lakeside Marina (with clams, algae and 6” of underlying sediment) into each microcosm, and operate them in the presence and absence of bottom barriers to provide biological and chemical data for comparative analysis (3) Inoculate replicate microcosms with fecal material from human and animal sources and track the decay of indicator bacteria and fecal source identifiers (Bacteroidales) using a series of well established quantitative genetic assays (4) Simulate a worst-case scenario where in addition to fecal material two bacterial pathogens are introduced into clam beds. Campylobacter jejuni and Salmonella enterica are water- and food-borne human pathogens known to cause gastroenteritis that thrive under microaerophilic (low oxygen) and anaerobic conditions, respectively. S. enterica also grows aerobically. (5) Use the relationships developed to establish whether fecal indicator bacteria measurements near covered clam beds adequately predict public health risks arising from exposure to potential pathogens (6) At regular intervals remove water samples from under the barriers and measure SRP and NH4. The hypotheses are: (1) The installation of bottom barriers changes environmental conditions in sediments and the overlying water by depleting oxygen levels and by increasing the pool of bioavailable nutrients. (2) Fecal indicator bacteria accumulating underneath bottom barriers are not the result of recent fecal contamination (3) Campylobacter jejuni and Salmonella enterica can reproduce underneath bottom barriers (4) In the absence of a point source of fecal pollution such as a sewage spill or malfunctioning septic tank system, human bacterial pathogens do not accumulate and multiply underneath bottom barriers (5) Fecal indicator bacteria accumulating underneath bottom barriers are inadequate predictors of the presence of bacterial human pathogens in the absence of fecal contamination (6) Internal nutrient loading does not significantly alter nutrient availability in the nearshore environment e. Approach, Methodology and Location of Research Previous work and preliminary results This is a laboratory measurement study and the results of the project will be useful to understand the impact of bottom barriers on the fate of fecal indicator bacteria (E. coli, Enterococcus and Bacteroidales) and bacterial pathogens in Lake Tahoe. The microcosms will be constructed by upgrading commercially available aquariums and operated in continuous flow and batch mode in the research laboratories at the Tahoe Research Center at Incline Village. The PI has extensive experience with flow-through microcosms to address research questions pertaining to the environmental fate and transport of microbial indicators and pathogens (e.g. Bae and Wuertz, in press; Schriewer et al. 2009; Wuertz et al. 2009). For example, the decay functions for Enterococcus and E. coli were determined in freshwater using approved culturing methods, quantitative PCR (qPCR) to detect a target gene sequence specific for Enterococcus, and a modification of qPCR (Bae and Wuertz 2009) to detect the target sequence only in viable cells (Fig. 1). Similarly, we have determined the decay functions of specific human pathogens like Campylobacter jejuni and Salmonella enterica and adenovirus (Fig. 2) and fecal source identifiers (Bacteroidales) for human, dog, cow and universally for all tested animals that carry Bacteroidales (Fig. 3). To summarize, prior work in the Wuertz laboratory has established that the microorganisms of interest are expected to die off in the water column leading to various 2-log reduction times (T99). The microcosm 4 approach will allow us to compare FIB and pathogen decay profiles in the benthos in the presence of clams with and without bottom barriers with those in the water column. Research by TERC (Schladow was PI) has shown that bottom barriers work efficiently at killing clams in Marla Bay and at Lakeside Marina. However, when barriers were removed levels of total coliforms and E. coli were highly elevated compared to other sites in the lake (Table 1). This observation led us to perform microbial source tracking analysis on a small set of samples that included biofilm (Cladophora algae growing close to clams) underneath the bottom barrier, water collected away from the bottom barrier at 5 m depth in Marla Bay (labeled background), and water at the Midlake sampling station, which served as a negative control. The Bacteroidales source tracking method has been widely used in California and is currently the preferred source tracking method in EPA studies. The human-specific genetic marker was absent in all samples and the universal fecal marker was also not detected at the control site (Table 2). The universal fecal marker was present in the biofilm Cladophora sample and also at a depth of 5 m in Marla Bay, indicating that there is some input of fecal material into Lake Tahoe. This result would be expected given that a variety of waterfowl carry Bacteroidales bacteria. Concurrent analysis of E. coli and total coliforms as measured by culture based methods was not possible for biofilm samples, but the sample taken at 5 m depth revealed only low concentrations of E. coli and fecal coliforms at 1 MPN/100 mL and 36 MPN/100 mL, respectively (Table 2). These recent analyses together with the monitoring results presented in Table 1 suggest that the elevated fecal indicator concentrations are indeed limited to areas covered by bottom barriers. Table 1 Fecal indicator bacteria (FIB) in samples taken at various times during with the bottom barrier pilot study. The 8/26 samples (barrier) are associated with water under the bottom barriers installed for clam control. Barriers were located at Marla Bay (southeast) and at Lakeside (more to the west). Yellow highlighting indicates elevated levels of FIB. Source: John Reuter, TERC Sampling location Sample date and time Method Marla Bay background Marla Bay in dredge plume Lakeside background Lakeside in active dredge plume 3/18/2009@ 1030 3/18/09 @ 1335 4/7/09 @ 0850 SM 9223B SM 9223B SM 9223B <1 1 <1 <1 <1 <1 4/7/09 @ 1000 SM 9223B 6.4 <1 Lakeside under dredge plume 4/7/09 @ 1030 SM 9223B <1 <1 Marla Bay background Marla Bay barrier moved 6/11/09 @ 1045 6/11/09 @ 1100 SM 9223B SM 9223B 1 2 <1 <1 Marla Bay under barrier 6/11/09 @ 1155 SM 9223B 2 <1 Marla Bay background Marla Bay multiple barrier removal 7/30/09 @1106 SM 9223B 1 <1 7/30/09 @1125 SM 9223B 201.4 <1 Marla Bay background 8/26/09 @ 1115 SM 9223B 35 16 Marla Bay near barrier 8/26/09 @ 1120 SM 9223B 63.1 38.4 Marla Bay under barrier 8/26/09 @ 1130 SM 9223B 1046 866.4 Lakeside background 8/26/09 @ 1215 SM 9223B 5.2 1 Lakeside under barrier 8/26/09 @ 1220 SM 9223B 121.1 1 5 Total Coliform E. coli MPN/100 ml MPN/100 ml Table 2 Universal and human-specific fecal Bacteroidales DNA in Lake Tahoe. Water samples were from a depth of 5 m in Marla Bay and Midlake; biofilm samples were of Cladophora outside the bottom barriers at Marla Bay. DNA from triplicate subsamples of each sample was purified. Location Universal Human-specific Bacteroidales Bacteroidales -1 (gc⋅ mL or gc⋅ mg-1 wet weight) Midlake 1 ND ND Midlake 2 ND ND Midlake 3 Marla-Bay Background 1 Marla-Bay Background 2 Marla-Bay Background 3 Biofilm 1 ND ND 6.36 x 102 ND 4.39 x 102 ND 1.22 x 103 ND 8.87 x 102 ND Biofilm 2 9.72 x 101 ND Biofilm 3 6.19 x 102 ND Total Coliform E. coli (MPN/100 ml) Not determined Not determined 35 1 Not determined Not determined ND: Not detected gc: Gene copies Study design Our approach relies on microcosms as testbeds to investigate the hypotheses formulated earlier. Experimental methods will involve both established methodology for total coliforms and E. coli used for monitoring by regulatory agencies and local authorities and selected molecular techniques to determine indicator and pathogen concentrations, which represent the monitoring tools the U.S. EPA is considering for its revised guidelines in 2012, and which the PI helped develop in his laboratory. For example, we will track genetic markers for human, dog- and cow-specific fecal pollution based on the order Bacteroidales and correlate results with those obtained by enumerating fecal indicator bacteria with traditional methods that rely on growing bacteria. We will also measure Enterococcus by qPCR, a method that has been suggested by EPA as the method of choice to monitor recreational water quality under the new guidelines being developed. Tracking real pathogens is important because it provides information that can be used by agencies to assess microbial risks .The combined use of all these tools will ensure that the results of the project will be relevant to agencies now and in the future. The phosphorus measurements will be performed in the TERC laboratory using the identical methods that TERC uses for the routine monitoring of Lake Tahoe and the inflowing streams. Four microcosms will be constructed from basic aquarium accessories and operated concurrently at the TERC. They will house an intact box core taken directly from regions considered typical for clam and attached algae concentrations. They will be exposed to the following conditions: I II III IV Core without bottom barrier Core with bottom barrier Core with bottom barrier after addition of mixture of human/animal feces Core with bottom barrier after addition of mixture of human/animal feces and cultures of Campylobacter jejuni and Salmonella enterica 6 Testbed I will serve as control to determine whether clams by themselves lead to any noticeable increase in fecal indicator concentrations. Testbed II will determine whether the application of bottom barriers leads to environmental conditions that release or support reproduction of indicator bacteria that have been filtered by clams or that have accumulated in algal mats. Testbed III will simulate a known fecal input from a point source. A mixture of untreated municipal wastewater and animal waste will be employed. Testbed IV will reflect a worst-case scenario where a point source of fecal material containing bacterial pathogens (Campylobacter and Salmonella) is released to clams under a bottom barrier. Groundwater from a well adjacent to Lake Tahoe (unchlorinated water) will be utilized for this study. All microcosms will be incubated indoors under controlled temperature and light (diurnal cycle) conditions while providing conditions of environmental stresses such as temperature and light penetration that are comparable to (Fig. 4). Each microcosm will be initially aerated using aquarium air pumps, which serve to maintain a typical dissolved oxygen concentration for surface water in the tank because the dissolved oxygen concentration is relatively low in groundwater. In a second experiment aeration pumps will be turned off to determine how fast anaerobic conditions will be established underneath bottom barriers under low flow conditions. Measurements for dissolved oxygen, pH, conductivity, salinity and temperature will be taken using YSI-55 and YSI-63. In addition, SRP and NH + 4 will be monitored. Measurements of all variables including FIB will be made daily for the first week following setup and after that once a week. Genetic analyses based on qPCR will be conducted in the PI’s laboratory at the UC Davis campus in Davis. Nutrient analyses will be performed at 3-7 day intervals. After exiting from the tanks, the water will be treated by UV irradiation and then discharged into a sewer to prevent accidental microbial contamination. Experiments will run during the summer for approximately 3 months in the first round and it is planned to perform a second round with some replication and additional variation based on the results obtained in the first round. A first-order kinetics model or two-stage exponential-plus-linear model will be used to fit the experimental data and to calculate the pathogen and indicator decay rates and T99 (time for 2-log reduction): Equation 1 Here, N is the gene copies number/mL of host-specific Bacteroidales at the time t, N0 and N1 is the initial concentration of host-specific Bacteroidales, y0 is a constant and k1, k2, and k3 are the decay rate constants. Statistical tools provided in the MINITAB® version 15 will be used for data analysis. The main and interaction effects of the variables tested will be analyzed by analysis of variance. f. Relationship of the research to previous relevant research, monitoring and/or environmental improvement efforts The proposed project builds on the research that has been conducted in the area of control of Asian clams in the last 18 months at Lake Tahoe. As part of the Basin’s response to the discovery of Asian clam, UC Davis and UNR researchers have studied the location, physiology and ecological impacts of this priority invasive species. In addition, with the support of basin agencies, this research team has conducted the 7 design and scientific monitoring of pilot scale experiments of potential control strategies. The recent observations of high concentrations of E. coli and dissolved phosphorus in these pilot scale experiments is what has directly led to this proposal. The PI (Wuertz) was contacted by the pilot project research team to enlist his expertise in understanding what was being observed, and several samples were sent down to his lab for preliminary analysis (described above). The co-PI (Schladow) is part of the pilot project research team, and will be interacting with Dr Wuertz to ensure that the box core samples are representative of the substrate of concern, and to ensure that the results of these two studies (and other related work) are being well communicated between the various research teams and the relevant agencies. This project, though simple in scope and short in its duration, is essential to ensuring that there are no (or at least acceptable) water quality consequences to the treatments being considered, and will provide essential information that will be required for general permitting. Without acquiring this information at this point, the eventual invasive species action plan may be delayed in the future. g. Strategy for engaging with managers Following an initial start-up workshop with the Asian Clam Workgroup (ACWG), we will report to the ACWG after approximately 6 months and towards the end of the project. Agencies represented in the ACWG include Tahoe Regional Planning Agency (TRPA), Tahoe Resource Conservation District TRCD, U.S. Fish and Wildlife Service (USFWS), Nevada Department of Wildlife (NDOW), Nevada Division of State Lands (NDSL) and the Lahontan Regional Water Quality Control Board (Lahontan). TERC is actively engaged with most monitoring programs in the Tahoe Basin (e.g. LTIMP, RSWMP) and we will also keep those groups apprised of results. By providing close interaction with the entire community of scientists performing aquatic invasives work at Lake Tahoe, we will ensure that the unintended water quality consequences of invasive species treatments are understood and fully communicated at all levels. h. Description of deliverables/products Report describing: 1. the effect of bottom barriers on the retention and amplification of indicator bacteria 2. the effect of bottom barriers on the retention and amplification of two bacterial pathogens 3. the effect of bottom barriers on release of phosphorus and formation of ammonium under the barriers 4. Estimates of the loadings of indicator bacteria, pathogens and nutrients due to the large-scale deployment of bottom barriers in Lake Tahoe. 5. Recommendations for the deployment of bottom barriers considering worst-case scenarios involving point sources for fecal contamination 8 III Schedule of Major Milestones/deliverables “Potential for Pathogen Growth, Fecal Indicator Growth and Phosphorus Release under Clam Removal Barriers in the Lake Tahoe Basin” - Project Schedule Months of project duration Task Start-up meeting and workplan Agency (ACWG) workshops Install & operate microcosm equipment Quarterly Rpt and Invoice 1 2 3 4 5 6 7 8 9 10 11 12 x x x x x x x x x x Drafts of publication manuscripts Draft Final Report x x x x x x Final Report and Invoice x 9 IV References Bae, S. and S. Wuertz. (2009) Discrimination of viable and dead fecal Bacteroidales bacteria by quantitative PCR with propidium monoazide. Appl. Environ. Microbiol. 75:2940-2944. Bae, S. and S. Wuertz. (2009) Rapid decay of host-specific fecal Bacteroidales cells in seawater as measured by quantitative PCR with propidium monoazide. Water Research, in press Robertson, D. M., Schladow, S. G. and Holdren, G. C. 2008. Long-term changes in the phosphorus loading to and trophic state of the Salton Sea, California. Hydrobiologia, 604, 21-36. U.S. EPA (2007). Criteria Development Plan & Schedule—Recreational Water Quality Criteria: U.S. EPA Offices of Water and Research and Development: Washington, D.C., United States. U.S. EPA (2007). Critical Path Science Plan for the Development of New or Revised Recreational Water Quality Criteria, U.S. EPA Office of Research and Development: Washington, D.C., United States. Vaughn, C. C. and C. C. Hakenkamp. (2001) The functional role of burrowing bivalves in freshwater ecosystems. Freshwater Biology 46:1431 1446. Wittmann, M., J. Reuter, J., G. Schladow, S. Hackley, B. Allen, S. Chandra, and A. Caires. (2008) Asian clam (Corbicula fluminea) of Lake Tahoe: Preliminary scientific findings in support of a management plan. Wittmann, M., S. Chandra, J. Reuter, G. Schladow, T. Thayer, N. Cartwright, D. Roberts, S. Chilton, D. Smith, K. Tisdale, D. Catalano, E. Harrison, and D. Oliver. (2008) Development of Asian clam control and monitoring plan strategies for Lake Tahoe. Report Schriewer, A., Bae, S., Rizvi, A., Sirikanchana, K., Wang, D. and Wuertz, S. (2009). Completion of environmental toolkit for fecal source tracking and pathogen analysis in stormwater. Report prepared for the Environmental Division of California Department of Transportation. Execution Date: Contract No. 43A0168. Task Order No.: 23. Wuertz S., Bombardelli F., Sirikanchana K., Schriewer A., Zamani K. (2009). Quantitative Pathogen Detection & Microbial Source Tracking Combined with Modeling the Fate and Transport of Bacteroidales in San Pablo Bay. Report prepared for NOAA/UNH Cooperative Institute for Coastal and Estuarine Environmental Technology. NOAA Grant Number NA06NOS4190167. 1 V. Figures Fig. 1. The decay curves in a freshwater microcosm for culturable E. coli or Enterococcus and the 23S rRNA gene of Enterococcus measured inside viable cells by PMA-qPCR and in the total sample by qPCR. n =3; the error bar represents standard deviation. (A) Culturable E. coli or Enterococcus cells under natural sunlight exposure. (B) Culturable E. coli or Enterococcus cells under dark conditions. Open and closed squares denote culturable Enterococcus and E. coli, respectively. (C) The gene copies number of Enterococcus measured by qPCR under sunlight exposure. (D) The gene copies number of Enterococcus measured by qPCR under dark conditions. Open circles represent viable Enterococcus cells measured by PMA-qPCR whereas closed circles represent total DNA including viable, dead cells and extracellular DNA. 2 Fig. 2 Survival of waterborne pathogens in a freshwater microcosm as measured by PMA-qPCR or qPCR in freshwater. n =3; the error bar represents standard deviation. The decay of Campylobacter jejuni under sunlight exposure (A) and dark conditions (B), and Salmonella enterica under sunlight exposure (C) and dark conditions (D). Open and closed circles denote target concentration (gene copies/ml) measured by PMA-qPCR and qPCR, respectively. The decay curve of adenovirus DNA is shown under sunlight exposure (E) and dark conditions (F). 3 Fig. 3. Survival of host-specific Bacteroidales cells measured by PMA-PCR and the persistence of DNA including viable/ dead cells and extracellular DNA as measured by qPCR under natural sunlight exposure in freshwater. Open and closed circles denote target concentration (gene copies/ml) measured by PMA-qPCR and qPCR, respectively. The dot line of DNA and the dash dot line were plotted using a first-order decay model and an exponential plus linear model, respectively. 4 Fig. 4. Microcosm setup to study effects of bottom barriers on microbial indicator and pathogen populations, and internal phosphorus loading. 5