Document 11212129
advertisement

Functions of the eukaryote-specific ribosomal protein
Asc1 /RACK1 in gene-specific translational activity
RH4NSE
MASSAC
By
NUSELS STITUTE
OF TECHNOLOGY
Mary Katherine Thompson
B.S. Biochemistry & Cell Biology
Rice University, 2008
LE
P 17 2015
Ll
3RARIES
Submitted to the Department of Biology In Partial Fulfillment of the Requirements for the
Degree of
Doctor of Philosophy
At the
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
September 2015
@ 2015 Massachusetts Institute Of Technology. All rights reserved
Signature redacted
Signature of Author:
Signature redacted
Department of Biology
September 4, 2015
Certified by:
Wendy V. Gilbert
Associate Professor of Biology
Signature redacted
Accepted by:
Thesis Supervisor
Michael Hemann
Associate Professor of Biology
Co-Chair, Graduate Committee
Functions of the eukaryote-specific ribosomal protein Asc1/RACK1
in gene-specific translational activity
By
Mary Katherine Thompson
Submitted to the Department of Biology on September 4, 2015 in Partial
Fulfillment of the Requirements for the Degree of Doctor of Philosophy in Biology
Abstract
Although the ribosome operates as a single molecular entity, it is composed of both
ribosomal RNAs and dozens of proteins. However, the individual contributions of most
ribosomal components to translational regulation are unknown. In Chapter 1, I will
review the current state of knowledge related to the functions of ribosomal proteins with
a focus on the RACK1 protein (Asc1 in yeast), a eukaryote-specific ribosomal protein
with many proposed functions in both cellular signaling and translation.
In Chapter 2, I will present evidence that the Asci protein is required for efficient
translation of a specific set of mRNAs with short open reading frames (ORFs), including
those that encode ribosomal proteins and nuclear-encoded mitochondrial components.
Consistent with these translation defects, ASC1 mutants are unable to grow in
conditions requiring full mitochondrial function. Asci -sensitive mRNAs are highly
associated with the translational closed-loop complex, a group of proteins that promotes
a loop-like conformation of the mRNA during translation by simultaneous interaction with
the 5' and 3' ends of the mRNA molecule. In wild type cells, mRNAs that associate
strongly with the translational closed-loop complex are much shorter than other ORFs.
Thus, I hypothesize that the closed-loop is preferentially formed and/or stabilized on
mRNAs with short ORFs, and that this process is enhanced by the presence of Asci on
the small ribosomal subunit. The dependence of closed-loop formation on ORF length
could also explain why short ORFs have notably higher translation efficiency than longer
ORFs, a trend I observed in data collected from several eukaryotes.
In Chapter 3, I will present evidence that the mammalian RACK1 protein is also required
for expression of mRNAs with short ORFs and for mitochondrial function in HeLa cells,
similar to my observations in yeast. These findings hint at a conserved role for the
Asc1/RACK1 protein in promoting the function of the closed-loop complex and the
translation of short ORFs, which encode a set of highly abundant proteins required for
central metabolic functions. Chapter 4 will discuss the biochemical and cell physiological
implications of these findings and suggest some avenues for future research.
Thesis Supervisor: Wendy V. Gilbert
Title: Associate Professor of Biology
2
Acknowledgements
I would like to thank all the enthusiastic supporters of my education and scientific
development over the many years. Firstly, I need to thank my family for always being
the people I turned to whenever I needed advice or encouragement during my PhD,
which was a lot. They never doubted that I could do it!
Next, I would like to thank my teachers. My first life sciences teacher Ms. Whitney for
kindling my interest in biology back in the late 90's. My high school chemistry and
biology teachers, Mr. Terry and Mr. Kelley, for being enthusiastic teachers and for
encouraging my interest in science. I would also like to thank my high school English
teacher Ms. Bailey for all the red ink she spilt in pursuit of teaching me to read and write
critically.
I would like to thank Bonnie Bartel for letting me work in her lab as an undergraduate
and for encouraging me to go to grad school. I'm sure that if I had not been so lucky to
stumble upon such a supportive and fun lab environment, I would have left science long
ago. I am also very fortunate to have been mentored by her extremely patient grad
student, Jeanne Rasbery, who was the perfect mentor for my skittish undergrad phase.
I am very thankful to Wendy for taking me on as one of her first grad students. I have
learned a lot from Wendy over the years - how to be charming, persistent, and
scientifically audacious. Wendy always respected my opinions and eventually I gained
the confidence to voice them (now she probably wishes I would shut up sometimes). I
hope I can continue to cultivate these traits as I move forward.
I'd also like to thank my thesis committee, Dave Bartel and Steve Bell, for scientific and
career advice over the years. I am also thankful to Allan Jacobson for agreeing to be on
my thesis defense committee and for his valuable scientific insight.
I'd like to thank all my labmates for many fun days both in and outside the lab. I am
especially indebted to the group of people that joined the lab around the same time as
me and shaped my early experiences there. Pavan Vaidyanathan, Thomas Carlile, and
Maria Rojas-Duran were generous with their time and knowledge. Grad students Boris
Zinshteyn and Josh Arribere were both good friends and invaluable scientific advisors. I
hope our paths will cross again someday. Perhaps on another 25-mile hike?
Finally, I would be remiss not to thank Coppe van Urk for all of his love and support over
the years. It was a blessing to go through grad school together. In addition to being a
great hiking, beer-drinking, and TV-watching partner, his eternal optimism is a constant
reminder to me to chill out and enjoy the science.
3
TABLE OF CONTENTS
Chapter I:.........................................................................................................................6
Introduction.....................................................................................................................6
Overview ..................................................................................................................................
The functions of the ribosom al subunits during translation..........................................
The translational closed-loop model...............................................................................
The functions of specific ribosom al proteins.................................................................
The RACK1 protein - a link between the ribosome and cellular signaling? .................
What is the ribosome-associated function of the RACK1 protein?.................................
Genetic analysis of the ASC1 locus ...............................................................................
Quantifying translation genome-wide: the road to a molecular phenotype...............
Do ribosomal proteins have functions in gene-specific translation? .........................
Thesis overview ....................................................................................................................
References ............................................................................................................................
7
7
11
13
16
20
24
26
29
30
32
Chapter II:......................................................................................................................42
Asci/RACK1 is required for efficient translation of short mRNAs .....................
42
Abstract .................................................................................................................................
Introduction ...........................................................................................................................
Results...................................................................................................................................
43
43
47
Loss of the Asc1 protein perturbs global translation ....................................................................
ASC1 'ribosome-binding' mutants associate with ribosomes and are largely functional................
Asc1 promotes translation of mRNAs with short open reading frames..........................................
The translational defects of ASC1 mutants are not a general consequence of perturbing the
rib o s o m e ...........................................................................................................................................
Loss of Asc1 impairs mitochondrial function in yeast.....................................................................
Discussion.............................................................................................................................
Experim ental Procedures ................................................................................................
References ............................................................................................................................
47
51
54
70
72
75
77
88
Chapter III:.....................................................................................................................95
RACK1 promotes the expression of small proteins required for mitochondrial
function in HeLa cells ..............................................................................................
95
Abstract .................................................................................................................................
Introduction ...........................................................................................................................
Results...................................................................................................................................
96
96
99
RACK1 knockdown alters total mRNA levels and ribosome association for many mRNAs.......... 99
RACK1 is required for the expression of mRNAs with short ORFs encoding proteins with functions in
the mitochondria and at the ribosome .............................................................................................
103
RACK1 is required for mitochondrial function in HeLa cells............................................................ 106
Future Directions ................................................................................................................
Discussion...........................................................................................................................
Experim ental Procedures ..................................................................................................
References ..........................................................................................................................
109
110
114
116
Chapter IV: ..................................................................................................................
122
Discussion and future directions .............................................................................
122
Overview ..............................................................................................................................
123
4
A role for ORF length-dependent translational regulation in evolution and cell
physiology ...........................................................................................................................
123
Are all m RNAs translated via the closed-loop?.................................. . ............. ........ ....... 124
The closed-loop as a specialized high-efficiency translation state .............................. 126
A model for mRNA/ORF length-dependent regulation of the closed-loop ................... 128
Future directions ................................................................................................................
132
References ..........................................................................................................................
135
Appendix I:..................................................................................................................139
Transcriptional and translational regulation of the FLO gene family in ribosomal
139
protein m utants ..........................................................................................................
140
Abstract ...............................................................................................................................
141
Introduction .........................................................................................................................
144
Results.................................................................................................................................
156
Discussion...........................................................................................................................
158
Experim ental Procedures ..................................................................................................
References ..........................................................................................................................
159
5
Chapter I:
Introduction
6
Overview
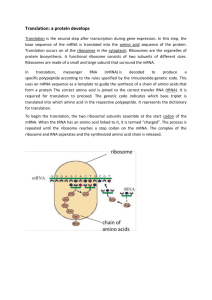
The ribosome is the molecular machine responsible for translating the information
contained in the genome into its ultimate manifestation, the proteins that form the
chemical and structural building blocks of the cell. Although the core chemical function
of peptide bond catalysis is conserved throughout life, domain-specific protein and RNA
components can modulate the efficiency or regulation of translation in different ways. In
this chapter, I will focus on the contributions of individual ribosomal proteins to the
regulation of translation in eukaryotes with further emphasis on RACK1, a eukaryotespecific ribosomal protein of the small ribosomal subunit.
The functions of the ribosomal subunits during translation
The eukaryotic ribosome is composed of the large (60S) and small (40S) subunits. Each
subunit has a distinct role in translation. One important function of the small subunit is to
recruit the mRNA during translation initiation via bridging interactions between the
ribosome-bound initiation factors and the mRNA-bound initiation factors. First, the
mRNA associates with the eIF4F cap-binding complex via interaction of the 5' 7methylguanosine (m 7 G) mRNA cap with the eIF4E component of eIF4F (Figure 1-1).
The eIF4F complex is held together by a large scaffolding protein eIF4G that mediates
interactions between several complexes in the initiation pathway (Pestova et al., 2007).
The recruitment of the eIF4F-mRNA complex to the small ribosomal subunit is an
elaborate process and some details differ between yeast and mammals. In mammals, a
small region in the central domain of eIF4G interacts with the ribosome-associated elF3
complex, and this interaction has been suggested to be a key step in cap-dependent
7
mRNA recruitment (Korneeva et al., 2005; Lamphear et al., 1995). However, this
domain is not conserved in yeast, and no association between these factors has ever
been detected (Marintchev and Wagner, 2005; Morino et al., 2000). It has been
suggested that another initiation factor, elF5, might act as a bridge between eIF4G and
the yeast ribosome by binding to the ribosome-associated elF3 complex and eIF4G
simultaneously (Asano et al., 2001), (Figure 1-1). However, mRNA recruitment still
occurs after elF5 depletion in yeast, which suggests that the elF5 bridging function must
be redundant with other mRNA recruitment mechanisms (Jivotovskaya et al., 2006).
Thus, whether the central process of mRNA recruitment differs drastically between
yeast and mammals or whether important interactions have been missed remains to be
seen.
8
40S recruitment
5'
eIF4E
m 7G cap
elF2-GTP
Met-tRNAym
_;F5
I
W
,
eIF4F complex
3,
ORF
elF3
40S subunit
2
scanning and start site selection
19
60S subunit
GTP
GDP
initiation factor dissociation
and 60S joining
-7,
7?
elongation
00
-a
termination and
ribosome recycling
2l
release factor
Figure 1-1: The functions of the ribosomal subunits during translation. (See next page.)
9
Figure 1-1: The functions of the ribosomal subunits during translation (previous page). During 40S
recruitment, the small ribosomal subunit and associated initiation factors bind to the elF4F cap-binding
complex via interactions with elF4G in mammals and elF5 and/or as yet unidentified factors in yeast. The
40S scans 5' to 3' along the 5' UTR until the elF2-associated Met-tRNAimet recognizes an AUG start
codon by base-pairing. This recognition event triggers a series of conformational alterations resulting in
elF2-GTP hydrolysis and release of most initiation factors, allowing 60S subunit joining. elF3 and possibly
elF4G remain associated with the small subunit during early elongation (P6yry et al., 2004). During
elongation, aminoacylated tRNA recognizes the codon presented in the ribosomal A-site and the peptide
is transferred onto the A-site tRNA. Elongation factors catalyze translocation of the ribosome to the next
codon. When the ribosome reaches a stop codon, release factor recognition mediates release of the
polypeptide from the ribosome. Ribosome recycling factors dissociate the 80S ribosome into free 40S and
60S subunits. For clarity, initiation factors not referred to in the text are omitted from the diagram and the
ribosomal subunits are drawn at smaller scale relative to the translation factors.
After mRNA recruitment, the small subunit scans 5' to 3' along the 5' UTR until it
reaches an AUG start codon (Figure 1-1). Base-pairing between the AUG and the
initiator tRNA (Met-tRNAiMet) stimulates GTP hydrolysis on the elF2 initiation factor and
the release of many small subunit-bound initiation factors, exposing the surface of the
small subunit for interaction with the large subunit (Jackson et al., 2010). After the
joining of the large ribosomal subunit, an aminoacyl-tRNA enters the ribosomal A-site
and peptide bond formation occurs. An elongation factor induces translocation of the
ribosome and a new A-site codon of the mRNA is presented. This process continues
until the ribosome encounters a stop codon. Ribosome release factors recognize the
stop codon and cause hydrolysis of the last aminoacyl-tRNA bond, releasing the
completed peptide. The 80S ribosome is dissociated into small and large subunits, and
the process can begin again (Dever and Green, 2012).
10
The translational closed-loop model
Although drawn in Figure 1-1 as a simple linear 5' to 3' process, biochemical and
microscopy evidence indicates the ability of the mRNA to adopt a circular loop-like form
during translation, which is mediated by the interaction of 5' and 3' mRNA elements with
a protein complex known as the closed-loop complex (Figure 1-2). The mRNA and
associated proteins in this circular form is known as the closed-loop mRNP, or simply
the 'closed-loop'.
Initial biochemical evidence for the closed-loop came from experiments in reticulocytes
in which it was noted that the rate of exchange of free inactive ribosomal subunits into
the translationally active fraction was lower than expected (Adamson et al., 1969;
Baglioni et al., 1969; Philipps, 1965). Combined with the visualization of loop-like
structures of polysomes by electron microscopy (Ladhoff et al., 1981; Mathias et al.,
1964), these experiments led to the conclusion that active ribosomes must be recycled
for translation on the same mRNA, thus going around the loop (Baglioni et al., 1969).
Over the next few decades, data supporting the existence of a circular form of mRNA
during translation amassed (Gallie, 1998). First, the poly(A) tail was suggested to be a
3' enhancer of translation initiation, indicating that it must somehow communicate with
the mRNA 5' end during initiation (Jacobson and Favreau, 1983). Several groups later
reported that the mRNA cap and poly(A) tail act synergistically to promote translation
both in vivo and in in vitro extract systems (Gallie, 1991; lizuka et al., 1994; Tarun and
11
Sachs, 1995), and this finding was explained as the cooperative function of factors
interacting with the 5' and 3' ends during mRNA recruitment (Preiss and Hentze, 1998;
Tarun and Sachs, 1995). These factors were eventually shown to be the elF4G
component of the elF4F cap-binding complex and the poly(A)-binding protein, PABP
(Pabi in yeast) (Tarun and Sachs, 1996; Wells et al., 1998). Hence, the model that
mRNAs circularize via the interaction of PABP with the poly(A) tail and elF4F with the 5'
cap was born. Although our current model of the closed-loop incorporates only
interactions between elF4G, PABP, and the mRNA, it will be interesting to determine
whether the ribosome or ribosome-bound factors function in closed-loop formation or in
closed-loop-dependent mRNA recruitment.
closed-loop form
linear form
5'
eIF4E
m7G
cap
ribosome
ORF
PABP
3'
PABP
closed-loop complex
closed-loop mRNP
Figure 1-2: The mRNA can adopt a circular closed-loop form during translation. Interaction of the
to
eIF4G component of the eIF4F complex with the poly(A) binding protein, PABP, can cause the mRNA
closedin
involved
factors
be translated in a circular form known as the closed-loop mRNP. The protein
loop formation (eIF4E, eIF4G, and PAPB) are known as the closed-loop complex.
12
The functions of specific ribosomal proteins
Although the catalytic function of peptide-bond formation is performed solely by the
ribosomal RNA (Nissen et al., 2000; Noller et al., 1992), the functional ribosome is
interwoven with a large group of proteins (55 in eubacteria and 80 in eukaryotes)
(Melnikov et al., 2012). Ribosomal proteins function both to chaperone the ribosomal
RNA during ribosome biogenesis and also to stabilize the mature ribosome structure
(Klein et al., 2004; Korobeinikova et al., 2012). In addition to their core structural roles,
ribosomal proteins can act as adapter molecules between the ribosome and translation
factors or other cellular complexes (Melnikov et al., 2012; Peisker et al., 2008). Many
ribosomal proteins are essential for life, suggesting that their absence may lead to gross
alterations in ribosomal RNA folding or translation, and non-essential ribosomal proteins
cause subtle to severe cellular and molecular phenotypes when depleted (Ferreira-
Cerca et al., 2005). The emerging picture suggests that each ribosomal protein has a
distinct role in ribosome biogenesis and/or mature ribosome function, as discussed in
more detail below.
Ribosomal proteins are incorporated into either the large or small ribosomal subunits
during ribosome biogenesis or maturation (Henras et al., 2008; Zemp and Kutay, 2007).
A handful of ribosomal proteins are required for crucial steps in ribosome production
(Ferreira-Cerca et al., 2005; Lo et al., 2010). However, most ribosomal proteins have no
known direct function in ribosomal RNA processing or ribosome assembly, perhaps
because of the inherent difficulty in separating bona fide protein-RNA interactions from
spurious associations for these highly abundant proteins (Henras et al., 2008).
13
Nevertheless, depletion of almost any small subunit protein in yeast results in ribosome
biogenesis defects specific for the small subunit (Ferreira-Cerca et al., 2005). This
finding suggests either that most small subunit ribosomal proteins have a specific
molecular function in the biogenesis of their associated subunit or that assembly
intermediates lacking these proteins are targeted for degradation by the quality control
machinery.
In addition to their functions in ribosome biogenesis, ribosomal proteins also have
diverse roles in mature ribosome function. Ribosomal proteins can bridge interactions
between the ribosome and translation factors, alter structural dynamics during
translation, or even interact with the mRNA directly. One particularly intriguing example
is the S1 ribosomal protein of prokaryotic ribosomes. At 68 kDa, it is over twice as large
as any other prokaryotic ribosomal protein and is required for translation start selection
(Subramanian, 1984). This mechanism is explained by its localization on the small
subunit that allows it to interact with 11 nucleotides upstream of the Shine-Dalgarno
sequence of mRNAs (Sengupta et al., 2001). Neither S1 nor the mechanism of
translation initiation is conserved between prokaryotes and eukaryotes. However, there
is evidence that several of the eukaryotic small subunit proteins interact with the mRNA
directly during translation initiation and could potentially influence its regulation (Pisarev
et al., 2008; Sharifulin et al., 2012). Alternatively, ribosomal proteins can mediate
interactions between the ribosome and translation factors. Several ribosomal proteins
interact with the large multi-subunit elF3 complex that coordinates multiple steps of
14
translation initiation, and loss of some of these proteins weakens the affinity of elF3 for
the ribosome (Chiu et al., 2010; Erzberger et al., 2014; Kouba et al., 2012; Szamecz et
al., 2008). Finally, ribosomal proteins can influence the structural dynamics of the
ribosome during translation. The Rpl10 protein acts as an allosteric switch that
coordinates a signal from the peptidyl-transfer center to the decoding center, making
decoding sensitive to the translocation state of the ribosome (Sulima et al., 2014).
Hence, in addition to their structural roles, ribosomal proteins have important functions
in nearly every aspect of translation. An interesting question for the field is whether each
of these functions is constituitive, or whether some of them might be subject to
biological regulation.
If functions of the mature ribosome can be regulated in response to cellular signaling,
then it should be possible to identify ribosomal proteins or accessory factors that can
integrate these signals at the ribosome. The most extensively studied ribosomal protein
in this regard is Rps6. The S6 kinase (S6K) phosphorylates Rps6 at five
phosphorylation sites in response to stimulation by the kinase and master growth
control regulator mTOR (Ruvinsky et al., 2005). It was thought that these phosphosites,
which are conserved throughout metazoans and partially conserved in yeast, might
perform an important function in translational control in response to growth signaling
(Ruvinsky and Meyuhas, 2006). This model was partially motivated by the finding that
S6K activation promotes the translation of a group of mRNAs encoding ribosomal
proteins and translation factors that contain a 5' terminal oligopyrimidine (5' TOP) motif
15
in their 5' UTRs (Jefferies et al., 1997). Despite decades of research, the molecular
mechanism by which the 5' TOP motif is targeted for translational regulation is still
unknown (Meyuhas and Dreazen, 2009). However, it is clear that phosphorylation of the
Rps6 protein plays no detectable role in the regulation of these mRNAs, although MEFs
in which RPS6 phosphosites are mutated grow slowly compared to wild type (Ruvinsky
et al., 2005). In yeast, mutation of orthologous RPS6 phosphosites causes no
detectable cellular phenotypes (Johnson and Warner, 1987). It remains to be seen
whether Rps6 phosphorylation might have a subtle yet undetected role in translational
regulation in response to growth signaling or whether, despite evolutionary
conservation, Rps6 phosphorylation has no role in regulating the ribosome.
The RACK1 protein - a link between the ribosome and cellular
signaling?
Another protein with proposed functions in signal integration at the ribosome is RACK1.
RACK1's name (Receptor for Activated C-Kinase) is derived from its first described
function as a protein that binds the activated form of protein kinase C (Mochlyrosen et
al., 1991; Ron et al., 1994). A cDNA clone containing the RACK1 sequence had already
suggested to encode a signaling protein due to its homology with the GP subunit of
trimeric G-proteins (Guillemot et al., 1989). Both RACK1 and GP belong to a class of
proteins defined by their signature tryptophan aspartate repeats (WD-repeats) and fold
into a p-propeller structure generally consisting of 7 repeats (Murzin, 1992; Neer et al.,
1994). We now know that the RACK1 protein differs structurally and functionally from
the Gp-subunit in several aspects. Firstly, RACKI does not possess the N-terminal a-
16
helix that interacts with the Gy-subunit of the trimeric G-protein complex, and likewise
GP does not interact with isoforms of PKC (Adams et al., 2011; Pitcher et al., 1992).
Another prominent difference is the presence of an expanded loop region in RACK1
between the last two propeller blades that forms a knob-like structure not found in GP or
other WD-repeat proteins (Coyle et al., 2009). Nevertheless, years before any RACK1
structures became available, the sequence of the RACK1 gene immediately suggested
that it might play important roles in scaffolding protein complexes, the major cellular
function assigned to WD-repeat proteins (Stirnimann et al., 2010).
Interestingly, most literature related to the RACK1 protein does not acknowledge its
function as a component of the ribosome. This may partly be due to the temporal order
of discoveries related to RACK1 function. RACK1 was not identified in the early studies
of ribosomal proteins that largely relied on 2D gel electrophoresis and Edman
degradation sequencing (Warner and Gorenstein, 1978), perhaps because of its
unusually large size and acidic nature for a ribosomal protein. An improved mass
spectrometry approach identified RACK1 as a ribosomal protein associated with the
small ribosomal subunit and a later cryo-EM study identified its ribosome binding site on
the solvent-exposed face of the small subunit (Link et al., 1999; Sengupta et al., 2004).
However, by this time a significant literature related to RACK1 signaling functions was
already beginning to emerge.
17
In the interim since RACK1's identification, it has been proposed to be involved myriad
signaling pathways and complexes, amounting to around 80 interaction partners
detected in human cells from low-throughput studies alone (Stark et al., 2006; Tyers,
2015). WD-repeat proteins are known to be promiscuous protein interactors, and the in
vivo relevance of many of these interactions remains untested (Stirnimann et al., 2010).
RACK1 binding partners are spread over a diverse group of proteins and signaling
pathways and do not share any defining mechanistic features; interaction with RACK1
enhances the activity of some binding partners while repressing others, making it
difficult to synthesize a coherent picture of RACK1's functions in signaling (Adams et al.,
2011). Nevertheless, several of RACK1's signaling interactions have been studied in
some detail and have demonstrated physiological relevance (Adams et al., 2011).
One of RACK1's best-characterized functions involves coordinating focal adhesion
assembly. Focal adhesions link the cell membrane to the extracellular matrix (ECM),
and they are dynamically formed and remodelled during cell migration (Wehrle-Haller
and Imhof, 2002). By interacting with P-integrin, insulin-like growth factor receptor, and
focal adhesion kinase (FAK), RACK1 integrates signaling within the focal adhesion
complex. RACK1 knockdown inhibits FAK activity and cell migration, supporting a key
role for RACK1 in this process (Hermanto et al., 2002; Kiely et al., 2009).
RACK1 also has a well-established function in the nervous system where it regulates
both the activity of the ligand-gated ion channel NMDA receptor and the transcription of
18
the neurotrophic factor BDNF in response to cAMP/PKA signaling (Yaka et al., 2002,
2003). In the basal state, RACK1 binds and inhibits the NMDA receptor. When
cAMP/PKA signaling is activated, RACK1 dissociates from the NMDA receptor and
translocates into the nucleus, where it activates the transcription of BDNF (Yaka et al.,
2002, 2003). Remarkably, administration of cell-penetrable RACK1 protein to adult mice
increases BDNF production, which attenuates alcoholism-like behaviors by upregulating
downstream dopamine signaling (Jeanblanc et al., 2006; McGough et al., 2004).
Fewer signaling functions have been suggested for the yeast ortholog of RACK1, Asci.
To start, Asci does not interact physically or genetically with the sole S. cerevisiae PKC
isoform (Pkcl) (Melamed et al., 2010), suggesting that PKC activation is not an
important function for Asci in yeast. One study proposed that Asci functions as the GPsubunit for Gpa2, the a-subunit of the trimeric G-protein required for glucose sensing in
yeast because none of the canonical P-subunits encoded in the yeast genome seemed
to fulfill this function (Zeller et al., 2007). However, this assignment is in conflict with
studies implicating two non-canonical Kelch repeat proteins as the missing Gp-subunit
(Batlle et al., 2003; Harashima and Heitman, 2002). Yet another study suggests that the
Kelch repeat proteins in fact regulate PKA directly and do not function as the GP-subunit
for Gpa2 (Peeters et al., 2006). In summary, the potential signaling functions of Asci in
yeast remain relatively mysterious.
19
One theoretical issue with RACK1's proposed functions as a signaling scaffold is its
high concentration relative to other signaling components (Ferrell, 2000; Levchenko et
al., 2000). At high relative concentrations, scaffolding proteins may inhibit signaling
pathways because they can sequester binding partners in incomplete complexes. This
idea has led the model that scaffold proteins need to operate within a specific
concentration range to be effective regulators (Levchenko et al., 2000). However, the
model is complicated by the fact that not all scaffolds may be readily available for
interaction with a specific binding partner due to their subcellular localization or
interaction with other partners (Witzel et al., 2012). In the case of RACK1, it is a
possibility that not all RACK1 protein is incorporated into ribosomes and that many of its
binding partners predominantly interact with a small pool of non-ribosome-associated
protein. Making sense of the many functions of the RACK1 protein and their potential
relationships to the ribosome is a difficult, yet important question for cell biology.
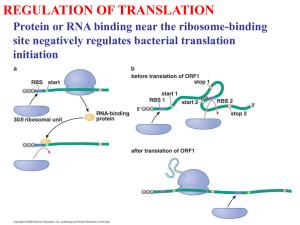
What is the ribosome-associated function of the RACK1 protein?
Because of RACK1's association with the small ribosomal subunit and its proposed
signaling functions, it is tempting to speculate that it may regulate translation in
response to cellular signaling (Nilsson et al., 2004). In this vein, some authors have
attempted to integrate a signaling function for RACK1 with its ribosomal protein identity
in mammalian cells (Angenstein et al., 2002; Arimoto et al., 2008; Ceci et al., 2003;
Grosso et al., 2008). One notable study proposed a signal-mediated function for RACK1
in ribosomal subunit joining (Ceci et al., 2003). They found that the PKC isoform PKCPII
promotes ribosomal subunit joining by phosphorylating the elF6 initiation factor, which
20
allows its release from the 60S subunit and subsequent subunit joining. They suggested
that RACK1 stimulates this process by providing a docking platform for PKCPII on a
nearby 40S subunit (Ceci et al., 2003). This model is based on the detection of a
physical interaction between RACK1 and elF6 in vivo and a RACKI -dependent
enhancement of PKCPII activity in a ribosomal subunit-joining assay in vitro. However,
their results do not rule out the possibility that RACK1 and PKCPll stimulate ribosomal
subunit joining independently. The absence of a physical interaction between RACK1
and PKC in yeast as well as the absence of an orthologous elF6 phosphorylation site
suggests that RACK1 -dependent elF6 release is not a deeply conserved mechanism for
ribosomal subunit joining (Melamed et al., 2010). Indeed, an alternative mechanism for
60S subunit joining was identified in yeast, which requires Efl1, a cytoplasmic GTPase
(Senger et al., 2001) and was later found to be conserved in mammalian cells, calling
into question the relevance of PKC-dependent 60S subunit joining (Finch et al., 2011).
Despite these apparent discrepancies, the interaction of RACK1 with PKCPII and the
association of both molecules with the ribosome suggests the intriguing possibility of
signal-dependent regulation of the translation machinery via RACK1.
Although no specific signaling functions for the Asci protein at the yeast ribosome have
been proposed, one study suggested that the ribosomal association of Asci could
regulate the translation of a subset of cellular mRNAs (Baum et al., 2004). The authors
reported that the Scpl 60 RNA-binding protein associates with yeast ribosomes via
binding to the Asci protein (Baum et al., 2004). Because Scpl60 interacts with specific
21
mRNAs (Hogan et al., 2008; Li et al., 2003), it was suggested that these mRNAs can be
targeted to the ribosome for translation by Scpl 60's interaction with Asci. Furthermore,
the fraction of Asci that is associated with ribosomes decreases during stationary
phase (the growth phase after exponential growth is complete and no additional growth
can occur because of nutrient exhaustion) (Baum et al., 2004). Based on this finding,
the authors postulated that Asci is released from the ribosome during stationary phase,
which should disrupt co-localization of Scpl 60 with the ribosome and inhibit translation
of Scpl 60 target mRNAs. A mechanism by which specific groups of mRNAs are brought
to the ribosome via interactions with ribosome-associated RNA binding proteins could
enable co-regulation of a common set of mRNAs bound by a specific RNA binding
protein in response to cellular signaling (Keene, 2007).
Finally, some insight into RACK1's potential regulatory functions in translation might be
gleaned from examination of available structural data. RACK1's location on the solventexposed side of the small ribosomal subunit puts it near the mRNA exit channel
(Sengupta et al., 2004), (Figure 1-3). Intriguingly, several eukaryote-specific ribosomal
proteins in addition to RACK1 and two eukaryotic ribosomal RNA expansion segments
occupy this region of the small subunit (Melnikov et al., 2012). Because the mRNA is
attached to the eIF4F cap-binding complex at its 5' end, it cannot be threaded through
the mRNA channel of the small subunit. Rather, interactions of the eIF4F complex with
the small subunit and its associated factors near the mRNA exit channel allow loading of
the small subunit onto the 5' UTR (Jackson et al., 2010). RACK1's direct neighbor,
22
Rps28, crosslinks to the -7 and -10 positions relative to the AUG start codon in
translation initiation complexes, demonstrating that the mRNA passes nearby the
RACK1 protein during its exit from the small subunit.
60S-binding side
solvent-exposed side
Figure 1-3: The position of RACK1 on the small ribosomal subunit. RACK1 binds the solventexposed side of the small ribosomal subunit near the mRNA exit channel. The small subunit shown is
from a mammalian pre-initiation complex (Lomakin and Steitz, 2013) juxtaposed with the size and position
of the elF3 complex on the small subunit obtained by cryo-EM of mammalian pre-initiation complexes
(Hashem et al., 2013). The relative size and location of elF4G is approximated from a previous cryo-EM
map in which elF4G was localized to the left arm of elF3 (Siridechadilok et al., 2005).
Ribosomal proteins near the mRNA exit channel can also interact with translation
initiation factors. The elF3 complex binds across the solvent-exposed face of the small
subunit, including a region near the mRNA exit channel (Hashem et al., 2013).
Interactions between elF3 and both RACK1 and RpsO have been detected (Kouba et
al., 2012; Szamecz et al., 2008). In addition, structural modeling of mammalian initiation
complexes places the eIF4G scaffold protein on the left arm of the eIF3 complex, close
to the mRNA exit channel and RACK1 (Siridechadilok et al., 2005), hinting at the
possibility that eIF4G could have additional contacts with the small subunit in addition to
23
its well-characterized interaction with eIF3 in mammalian ribosomes (Figure 1-3).
Together, these structural data suggest that RACK1 could function in mRNA
recruitment, either by interacting with translation initiation factors or even by binding the
mRNA directly.
Genetic analysis of the ASCi locus
The study of the ribosomal functions of the RACK1 protein has largely focused on
RACK1's yeast ortholog, Asci. Because the Asci protein may have ribosomeindependent functions in the cell, mutants of Asci with decreased affinity for the
ribosome have been designed to assess its ribosome-specific functions (Figure 1-4).
The assumption is that these mutants should both substantially reduce the in vivo
association of the Asci protein with the ribosome and allow non-ribosome-associated
protein to continue its potential cellular signaling activities off the ribosome. The first
mutant to be developed was the R38D K40E (DE) mutant. This mutant was designed
based on a cryo-EM structure of Asc1 in complex with the ribosome and the amino acid
substitutions disrupt electrostatic interactions with the ribosomal RNA via chargeswitching at the ribosome-binding interface (Sengupta et al., 2004). A second mutant
was isolated in a forward genetic screen for mutants in no-GO decay, a ribosomeassociated RNA quality control mechanism (Kuroha et al., 2010). This mutant, D109Y,
may disrupt Asci ribosome binding by perturbing its interaction with a nearby ribosomal
protein, Rps17 (Figure 1-4).
24
RACK1
Figure 1-4: The ribosome-binding interface of RACKI. The binding interface of the Asc1/RACK1
protein with the small ribosomal subunit is shown and the positions of residues mutated in the ribosomebinding mutants are indicated. R38D and K40E are thought to disrupt binding with the ribosomal RNA,
whereas D109Y likely alters the interaction between RACK1 and Rpsl7. The structure shown is derived
from yeast ribosomes (Ben-Shem et al., 2011).
Despite the existence of ribosome-binding mutant alleles, most of our knowledge about
Asci function in vivo was obtained using a deletion of the entire ASC1 locus (ascA).
This approach is additionally problematic because the ASC1 locus contains a small
nucleolar RNA (snoRNA) snR24, that acts as a guide for 2'-O-methylation of three
closely spaced positions on the large ribosomal subunit (C1437, C1449, C1450)
(Samarsky and Fournier, 1999), thus deletion of the ASC1 locus causes a large
ribosomal subunit biogenesis defect. One previous study showed that the snR24
snoRNA and not the Asci protein is required for large ribosomal subunit biogenesis
because a strain lacking only SNR24 displays a severe temperature-sensitive ribosome
biogenesis defect (Li et al., 2009). Because all other studies of Asci function in yeast to
date have used the asciA locus deletion, many functions assigned to Asci could in fact
25
be linked to the SNR24 gene and not directly to the function of the Asci protein.
Therefore, genetic separation of the function of the Asci protein and the snR24 snoRNA
is necessary to accurately characterize the functions of the Asci protein in translation,
and the ribosome binding mutants should allow the specific contribution of ribosome-
bound Asci to be assessed.
Quantifying translation genome-wide: the road to a molecular
phenotype
To address the hypothesis that specific translation factors and ribosomal proteins such
as RACK1 have mRNA-specific functions in translation (Dever, 2002), we must identify
mRNA targets whose translation is affected by the level or activity of that factor. This
question is theoretically similar to the one posed by the search for transcription factor
binding sites in the genome (Valouev et al., 2008), although it is made more
complicated by the fact that we do not have the expectation that each translation factor
will physically interact with the mRNA in the way that a transcription factor is expected to
bind the DNA promoter regions of its targets. Rather, the mRNA specificity of translation
factors may result from their contributions to the overall structure of translation initiation
complexes or the ribosome, or by altering the specificity of the translation apparatus in a
way not reliant on a simple cis-acting mRNA element.
-
An additional challenge is that translational measurements require two components
quantitation of both the ribosome-associated mRNA pool as well as the total mRNA
pool. One approach is to subject polysome-associated mRNAs to microarray analysis in
26
parallel with a sample derived from total mRNA or non-polysomal mRNA (Arava, 2003),
(Figure 1-5). A newer technique, ribosome footprint profiling (Ribo-Seq) is based on
ribosomal protection of mRNA fragments after RNAse digestion (Ingolia et al., 2009).
Unlike microarray-based techniques, Ribo-Seq provides nucleotide-level precision that
allows assignment of the position of the ribosome on mRNAs with subcodon resolution.
This information can be used to detect events such as ribosomal stalling and
frameshifting (Guydosh and Green, 2014; Michel et al., 2012). Both polysome
microarray analysis and Ribo-Seq can be used to quantify mRNA-specific translation
activities on a genome-wide scale. In theory, analysis of this data should allow detection
of translation factor-specific response elements present in mRNAs.
polysome microarray/sequencing
Ribo-Seq
mRNP
80S
RNAse
treatment
60S
808
ribosomal footprint
polysomes
40S
analyze polysomal and nonpolysomal mRNAs
(microarray or RNA-Seq)
analyze ribosome footprints
(RNA-Seq)
Figure 1-5: Genome-wide techniques for quantifying translation. Two techniques for genome-wide
quantification of mRNA-specific translation activity. In polysome microarray/sequencing (left) mRNAs are
isolated from intact polysomes and compared to either a non-polysomal or total mRNA sample to quantify
translation. In Ribo-Seq (right), cell extract is digested with RNAse to degrade non-translating RNA and
only the ribosome-protected footprints are sequenced. A sample prepared from total mRNA is analyzed in
parallel to quantify translation for each mRNA.
27
Using these techniques, several studies have drawn conclusions about the substrate
specificity of translation factors on a genome-wide scale. Most studies to date have
focused on translation initiation factors rather than ribosomal proteins, perhaps because
of the clear expectation that defects in these factors should adversely affect translation
initiation. One interesting question has been whether the eIF4F mRNA cap-binding
complex and the eIF4E-binding protein translation inhibitors (4E-BPs) regulate the
translation of specific mRNAs. One would predict that depletion of the eIF4F complex
should increase the relative translational activity of mRNAs that can be translated via
cap-independent mechanisms. In support of this prediction, a small subset of mRNAs
are resistant to translational repression after Polio virus infection during which the eIF4F
complex is inactivated. At least two of these mRNAs contain internal ribosome entry site
(IRES) elements that do not require the eIF4F complex for ribosome recruitment
(Johannes et al., 1999). On the other hand, given the requirement of almost all mRNAs
for eIF4F, one would expect that 4E-BPs should repress the translation of most mRNAs
by sequestering capped mRNAs and preventing their association with the eIF4F
complex (Richter and Sonenberg, 2005). In reality, however, mRNAs containing 5' TOP
motifs are targeted for 4E-BP-mediated repression much more effectively than other
mRNAs when assessed by ribosome footprint profiling (Thoreen et al., 2012). These
findings highlight the intricacies of translation initiation factor substrate specificity, even
for a single initiation factor complex.
28
Another interesting direction has been the characterization of functions for specific
subunits of the elF3 complex. In yeast, the elF3 complex is composed of 6 subunits,
whereas in metazoans, the elF3 complex contains the orthologous subunits and several
additional subunits (Hinnebusch, 2006). The complexity and evolutionary diversification
of the elF3 complex suggests that it may perform specific translational regulatory
functions in different species. For example, the non-essential subunit elF3h is required
for efficient translation of mRNAs containing upstream open reading frames (uORFs) in
Arabidopsis and for translation of several mRNAs required for neural development in
Zebrafish (Choudhuri et al., 2013; Kim et al., 2007). Because elF3 is thought to interact
with some mRNAs directly (Block et al., 1999; Kolupaeva et al., 2005), another
approach to addressing the mRNA specificity of this complex is to identify mRNA
binding partners of elF3 subunits. Different subunits interact with specific sets of
mRNAs, and in some cases these interactions can cause translational enhancement or
repression of the target mRNAs (Lee et al., 2015; Zhou et al., 2005).
Do ribosomal proteins have functions in gene-specific translation?
In contrast, little work has been done to characterize the functions of ribosomal proteins
in translation at the genome-wide scale. However, specific case studies hint that some
ribosomal proteins have functions in gene-specific translation. Several examples have
emerged in which viral mRNAs require specific ribosomal proteins for translation. Both
Rps25 and RACK1 are required for translation of the HCV viral IRES (Landry et al.,
2009; Majzoub et al., 2014), a finding that can be structurally rationalized by the close
proximity of Rps25 and RACK1 to the binding site of the HCV IRES on the small
29
ribosomal subunit (Boehringer et al., 2005). Thus, RACK1 and Rps25 may physically
interact with the HCV IRES or change the conformation of the IRES docking site if
absent from the ribosome. The translation of some cellular mRNAs can also be
regulated by specific ribosomal proteins. Mutation of Rpl38 causes developmental
abnormalities in mice that can be explained by reduced translation of the Hox family of
developmentally-regulated mRNAs (Kondrashov et al., 2011). Because these mRNAs
contain 5' UTR elements that harbor IRES activity, Rpl38 may function in a specialized
translation initiation pathway utilized by the Hox mRNAs (Xue et al., 2014).
Finally, some groups of ribosomal proteins cause similar phenotypes when mutated,
suggesting that the mutants share similar underlying molecular defects. For example, 14
distinct mutations in large ribosomal subunit proteins in yeast lead to lifespan extension,
and haploinsufficiency of 11 distinct ribosomal proteins causes Diamond-Blackfan
Anemia in humans (Ruggero and Shimamura, 2015; Steffen et al., 2008). The
phenotypic similarities shared between groups of ribosomal proteins mutants hint at the
existence of translational regulons that are sensitive to the levels of specific ribosomal
proteins or complexes, such as the large ribosomal subunit.
Thesis overview
In this thesis, I explore the translational functions of the Asc1/RACK1 protein. First, I
performed Ribo-Seq on a series of yeast ASC1 mutants in which the Asci protein is
either not expressed or has reduced ribosome-binding affinity. This quantitative
genome-scale analysis allowed me to identify translationally co-regulated groups of
30
mRNAs that display translational defects in ASC1 mutants and therefore normally
require Asci for efficient translation. I identified a trend in which mRNAs with short open
reading frames (ORFs) are translated more poorly in ASC1 mutants. I validated this
result using a reporter system that I designed to measure the effect of ORF length on
protein production in vivo. Using recent measurements of translation initiation factor
association with cellular mRNAs (Costello et al., 2015), I found that mRNAs associated
with the translational closed-loop complex have much shorter ORFs than average
(median ~500 nt vs. -1100 nt), and that closed-loop association is more strongly
predictive of Asci sensitivity than ORF length. We hypothesize that the preferential
association of short ORFs with the closed-loop complex may privilege their translation
and thus explain the high global correlation between ORF length and translation
efficiency that has been observed in many eukaryotes (Arava et al., 2003; Guo et al.,
2015), and our evidence suggests that the Asci protein promotes the formation or
function of these closed-loop complexes. Differential association of mRNAs with the
closed-loop complex has important implications for biological regulation because
mRNAs with short ORFs are heavily biased towards specific functional groups, such as
those encoding ribosomal proteins and components of the mitochondrial respiratory
complexes. Consistent with these translation defects, ASC1 mutants are unable to grow
in carbon sources that require mitochondrial respiration. Intriguingly, knockdown of
RACK1 in HeLa cells also causes a respiratory growth defect and decreased synthesis
of small proteins. These results point towards a conserved function for the Asc1/RACK1
31
protein in promoting the translation of mRNAs with short ORFs that perform a core set
of metabolic functions across eukaryotes.
References
Adams, D.R., Ron, D., and Kiely, P.A. (2011). RACK1, A multifaceted scaffolding
protein: Structure and function. Cell Commun. Signal. 9, 22.
Angenstein, F., Evans, A.M., Settlage, R.E., Moran, S.T., Ling, S.C., Klintsova, A.Y.,
Shabanowitz, J., Hunt, D.F., and Greenough, W.T. (2002). A receptor for activated C
kinase is part of messenger ribonucleoprotein complexes associated with polyA-mRNAs
in neurons. J Neurosci 22, 8827-8837.
Arava, Y. (2003). Isolation of Polysomal RNA for Microarray Analysis. In Functional
Genomics SE - 6, M. Brownstein, and A. Khodursky, eds. (Humana Press), pp. 79-87.
Arava, Y., Wang, Y., Storey, J.D., Liu, C.L., Brown, P.O., and Herschlag, D. (2003).
Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc
Natl Acad Sci U S A 100, 3889-3894.
Arimoto, K., Fukuda, H., Imajoh-Ohmi, S., Saito, H., and Takekawa, M. (2008).
Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK
pathways. Nat Cell Biol 10, 1324-1332.
Asano, K., Shaiev, A., Phan, L., Nielsen, K., Clayton, J., Vainsek, L., Donahue, T.F.,
and Hinnebusch, A.G. (2001). Multiple roles for the C-terminal domain of eIF5 in
translation initiation complex assembly and GTPase activation. EMBO J. 20, 23262337.
Batlle, M., Lu, A., Green, D.A., Xue, Y., and Hirsch, J.P. (2003). Krhl p and Krh2p act
downstream of the Gpa2p G(alpha) subunit to negatively regulate haploid invasive
growth. J. Cell Sci. 116, 701-710.
Baum, S., Bittins, M., Frey, S., and Seedorf, M. (2004). Ascip, a WD40-domain
containing adaptor protein, is required for the interaction of the RNA-binding protein
Scp1 60p with polysomes. Biochem J 380, 823-830.
Ben-Shem, A., Garreau de Loubresse, N., Melnikov, S., Jenner, L., Yusupova, G., and
Yusupov, M. (2011). The Structure of the Eukaryotic Ribosome at 3.0 A Resolution.
Science 334, 1524-1529.
32
Block, K.L., Vornlocher, H.P., and Hershey, J.W.B. (1999). Characterization of cDNAs
encoding the p44 and p35 subunits of human translation initiation factor elF3. J. Biol.
Chem. 273, 31901-31908.
Boehringer, D., Thermann, R., Ostareck-Lederer, A., Lewis, J.D., and Stark, H. (2005).
Structure of the hepatitis C virus IRES bound to the human 80S ribosome: Remodeling
of the HCV IRES. Structure 13,1695-1706.
Ceci, M., Gaviraghi, C., Gorrini, C., Sala, L.A., Offenhauser, N., Marchisio, P.C., and
Biffo, S. (2003). Release of elF6 (p27BBP) from the 60S subunit allows 80S ribosome
assembly. Nature 426, 579-584.
Chiu, W.-L., Wagner, S., Herrmannov&, A., Burela, L., Zhang, F., Saini, A.K., ValAsek,
L., and Hinnebusch, A.G. (2010). The C-terminal region of eukaryotic translation
initiation factor 3a (elF3a) promotes mRNA recruitment, scanning, and, together with
elF3j and the elF3b RNA recognition motif, selection of AUG start codons. Mol. Cell.
Biol. 30, 4415-4434.
Choudhuri, A., Maitra, U., and Evans, T. (2013). Translation initiation factor elF3h
targets specific transcripts to polysomes during embryogenesis. Proc. Natl. Acad. Sci.
U. S. A. 110, 9818-9823.
Costello, J., Castelli, L.M., Rowe, W., Kershaw, C.J., Talavera, D., Mohammad-Qureshi,
S.S., Sims, P.F.G., Grant, C.M., Pavitt, G.D., Hubbard, S.J., et al. (2015). Global mRNA
selection mechanisms for translation initiation. Genome Biol. 16, 10.
Coyle, S.M., Gilbert, W. V, and Doudna, J.A. (2009). Direct link between RACK1
function and localization at the ribosome in vivo. Mol Cell Biol 29, 1626-1634.
Dever, T.E. (2002). Gene-specific regulation by general translation factors. Cell 108,
545-556.
Dever, T.E., and Green, R. (2012). The elongation, termination, and recycling phases of
translation in eukaryotes. Cold Spring Harb. Perspect. Biol. 4, 1-16.
Erzberger, J.P., Stengel, F., Pellarin, R., Zhang, S., Schaefer, T., Aylett, C.H.S.,
Cimermanbi6, P., Boehringer, D., Sali, A., Aebersold, R., et al. (2014). Molecular
architecture of the 40S-elF1-elF3 translation initiation complex. Cell 158, 1123-1135.
Ferreira-Cerca, S., P611, G., Gleizes, P.E., Tschochner, H., and Milkereit, P. (2005).
Roles of eukaryotic ribosomal proteins in maturation and transport of pre-1 8S rRNA and
ribosome function. Mol. Cell 20, 263-275.
Ferrell, J.E. (2000). What do scaffold proteins really do? Sci. Signal. 2000, pel.
33
Finch, A.J., Hilcenko, C., Basse, N., Drynan, L.F., Goyenechea, B., Menne, T.F.,
Fernandez, A.G., Simpson, P., D'Santos, C.S., Arends, M.J., et al. (2011). Uncoupling
of GTP hydrolysis from elF6 release on the ribosome causes shwachman-diamond
syndrome. Genes Dev. 25, 917-929.
Grosso, S., Volta, V., Sala, L.A., Vietri, M., Marchisio, P.C., Ron, D., and Biffo, S.
(2008). PKCbetall modulates translation independently from mTOR and through
RACK1. Biochem J 415, 77-85.
Guillemot, F., Billault, A., and Auff ray, C. (1989). Physical linkage of a guanine
nucleotide-binding protein-related gene to the chicken major histocompatibility complex.
Proc NatI Acad Sci U S A 86, 4594-4598.
Guo, J., Lian, X., Zhong, J., Wang, T., and Zhang, G. (2015). Length-dependent
translation initiation benefits the functional proteome of human cells. Mol. Biosyst. 11,
370-378.
Guydosh, N.R., and Green, R. (2014). Dom34 Rescues Ribosomes in 3' Untranslated
Regions. Cell 156, 950-962.
Harashima, T., and Heitman, J. (2002). The Galpha protein Gpa2 controls yeast
differentiation by interacting with kelch repeat proteins that mimic Gbeta subunits. Mol
Cell 10, 163-173.
Hashem, Y., Des Georges, A., Dhote, V., Langlois, R., Liao, H.Y., Grassucci, R.A.,
Hellen, C.U.T., Pestova, T. V., and Frank, J. (2013). Structure of the mammalian
ribosomal 43S preinitiation complex bound to the scanning factor DHX29. Cell 153.
Henras, a K., Soudet, J., G6rus, M., Lebaron, S., Caizergues-Ferrer, M., Mougin, a, and
Henry, Y. (2008). The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell.
Mol. Life Sci. 65, 2334-2359.
Hermanto, U., Zong, C.S., Li, W., and Wang, L.H. (2002). RACK1, an insulin-like growth
factor I (IGF-1) receptor-interacting protein, modulates IGF-1-dependent integrin
signaling and promotes cell spreading and contact with extracellular matrix. Mol Cell
Biol. 22, 2345-2365.
Hinnebusch, A.G. (2006). elF3: a versatile scaffold for translation initiation complexes.
Trends Biochem Sci 31, 553-562.
Hogan, D.J., Riordan, D.P., Gerber, A.P., Herschlag, D., and Brown, P.O. (2008).
Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting
an extensive regulatory system. PLoS Biol 6, e255.
34
Ingolia, N.T., Ghaemmaghami, S., Newman, J.R., and Weissman, J.S. (2009). Genomewide analysis in vivo of translation with nucleotide resolution using ribosome profiling.
Science 324, 218-223.
Jackson, R.J., Hellen, C.U.T., and Pestova, T. V (2010). The mechanism of eukaryotic
translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 11, 113127.
Jeanblanc, J., He, D.-Y., McGough, N.N.H., Logrip, M.L., Phamluong, K., Janak, P.H.,
and Ron, D. (2006). The dopamine D3 receptor is part of a homeostatic pathway
regulating ethanol consumption. J. Neurosci. 26, 1457-1464.
Jefferies, H.B.J., Fumagalli, S., Dennis, P.B., Reinhard, C., Pearson, R.B., and Thomas,
G. (1997). Rapamycin suppresses 5'TOP mRNA translation through inhibition of
p70(s6k). EMBO J. 16, 3693-3704.
Jivotovskaya, A. V, Valtsek, L., Hinnebusch, A.G., and Nielsen, K.H. (2006). Eukaryotic
translation initiation factor 3 (elF3) and elF2 can promote mRNA binding to 40S
subunits independently of eIF4G in yeast. Mol. Cell. Biol. 26, 1355-1372.
Johannes, G., Carter, M.S., Eisen, M.B., Brown, P.O., and Sarnow, P. (1999).
Identification of eukaryotic mRNAs that are translated at reduced cap binding complex
eIF4F concentrations using a cDNA microarray. Proc Natl Acad Sci U S A 96, 1311813123.
Johnson, S., and Warner, J. (1987). Phosphorylation of the Saccharomyces cerevisiae
equivalent of ribosomal protein S6 has no detectable effect on growth. Mol. Cell. Biol. 7,
1338-1345.
Keene, J.D. (2007). RNA regulons: coordination of post-transcriptional events. Nat. Rev.
Genet. 8, 533-543.
Kiely, P.A., Baillie, G.S., Barrett, R., Buckley, D.A., Adams, D.R., Houslay, M.D., and
O'Connor, R. (2009). Phosphorylation of RACK1 on tyrosine 52 by c-Abl is required for
insulin-like growth factor I-mediated regulation of focal adhesion kinase. J. Biol. Chem.
284, 20263-20274.
Kim, B.-H., Cai, X., Vaughn, J.N., and von Arnim, A.G. (2007). On the functions of the h
subunit of eukaryotic initiation factor 3 in late stages of translation initiation. Genome
Biol. 8, R60.
Klein, D.J., Moore, P.B., and Steitz, T.A. (2004). The roles of ribosomal proteins in the
structure assembly, and evolution of the large ribosomal subunit. J Mol Biol 340, 141177.
35
Kolupaeva, V.G., Unbehaun, A., Lomakin, I.B., Hellen, C.U.T., and Pestova, T. V
(2005). Binding of eukaryotic initiation factor 3 to ribosomal 40S subunits and its role in
ribosomal dissociation and anti-association. RNA 11, 470-486.
Kondrashov, N., Pusic, A., Stumpf, C.R., Shimizu, K., Hsieh, A.C., Xue, S., Ishijima, J.,
Shiroishi, T., and Barna, M. (2011). Ribosome-mediated specificity in Hox mRNA
translation and vertebrate tissue patterning. Cell 145, 383-397.
Korneeva, N.L., First, E.A., Benoit, C.A., and Rhoads, R.E. (2005). Interaction between
the NH2-terminal domain of eIF4A and the central domain of eIF4G modulates RNAstimulated ATPase activity. J. Biol. Chem. 280,1872-1881.
Korobeinikova, a V, Garber, M.B., and Gongadze, G.M. (2012). Ribosomal proteins:
structure, function, and evolution. Biochemistry. 77, 562-574.
Kouba, T., Rutkai, E., Karaskova, M., and Vala ek, L.S. (2012). The elF3c/NIP1 PCI
domain interacts with RNA and RACK1/ASC1 and promotes assembly of translation
preinitiation complexes. Nucleic Acids Res. 40, 2683-2699.
Kuroha, K., Akamatsu, M., Dimitrova, L., Ito, T., Kato, Y., Shirahige, K., and Inada, T.
(2010). Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent
translation arrest. EMBO Rep 11, 956-961.
Lamphear, B.J., Kirchweger, R., Skern, T., and Rhoads, R.E. (1995). Mapping of
functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with
picornaviral proteases. Implications for cap-dependent and cap-independent
translational initiation. J Biol Chem 270, 21975-21983.
Landry, D.M., Hertz, M.I., and Thompson, S.R. (2009). RPS25 is essential for
translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes Dev. 23,
2753-2764.
Lee, A.S.Y., Kranzusch, P.J., and Cate, J.H.D. (2015). elF3 targets cell-proliferation
messenger RNAs for translational activation or repression. Nature.
Levchenko, A., Bruck, J., and Sternberg, P.W. (2000). Scaffold proteins may
biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its
threshold properties. Proc. NatI. Acad. Sci. U. S. A. 97, 5818-5823.
Li, A.M., Watson, A., and Fridovich-Keil, J.L. (2003). Scp160p associates with specific
mRNAs in yeast. Nucleic Acids Res 31, 1830-1837.
Li, Z., Lee, I., Moradi, E., Hung, N.-J., Johnson, A.W., and Marcotte, E.M. (2009).
Rational Extension of the Ribosome Biogenesis Pathway Using Network-Guided
Genetics. PLoS Biol 7, e1000213.
36
Link, A.J., Eng, J., Schieltz, D.M., Carmack, E., Mize, G.J., Morris, D.R., Garvik, B.M.,
and Yates, J.R. (1999). Direct analysis of protein complexes using mass spectrometry.
Nat Biotechnol 17, 676-682.
Lo, K.Y., Li, Z., Bussiere, C., Bresson, S., Marcotte, E.M., and Johnson, A.W. (2010).
Defining the pathway of cytoplasmic maturation of the 60S ribosomal subunit. Mol. Cell
39,196-208.
Lomakin, I.B., and Steitz, T.A. (2013). The initiation of mammalian protein synthesis and
mRNA scanning mechanism. Nature 500, 307-311.
Majzoub, K., Hafirassou, M.L., Meignin, C., Goto, A., Marzi, S., Fedorova, A., Verdier,
Y., Vinh, J., Hoffmann, J.A., Martin, F., et al. (2014). RACK1 Controls IRES-Mediated
Translation of Viruses. Cell 159, 1086-1095.
Marintchev, A., and Wagner, G. (2005). eIF4G and CBP80 share a common origin and
similar domain organization: Implications for the structure and function of eIF4G.
Biochemistry 44, 12265-12272.
McGough, N.N.H., He, D.-Y., Logrip, M.L., Jeanblanc, J., Phamluong, K., Luong, K.,
Kharazia, V., Janak, P.H., and Ron, D. (2004). RACK1 and brain-derived neurotrophic
factor: a homeostatic pathway that regulates alcohol addiction. J. Neurosci. 24, 1054210552.
Melamed, D., Bar-Ziv, L., Truzman, Y., and Arava, Y. (2010). Asci supports cell-wall
integrity near bud sites by a Pkc1 independent mechanism. PLoS One 5, el 1389.
Melnikov, S., Ben-Shem, A., Garreau de Loubresse, N., Jenner, L., Yusupova, G., and
Yusupov, M. (2012). One core, two shells: bacterial and eukaryotic ribosomes. Nat.
Struct. Mol. Biol. 19, 560-567.
Meyuhas, 0., and Dreazen, A. (2009). Ribosomal protein S6 kinase from TOP mRNAs
to cell size. Prog. Mol. Biol. Transl. Sci. 90, 109-153.
Michel, A.M., Choudhury, K.R., Firth, A.E., Ingolia, N.T., Atkins, J.F., and Baranov, P. V.
(2012). Observation of dually decoded regions of the human genome using ribosome
profiling data. Genome Res. 22, 2219-2229.
Mochlyrosen, D., Khaner, H., and Lopez, J. (1991). Identification of intracellular receptor
proteins for activated protein-kinase-c. Proc NatI Acad Sci U S A 88, 3997-4000.
Morino, S., Imataka, H., Svitkin, Y. V, Pestova, T. V, and Sonenberg, N. (2000).
Eukaryotic translation initiation factor 4E (eIF4E) binding site and the middle one-third of
eIF4GI constitute the core domain for cap-dependent translation, and the C-terminal
one-third functions as a modulatory region. Mol. Cell. Biol. 20, 468-477.
37
Murzin, A.G. (1992). Structural principles for the propeller assembly of beta-sheets: the
preference for seven-fold symmetry. Proteins 14, 191-201.
Neer, E.J., Schmidt, C.J., Nambudripad, R., and Smith, T.F. (1994). The ancient
regulatory-protein family of WD-repeat proteins. Nature 371, 297-300.
Nilsson, J., Sengupta, J., Frank, J., and Nissen, P. (2004). Regulation of eukaryotic
translation by the RACK1 protein: a platform for signalling molecules on the ribosome.
EMBO Rep 5,1137-1141.
Nissen, P., Hansen, J., Ban, N., Moore, P.B., and Steitz, T.A. (2000). The structural
basis of ribosome activity in peptide bond synthesis. Science 289, 920-930.
Noller, H.F., Hoffarth, V., and Zimniak, L. (1992). Unusual resistance of peptidyl
transferase to protein extraction procedures. Science 256, 1416-1419.
Peeters, T., Louwet, W., Gelad, R., Nauwelaers, D., Thevelein, J.M., and Versele, M.
(2006). Kelch-repeat proteins interacting with the Galpha protein Gpa2 bypass
adenylate cyclase for direct regulation of protein kinase A in yeast. Proc. Natl. Acad.
Sci. U. S. A. 103,13034-13039.
Peisker, K., Braun, D., W6lfle, T., Hentschel, J., Funfschilling, U., Fischer, G.,
Sickmann, A., and Rospert, S. (2008). Ribosome-associated complex binds to
ribosomes in close proximity of Rpl31 at the exit of the polypeptide tunnel in yeast. Mol.
Biol. Cell 19, 5279-5288.
Pestova, T., Lorsch, J.R., and Hellen, C.T. (2007). The mechanism of translation
initiation in eukaryotes. in Translational Control in Biology and Medicine, M.B. Mathews,
N. Sonenberg, and J.W.B. Hershey, eds. (Cold Spring Harbor Laboratory Press), pp.
87-128.
Pisarev, A. V, Kolupaeva, V.G., Yusupov, M.M., Hellen, C.U.T., and Pestova, T. V
(2008). Ribosomal position and contacts of mRNA in eukaryotic translation initiation
complexes. EMBO J. 27, 1609-1621.
Pitcher, J.A., Inglese, J., Higgins, J.B., Arriza, J.L., Casey, P.J., Kim, C., Benovic, J.L.,
Kwatra, M.M., Caron, M.G., and Lefkowitz, R.J. (1992). Role of beta-gamma-subunits of
G-proteins in targeting the beta-adrenergic-receptor kinase to membrane-bound
receptors. Science 257, 1264-1267.
P6yry, T.A.A., Kaminski, A., and Jackson, R.J. (2004). What determines whether
mammalian ribosomes resume scanning after translation of a short upstream open
reading frame? Genes Dev. 18, 62-75.
38
Richter, J.D., and Sonenberg, N. (2005). Regulation of cap-dependent translation by
eIF4E inhibitory proteins. Nature 433, 477-480.
Ron, D., Chen, C.H., Caldwell, J., Jamieson, L., Orr, E., and Mochly-Rosen, D. (1994).
Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of
G proteins. Proc. Nati. Acad. Sci. U. S. A. 91, 839-843.
Ruggero, D., and Shimamura, A. (2015). Marrow failure: a window into ribosome
biology. 124, 2784-2793.
Ruvinsky, I., and Meyuhas, 0. (2006). Ribosomal protein S6 phosphorylation: from
protein synthesis to cell size. Trends Biochem. Sci. 31, 342-348.
Ruvinsky, I., Sharon, N., Lerer, T., Cohen, H., Stolovich-Rain, M., Nir, T., Dor, Y.,
Zisman, P., and Meyuhas, 0. (2005). Ribosomal protein S6 phosphorylation is a
determinant of cell size and glucose homeostasis. Genes Dev. 19, 2199-2211.
Samarsky, D.A., and Fournier, M.J. (1999). A comprehensive database for the small
nucleolar RNAs from Saccharomyces cerevisiae. Nucleic Acids Res. 27, 161-164.
Senger, B., Lafontaine, D.L.J., Graindorge, J.S., Gadal, 0., Camasses, A., Sanni, A.,
Garnier, J.M., Breitenbach, M., Hurt, E., and Fasiolo, F. (2001). The nucle(ol)ar Tif6p
and Efl p are required for a late cytoplasmic step of ribosome synthesis. Mol. Cell 8,
1363-1373.
Sengupta, J., Agrawal, R.K., and Frank, J. (2001). Visualization of protein S1 within the
30S ribosomal subunit and its interaction with messenger RNA. Proc. NatI. Acad. Sci. U.
S. A. 98, 11991-11996.
Sengupta, J., Nilsson, J., Gursky, R., Spahn, C.M., Nissen, P., and Frank, J. (2004).
Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by
cryo-EM. Nat Struct Mol Biol 11, 957-962.
Sharifulin, D., Khairulina, Y., Ivanov, A., Meschaninova, M., Ven'yaminova, A., Graifer,
D., and Karpova, G. (2012). A central fragment of ribosomal protein S26 containing the
eukaryote-specific motif YxxPKxYxK is a key component of the ribosomal binding site of
mRNA region 5' of the E site codon. Nucleic Acids Res. 40, 3056-3065.
Siridechadilok, B., Fraser, C.S., Hall, R.J., Doudna, J.A., and Nogales, E. (2005).
Structural roles for human translation factor elF3 in initiation of protein synthesis.
Science 310,1513-1515.
Stark, C., Breitkreutz, B.-J., Reguly, T., Boucher, L., Breitkreutz, A., and Tyers, M.
(2006). BioGRID: a general repository for interaction datasets. Nucleic Acids Res. 34,
D535-D539.
39
Steffen, K.K., MacKay, V.L., Kerr, E.O., Tsuchiya, M., Hu, D., Fox, L.A., Dang, N.,
Johnston, E.D., Oakes, J.A., Tchao, B.N., et al. (2008). Yeast life span extension by
depletion of 60s ribosomal subunits is mediated by Gcn4. Cell 133, 292-302.
Stirnimann, C.U., Petsalaki, E., Russell, R.B., and Mueller, C.W. (2010). WD40 proteins
propel cellular networks. TRENDS Biochem. Sci. 35, 565-574.
Subramanian, A.R. (1984). Structure and functions of the largest Eschedchia coli
ribosomal protein. Trends Biochem. Sci. 5, 491-494.
Sulima, S.O., Gulay, S.P., Anjos, M., Patchett, S., Meskauskas, A., Johnson, A.W., and
Dinman, J.D. (2014). Eukaryotic rpL1 0 drives ribosomal rotation. Nucleic Acids Res. 42,
2049-2063.
Szamecz, B., Rutkai, E., CuchalovA, L., Munzarova, V., Herrmannova, A., Nielsen, K.H.,
Burela, L., Hinnebusch, A.G., and Valasek, L. (2008). elF3a cooperates with sequences
5' of uORF1 to promote resumption of scanning by post-termination ribosomes for
reinitiation on GCN4 mRNA. Genes Dev 22, 2414-2425.
Thoreen, C.C., Chantranupong, L., Keys, H.R., Wang, T., Gray, N.S., and Sabatini, D.M.
(2012). A unifying model for mTORC1 -mediated regulation of mRNA translation. Nature
485,109-113.
Tyers, M. (2015). BioGRID. http://thebiogrid.org/. Accessed June 25, 2015.
Valouev, A., Johnson, D.S., Sundquist, A., Medina, C., Anton, E., Batzoglou, S., Myers,
R.M., and Sidow, A. (2008). Genome-wide analysis of transcription factor binding sites
based on ChiP-Seq data. Nat Methods 5, 829-834.
Warner, J.R., and Gorenstein, C. (1978). The ribosomal proteins of Saccharomyces
cerevisiae. In Methods in Cell Biology, pp. 45-60.
Wehrle-Haller, B., and Imhof, B.A. (2002). The inner lives of focal adhesions. Trends
Cell Biol. 12, 382-389.
Witzel, F., Maddison, L., and Blthgen, N. (2012). How scaffolds shape MAPK
signaling: What we know and opportunities for systems approaches. Front. Physiol. 3
DEC.
Xue, S., Tian, S., Fujii, K., Kladwang, W., Das, R., and Barna, M. (2014). RNA regulons
in Hox 5' UTRs confer ribosome specificity to gene regulation. Nature.
Yaka, R., Thornton, C., Vagts, A.J., Phamluong, K., Bonci, A., and Ron, D. (2002).
NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc.
Natl. Acad. Sci. U. S. A. 99, 5710-5715.
40
Yaka, R., He, D.-Y., Phamluong, K., and Ron, D. (2003). Pituitary adenylate cyclaseactivating polypeptide (PACAP(1-38)) enhances N-methyl-D-aspartate receptor function
and brain-derived neurotrophic factor expression via RACK1. J. Biol. Chem. 278, 96309638.
Zeller, C.E., Parnell, S.C., and Dohlman, H.G. (2007). The RACK1 ortholog Asci
functions as a G-protein beta subunit coupled to glucose responsiveness in yeast. J Biol
Chem 282, 25168-25176.
Zemp, I., and Kutay, U. (2007). Nuclear export and cytoplasmic maturation of ribosomal
subunits. FEBS Lett. 581, 2783-2793.
Zhou, C., Arslan, F., Wee, S., Krishnan, S., Ivanov, A.R., Oliva, A., Leatherwood, J., and
Wolf, D.A. (2005). PCI proteins elF3e and elF3m define distinct translation initiation
factor 3 complexes. BMC Biol. 3,14.
41
Chapter II:
Asc1/RACK1 is required for efficient translation of
short mRNAs
42
Abstract
Translation is a core cellular process carried out by a highly conserved macromolecular
machine, the ribosome. There has been remarkable evolutionary adaptation of this
machine through the addition of eukaryote-specific ribosomal proteins whose individual
effects on ribosome function are largely unknown. Here we show that eukaryote-specific
Asci/RACK1 is required for efficient translation of mRNAs with short open reading
frames that comprise a significant proportion of the transcriptome and show greater than
average translational efficiency in diverse eukaryotes. ASC1 mutants compromise
translation of mRNAs in specific functional groups, including those encoding
cytoplasmic and mitochondrial ribosomal proteins, and display cellular phenotypes
consistent with their gene-specific translation defects. ASC1-sensitive mRNAs are
preferentially associated with the translational closed-loop complex, and depletion of the
central closed-loop factor, elF4G, mimics the translational defects of ASC1 mutants.
Together, our results reveal a role for Asci /RACK1 in a length-dependent initiation
mechanism optimized for efficient translation of genes with important housekeeping
functions.
Introduction
Ribosomes are universal protein-synthesizing machines that are highly conserved in
their structure and function through all kingdoms of life. However, each domain of life
has evolved unique ribosomal proteins that are added to the conserved core. The
fundamental tasks of ribosomes - deciphering the genetic code and synthesizing
43
peptide bonds - are the same in all organisms, so the functions of these 'extra'
ribosomal proteins are intriguing, yet almost entirely unknown.
Eukaryotic ribosomes contain 13 domain-specific proteins that may play roles in
translation initiation, which is both more complicated and more highly regulated in
eukaryotes than in prokaryotes (Ban et al., 2014; Sonenberg and Hinnebusch, 2009).
Recruitment of prokaryotic ribosomes to mRNAs requires only three initiation factors,
IF1, 2, and 3, and relies on base-pairing between the RNA of the small ribosomal
subunit and the anti-Shine-Delgarno sequence of the mRNA (Boelens and Gualerzi,
2002). In contrast, canonical translation initiation in eukaryotes requires at least 12
initiation factors and proceeds by a complex series of steps beginning with recognition
of the mRNA 5' cap structure, followed by unwinding of mRNA secondary structure,
recruitment of the small (40S) ribosomal subunit, scanning, recognition of the initiation
codon, and finally, joining of the large (60S) ribosomal subunit to form a functional
ribosome (Aitken and Lorsch, 2012). Although the eukaryotic ribosome is generally
considered to be a passive player during canonical initiation, several of its proteins have
been implicated in mRNA recruitment. For example, Rpl38 is required for the translation
of the Hox body-patterning genes during embryonic development, allowing
spatiotemporal regulation of gene expression through translational control (Kondrashov
et al., 2011). Other proteins including Rps25, Rpl40, and RACK1 are essential for the
translation of viral mRNAs that are recruited to the ribosome via alternative initiation
44
pathways (Cherry et al., 2005; Landry et al., 2009; Lee et al., 2013; Majzoub et al.,
2014).
The eukaryote-specific ribosomal protein RACK1 is a WD40-repeat propeller protein
that binds the solvent-exposed face of the 40S subunit near the mRNA exit channel, in
close proximity to proteins that contact the mRNA during translation initiation (Pisarev et
al., 2008; Sengupta et al., 2004). In addition to its function as a core ribosomal protein,
RACK1 has been found in complex with several proteins involved in signal transduction
including protein kinase C, Src kinase, and cAMP phosphodiesterase, among many
others (Adams et al., 2011). The location of RACK1 on the ribosome together with its
interactions with signaling proteins suggests a possible role in conveying stimulusdependent information to the translation machinery (Nilsson et al., 2004). However,
signaling pathways in yeast and human have diverged significantly compared to genes
required for ribosomal function (Stuart et al., 2003), suggesting that RACK1 might have
another, more conserved function during translation.
Loss of RACK1 causes widespread and pleiotropic defects in many organisms. Deletion
of the RACK1 homolog in budding yeast, ASC1, leads to slow growth, loss of invasive
growth, loss of cell wall integrity, and decreased 60S subunit levels, among many
described effects (Li et al., 2009; Melamed et al., 2010; Valerius et al., 2007; Yoshikawa
et al., 2011). In metazoans, RACK1 is required for cell migration, neural tube closure,
and control of post-synaptic excitation in the brain (Kiely et al., 2009; Ron et al., 1999;
45
Wehner et al., 2011; Yaka et al., 2002). These cellular functions may explain why
homozygous RACK1 loss-of-function mutations cause early developmental lethality in
mouse and flies (Kadrmas et al., 2007; Volta et al., 2013). However, it is not known
whether and how the effects of RACK1 on ribosome function contribute to the myriad
cellular and organismal phenotypes observed in RACK1/ASC1 mutants (Gibson, 2012).
Here we have examined the translational functions of Asc1/RACK1 genome-wide by
ribosome footprint profiling in yeast ASC1 mutants. We show that Asci is required for
the efficient translation of mRNAs with short open reading frames (ORFs), including
those encoding cytoplasmic and mitochondrial ribosomal proteins. This requirement is
specific as depletion of other ribosomal proteins does not cause similar translation
defects. Using a series of translational reporters we demonstrate that ORF length per se
determines translational sensitivity to Ascl, thus confirming a role for Asci in the
translational privileging of mRNAs with short ORFs, which is a dominant trend in
genome-wide translational efficiency data from diverse eukaryotes. Remarkably, an
mRNA's association with proteins that mediate the formation of a 'closed-loop' during
translation - elF4E, elF4G, and Pabi - is an even better predictor of Asc1 -sensitivity
than ORF length, strongly suggesting a role for Asci in the function of the translational
closed-loop complex. Consistent with this prediction, we find that depletion of the central
closed-loop factor, elF4G, mimics the translational effects of mutating ASC1. Finally, we
show that loss of ASC1 renders cells unable to use alternative carbon sources that
require enhanced mitochondrial function, demonstrating the functional significance of
46
translational perturbation in ASC1 mutants. Together, our results reveal a role for Asci
in the enhanced translation of short ORFs and establish a direct connection between
gene-specific effects of Asci on translation and defects in cellular physiology.
Furthermore, because mitochondria are essential for energy generation and regulation
of many cellular networks, our results suggest that the pleiotropic phenotypes
associated with the Asci /RACK1 protein should be re-examined in the context of
mitochondrial health.
Results
Loss of the Asci protein perturbs global translation
The ASC1 locus encodes two distinct gene products - the Asci protein and an intronic
small nucleolar RNA, snR24. Because snR24 directs 2'-O-methylation of 25S rRNA at
positions C1437, C1449, and C1450, some of the reported phenotypes of ASC1 null
mutants (asciA) could be due to effects of deleting SNR24 on ribosome biogenesis or
function. In addition, Asci/RACK1 may have functions off the ribosome (Baum et al.,
2004; Coyle et al., 2009; Warner and McIntosh, 2009). We therefore created an allelic
series of yeast mutants with altered Asci function to enable direct comparison of the
cellular and translational effects of Asci/RACK1 (Figure 2-1A). We created protein null
alleles by mutating the start codon of ASC1 to a stop codon (asci-MiXand asc-E5X),
which abolished Asci protein expression but maintained wild type levels of SNR24
(Figure 2-1 B,C). Although bulk polysomes appeared normal in each of these strains,
both asciA and asci-MiX showed reduced levels of free 60S subunits (Figure 2-11D).
Similar defects in asclA were previously attributed solely to loss of snR24 (Li et al.,
47
2009), but our results indicate that the Asci protein has a separate function in 60S
biogenesis or quality control that is distinct from that of snR24. Restoring SNR24
expression did rescue the temperature-sensitive polysome defect of the asciA mutant in
agreement with previous observations (Figure 2-2A-D) (Li et al., 2009), however the
asc 1-M1X mutant maintained lower levels of 60S subunits even at the normal growth
temperature. The slight discrepancy between our results and the literature may stem
from differences in strain background (Sigma vs. S288C) and/or growth temperature
(300C vs. 370C). Both asci-MiX and ascL grow slowly under standard laboratory
conditions, whereas a mutant lacking only snR24 grows as well as wild type, further
demonstrating the importance of the Asci protein (Figure 2-2E).
48
R38D K40E (DE)
A
MIX E5X
D1O9Y
C 3.0
B
2.5
SNR24
2.0
a-Ascl
.4
a-Pgkl
--
qMMV-WjMW- 40MM"0010,
1.5
TC
1.0
I-
0.5
<,
0.0
0
D
ASC1
M
I
D109Y
DE
WT
E5X
MiX
asciA tAintron
80S
80S
80S
80S
MiX
P/M=2.00+/-0.03
60/40=1.98+/-0.03
D109Y
P/M=1.69+/-0.18
WT
P/M=1.83+/-0.18
60/40=9.09+/-0.32
60/40=6.59+/-0.09
asclt
P/M=1.75+/-0.06
60/40=1.57+/-0.32
Figure 2-1: The ASC1 allelic series. (A) Gene model of ASC1, showing the SNR24 snoRNA and
locations of protein null (M1X and E5X) and ribosome binding (DE and D109Y) mutations. (B) Asci
protein levels quantified by Western blot. Pgkl blot on the same membrane is shown as a loading control.
Dilutions of the WT sample are shown on the left. (C) ASC1 mRNA and SNR24 snoRNA levels quantified
by qRT-PCR. Levels were normalized to ACT1 mRNA levels. Error bars represent s.d. from three
technical replicates. (D) Polysome profiles of the ASC1 mutants at 30 *C. The polysome/monosome (P/M)
ratio and 60S/40S (60/40) are shown with s.d. from two biological replicates.
A
80SWT
B
M1x
C
asciA
D
asclAintron
E
WT
DE
80
8 -
D1 09Y
MiX
asc1A
YPAD, 370 C
hintron
Figure 2-2: The MiX mutation rescues the temperature-sensitive ribosome biogenesis defect of
ascA (A-D) Polysomes of ASC1 mutants grown at 37 0C, showing halfmer formation in mutants lacking
SNR24 (inset), but rescued in asc1-MiX. (E) Growth of the ASC1 mutants on YPAD plates at 30C.
Growth of 5-fold dilutions of the culture is shown.
49
To define the translational function of Asci, we subjected the ASC1 mutants to
ribosome footprint profiling and RNA-seq. Together, these techniques allow
quantification of ribosome densities transcriptome-wide and can be used to infer
changes in gene-specific translation activity (Ingolia et al., 2009). Loss of the Asci
protein caused changes in translation activity for many genes as measured by
translational efficiency (TE) - the number of ribosomal footprints normalized by the
number of total RNA fragments for each RNA (Figure 2-3A,B). The magnitude and
pervasiveness of translation changes in asci-MiX and ascliA are notable given the
normal appearance of bulk polysomes in ASC1 mutants (Figure 2-1 D). Thus
superficially normal polysomes can conceal significant perturbations of cellular
translation. Translation efficiency changes in asciA and ascl-MlX are well correlated
(r=0.89, Figure 2-3C), indicating that the Asc1 protein rather than the snR24 snoRNA is
responsible for most translational defects of the asciA mutant. Together, these results
demonstrate that the lack of Asci substantially alters the translational landscape of
yeast cells.
50
B 0.08
A
-
0.07
translation efficiency (TE)=
ribosome-protected mRNA fragements
error
replicate
miM1#1r=0.89
C
0.06
Wt-
109 2A TE (mutant /WT)
-
=2
asc1A #1
0.05
asclA#2
0~ 0.04
total mRNA abundance
0.030
0.02
-1.5
-1.0
-0.5
0.0
0.5
1.0
1092 A TE (mutant / WT)
j
1.5
-
'
0.00
-
0.01
ascIA
Figure 2-3: Loss of the Asci protein causes widespread changes in translation efficiency. (A)
Calculation of translation efficiency as the ratio of ribosome-protected mRNA fragments to total mRNA
abundance. (B) Distribution of changes in TE comparing two biological replicates from WT cells (i.e.
replicate error) or ascl-M1X or asclA to its corresponding WT comparison. #1 and #2 denote biological
replicate experiments. (C) Scatterplot of TE changes between the two ASC1 null mutants. The Pearson
correlation coefficient is shown.
ASCI 'ribosome-binding'mutants associate with ribosomes and are largely
functional
Next we examined isogenic yeast strains that express normal levels of Asci protein with
perturbed association to the ribosome. Asci is a WD-repeat protein that interacts with
helices 39 and 40 of the 18S rRNA primarily through its N-terminal blade (Sengupta et
al., 2004). Directed mutation of several basic residues in this region interferes with the
ribosome-binding capacity of the protein, with the strongest defect observed in the
R38D K40E (DE) mutant (Coyle et al., 2009; Sengupta et al., 2004). Another Asci
ribosome binding mutant, D1 09Y, was discovered serendipitously in a forward genetic
screen for mutants with defects in no-go decay, a ribosome-associated RNA quality
control mechanism (Kuroha K et al., 2010). These mutant proteins were expressed at
near wild type levels (Figure 2-1 B), and both mutations substantially decreased cosedimentation of Asci with ribosomes in sucrose gradients, with D1 09Y having a
51
markedly stronger effect (Figure 2-4A) that is consistent with previous reports (Kuroha K
et al., 2010).
The D1 09Y strong ribosome-binding mutant showed translational defects that, although
correlated with those observed in the ASC1 null mutants (r=0.43, p=10 2 21 for asclA;
r=0.42, p=10 20 4 for asci-MiX), were much smaller in magnitude (Figure 2-4B), whereas
the DE mutant showed almost negligible effects on translation (Figure 2-4C). These
findings suggest that either Asci primarily affects translation from a location off the
ribosome, or that the ribosome-binding assay underestimates the extent of in vivo
association of the D109Y and DE mutant proteins because ribosome-bound factors can
dissociate during ultracentrifugation (Valasek et al., 2007). To test this second
possibility, we performed formaldehyde crosslinking before ultracentrifugation. In the
presence of crosslinking, we observed significant co-sedimentation of the DE and
D1 09Y proteins with polysomes (Figure 2-4D). Crosslinking is not quantitative (Orlando,
2000); thus this assay underestimates the extent of ribosome binding by the mutant
Asci proteins in vivo, which are likely much closer to wild type than previously
appreciated.
An important implication of these findings is that phenotypic differences between
'ribosome-binding' alleles and ASC1 null mutants likely reflect different degrees of
perturbing ribosome function and do not constitute evidence for 'extra-ribosomal' activity
by Asci /RACK1. We attempted to generate stronger ribosome binding-defective alleles
52
by combining multiple mutations, but the only mutant that we could generate with
additionally reduced ribosome binding compared to the DE mutant was expressed at
very low levels potentially due to misfolding (Figure 2-5A,B). Given the overall
correlation between D1 09Y and ASC1 null alleles for translation changes transcriptomewide, we infer that many of the translational changes in asci-MiX and asciA are likely
to be caused by direct effects of Asci on ribosome function.
A
B
- crosslinking
DE
WT
80S
80S
log, A TE (mutant / WT)
r=0.42
D109Y
r
=0.43
80S
1.r
Q0-
-1
0
-1
D
C
+ crosslinking
NT
DE
80S
log 2 A TE (mutant / WT)
D109Y
80S
r=
9 0%U
-1
-1
a-Asc
0
asclA
0.11
r = 0.10
1
-
1-
80S
I
asc i-MIX
-
a-Ascl
1
II,
0
asc1-M1X
1
0
-1
0
ascIA
Figure 2-4: Asci 'ribosome-binding' mutants retain ribosomal association in vivo. (A) Association
of Asc1 mutant proteins with the ribosome assayed by Western blot of fractions isolated after velocity
gradient sedimentation. (B, C) Scatterplot of TE changes between the two ASC1 null mutants and the
ascl-D109Y and ascl-DEribosome-binding mutants. The Pearson correlation coefficients are shown. (D)
The same as (A) but proteins were crosslinked with formaldehyde in vivo before sample processing.
53
1
non-polysoma
A
asci+ ASCI-
B
polysomal
WT
DE
D5E
D109Y
WT
DE
Q-Ascl
+
$
D5E
Q-Rpp2A/B
WT
DE= R38D, K40E
D5E= R38D, K40E, K62E, K87E, K90E, K102E
*cross-reacting band
Figure 2-5: An ASC1 mutant with additional amino acid substitutions to disrupt ribosome binding
is not expressed. (A) Polysome Western blots show decreased polysomal association of the D5E
mutant compared to the DE mutant. (B) Western blot for total Asc1 protein levels in the ribosome-binding
mutants. Equal amounts of total protein were loaded in each lane. ASC1 alleles for this series of
experiments were expressed from a low-copy vector in the ascii strain. Part (A) is not directly
comparable to part (B) because the blots were overexposed to allow visualization of very faint bands in
the polysomal region.
Asci promotes translationof mRNAs with short open reading frames
To probe the mechanism by which Asci promotes translation of specific RNAs, we
searched for shared attributes among mRNAs with decreased TE in the asc1-MiX
mutant. We first addressed the hypothesis that Asci enhances the translation of mRNA
targets of the Scpl 60 RNA-binding protein, which associates with polysomes only in the
presence of Asci (Baum et al., 2004). A second RNA-binding protein, Bfr1, also
associates with polysomes, possibly via its interaction with Scpl 60 (Lang and FridovichKeil, 2000). A previous study found that Asci dissociates from the ribosome during
stationary phase and suggested that regulated loss of ribosomal Asci would repress
the translation of Scp1 60-target mRNAs during this growth phase (Baum et al., 2004).
54
However, neither Scpl 60-associated mRNAs nor Bfrl -associated mRNAs had notably
decreased translation in the asci-MiX mutant during mid-log phase growth (Figure 26A). Furthermore, our experiments do not support the model of regulated ribosome
dissociation for Asci during stationary phase because Asci remained almost
completely ribosome associated during both post-diauxic growth (OD 6) and stationary
phase (OD 10) (Figure 2-6B).
A 1.0 0.
B
OD 6
-8
LI)O
00.6
S-
-
mp
-
--
a-Ascl
0.4
OD 10
0.2
C'j
-2.0 -1.5 -1.0 -0.5 0.0 0.5 1.0
1092 ATE (ascl-M1XIWT)
1.5
2.0
- all genes (n=5413)
- Scpl 60 targets (n=2205) p=0.41
- Bfrl targets (n=1409) p=7.7e-03
a-Asc1
Figure 2-6: Asc1 does not enhance the translation of Scpl60 target mRNAs or dissociate from the
ribosome during stationary phase. (A) Cumulative distribution of translational efficiency changes for
Scp160 and Bfrl target mRNAs in the ascl-MiXmutant. mRNA targets for Scpl60 and Bfrl are taken
from (Hogan et al., 2008). P-values are from the Kolmogorov-Smirnov test. (B) Polysome Western blot
showing continued association of the Asc1 protein with ribosomes during post-diauxic (OD 6) and
stationary phase (OD 10) growth.
We then took a more open-ended approach to identifying the molecular basis of
translational sensitivity to loss of Asci. Motif analysis of 5' UTRs revealed the presence
of a U-rich sequence in mRNAs sensitive to loss of Asci (Figure 2-7A), but not found in
-
mRNAs resistant to loss of Asci. However, this motif was present in only 11 % of Asci
55
sensitive mRNAs and so cannot be required generally for translational enhancement by
Asci. We next examined various physical properties of Asci -sensitive mRNAs (Figure
2-7B). Among the tested attributes, ORF length was notably well-correlated with ATE in
ascl-M1X(r=0.27, p=1 084 , Figure 2-7B) and ORFs < 500 nts were the most strongly
affected (Figure 2-8A). Short ORFs are highly translated in wild type cells (Figure 2-8B
and (Arava et al., 2003)), an effect that has been hypothesized to reflect a higher rate of
translation initiation on short mRNAs for reasons that are mechanistically mysterious
(Figure 2-8C and (Arava et al., 2005; Shah et al., 2013)). Because short ORFs are
among the most highly expressed, the loss of RACK1/Asc1 significantly alters the gene
expression landscape of the cell.
56
A
2
5' UTRs of mRNAs with decreased TE in ascl-MlX
to
51
3
B
attribute
Spearman r
(asci-MIX ATE vs.
attribute)
p-value
wild type protein level
0.103
1.49e-2
wild type TE
-0.091
1.55e-10
tRNA adaptation index
0.023
1.17e-1
5' UTR length
-0.004
7.73e-1
3' UTR length
0.079
9.62e-8
ORF length
0.272
3.05e-84
5' UTR folding energy
0.030
3.97e-2
3' UTR folding energy
-0.077
1.83e-7
poly(A) tail length
0.029
7.38e-2
Figure 2-7: Identification of properties of Asci -sensitive mRNAs. (A) Motif analysis of 5' UTRs of
genes with decreased TE in asci-MiX, defined as having a z-score s -1. (motif present in 37/325 genes,
E-value= 8.3e-1 1). (B) Properties of Asc1 -sensitive mRNAs. Gene or mRNA attributes were correlated
with ATE in the asci-MiX mutant. The Spearman correlation coefficients and p-values are shown.
57
A
r = O.27
40
B 'o
r = -0.50
'
fast initiation
'
w
20
C
0-5
..
~~..!*'!**%
t
slow initiation
00
W
,,.
-0.
:.~'
4-20
w
TE,> TE,
0P
fraction of genes
_401
0.2
500
0.4
1000
0.6
1500
0.8
2000
10
4000
ORF length (nt)
-1 0
-
~..
1000
2
2000
0
4000
5000
60O
ORF length (nt)
(A) Relationship between ORF
Figure 2-8: Asc1 is required for efficient translation of short ORFs.
percent change in TE for
length and TE changes in asci-MiX. The values shown represent the average
to the point at which the
bins of 100 genes arranged by length. The ORF lengths shown correspond
areas represent +/- 1 s.d. from the
average ORF length of the bin exceeds the indicated value. Shaded
between ORF length and
average change. The ASC1 gene is excluded from the plot. (B) Relationship
Spearman correlation coefficient
translational efficiency in wild type yeast cells (data from this study). The
on short mRNAs compared to
is shown. (C) Model showing the expected effect of a higher initiation rate
long mRNAs on translation efficiency measurements.
predictive
We then sought to determine whether ORF length or transcript length is more
type
of translational efficiency. ORF length was slightly more predictive of wild
r=-0.09
translation efficiency than transcript length, (Figure 2-9A-E, partial correlation
transcript boundary
(p=1 0-8) vs. r=0.03 (p=1 0-1)). For simplicity, and because
metric in
annotations are not available for all yeast genes, we have used the ORF length
of the 3'
subsequent analyses. However, it is interesting to note that adding the length
length
UTR, but not the 5' UTR, to each ORF, slightly improved the correlation between
This observation
and ATE in asc1-MiX (Figure 2-9F-1, r=0.28 (10-78) vs. r=0.26 (10~70)).
the start
suggests that ribosome recruitment may be sensitive to the distance between
codon and the poly(A) tail in an Asci -dependent manner.
58
A
1-0
w
LL
1.0
B
r= -0-49
p= 4.66e-273
05
r= -0.49
p=1.07e-262
.a
0.!
0.5.
0)-
0.0-
50OOr
C
r= 0.98
p= 0.00e+00
4000
S3000
C
I
'
VA: .
200C
;-
0 -0.5-
.
o -0.5
100C
-1.e
1000
2000
3000
4000
0
-1
5000
1000
ORF length (nt)
D
1c
2000
3000
4000
5000
E
r= -0 09
p= 1. 180-08
t
-0
1000
2000
r= 0.03
p= 5.76e-02
C
-0
-
C 05
0
u--
.
0
0)
0>
-0 C
0
2 0400
.
residual
'
r=
G
0 26
200
400 600
800 1000
1 5
p- 3 69e-70
Sr= 0 25
p= 4 19e-65
1.0
-
1.c
0
residual
(transcript length vs. ORF length)
(ORF length vs. transcript length)
--
00 -200
-
?1 )00-800 -600 -400 -200
--
.-
-
0
F
-
0
0, 5
05
-N
00
U)
C -0.5
S00
-
-
LL!-0 '5
-1 0
0j) -1'0
0
0
-1, '1
0
1000
2000
3000
4000
50 0
1000
15
H
r= 0.25
P- 5 09e-65
-
05 -
2000
300
4000
5000
transcript length (nt)
ORF length (nt)
I
15,S
-
r=
0,28
9,93e-79
-p=
1.0 05
-X.
00
-
00
S-0.5
-
L -0.5
)-10
0
0
0
1000
2000
3000
4000
ORF + 5' UTR length (nt)
5000
0
1000
2000
3000
4000
5000
ORF + 3' UTR length (nt)
Figure 2-9: Relationship between translation efficiency, ORF length, transcript length.
59
3000
4000
ORF length (nt)
transcript length (nt)
5000
Figure 2-9: Relationship between translation efficiency, ORF length, and transcript length
(previous page). (A-E) Partial correlation analysis showing the relationship between ORF length,
transcript length, and TE. (A) TE vs. ORF length (B) TE vs. transcript length (C) transcript length vs. ORF
length (D) TE vs. ORF length, partial correlation controlling for transcript length (E) TE vs. transcript
length, partial correlation controlling for ORF length. (F-1) Correlations between ATE in asci-MiX and the
lengths of the indicated transcript regions: (F) ORF length, (G) transcript length, (H) ORF length + 5' UTR
length (1) ORF length + 3' UTR length. Length values are the median reported values from (Pelechano et
al., 2014). Spearman correlation coefficients are shown between the indicated values or the residuals
after linear regression.
To test whether length per se, and not some other feature common to short mRNAs, is
responsible for Asci -sensitive translation, we generated constructs with identical
regulatory regions (promoter, 5' UTR, 3' UTR) that differed only in the length of the ORF
(Figure 2-1 OA). These ORF length reporters contain a variable number of repeats of the
127 domain from the human cardiac protein titin, which has been used extensively in
studies of protein folding because the small globular domains fold and unfold
independently of each other (Hoffmann and Dougan, 2012). This modular architecture
allows the construction of proteins of different lengths that resemble linear chains and
minimizes the potential for differential protein folding or stability between the long and
short proteins. A C-terminal epitope tag allowed us to quantify the translational
efficiency (protein/mRNA) of each construct (Figure 2-10B). Remarkably, the
translational efficiency of the shortest ORF (~300 nt) was almost 2-fold lower in the
asci-MiX mutant compared to the longest ORF (-2400 nt) with the intermediate ORF
length constructs displaying intermediate effects (Figure 2-10C). Together, our results
establish a role for Asci in the translational advantage of short mRNAs. Given that ORF
length is anti-correlated with translational efficiency in diverse eukaryotes (Figure 21OD-F, data from (Guo et al., 2010; Stadler and Fire, 2013)), this function of
Asc1/RACK1 may be conserved.
60
A
monomer
~tag
~
-300nt
dimer
~
-000 nt
tetra mer
~
-1200 nt
octamer
-2400 nt
µg pooled extract
B
WT
c
asc1-M1X
*
kDa
102
76
52
38
31
24
17
12
Red =a-VS (tag)
Green = a-Pgk1 (loading control)
D
UJ
I-
1.0
C. elegans
r= -0.36
P= O.OOe+OO
M. musculus
E
r= -0.43
p: O.OOe+OO
O>
r= -0.6 1
p;;;;O.OOe+OO
w
LU
I5!
H. sapien~
F
I-
o.o
.S?
-0 .5
- 0.5
_, 00
1000 2000 3000 4000 5000 6000
OR F length (nt)
-1 .00
1000 2000 3000 4000 5000 6000
OR F length (nt)
Figure 2-10: ORF length determines mRNA sensitivity to Asc1.
(A) Diagram of ORF length reporter constructs. The 127 monomer is used to make proteins corresponding
to a range of ORF lengths, each fused to a C-terminal VS epitope tag. (B) Representative Western blot
showing polyl27-expression in WT and asc1-M1X cells. The standard curve is a dilution series of the
different proteins expressed in WT cells and mixed at a ratio calibrated to allow accurate quantification of
each size protein. The polyprotein bands display variable brightness due to transfer efficiency and
membrane binding differences between proteins of different molecular weights (Bolt and Mahoney, 1997).
Therefore, we quantify the relative difference between mutant and WT at each protein size and cannot
draw conclusions about absolute protein concentrations across the molecular weight range from Western
blotting analysis. (C) Result of ORF length reporter experiment. TE is calculated as the protein (VS
tag/Pgk1) to mRNA ratio (VS mRNA/188) and the ~TE (ratio between mutant and WT) is shown. Relative
protein concentration was obtained from quantitative Western blotting and mRNA concentration from
61
qRT-PCR. *p<0.05, one-tail t-test (monomer vs. octamer). For dimer vs. octamer, p=0.067. Error bars are
SEM from 4 biological replicates. (D-F) Scatterplots showing the relationships between TE and ORF
length in diverse eukaryotes: C. elegans, dauer stage (Stadler and Fire, 2013) (D), M. musculus,
neutrophils (Guo et al., 2010) (E), and H. sapiens, HeLa cells (Guo et al., 2010) (F).
How might short ORFs be both translationally privileged and sensitive to loss of Asc1?
Asci's position near the mRNA exit channel places it in close proximity to translation
initiation factors that interact with the mRNA during initiation, including elF3 and eIF4G
(Figure 1-2). eIF4G has a well-characterized role in promoting a circularized form of the
mRNA in which the 5' and 3' regions of the mRNA are bundled together via the
interaction between the eIF4G protein, associated with the mRNA 5' cap through the
elF4F complex, and Pabi, an RNA-binding protein that binds the poly(A) tail. The
mRNA in this conformation is known as the closed-loop, and closed-loop formation is
thought to enhance translation (Kahvejian et al., 2001). We hypothesized that mRNAs
with short ORFs might form closed-loop structures more efficiently than mRNAs with
longer ORFs, and that Asc1 could promote the function of the closed-loop in translation.
According to this model, mRNAs with short ORFs should be more highly associated with
the closed-loop factors - elF4E, elF4G, and Pabi - than other mRNAs. To test this
prediction, we analyzed data quantifying the association of specific mRNAs with the
closed-loop factors and the elF4E binding proteins (4E-BPs) by RNA
immunoprecipitation and sequencing (Costello et al., 2015). We grouped mRNAs into
'closed-loop', 'strong closed-loop', and 'other' categories based on the following
enrichment profiles: 'Strong closed loop' mRNAs are enriched in immunoprecipitations
of elF4E, elF4G, and Pabi and de-enriched in immunoprecipitations of the 4E-BPs,
62
which should not be associated with mRNAs in closed-loops because 4E-BPs and
elF4G compete for binding to elF4E (Haghighat et al., 1995). 'Closed-loop' mRNAs
have similar enrichment profiles as 'strong closed-loop' mRNAs, but are not de-enriched
for association with the 4E-BPs. Remarkably, we found that closed-loop-enriched
mRNAs were dramatically shorter than other mRNAs (median ORF lengths= 489, 774,
and 1694 nt for 'strong closed-loop', 'closed-loop', and 'other' mRNAs, respectively),
regardless of whether ribosomal proteins were included in the analysis (Figure 2-11 A,B,
data from (Costello et al., 2015)). The fact that closed-loop-associated mRNAs are
much shorter than other mRNAs provides a biochemical explanation for the preference
for higher translation of mRNAs with short ORFs observed here and previously (Arava
et al., 2003). Furthermore, loss of Asci or eIF4G depletion similarly decreased the
translation of closed-loop-associated mRNAs, even after accounting for the relationship
between ORF length and ATE or polysome association in these mutants by linear
regression, indicating that closed-loop association has additional predictive power for
sensitivity to Asci and eIF4G (Figure 2-11 C-F, Figure 2-12). These results suggest that
Asci is important for closed-loop formation or stability, or for closed-loop-dependent
ribosome recruitment.
63
A 0.16
a
T
0.14
B 0.18
strong closed-loop (n=375)
closed-loop (n=586)
other (n=1722)
0.12
0.14
no RPs (n=263)
U)
0.12
no RPs (n=572)
no RPs (n=1669)
I-
0.10
g 0.10
0.08
c
0
.2
0.06
2 (n=195)
3A (n=234)
3B (n=141)
4A (n=586)
4B (n=1000)
4C (n=337)
0.08
0.04
0.04
0.02
0.02
0.00
500 1000 1500 2000 2500 3000 3500
ORF length (nt)
0.09
D
0 10
.
r
LI
0.08
0.06
(0
-
0.04
0.06
-
0.05-
.2 0.04
-
0
C
0
0)
0
C
Group 1 (n=190)
Group 2 (n=195)
Group 3A (n=234)
Group 3B (n=141)
-
0.07
OuV 1uuu 1OVu zuuu zOuu suVu sOuu
ORF length (nt)
strong closed-loop (n=375)
closed-loop (n=586)
other (n=1722)
ORF length corrected
0.08
0)
1 (n=190)
a 0.06
0.00
C
Group
Group
L Group
Group
Group
M
Group
Group
0.16
F
M
Group 4A (n=586)
Group 48 (n=1 000)
Group 4C (n=337)
U
0
0.03
0.02
-
0.02
0.01
0.00
-40
-20
0
0.00 W
-40
40
20
strong closed-loop (n=390)
closed-loop (n=626)
0.06
4
zu
0.09
4U
Group 1 (n=193)
Group 2 (n=196)
E Group 3A (n=245)
Group 3B (n=145)
Group 4A (n=626)
-
F
0.08
other(n=1742)
ORF length corrected
0.05
U
-
E 0.07
-2
% A TE (asc1-M1X / WT)
% ATE (ascl-MlX / WT)
-
0.07
0.06
0.04
0)
C
0'
0.03
C
Group 4B (n=1015)
a
0.05
Group 4C (n=338)
0.04
0.03
-
& 0.02
0.02
0.01 1-
0.00 M
-40
0.01
0.00
-40
zu
% ATE (elF4G-depletlon / WT)
-ZU
u
-20
0
20
40
% A TE (eIF4G-depletion / WT)
Figure 2-11: Asc1 is required for translation of closed-loop complex associated mRNAs. Closedloop associations for mRNAs are taken from (Costello et al., 2015), in which mRNAs were subdivided by
hierarchical clustering into groups with similar translation factor enrichment profiles. (B, D, F) show the
original groups of factor enrichment from (Costello et al., 2015). In (A, C, E), we reassigned group names
based on their factor enrichment profiles. 'Strong closed-loop' is the combination of Group 3A and 3B
64
which are highly enriched with eIF4G, eIG4E, and Pabi and de-enriched for the 4E-BP repressors.
Because 4E-BPs prevent the interaction of eIF4E with eIF4G, 4E-BP association should be mutually
exclusive with closed-loop formation. 'Closed-loop' is Group 4A, which has a similar enrichment profile to
Groups 3A and 3B but also has some enrichment for the 4E-BP repressors. Association of 4E-BPs with
Group 4A mRNAs may indicate that a subset of these mRNAs is found in translationally repressed
complexes, outside of the closed-loop. All other groups were combined to form the category 'other'. (A, B)
The relationship between mRNA closed-loop complex association and ORF length. Strong closed-loopassociated and closed-loop associated mRNAs have shorter ORFs than other mRNAs (p=10-1 72 and 10-15
for strong closed-loop and closed-loop groups vs. other mRNAs, respectively). The dotted lines show the
ORF length distributions with ribosomal protein genes excluded. (C, D) Closed-loop-associated mRNAs
have decreased TE in ascl-M1X(p=10~ 1 and 10-42 for strong-closed loop and closed-loop vs. other
mRNAs, respectively). (E,F) Closed-loop-associated mRNAs have decreased TE after eIF4G depletion
(p=10-70 and 10-73 for strong closed-loop and closed-loop vs. other mRNAs, respectively. Data from (Park
et al., 2011)). In (C) and (E), the dotted lines show the results after accounting for the relationship
between ORF length and ATE in asci-MiX and after eIF4G depletion using linear regression. For asciMiX, ORF length corrected p-values are 10-30 and 10~14 for strong closed-loop and closed-loop groups,
respectively. For eIF4G depletion, ORF length corrected p-values are 10-17 and 10-2 for strong closedloop and closed-loop groups, respectively. P-values are from the one-sided Mann-Whitney U test.
0
40
M
0
r=
0.53
C
CR
20
be
.
-a
0
124
00
0 -20
1.I
..
ORF length
(nt)
Figure 2-12: e1F4G depletion decreases translation of short ORFs. Relationship between ORF length
and changes in mRNA polysome association after eIF4G depletion (data from (Park et al., 2011)). Plot
parameters are as described for Figure 2-8A.
What are the potential consequences of impairing translation of short ORFs? Using
gene ontology analysis, we found that transcripts annotated to the category 'ribosomal
subunit' had significantly decreased TE in the ASC1 null mutants (M1X, p=1 0-35, Figure
65
2-13A). This category is composed of short ORFs encoding both cytoplasmic and
mitochondrial ribosomal proteins (RPs, MRPs), which both displayed -20% decreased
TE in ASC1 null mutants (M1X, p=10- 37 for RPs and p=1010 for MRPs; ascA, p=1 o-3
for RPs and p=10 10 for MRPs, respectively, Figure 2-13B). As the median RP and MRP
ORF lengths are 434 and 716 nt, respectively, their translational defects are within the
range predicted by their length. Indeed, removing RP and MRP genes does not
significantly alter the global relationship between ATE and ORF length in asc1-MiX
(r=0.23, p=1 0-58, Figure 2-13C) indicating that all classes of genes with short ORFs
have decreased TE in asci-MiX. Although these GO categories were the clear outliers,
most GO categories with short median ORF lengths also displayed decreased TE in the
ASC1 null mutants, including several additional groups of genes whose protein products
function in mitochondria (Figure 2-13D). Because short ORF length is associated with
-
specific functional categories, loss of Asci - or, potentially, modulation of its activity
can lead to functionally coherent changes in gene expression.
66
75
B
-
45
asciA
30
I
.
F
15
0
E
-15
-30
10
-45
5
0
D109Y
60
ascIA
Iii,
.4 H-
-60
-75
ribosomal
subunit
C 40
D
ascl-M1Xr= 0.27
no RPs r= 0.23
n= 132
1 Or
00
5
0
0
S
0
U-,
-.
-
~
* *
0
0
~D0Q~~
W~;*,~j~~-.= jS
.1
S
-5
6565
~0
0
*
-20
MRP
n= 69
RP
all genes
n= 4870
cytosolic mitochondrial
ribosome ribosome
*15
.
.0.
.0-20
-J
S
8
%oft
I
a.
for
10
0
-
30
D109Y
MiX
fraction of genes
0.2
500
0.4
1000
0.6
1.0
0.8
1500 2000
4000
*
*
*
-20
-25
ORF length (nt)
500
1000
all
mitochondrial
ribosomal
1500
2000
median ORF length (nt)
Figure 2-13: Loss of Asc1 causes decreased translational efficiency of cytoplasmic and
mitochondrial ribosomal protein mRNAs. (A) GO Component category enrichments for mRNAs with
decreased TE in the ASC1 mutants. GO categories related to the top category 'ribosomal subunit' for the
asc1-MiX mutant are displayed. (B) Violin plot showing the decreases in TE for the cytosolic ribosomal
protein (RP) and mitochondrial ribosomal protein (MRP) gene sets in the ASC1 mutants. The violin shape
represents a kernel density estimation and the top and bottom of the plot extend to the most extreme data
3
point within 1.5x of the inner quartile range. Midlines represent the medians. ***p<1 0-1, *p<1 0 *p<10
(C) Relationship between ORF length and TE change in asci-MiX showing that excluding RPs and
MRPs from the analysis ('no RPs') does not change the results. Plot made as in Figure 2-8A. (D) The
percentage change in TE for all GO component categories containing >20 genes and arranged with equal
spacing by median ORF length. The x-axis denotes the point at which the median ORF length of the
group exceeds the indicated value. Categories related to mitochondrial or ribosomal functions are
highlighted.
We noted that RP and MRP mRNAs decreased in both the total RNA pool and the
ribosome-protected footprint (FP) pool (Figure 2-14A, B). The additional decrease in the
FP pool shows that these mRNA substrates are translationally disadvantaged in the
ASC1 mutants. In support of this interpretation, qRT-PCR analysis of polysome gradient
67
fractions demonstrated that representative MRP mRNAs associated with fewer
ribosomes in ascl-M1X (Figure 2-14C,D), which specifically indicates a defect in
translation initiation. Because inhibiting translation initiation can induce mRNA
degradation (Coller and Parker, 2005; LaGrandeur and Parker, 1999; Schwartz and
Parker, 1999), decreased translation may account for the reduction in total mRNA levels
although we cannot exclude the possibility of transcriptional effects or translationindependent effects of Asci on mRNA stability. We note that our translation efficiency
measurements are correlated with steady-state mRNA half-life estimates using noninvasive metabolic labeling approaches (r=0.43, p=10-194, Figure 2-15A, data from
(Miller et al., 2011) and r=0.39, p=10-168, Figure 2-15B (Neymotin et al., 2014)),
consistent with the hypothesis that the decay rates of mRNAs are coupled to their
translational status. The same trends of decreased TE for the RP and MRP genes were
observed using an rRNA-depletion strategy instead of poly(A) selection (Figure 215C,D), ruling out a significant effect of poly(A) tail length on our ATE calculations
(Subtelny et al., 2014). Thus, Asci is required for efficient translation of short ORFs,
which includes most cytosolic and mitochondrial ribosomal proteins.
68
B
A 3
.:
'.
75
D109Y
60
2
M
45
increased TE
asciA
30
**i'
15
-
r= 0.74
-
-.
-
CL
U
U-
6%
decreased TE
-
0
[ RP
-30
-60
2
1
0
-1
-2
-3
-75
n,-A
.
I
-a-
MRP2
D
wr
asci-MIX
MRP
RP
n=1 32
n=4880
n=69
0.4-
YML6
ascl-MIX
+
0.3-
0.20-
E
E
C
0
all genes
log 2 A total mRNA (ascl-MIX / WT)
+
z
-15
-45
-2
C
0
0.2-
C
0.1-
0.1-
.1=0a
U.0
1
2
3
4
5
6
mRNP 40S 60S 8OS
7
8
9
0. :
10 11 12 fraction
polysomes
1
2
3
4
5
6
mRNP 40S 60S 80S
7
8
9
10 11 12
fraction
polysomes
Figure 2-14: Asci -sensitive mRNAs decrease in both translation efficiency and total mRNA levels.
(A) Scatterplot showing the decrease in both the footprint (FP) and total RNA pool for RP and MRP
mRNAs. The Pearson correlation coefficient in shown. (B) As in Figure 2-13B, but with the change in the
ribosome footprint pool (FP) shown instead of TE. (C, D) Polysome qRT-PCR showing decreased
association of MRP genes with heavy polysomes. Values are normalized to an RNA spike-in control in
each fraction and then set so that the sum of all fractions=1.
69
30'
A
30
B
r= 0.43
p= 1 25e-194
2.5[
2,5
E,
2.0
2.0
1.5
1.5
I'D
F
.cc*
ZE
.s.
E
Oi
0.5 l_
0-3.0I
r= 0.39
p= 3.11e-168
1.0
L4
0.5
0
OC
-2-5
-2.0 -1.5 -1.0
0.0
-0.5
0.5
10
3.0 -2.5 -2.0 -1.5 -10 -0.5 0.0
M
M
45
D2
polyA selection
2.0
1.5
Rlbo-Zero
0
LU -15
.0-30
-45
-60
e
Al (r=0.84)
RP (r= 0.98)
.~1.0-
all genes
n=4924
RP
n=132
-
0.0 -
-0w
.
i'$4 I
14
0.5
-
15
.
&
Cr
30
1 0
109, wildtype TE
log,O wild type TE
C 60
0.5
I.-
< -1.5
- 2.0
-2.0 -1.5 -1.0 -0.5 0.0 0.5 1.0 1.5 2.0
1092 ATE (ascl-M1X/ WT) with poly(A) selection
MRP
n=69
and the effect of different rRNA
Figure 2-15: The relationship between TE and mRNA half-lives
from our data correlated with steadydepletion strategies on ATE measurements. (A, B) TE values
labeling from (Miller et al., 2011) (A) or
state mRNA half-life measurements obtained using 4-thiouridine
are shown. (C) Violin plot showing TE
(Neymotin et al., 2014) (B). The Spearman correlation coefficients
selection or rRNA subtraction
changes of the RP and MRP groups in asci-MiX using either polr(A)
p<10-9, *p<10 -2. (D) Scatterplot
(Ribo-Zero) during preparation of the total RNA libraries. ***p<10~ ,
subtraction or poly(A) selection.
showing well-correlated global changes in ATE whether using rRNA
of
The translationaldefects of ASC1 mutants are not a general consequence
perturbing the ribosome
The mRNAs that are sensitive to the loss of Asc are among the most efficiently
of
translated in a cell. We therefore considered the possibility that reduced translation
To assess
these mRNAs might be a general consequence of perturbing the ribosome.
two
the specificity of the translational phenotypes of ASC1 null mutants, we tested
deletes one
additional ribosomal protein mutants, rpI23bA and rppa4 each of which
70
paralog encoding a core ribosomal protein. Like asci-MiX, rpI23bA and rpplaA show
reduced growth on glucose and decreased 60S subunit levels (Figure 2-16A, B).
However, rpI23bA and rppla,6 showed little similarity to asc1-MiX in their translational
dysregulation genome-wide (r=0. 18 and 0.07, respectively, Figure 2-16C), and they did
not display decreased TE of RP and MRP transcripts (Figure 2-16D). Because the
growth and bulk translation phenotypes of rpI23bA and rppiaA are more severe than
asci-MiX, any shared defects in gene-specific translation should have been readily
detected. Thus, decreased translation of RP genes is not a general feature of slowgrowing mutants or those with decreased 60S subunit levels.
6
-WT
asci-MIX
5
rpI23bd
Co
WT
P/M=1.83+/-0.18
60/40=9.09+/-0.32
rpplaA
4
0
0
B
growth in glucose
A 7
rppiaA
P/M=1.92+/-0.28
60/40=2.19+/-0.51
rpI23bA
P/M=3.06+/-0.30
60/40=0.79+/-0.14
80S
3j
80S
80S
21
0 2
4
6
8
time (hours)
10
12
D
r= 0. 18
C
75
60
45
0.
30
15
0
--
r= 0.07
3.
r= 0.82
0
-15
0
-30
<0
0.
0-
ascl-MIX
-45
M
M
-60
-3
3
-3---3
0
-
0
asc1 -MiX
rpI23bA
3
-75
all genes
n= 4924
RP
n= 132
rpI23bA
rpplaA
MRP
n= 69
Figure 2-16: Other ribosomal protein mutants with decreased 60S levels do not share
translational phenotypes with the ASC1 mutants. (A) Growth curve comparing growth of asci-MiX
with other RP mutants rpI23bA and rpplaA at 30 OC in glucose. (B) Polysome profiles of the rpI23aA and
rppiaA mutants at 30 OC. The polysome/monosome (P/M) and 60S/40S (60/40) ratios are shown with s.d.
from two biological replicates. (C ) Correlations between ATE among rpI23bA, rppiaA, and asci-MiX.
The Pearson correlation coefficient is shown. (D) Violin plots showing the change in TE for the cytosolic
ribosomal protein (RP) and mitochondrial ribosomal protein (MRP) gene sets in asci-MiX, rpI23bA and
rppiaA. Violin plot parameters are described in Figure 2-13B.
71
Loss of Asci impairs mitochondrialfunctionin yeast
To assess the physiological significance of gene-specific translation defects in ASC1
mutants, we looked for phenotypes related to gene categories with significantly impaired
translation. In particular, the requirement of Asci for efficient MRP translation suggested
the possibility of impaired mitochondrial function in ASC1 mutants. To assess
mitochondrial health, we measured growth on the non-fermentable carbon source
glycerol, which requires the activity of the mitochondrial respiratory chain to generate
energy (Dimmer et al., 2002). When shifted to glycerol-containing media, wild type yeast
resumed rapid growth after an initial adaptation phase, but the asci-MiX mutant
completed only -3 doublings before ceasing growth (Figure 2-17). In contrast, the
rpI23bA and rppiaA mutants grew better in glycerol than asc1-MiX, demonstrating the
specificity of this phenotype (Figure 2-17). Consistent with our results, a proteomic
survey of asciA cells showed a shift away from respiration and towards fermentative
metabolism (Rachfall et al., 2012). Because mitochondrial ribosomes are required for
mitochondrial biogenesis and function, it is plausible that the growth and metabolic
defects of ASC1 mutants are consequences of the translation defects observed for MRP
genes.
72
in glycerol
5 -growth
4
WT
asc-MIX
-
rpplab
-
3
2
0
5
10
15
20
25
30
time (hours)
Figure 2-17: Asc1 is required for adaptation to a non-fermentable carbon source. Growth curves of
cells after a shift from glucose-containing media to fresh media containing glycerol. Compare to Figure 216A in which cells were grown in glucose. Curves are averages of two biological replicates, error bars are
s.d.
Given the severe growth defects of ASC1 null mutants in glycerol, we wondered
whether the moderate impairment of MRP translation observed in glucose would worsen
under conditions of increased MRP expression. Adaptation to growth in non-fermentable
carbon sources or low glucose is accompanied by a rewiring of the transcriptional
network in yeast (Galdieri et al., 2010) and widespread reprogramming of translation
(Vaidyanathan et al., 2014). To investigate whether the glycerol growth defect of asciM1X is linked to inadequate translational adaptation, we examined translation genomewide 6 hours after transfer from glucose to glycerol, a point just before the resumption of
rapid growth in wild type cells (Figure 2-17) and coincident with the recovery of
polysomes after the initial collapse upon glucose withdrawal (Figure 2-18A). In the asciM1X mutant, polysomes recovered only partially (Figure 2-18B). Moreover, the
ribosome-associated pool was strongly depleted of MRP mRNAs compared to wild type
(Figure 2-18C). However, the magnitude of translational defect (ATE) for this class of
mRNAs was similar in both glucose and glycerol, supporting a constitutive rather than
regulatory role for Asci in translation of MRP mRNAs (Figure 2-18C). The fact that
73
asc1-MiX shows a bulk translation defect in glycerol but not in glucose may reflect the
fact that MRP mRNAs make up a larger portion of the translatome in glycerol. Thus, the
cellular context is an important factor in determining the phenotypic consequences of
as
translational perturbations in asci-MiX and likely in other ribosomal protein mutants
well. Taken together, our results suggest an important role for Asci in supporting
cellular respiration by promoting synthesis of mitochondrial ribosomal proteins.
A
80S
WT 0 hrs
BOS P/M=188+1-0.04
WT 60.20
P/M=1.42+/-0.20
P/M=O5+/-0,06
80S
B
80S
M1X 3 hrs
P/M=0.49+/-0.04
7560-
growth in g1 ucos e
FP
total mRNA
TE
6
-
45
x
30
15-
M FP
=ZI total mRNA
M TE
75-
60
45
30
45
,,
-15-30
-45
-15
-30
-45
-60
-60
75 r
-90.
-75 --90
growth in glycerol
90
-
90
MiX 6 hrs
P/M=0.91+/-0.01
-
C
80S
-
M1X 0 hrs
80S P/M=1.88+/-0.09
all genes
n=4960
MRP
n=69
all genes
n=5121
MRP
n=69
proteins in the asci-MiX
Figure 2-18: The translational defects of the mitochondrial ribosomal
profiles of WT (A) and ascimutant are not augmented during respiratory growth. (A, B) Polysome
were shifted at OD=0.5. The
M1X (B) yeast after a shift from glucose to glycerol-containing media. Yeast
from two biological replicates.
polysome/monosome (P/M) and 60S/40S (60/40) ratios are shown with s.d.
74
(C) Violin plot of the FP, total, and TE changes in asci-MiXfor MRP transcripts during growth in glucose
(left, FP and TE data is also represented in Figure 2-13B and 2-14B) or after 6 hours of growth in glycerol
(right). Violin plot parameters are as described for Figure 2-13B.
Discussion
Here we have demonstrated that the eukaryote-specific ribosomal protein Asci /RACK1
is required for efficient translation of short mRNAs, a category that includes functionallyrelated groups of genes required for vital cellular processes (e.g. cytoplasmic and
mitochondrial ribosomal proteins). A correlation between ORF length and translation
efficiency or ribosome density has been observed since the advent of genome-wide
translation profiling (Arava et al., 2003; Ingolia et al., 2009) and we observed this
relationship in data collected from diverse eukaryotes including yeast, nematodes, mice,
and humans (Guo et al., 2010; Stadler and Fire, 2013). To account for this trend, it was
proposed that the rate of translation initiation is higher for short ORFs (Arava et al.,
2005; Shah et al., 2013), but the mechanism(s) underlying length-dependent initiation
rate differences were unknown.
It has been suggested that the increased probability of mRNA circularization by diffusion
could make translation initiation more efficient on short mRNAs (Chou, 2003; Guo et al.,
2015). Our results add an additional nuance to these physical models - the presence
of a ribosome-dependent regulatory mechanism that specifically enhances the
translation of short mRNAs/ORFs by promoting the formation and/or function of the
closed-loop. Our analyses reveal a clear trend that short ORFs preferentially associate
with closed-loop factors in vivo, and consistent with these observations, short mRNAs
75
form more stable closed-loop complexes than longer mRNAs in vitro (Amrani et al.,
2008). A challenge for the future will be to determine how the mRNA, the closed-loop
factors, and the ribosome cooperate to privilege the translation of short ORFs.
How might Asc1/RACK1 promote closed-loop formation? RACK1's placement on the
solvent-exposed side of the head of the small subunit puts in it close proximity to the
mRNA exit channel, in a position with the potential to interact with the mRNA-bound
closed-loop factors during initiation. Intriguingly, eIF4G co-purifies with Asci from yeast
lysates under stringent conditions in which most other initiation factors do not (Gavin et
al., 2002, 2006), suggesting that Asci may interact directly with the closed-loop via
eIF4G. Our results also raise the possibility that translation of many mRNAs could be
co-regulated by mechanism(s) that target Asci /RACK1's function in closed-loopdependent initiation. Moving forward, it will be important to determine how the many
signaling pathways that have been linked to Asc1/RACK1 impact the translation of
closed-loop-dependent mRNAs.
More generally, our study highlights the fact that individual ribosomal proteins can
contribute to efficient translation of subsets of mRNAs with important consequences for
cellular physiology. In particular we show that loss of the non-essential ribosomal
protein Asc1 /RACK1 causes a concerted decrease in MRP expression that is likely
linked to mitochondrial defects. Given the central role of mitochondria in energy and
metabolite production in eukaryotic cells, it is not surprising that mitochondrial defects
76
elicit pleiotropic consequences (Calvo and Mootha, 2010; Fleming et al., 2001; Kushnir
et al., 2001; Shoffner et al., 1990; Vafai and Mootha, 2012). In light of our findings,
many of Asci/RACK1's ascribed cellular functions should be re-evaluated for potential
connections to mitochondrial dysfunction. Finally, it is intriguing that several distinct
mutations in human ribosomal proteins and ribosome biogenesis factors result in
anemia, the cause of which is currently the source of much debate (Freed et al., 2010;
Narla et al., 2011). Given that many forms of heritable anemia have been traced to
defects in mitochondrial iron metabolism (Dailey and Meissner, 2013; Huang et al.,
2011), it will be interesting to see whether nuclear-encoded mitochondrial mRNA
translation is affected in these diseases and whether these defects contributes to
pathogenesis.
Experimental Procedures
Plasmid construction
The 127 domain monomer cDNA from human cardiac titin was a generous gift from Julio
Fernandez. The 127 monomer was fused to a serine-glycine linker (SGGGGG) followed
by the V5 epitope tag. Longer constructs were made using the iterative subcloning
method that relies upon the compatible cohesive ends of BamHI and BgllI and results in
an arginine-serine linker added between individual domains (Hoffmann and Dougan,
2012). 127 proteins were expressed in yeast from a low-copy plasmid (pRS415) under
the Gal promoter.
77
Yeast strain constructionand growth
Yeast were cultivated in liquid or on solid (2% agar) YPAD media (yeast extract,
peptone, dextrose (2% w/v) supplemented with adenine hemisulfate). Liquid cultures
were grown with rapid agitation and harvested at OD 0.6-0.9 or 0.6-0.7 (for Ribo-Seq
experiments). For glycerol shift polysome experiments, yeast were grown to mid-log
phase (OD 0.5-0.6) in YPAD and then media was removed by brief centrifugation and
replaced with YPAG (YPA + 3% (w/v) glycerol). For the yeast growth curves, yeast were
diluted from saturated cultures into fresh media and allowed to double 1-2 times before
rediluting to an OD of 0.1 in glucose or glycerol-containing media. For the 127 protein
experiments, yeast were grown overnight in SC-Leu and diluted to an OD of 0.2 in
YPAGal (2% galactose), then grown for 8 hours before harvest. Deletion strains of the
ASC1, RPL23B, and RPP1A loci were obtained by homologous recombination using the
pFA6a-kanMX6 plasmid as a template and PCR product adding 40 nt of homology to
each side of the kanMX6 cassette (Longtine MS et al., 1998). Isolates were confirmed
by PCR. Integration of mutant ASC1 alleles was performed using the two-step gene
replacement strategy. First, the URA3 marker was integrated at the ASC1 locus.
Subsequently, ASC1 mutant alleles were amplified by PCR from plasmid templates and
integrated into ascl::URA3 strain at the ASC1 locus. Isolates were identified by 5-FOA
resistance and correct integration was confirmed by sequencing. All strains were
constructed in the Sigmal 278b strain background.
Polysome analysis
78
Cycloheximide (CHX, Sigma-Aldrich) was added to a final concentration of 0.1 mg/ml to
cells and incubated an additional 2 minutes at the growth temperature with shaking.
Cells were rapidly cooled and washed with polysome lysis buffer (PLB: 20 mM HepesKOH, pH 7.4, 2 mM Mg acetate, 100 mM K acetate, 3 mM DTT, 0.1 mg/ml CHX + 1%
Triton X-1 00). Formaldehyde crosslinking experiments were performed as described
(Valcsek et al., 2007). 10-15 OD260 units were loaded on 10-50% sucrose gradients in
polysome gradient buffer (PGB: PLB -Triton) and centrifuged in an SW 41 rotor
(Beckman Coulter) at 35,000 rpm for 3 hours. Fractions were collected from the top
using a BioComp Gradient Station (Biocomp Instruments). A254 traces were quantified
with a custom script. Minima were identified and used as boundaries for each peak.
Values are the integral under the curve to the baseline, which was set as a line
connecting the lowest minimum in the first half of the trace with the lowest minimum in
the second half of the trace. This adjustment produced the best agreement between
biological replicates and was robust to experimental variation in baseline height.
Ribosome footprintprofiling
Ribo-Seq was performed essentially as described (Ingolia et al., 2009) with the following
modifications: monosomes were isolated manually from 10 - 50% sucrose gradients. 50
A260 units were digested with 750 U of RNAse I (Ambion). Selective precipitation was
used to enrich for small RNA fragments prior to size selection of 28mers on denaturing
gels. In brief, RNA samples were resuspended in GuHCI buffer (8 M guanidine HCI, 20
mM MES hydrate, 20 mM EDTA) and brought to 33% ethanol before binding to a silica-
79
based column (Zymoprep-V, Zymoresearch) to precipitate and remove large RNAs. The
eluate was brought to 70% ethanol to precipitate small RNAs. Total RNA for
accompanying RNA-Seq samples was isolated from the same cell extracts used for
footprint library generation using the hot acid phenol method. Poly(A) selection was
performed using oligo-dT cellulose (Sigma-Aldrich or NEB) as previously described
(Sambrook et al., 2001). For experiments using rRNA-depletion to enrich for coding
transcripts, the Ribo-Zero kit (Epicentre) was used. The asci-DE and matched WT
libraries were constructed using poly(A) tailing to capture the small RNA fragments as
previously described (Ingolia et al., 2009). For all other libraries, we ligated a preadenylated 3' adaptor (5Phos/TGGAATTCTCGGGTGCCAAGG/3ddC/) to the fragments
using T4 RNA Ligase 1 (NEB). First strand synthesis was performed with Superscript Ill
(Life Technologies) using primer OJA225
(/5Phos/GATCGTCGGACTGTAGAACTCTGAACCTGTCGGTGGTCGCCGTATCATT/iS
p1 8/CACTCA/iSp1 8/GCCTTGGCACCCGAGAATTCCA).
cDNA was amplified using
primer oNT1230 (AATGATACGGCGACCACCGA) and
(CAAGCAGAAGACGGCATACGAGATXXXXXXGTGACTGGAGTTCCTTGGCACCCGA
GAATTCCA), where XXXXXX denotes a six-nucleotide barcode used to distinguish
samples run in the same lane. Samples were run on an Illumina HiSeq 2000 instrument
or an Illumnina GAIIx.
qRT-PCR
RNA was extracted using the hot acid phenol method. RNA was treated with TURBO
DNase (Life Technologies). First strand synthesis was performed with AMV Reverse
80
Transcriptase (Promega) using an anchored oligo-dT primer (for coding transcripts) or a
random hexamer primer (for SNR24). Quantitative PCR was performed with SYBR Fast
reagents (Kapa Biosystems) using a Roche Lightcycler 480. For polysome qRT-PCR,
20 ng of a reporter mRNA encoding firefly luciferase (FLUC) was spiked in to each
polysome fraction before RNA extraction. Relative concentrations obtained for each
gene-specific primer were normalized to the corresponding FLUC concentration in that
fraction. Plots are shown such that the total value across all fractions=1. Primers
amplifying 18S rRNA were used to verify the expected distribution of ribosomes in each
gradient.
Western blotting
Total protein levels were determined using the BCA assay (Thermo Scientific). For total
Asci level quantification, 1 pg of total protein obtained by TCA precipitation followed by
cell lysis was loaded onto 12% SDS-PAGE gels. The membrane was blotted with aAsci (prepared by our lab) and a-Pgkl (Life Technologies 22C5D8) as a loading
control. For polysome Westerns, the same volume of each fraction was loaded per well.
Blots were overexposed to show the remaining ribosome-associated protein for the
ribosome-binding mutants. After secondary antibody incubation, blots were incubated
with ECL (GE) and exposed to X-ray film. For quantitative fluorescent Western blotting
experiments, the ECL Plex kit (GE) was used in combination with the mouse a-Pgkl
(Life Technologies 22C5D8) and rabbit a-V5 (Sigma-Aldrich V8137). Blots were
scanned with a Typhoon imager (FLA 9500, GE) and quantified using ImageQuant
81
software using the automatic quantification protocol according to the manufacturer's
instructions.
Read Mapping and positionalalignment
Yeast reads were aligned to the sigmal278b (Dowell et al., 2010) genome downloaded
from SGD on June 29, 2014. We used Tophat to map first to annotated splice junctions
and then to the genome. We used only uniquely mapping reads for all downstream
analyses. Because RFP reads generally start 12 nt upstream of start codons and end
18 nt upstream of stop codons (Ingolia et al., 2009), we used only reads mapping within
these boundaries. Additionally, to avoid potential variability that can be present at the 5'
end of mRNAs, we excluded the first 30 codons from counting for quantification of gene
expression.
Accession numbers
Sequencing data has been deposited in the NCBI GEO database under accession
number GSE61753.
Gene expression analyses
For comparisons between libraries, we used normalized values obtained from running
count data through the DE-Seq package (Anders and Huber, 2010) because quantilebased normalization is less affected by sample variability and changes in the length
distribution of the mRNA pool than RPKM values (Wagner et al., 2012). For gene
82
expression measurements, we only included genes for which there were at least 128
mapping reads total among the libraries used for the analysis (Ingolia et al., 2009). All
analyses were performed with custom Bash and Python scripts written in-house. Data in
figures represent the average of two biological replicates. Figures were constructed
using Matplotlib (Hunter, 2007) and Prism.
Analysis of TE in other organisms
For correlations of TE with ORF length shown in Figure 2-1 OD-F, processed data files
were downloaded from GEO and used to calculate TE. Only genes for which the pooled
reads or scaled reads (for (Stadler and Fire, 2013)) from footprint and total RNA libraries
reached 128 reads were included.
Pathway Analysis
To identify groups of genes with significantly altered TE in yeast mutants, we used the
Mann Whitney U test and report one-sided p-values for groups of genes with
significantly altered TE each condition. For this analysis, we included all genes without
filtering for read cutoff and added a pseudocount of one read in cases where >1 read
was detected in some but not all libraries.
Motif finding
We used MEME (Bailey and Elkan, 1994) to identify motifs present in 5' UTRs of the
selected groups of mRNAs. 5' UTR boundaries were taken from the median UTR length
83
reported in (Pelechano et al., 2014). UTRs < 8 nt were excluded from the motif
analysis. WebLogo (Crooks et al., 2004) was used to generate sequence logos.
mRNA attributes
5' and 3' UTR lengths were taken as the median length from (Pelechano et al., 2014).
UTR folding energies are minimum free energies (MFEs) calculated by running these
sequences through RNAfold (Gruber et al., 2008) with temperature set to 30 0C and
otherwise default parameters. Translation adaptation index values per gene were kindly
provided by Eckhard Jankowsky and colleagues using values from (Tuller et al., 2010).
Poly(A) tail lengths were taken from (Subtelny et al., 2014).
Contributions
This chapter is based on a manuscript in currently in review. Mary K. Thompson and
Wendy V. Gilbert wrote the chapter. M.K.T performed experiments and bioinformatics
analyses. Maria F. Rojas-Duran provided technical assistance for the experiments
shown in Figure 2-18.
Yeast strains used in this study
Strain
YWG25
Genotype
MATa ura3-52 his3AO leu2AO trplAO
YWG1034
MATa ura3-52
asc1::kan
MATa ura3-52
asc1::kan
MATa ura3-52
asc1::kan
MATa ura3-52
YWG1035
YWG1036
YWG1062
Plasmid
Source
This study
his3AO leu2A0 trplAO
This study
his3AO leu2AO trplAO
This study
his3AO leu2AO trplAO
This study
his3AO leu2AO trplAO
This study
84
Notes
Isogenic WT
Ribo-Seq
replicate#2
Ribo-Seq
replicate#1
Ribo-Seq
YWG 1480
rpl23b::kan
MA Ta ura3-52 his3AO Ieu2AO trpl1,O
rpI23b::kan
MA Ta ura3-52 his36O leu2A0 trp1AO
rpI23b::kan
MA Ta ura3-52 his3AO leu2AO trp1AO
rpp 1a::kan
MA Ta ura3-52 his3AO leu2A0 trp1AO
rpp 1a::kan
MA Ta ura3-52 his3UO Ieu2AO trp1AO
rpp 1a::kan
MA Ta ura3-52 his3AO leu2AO trp1AO
ascl-R38D K40E
MA Ta ura3-52 his3AO leu2AO trp1AO
ascl-R38D K40E
MA Ta ura3-52 his3AO Ieu2AO trp 1AO
ascl-R38D K40E
MATa ura3-52 his3AO leu2AO trplAO
ascl-R38D K40E
MA Ta ura3-52 his3AO leu2A0 trp1AO
ascl-D109Y
MA Ta ura3-52 his3AO leu2AO trp1AO
ascl-D109Y
MA Ta ura3-52 his3AO leu2AO trp 1AO
ascl-D109Y
MA Ta ura3-52 his3A0 leu2AO trp1AO
ascl-MlX
MA Ta ura3-52 his3AO leu2A0 trp1AO
asc1-M1X
MA Ta ura3-52 his3AO leu2AO trp1AO
asc1-M1X
MA Ta ura3-52 his3AO leu2A0 trp1AO
asc1-E5X
MA Ta ura3-52 his3AO leu2AO trp 1AO
asc1-E5X
MA Ta ura3-52 his3AO leu2AO trp1AO
asc1-E5X
MA Ta ura3-52 his3AO leu2AO trp 1AO
asc 1-Aintron
MA Ta ura3-52 his3AO leu2AO trp 1AO
asc1-Aintron
MA Ta ura3-52 his3AO leu2AO trp1A0
asc 1-/Aintron
MA Ta ura3-52 his3AO leu2A0 trp 1AO
pWG735
This study
YWG1481
MA Ta ura3-52 his3AO leu2A0 trp1AO
pWG735
This study
YWG1482
MA Ta ura3-52 his3AO leu2A0 trp1A0
pWG735
This study
YWG1483
MA Ta ura3-52 his3AO leu2A0 trp1AO
pWG740
This study
YWG1484
MA Ta ura3-52 his3AO leu2A0 trp1AO
pWG740
This study
YWG1063
YWG1064
YWG1071
YWG1072
YWG1073
YWG1247
YWG1115
YWG 1120
YWG 1125
YWG1114
YWG1119
YWG 1124
YWG1116
YWG 1121
YWG1 126
YWG 117
YWG 1122
YWG1 127
YWG 1118
YWG 1123
YWG 1128
85
replicate#2
This study
This study
Ribo-Seq
replicate#1
This study
This study
This study
Coyle et al.,
2009
This study
Ribo-Seq
replicate#1
Ribo-Seq
replicate#2
Ribo-Seq
replicate#1,#2
This study
This study
This study
This study
Ribo-Seq
replicate#1
Ribo-Seq
replicate#2
This study
This study
This study
Ribo-Seq
replicate#1
Ribo-Seq
replicate#2
This study
This study
This study
This study
This study
This study
This study
polyprotein
exps.
polyprotein
exps.
polyprotein
exps.
polyprotein
exps.
polyprotein
sdxe uielo.JdAIod
SeOON
Apnjs Siq14
sdxe
sdxe
Apnis siq~
tt7,L!Md
ttLDMd
L00
Ap
99
Apnis SitLi
aoinoS
bel ou-,LI-lV!Dd
SIuO~uOD
. s L tt7LoMd
Apnis S!L4
_L3,!Dd
tLqd
Apnis S!Li
_L3,!Dd
APfl1s s!LIIjLO~
Aprns s!Lfl
Ap
ecL!Md
P!Wseld
Apnis siqj ui pesn Sp!WseId
sdxe
uiefldAIod
ui~gloidAlod
u -sodxo
uialoidAlod
sdxe
uieloidAlod
-sdxe
uieloidAIod
xIP,-Iose
071dj4l oi~jnel ovesyiL 3g-cein eLk
~
1EM
_xkV-i-3 Ose
.M
~
09OM
Oxtiyovne
VC.L J-WlseA
7nlOCoqgCj xA-eI~V
61.9 LDMA
Oriidii ovine, o~syq e'g-Cein eiVV
1.M
X1LDd OPdu vgeocsIqo-snev
GOM
Lk
gen
OM
L
se,
X
LL9DM
_kV
9ci
07dlo.nlvoy
oie
Oi~
07404nlOCq
-sdxe
uieioidAiod
d~
o7L!Dmd I~
~sq&-w
1. 1.)
OVdlowe vs'1,k-~n~vvv
xuA,-v'tsJ 7ne 70y 7gCene VV 9L9OM
I .M
y-ton eiv' I
vnoc~
&
xtyv-tose
Ani i
silo dxed
017tdJJ ove'nel 07CSIq i-cwen L,_VipV
~ Ap . si O7LDmd
s!~Odxe
xtv-i-ose
silo dxlo pi
OV t dJ 01owne, c17,syq ig-cein e~v' L. Vk
U -sOdxIe _psSL4 ot7L-Dmd
silodxeo Ans iqxtkv-tose
Ot!dil 017gnel 017CSYi ag-cein elVAI 3
. _iL scL!DMd
s!Odxe ~ Ap
xtpv-tose
siiodxlo pi
Or?! dil ov4Fnel 017CS.ILI -cwen eIVyN L.u! sodIe Ap . _Lt SCL!DMd
Xtyv-tose
siiodxed Ani i
eLVV
9 LDMd 017cb t ovl07nel 01CS.Iq 0-cen
s~~odxeo
. sL
U~~a~O.JdAIuod 0prn St4~LDd O dJl 1onel ,vCSIL 9-cejn elVAI
Apnis s!Lfl~L~
Apnjs S!Lli
uieoidAIod
Apnis s!1iq
._
Apnjs siqu
._
Apnis S!1
uielo~dAlod
-sdxe
uieloidAlod
sdxe
uieloidAlod
sdxe
uieloidAlod
sdxe
uialoidAlod
Apnis siqI
Apnjs s~I I
uiegloidAiod
Apnjs s!Ll~7Dd
uieloidAIod
U *sOdIe
_psSL4
Cod
Dd
t~m
7Dd
t,!m
11L~
t!m
77DdOiiz
~d~onocjI
l
0t
O?
une
7IL
~cn~v~
gveneLV
i v'e jsI
9C~l~~V
Vtdl0ee V~qugci Lk
~jj
we qsqj-ei
_
gci
7~e 1C~
_
C!djonocsq~c~
Vtdl07nlO7SqGcj
VdJono~~q~g~nev~
Vtd
pi~
y
VSII~Cf
we
V 9 DMA
S -SDMA
1.9 1-DMA
LG DMA
01.9 LOMA
L6t 1.DMA
-M
9t 9VOM
9t
.1
81
L1
IIJ
weoc.qJ-ene~j'j
OVtdiz owne, OVCS~i Jg9-cein eiVp,
-M t M
.M
6,OM
01 8,OM
.M
61
9CJlQLIJ
7DdOtd4oni07S.L
O~J
7DdO4~
ot7LDMd
.M
.M
.M
91.M
98t7 1.DMA
IdxI
pWG735
pWG740
pWG742
pWG744
pWG792
pWG793
pWG794
pWG795
pWG796
pGAL-127-monomer-V5 tag
pGAL-127-dimer-V5 tag
pGAL-127-tetramer tag
pGAL-127-octamer tag
pRS316-ascl-R38D K40E
pRS316-ascl-D109Y
pRS316-ascl-M1X
pRS316-ascl-E5X
pRS316-asclAintron
This study
This study
This study
This study
This study
This study
This study
This study
This study
polyprotein
polyprotein
polyprotein
polyprotein
exps.
exps.
exps.
exps.
Oligonucleotidesused for quantitative PCR
Primer
ACT1_RTF
ACT1_RTR
ASC1_RTF
ASC1_RTR
FLUCRTF
FLUCRTR
MRP2_RTF
MRP2_RTR
PGK1_RTF
PGK1_RTR
SNR24_RTF
SNR24_RTR
V5_RTF
V5_RTR
YML6_RTF
YML6_RTR
18SRTF
18SRTR
Sequence
TTCTGAGGTTGCTGCTTTGG
CTTGGTGTCTTGGTCTACCG
ATGTTTGGCCACTTTGTTGG
GTTACCGGCAGAAATGATGG
GTACCAGAGTCCTTTGATCGTGA
ACCCAGTAGATCCAGAGGAATTC
AATAGGTGCGTGGACTCTGG
CTGGCAAATTACCCTTCAGAGC
CAAAGGCTAAGACCATTGTC
CACCAGTAGAGACATGGGAG
TTGCTACTTCAGATGGAACTTTG
TCAGAGATCTTGGTGATAATTGG
AGATCTTCCGGAGGCGGG
GGATCTATTACGTAGAATCGAGACC
AGAGTAGGCGCCTCAAATCC
TTGGAGAGTTAGCATCCCCG
TGGCGAACCAGGACTTTTAC
CCGACCGTCCCTATTAATCAT
Acknowledgements
We thank N. Ingolia, J. Weissman, D. Bartel, and members of the Gilbert lab for
discussion; and B. Zinshteyn and P. Vaidyanathan for discussion and scripts. The
sequencing was performed at the BioMicro Center under the direction of S. Levine. This
work was supported by grants from the National Institutes of Health (GM094303,
87
GM081399) to W.V.G. This work was supported in part by the NIH Pre-Doctoral
Training Grant T32GM007287.
References
Adams, D.R., Ron, D., and Kiely, P.A. (2011). RACK1, A multifaceted scaffolding
protein: Structure and function. Cell Commun. Signal. 9, 22.
Aitken, C.E., and Lorsch, J.R. (2012). A mechanistic overview of translation initiation in
eukaryotes. Nat. Struct. Mol. Biol. 19, 568-576.
Amrani, N., Ghosh, S., Mangus, D.A., and Jacobson, A. (2008). Translation factors
promote the formation of two states of the closed-loop mRNP. Nature 453, 1276-1280.
Anders, S., and Huber, W. (2010). Differential expression analysis for sequence count
data. Genome Biol. 11, R106.
Arava, Y., Wang, Y., Storey, J.D., Liu, C.L., Brown, P.O., and Herschlag, D. (2003).
Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc
Natl Acad Sci U S A 100, 3889-3894.
Arava, Y., Boas, F.E., Brown, P.O., and Herschlag, D. (2005). Dissecting eukaryotic
translation and its control by ribosome density mapping. Nucleic Acids Res. 33, 24212432.
Bailey, T.L., and Elkan, C. (1994). Fitting a mixture model by expectation maximization
to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2, 28-36.
Ban, N., Beckmann, R., Cate, J.H., Dinman, J.D., Dragon, F., Ellis, S.R., Lafontaine,
D.L., Lindahl, L., Liljas, A., Lipton, J.M., et al. (2014). A new system for naming
ribosomal proteins. Curr. Opin. Struct. Biol. 24, 165-169.
Baum, S., Bittins, M., Frey, S., and Seedorf, M. (2004). Asci p, a WD40-domain
containing adaptor protein, is required for the interaction of the RNA-binding protein
Scp1 60p with polysomes. Biochem J 380, 823-830.
Boelens, R., and Gualerzi, C.O. (2002). Structure and Function of Bacterial Initiation
Factors. Curr. Protein Pept. Sci. 3, 107-119.
Bolt, M.W., and Mahoney, P.A. (1997). High-efficiency blotting of proteins of diverse
sizes following sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal.
Biochem. 247,185-192.
88
Calvo, S.E., and Mootha, V.K. (2010). The mitochondrial proteome and human disease.
Annu. Rev. Genomics Hum. Genet. 11, 25-44.
Cherry, S., Doukas, T., Armknecht, S., Whelan, S., Wang, H., Sarnow, P., and
Perrimon, N. (2005). Genome-wide RNAi screen reveals a specific sensitivity of IREScontaining RNA viruses to host translation inhibition. Genes Dev. 19, 445-452.
Chou, T. (2003). Ribosome recycling, diffusion, and mRNA loop formation in
translational regulation. Biophys. J. 85, 755-773.
Coller, J., and Parker, R. (2005). General translational repression by activators of
mRNA decapping. Cell 122, 875-886.
Costello, J., Castelli, L.M., Rowe, W., Kershaw, C.J., Talavera, D., Mohammad-Qureshi,
S.S., Sims, P.F.G., Grant, C.M., Pavitt, G.D., Hubbard, S.J., et al. (2015). Global mRNA
selection mechanisms for translation initiation. Genome Biol. 16, 10.
Coyle, S.M., Gilbert, W. V, and Doudna, J.A. (2009). Direct link between RACK1
function and localization at the ribosome in vivo. Mol Cell Biol 29, 1626-1634.
Crooks, G.E., Hon, G., Chandonia, J.M., and Brenner, S.E. (2004). WebLogo: A
sequence logo generator. Genome Res. 14, 1188-1190.
Dailey, H. a, and Meissner, P.N. (2013). Erythroid heme biosynthesis and its disorders.
Cold Spring Harb. Perspect. Med. 3, aOl 1676.
Dimmer, K.S., Fritz, S., Fuchs, F., Messerschmitt, M., Weinbach, N., Neupert, W., and
Westermann, B. (2002). Genetic basis of mitochondrial function and morphology in
Saccharomyces cerevisiae. Mol. Biol. Cell 13, 847-853.
Dowell, R.D., Ryan, 0., Jansen, A., Cheung, D., Agarwala, S., Danford, T., Bernstein,
D.A., Rolfe, P.A., Heisler, L.E., Chin, B., et al. (2010). Genotype to phenotype: a
complex problem. Science 328, 469.
Fleming, M.D., Campagna, D.R., Haslett, J.N., Trenor, C.C., and Andrews, N.C. (2001).
A mutation in a mitochondrial transmembrane protein is responsible for the pleiotropic
hematological and skeletal phenotype of flexed-tail (f/f) mice. Genes Dev. 15, 652-657.
Freed, E.F., Bleichert, F., Dutca, L.M., and Baserga, S.J. (2010). When ribosomes go
bad: diseases of ribosome biogenesis. Mol. Biosyst. 6, 481-493.
Galdieri, L., Mehrotra, S., Yu, S., and Vancura, A. (2010). Transcriptional regulation in
yeast during diauxic shift and stationary phase. OMICS 14, 629-638.
89
Gavin, A.-C., B6sche, M., Krause, R., Grandi, P., Marzioch, M., Bauer, A., Schultz, J.,
Rick, J.M., Michon, A.-M., Cruciat, C.-M., et al. (2002). Functional organization of the
yeast proteome by systematic analysis of protein complexes. Nature 415, 141-147.
Gavin, A.-C., Aloy, P., Grandi, P., Krause, R., Boesche, M., Marzioch, M., Rau, C.,
Jensen, L.J., Bastuck, S., Dimpelfeld, B., et al. (2006). Proteome survey reveals
modularity of the yeast cell machinery. Nature 440, 631-636.
Gibson, T.J. (2012). RACK1 research - ships passing in the night? FEBS Lett. 586,
2787-2789.
Gruber, A.R., Lorenz, R., Bernhart, S.H., Neub6ck, R., and Hofacker, I.L. (2008). The
Vienna RNA websuite. Nucleic Acids Res. 36.
Guo, H., Ingolia, N.T., Weissman, J.S., and Bartel, D.P. (2010). Mammalian microRNAs
predominantly act to decrease target mRNA levels. Nature 466, 835-840.
Guo, J., Lian, X., Zhong, J., Wang, T., and Zhang, G. (2015). Length-dependent
translation initiation benefits the functional proteome of human cells. Mol. Biosyst. 11,
370-378.
Haghighat, A., Mader, S., Pause, A., and Sonenberg, N. (1995). Repression of capdependent translation by 4E-binding protein 1: competition with p220 for binding to
eukaryotic initiation factor-4E. EMBO J. 14, 5701-5709.
Hoffmann, T., and Dougan, L. (2012). Single molecule force spectroscopy using
polyproteins. Chem. Soc. Rev. 41, 4781.
Hogan, D.J., Riordan, D.P., Gerber, A.P., Herschlag, D., and Brown, P.O. (2008).
Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting
an extensive regulatory system. PLoS Biol 6, e255.
Huang, M.L.-H., Lane, D.J.R., and Richardson, D.R. (2011). Mitochondrial mayhem: the
mitochondrion as a modulator of iron metabolism and its role in disease. Antioxid.
Redox Signal. 15, 3003-3019.
Hunter, J.D. (2007). Matplotlib: A 2D Graphics Environment. Comput. Sci. Eng. 9.
Ingolia, N.T., Ghaemmaghami, S., Newman, J.R., and Weissman, J.S. (2009). Genomewide analysis in vivo of translation with nucleotide resolution using ribosome profiling.
Science 324, 218-223.
Kadrmas, J.L., Smith, M.A., Pronovost, S.M., and Beckerle, M.C. (2007).
Characterization of RACK1 function in Drosophila development. Dev. Dyn. 236, 22072215.
90
Kahvejian, A., Roy, G., and Sonenberg, N. (2001). The mRNA closed-loop model: the
function of PABP and PABP-interacting proteins in mRNA translation. Cold Spring Harb.
Symp. Quant. Biol. LXV.
Kiely, P.A., Baillie, G.S., Barrett, R., Buckley, D.A., Adams, D.R., Houslay, M.D., and
O'Connor, R. (2009). Phosphorylation of RACK1 on tyrosine 52 by c-Abl is required for
insulin-like growth factor I-mediated regulation of focal adhesion kinase. J. Biol. Chem.
284, 20263-20274.
Kondrashov, N., Pusic, A., Stumpf, C.R., Shimizu, K., Hsieh, A.C., Xue, S., Ishijima, J.,
Shiroishi, T., and Barna, M. (2011). Ribosome-mediated specificity in Hox mRNA
translation and vertebrate tissue patterning. Cell 145, 383-397.
Kuroha K, Akamatsu M, Dimitrova L, Ito T, Kato Y, Shirahige K, and Inada T (2010).
Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent translation
arrest. EMBO Rep. 11, 956-961.
Kushnir, S., Babiychuk, E., Storozhenko, S., Davey, M.W., Papenbrock, J., De Rycke,
R., Engler, G., Stephan, U.W., Lange, H., Kispal, G., et al. (2001). A mutation of the
mitochondrial ABC transporter Stal leads to dwarfism and chlorosis in the Arabidopsis
mutant starik. Plant Cell 13, 89-100.
LaGrandeur, T., and Parker, R. (1999). The cis acting sequences responsible for the
differential decay of the unstable MFA2 and stable PGK1 transcripts in yeast include the
context of the translational start codon. RNA 5, 420-433.
Landry, D.M., Hertz, M.I., and Thompson, S.R. (2009). RPS25 is essential for
translation initiation by the Dicistroviridae and hepatitis C viral IRESs. Genes Dev. 23,
2753-2764.
Lang, B.D., and Fridovich-Keil, J.L. (2000). Scp160p, a multiple KH-domain protein, is a
component of mRNP complexes in yeast. Nucleic Acids Res. 28,1576-1584.
Lee, A.S., Burdeinick-Kerr, R., and Whelan, S.P.J. (2013). A ribosome-specialized
translation initiation pathway is required for cap-dependent translation of vesicular
stomatitis virus mRNAs. Proc. NatI. Acad. Sci. U. S. A. 110, 324-329.
Li, Z., Lee, I., Moradi, E., Hung, N.-J., Johnson, A.W., and Marcotte, E.M. (2009).
Rational Extension of the Ribosome Biogenesis Pathway Using Network-Guided
Genetics. PLoS Biol 7, e1000213.
Longtine MS, McKenzie A, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P,
and Pringle JR (1998). Additional modules for versatile and economical PCR-based
gene deletion and modification in Saccharomyces cerevisiae. Yeast 14, 953-961.
91
Majzoub, K., Hafirassou, M.L., Meignin, C., Goto, A., Marzi, S., Fedorova, A., Verdier,
Y., Vinh, J., Hoffmann, J.A., Martin, F., et al. (2014). RACKI Controls IRES-Mediated
Translation of Viruses. Cell 159, 1086-1095.
Melamed, D., Bar-Ziv, L., Truzman, Y., and Arava, Y. (2010). Asci supports cell-wall
integrity near bud sites by a Pkc1 independent mechanism. PLoS One 5, el 1389.
Miller, C., Schwalb, B., Maier, K., Schulz, D., Dimcke, S., Zacher, B., Mayer, A., Sydow,
J., Marcinowski, L., D6lken, L., et al. .(2011). Dynamic transcriptome analysis measures
rates of mRNA synthesis and decay in yeast. Mol. Syst. Biol. 7, 458.
Narla, A., Hurst, S.N., and Ebert, B.L. (2011). Ribosome defects in disorders of
erythropoiesis. Int. J. Hematol. 93, 144-149.
Neymotin, B., Athanasiadou, R., and Gresham, D. (2014). Determination of in vivo RNA
kinetics using RATE-seq. RNA 20,1645-1652.
Nilsson, J., Sengupta, J., Frank, J., and Nissen, P. (2004). Regulation of eukaryotic
translation by the RACK1 protein: a platform for signalling molecules on the ribosome.
EMBO Rep 5, 1137-1141.
Orlando, V. (2000). Mapping chromosomal proteins in vivo by formaldehydecrosslinked-chromatin immunoprecipitation. Trends Biochem. Sci. 25, 99-104.
Park, E.-H., Zhang, F., Warringer, J., Sunnerhagen, P., and Hinnebusch, A.G. (2011).
Depletion of elF4G from yeast cells narrows the range of translational efficiencies
genome-wide. BMC Genomics 12, 68.
Pelechano, V., Wei, W., Jakob, P., and Steinmetz, L.M. (2014). Genome-wide
identification of transcript start and end sites by transcript isoform sequencing. Nat.
Protoc. 9, 1740-1759.
Pisarev, A. V, Kolupaeva, V.G., Yusupov, M.M., Hellen, C.U.T., and Pestova, T. V
(2008). Ribosomal position and contacts of mRNA in eukaryotic translation initiation
complexes. EMBO J. 27,1609-1621.
Rachfall, N., Schmitt, K., Bandau, S., Smolinski, N., Ehrenreich, A., Valerius, 0., and
Braus, G.H. (2012). RACKI/Ascip, a ribosomal node in cellular signaling. Mol. Cell.
Proteomics.
Ron, D., Jiang, Z., Yao, L., Vagts, A., Diamond, I., and Gordon, A. (1999). Coordinated
movement of RACK1 with activated betallPKC. J. Biol. Chem. 274, 27039-27046.
Sambrook, J., Fritsch, E.F., and Maniatis, T. (2001). Molecular Cloning: A Laboratory
Manual.
92
Schwartz, D.C., and Parker, R. (1999). Mutations in translation initiation factors lead to
increased rates of deadenylation and decapping of mRNAs in Saccharomyces
cerevisiae. Mol. Cell. Biol. 19, 5247-5256.
Sengupta, J., Nilsson, J., Gursky, R., Spahn, C.M., Nissen, P., and Frank, J. (2004).
Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by
cryo-EM. Nat Struct Mol Biol 11, 957-962.
Shah, P., Ding, Y., Niemczyk, M., Kudla, G., and Plotkin, J.B. (2013). Rate-limiting steps
in yeast protein translation. Cell 153, 1589-1601.
Shoffner, J.M., Lott, M.T., Lezza, A.M., Seibel, P., Ballinger, S.W., and Wallace, D.C.
(1990). Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a
mitochondrial DNA tRNA(Lys) mutation. Cell 61, 931-937.
Sonenberg, N., and Hinnebusch, A.G. (2009). Regulation of translation initiation in
eukaryotes: mechanisms and biological targets. Cell 136, 731-745.
Stadler, M., and Fire, A. (2013). Conserved translatome remodeling in nematode
species executing a shared developmental transition. PLoS Genet. 9, e1003739.
Stuart, J.M., Segal, E., Koller, D., and Kim, S.K. (2003). A gene-coexpression network
for global discovery of conserved genetic modules. Science 302, 249-255.
Subtelny, A.O., Eichhorn, S.W., Chen, G.R., Sive, H., and Bartel, D.P. (2014). Poly(A)tail profiling reveals an embryonic switch in translational control. Nature 508, 66-71.
Tuller, T., Waldman, Y.Y., Kupiec, M., and Ruppin, E. (2010). Translation efficiency is
determined by both codon bias and folding energy. Proc. Natl. Acad. Sci. U. S. A. 107,
3645-3650.
Vafai, S.B., and Mootha, V.K. (2012). Mitochondrial disorders as windows into an
ancient organelle. Nature 491, 374-383.
Vaidyanathan, P.P., Zinshteyn, B., Thompson, M.K., and Gilbert, W. V (2014). Protein
kinase A regulates gene-specific translational adaptation in differentiating yeast. RNA
20, 912-922.
ValAsek, L., Szamecz, B., Hinnebusch, A.G., and Nielsen, K.H. (2007). In vivo
stabilization of preinitiation complexes by formaldehyde cross-linking. Methods
Enzymol. 429,163-183.
Valerius, 0., Kleinschmidt, M., Rachfall, N., Schulze, F., L6pez Marn, S., Hoppert, M.,
Streckfuss-B6meke, K., Fischer, C., and Braus, G.H. (2007). The Saccharomyces
93
homolog of mammalian RACK1, Cpc2/Ascl p, is required for FLOl 1 -dependent
adhesive growth and dimorphism. Mol Cell Proteomics 6,1968-1979.
Volta, V., Beugnet, A., Gallo, S., Magri, L., Brina, D., Pesce, E., Calamita, P., Sanvito,
F., and Biffo, S. (2013). RACKI depletion in a mouse model causes lethality,
pigmentation deficits and reduction in protein synthesis efficiency. Cell. Mol. Life Sci. 70,
1439-1450.
Wagner, G.P., Kin, K., and Lynch, V.J. (2012). Measurement of mRNA abundance
using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci.
131, 281-285.
Warner, J.R., and McIntosh, K.B. (2009). How common are extraribosomal functions of
ribosomal proteins? Mol Cell 34, 3-11.
Wehner, P., Shnitsar, I., Urlaub, H., and Borchers, A. (2011). RACK1 is a novel
interaction partner of PTK7 that is required for neural tube closure. Development 138,
1321-1327.
Yaka, R., Thornton, C., Vagts, A.J., Phamluong, K., Bonci, A., and Ron, D. (2002).
NMDA receptor function is regulated by the inhibitory scaffolding protein, RACKI. Proc.
NatI. Acad. Sci. U. S. A. 99, 5710-5715.
Yoshikawa, K., Tanaka, T., Ida, Y., Furusawa, C., Hirasawa, T., and Shimizu, H. (2011).
Comprehensive phenotypic analysis of single-gene deletion and overexpression strains
of Saccharomyces cerevisiae. Yeast 28, 349-361.
94
Chapter III:
RACK1 promotes the expression of small proteins
required for mitochondrial function in HeLa cells
95
Abstract
Ribosomes regulate the production of proteins, which ultimately determine cell fate. The
group of proteins that associates with the ribosome has diverged throughout evolution
and can be divided into two subcategories: a core set of proteins found in all life and
those present only in a specific domain or organism. It is thought that domain-specific
proteins may play specialized regulatory roles in translation (Melnikov et al., 2012; Xue
and Barna, 2012). Here we assess the translational functions of the eukaryote-specific
ribosomal protein, RACK1, in HeLa cells by ribosome footprint profiling. RACK1
knockdown causes largely concomitant changes in mRNA expression levels and
ribosome association for most mRNAs, suggesting either a lack of RACK1-dependent
translational regulation or tight coupling between translation and RNA decay in this
system. Intriguingly, the same functional classes of mRNAs affected in yeast lacking
Ascl, the yeast RACK1 ortholog (Chapter 2), display decreased steady-state mRNA
levels after RACK1 knockdown in HeLa cells. These mRNAs have short open reading
frames and are functionally biased towards ribosomal proteins and nuclear-encoded
mitochondrial proteins. Consistent with these defects, siRACK1 cells display reduced
proliferation in conditions requiring full mitochondrial function. Our results argue for a
conserved eukaryotic function of the RACK1 protein in promoting the expression of
small proteins, which are essential for core cellular metabolism.
Introduction
Ribosomes are universal protein-synthesizing machines. However, the group of
ribosomal proteins that associate with the ribosome in each domain of life has diverged
96
such that some proteins are present only in one domain. The RACK1 (Receptor for
Activated C-Kinase) protein is a ribosomal protein found only on the eukaryotic
ribosome but conserved from yeast to human. RACK1's position on the head of the
small ribosomal subunit, near the mRNA exit channel and the interaction surface of the
eukaryotic translation initiation factors elF3 and eIF4G make it a prime candidate for an
important regulatory role in translation initiation (Hashem et al., 2013; Siridechadilok et
al., 2005), (Chapter 1). Indeed, our previous study found that the ortholog of RACK1 in
yeast, Ascl, is required for the translation of mRNAs associated with the translational
closed-loop complex, which preferentially targets mRNAs with short open reading
frames (ORFs) for translational enhancement (Chapter 2). Because the translational
closed-loop is a conserved pathway (Preiss and Hentze, 1999), we wondered whether
the function of RACK1 in the translation of short ORFs might be likewise conserved
from yeast to human.
Despite its identity as a core ribosomal protein (Ben-Shem et al., 2011; Sengupta et al.,
2004), RACK1 has largely been studied for its functions in regulating signaling pathways
and mediating protein-protein interactions in large complexes (Adams et al., 2011). A
WD-repeat protein that adopts a P-propeller fold, RACK1 shares close structural
homology with the Gp-subunit of trimeric G-proteins (Coyle et al., 2009; Guillemot et al.,
1989). By interacting with multiple molecules through different blades of the propeller
structure, the RACK1 protein can act as a scaffold to bring different binding partners
together as part of a larger complex. RACK1 can also regulate the activity of signaling
97
effectors, either directly, or by scaffolding the interaction between the signaling protein
and its regulator (Smith, 2008; Xu and Min, 2011).
RACKI's functions in mammalian biology are numerous. Although it was first isolated as
a binding partner of the activated form of protein kinase C (Mochlyrosen et al., 1991), it
has since been identified as an interactor of almost 80 proteins from low-throughput
studies in human cells alone (Tyers, 2015) and implicated as a regulator of various
pathways in both nontransformed and cancer cells (Adams et al., 2011; Gandin et al.,
2013; Li and Xie, 2014). It acts as a scaffold protein in PKC, PKA, and focal adhesion
signaling pathways and regulates processes as diverse as cell migration and the
circadian rhythm (Hermanto et al., 2002; L6pez-Bergami et al., 2005; Robles et al.,
2010; Serrels et al., 2010). Association with RACK1 activates some binding partners
and inhibits others. For example, RACK1 binding inhibits Src kinase, but activates
PKCPII (Chang et al., 1998; Ron et al., 1994). The ability of scaffolding proteins to
activate or inhibit their binding partners depends on their cellular locations and
concentrations relative to their interaction partners (Levchenko et al., 2000; Witzel et al.,
2012), thus it is important to consider whether the ribosome might act as a sink titrating
RACK1 away from its other binding partners, or whether those interactions occur in the
context of the ribosome. However, signaling pathways are evolving much more rapidly
than the ribosome (Stuart et al., 2003), and thus none of RACK1's characterized
mammalian signaling functions were relevant for our previous study in which we
addressed the function of Asci in yeast (Chapter 2). Because we were interested in
98
identifying deeply conserved functions of the Asci/RACK1 protein, we focused our
studies on RACK1's effects on translation.
We previously assessed the translational functions of the yeast ortholog of RACK1,
Asci, by ribosome footprint profiling. We observed that mRNAs with short ORFs display
both decreased translational efficiency and decreased steady-state mRNA levels in
yeast ASC1 null mutants (Chapter 2). Here we show that this same group of mRNAs
decreased in steady-state mRNA levels after knockdown in HeLa cells, with no effect on
their translational efficiencies. We hypothesize that RNA decay downstream of
translational repression may explain the lack of observed translation defects for these
mRNAs. As in yeast, RACK1 -sensitive mRNAs encode ribosomal proteins and nuclearencoded mitochondrial proteins. Consistent with these defects, RACK1 knockdown
caused decreased proliferation in conditions requiring full mitochondrial function. Our
results point towards a conserved function for the Asc1 /RACK1 protein in promoting the
expression of small proteins, which are required for cell growth and respiration.
Results
RACK1 knockdownalters total mRNA levels and ribosome association for many
mRNAs
To assess the effects of RACK1 function on translation, we performed ribosome
footprint profiling on HeLa cells after RACK1 knockdown. Two independent siRNAs
targeting different exons were used to knockdown the RACK1 gene, and we achieved a
40-50% knockdown efficiency after 48 hours compared to a control siRNA not targeting
RACK1 (Figure 3-1A). At the mRNA level, siRACK1 knockdown was effective (~80%,
99
Figure 3-1 B). It may be difficult to achieve higher knockdown efficiency because of the
high protein stability of ribosomal proteins and the dilution effects of cell division on
siRNA concentration (Dorsett and Tuschl, 2004; Schwanhausser et al., 2011).
To gain insight into RACK1's potential functions in translation, we looked at changes in
gene-specific expression levels after RACK1 knockdown compared to the distribution of
changes obtained by comparing the siControl replicate experiments to each other
('siControl replicate error', Figure 3-1 C-E). Although RACK1 knockdown clearly affected
mRNA-specific expression levels (Figure 3-1 C,D), the translation efficiencies of most
mRNAs were largely unaltered (Figure 3-1 E). Ribo-Seq biological replicates after
RACK1 knockdown were less reproducible than Ribo-Seq biological replicates after the
control knockdown and all total mRNA replicate experiments (r 2 =0.93 vs. r2 >0.98 for all
other experiments) which may explain why there appears to be a wider distribution of
ribosomal footprint changes after siRACK1 #1 knockdown compared to the distribution
of changes in the total mRNA pool after knockdown (Figure 3-1 C,D). We then examined
the relationship between changes in total mRNA levels and in ribosomal footprints for
each mRNA after RACK1 knockdown. In contrast to the effects that we observed
previously for yeast, changes in gene expression after RACK1 knockdown in HeLa cells
largely move in the same direction with the same magnitude for both total mRNA levels
and ribosomal footprints (Figure 3-1 F). Because genome-wide measurements of
translation efficiency are correlated with mRNA half-lives (Chapter 2), and inhibition of
translation initiation can lead to mRNA decay in yeast (LaGrandeur and Parker, 1999;
100
Muhlrad and Parker, 1999; Schwartz and Parker, 1999), we speculate that some
changes in mRNA-specific translation after RACK1 knockdown are invisible due to
mRNA decay of non-translating mRNAs. We therefore suggest three possible models:
1) that RACK1 knockdown alters the transcription of the affected mRNAs rather than
their translation, 2) that RACK1 knockdown decreases the stability of these mRNAs
independently of any translational effects, or 3) that RACK1 knockdown primarily
causes a translational defect for these mRNAs, but their total mRNA levels are tightly
coupled to their mRNA-specific translational activities due to decay of non-translating
mRNAs. In this case, no changes in translational efficiency would be observed because
of the necessity of using the total mRNA pool for normalization (Figure 3-1 G).
To investigate a potential link between translational repression of RACK1 -sensitive
mRNAs and mRNA decay, it will be interesting to quantify mRNA half-lives in
mammalian cells concomitant with RACK1 knockdown (see Future Directions section).
For now we describe the function of RACK1 in the 'expression' of specific mRNAs. We
further focus our attention on the ribosomal footprints, because they generally mirror the
total mRNA pool after RACK1 knockdown and are proportional to protein synthesis
rates (Ingolia et al., 2009).
101
%N
4~%
A
~p
4,0
-'0" Qo
0
M ribosome footprints
-
p
0,25X 0.5X 1X
B
[~ total mRNA
2-20- 13-Actin
Za40-
E
-
0. 12
S0.
-W80#100
RACK1 level
(% of matched control): 100 100 45
C 0. 14
9-60-
()o
-RACK1
siRACK1: #1
50
siControl replicate error
(a= 0.20)
D
siRACK1#1 (a = 036)
sRACK1#2 (a = 031)
012
#1
siControl replicate error
(0=0.18)
.4siRACK1#1 (o = 0.30)
siRACK1#2(o=0.26)
0 16
0.14-
#2
0.10-
S0. 08
0
0
C 0. 06
C
0.04
04 02-
002
0. 00
-1.0
-.
1092
0.00
-1.0
1.0
0.5
0.0
5
E
012~
0.10.
0
C0 008-
C
60.04-
0.02
-0.5
0.0
0.5
1.0
-1'0
F 1
G
r
-0.5
0.0
0.5
1.0
1092 A TE
1092 A total mRNA
A FP
siControl replicate error
(0 = 0.24)
siRACK1i1 (0=0.31)
siRACK1#2 (o=0.29)
o 0.06
0.08-
~006
0. 04
#2
siControl
0.70
1 .0
0
0
0 5-
cc
.r
Z
0
siRACK1
l
-0 5
1 0
.5
15
-10
05
00
0.5
10
1.5
decay of non-translating mRNAs?
log2 A total mRNA (siRACK1/siControl)
Figure 3-1: RACK1 knockdown causes concomitant changes in the levels of ribosomal footprints
and total mRNA for most mRNAs. (A) Knockdown of RACK1 at the protein level in HeLa cells 48 hours
after transfection from the two replicates used for ribosome footprint profiling is shown. A dilution series of
the siControl#1 sample is shown at left. RACK1 level is normalized to P-Actin and then to the RACK1/pActin value for the matched control sample. (B) Knockdown of RACK1 mRNA as assessed by ribosome
footprint profiling and RNA-Seq. (C-E) Distribution of changes in ribosomal footprints (C, AFP), total
mRNA levels (D, Atotal mRNA) or translation efficiency (E, ATE) comparing two biological replicate
experiments using the control siRNA (i.e. replicate error) or siRACK1 #1 or siRACK1 #2 to their
corresponding siControl experiment. The standard deviation (CT) is indicated. (F) Scatterplot showing
relationship between the change in ribosomal footprints and total mRNA levels for each mRNA after
RACK1 knockdown. The red line (y=x) indicates perfectly matched changes in both pools. The Pearson
correlation coefficient is shown. (G) Model for coupling between translational repression and mRNA decay
102
after RACK1 knockdown. Decreased translation initiation on RACK1 -sensitive mRNAs allows their
targeting by the mRNA decay machinery, thus masking effects of RACK1 on translational efficiency.
RACK1 is required for the expression of mRNAs with short ORFs encoding
proteins with functionsin the mitochondriaand at the ribosome
We previously observed that loss of the RACK1 ortholog, Ascl, in yeast decreases the
translation efficiency of mRNAs that contain short ORFs (Chapter 2). To assess whether
this function is conserved from yeast to humans, we analyzed the relationship between
ORF length and mRNA expression level changes after RACK1 knockdown. Changes in
mRNA-specific expression levels after RACK1 knockdown were positively correlated
with ORF length (r= 0.19, p= 10-71) (Figure 3-2A,B), thus mirroring the effects we
observed earlier in yeast. As expected based on our global analysis of total mRNA
levels and ribosome association (Figure 3-1 F), these effects were observed at both the
total mRNA and ribosomal footprint levels, and thus did not appear as translation
efficiency defects (Figure 3-2C).
103
A
30
r
B
r = 0.19
r = 0.21
30,
201-
20
0
10
10
0
0
U
cc
q
0
0
0
0
0
40ft
4
A
E
-10
-10
Co
-201l
fraction of genes
-20
-30
-30
5500
1500 2000
1000
500
C
0.2
1.0
0.8
0.6
0.4
0.2
ORF length (nt)
30
i
500
0.4
0.6
1000
fraction of genes
1500
0.8
10
2000
5500
ORF length (nt)
r = O.0O
20
:L
,
0
10
0
0
0.'oa
o
*
0
%*~
6
0
-0g
Q
a-10
-20
02
'i I-linea
-30
06
0.4
'
500
fraction08of genes1,0
I
1000
1500
2000
5500
OR F length (nt)
Figure 3-2: RACKI knockdown decreases the expression of mRNAs with short ORFs. (A-C)
Relationship between ORF length and mRNA-specific changes in ribosomal footprints (A, AFP), total
mRNA levels (B, Atotal mRNA), and translation efficiency (C, ATE) after RACK1 knockdown. The values
shown represent the average percent change for bins of 100 genes arranged by length. The ORF lengths
shown correspond to the point at which the average ORF length of the bin exceeds the indicated value.
Shaded areas represent +/- 1 s.d. from the average change. The RACK1 gene is excluded from the plot.
We then analyzed the functional groups affected by RACK1 knockdown. RACK1
knockdown decreased the expression of mRNAs encoding cytoplasmic and nuclearencoded mitochondrial ribosomal proteins by ~10% (Figure 3-3A). Gene ontology
analysis of mRNAs displaying decreased ribosomal footprints after RACK1 knockdown
104
likewise revealed an enrichment for genes encoding components of cytoplasmic and
mitochondrial ribosomes (Figure 3-3B). In addition to the mitochondrial ribosomal
protein genes, other groups of genes with functions in the mitochondria, such as those
that form mitochondrial respiratory complexes, were also enriched among the genes
with decreased expression after RACK1 knockdown (Figure 3-3B). Because protein
components of the mitochondrial respiratory chain are also encoded by short ORFs
(median length ~400 nt), it is not surprising that RACK1 knockdown decreases their
expression levels to a similar degree as the cytoplasmic and mitochondrial ribosomal
protein genes (median length ~500 nt, Figure 3-3C). Therefore, despite the relatively
subtle effects of RACK1 knockdown on the expression levels of mRNAs whose protein
products function in the mitochondria (10-15% reduction in steady-state mRNA levels,
Figure 3-3C), it is feasible that this level of reduction could cause substantial phenotypic
defects by lowering protein production from a large group of functionally-related mRNAs.
105
B
A
60
FP
total mRNA
45
mt. inner membrane
ribosome
rbs
~TE
TE
0
30
C-
GO categories with decreased FP (siRACK1 /siControl)
respiratory chain
<
mtb matrix
cytosolic ribosoe
-
proteasome complex
0 -30
-
spliceosomal complex
p -45
mt. ribosome
RP
MRP
n=9136
n=69
n=75
10
0
5
-log, pvalue
10
15
.
C
all genes
00
00
tiooa
5
-0
su0
*
1***
i (46n)
l
*so
1*
e.~ riosma
mitohonriaalsuFuni
rioso n ( n)a
e
ribosom
rib8 soma)
Figure 3-3: RACK1I knockdown decreases the expression of mRNAs encoding ribosomal proteins
and mitochondrial components. (A) Violin plot showing decreases in ribosomal footprints (FP), total
mRNA levels (total mRNA), and translation efficiency (TE) for cytoplasmic ribosomal protein (RP) and
nuclear-encoded mitochondrial ribosomal protein (MRP) mRNAs in HeLa cells after RACK1 knockdown.
(B) Top GO component enrichment categories for genes with decreased ribosomal footprints (FP) in
HeLa cells after RACK1 knockdown. Categories were manually pruned to remove redundant terms. (C)
The average percentage change in ribosomal footprints for genes in all GO component categories
containing >20 genes and arranged with equal spacing by median ORF length. The x-axis denotes the
point at which the median ORF length of the group exceeds the indicated value. Categories related to
mitochondrial or ribosomal functions are highlighted. Specific categories are indicated with their median
ORF lengths.
RACK1 is requiredfor mitochondrialfunction in HeLa cells
To assess the functional consequences of the observed decrease in expression of
nuclear-encoded mitochondrial genes in siRACK1 R s in Hea cellsater Ces to grow in
galactose-containing media and measured their proliferation. Galactose cannot be
106
efficiently converted to glucose but is required in the media to maintain osmolarity
similar to glucose (Holden et al., 2003). Therefore, proliferation in galactose measures
the cells' ability to use alternative carbon sources that require mitochondrial respiration
rather than glycolysis and can be used as a general measure of mitochondrial health
(Gohil et al., 2010; Rossignol et al., 2004). RACK1 knockdown decreased the
proliferation of HeLa cells grown in galactose relative to glucose by 20-30%, indicating
compromised respiratory metabolism associated with RACK1 knockdown (Figure 3-4A).
We hypothesize that reduced synthesis of a large class of small proteins with functions
in the mitochondria is responsible for the defects in respiratory growth that we observe
after RACK1 knockdown (Figure 3-4B).
107
A
120
110
100
ZC)90
~80
o
70
,5040
0~
0
30
'-
20
$
10
0
siControl
siRACK1 #1
siRACK1 #2
mitochondrial ribosomes
B
go
ETC
RACK1
5,.-
MRPs
& other small proteins
5-
mitochondrial protein synthesis 4
respiratory capacity 4
Figure 3-4: RACK1 is required for cell proliferation in respiratory conditions. (A) Reduced
media
proliferation of siRACK1-transfected HeLa cells after a shift from glucose- to galactose-containing
with
replicates
three
of
mean
the
are
Values
100%.
to
compared to siControl. The siControl value was set
Cytoplasmic
cells.
RACK1-depleted
in
s.d. shown. *p<0.05. (B) Model for mitochondrial dysfunction
proteins
ribosomes containing RACK1 (in siControl HeLa cells) synthesize MRP proteins and other small
into the
and
ribosomes
mitochondrial
into
that are imported into mitochondria and thereafter incorporated
mitochondrially-encoded
the
synthesize
turn
in
electron transport chain, etc. Mitochondrial ribosomes
fewer
proteins of the electron transport chain. In siRACK1 HeLa cells, ribosomes lacking RACK1 make
reduced
concomitantly
and
translation
mitochondrial
MRPs and other small proteins, resulting in reduced
respiration. MRP= mitochondrial ribosomal protein, ETC= electron transport chain.
108
Future Directions
The decreased steady-state mRNA levels that we observe for mRNAs with short ORFs,
including those that encode ribosomal proteins and mitochondrial components, could be
explained by several mechanisms. Perhaps the two most intuitive explanations are
either that loss of RACK1 decreases the synthesis rate (i.e. via reduced transcription) or
increases the decay rate of mRNAs with short ORFs. However, the similarity of these
results to those observed after loss of the Asci protein in yeast (Chapter 2) hints at a
common mechanism for the Asc1/RACK1 protein in promoting the expression of short
ORFs via its effect on their translation, potentially by promoting the function of the
translational closed-loop complex (Chapter 2). If decreased translation of short ORFs
after RACKI knockdown in HeLa cells caused a stoichiometric decrease in mRNA
stability, then no change in translation efficiency for these mRNAs would be observed.
To test this model, we could quantify mRNA stability after RACK1 knockdown in parallel
with our translational measurements. If our model is correct, then we should observe a
decrease in the stability of mRNAs with short ORFs after RACKI knockdown
concomitant with their loss from the ribosomal footprint pool.
Note that this is not a perfect experiment because correlation does not imply causation,
and there could be a mechanism (or mechanisms) that represses short ORF
transcription and decreases the stability of the resulting mRNAs independently. Indeed,
several studies now suggest that determinants of mRNA stability are imprinted on the
mRNA during transcription (Goler-Baron et al., 2008; Haimovich et al., 2013; Trcek et
109
al., 2011). Alternatively, RACKI knockdown could decrease the stability of the RP and
MRP mRNAs independently of any effects on translation, which could lead to the
decreased steady-state mRNA levels that we observe. However, given the genomewide correlation between translation efficiency and mRNA stability that we detected in
yeast (r-0.4 for mRNA half-life vs. translation efficiency, Chapter 2) and the specific
examples of translationally-inhibited mRNAs that exhibit decreased mRNA stability
(LaGrandeur and Parker, 1999; Muhlrad and Parker, 1999; Schwartz and Parker, 1999),
it is tempting to speculate that translational efficiency is a major causative determinant
of mRNA stability genome-wide, both at steady-state and during stress conditions. Now
that metabolic labeling approaches to measuring mRNA stability are becoming more
commonly used (Tani and Akimitsu, 2012), it should be feasible for many future studies
to measure mRNA stability in tandem with translation efficiency. It will be interesting to
observe the coupling (or lack thereof) between translation and mRNA stability in
different organisms and in response to different stress conditions.
Discussion
Here we have shown that the eukaryote-specific ribosomal protein RACK1 has a
function in promoting the expression of a group of small proteins with important roles in
translation and mitochondrial health. Because similar effects were observed in ASC1
yeast mutants, we suggest that the Asc1 /RACK1 ribosomal protein has a deeply
conserved function in promoting the expression of these mRNAs. One discrepancy
between the results presented here and Chapter 2 is that in yeast ASC1 mutants, the
RP and MRP mRNAs decrease twice as much in the ribosomal footprint pool compared
110
to the total mRNA pool, which causes them to have an apparent translation defect in
addition to decreased steady-state mRNA levels. This pattern of homodirectional
changes for the total mRNA pool and the ribosome-associated pool has been observed
in several previous studies where both pools of RNA were quantified during a stress
condition (Halbeisen and Gerber, 2009; Lackner et al., 2012; Preiss et al., 2003;
Smirnova et al., 2005; Stadler and Fire, 2013). Others have rationalized this effect as
'potentiation', whereby regulation at the translational level mirrors transcriptional
regulation and provides robustness to gene regulation (Preiss et al., 2003). However,
the mechanism underlying potentiation is currently unclear. One suggestion is that
during transcription, mRNAs are packaged into mRNP complexes whose components
can determine the decay rate, and possibly the translational activity, of their associated
mRNAs (Haimovich et al., 2013; Harel-Sharvit et al., 2010; Preiss et al., 2003). Such a
system could allow coordinated regulation of transcription, translation, and RNA decay
in response to stress conditions and could manifest as homodirectional changes in
translation and mRNA levels.
A second, perhaps more parsimonious, possibility is that RNA stability is linked to
translation efficiency for some or most mRNAs, such that a block in translation initiation
results in mRNA decay. Specific examples of this phenomenon have been described for
yeast (LaGrandeur and Parker, 1999; Muhlrad and Parker, 1999; Schwartz and Parker,
1999), but not yet in human cells. This idea is also supported by our finding of a
genome-wide correlation between translation efficiency and mRNA stability in yeast,
111
although it is possible that this correlation could be explained by distinct factors, such as
independent regulation of translation efficiency and mRNA stability in a homodirectional
manner.
It should also be noted that some cases of translational inhibition are not accompanied
by mRNA decay. For example, the mammalian mRNA encoding ferritin is stable and
translationally repressed in conditions where the iron-regulatory protein (IRP) is bound
to the iron response element (IRE) present in its 5' UTR (Thomson et al., 1999).
Additionally, experiments in yeast have shown that inhibiting translation via insertion of
hairpins or other translation inhibitory elements in the 5' UTRs of reporter mRNAs does
not have a uniform effect on mRNA stability. Although the stable PGK1 mRNA is
destabilized by addition of translation inhibitory elements, unstable reporter mRNAs are
not destabilized by these elements, and some even become more stable after the
addition of translation inhibitory elements (Beelman and Parker, 1994; Linz et al., 1997;
Muhlrad et al., 1995; Sagliocco et al., 1994; Vega Laso et al., 1993). These studies,
although focused on only a handful of reporter mRNAs, clearly demonstrate that the
relationship between translation and mRNA stability is not a straightforward link.
However, it remains possible that certain classes of mRNAs (for example, the closedloop-associated mRNAs) could have mRNA stabilities that are sensitive to their
translational statuses.
112
Whatever the ultimate mechanism, it is clear that Asc1/RACK1 is required for high
expression levels of proteins that function in mitochondria and for growth in conditions
requiring high levels of respiration in both yeast (Chapter 2) and human cells. It is
interesting that RACK1 overexpression activates the intrinsic apoptotic pathway which is
mediated by mitochondrial signaling (Mamidipudi and Cartwright, 2009). Taken together
with our findings, the evidence suggests that mitochondria are highly sensitive to the
level of RACK1. The complex effects of RACK1 protein levels on mitochondria may help
explain why RACK1 has been proposed to be an oncogene in some studies and a
tumor suppressor in others (Al-Reefy et al., 2010; Cao et al., 2010). One possibility is
that an optimal level of RACK1 is required to promote mitochondrial function and cell
growth, but very high levels of RACK1 lead to apoptosis through imbalanced production
of nuclear-encoded mitochondrial proteins and activation of apoptotic signaling.
If the effects of RACK1 function on mitochondria and respiratory growth are downstream
of RACK1's proposed function in the translational closed-loop complex (Chapter 2), then
it may be possible to target the translation of these mRNAs specifically for therapeutic
intervention. Analogous to mTOR inhibitors, which repress the translation of 5' terminal
oligopyrimidine (5' TOP) mRNAs via downstream activation of the eIF4E-BP
translational repressors (Thoreen et al., 2012), molecules targeting the interaction of
RACK1 with the closed-loop complex factors might specifically knockdown the
translation of mRNAs with short ORFs, including those encoding ribosomal proteins and
mitochondrial components. Because cancer cells exhibit a stronger bias towards
113
translation of short ORFs than other cell lines (Wang et al., 2013), targeting the
translation of short ORFs might constitute a previously unexploited therapeutic
vulnerability.
Experimental Procedures
Cell growth and transfection
HeLa (human cervical adenocarcinoma, CCL-2, ATCC) cells were cultured in DMEM
(D6429, Sigma) supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals).
Cells were grown under standard laboratory conditions at 370C with 5% C02. For siRNA
transfection, cells were transfected for 48h with siRNA (20 nM final concentration) using
Lipofectamine 2000 (Life Technologies) following the manufacturer's protocol. Silencer
select negative control siRNA (catalog number 4390843, Life Technologies) was used
as the control and Silencer Select GNB2L1 #1 siRNA (Cat#4392420, ID#s20341) and
Silencer Select GNB2L1 #2 siRNA (Cat#4392420, ID#s20342) were used to knockdown
RACK1. For the glucose to galactose media shift experiments HeLa cells were
transfected with siRNAs (20 nM final concentration) in triplicate using the transfection
reagent HiPerfect (Qiagen) following the manufacturer's protocol. 48h after transfection,
cells were washed three times in PBS (14040-117, Gibco) prior to an additional 24h
incubation with DMEM media (11966-025, Gibco) containing 10% dialyzed FBS (Life
Technologies), 1 mM sodium pyruvate, and either 4500 mg/L of galactose or 4500 mg/L
of glucose. 24h after media change, cells were trypsizined and counted using a
hemocytometer.
114
Ribosome footprintprofiling
The growth media was removed from cells by aspiration and fresh media containing 0.1
mg/ml CHX was added to the cells and incubated for 10 minutes at 370C. Cells were
then washed in PBS + CHX and resuspended in lysis buffer (20 mM Tris-HCI, pH 7.5,
10 mM MgCI2, 200 mM KCl, 1% Triton X-100, 1 mM DTT, 0.1 mg/ml CHX, 1 EDTA-free
protease inhibitor tablet/1 0 ml). Cells were lysed by passing through a 25-gauge needle,
and lysate was cleared by centrifugation at 1300 xg. 10 A260 U of extract were digested
with 0.5 U/pl of RNAse I (Ambion). Lysate was loaded onto 10-50% sucrose gradients in
buffer (20 mM HEPES-KOH, pH 7.4, 5 mM MgCI2, 100 mM KCI) and centrifuged 2.5 hrs
at 36,000 xg in an SW 41 rotor and a fraction corresponding to the monosome peak was
collected manually. Total RNA for accompanying RNA-Seq samples was isolated from
the same cell extracts used for footprint library generation using QlAzol (Qiagen),
according to the manufacturer's instructions. Library construction was completed as
described in Chapter 2.
Western blotting
For quantification of RACK1 knockdown in HeLa cells, 12 pg of total protein from HeLa
lysate was loaded onto a 12% SDS-PAGE gel. The membrane was blotted with a
mixture of a-RACK1 (Cell Signaling D59D5) and a-beta-Actin (Sigma-Aldrich A5441).
After secondary antibody incubation, blots were incubated with ECL (GE) and exposed
to X-ray film. Western blots were quantified with ImageJ. Values were derived by
comparison of each lane to a standard curve loaded on the same gel.
115
RNA-Seq data processing
HeLa reads were mapped with Bowtiel allowing up to 2 mismatches to a database of
spliced transcripts (hg19 sequence, and transcripts downloaded from UCSC on August
1, 2012) containing the transcript with the longest coding sequence. We used only
uniquely mapping reads for all downstream analyses. All other processing steps were
performed as described in Chapter 2.
Contributions
This chapter is based on an ongoing project. Kristen M. Bartoli optimized RACK1
knockdown and did most HeLa cell experiments. Mary K. Thompson did most molecular
biology experiments including ribosome footprint profiling. Mara F. Rojas-Duran
provided technical assistance. M.K.T. wrote the chapter and performed bioinformatic
analyses.
References
Adams, D.R., Ron, D., and Kiely, P.A. (2011). RACK1, A multifaceted scaffolding
protein: Structure and function. Cell Commun. Signal. 9, 22.
Al-Reefy, S., Osman, H., Jiang, W., and Mokbel, K. (2010). Evidence for a pro-apoptotic
function of RACKI in human breast cancer. Oncogene 29, 5651; author reply 5652.
Beelman, C.A., and Parker, R. (1994). Differential effects of translational inhibition in cis
and in trans on the decay of the unstable yeast MFA2 mRNA. J. Biol. Chem. 269, 96879692.
Ben-Shem, A., Garreau de Loubresse, N., Melnikov, S., Jenner, L., Yusupova, G., and
Yusupov, M. (2011). The Structure of the Eukaryotic Ribosome at 3.0 A Resolution.
Science 334, 1524-1529.
116
Cao, X.X., Xu, J.D., Liu, X.L., Xu, J.W., Wang, W.J., Li, Q.Q., Chen, Q., Xu, Z.D., and
Liu, X.P. (2010). RACK1: A superior independent predictor for poor clinical outcome in
breast cancer. Int J Cancer 127, 1172-1179.
Chang, B.Y., Conroy, K.B., Machieder, E.M., and Cartwright, C.A. (1998). RACK1, a
receptor for activated C kinase and a homolog of the beta subunit of G proteins, inhibits
activity of src tyrosine kinases and growth of NIH 3T3 cells. Mol. Cell. Biol. 18, 32453256.
Coyle, S.M., Gilbert, W. V, and Doudna, J.A. (2009). Direct link between RACK1
function and localization at the ribosome in vivo. Mol Cell Biol 29, 1626-1634.
Dorsett, Y., and Tuschl, T. (2004). siRNAs: applications in functional genomics and
potential as therapeutics. Nat. Rev. Drug Discov. 3, 318-329.
Gandin, V., Senft, D., Topisirovic, I., and Ronai, Z.A. (2013). RACK1 Function in Cell
Motility and Protein Synthesis. Genes Cancer 4, 369-377.
Gohil, V.M., Sheth, S.A., Nilsson, R., Wojtovich, A.P., Lee, J.H., Perocchi, F., Chen, W.,
Clish, C.B., Ayata, C., Brookes, P.S., et al. (2010). Nutrient-sensitized screening for
drugs that shift energy metabolism from mitochondrial respiration to glycolysis. Nat.
Biotechnol. 28, 249-255.
Goler-Baron, V., Selitrennik, M., Barkai, 0., Haimovich, G., Lotan, R., and Choder, M.
(2008). Transcription in the nucleus and mRNA decay in the cytoplasm are coupled
processes. Genes Dev. 22, 2022-2027.
Guillemot, F., Billault, A., and Auffray, C. (1989). Physical linkage of a guanine
nucleotide-binding protein-related gene to the chicken major histocompatibility complex.
Proc Natl Acad Sci U S A 86, 4594-4598.
Haimovich, G., Choder, M., Singer, R.H., and Trcek, T. (2013). The fate of the
messenger is pre-determined: A new model for regulation of gene expression. Biochim.
Biophys. Acta - Gene Regul. Mech. 1829, 643-653.
Halbeisen, R.E., and Gerber, A.P. (2009). Stress-Dependent Coordination of
Transcriptome and Translatome in Yeast. PLoS Biol 7, e105.
Harel-Sharvit, L., Eldad, N., Haimovich, G., Barkai, 0., Duek, L., and Choder, M. (2010).
RNA polymerase 11 subunits link transcription and mRNA decay to translation. Cell 143,
552-563.
Hashem, Y., des Georges, A., Dhote, V., Langlois, R., Liao, H.Y., Grassucci, R.A.,
Hellen, C.U.T., Pestova, T. V., and Frank, J. (2013). Structure of the mammalian
117
ribosomal 43S preinitiation complex bound to the scanning factor DHX29. Cell 153,
1108-1119.
Hermanto, U., Zong, C.S., Li, W., and Wang, L.H. (2002). RACK1, an insulin-like growth
factor I (IGF-1) receptor-interacting protein, modulates IGF-1-dependent integrin
signaling and promotes cell spreading and contact with extracellular matrix. Mol Cell
Biol. 22, 2345-2365.
Holden, H.M., Rayment, I., and Thoden, J.B. (2003). Structure and Function of Enzymes
of the Leloir Pathway for Galactose Metabolism. J. Biol. Chem. 278, 43885-43888.
Ingolia, N.T., Ghaemmaghami, S., Newman, J.R., and Weissman, J.S. (2009). Genomewide analysis in vivo of translation with nucleotide resolution using ribosome profiling.
Science 324, 218-223.
Lackner, D.H., Schmidt, M.W., Wu, S., Wolf, D. a, and Bahler, J. (2012). Regulation of
transcriptome, translation, and proteome in response to environmental stress in fission
yeast. Genome Biol. 13, R25.
LaGrandeur, T., and Parker, R. (1999). The cis acting sequences responsible for the
differential decay of the unstable MFA2 and stable PGK1 transcripts in yeast include the
context of the translational start codon. RNA 5, 420-433.
Levchenko, A., Bruck, J., and Sternberg, P.W. (2000). Scaffold proteins may
biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its
threshold properties. Proc. Nati. Acad. Sci. U. S. A. 97, 5818-5823.
LI, J.-J., andJ Xie, D. (2 I14). RA.K1, a versatile IIUU II LcnILCI.
n.JiI
UyeII
1-9.
Linz, B., Koloteva, N., Vasilescu, S., and McCarthy, J.E. (1997). Disruption of ribosomal
scanning on the 5'-untranslated region, and not restriction of translational initiation per
se, modulates the stability of nonaberrant mRNAs in the yeast Saccharomyces
cerevisiae. J. Biol. Chem. 272, 9131-9140.
L6pez-Bergami, P., Habelhah, H., Bhoumik, A., Zhang, W., Wang, L.H., and Ronai, Z.
(2005). Receptor for RACK1 mediates activation of JNK by protein kinase C. Mol. Cell
19, 309-320.
Mamidipudi, V., and Cartwright, C.A. (2009). A novel pro-apoptotic function of RACK1:
suppression of Src activity in the intrinsic and Akt pathways. Oncogene 28, 4421-4433.
Melnikov, S., Ben-Shem, A., Garreau de Loubresse, N., Jenner, L., Yusupova, G., and
Yusupov, M. (2012). One core, two shells: bacterial and eukaryotic ribosomes. Nat.
Struct. Mol. Biol. 19, 560-567.
118
Mochlyrosen, D., Khaner, H., and Lopez, J. (1991). Identification of intracellular receptor
proteins for activated protein-kinase-c. Proc Natl Acad Sci U S A 88, 3997-4000.
Muhlrad, D., and Parker, R. (1999). Recognition of yeast mRNAs as "nonsense
containing" leads to both inhibition of mRNA translation and mRNA degradation:
implications for the control of mRNA decapping. Mol. Biol. Cell 10, 3971-3978.
Muhlrad, D., Decker, C.J., and Parker, R. (1995). Turnover mechanisms of the stable
yeast PGK1 mRNA. Mol. Cell. Biol. 15, 2145-2156.
Preiss, T., and Hentze, M.W. (1999). From factors to mechanisms: Translation and
translational control in eukaryotes. Curr. Opin. Genet. Dev. 9, 515-521.
Preiss, T., Baron-Benhamou, J., Ansorge, W., and Hentze, M.W. (2003).
Homodirectional changes in transcriptome composition and mRNA translation induced
by rapamycin and heat shock. Nat. Struct. Biol. 10, 1039-1047.
Robles, M.S., Boyault, C., Knutti, D., Padmanabhan, K., and Weitz, C.J. (2010).
Identification of RACK1 and protein kinase Calpha as integral components of the
mammalian circadian clock. Science 327, 463-466.
Ron, D., Chen, C.H., Caldwell, J., Jamieson, L., Orr, E., and Mochly-Rosen, D. (1994).
Cloning of an intracellular receptor for protein kinase C: a homolog of the beta subunit of
G proteins. Proc. Natl. Acad. Sci. U. S. A. 91, 839-843.
Rossignol, R., Gilkerson, R., Aggeler, R., Yamagata, K., Remington, S.J., and Capaldi,
R.A. (2004). Energy Substrate Modulates Mitochondrial Structure and Oxidative
Capacity in Cancer Cells. Cancer Res. 64, 985-993.
Sagliocco, F.A., Zhu, D., Vega Laso, M.R., McCarthy, J.E., Tuite, M.F., and Brown, A.J.
(1994). Rapid mRNA degradation in yeast can proceed independently of translational
elongation. J. Biol. Chem. 269,18630-18637.
SchwanhAusser, B., Busse, D., Li, N., Dittmar, G., Schuchhardt, J., Wolf, J., Chen, W.,
and Selbach, M. (2011). Global quantification of mammalian gene expression control.
Nature 473, 337-342.
Schwartz, D.C., and Parker, R. (1999). Mutations in translation initiation factors lead to
increased rates of deadenylation and decapping of mRNAs in Saccharomyces
cerevisiae. Mol. Cell. Biol. 19, 5247-5256.
Sengupta, J., Nilsson, J., Gursky, R., Spahn, C.M., Nissen, P., and Frank, J. (2004).
Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by
cryo-EM. Nat Struct Mol Biol 11, 957-962.
119
Serrels, B., Sandilands, E., Serrels, A., Baillie, G., Houslay, M.D., Brunton, V.G., Canel,
M., Machesky, L.M., Anderson, K.l., and Frame, M.C. (2010). A complex between FAK,
RACK1, and PDE4D5 controls spreading initiation and cancer cell polarity. Curr. Biol.
20,1086-1092.
Siridechadilok, B., Fraser, C.S., Hall, R.J., Doudna, J.A., and Nogales, E. (2005).
Structural roles for human translation factor elF3 in initiation of protein synthesis.
Science 310,1513-1515.
Smirnova, J.B., Selley, J.N., Sanchez-Cabo, F., Carroll, K., Eddy, A.A., McCarthy,
J.E.G., Hubbard, S.J., Pavitt, G.D., Grant, C.M., and Ashe, M.P. (2005). Global gene
expression profiling reveals widespread yet distinctive translational responses to
different eukaryotic translation initiation factor 2B-targeting stress pathways. Mol. Cell.
Biol. 25, 9340-9349.
Smith, T.F. (2008). Diversity of WD-repeat proteins. Subcell. Biochem. 48, 20-30.
Stadler, M., and Fire, A. (2013). Conserved translatome remodeling in nematode
species executing a shared developmental transition. PLoS Genet. 9, el 003739.
Stuart, J.M., Segal, E., Koller, D., and Kim, S.K. (2003). A gene-coexpression network
for global discovery of conserved genetic modules. Science 302, 249-255.
Tani, H., and Akimitsu, N. (2012). Genome-wide technology for determining RNA
stability in mammalian cells: Historical perspective and recent advantages based on
modified nucleotide labeling. RNA Biol. 9.
Iomson, A.MA., Rogers, J.T., and Leedman, P.J. (1999). iron-regulatory proteins, ironresponsive elements and ferritin mRNA translation. Int. J. Biochem. Cell Biol. 31, 11391152.
Thoreen, C.C., Chantranupong, L., Keys, H.R., Wang, T., Gray, N.S., and Sabatini, D.M.
(2012). A unifying model for mTORC1 -mediated regulation of mRNA translation. Nature
485,109-113.
Trcek, T., Larson, D.R., Mold6n, A., Query, C.C., and Singer, R.H. (2011). Singlemolecule mRNA decay measurements reveal promoter-regulated mRNA stability in
yeast. Cell 147,1484-1497.
Tyers, M. (2015). BioGRID. http://thebiogrid.org/. Accessed June 25, 2015.
Vega Laso, M.R., Zhu, D., Sagliocco, F., Brown, A.J., Tuite, M.F., and McCarthy, J.E.
(1993). Inhibition of translational initiation in the yeast Saccharomyces cerevisiae as a
function of the stability and position of hairpin structures in the mRNA leader. J. Biol.
Chem. 268, 6453-6462.
120
Wang, T., Cui, Y., Jin, J., Guo, J., Wang, G., Yin, X., He, Q.-Y., and Zhang, G. (2013).
Translating mRNAs strongly correlate to proteins in a multivariate manner and their
translation ratios are phenotype specific. Nucleic Acids Res. 41, 4743-4754.
Witzel, F., Maddison, L., and Blthgen, N. (2012). How scaffolds shape MAPK
signaling: What we know and opportunities for systems approaches. Front. Physiol. 3
DEC.
Xu, C., and Min, J. (2011). Structure and function of WD40 domain proteins. Protein Cell
2, 202-214.
Xue, S., and Barna, M. (2012). Specialized ribosomes: a new frontier in gene regulation
and organismal biology. Nat. Rev. Mol. Cell Biol. 13, 355-369.
121
Chapter IV:
Discussion and future directions
122
Overview
In Chapter 2, I presented evidence that the eukaryote-specific ribosomal protein
Asci /RACK1 promotes the formation or function of the translational closed-loop
complex and that this pathway normally acts to enhance the translation of mRNAs with
short open reading frames (ORFs). In this chapter I will discuss the physiological and
evolutionary ramifications of this work. I will then review our current knowledge of the
translational closed-loop and present a model for ORF length-dependent translational
enhancement via the closed-loop that could be modulated by RACKI and translation
initiation factors. Finally, I will suggest some experimental approaches that could be
employed to test this model in future work.
A role for ORF length-dependent translational regulation in evolution
and cell physiology
Why would the cell want to privilege the translation of short ORFs? Despite the large
evolutionary distances and variable genomic complexity between different eukaryotic
species, the length of their ORFs is largely the same, which may imply that there are
some physical limitations to protein size that act to keep ORFs short (Wang et al., 2005;
Xu et al., 2006). Although some proposals have focused on maintaining translational
accuracy and protein folding fidelity (Drummond and Wilke, 2008; Guo et al., 2015),
another obvious advantage of short ORFs is the energetic efficiency of translation per
mole of protein (Akashi, 2003; Warringer and Blomberg, 2006). In the same way that
mRNAs encoding highly expressed proteins display codon usage that is biased towards
abundant tRNA species (Quax et al., 2015), selection to minimize the number of amino
123
acids per highly expressed protein would minimize production costs and maximize their
rates of production. Favoring the translation of short ORFs could also be a way to
ensure robust expression for a particular set of proteins across many tissue types,
consistent with the observation that ubiquitously expressed mRNAs tend to have short
ORFs (Eisenberg and Levanon, 2003). It is intriguing to note that translation in cancer
cells is skewed towards even higher production of small proteins relative to other
proteins, as this might represent a way to maximize cellular growth potential while
minimizing the energetic costs associated with protein production (Guo et al., 2015;
Wang et al., 2013). These observations suggest that a simple mechanism that favors
the translation of short ORFs should have evolutionary benefits and provide a base level
of translational regulation upon which more complicated regulatory schemes can be
built.
In Chapter 2, I presented evidence that the higher translation of short ORFs stems from
their preferential association with the translational closed-loop complex. In the next
sections, I will review our evidence and current understanding of the closed-loop model
and present a biochemical hypothesis to explain preferential closed-loop formation on
short ORFs, with important implications for translational regulation of genes in different
functional classes.
Are all mRNAs translated via the closed-loop?
In chapter 1, I introduced the closed-loop model, a widely-accepted addition to the
canonical translation initiation pathway in eukaryotes that involves interaction of the 5'
124
and 3' ends of the mRNA with the protein components of the closed-loop, elF4G and
PABP (Kahvejian et al., 2001). Genetic and biochemical evidence clearly demonstrate
that elF4G and PABP can interact and that their interaction enhances the translation of
reporter mRNAs (Preiss and Hentze, 1998; Tarun and Sachs, 1995, 1996; Wells et al.,
1998). These observations have been taken as evidence for closed-loop formation and
promoted as a general translation initiation pathway for all mRNAs (Gallie, 1998).
However, some studies suggest that cap-poly(A) synergy may not require closed-loop
formation per se. PABP can promote translation in trans (that is for an RNA without a
poly(A) tail) (Borman et al., 2002; Munroe and Jacobson, 1990). This observation
makes sense in light of biochemical data showing that PABP enhances the affinity of
the elF4F complex for the mRNA cap even in the absence of a poly(A) tail (Borman et
al., 2000; Haar et al., 2000). Thus it is unclear to what extent cap-poly(A) synergy can
be interpreted as proof of closed-loop formation. Additionally, adding a cap and poly(A)
tail to an mRNA may promote mRNA stability in a synergistic manner and it is unclear in
some studies to what extent the synergistic effect on reporter expression can be
assigned primarily to translational enhancement instead of RNA stability (Gallie, 1991;
lizuka et al., 1994).
Even without these potential confounding effects, our knowledge of the translational
closed-loop is nevertheless based on a handful of reporter RNAs that were likely
selected for their moderate to high translational activity. How do these principles hold up
globally? A recent genome-wide study that quantified mRNA enrichment with factors of
125
the eIF4F complex and PABP found that most translated mRNAs interact with PABP,
but only a small subset interact highly with both the eIF4F complex and PABP
simultaneously (Costello et al., 2015). They speculate that the closed-loop complex is
only required for translation of a subset of mRNAs and that other pathways exist for the
translation of most mRNAs. It is also possible that many non-closed-loop mRNAs
associate with both PABP and the eIF4F complex in vivo, but that in the absence of
closed-loop formation, the eIF4F complex is less stably associated with the mRNA and
dissociates during immunoprecipitation, thus leaving only PABP associated with the
poly(A) tail. Indeed, PABP has several potential functions that are not obviously linked
to closed-loop formation, including 60S subunit joining and protection of the poly(A) tail
from nucleases (Decker and Parker, 1993; Kahvejian et al., 2005; Mangus et al., 2003;
Munroe and Jacobson, 1990; Sachs and Davis, 1989, 1990). Perhaps it is one of these
functions through which PABP acts as a 'general' translation factor. The PABP-eIF4G
interaction within the closed-loop might be a more specialized high-efficiency translation
pathway that selectively promotes the translation of a class of highly translated short
ORFs associated with growth and metabolism.
The closed-loop as a specialized high-efficiency translation state
In the closed-loop model of translation ribosomes can in theory reinitiate translation on
the same mRNA molecule after dissociation because the start and stop codon would be
brought in closer proximity by circularization, a process called intrapolysomal reinitiation
(Kopeina et al., 2008). Therefore closed-loop mRNAs would be expected to have higher
translation efficiency than linear mRNAs because of a higher local concentration of
126
ribosomal subunits near the start codon after termination. In this context, it is interesting
to consider whether the start and stop codons might be brought closer together than the
5' and 3' termini of the mRNA in the closed-loop, which would allow the small subunit to
bypass 5' UTR scanning and associated ATP consumption (Kopeina et al., 2008). In the
next section, I will discuss a detailed model for closed-loop formation that includes a
mechanism for anchoring the closed-loop near the start and stop codons. Closed-loop
anchoring at start and/or stop codons would be expected to make the closed-loop
complex more strongly dependent on ORF length, rather than transcript length. Such a
mechanism could explain why we see stronger partial correlations between ORF length
and translational efficiency than between transcript length and translational efficiency in
our data (Chapter 2), although experimental manipulations of ORF and UTR lengths
independently will be needed to substantiate this model.
Intrapolysomal reinitiation directly from the stop codon to the start codon on a subset of
mRNAs that form closed-loops would also have important regulatory implications. Many
cis-acting mechanisms of translation inhibition rely on the small subunit scanning
through the 5' UTR. A reinitiating ribosome that could be directly shunted to the main
ORF's start codon would be impervious to diversion by upstream open reading frames
or the scanning block of a strong RNA secondary structure (Abaeva et al., 2011;
Wethmar, 2014). Thus, mRNAs found predominantly in the closed-loop form would be
expected to be more resistant to inhibition by these elements if the number of reinitiation
events within the closed-loop exceeded the number of initiation events outside of the
127
closed-loop. Therefore, in addition to boosting the rate of initiation by increasing the
local concentration of ribosomal subunits, intrapolysomal reinitiation may also increase
the translational efficiency of an mRNA by immunizing it against many common forms of
translational inhibition.
A model for mRNA/ORF length-dependent regulation of the closedloop
One explanation for ORF length-dependent preferences to form or maintain mRNA
closed-loops relies on biophysical constraints and not on specific interactions between
the ribosome and translation factors. Because shorter mRNAs have a higher probability
of their 5' and 3' ends colliding by diffusion, their rate of circularization should be higher
than for longer mRNAs (Guo et al., 2015). One study modeled the mRNA as a worm-like
chain and calculated the circularization time for mRNAs of different lengths. Their model
predicts a nearly exponential increase in circularization time as a function of mRNA
length, with longer mRNAs (>~2000 nt) taking over a minute to circularize (Guo et al.,
2015). By comparing calculated circularization times to the rates of other steps in the
translation initiation pathway determined in an extract system (Lorsch and Herschlag,
1999), the authors postulate that mRNA circularization could be the rate-limiting step in
translation initiation for some mRNAs.
One assumption here is that circularization needs to occur before translation can begin,
but what if other faster steps of initiation are actually in competition with mRNA
circularization? If the closed-loop complex is only able to form at a certain stage of
128
translation initiation, then at a certain mRNA length, other steps of the initiation pathway
will compete better for this intermediate and circularization will not occur. In this version
of the model, there will be a maximum mRNA length at which the closed-loop can form,
thus limiting closed-loop complexes to short mRNAs exclusively. Another possibility is
that circularization occurs on all mRNAs but that each mRNA is in equilibrium between
the open linear and closed-loop forms. If the circularization rate is higher for short
mRNAs but the loop-opening rate is similar across all mRNAs, then short mRNAs would
be expected to spend more time in the closed-loop state. In either version of the model,
any mutation that affects the stability of the closed-loop would be expected to
disproportionally affect short mRNAs. I favor the competition model because of
evidence that cap-poly(A) synergy occurs only in the presence of competition for limiting
translation factors and ribosomal subunits (Michel et al., 2000; Preiss and Hentze, 1998;
Rifo et al., 2007). Because most of these experiments were done using relatively long
luciferase mRNAs (~1700 nt ORF length), it seems likely that their average
circularization times would fall in the range in which closed-loop formation could occur
only if the relative rates of later steps in the initiation pathway were lowered by depletion
of the relevant translation factors via competition.
At which step(s) in translation initiation can the closed-loop complex form? A previous
study indicated that closed-loop formation occurs either before or at the 48S complex
step of the initiation pathway (the mRNA bound to the 40S subunit) due to the formation
of the closed-loop in the presence of GMP-PNP, which prevents 60S subunit joining
129
(Amrani et al., 2008). Based on our evidence from Chapter 2 that RACK1 and eIF4G
promote the function of the closed-loop, we suggest that formation of the closed-loop
occurs at the 48S complex step and not at an earlier pre-ribosome binding step (Figure
4-1A). Perhaps 40S-RACK1 enhances eIF4G's affinity for PABP and therefore
increases the probability of productive eIF4G-PABP complex formation after diffusioncontrolled collision occurs on short mRNAs. Another possibility is that the presence of
RACK1 on the 40S could promote the formation of a more stable version of the closedloop that persists even after the 40S-eIF4F association is broken during the transition to
translation elongation. In either scenario, the formation and/or stability of the closed-loop
is dependent on the interaction between the 40S ribosome and the eIF4F complex.
Therefore, formation of a stable closed-loop will be in competition with 60S subunit
joining (or a later step in the translation pathway) because at later stages of translation
the ribosome is no longer associated with the eIF4F complex (Figure 4-1 B). In effect, for
short mRNAs the high local concentration of PABP relative to 60S drives closed-loop
formation whereas for long mRNAs the lower local concentration of PABP cannot
compete with 60S subunit joining, and the probability of closed-loop formation on these
mRNAs becomes very low.
130
B
A
large subunit
eV ''
ORF
RACK(
small subunit
RACK1
eIF4F
cap-binding complex
PABP
PABP
(low PABP affiinity)
(high PABP affiinity)
short mRNAs: [PABP], > [60S]
long mRNAs: [PABP.1< [60S]
The presence of RACK1
Figure 4-1: A model for ribosome-dependent closed-loop formation. (A)
productive collision event
a
of
probability
the
increases
which
enhances the affinity of elF4G for PABP,
step in translation
downstream
a
with
competition
in
is
step
this
between PABP and elF4G. Because
of forming the
probability
low
a
have
will
mRNAs
longer
joining),
initiation (here shown as 60S subunit
(B).
occurs
closed-loop before this collision
A ribosome-dependent model for closed-loop formation also suggests a mechanism by
which the start and stop codons could be brought into proximity to facilitate
intrapolysomal reinitiation. If eIF4G remained near the start codon after closed-loop
formation, then the 5' side of the closed-loop could be anchored near the start codon.
Because PABP interacts with the ribosome release factor eRF3 (Cosson et al., 2002;
Uchida et al., 2002), it seems possible that a terminating ribosome associated with
eRF3 could induce a conformation of the closed-loop whereby the 3' side of the closedloop becomes anchored at the stop codon. In support of this possibility, one study
identified a second state of the closed-loop that is dependent on the interaction between
PABP, eRF3, and an 80S ribosome (Amrani et al., 2008). Therefore, it is feasible to
of
construct a model in which the closed-loop is initially formed before the pioneer round
131
translation at the 48S step but then is remodeled after the first termination event by
PABP and eRF3, thereby creating a form of the closed-loop in which the start and stop
codons are spatially juxtaposed, facilitating intrapolysomal reinitiation.
Future directions
Our model makes several predictions, some of which are testable with current tools.
First, it will be important to determine whether RACK1 binding to eIF4G alters its affinity
with PABP, alone and in the context of the ribosome. Affinities could first be measured
with recombinant proteins, and later in the presence of salt-washed ribosomes from the
ASC1 null yeast strain (to obtain ribosomes lacking both eIF4G and RACK1). Secondly,
our hypothesis predicts that the presence of RACK1 will promote formation of more
stable closed-loop complexes that will preferentially form on shorter ORFs. It has
already been shown that mRNAs with shorter ORFs form more stable closed-loops than
longer mRNAs by ribosome toeprinting (Amrani et al., 2008). This study used the
ribosome toeprinting assay in the presence or absence of a cap analog inhibitor to
assess closed-loop stability based on the assumption that stable closed-loops should be
resistant to cap analog due to the high affinity of the eIF4F-PABP complex for the
mRNA cap (Amrani et al., 2008), (Figure 4-2). By repeating these experiments in the
presence and absence of RACK1, we could determine the contribution of the RACK1
protein to closed-loop stability on short ORFs.
132
closed-loop
non-closed-loop
5'm 7G cap
elF4F complex
PABP
cap analog (inhibitor)
+
r+
reverse transcrIptIon
toeprint
*32P
-32P
Figure 4-2: The ribosome toeprinting assay monitors closed-loop stability. Cap analog competes
with the mRNA 5' m 7G cap for binding to the elF4F complex, which is required for cap-dependent
association of the ribosome with the mRNA. Because of the enhanced affinity of PABP-eIF4F for the
mRNA cap, closed-loop mRNAs should be more resistant to the presence of cap analog than non-closedloop mRNAs. The assay is performed by primer extension in the presence of cycloheximide, which stalls
80S ribosomes on the AUG start codon. Toeprints are identified as primer extension products of the
correct size.
There are also many questions about the translational closed-loop that are impossible to
address using current tools. It is interesting to consider if and how the closed-loop might
be maintained or stabilized after its initial formation, but it is difficult to distinguish the
133
pioneer round of translation from later rounds using bulk assays. It is also difficult to
measure the contribution of the ribosomal subunits to closed-loop formation or stability
because ribosome toeprinting by definition relies on the association of the mRNA with
the ribosome. Many of these hypotheses await the development of an assay that can
directly measure closed-loop formation and its correlation with ribosome association.
Perhaps a single-molecule FRET reporter system could bridge this gap (Figure 4-3). A
similar strategy has already been used to monitor the temporal order of events in mRNA
splicing (Crawford et al., 2013; Hoskins et al., 2011). By correlating FRET states
dependent on the eIF4G-PABP interaction with arrival and departure of the small
subunit, one could discern whether the small subunit binds the mRNA before closedloop formation, as in our model (Figure 4-1A). Temporal comparisons may also be able
to provide some information about the pioneer round versus later rounds of translation
with respect to the stability of the closed-loop.
134
non-closed-loop
closed-loop
ORF
40S ribosome
RACK1
m
elF4F
cap-binding complex
PABP
fluorophores
i
low FRET
I
biotin:streptavidin
high FRET
glass slide
Figure 4-3: A potential single-molecule FRET system to monitor the temporal and spatial
relationships between eIF4G, PABP, and the small ribosomal subunit (40S ribosome).
References
Abaeva, l.S., Marintchev, A., Pisareva, V.P., Hellen, C.U.T., and Pestova, T. V (2011).
Bypassing of stems versus linear base-by-base inspection of mammalian mRNAs
during ribosomal scanning. EMBO J. 30,115-129.
Akashi, H. (2003). Translational selection and yeast proteome evolution. Genetics 164,
1291-1303.
Amrani, N., Ghosh, S., Mangus, D.A., and Jacobson, A. (2008). Translation factors
promote the formation of two states of the closed-loop mRNP. Nature 453, 1276-1280.
Borman, A.M., Michel, Y.M., and Kean, K.M. (2000). Biochemical characterisation of
cap-poly(A) synergy in rabbit reticulocyte lysates: the elF4G-PABP interaction increases
the functional affinity of elF4E for the capped mRNA 5'-end. Nucleic Acids Res. 28,
4068-4075.
Borman, A.M., Michel, Y.M., Malnou, C.E., and Kean, K.M. (2002). Free poly(A)
stimulates capped mRNA translation in vitro through the eIF4G-poly(A)-binding protein
interaction. J. Biol. Chem. 277, 36818-36824.
135
Cosson, B., Couturier, A., Chabelskaya, S., Kiktev, D., Inge-Vechtomov, S., Philippe,
M., and Zhouravleva, G. (2002). Poly(A)-binding protein acts in translation termination
via eukaryotic release factor 3 interaction and does not influence [PSI(+)] propagation.
Mol. Cell. Biol. 22, 3301-3315.
Costello, J., Castelli, L.M., Rowe, W., Kershaw, C.J., Talavera, D., Mohammad-Qureshi,
S.S., Sims, P.F.G., Grant, C.M., Pavitt, G.D., Hubbard, S.J., et al. (2015). Global mRNA
selection mechanisms for translation initiation. Genome Biol. 16, 10.
Crawford, D.J., Hoskins, A. a, Friedman, L.J., Gelles, J., and Moore, M.J. (2013).
Single-molecule colocalization FRET evidence that spliceosome activation precedes
stable approach of 5' splice site and branch site. Proc. Natl. Acad. Sci. U. S. A. 110,
6783-6788.
Decker, C.J., and Parker, R. (1993). A turnover pathway for both stable and unstable
mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev. 7, 16321643.
Drummond, D.A., and Wilke, C.O. (2008). Mistranslation-induced protein misfolding as a
dominant constraint on coding-sequence evolution. Cell 134, 341-352.
Eisenberg, E., and Levanon, E.Y. (2003). Human housekeeping genes are compact.
Trends Genet. 19, 362-365.
Gallie, D.R. (1991). The cap and poly(A) tail function synergistically to regulate mRNA
translational efficiency. Genes Dev. 5, 2108-2116.
r%
A
.9
..
i.I
I~O.A
-r1[1.
Uefe
Gallie,i.~iii,
U.r.. (1998).
A LIale UI
of LWU
wo Lermifni:
ee26ZIO0,
e%.~I-
rn
/A~.~
L
A-.
1
-11
I.
Guo, J., Lian, X., Zhong, J., Wang, T., and Zhang, G. (2015). Length-dependent
translation initiation benefits the functional proteome of human cells. Mol. Biosyst. 11,
370-378.
Haar, T. Von Der, Ball, P.D., Mccarthy, J.E.G., and von Der Haar, T. (2000).
Stabilization of Eukaryotic Initiation Factor 4E Binding to the mRNA 5 -Cap by Domains
of eIF4G. J. Biol. Chem. 275, 30551-30555.
Hoskins, A.A., Friedman, L.J., Gallagher, S.S., Crawford, D.J., Anderson, E.G.,
Wombacher, R., Ramirez, N., Cornish, V.W., Gelles, J., and Moore, M.J. (2011).
Ordered and dynamic assembly of single spliceosomes. Science 331, 1289-1295.
lizuka, N., Najita, L., Franzusoff, A., and Sarnow, P. (1994). Cap-dependent and capindependent translation by internal initiation of mRNAs in cell extracts prepared from
Saccharomyces cerevisiae. Mol. Cell. Biol. 14, 7322-7330.
136
Kahvejian, A., Roy, G., and Sonenberg, N. (2001). The mRNA closed-loop model: the
function of PABP and PABP-interacting proteins in mRNA translation. Cold Spring Harb.
Symp. Quant. Biol. LXVI.
Kahvejian, A., Svitkin, Y. V, Sukarieh, R., Boutchou, M.M., and Sonenberg, N. (2005).
Mammalian poly(A)-binding protein is a eukaryotic translation initiation factor , which
acts via multiple mechanisms. Genes Dev. 104-113.
Kopeina, G.S., Afonina, Z.A., Gromova, K. V., Shirokov, V.A., Vasiliev, V.D., and Spirin,
A.S. (2008). Step-wise formation of eukaryotic double-row polyribosomes and circular
translation of polysomal mRNA. Nucleic Acids Res. 36, 2476-2488.
Lorsch, J.R., and Herschlag, D. (1999). Kinetic dissection of fundamental processes of
eukaryotic translation initiation in vitro. EMBO J 18, 6705-6717.
Mangus, D.A., Evans, M.C., and Jacobson, A. (2003). Poly(A)-binding proteins:
multifunctional scaffolds for the post-transcriptional control of gene expression. Genome
Biol. 4, 223.
Michel, Y.M., Poncet, D., Piron, M., Kean, K.M., and Borman, A.M. (2000). Cap-poly(A)
synergy in mammalian cell-free extracts. Investigation of the requirements for poly(A)mediated stimulation of translation initiation. J. Biol. Chem. 275, 32268-32276.
Munroe, D., and Jacobson, A. (1990). mRNA poly(A) tail, a 3' enhancer of translational
initiation. Mol. Cell. Biol. 10, 3441-3455.
Preiss, T., and Hentze, M.W. (1998). Dual function of the messenger RNA cap structure
in poly(A)-tail-promoted translation in yeast. Nature 392, 516-520.
Quax, T.E.F., Claassens, N.J., S611, D., and van der Oost, J. (2015). Codon Bias as a
Means to Fine-Tune Gene Expression. Mol. Cell 59, 149-161.
Rifo, R.S., Ricci, E.P., Decimo, D., Moncorg6, 0., and Ohlmann, T. (2007). Back to
basics: The untreated rabbit reticulocyte lysate as a competitive system to recapitulate
cap/poly(A) synergy and the selective advantage of IRES-driven translation. Nucleic
Acids Res. 35.
Sachs, A.B., and Davis, R.W. (1989). The poly(A) binding protein is required for poly(A)
shortening and 60S ribosomal subunit-dependent translation initiation. Cell 58, 857867.
Sachs, A.B., and Davis, R.W. (1990). Translation initiation and ribosomal biogenesis:
involvement of a putative rRNA helicase and RPL46. Science 247, 1077-1079.
137
Tarun, S.Z., and Sachs, A.B. (1995). A common function for mRNA 5' and 3' ends in
translation initiation in yeast. Genes Dev. 9, 2997-3007.
Tarun, S.Z., and Sachs, A.B. (1996). Association of the yeast poly(A) tail binding protein
with translation initiation factor eIF-4G. EMBO J. 15, 7168-7177.
Uchida, N., Hoshino, S.-l., Imataka, H., Sonenberg, N., and Katada, T. (2002). A novel
role of the mammalian GSPT/eRF3 associating with poly(A)-binding protein in
Cap/Poly(A)-dependent translation. J. Biol. Chem. 277, 50286-50292.
Wang, D., Hsieh, M., and Li, W.-H. (2005). A general tendency for conservation of
protein length across eukaryotic kingdoms. Mol. Biol. Evol. 22, 142-147.
Wang, T., Cui, Y., Jin, J., Guo, J., Wang, G., Yin, X., He, Q.-Y., and Zhang, G. (2013).
Translating mRNAs strongly correlate to proteins in a multivariate manner and their
translation ratios are phenotype specific. Nucleic Acids Res. 41, 4743-4754.
Warringer, J., and Blomberg, A. (2006). Evolutionary constraints on yeast protein size.
BMC Evol. Biol. 6, 61.
Wells, S.E., Hillner, P.E., Vale, R.D., and Sachs, A.B. (1998). Circularization of mRNA
by Eukaryotic Translation Initiation Factors. Mol. Cell 2, 135-140.
Wethmar, K. (2014). The regulatory potential of upstream open reading frames in
eukaryotic gene expression. Wiley Interdiscip. Rev. RNA.
Xu, L., Chen, H., Hu, X., Zhang, R., Zhang, Z., and Luo, Z.W. (2006). Average gene
lengtII
lb IIlIIly
UUInseVed InI pIUkrdIyULtb
dII
eUIdIyULkayb
two kingdoms. Mol. Biol. Evol. 23,1107-1108.
138
iIU UIVeryb Uiniy Utwe1
lu
Appendix I:
Transcriptional and translational regulation of the FLO
gene family in ribosomal protein mutants
139
Abstract
Saccharomyces cerevisiae yeast has adapted to grow in a range of environmental
conditions that require elegant coordination of gene expression programs. In response
to nutrient deprivation, yeast transcriptionally upregulate the expression of the FLO
gene family, which encode cell-wall glycoproteins that enable surface adhesion, an
adaptive response. Deletion of three distinct ribosomal proteins ablates FLO-dependent
phenotypes. To investigate a potential connection between FLO gene regulation and
translation, we performed ribosome footprint profiling of the FLU ribosomal protein
mutants. All FLO- ribosomal protein mutants display virtually identical effects on the
expression of FLO10 and FLO11, the only FLO genes expressed in the sigmal28b
FLO+ laboratory yeast strain. Although both FLO10 and FLO1 1 are tightly repressed in
these mutants (~1 00-fold), FLO10 is translationally repressed whereas FLO1 1 appears
to be repressed via both decreased mRNA levels and decreased translation levels. The
FLO1 1 promoter is necessary and sufficient for repression repression of the FLO 1I
locus in a ribosomal protein mutant, indicating that either transcriptional shutoff and/or
alternate 5' UTR isoform production causes FLO1 1 repression. Polysome analysis
indicated that the majority of FLO10 mRNA co-migrates with monosomes in a ribosomal
protein mutant suggesting that it is being actively translated in a region outside its
annotated open reading frame. Conserved upstream open reading frames (uORFs) are
present upstream of both FLO10 and FLO1 1, suggesting that alternate transcript start
sites in ribosomal protein mutants may produce longer 5' UTRs that divert the ribosome
from the canonical translation start sites and cause translational repression of both
140
FLO10 and FLO1 1. We hypothesize that these ribosomal protein mutations induce
epigenetic silencing of the FLO promoters equivalent to silencing upon PKA inhibition,
which is known to fully repress both FLO10 and FLO1 1 (Halme et al., 2004).
Conservation of uORFs upstream of the FLO genes may act as a failsafe mechanism to
ensure full silencing during inhibitory conditions, or alternatively, they may allow lowlevel production of FLO proteins in response to a starvation signal while epigenetic
activation and transcription of the long FLO genes occurs.
Introduction
Although yeast are not capable of the complex behaviors characteristic of multicellular
organisms, they sense and respond to a plethora of environmental signals that allow
them to adapt quickly to extracellular conditions. Nutrient availability is of paramount
importance for yeast, as each cell must have access to sufficient carbon and nitrogen
sources (Zaman et al., 2008). Rapidly dividing yeast in nutrient-rich conditions undergo
bipolar cell division, in which the daughter cell may emerge from either side of the
mother followed by cytokinesis and physical separation. During nutrient limitation, yeast
switch to a unipolar budding pattern in which successive daughters emerge from the
same end of the mother cell without physical separation to enable directional growth,
eventually forming a filament of connected yeast cells (Cullen and Sprague, 2012).
These cells express a cell-wall glycoprotein Flo 1 that allows them to adhere to and
penetrate through solid growth media, a process known as invasive growth in haploid
yeast (Cullen and Sprague, 2012). Invasive growth allows yeast to forage for potential
nutrients and may have emerged as an advantageous trait in wild yeast whose major
141
habitat is the surface of fruit. A related gene, FLO10, is expressed in similar
environmental conditions and encodes a glycoprotein that mediates cell-cell adhesion.
Mutations in most laboratory strains of yeast prevent expression of the entire FLO
family, thus we use the FLO- yeast strain, sigmal 278b, for our studies. Sigmal 278b
expresses FLO1 1 highly and FLO10 moderately, but does not express other members
of the FLO family (FLO1, FLO5, FLO9) that are expressed in some wild and industrial
strains of yeast (Verstrepen et al., 2003; Zhao and Bai, 2009).
The regulation of FLO1 1 has been extensively studied, as it possesses one of the
largest (- 3 kb) and most complex promoters in yeast which responds to input from at
least six signaling pathways including the Kssl MAPK pathway, the cAMP/PKA
pathway, the TOR pathway, the Snf 1 glucose-sensing pathway, the Rimi 01 pH-sensing
pathway, and the RTG mitochondrial retrograde signaling pathway (Cutler et al., 2001;
Jin et al., 2008; Kuchin et al., 2002; Lamb and Mitchell, 2003; Rupp et al., 1999).
Mutations that negatively affect the activity of these signaling pathways reduce
expression of the FLOl 1 mRNA. Inhibition of PKA signaling also results in epigenetic
silencing of both the FLO10 and FLO1 1 promoters (Halme et al., 2004). The ability of
FLO promoters to undergo epigenetic silencing suggests that bulk measurements of
FLO mRNA expression levels may more closely reflect the fraction of cells with active
promoters, rather than the average promoter activity among all cells. Hence, regulation
of the FLO1 1 promoter serves as important model for the integration of several signaling
pathways into transcriptional output.
142
Despite this rich literature, a screen of the sigmal 278b deletion collection revealed 239
mutants severely defective for FLO phenotypes (FLO~), many with no clear connection
to known FLO regulators (Ryan et al., 2012). Five of the FLO- mutants are deletions of
ribosomal protein genes. Because most studies of FLO gene regulation have focused
on the transcriptional rather than post-transcriptional level, we reasoned that ribosomal
protein mutants with altered FLO phenotypes might provide a window into potential
post-transcriptional regulatory mechanisms that may have been overlooked.
Here we characterized each FLO ribosomal protein mutant by ribosome footprint
profiling. We found that both FLO10 and FLO1 1 expressionwere dramatically repressed
in these mutants (-1 00-fold). Although both mRNAs showed evidence of translational
repression, the bulk of their repression came from decreased mRNA levels for FLOl 1
and decreased translation levels for FLO10. The FLO10 mRNA showed evidence of
translation at a density of one ribosome per mRNA, probably in a region outside of its
annotated open reading frame. The presence of conserved upstream open reading
frames (uORFs) upstream of both FLO10 and FLO1 1 suggests a mechanism by which
both mRNAs may be translationally repressed during inhibitory environmental conditions
that may be mimicked by altered signaling in the ribosomal protein mutants. uORFs of
the FLO gene family may act as a failsafe mechanism to prevent inappropriate protein
synthesis in conditions that silence FLO promoters. We speculate that these uORFs
may also provide a mechanism to quickly produce low to moderate levels of protein
143
during the relatively slow transition period to FLO promoter activation and transcription
of the FLO genes, which are among the longest genes in yeast.
Results
A screen of the sigma1278b deletion library identified 239 mutants severely defective for
haploid invasive growth (Ryan et al., 2012). Five of these mutants, asc1A, rpI23bA,
rpI35aA, rpplaA, and rpsl7aA are deletions of ribosomal protein genes. Although five
mutants does not represent a statistically significant overrepresentation, we
nevertheless reasoned that dissection of the molecular defects of these mutants might
uncover translational regulation of the FLO gene family. Additionally, because most
ribosomal proteins in yeast are encoded by two paralogs, many single paralog mutants
do not display severe cellular phenotypes due to genetic redundancy (Parenteau J et
al., 2011; Steffen et al., 2008). Thus it is likely that other ribosomal proteins in addition
to those investigated here may be essential for invasive growth.
We first assessed the phenotype of each FLO- ribosomal protein mutant for invasive
growth defects. asc1A, rpI23bA, rpI35a.A and rpp1aA conferred strong defects in
invasive growth, but rpsl7aA showed only a mild defect (Figure Al-1A). We therefore
chose to focus our analysis on ascA, rpI23bA, and rpplaA based on the severity of
their invasive growth defects and their relatively normal growth rates in rich media
(Figure Al-1 A). For experiments related to Asci, we also present data obtained from a
mutant in which the start codon is mutated to a nonsense codon (ascl-M1X), which
144
ablates the expression of the Asci protein but maintains expression of its intronic
snoRNA, SNR24 (Chapter 2).
To identify translational defects present in asc1A, rpI23bA, and rpplaA, we conducted
ribosome footprint profiling of these mutants. Although rpI23bA and rpplaA have similar
translational defects, asc1-MiX translational defects are largely non-correlated with
those of rpI23bA and rpplaA (Chapter 2). However, both FLO10 and FLO1 1 showed
similar switch-like repression in each mutant (Figure Al-1 B-D). FLO1 1 decreased -50fold in the total mRNA pool but showed evidence of ~2-fold additional translational
repression (Figure Al-1 E, F). In contrast, FLO10 total mRNA levels were decreased only
-2-fold, but its translation was dramatically repressed -50-fold (Figure Al-1 E, F). Other
members of the FLO family, FLOl, FLO5, and FLO9, are very poorly expressed in our
wild type strain and were not pursued further here.
145
A
'59'
before wasafter wash
D
5
R
Ib
C.,
0
0
0
0~
-5
-5
-5
*FL010
-
FLO10
FLO11
FLOU1
ASCI'
.FLO11
FLOW0
RPP1A
PL23B
-10
-
a
a
5
I
10
-g5
-1
1og 2 6totaI mRNA (rp#23bA
log, Atotal mRNA (ascl-A41XfT)
F
E
800-m
0 -0
*6
400-
ME
0A
5
-5
10
T)
0
5
10
log, Motal (rp I aNWT)
C
FO
80-
m FL0ll
O1 1
L 1
LiiFLOIlI
z
<6040-
200-
0/2
'Ni
0*20
Invasive
Figure Al-1: Cellular and molecular FLO phenotypes of the ribosomal protein mutants. (A)
invasionfor
screen
throughput
high
a
in
growth assay for FLO ribosomal protein mutants identified
expression of
defective mutants (Ryan et al., 2012). (B-D) The effect of asclA, rpI23bA and rpplaA on
showing
plots
Bar
F)
(E,
pools.
mRNA
total
FLO10 and FLO1 1 in both the ribosomal footprint (FP) and
Error
levels.
(F)
mRNA
total
and
(E)
footprint
the fold repression of FLO10 and FLO1 1 at the ribosomal
bars are s.d. from two biological replicates.
Because FLO1 1 repression appeared to be largely transcriptional in the ribosomal
protein mutants, we assessed the function of the FLO 11 promoter in repression. The
FLO1 1 promoter is subject to complex regulation (Cullen and Sprague, 2012), and the
activity of one or multiple promoter input pathways may be altered in the ribosomal
protein mutants. We first asked whether the promoter region is required for repression
146
of FLO1 1 expression in cells lacking Asc1. Expression of FLO1 1 under the TEF1
promoter restored haploid invasive growth in asciA mutants (Figure AI-2A, B). Likewise,
integration of GFP and an alternate 3' UTR into the FLO1 1 genomic locus represses the
expression of GFP in the asciA mutant (Figure AI-2C, D). Thus, the FLO1 1 promoter is
necessary and sufficient for repression of the FLOl 1 locus in the asciA mutant.
Although less is known about the regulation of the FLO10 promoter, it is also silenced
by inactivation of the PKA signaling pathway (Halme et al., 2004). Thus, it is possible
that both FLO10 and FLO1 1 promoters respond to loss of the ribosomal proteins via
inhibition of the PKA pathway or another pathway that affects the expression of both
FLO10 and FLO11.
Next, we sought to validate the translational defect of FLO10 in the asc1-MiX mutant by
polysome RT-qPCR. In wildtype cells, FLO10 mRNA is associated with polysomes,
consistent with active translation of its long open reading frames (3.5 kb). However, in
the asci-MiX mutant where FLO10 is translationally silent, it does not accumulate in
the non-translating mRNP fraction. Rather, it co-migrates with the monosome peak,
which suggests that is being translated at a density of one ribosome per mRNA
molecule (Figure Al-3).
147
WT
B
A
WT
asc1A
+pPTEFi-FLO11
asc1A
before wash
after wash
D
C
86-
VVT
-4LL
39X
asc1A
Figure AI-2: The FLO11 promoter is necessary and sufficient for FLO11 repression in ascA (A)
Invasive growth assay in WT and asciA cells. (B) Invasive growth assay in WT and asclA cells
expressing FLO1 1 under the TEF1 promoter. (C) Expression of GFP from the FLO1 1 locus in WT and
ascl1d cells. (D) Same as (C), but with GFP levels quantified by spectrophotometer. Error bars are s.d.
from technical replicates of a representative experiment.
148
0.5FLO10mRNA
0.
0.4o
0.
z
c
0.3-
o
0.2-
asc1-MIX
E
.0
0.0
-
0.1-
1 2 3 4 5 6 7
mRNP 40S 60S 80S
8
9 10 11 12
fraction
polysomes
Figure AI-3: FLO10 is monosome-associated in the asc1-MiX mutant. Polysome qRT-PCR for
FLO10 mRNA in WT and asci-MiX. Values are normalized to an RNA spike-in control in each fraction
and then set so that the sum of all fractions=1.
To explain this discrepancy, we looked upstream of the FLO10 start codon for a
potential upstream open reading frame (uORF). Within the region 200 nt upstream of
FLO10, uORFs are present at -181, -174 and -116 nt relative to the start codon of the
main ORF (Figure AI-4). We also looked upstream of FLO1 1, reasoning that FLO10 and
FLO1 1 might be subject to similar control in these mutants and also seeking to explain
our finding that the FLO1 1 mRNA is slightly translationally repressed in the ribosomal
protein mutants (~2-fold, Figure Al-1 E, F). The region upstream of FLO1 1 contains one
uORF at -60 nt relative to the start codon of the main ORF (Figure AI-4). Both FLO10
and FLO1 1 uORFs extend until -40 nt upstream of the main ORF and this pattern is
conserved across four yeast species (Figure AI-4). The conservation of FLO10 and
FLO1 1 uORFs is notable, as only ~20% of uORFs are conserved across yeast species
(Cvijovi6 et al., 2007).
149
FLO10 (200 nt upstream)
uORF3 (-116, 10 codons)
uORF1 (-181, 46codons) uORF2(-174, 3codons)
ct-tttttagactct----------agtataactagc-aagt---atacata-tttgtcaaaac
macPpr atatatttccttgtgaagaatgattcatgactgaaat-ttttgacgttcttct-ctgtcaaatgcaactaccaaaat---atatata-tttgtcaaaac
gtacttttgccgttaaataatactaat-aaa---sacMik agatgtttctttgtgaagaotgattg
macay aattgtttt-ttgtgaagaatggccaacgccttaattatttcgttaatatcg agcatcaaat---actggc-cagttccgcacatattttgctcaaaacatgctatatcc
asCer atttattctgttgtaacgaatgattc
43 nt
sacCr
acPar
cMik
gagacacttctt--ttacgttccactgtttcgagtttacgttgaa-gatttgttttagggtgtta--atcaaagaacaacaaataaa-aaATG
gagactctcctt--ttacgtctcacaatttcaagtttatgttgaacggttctttttggaggcttattatcatagaataacaaaccaa-aaATG
agctcttgctttagaatggttattactttgaacaacaaaccaa-atATA
ma80ay cgatgtccttaacatatataaagcactccttacttttgtttcttattaagaagtttttctcgaaagttcttcttagaaaagtttgtcaacattgaataacaaaccaagaaAAG
FLO11 (100 nt upstream)
uORFI
macCer gtat-aaaaagcaccctattcatcagttattatccctcgt
sacPar atataaaaaagcatcccattcatcagttataatctcttgt
amcilk gtat-aaaaaqcgctatattcatccataat
acBay gtat-aaaaacccttatattcattattaataatatcttata
(-60. 5 codons)
c
45 nt
-a
g
aatatacttttgtag---gcctc aaa
atccatatacgcacactATG
tataatatacttttgtaa---gcctcaaaaatccatataccacaccATG
atatacttttgtagtaagettcaaaaacceatatacgcataccATG
tgaaatgttttttgtaataagcttcaaaaatccataaaacacaccATG
Figure Al-4: The presence of conserved uORFs near the translation start sites of FLO10 and
FLO11. The genomic regions upstream of FLO10 and FLO1 1 are shown. ORFs are highlighted in
different colors to distinguish between different reading frames. red=+O, yellow=+1, blue=+2, with respect
to the frame of the main ORF for each species. The distance between the stop codon of the last S.
cerevisiae uORF and the main ORF is indicated. sacCer= S. cerevisiae, sacPar= S. paradoxus, sacMik=
S. mikatae, sacBay= S. bayanus.
Although we looked for evidence of uORF translation in the ribosomal protein mutants
by ribosome footprint profiling, low read coverage precluded confident assignment of
translation at any uORF. In the future, the functionality of the FLO uORFs could be
explored by fusing the FLO gene 5' UTRs to a reporter gene and assessing the effect of
each uORF on translation of the reporter. The fact that the FLO10 mRNA is present at
similar levels in both wild type and the ribosomal protein mutants suggests that the
mRNA probably maintains similar stability across these strains. This is interesting, as
translation in uORFs is expected to activate the nonsense-mediated decay (NMD)
pathway (Oliveira and McCarthy, 1995). However, certain uORFs that promote
reinitiation at the downstream ORF during stress conditions, such as those present in
the 5' UTRs of GCN4 and YAP1, contain stabilizer elements that prevent targeting by
NMD (Ruiz-Echevarria and Peltz, 2000; Vilela and McCarthy, 2003). Therefore, the
150
probable stability of the translationally repressed FLO10 mRNA suggests a functional
role for its uORFs and could indicate that the FLO10 mRNA is stabilized because
certain stress conditions allow reinitiation at the main ORF and rapid production of Flo 0
protein.
We hypothesize that altered signaling in the ribosomal protein mutants, perhaps via
repression of the PKA pathway, causes silencing of the FLO10 and FLO1 1 promoters
which represses transcription from a CDS-proximal transcription start site. Leaky
transcription from a region further upstream could then produce transcripts that possess
longer 5' UTRs. Note that we cannot assign transcript boundaries from our RNA-Seq
data due to the presence of pervasive non-coding transcription upstream of FLO10 and
FLO1 1 (Bumgarner et al., 2009). 5' RACE analysis of FLO10 and FLO1 1 in wildtype
cells supports the existence of long 5' UTR isoforms (~250 nt for FLO10 mRNA and
~200 nt for FLO1 1 mRNA), but this analysis should be repeated for the ribosomal
protein mutants to determine whether longer 5' UTRs are produced in the mutants
(Figure 5). RNAse protection assays could then be used to determine the abundance of
these isoforms in wildtype, the ribosomal protein mutants, and different environmental
conditions quantitatively.
151
ladder:
1 kb 100 bp
1 kb
0.5 kb
0.1 kb
IN
IN OUT
OUT
-TAP
+TAP
FLO10
IN
OUT
IN
OUT
-TAP
+TAP
FLO11
Figure Al-5 5' RACE for FLO10 and FLO11 mRNAs in wildtype yeast. The +TAP and -TAP control
reactions are shown. In the absence of TAP, uncapped mRNAs will be cloned by the RACE procedure.
The outer (OUT) and inner (IN) RACE reactions are also shown. The asterisks indicate final RACE
products. Because -50 nt is added by the inner RACE, the approximate 5' UTR sizes can be determined
by subtracting 50 nt from the size indicated by the ladder.
Although they are not expressed in sigmal 278b, FLO1, FLO5, and FLO9 also harbor
conserved uORFs upstream of their start codons that may be functional in wild and
industrial yeast strains that express these flocculins (Figure 6). Together, the
conservation of FLO family uORFs suggests that they may play important roles in the
regulation of FLO-family phenotypes during adaptation to nutrient limitation. We prevent
three, not mutually exclusive, models to explain conservation of the FLO uORFs (Figure
7). In the most simple 'failsafe' model, uORF inclusion in a longer 5' UTR may simply
prevent inappropriate expression of a FLO gene during inhibitory conditions when the
152
promoter is silenced. In a second 'just-in-case' model, uORFs could allow inducible
translation of a FLO ORF in response to a starvation signal, analogous to translational
activation of the GCN4 mRNA by reinitiation downstream of its uORFs during amino
acid starvation (Hinnebusch, 2005). Finally, in a 'tuner' model, uORF-containing
transcripts could allow intermediate expression levels in response to weak signals that
do not fully activate the promoter but allow reinitiation downstream of the uORFs. Future
experiments will test these models to determine the contribution of the FLO gene
uORFs to FLO-dependent phenotypes.
153
FLO1 (200 nt upstream)
uORF1 (-120, 21 codons)
acCer ttaatc-tattogcatacttacgctgtaggaacattttattattaggatccgacta-ctgcctacatatttattcggaaggi
acgtgcaccgtagggtcattttattattagaggccaactagttgagcaaatattcattcaaaaac
scPr ttattctgcttg--ggaagacac-----ttotgcaagattgtgaata
CaMik --------------------------------------------------------------------------atgtttttgaagacacgatgttttctcagtgttcttaaca
say -------------------------------------------------------------------
57 nt
moCor
-c--tctcttccgggttcttatttttaattcttgtcaccagtaaacagaacatccaaaA-TG
-cagtaaacaaatatccaaaaAATG
aCPor
macMik tataaaaaggcgcca-atttgcatctttatctc-ttt-----aaaa--tttccttaaagttttcttacacctttatttcaca--aaagaggtcaaccaaagA-TG
macBay tataaaaag--ggcggttt---tacttttgttt-gttgagtatagagtttttctcgaagatctaacttactttttttgtcaacatag
FLO5
(200 nt
upstream)
uORF1 (-177, 29codons)
sacCr
ga-agttectctgg--tcaaatttt
acPar ga-agttcctc---------------------atact----------tctttcacggtcttataactttgcaattactgaa---ggttgtagtocttgctcc--------atatt
asck gagaatttctt-----------------atgaaatatttac -------actttcacagtcttatcattttagaaacactgaaggttggttgtagtcttctgcttcagaaaagcaatatt
saclay
gg-aatatcgctggaattgttttatcgtcggagacaattcgcgttcaaattattcacagtagtat-------------------------------------------------------
90 nt
24 n
uORF3 (-37. oit-of-frame overlap with main ORF)
uORF2 (-68. 15 codons)
ttgattagcaccactaa----------aaaaaATG
-- aaatataaaaagagcaccctc
sacCer
tatcgctactgg----------aaaaaATG
sacPar tggacaa-ataataaaaatataaaaagagcaccctc
actik ttgataa-ataaaggacatataaaaagaacgccctcatqctttcqtttctccaccgtcac--------------ttttcttctaatagatcatcactactgaacaattaqccaaaaaATG
acBay --------------- acatataaaaagggcggttt-tattttgtttgttgagtatagagtttttctcgaagatctaacttactttttttgtcaacataga----------aaaaaATG
FLO9 (200 nt upstream)
uORF1 (-109, 21 codons)
macCer tttttctttttaaaggtattcgatttctcctt--tgctaacacattatcctgccaaattatt-ctaccttcttatatattacataaaatt
sacPar atattttctttcqagaagct---------------------cacatcatcgcatcggattaat-ctgcctgcgtatatattacttaaaacacgatgacactcaaaatcc
maoclk tcttccggttt--aagataatcatacccccacta--tgtagaagtattgtcccaccaaattatt-tcatctgcgtatatattacatcaat
sac!ay atgttttgtac--ggtatatttcatgccttacttactacaaacatattatcgcatcgaaccgttatcadttcataattattacgcatacgac
86 nt
sacCer
Super
80CMik
mascay
-------aacataacgttcttccttcttattta-caggtacgctctttaaa-----ttgcaatttaaa-aagaacaat-----tgtacaataaaagccccaaaaATO
_
---- tttqcaatttaaa-gcagacaat-----tgttcaataaaagccccaacaAAA
ag----ttcgcaatctaaa-actaatgat-----tgtaaaataaac-tcccaactATG
tagca-ccctaaccATG
Figure AI-6: uORFs present upstream of the other FLO genes. Parameters are as described for
Figure AI-4.
154
'fallsafe'
constituitive repression of leaky mRNA production
x
3'
5'
'just-in-case'
inhibitory conditions
activating conditions (early protein production during promoter activation)
3'
51
'tuner'
modulation of reinitiation frequency without or in addition to promoter regulation
Figure AI-7: Models for functions of FLO family uORFs.
155
Discussion
Here we have shown that mutations in several distinct ribosomal protein genes cause
repression of the FLO gene family via mechanisms that affect both the total mRNA
levels and translational levels of the FLO mRNAs. One protein, Ascl, is a small
ribosomal subunit protein and the other proteins, Rpl23b and Rppl a bind the large
subunit. Although mutations in these loci cause largely distinct translational
consequences (Chapter 2), all of them share quantitatively similar repression of the FLO
genes. Of note, each of these mutants displays decreased 60S ribosomal subunit levels
(Chapter 2). It would be interesting to see whether other ribosomal protein mutants with
severely decreased 60S subunit levels exhibit similar defects in FLO gene expression,
or whether the regulatory mechanisms examined here are specific to this subset of
ribosomal protein mutants. Another possibility, which we think more likely, is that the
phenotypes are not related to the function of the ribosome at all, but that this type of
ribosome dysfunction feeds into one of the siqnaling pathways that regulates the Flfi10
and FLO1 1 promoters. Promoter regulation in these mutants may result in
transcriptional silencing and/or use of a more distal transcription start site to produce
transcripts containing uORFs. We think that a good candidate for the affected pathway
in the ribosomal protein mutants is the PKA pathway, as the activity of this pathway is
more tightly coupled to FLO1 1 expression than other pathways (Vinod et al., 2008). In
addition, a simple hypothesis would posit that both FLO10 and FLO1 1 are regulated by
the same signal in the ribosomal protein mutants. Thus it is attractive to consider the
possibility that the PKA pathway is inactivated in the ribosomal protein mutants because
156
PKA inactivation is already known to repress both FLO10 and FLO1 1 via recruitment of
the Sfl1 repressor to the promoter regions and downstream epigenetic silencing (Halme
et al., 2004).
Looking beyond the FLO family, low-level production of translationally-activatable
mRNAs from epigenetically-silenced promoters (the 'just-in-case' model, Figure Al-7)
could present a mechanism to provide finer scale temporal control over cell fate
decisions. With the burgeoning use of ribosome footprint profiling to interrogate
translational regulation during development, potential regulatory uORFs could be
identified by searching for uORFs that have increased ribosome occupancy during a
developmental transition or stress response in concert with the appearance of longer 5'
UTRs, which can now be assessed with one of many genome-wide sequencing
techniques (Arribere and Gilbert, 2013; Ni et al., 2010; Pelechano et al., 2014). In fact,
increased uORF usage during developmental transitions has already been observed in
both yeast and mammalian cells (Brar et al., 2012; Ingolia et al., 2011). It has been
hypothesized that the increased uORF usage observed during these transitions is a
result of altered fidelity of start codon recognition during these conditions, which would
act to produce small peptides from the upstream uORFs that could have important
developmental functions (Brar et al., 2012; Ingolia et al., 2011). An alternate possibility
is that longer transcript isoforms are produced during these stages, and that the uORFs
either provide an additional level of regulation via downstream reinitiation or act to
translationally silence mRNAs that are produced from silent promoters. Whether 'leaky'
157
production of uORF-containing mRNAs from silenced promoters represents a robust
solution to imperfect transcriptional silencing or a more complex regulatory logic
remains to be seen.
Experimental Procedures
Yeast growth and invasive growth assays
For most assays, yeast were grown to mid-log phase (OD -0.6). For microscopy
analysis of yeast expressing pFLO11-GFP, yeast were grown to post-diauxic phase
(OD -5). Invasive growth assays were performed by spotting yeast onto solid media,
growing for 2 days and then washing the top layer of yeast growth off under a rapid
stream of water. Invasive strains form mats that penetrate the media and remain
attached to the plate after washing. For pTEF1-FLO1 1 expression, experiments were
done in SC-Ura to maintain selection for the plasmid. The plasmid containing pTEF1FLO11 (BWG 697) was a generous gift from Brian Chin.
Sequence data analysis
Ribosome footprint profiling was described in Chapter 2. For this appendix, we mapped
reads to the s288c genome, downloaded from SGD on Sept. 2, 2011, because the
sigmal278b genome available from SGD does not include the FLO family genes. For
the uORF conservation analysis, we downloaded sequences corresponding to 200 nt
upstream of each FLO gene from the UCSC genome browser. Only the species for
which sequence data was available in these regions were included.
158
5'RACE
The FirstChoice RLM-RACE kit (Ambion) was used to amplify cDNA made from capped
5' mRNA ends using RNA isolated from wildtype sigmal 278b yeast grown to mid-log
phase (OD 0.6) in YPAD.
Polysome Analysis
Polysome qRT-PCR was performed as described in Chapter 2. qRT-PCR primer
sequences are: FLO10 (F: CTACACAACACCCACCAACG, R:
GGCAACATTACCTCCAACCG), FLO 11: (F: CTGGCGAAACAACAACTGG
, R: AATAGGGTTGGTTGCTGTGG).
References
Arribere, J.A., and Gilbert, W. V. (2013). Roles for transcript leaders in translation and
mRNA decay revealed by transcript leader sequencing. Genome Res. 23, 977-987.
Brar, G.A., Yassour, M., Friedman, N., Regev, A., Ingolia, N.T., and Weissman, J.S.
(2012). High-Resolution View of the Yeast Meiotic Program Revealed by Ribosome
Profiling. Science 335, 552-557.
Bumgarner, S.L., Dowell, R.D., Grisafi, P., Gifford, D.K., and Fink, G.R. (2009). Toggle
involving cis-interfering noncoding RNAs controls variegated gene expression in yeast.
Proc. Natl. Acad. Sci. U. S. A. 106,18321-18326.
Cullen, P.J., and Sprague, G.F. (2012). The regulation of filamentous growth in yeast.
Genetics 190, 23-49.
Cutler, N.S., Pan, X., Heitman, J., and Cardenas, M.E. (2001). The TOR signal
transduction cascade controls cellular differentiation in response to nutrients. Mol Biol
Cell 12, 4103-4113.
159
Cvijovid, M., Dalevi, D., Bilsland, E., Kemp, G.J.L., and Sunnerhagen, P. (2007).
Identification of putative regulatory upstream ORFs in the yeast genome using heuristics
and evolutionary conservation. BMC Bioinformatics 8, 295.
Halme, A., Bumgarner, S., Styles, C., and Fink, G.R. (2004). Genetic and epigenetic
regulation of the FLO gene family generates cell-surface variation in yeast. Cell 116,
405-415.
Hinnebusch, A.G. (2005). Translational regulation of GCN4 and the general amino acid
control of yeast. Annu. Rev. Microbiol. 59, 407-450.
Ingolia, N.T., Lareau, L.F., and Weissman, J.S. (2011). Ribosome profiling of mouse
embryonic stem cells reveals the complexity and dynamics of mammalian proteomes.
Cell 147, 789-802.
Jin, R., Dobry, C.J., McCown, P.J., and Kumar, A. (2008). Large-scale analysis of yeast
filamentous growth by systematic gene disruption and overexpression. Mol Biol Cell 19,
284-296.
Kuchin, S., Vyas, V.K., and Carlson, M. (2002). Snf1 protein kinase and the repressors
Nrgl and Nrg2 regulate FLO1 1, haploid invasive growth, and diploid pseudohyphal
differentiation. Mol Cell Biol 22, 3994-4000.
Lamb, T.M., and Mitchell, A.P. (2003). The transcription factor Rim101p governs ion
tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and
SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23, 677-686.
NiT, Corcoran, D.L., Rach, E.A., Song,
,
ao,
T.,
Ohler, U., and Zhu,
J. (2010). A paired-end sequencing strategy to map the complex landscape of
transcription initiation. Nat. Methods 7, 521-527.
Oliveira, C.C., and McCarthy, J.E.G. (1995). The relationship between eukaryotic
translation and mRNA stability. A short upstream open reading frame strongly inhibits
translational initiation and greatly accelerates mRNA degradation in the yeast
Saccharomyces cerevisiae. J. Biol. Chem. 270, 8936-8943.
Parenteau J, Durand M, Morin G, Gagnon J, Lucier JF, Wellinger RJ, Chabot B, and
Elela SA (2011). Introns within ribosomal protein genes regulate the production and
function of yeast ribosomes. Cell 147, 320-331.
Pelechano, V., Wei, W., Jakob, P., and Steinmetz, L.M. (2014). Genome-wide
identification of transcript start and end sites by transcript isoform sequencing. Nat.
Protoc. 9, 1740-1759.
160
Ruiz-Echevarria, M.J., and Peltz, S.W. (2000). The RNA binding protein Pubi
modulates the stability of transcripts containing upstream open reading frames. Cell
101, 741-751.
Rupp, S., Summers, E., Lo, H.J., Madhani, H., and Fink, G. (1999). MAP kinase and
cAMP filamentation signaling pathways converge on the unusually large promoter of the
yeast FLO1 1 gene. EMBO J 18,1257-1269.
Ryan, 0., Shapiro, R.S., Kurat, C.F., Mayhew, D., Baryshnikova, A., Chin, B., Lin, Z.-Y.,
Cox, M.J., Vizeacoumar, F., Cheung, D., et al. (2012). Global Gene Deletion Analysis
Exploring Yeast Filamentous Growth. Science 337,1353-1356.
Steffen, K.K., MacKay, V.L., Kerr, E.O., Tsuchiya, M., Hu, D., Fox, L.A., Dang, N.,
Johnston, E.D., Oakes, J.A., Tchao, B.N., et al. (2008). Yeast life span extension by
depletion of 60s ribosomal subunits is mediated by Gcn4. Cell 133, 292-302.
Verstrepen, K.J., Derdelinckx, G., Verachtert, H., and Delvaux, F.R. (2003). Yeast
flocculation: what brewers should know. Appl. Microbiol. Biotechnol. 61, 197-205.
Vilela, C., and McCarthy, J.E.G. (2003). Regulation of fungal gene expression via short
open reading frames in the mRNA 5' untranslated region. Mol. Microbiol. 49, 859-867.
Vinod, P.K., Sengupta, N., Bhat, P.J., and Venkatesh, K. V. (2008). Integration of global
signaling pathways, cAMP-PKA, MAPK and TOR in the regulation of FLO1 1. PLoS One
3, e1663.
Zaman, S., Lippman, S.I., Zhao, X., and Broach, J.R. (2008). How Saccharomyces
Responds to Nutrients. Annu. Rev. Genet. 42, 27-81.
Zhao, X.Q., and Bai, F.W. (2009). Yeast flocculation: New story in fuel ethanol
production. Biotechnol. Adv. 27, 849-856.
161