The Purification and Characterization of ... Chitinase and Chitobiase Enzymes Produced ... EF4a
advertisement

The Purification and Characterization of the
Chitinase and Chitobiase Enzymes Produced by
EF4a
Sheila ~I. Bailey
November 20, 1986
Submitted in =ul=illment
of 5-fonors Thesis
requirements.
.Chitin, predominantly a polymer of B(1-4) linked
N-Acetyl-D-glucosamine molecules, is found within the cell walls
of funqi, bacteria, the exoskeletons of insects, and the
coverlngs of crustacea.
Chitin is degraded by two classes of
enzymes, the chi tinases and the chi tobiases.
Chitinases primarily
cleave chitin into the dimer known as chitobiose and other
soluble chitodextrins.
studies inVOlving the chitinase of
serratia marcescens have indicated that its activity relies on
the acetyl group of the N-acetylglucosamine molecule.
The enzyme
has no activity against modified chitin whose acetyl groups have
been replaced by H or OH groups (12).
I'ihether or not the chi tobiase
enzyme has the same specificity has not been determined.
This enzyme degrades the chitobiose and other sOluble chitodextrins
produced by the action of chitinase into the monomer C0-acetylD-glucosamine molecules.
Chitinases have been isolated from a variety of organisms.
Their molecular weights have been determined by electrophoretic
techniques, column chromatography, and density centrifugation.
The mOlecu;"ar weights of serratia, Aeromonas, Vibrio, and several
Streptomyces species have been determined as 52,000 and 58,000;
110,000; 63,000; 30,000 and 56,000 daltons respectively (16)(20)
(14)(6)(3)(2).
Chitinases have also been isolated from sources
such as Yarn, I;)heat germ, Spider, and Stable fly l1ith the following
respective '1101ecular weights 33,500; 30,000;
48,500 dal-oons
48,000~4,000
and
(19)\~0)(11)(4).
>Jot a lot of regard has been given to the chitobiase enzyme
I1hich is normally a part of the chitin degradation systeM.
Isolat~ons
from Streptomyces and Aeromonas species indicate
molecular weights of 50,000 and 105,000, respectively (5) (3)(20).
The Spider produces a chitobiase having a molecular weight
of lOB,000:!;.5,500 daltons I1hich lS very similar to the chitobiase
of the .'\eromonas species(;j,D).
It should also be noted that the
molecular weights of the chitobiases are very close to those
found cor the chitinases in those systems.
Purification procedures attempt to separate these two
enzyr:les which is difficult due to :nolecular :(eight similctrities
and the substrate of the chitobiase enzYr:le is the product of
the chi tinase enzyme s action.
1
Some chi tinase purif iea tion
procedures have led to over a five cold reduction in the level
of chitobia'3e activity found in the pur~:'ied extract.
Total
elimination of chitoblase activity has not oeen accomplished;
some researchers theorize that this activity lS intrinsic to
the chitinase enzyme (16) .
."ttempts have been made to isolate the chitinase and
chitobiase '2nzyme produced from yet another source, a oacterial
EF~a.
pond isolate identified by CDC as an
:his paper describes
the purification procedures used In the separation of these
enzymes and the methods er:lployed to deterr:line their molecular
weights.
Methods
Enz::'-me purification.
Cultures of Ef4a were :naintained on chitin
agar plates and gro\in in mineral salt chitin broths as descrioed
O'{ Sr:mcker (18).
Broth cultures "ere :nonitored throughout their
gro\ith and harvested by centrifugation at 10,OOOrpm once the
culture ent.ered the stationary phase.
:he supernate was then
subjected teo ....... ultrafiltration using a size exclusion filter
which alloved passage of proteins liith molecular weights less
than lL:,l)OU daltons.
The retentate was then purified by the
."f:inity Chromatography method descrioed by Jeuinaux(f3).
~ethod
rel~es
on the binding of the
c~itinase
This
enzyme to the
insoluble chitin particles followed oy decantation of the
un00uncl proteins.
The resuspended pellet is then allowed to
digest the chitin resulting in unbound chicinase.
Assays.
?rotein concentraion was determined using the Biorad
prot:eln dy,= and by aiJsorption at 28lJnr1.
Bovine SerUr:l Alour:lin as a standard.
BOch methods employed
3
Chitinase activity was deter!011ned by the method of ololano( 'l).
:;::n this reaction,
. Sml of enzyrc,e is reacted with 20,1.11 of tritiated
o
.
The reaction 1S stopped by the
chitin for one hour at 37 C.
addition of .2ml of a llJ% solution of TCA.
Ea·ch sample is then
filtered to remove any undigested particulate chitin.
The
filtrate 1S suspended in liquid scintillation fluid and counted.
The level of chitinolyitic activity is determined
~y
the
radioactivity of the soluble chitodextrins present within the
filtrate.
Chitobiase activity was determined colorimetrically by warming
0
.lml of enzyme and .lml of 'Ia~P04 buffer to 37 C followed by
the addit10n of 1ml of a solution of 20mg of p-Nitrophenyl-Nacetyl-B-D-glucosaminide dissolved in 100ml of water also
0
warmed to 37 0 C.
After inCUbation at 37 C for 10 minutes, the
reaction i'3 halted by the addition of 2ml of .1N [.ra Co . Absorbance
2
3
was measured at 420nm.
Electropho resis.
Polyacrylamide gel electrophoresis was carried
out under denaturing conditions using SDS.
polyacrylaclide and
l·.·~SDS.
The gels Here 12%
Protein samples were denatured by
suspending .3ml of sample in Iml of a buffer solution consisting
.5~jtris-Cl
of clml distilled water, 1ml
1.61'11 of a 10% SDS solution,
.8ml glycerol,
.4ml 2-"lercaptoethanol and
of a .'J5;£ solution of Bromophenol blue.
2Ur~'\
then run ',1ith a current of
pH b.8,
.2ml
The 1.5mm slabs were
per gel through the stacking gel,
increasing the current to 3llru'\ per gel once the separating gel
was reached. An electrode Duffer consisting of 3g Tris base,
1cl.4g of :lycine, and 19 of SilS, wa-s adjusted to pH S.3 1n one
liter of ,{ater was used for conduction of the current.
Gels
,,,ere stained with Commassie Brilliant Blue Rand destained by
acetic acid solution.
:~,
values ',Jere determined by dlviding
the distance traveled by the protein by the distance traveled
by the trctcking dye.
Th~
following standards \Vere used for
determination of Dolecular 'Jeights of the sample proteins:
Sovine seJ:-ur.l albuf:1in tJt),.,JUUc,
36,O(Jlld, ':arbonic anhydrase
~rypsln inhibitor
2U,100d,
2gg albunin 4S,OUUd,
2~,00l)d,
"IrYPslnugen
~nd ALactal~urnin
C;'lyceraldehyde-3-;E'
24,000d,
14,200d.
4
;~ll enzyme
~:amples
'..Jere reacted
\,;i th
the sample 0uf~er for a
periud of 3-·4 hours at ruuf.1 ter.tperature 'Jr :ur S r~,inutes in
a ~8oC water bath.
Column chroroatography \,as carried out using a column packed by
fSK EN 55 fractogel
gravity and also by a peristaltic pump.
was used as the packing material based on its size exclusion
separating abilities.
eluants.
Results
Ultrafiltration of Crude supernate followed by affinity
chromatography resulted in a 45 fold purification of the chitinase.
See fable 1.
'ehe majority of chitobiase activity vas decanted
~\
after the first centrifugation in the :'Iffinity 'Clrocedure.
lot of chitinase activity was also
los~
in this supernate.
It is interesting to note. crudes vhich vere frozen for a brief
period. about 5 days. prior to ultrafiltration. had very high
Crudes which vere
chitobiase activity in the first supernate.
only refrigerated for an equivalent length of tirc,e. shoved less
chitobiase activity overall.
Cultures grovn for longer periods
of time also exhibited lover levels of chitobiase activity.
Although in :'I crude form. the chitobiase activity found
within the affinity chromatography supernate 1 has been main-
tained for over 5 months by refrigeration with little loss in
activity.
~he
retentate sample used for trl.e source
for the affinity procedure also
exhibl~ed
a
(J:
ver~-
activity as well as a high chitinase activity.
crude enzyme
~ligh
chitobiase
~reezing
of this
sample for several months resulta:! in ~ loss of alroost all
chitobiase activity but a retention of
chitinase activity.
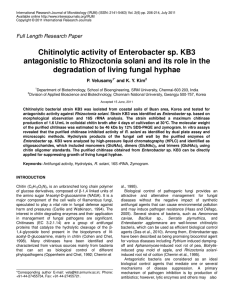
SDS electrophoretic studies of fresh retentate samples
exhibit numerous protein Dands \<.-ith
3.
-:ery pror:1inant band at 42,OOOd.
5
TaDle 1:
2nzyme Ptlrification
Sample
Protein
mg/ml
'v'olume
ml
13.8
54'l
66
45
4433
98
.162
3'l88
24620
124mg
94
3.26
•
.:..
j
Purity
Specific
1)8098
Ii 189
5.22
,~
460
'-8
Crude
RetentClte
Affinity
Sup. 1
~ctivity*
Total
Total
1
.12
.18
~=\ffinity
.00'l
lS
Product
44.8
*Activies are in thousands.
This band was also observed with electrophoretlc studies done
a fte r sevet:a 1 months 0 f fro zen sto rage.
The majority of other
bands observed when ;:he sample \las =resh wet:e either greatly
dimini~3hed
in their intensity or absenCe totally.
A band Clt ;:his
loc3.tion of 42,OOOd is also present in the :irst affinit..y chrorn-
atography supernate.
Application 0= the affini;:y supernate
to 3:; TSI{ flh 55 column elutes a number of chi;:inolytic fractions
which -crail off in their activity.
SDS electrophoresis of these
chitinolytic fractions also produced a band at the 42,OOOd
location. Fig.
1,
2.
,'in affinity chromatography product when :resh also exhibited
a band at the 42,OOOd position, as well as, one at 35,OOOd.
The 35,OOOd band was solely observed ln the affinity chromatography
products obtained from other crudes.
This band was also seen
in electrophoresis of the retentate and affinity supernate 1.
Iio\~-ever,
'i~-as
in -::hese t",,ro samples the 42 I 'JUOd band
h~{
::ar
12 rger.
To ensure that the 35,OOOd band was not solely an impurity
SODe how concentrated by the affinity chromatography procedure,
a product was placed on ;:he colur:m.
~Dsorption
the :rClctions revealed a single peak eluted.
at 28(Jnm of
Assays for chitinase
activit}' in the fractions comprising the peak were positive.
ColUl~n
chromatography Tw-as also utilized in .J.tter:1p-c.s to
isolute the chitobiase
affinit}-
Fig.
chro~atography
enzy~e.
~he
:irst supernate
0:
;:he
procedure was employed as a source
Like ::he chitinase enzYT:1e, the
c~itobiase
~or
enz=-':-:1e 'w·as
3.
~o
"
'll>
10
.
\
\.
/1)- .
~
~
5D+
~
• _4i.,c-..o
...+I
,.
- 1,,
_ 'fl, uoO
~e
~
~"
J
J
'_3!i,c-ec
jj~
•
I
i
~'.
!
-'., u...LI
\.
\
•
i
.1
. .3
.2.
'\
.5.~
.1
.'g
.9
/.0
"-'
iI.,
Fig.
Fig 1. SDS electrohporesis of a
retentate "hen fresh. The 42,000
band "as large and retained after
frozen storage. The 35, ODD band
was also exhibited at both tines.
.;-!
~
'-",
~
,0
-!.
...i.
~
-
'.
:i.•
.C).DI'6
I
.t·,,,,
;-
:
.,.1-
.Ol~
,a ,21
.
.
i
'"
;
1:
u
~
0
i'
-
;D
CI;l.
"
,u
'\
.01
"
8
!
"
.008
!>-i~
I (
2
• '.
'"
\
'0
"f
"r
)-..
.oolt:
!/v
\\
,.
1
19 3.
2. SDS electro~horesis of
the products of
affinity chromatography.
Product 4 exhicited bott
bands all others only
showed the 35,000 band.
1.co-l
I
.oc;..
\
00
,G',
(
I
go
f.u....h!~ 'lc\w,Q. ',."
11\.\
Colurnn
. . chromatography using .Ol:! ::a,PD. buf::'or to elute
,Clffl.nlty chromatography product tvo ::'rein the ~'SJ" i-ili ~:s ,colunn.
~
~
7
eluted
over several fractions.
The elutlon of chitobiase was
always prior to chltinase, but a region of overlap existed
~,
where fractions exhibited SOme activity of both enzymes. Fig
o.
Attempts to determine the molecular weight of the chitobiase
enzyme by subjecting fractions high In chitobiase activity to
SDS electrophoresis were unsuccessful.
20
~
co'
Ii>
~
l..
~.
19.
;)
1(,
3,
r-
0
n
,z8
""
,,"
-"
~
~
IZ
>
~
='- •
<I
fl'
jl
....
0
:r:
'0
,I.
~,
r
8
:'St<:
~i\";'
':) =)
n
."
. il
;.
~
~
u
t~
J;r,r
.z
chromatograph)
gravity
and .1H
~a2Po4 buffer. The
chltlnase and
ehitooiase enzyr:les
both trail over
several fractions.
:r
;..:.
Iq
Colucn
using 3
packed
column
~
,1l'O
;;
w
~
.
·'1
15
-1
Fig.
5.
,'1-
20
(~
~
,
~.~
chromatography
o~ affinity supernate
.:1>" " I ,.\Ja
'
,
ff er
.',,11.'>.
1COl) 7±
ou
2
-1
~,..s u.s-e-e, t fie co 1 umn
.3!
was still under aravitv I:
pc. eking, b~t had ~ been
~ ~ I.$"~
rE'packed Slnce the datil-:r:
91-esented in Fig ~
,zwas obtained.
~his
obtained better
,,5sE'par}Slation of the t,,.{o
enzymes.
.~(~clumn
1I
....I
1'\
,~
~
-Iq
~ 12.
~
i
J-
\/
J...\c
,,
t~
-
,
/,
/"
-
':t V
15
.00
I
~
/
~
...
,~
"v
.' ,.5
::.
-~
, ..c-
-,
,""
,~o
,
~
-'
Discussion
~olecular
weight studies of the various fractions obtained
during the chitinase purification procedure indicate the presence
of t\{O chitinases havlng molecular \,eights of cl2,JOO and 35,,)00d.
The absence of the 42,000d band in affinity chro[C.atography
products which were frozen prior to
elec~rophoresis
possibly
indicates this enzyme is more temperatGre sensitive or the 35,UOOd
band represents a chitinase with a greater
substrate.
speci~icity
for the
The later may be indicated since the 35,000d band
vas present In all affinity
chromatogra,~hy
produces obtained,
as I-Iell as, In the retentate and affinicY supern,,::e 1.
The 42,IJOOd
band also persisted in retentate samples I-Ihich had been frozen
for prolonged periods of time.
HOl-lever, the si=e of the band
Further evidence for Loe 42, clOO band
was some'i"hat diminished.
fr~ctions
being a chitinase comes from the chitinolytlc
obtained
by column chromatography of the affinity chromato1graphy super-
.1,·,
Using a Na po buffer at pH i :'it either 3.
or a . Jle!
2 4
concentratio~ =ractions exhibiting chicinolytic activity produced
nate 1.
bands at 42., JUDd \{hen
subjected to SDS electrophoresis.
An
early column fractionation attempt done \{hen the colunn had been
packed oy 9ravi ty, produced a fraction havin9 c!",i tinolytic
activity but I-Ihich produced bands at B3,OOOd, 55,OOOd, 42,SOOd
and 26,75Ud \{hen subjected to electrophoresis.
fraction arter several I-Ieeks of storage in the refrigerator
retained it,s chitinolytic activity but ,chen electrophoresied
exhlbited only the band at 42,000d.
evidence for
~~e
\.,-i thin other syster..s,
existence of several chitinases
molecular weights exists.
Research in'.-olving
indicar..ed the presence of
~aving
~r~:'itia
'title
siDilar
and
.)r more
chi tinases "i t:1 close molecular weights :.2) i. 3) .
Although unable to isolate the chitobiase enzyme by SDS
e leccrophoresi s,
the fact that it eluted in close
proximit~-
to
the chitinase enzyme on a size exclusicn column ':"::1dicates the
closeness of their molecular 'iv-eights.
-
techniques on
-3.
Bio-(~el
?
150 column,
Using gel
~iltration
-'.:.:'1e chitin::.se,
chitobiase,
'J
al:~ost
and chitcosan<lse were eluted
together \{ith molecul<lr
\{eights ranglng from ~0-5u,l)()uu '2).
,jitobiase <lnd chitinase
enzymes with close molecular '.eights have <llso been reported
for
A~ro~onas
(20).
The observed stabili'cy of the
wnditions is similar to yeasts.
yeast
c~itinases
s~able
remained
~l'ija
chitinase under frozen
Correa found that purified
for several months when frozen
at 0-SoC in a sodium citrate Duf~er with .02% sodium azide (5).
Chitotiase activity
~s
also retained for several months
if samples exhibiting this activity are refrigerated.
Freezing
leads to a great reductien in the activity of this enzyme.
For the conclusions dralin in this paper to be acceptable
\{i thout question,
electro!=,hore1OlC studies under nondenaturing
conditions need to be carried out.
have been c.nsuccessful.
AtteMpts at this so far
"resumablv
bec<luse the Droteins
"ere
1
-
denatured c.ue to overheating
0:
the glass surrounding the slab gels.
Disc electrophoretic studies sho\{ed no bands at alL possibly
due to errors in gel content or migration of the protein to the
anode or cc.thod causing elution from the gel.
A Streptomyces
griseus chitinase has been observed to separate into two different
chitinolytic proteins one migrating to the anode,
the cathodE' at pH 7.(12)
the other to
?ossibly, a similar Mechanlsm is happening
IJithin our system resulting in the absence of any bands
under nondEmaturing condill\.tions.
It liQuId also be interesting to measure the amount of activity
lost over time by freezing and refrigeration of highly chitinolytic
samples, highly chitobiotic
~ctivities
of both
enzy~es.
sample~
= ',iQuld
and samples containing
anticipate retention of
chitinase activity with loss of chitobiase activity in a more
purified sample than the retentate when the saMple is subjected
to
~reezinq.
Some data was acgulred which indicated the loss
of chitinolytic activity over a period of refrigeration,
it
liGule be interesting to cGmpare the loss of activity under frozen
conditions with this and ~lth retention of chitobiase activity.
~hitobiase may be
~he more
stable enzyme under the tnilder te~perature.
Acknowledger:1ents;
I thank Dr.
:,~arnes
for the use of his laboratory equipment and
Ball State Jniversity for their financial support by offering
undergraduate research grants.
-
Li te ru t ure c:i ted
,.
Berger, L. and 2eynolds, J . 1958.
"The Chltinase System
ofc_ Strain of Streptomvces griseus."
Biochemica et
Biophvsica Acta 29: pg 522-~34.
)
Beyer,:'1. and DieJemann. l-)85.
"The Chitinase System of
Streptomyces sp. ATCC ll238 and Its Significance for
cuncra 1 Ce 11 \'ia 11 Degrada tlon. "
,\polied :'ficrobiologv
and Biotechnology 23: pg 104-6.
3.
Charpentier, :·~adeleine and Percheon, Fracois. 1983.
liThe
Chi t,in-Degrading Enzyme System of a Strep~omyces Species."
IntE~rnational ,Journal of 3iochemistrv 15( 3) pg 280-92.
4.
Chen, AndreI; C; 2-layer, 2ichard; DeLoach, John. 1982.
Purification and Char~cterization of Chitin~se from
the Stable Fly Stomoxys calcltras."
Archives of Biochemistry
and Blophysics 216(1) 99 314-21.
5.
Correa" Julieta; Elango, ::arayanasany; and ?olachecK, Itzach
Cab:.b, Enrico. 1982. "Endochitlnase, a :'!annan ) .. ssociated
Enzyme :rom Saccharomyces cerevisiLie. II
,--,-:-curnal of Bioloaical
Chemistry 257(3) pg 1392-Ll/.
6.
Hara, S. 1982. IIPuri.:ication and Characteri::::ation of Chitinase
?roduced by Streptomyces erthraeus." in Chi tin and
Chitosan International Conference on Chitln and Chitosan
pg. 125- 1 3 () .
7.
Hortl;ig, :-[arc; REid, ,John; Ogrydziak, David. »84. "Genetic
Improvement of Chitinase Production by serratia :narcescens.
Chi-oin, Chitosan and Related Enzymes.
Ed. oy John
~ikakis.
Academic Press, Inc.
Orlando, =T
09 191-208.
8.
Jeunla-xx:, Ch2rles. 1966. "Chitinases." in :'lethods of
_:;.cademic Press: New- York, ~'JY P9 644-54.
Enzv:-:-,oloo"\' "1./018.
9.
>~olano,
II
~~esus;
Duran, .2\.ngel and C3iJib, Enrico. l~j~-;.
"_:;'
Rapid 3nd sensitive Assay for {:~litinase Csing Tr~tiated
Chitin.
~\nalyticd1 Jiochemistry 83. 99 ,)....;8-:5G.
II
1U.
'?olacheck, Itzach; =,,'uran, ~:;'ngel and \"::abiD,
:"::179.
I'~;;'n Endo-chitinase from L'hea.t Ger::l:
.:\"ctivi"c.y on :'J3.scent and ?reformed Chitin. II
Journal of
3iological Chemistrv . .254 (11 J.
pg. ~901- --;.
:'!olanu,
,~esus;
~nric{J.
11.
~\lomr:1sen,
~homas. l'-:18U.
"Chitinase and 3-~J-_':'cetylglucosaminidase
from t.:'le di';Jestive Fluid of the Spider CUDiennius salei.
3iochi~ic3 et Biophysic~ Acta t)12
pg 361-372.
II
12.
~~nreal,
Jai~e and Reese, Elwyn.
:969.
I'The Chitinase of
Serratia :':'"'.Clrcescens.
1,':::Clnadian .~ournal of ~·:icroDioloa\'
II
Ijl
;;9
,-jGlj-':Jb.
13.
:'luzzare'.li, R. ,\.1971. Chitin. ?ermagon Press: Elmsford, :JY
]:g. 3US.
14.
Uhtakara, Akira; Mitsutomi, Masau; Uchida, Yasushi. 1979.
"l?ur~~fication
?roper~ies of Chitinase from a
~aurnal of Fermentation Technology
and Some
Vibrio species."
pg. 1 b0- 77.
15.
Ortiz,
,Ii
'~.i
l:;illespie,
Berkeley,
R.
1972.
57(3).
!IAn Exo-B-N-Acetyl-
glucosaminidase from Bacillus subtilis B:
Extraction
and ?urification."
Biochimica et Biophvsica Acta 289 pg 174-86.
lb.
Ro,,,enaj Cabin, Enrico. 1982.
"Serratia marcescens
Chitinase:
One Step Purification and Use for the
Deter:-:1ination of '=~1itin.
L~nalYL.ical Biochemistrv 127
pg. 'clU2-12.
2oberts,
II
17.
Skujins, J; Pukite, .";:'lcLaren. 1970.
"Chitinase of
Streptomyces sp:
?urification and Properties."
Znzymologia 39(6). pg 353-37u.
lB.
Smucker, Richard. 1984. "Effects af Phosphate on Streptomyces
grlseus Production of Chitinase." in Chitin, Chitosan, and
Related Enzymes.
Academic ?ress, Urlando, FL pg 397-U6.
19. Tsuk3moto, Tsuyoshi;
~oga,
~orino-~atsushize,
i:TIot,Q, Taiji.
1984.
Daizo; Ide, Akio; Ishibashi, Takuya
~litsujii Yagishita, Kazuyoshi; and
"Purific3.t.ion and Some ?roperties
of Chitinases fro~ Yam, Discorea oooosita thumb."
."aricultural Bioloaical ChemistrY :18(4) pg 931-39.
20.
>:inorui l'~izushina, Keija; Amatatsu, Tomoko; Ando,
Aki,:azu; Yamashita, 'Iinoru. ~9B6.
"Purification and
Characterization of Chitinase and chitobiase Produced
by l\eromonas hydroohila." C:-ournal of ,:;eneral and Applied
Microbiology 32. pg 25-38.
":{abuki,