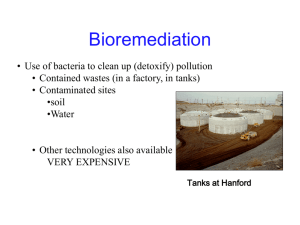
Conceptual Design of an In-Situ Sequential Anaerobic/Aerobic
advertisement

Conceptual Design of an In-Situ Sequential Anaerobic/Aerobic Bioremediation Scheme for Chlorinated Solvents in the Landfill-1 Plume at the Massachusetts Military Reservation by Farnaz Saboori Haghseta B.S. Chemical Engineering Massachusetts Institute of Technology, 1996 Submitted to the Department of Civil and Environmental Engineering in Partial Fulfillment of the Requirements for the Degree of MASTER OF ENGINEERING IN CIVIL AND ENVIRONMENTAL ENGINEERING at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY JUNE 1997 copyright 0 1997 Farnaz Saboori Haghseta. All rights reserved. The author hereby grants MIT permission to reproduce and to distribute publicly paper and electronic copies of this thesis document in whole and in part. -Farnaz Sabuoori Haghseta Department of Civil and Environmental Engineering May 9, 1997 Signature of Author 0 Certified by r I v I Fernando Miralles-Wilhelm Lecturer, Department o Civil and Environmental Engineering Accepted by - -~--~ V V Joseph SSussman Chairman, Departmental Committee on Graduate Studies OF 'C•H-••'LY JUN 2 41997 Eng. Conceptual Design of an In-Situ Sequential Anaerobic/Aerobic Bioremediation Scheme for Chlorinated Solvents in the Landfill-1 Plume at the Massachusetts Military Reservation by Farnaz Saboori Haghseta Submitted to the Department of Civil and Environmental Engineering on May 9, 1997 inPartial Fulfillment of the Requirements for the Degree of Master of Engineering inCivil and Environmental Engineering ABSTRACT Chlorinated compounds represent the most prevalent organic groundwater contaminants in the country. Until recently, bioremediation had not been considered a viable option for the treatment of chlorinated solvents. There is now sufficient theoretical and experimental information to suggest that in-situ bioremediation can be utilized to effectively degrade chlorinated compounds. A conceptual design is proposed for the in-situ bioremediation of tetrachloroethylene (PCE) and trichloroethylene (TCE). These contaminants have been detected in LF-1, a plume emanating from the Main Base Landfill at the Massachusetts Military Reservation in Cape Cod, Massachusetts. The conceptual design relies on the establishment of sequential anaerobic and aerobic biozones to reduce the local maximum PCE and TCE concentrations. These biozones are created by the injection of essential components of bioremediation, which include nutrients, growth substrates, and oxygen. The delivery of these agents to the areas of highest measured PCE and TCE concentration is necessary for the successful biodegradation of these contaminants in the LF-1 plume. Design parameters include the placement of injection wells to establish the aerobic and anaerobic biozones, the radii of influence of the wells, selected substrates and nutrients, and the concentrations and flow rates for the injected nutrients, oxygen, and substrates. In order to induce the biodegradation processes, the design requires the following to be injected at key locations in the plume: ammonium and phosphate as nutrients for the microorganisms, methane substrate for the aerobic microorganisms, methanol substrate for the anaerobic microorganisms, and oxygen for the stimulation of microorganisms in the aerobic biozones. The estimated total time for bioremediation of TCE and PCE to 5 pg/L (the Maximum Contaminant Level for TCE and PCE) ranges from five to fourteen years, depending upon the extent of contamination and the background conditions at the particular location in the plume. The bioremediation of chlorinated solvents is a promising treatment technology. However, the lack of full-scale implementation to date leaves many questions to be answered, particularly regarding the cost-effectiveness of the process. This conceptual design serves as a basis for the development of pilot tests which can more accurately assess the effectiveness and feasibility of this technology. Thesis Supervisor: Fernando Miralles-Wilhelm Title: Lecturer of Civil and Environmental Engineering ACKNOWLEDGEMENTS My deepest and warmest gratitude goes to my family and friends for their love and support, especially during the past five years at MIT. I would like to thank Professor Fernando Miralles- Wilhelm, whose kindness, direction, and valuable input was instrumental in the completion of my thesis. I would like to thank Professor David Marks and Shawn Morrisey for their constant support and encouragement, and for their invaluable advice and guidance during the hectic job search process. I am also grateful to my fellow LF-1 team members, Mia Lindsey, Roberto Le6n, Becky Kostek, and Mandeera Wagle, who made the experience of writing a thesis as enjoyable as it could possibly be. TABLE OF CONTENTS 1. INTRODUCTION ............................................................................................................................... 7 1.1 PROBLEM STATEMENT ........................................................................................................................... 1.2 OBJECTIVE...................................................................................................7 1.3 SCOPE OF THESIS..............................................................................................................................8 9 2. SITE DESCRIPTION ......................................................................................................................... 2.1 2.2 2.3 2.4 2.5 LOCATION AND HISTORY..................................................................................................................9 GEOLOGY . ................................................. HYDROGEOLOGY.................................................................................................................................. ENVIRmONMENTAL POLLUTION AT MMR .................................................. ....................... CHARACTERISTICS OF THE MAIN BASE LANDFILL............................................. 10 11 12 12 2.5.1 Landfilled Materials..................................................................................................................... 14 2.5.2 CurrentStatus of the Landfill....................................................................................................... 15 2.5.3 Contaminant Plume Resultingfrom the Landfill........................................... ............................ 15 2.5.3.1 Current Status of LF-I 17 ...................................................................................................................................... 18 3. BIOREMEDIATION ........................................................................................................................... 3.1 DEFINITION .................................................................................................................................... 18 18 3.2 MICROORGANISMS .................................................................................................................... 19 3.3 ENVIRONsMENTAL REQUIREMENTS ................................................................................................. .................................. 20 3.3.1 Nutrients............................................................................................... 3.3.2 Electron Acceptor ................................................................................................................... 21 ..................................... 22 3.3.3 Substrate............................................................................................ 3.3.4 Other Environmental Conditions............................................................................................24 3.3.4.1 Tem perature............................................................................................................................................24 .................................. . ........25 3.3.4.2 pH ....................................................................................................... 3.3.4.3 Concentration Range of Contaminant .............................................................................................. 25 26 3.4 IN- SITU BIOREMEDIATION............................................................................................................. 3.4.1 Advantages over Remedial Alternatives................................................................................. 26 ...... 28 4. CHARACTERIZATION OF THE LF-1 PLUME ........................... 4.1 4.2 4.3 4.4 4.5 PLUME CHARACTERIZATION .......................................................................................................... 28 SPATIAL DISTRIBUTION OF CONTAMINATION ................................................................................. 28 SPATIAL DISTRIBUTION OF NUTRIENTS .......................................................................................... 32 SPATIAL DISTRIBUTION OF OXYGEN .............................................................................................. 34 SPATIAL DISTRIBUTION OF SUBSTRATES........................................................................................36 4.6 SPATIAL DISTRIBUTION OF MICROORGANISMS............................................................................... 36 4.7 SPATIAL DISTRIBUTION OF TEMPERATURE AND PH................................... ....... ................ 36 5. SEQUENTIAL ANAEROBIC/AEROBIC DEGRADATION OF CHLORINATED SOLVENTS .37 5.1 5.2 5.3 5.4 BIODEGRADATION OF ORGANIC COMPOUNDS ................................................................ 37 40 TCE: AEROBIC COMETABOLIC DEGRADATION ....................................................... PCE: ANAEROBIC COMETABOLIC DEGRADATION.............................................................................44 47 COMBINATION OF ANAEROBIC AND AEROBIC PROCESSES.............................. .............. 6. DEVELOPMENT OF CONCEPTUAL DESIGN ..................................................... 6.1 6.2 6.3 6.4 6.5 BASIS FOR CONCEPTUAL DESIGN ................................................................................................... SUBSTRA-TE REQUIREMENT .................................................................................................... OXYGEN REQUIREMENT ..................................................................................................................... BACTERIA ............................................................................................ REQUIREMENTS........................... ............................................................................... NUTRIENT 4 48 48 48 49 50 51 6.6 ANALYTICAL MODELS ......................................................................................................................... 51 6.6.1 Injection Wells..............................................................................................................................51 ............................................. 55 6.6.2 Biodegradation.......................................................................... .................................. 57 7. CO N CEPTU A L D ESIG N ............................................................................... 7.1 PLACEMENT OF INJECTION WELLS ................................................................ 7.2 INJECTION PARAM ETERS .................................................................................. ................................. 57 .............................. 60 ..... 60 7.2.1 Injection Contents............ ........................................................................................ ......... ......... 61 7.2.2 Injection Flowrates and Concentrations ..................................................... 7.3 ESTIMATION OF BIOREMEDIATION TIME ........................................... ............................................. 66 8. CO N CLU SIO N ............................................................................................... ................................... 68 8.1 SUMMARY OF CONCEPTUAL D ESIGN ............................................. ................................................. 68 ................................... 69 8.2 POTENTIAL PROBLEM S ................................................................................ ..................................... 69 8.2.1 Clogging............................................................................................ 8.2.2 Intermediate Toxicity.................................................................................................................... 70 8.2.3 Competitive Inhibition.................................................................................................................. 70 8.3 RECOMM ENDATIONS FOR FUTURE WORK .................................................................. .................... 71 ............................................ 73 8.4 SUM MARY .......................................................................................... 9. REFEREN CES ................................................................................................ 10. A PPEND IC ES........................................................................................... .................................. 74 ....................................... 78 10.1 APPENDIX A: DISSOLVED OXYGEN CONCENTRATION DATA FOR LF-1 (OPTECH, 1996b) ................... 79 10.2 APPENDIX B: EXPLANATION FOR EQUATIONS 7-1 AND 7-2 ............................................ 83 10.3 APPENDIX C: SPREADSHEET CALCULATIONS FOR CONCEPTUAL DESIGN........................................84 LIST OF FIGURES FIGURE 2-1 FIGURE 2-2 FIGURE 2-3 FIGURE 2-4 FIGURE 3-1 LOCATION OF M M R.............................................................................. ............................... 9 GEOLOGY OF UPPER CAPE COD .................................................................................................. 10 LOCATION OF LANDFILL CELLS (CDM FEDERAL PROGRAMS, 1995).......................................... 13 MAP OF LF-1 PLUME INUPPER CAPE COD.................................................................................. 16 UTILIZATION OF THE ORGANIC CONTAMINANT FOR ENERGY AND CELL PRODUCTION (NATIONAL RESEARCH COUNCIL, 1993) ................................................................................................................... 21 FIGURE 3-2 ENZYME REACTIONS AS "LOCK AND KEY" (NYER, 1992).................................. ...... 23 FIGURE 3-3 TEMPERATURE RANGES FOR THREE CLASSES OF BACTERIA (NYER, 1992) ............................. 24 FIGURE 3-4 TYPICAL BIOREMEDIATION SCHEME IN THE SATURATED ZONE (KERR, 1994)............................26 FIGURE 4-1 PCE ISOCONCENTRATION CONTOUR MAP (CDM FEDERAL PROGRAMS CORPORATION, 1995)..30 FIGURE 4-2 TCE ISOCONCENTRATION CONTOUR MAP (CDM FEDERAL PROGRAMS CORPORATION, 1995).31 FIGURE 4-3 SPATIAL DISTRIBUTION OF DISSOLVED OXYGEN AND OTHER PARAMETERS (OPTECH, 1996a)35 FIGURE 5-1 IMPORTANT ELECTRON ACCEPTORS IN PRIMARY BIOTRANSFORMATIONS (KERR, 1995)...........38 FIGURE 5-2 PATHWAY FOR THE METHANOTROPHIC UTILIZATION OF METHANE (SEMPRINI ET AL, 1991 a)...41 FIGURE 5-3 EPOXIDATION OF TCE INCOMETABOLIC DEGRADATION (UNIVERSITY OF MINNESOTA BIOCATALYSIS/BIODEGRADATION DATABASE, 1997) .................................. 42 FIGURE 5-4 ABIOTIC HYDROLYSIS OF THETCE EPOXIDE IN COMETABOLIC DEGRADATION (UNIVERSITY OF MINNESOTA BIOCATALYSIS/BIODEGRADATION DATABASE, 1997)............................... ....... 43 FIGURE 5-5 HETEROTROPHIC DEGRADATION OF INTERMEDIATE AS THE FINAL STEP IN COMETABOLIC DEGRADATION (UNIVERSITY OF MINNESOTA BIOCATALYSIS/BIODEGRADATION DATABASE, 1997).....43 FIGURE 5-6 RELATIONSHIP BETWEEN DEGREE OF CHLORINATION AND AEROBIC AND ANAEROBIC DEGRADATION RATES (KERR, 1994) ................................................................................................. 45 FIGURE 5-7 IDEAL SCHEMATIC FOR SEQUENTIAL ANAEROBIC/ AEROBIC DEGRADATION OF PCE AND TCE.47 FIGURE 6-1 INJECTION WELL SCHEMATIC USING PARAMETERS INWELTY-GELHAR SOLUTION..................53 FIGURE 6-2 DIMENSIONLESS BREAKTHROUGH CURVE FOR CONTINUOUS INJECTION AND CONSTANT DISPERSIVITY (WELTY AND GELHAR, 1994)..................................................................................... 54 FIGURE 7-1 INJECTION WELL PLACEMENT.....................................................................................................58 FIGURE 7-2 CONCEPTUAL DESIGN OF INJECTION WELLS ........................................... ......... 59 FIGURE 8-1 BIOREMEDIATION WITH SEQUENTIAL ANAEROBIC/ AEROBIC BIOZONES ................................. 68 LIST OF TABLES TABLE 2-1 TABLE 3-1 TABLE 3-2 TABLE 4-1 LANDFILLED MATERIALS (CDM FEDERAL PROGRAMS, 1995) ................................................. 14 MICROORGANISM POPULATION DISTRIBUTION IN SOIL AND GROUNDWATER (NYER, 1992)....... 19 MOLECULAR COMPOSITION OF ABACTERIAL CELL (NYER, 1992)...........................................20 CONTAMINANT MAXIMUM CONCENTRATIONS AND MCLS (CDM FEDERAL PROGRAMS CORPORATION, 1995)........................................................................................................... ................ 28 TABLE 4-2 NITRATE VALUES FOR LF-1 (OPTECH, 1996a) ...................... .................... 33 TABLE 5-1 POTENTIAL FOR CHLORINATED ALIPHATIC HYDROCARBON BIODEGRADATION AS A PRIMARY SUBSTRATE OR THROUGH COMETABOLISM (KERR, 1994) ........................................................... 39 TABLE 6-1 DEGRADATION RATE ESTIMATES FOR PCE AND TCE ......................................................... 55 TABLE 7-1 SUMMARY OF INJECTION CONTENTS, CONCENTRATIONS, AND FLOWRATES.................. 66 TABLE 7-2 SUMMARY OF BIOREMEDIATION TIME ESTIMATES...........................................67 1. INTRODUCTION 1.1 Problem Statement The main base landfill at the Massachusetts Military Reservation (MMR) on Cape Cod, Massachusetts has served as MMR's primary solid waste disposal facility since 1941. Unregulated hazardous material disposal activities from 1941 to 1984 resulted in a groundwater contaminant plume migrating from the landfill, containing chlorinated solvents, such as tetrachloroethylene (PCE) and trichloroethylene (TCE), at levels above statutory primary drinking water standards. Remediation of the contaminant plume to concentrations below the Maximum Contaminant Levels (MCLs) is necessary in order to prevent potential adverse effects of the contaminants to human health and the environment. 1.2 Objective The objective of this thesis is to develop a conceptual design for the in-situ bioremediation of chlorinated solvents in the LF-1 plume. The design will rely on the sequential anaerobic/aerobic degradation of PCE and TCE. Design parameters will include the placement of wells to establish the aerobic and anaerobic biozones, the radii of influence of the wells, selected substrates and nutrients, as well as recommended concentrations and flow rates for the injected nutrients, oxygen, and substrates. The total time for remediation of the plume to the MCLs will also be estimated. 1.3 Scope of Thesis This thesis describes a conceptual design for the in-situ bioremediation of chlorinated solvents in the LF-1 plume at the Massachusetts Military Reservation, located in Upper Cape Cod. Chapter 2 provides an overview of the geology and hydrogeology of Upper Cape Cod. It also includes a description of the location, history, and pollution issues of the MMR, as well as an introduction to the main base landfill at MMR. Chapter 3 provides an overview of bioremediation, including basic definitions, the necessary components for successful bioremediation, and the advantages of in-situ bioremediation over common cleanup techniques such as pump-and-treat and air stripping. Chapter 4 is a detailed examination of the subsurface conditions at LF-1. The distribution of essential bioremediation components throughout the LF-1 plume is described. This chapter will provide the basis for the conceptual design. Chapter 5 explains the processes of aerobic and anaerobic degradation, and the utilization of these processes in concert to effectively degrade trichloroethylene (TCE) and tetrachloroethylene (PCE). Chapter 6 discusses the data gathered from various literature sources which are used to develop the conceptual design. Furthermore, the chapter describes the analytical equations utilized in the conceptual design. Chapter 7 provides the resulting conceptual design, including injection well placement, injection contents, injection flowrates and concentrations, and total bioremediation time. Finally, Chapter 8 will summarize the results of the conceptual design. Potential problems with the design are also discussed, along with recommendations for future work which could improve the feasibility and costeffectiveness of the design. 2. SITE DESCRIPTION 2.1 Location and History The Massachusetts Military Reservation (MMR) is located in the upper Western portion of Cape Cod, Massachusetts, as shown in Figure 2-1. The MMR occupies approximately 22,000 acres within the towns of Bourne, Sandwich, Mashpee, and Falmouth in Barnstable County. 0 Figure 2-1 Location of MMR Military activity began at the base in 1911, but the bulk of the activity has occurred since 1935, with operations by the U.S. Army, U.S. Navy, U.S. Coast Guard, U.S. Air Force, Massachusetts Army National Guard, and U.S. Air National Guard. The heaviest activity at MMR occurred from the 1940s, when U.S. Army activities intensified due to World War II, to the 1960s and 1970s, when the U.S. Air Force maintained heightened aircraft operations. (CDM Federal Programs Corporation, 1995) 2.2 Geology Three types of major geologic formations make up the stratigraphy of the Upper Cape: the Mashpee Pitted Plain (MPP), the Buzzard's Bay Moraine (BBM), and the Sandwich Moraine (SM), shown in Figure 2-2. .r IULLzII n.. 1- -.. C3......:.. Ll...:.. Outwa! Buzzard's E Moraine Figure 2-2 Geology of Upper Cape Cod The BBM and SM units have not been characterized in detail, and are reliably known only to consist of poorly sorted sand and gravel with localized deposits of silt and clay. (Masterson et al., 1994, 1996) To the northwest of the BBM lies the Buzzard's Bay Outwash (BBO). The BBO is thought to simply be a continuation of the MPP, with similar soil layers and properties. (Masterson et al., 1996) The MPP, which lies between the BBM and SM, consists of fine to coarse- grained sands that form a broad outwash plain. The bedrock in Upper Cape Cod lies approximately 300 feet below ground surface. (CDM Federal Programs Corporation, 1995) 2.3 Hydrogeology Upper Cape Cod, including MMR, has a single groundwater system which is an unconfmed aquifer. The aquifer, which ranges from 50 to 175 feet in thickness, is recharged by infiltration from precipitation. It is bounded by the Atlantic Ocean on three sides, with groundwater discharging into Cape Cod Bay on the north, Buzzards Bay on the west, and Nantucket Sound on the south. The water table, which resembles a mound, has its high point beneath the northern portion of MMR, near the site of the FS-12 plume. The hydraulic gradient across the MMR ranges from 0.0014 to 0.0018 ft/ft. (CDM Federal Programs Corporation, 1995) The hydraulic conductivity of the MPP outwash soils are as high as 380 ft/day. The hydraulic conductivity of the fine-grained sediments underlying the outwash are only 2 to 10% of that value. Most of the regional groundwater flow occurs in the upper, coarse sand and gravel layer. Horizontal flow velocities range from 1 to 3.4 ft/day. (CDM Federal Programs Corporation, 1995) 2.4 Environmental Pollution at MMR In 1982, the Department of Defense developed the Installation Restoration Program, whose purpose was to investigate and clean up environmental pollution at MMR facilities. The MMR was placed on the United States Environmental Protection Agency (USEPA) National Priorities List (NPL) in November 1989, indicating that the contamination at the reservation posed a serious threat to human health and the environment. Since May 1996, the Installation Restoration Program has been managed by the Air Force Center for Environmental Excellence (AFCEE). A series of remedial studies and activities are currently being conducted under the Program in accordance with the guidelines and procedures of the USEPA Superfund Program and the National Contingency Plan (NCP). To date, the Installation Restoration Program has spent over $130 million on investigation and cleanup at the reservation. Currently, 78 separate sites at the MMR are identified as environmental contamination sources, including fuel and chemical spill areas, landfills, coal yards, storm drains, and firefighter training areas, and 10 major groundwater contaminant plumes, including the main base landfill plume, known as LF-1. (Massachusetts Department of Environmental Protection, 1996) 2.5 Characteristicsof the Main Base Landfill The main base landfill covers approximately 100 acres in the southwestern section of the Massachusetts Military Reservation. It was used by the Army, Air Force, other military branches, and the towns of Bourne, Falmouth, and Sandwich as a place of disposal from 1940-1984. Unregulated and unmonitored disposal to the landfill was a common occurrence. (Kostek, 1997) The landfill can be broken up into 6 distinct cells: 1947, 1951, 1957, 1970, post1970, and Kettle Hole, as seen in Figure 2-3. d - ~WWlROM rT.C PHOMInAP") ccw~lm roavxncm 2 PISET RAbMA2LOCEFICIS (UGA.eMUOL Figure 2-3 Location of Landfill Cells (CDM Federal Programs, 1995) wei ,0 m 0SAL 1ffl The cells are named for the date in which they were last used. The post-1970 cell ceased to be used in June 1989. The Kettle Hole is a naturally occurring formation which was used for dumping waste for many years. The highest activity at the base occurred from 1940-1946, when it was under Army control and from 1955-1970, when it was under Air Force control. It is believed that these times of highest activity coincided with the highest rate of waste generation and disposal. All of the information about the landfill was obtained from interviews of base employees during the preliminary records search (Hazwrap, 1987). This is the only source of information about the landfill since no written records were kept about waste disposal methods. 2.5.1 Landfilled Materials Many of the base activities generated wastes. The main wastes that were produced and believed to have been disposed of in the landfill are listed in Table 2-1. Table 2-1 Landfilled Materials (CDM Federal Programs, 1995) Wastes General refuse Herbicides Transformer oils Blank small arms ammunition Paint thinners DDT powder Municipal sewage sludge Fuel tank sludge Solvents Fire extinguisher fluids Paints Batteries Hospital wastes Coal fly ash Possible live ordnance These wastes are believed to have been disposed of by the trench method for landfilling, as indicated by recent waste disposal practices and the surface topography at the older cells. The waste was buried in linear trenches and covered daily with the soil that was excavated from the trench. (CDM Federal Programs, 1995) 2.5.2 Current Status of the Landfill Since 1989, when waste disposal in the post-1970 cell was ceased, all MMR waste has been sent to the SEMASS incinerator in Rochester, MA for disposal. Small quantities of demolition debris are still disposed of in the Kettle Hole. (CDM Federal Programs, 1995) Currently, the landfill is mostly covered with vegetation native to the area. The 1970, post-1970, and Kettle Hole cells have been capped to prevent any further contamination from migrating from the landfill. An alternate closure has been recommended for the 1947, 1951, and 1957 cells. Alternate closure involves leaving the waste in the cells intact beneath a vegetative cover and providing groundwater monitoring and landfill surface maintenance for thirty years. Post- closure management plans are being developed for these cells. (Leon, 1997) 2.5.3 Contaminant Plume Resulting from the Landfill The landfill was identified as a potential contaminant source in 1979 when sampling at a drinking water well downgradient of the landfill demonstrated volatile organic carbon (VOC) concentrations exceeding drinking water standards (Metcalf & Eddy, 1983). This discovery led to further sampling for the assessment of the nature and extent of the contamination due to the landfill. Some sampling wells were placed within the boundaries of the landfill, but not directly within any of the cells, due to the possible presence of buried live ordnance in the landfill. The sampling determined that there is a VOC plume that extends to the southwest of the landfill, beyond the MMR boundaries, as shown in Figure 2-4. Figure 2-4 Map of LF-1 Plume in Upper Cape Cod The plume begins at a depth of 40 feet below the landfill. In October 1995 the LF-1 plume was measured to be approximately 16,500 feet long in the southwest direction, 6000 feet wide, and over 90 feet thick. By this same time over 22 billion gallons of groundwater had been contaminated by the plume. The velocity of the LF-1 plume is estimated to be 544 feet/year. The main VOCs of concern in the plume are trichloroethylene (TCE) and tetrachloroethylene (PCE). Carbon tetrachloride (CC14) has also been found in many of the wells, but not on a consistent basis. The VOC plume exceeds the Maximum Contaminant Levels (MCLs) of 5 parts per billion (ppb) for groundwater. 2.5.3.1 Current Status of LF-1 A groundwater extraction, treatment and reinjection (ETR) system was at 60% design for the LF-1 plume in January 1996, but was put on hold due to unacceptable groundwater pumping rates. The ETR pilot test system had been proposed by AFCEE for the southern lobe of LF-1, with a scheduled construction startup in June 1997 and system startup in March 1998. This project has now been indefinitely delayed. A recirculating well pilot test system was proposed by AFCEE for the northern lobe of LF-1. It was scheduled for construction startup in December 1996 and system startup in May 1997, but this project has also been indefinitely delayed. (Massachusetts Department of Environmental Protection, 1996) Currently, no remedial actions are being implemented for the LF-1 plume. The nature and extent of the contamination require that a remediation system be installed as quickly as possible, to prevent further spreading of the plume. This thesis examines an insitu bioremediation design scheme as an alternative to ETR and recirculating wells for the remediation of LF-1. 3. BIOREMEDIATION 3.1 Definition Bioremediation involves the use of microorganisms to convert contaminants to less harmful species in order to remediate contaminated sites. Microorganisms usually degrade organic compounds to obtain energy that is conserved in the carbon-carbon bonds of the compounds, and to use the organic carbon as building blocks for new microbial cells. 3.2 Microorganisms Bacteria, viruses, fungi, algae, protozoa, and metazoa are among the microorganisms that exist on earth. Microorganisms can be classified into two general categories of cell structure: eucaryotic or procaryotic. Eucaryotic microorganisms, which have a relatively complex cell structure, include fungi, algae, and protozoa. With a more simple cell structure, prokaryotic microorganisms include bacteria. Protozoa and metazoa do not have important degradative roles, and are not usually considered for bioremediation applications. (Nyer, 1992) Fungi comprise a diverse group of organisms, such as molds, mildews, and mushrooms, but are predominately located in soil or dead plant material. Bacteria have been on earth for 3 billion years. They are the most prevalent and diverse microorganisms on earth. Approximately 85% of bacterial existence to date occurred before the continental plates began to separate, thereby providing the organisms with a long time to evolve, adapt, and disperse. (National Research Council, 1993) Bacteria have excellent adaptation and survival capabilities, which make them an ideal candidate for bioremediation, since the contaminated sites often have less than optimal conditions. These characteristics help to explain why bacteria usually outnumber the other organisms found in soil and groundwater, as shown in Table 3-1. Table 3-1 Microorganism Population Distribution in Soil and Groundwater (Nyer, 1992) ORGANISM _ Bacteria Fungi Algae Bacteria Bacteria POPULATION SIZE :_ _(TYPICAL) Surface Soil (cells/g soil) 0.1-1 billion 0.1-1 million 10,000-100,000 Subsoil (cells/g soil) 1,000-10,000,000 Groundwater (cell/mL) 100-200,000 POPULATION SIZE (EXTREME) > 10 billion 20 million 3 million 200 million 1 million 3.3 Environmental Requirements In order for biodegradative processes to occur, the microorganisms require the presence of certain nutrients, electron acceptors, and substrates. Several other environmental conditions also affect the effectiveness of the microorganisms' degradative capabilities. 3.3.1 Nutrients The molecular composition of the bacterial cell is an indicator of the bacteria's requirements for growth. Approximately 80-90% (by weight) of the cell is composed of water, which is therefore the main nutrient for the cells. Table 3-2 shows that the solid portion of the cell is composed mostly of carbon, oxygen, nitrogen, hydrogen, and phosphorus. Table 3-2 Molecular Composition of a Bacterial Cell (Nyer, 1992) Element Percentage of Dry Weight Carbon Oxygen Nitrogen Hydrogen Phosphorus Sulfur Potassium Sodium Calcium Magnesium 50 20 14 8 3 1 1 1 0.5 0.5 Chlorine 0.5 Iron Others 0.2 -~0.3 The microbes derive carbon from the organic compounds they destroy. Oxygen and hydrogen are provided by the groundwater. The other major nutrients required by the microorganisms for growth and energy are nitrogen and phosphorus. The most common sources of nitrogen are ammonia and nitrates. Ammonia can be directly utilized for amino acid synthesis, while nitrates are reduced to ammonia and then assimilated into the synthesis. Phosphorus, mostly in the form of phosphates, is used by bacteria to synthesize phospholipids and nucleic acids, as well as for the energy transfer reactions of adenosine triphosphate (ATP). An adequate supply of these nutrients must be available to the microorganisms for the bioremediation process to be effective. 3.3.2 Electron Acceptor In order for microorganisms to transform nutrients to forms that are useful for incorporation into cells and synthesis of cellular material, microorganisms need a source of energy. Microbes derive much of their energy from oxidation- reduction reactions, where a transfer of electrons from an electron donor to an electron acceptor occurs, thereby resulting in the release of energy. Figure 3-1 demonstrates the common utilization of organic contaminants as the electron donor. Organic Contaminant Figure 3-1 Utilization of the Organic Contaminant for Energy and Cell Production (National Research Council, 1993) There are a number of different compounds that can act as electron acceptors, including oxygen (O2), nitrate (NO'), iron oxide (Fe(OH)3), sulfate (SO4"2), and carbon dioxide (CO 2). Aerobic bacteria can only utilize oxygen as an electron acceptor, while anaerobic bacteria use the other compounds as electron acceptors. Oxygen is the optimal electron acceptor because microorganisms can derive the most energy from oxidation-reduction reactions with oxygen. Sulfate and carbon dioxide provide the least amount of energy to the microbes. Figure 5-1 illustrates the difference in electron acceptors. 3.3.3 Substrate Electron donors which participate in microbe-catalyzed oxidation-reduction reactions are also called substrates. Reactions are catalyzed when the substrate collides and binds to the active site of an enzyme. Enzymes are proteins, produced by the bacteria, which perform highly specific reactions. A bacterial cell contains approximately 1000 enzymes. (Nyer,1992) Substrate activation allows for the enzyme to react and produce products and restore the enzyme. Enzyme reactions can be understood with the lock and key representation. Substrate Enzyme: Active Site on Surface Free Enzyme Figure 3-2 Enzyme Reactions as "Lock and Key" (Nyer, 1992) Figure 3-2 shows how only an enzyme with the right shape can function as a key for the oxidation-reduction reactions. Although it is not shown on Figure 3-2, the fit between the enzyme and substrate must be three-dimensional and precise. When microorganisms consume a compound to satisfy its energy and cell growth needs, the compound is considered a primary substrate. This is the usual process for organic decomposition in nature. However, in the environment, the microorganisms will not directly utilize organic compounds that do not provide them with significant energy for growth. However, some of these organic compounds can be biotransformed by microorganisms as secondary substrates through a process known as cometabolic transformation, which is explained in sections 5.2 and 5.3. 3.3.4 Other Environmental Conditions There are other factors which will affect the biodegradation process, including temperature, pH, and the concentration range of the contaminant. 3.3.4.1 Temperature Temperature is an important microorganism growth factor. Every microorganism has a minimum temperature below which growth does not occur, a maximum temperature above which the proteins and cellular components of the microbes will become inactivated, and an optimum temperature at which growth is the most rapid. There are three categories of bacteria, thermophiles, mesophiles, and psychrophiles, which thrive at different temperatures, as shown in Figure 3-3. Microorganisms that are cultivated in aquatic environments are mostly mesophiles, which grow in a range of 10"C to 45TC. 3.0 4 1.0 Growth Rate (Generations/hr) 0.3 .0. 10 20 30 40 50 60 Temp•rmtureoc Figure 3-3 Temperature Ranges for Three Classes of Bacteria (Nyer, 1992) 70 When designing a bioremediation scheme, it should be noted that since these microbes can grow at a wide range of temperatures, large temperature fluctuations pose a greater threat to biological activity than the maintenance of a particular temperature. 3.3.4.2 pH The optimal pH for microbial growth is dependent on the specific microorganisms and their biological pathways. Many enzymes can only be produced by microbes in the proper pH range. Aerobic microorganisms are usually able to tolerate a wider pH range, whereas many anaerobic bacteria grow efficiently in a very narrow pH range. The optimal pH for bacteria in groundwater is between 6.5 and 7.5, values which are close to their intracellular pH. (Nyer, 1992) 3.3.4.3 Concentration Range of Contaminant When contaminants in the groundwater are secondary substrates, whereby the contaminant and the primary substrate are degraded by the same enzyme, the contaminant and primary substrate can compete for the enzyme's active sites. This process, known as competitive inhibition, will occur if the contaminant concentration is too high. The maximum allowable concentration for no inhibition depends on the specific compound, but ranges between 10 mg/L and 100 mg/L for chlorinated compounds. (Kerr, 1994) High primary substrate concentrations have also been found to significantly inhibit the degradation of contaminants, as explained in section 8.2.3. 3.4 In- Situ Bioremediation In-situ bioremediation of contaminants in groundwater allows for the on- site destruction of contaminants. The in-situ system, depicted in Figure 3-4, typically consists of extraction wells, where the extracted groundwater is treated with substrate. electron acceptor, and nutrients, and injection wells which are used to reinject the treated water. ScOur= GrCmd-Waft Tratment Nutrients r I r M Ionng Well -i ll Gound.WaLer Gradt 'W -- W Nknimoing Well Iw m m Recovery Well mrýW Soil Contammation GWW Figure 3-4 Typical Bioremediation Scheme in the Saturated Zone (Kerr, 1994) 3.4.1 Advantages over Remedial Alternatives In-situ bioremediation has the potential to provide a more effective and inexpensive alternative to traditional remediation methods. It can completely destroy the contaminant rather than transfer it to another medium, which is common in re=ediation technologies like air stripping. It typically requires less treatment time than pump-andtreat remediation. It also offers the opportunity to use the subsurface as a bioreactor, whereby the necessary bioenhancers are injected into the contaminated groundwater and the remediation occurs in the aquifer, which eliminates the need to pump large quantities of water to the surface. 4. CHARACTERIZATION OF THE LF-1 PLUME 4.1 Plume Characterization In order for bioremediation of the contaminants to occur, microorganisms require the presence of certain substances and conditions. Examination of the characteristics of the LF-1 plume will determine what additional substances and conditions are needed to stimulate or enhance bioremediation. 4.2 Spatial Distributionof Contamination The two main contaminants of concern at LF-1 are tetrachloroethylene (PCE) and trichloroethylene (TCE). As Table 4-1 shows, both contaminants are present at levels which significantly exceed Maximum Contaminant Levels (MCLs). Table 4-1 Contaminant Maximum Concentrations and MCLs (CDM Federal Programs Corporation, 1995) COMPOUND MAXIMUM DETECTED (pg/L) PCE TCE 65 64 MCL _1_gfL) 5 5 The TCE and PCE isoconcentration contour maps (Figures 4-1 and 4-2) provide the spatial distribution of the contaminants in the LF-1 Plume. The PCE plume contains three areas of highest measured concentration, located at monitoring wells GB22, MW35, and MW103, with average concentrations of 30 jg/L, 48 pLg/L and 65 pg/L, respectively. The TCE plume contains three areas of highest measured concentration, located at monitoring wells MW37A, MW38, and MW31, with average concentrations of 19 lag/L. 26 ýtg/L, and 64 ipg/L, respectively. ~LY 1"-500 -)---C •1; n IR'IM 4p brr * FI~ - nnrsurm mpr y fiu ra0mr w Figiurc 4-1 II'CE Isoconlccntrationl Contour Malp (CI)M Fcdcrnl Progrlams Corporation, 1995) 30 KEY NOTES: S4LC1TD CItOA#4ALtD rr~ gIjM OVERHEAD ELECTRIC POWERLINES ~~itLxDw I' -, alawnm pe inmcoeen s* e Figure 4-2 TCE Isoconcentration Contour Map (CDM Federal Programs Corporation, 1995) 31 4.3 Spatial Distributionof Nutrients The only available nutrient data demonstrates the distribution of nitrate throughout the plume. Since no phosphate data is available, it will be assumed that the plume is depleted of phosphorus, for design purposes. Further design modifications would require a detailed analysis of background phosphorus concentrations in the plume. Most of the plume has a sufficient supply of nutrients in the form of nitrate, with only ten, out of seventy four, monitoring wells measuring non-detectable levels. The detectable concentration of nitrate is shown in Table 4-2 to range between 0.07 mg/L (at MW-22) and 1.20 mg/L (at MW-35). This conceptual design assumes that the background concentration of nitrate remains constant, so that a nitrogen source is only added to locations where the background levels are below the requirements of the bacteria, which are indicated in section 6.5. Table 4-2 Nitrate Values for LF-1 (OPTECH, 1996a) _ Nitrated Site Well Nwnber Nttrite (mOtI) Up'grdient f Fence I LF.I MW.707 0.09 MW-17A MW.17B MW-19 Well Fence 1.1 MW-9 I..I MW-20A LE-1 MW.208B IE-I MW-O0C LF-I LFI.1 MW-20Z CS-9 : MW-I MW-35 CS-Ia MW.36 CS-la MW-61 LF I MW.-71 LF-I MW-705 LF. ND 0.27 0.10 0.60 0.22 0.85 0.09 0.23 ND ND 0389 Well Fence 1.2 CS-10 L.lI MW-48 MW.701A Nitram/ Nltrite (m•nl) 0.25 0.25 LF1CS-10 CS-10 CS-10 LF-I LF.I LF-I LF-1 LF.I WT-25 MW-42A MW-42B MW42C MW.103A MW-103B MW-t03Z MW.104A MW-104B 0.46 ND 0.42 033 0.46 0.23 0.14 0.60 0.54 Between Fences 2 & 3 LF-I LF-I LF-I MW-25 MW-25A MW-26 LF-. MW-28Z LF-I MW-29 LF-I MW.31A LF-I MW.31B LF-I MW.31C LF.I MW-601A LF-I MW-601B LF. MW.601C LF-I MW-602B LF.I MW-602C Site se Well Nmiber 0.J8 0.96 0.32 030 0.13 0.23 0.03 0.08 0.70 0.47 0.59 1.10 0.67 0.19 LF*I LF-I LF-I LF-I LF-I LF-I LF-1 LF-I LF-I LF-I LF-I GB-20 MW-38 MW-38A MW-38Z MW.39A MW-40 MW-40A MW-l4 MW-.4 MW-S5 MW.47 LF.I LF-I II I- MW.22A MW-23 MW-24A MW-24B MW-32 MW-33 MW-34 0.07 0.77 ND ND 0.60 0.48 0.78 .F-I LF-I LF-I LF-I LF.I LFl.I , ,I MW.35 MW-36B MW-37A MW-43 0G-22 WT-26 1.20 0.92 030 0.80 0.21 ND I i r 0.36 ND 0.72 0.18 0.29 0.23 0.75 029 032 0.71 0.47 MW-6 MW-46A 0.62 0.13 MW-50A MW-50B MW-51 MW-52 MW-53 0.62 0.40 0.62 059 0.37 037 Well Fence 5 LE-I LE-I LF-I LF-I LF-l LF-I MW.54 Other Wells LI.1 LF-I .LF-I LF.I LF-I LF-I LF-I (mi,) Between Fences 4 &5 Well Fence Well Fenc 2 itrtt Well Fence 4 Well Fence 2 (co•.) Wel Fence I LF-I LF-I LF-I Site Well Nmnber __ LF-I LF-I WT-28 WT.29 4.4 Spatial Distributionof Oxygen The plume overall is fairly aerobic, with values ranging from non-detectable to approximately 10 mg/L. For design purposes, the wells which do not have measured dissolved oxygen levels will be considered anaerobic, however a more detailed design will require the assessment of oxygen levels at those locations. The spatial distribution of dissolved oxygen is illustrated in Figure 4-3; additional dissolved oxygen data from monitoring wells is shown in Appendix A. Figure 4-3 Spatial Distribution of Dissolved Oxygen and Other Parameters (OPTECH, 1996a) 35 4.5 Spatial Distributionof Substrates The only primary growth substrate that has been detected in the plume is toluene, which was present in low concentrations (0.15 pg/L to 7.1 gg/L) at a few wells. (CDM Federal Programs Corporation, 1995) Other substrates, including phenol, were not detected. Based on this information, the design will be developed with the assumption that there is no primary substrate present in the plume. 4.6 Spatial Distributionof Microorganisms In this bioremediation design of the LF-1 plume, it is assumed that there is a viable microbial population in the groundwater, and the bacteria are the main source of microorganisms in the aquifer. The assumption of viability is valid, given that historically less than 1% of sites have been found to lack a viable microorganism community capable of biodegrading a wide range of hydrocarbons. (Kerr, 1994) 4.7 Spatial Distributionof Temperature and pH The temperature in the plume ranges between 10*C and 20*C, which is within the temperature range recommended for optimal bioremediation. (See Section 3.3.4.1) The pH of the plume ranges between 5.5 and 7, which is in the optimal range for the microorganisms to perform the biodegradation processes. (See Section 3.3.4.2) Figure 4-3 illustrates the spatial distribution of temperature and pH in the LF-1 plume. 5. SEQUENTIAL ANAEROBIC/ AEROBIC DEGRADATION OF CHLORINATED SOLVENTS 5.1 Biodegradation of Organic Compounds Organic compounds can be biotransformed by microorganisms through two main processes: (1) primary substrate utilization or (2) cometabolism. When the organic compound is used as a primary substrate, the microorganism consumes the compound because it derives food, in the form of organic carbon, and energy, in the form of reduction-oxidation reactions, from the process. The microorganisms convert the compound into harmless products, primarily CO,, cell mass, inorganic salts, and water. The microorganisms are driven to degrade the organic compounds due to their need for utilizing reduced forms of nutrients for nutrient incorporation into cells and for the synthesis of cell polymers. The reduction of nutrients requires energy and electrons, which the microorganisms acquire from the degradation of organic compounds. When the organic compound is utilized as a primary substrate, it becomes the electron donor in a reduction-oxidation reaction involving a terminal electron acceptor, which can differ depending on whether or not the conditions are aerobic or anaerobic, as demonstrated by Figure 5-1. 1.0 C 0 Aerobic (Oxygen as ctionn Acceptor) ( 0o,+4H+ +4.- - 2H 2 2NO; + 12H+ +10e -+ N2 + 6H2 0 >0.5 - MnO2(s)+HCO, +3H +2e I< - IMnCO - C= C 0 3(s)+ 2H20 C E = So + 0 FeOOH(s) +HC +2H+ 2H' - -+ C= il FeCO 3 (s) + 2H 2 0 - SO' + 9H+ + 8e HS' + 4H 0 CCO. + 8H+ + 8es -+ CH4 + 2H2 TypicalPrinmary Substrates (Elecron onors) 2C02 +8H +8e- - CH 3COOH +2H 20 2H* + 290-+ H2 Figure 5-1 Important Electron Acceptors in Primary Biotransformations (Kerr, 1995) This transfer of electrons provides energy for the reduction of nutrients and the resulting synthesis of cellular material or incorporation into cells. The following is an example of an organic compound, benzene, being used as a primary substrate for growth by microorganisms, which was illustrated in Figure 3-1: C6H6 + 7.5 0 2 C6H 6+ 1.5 HC03- + 1.5 NH44 6 CO2+ 3 H 20 1.5 C5H70 2N + 1.5 H20 In the above example, 02 is the terminal electron acceptor and CsH 70 2N is the synthesized cellular material. A portion of the organic contaminant is used as a primary energy source that is converted to end products, and another portion is used to synthesize the cellular material. Few chlorinated aliphatic hydrocarbons are utilized by microorganisms as a primary growth substrate. Table 5-1 illustrates that dichloromethane (CH2CI2), 1-2 dichloroethane (CH 2C1CH2C1), chloroethane (CH 3CHC1), and vinyl chloride (CH2=CHCI) are the few exceptions which have been shown to be available as primary growth substrates. Table 5-1 Potential for Chlorinated Aliphatic Hydrocarbon Biodegradation as a Primary Substrate or Through Cometabolism (Kerr, 1994) Primary Substrate Cometabolism Compound Name Chemical Aerobic Anaerobic Aerobic Anaerobic Potential Potential Carbon Tetrachloride Chloroform Dichloromethane Trichloroethane 1,1 Dichloroethane Formula CCI 4 CHCl 3 CH2 Cl 2 CH 3 CCl 3 CH 3 CHCI2 Potential XXXX XX Yes Yes Potential 0 X XXX X X 1,2 Dichloroethane CH2CICH 2CI Yes X X Yes XX XXXX XX Chloroethane CH 3CH 2 CI Tetrachloroethylene CC12=CC12 0 XXX Trichloroethylene Dichloroethylene 1,1 Dichloroethylene CHCI=CC12 CHCI=CHCI CH2=CCI2 XXX XXX X XX XX XX Vinyl Chloride CH2=CHCI XXXX X Yes O:No real potential, X-XXXX: Little to excellent potential, Bold: PCE and TCE. These findings suggest that only the less chlorinated compounds are used by microorganisms for energy and growth. The majority of chlorinated aliphatic hydrocarbons are biodegraded by the second process, known as cometabolic degradation. 5.2 TCE: Aerobic Cometabolic Degradation Cometabolic degradation occurs when a compound is inadvertently degraded by an enzyme that is produced by the microorganism for other purposes. It is a fortuitous process since the microorganisms derive no direct benefit from the degradation, and it might even be harmful to them. Many common chlorinated contaminants in the groundwater can be cometabolically biodegraded. Even though trichloroethylene (TCE, CCI,=CHCI) can not serve as a primary growth substrate, it can be cometabolically transformed by methanotrophic bacteria, microorganisms that oxidize methane for energy and growth. The reaction occurs in an aerobic environment, where TCE is the electron donor and 02 is the terminal electron acceptor. When the primary growth substrate methane is available to the bacteria, the bacteria will produce an enzyme called methane monooxygenase (MMO) which catalyzes the initial oxidation of methane to methanol, as shown in Figure 5-2. Energy is derived from that reaction and new enzymes are formed to drive the oxidation process to continue, whereby formaldehyde, formate, and ultimately carbon dioxide is produced. CELL CONSTITUENTS NADH E t2 £1 4 CH CH NAD N 1HCHO " 7 XH X NAD EA N CO: * H20 HC00Hn NADH NA NAD NADHH El - METHANE MONOOXYGENASE (E :METHANOL DEHYOROGENASE OR ALCOHOL OxiDASE Et : FORMALDEHYDE DEHYDROGENASE 4 : FORMATE DEHYOROGENASE x x A PROTON AND ELECTRON CARRIER Figure 5-2 Pathway for the Methanotrophic Utilization of Methane (Semprini et al, 1991a) The MMO enzyme has a low substrate specificity and is therefore capable of initiating the oxidation of many halogenated compounds, including TCE. MMO is a dinuclear iron enzyme which catalyses the conversion of TCE into an epoxide by breaking its own oxygen-oxygen bond and inserting one oxygen molecule into a carbon-hydrogen bond. Figure 5-3 demonstrates the epoxidation process of TCE by MMO. 'YFe, IYFe- '10 C•I j IIIFe, OH IIIFe' IVFeOH 0 OH HCI IIIFe, C III'Fe H IIIF e'O FeH C I •poxidation CI iIiFe •' OH H CI Figure 5-3 Epoxidation of TCE in Cometabolic Degradation (University of Minnesota Biocatalysis/Biodegradation Database, 1997) The epoxidation of TCE by MMO is followed by the abiotic hydrolysis of the highly unstable epoxide into nonvolatile compounds, as shown in Figure 5-4. Cl 0 CI O 5% Dichloroacetate CO methane monooxygenase 0 53% C T E epode TCE eposide 2 H2 O0 2 M11 35% Formate + 0 0 5% Glyoxylate Figure 5-4 Abiotic Hydrolysis of the TCE epoxide in Cometabolic Degradation (University of Minnesota Biocatalysis/Biodegradation Database, 1997) Finally, the compounds are biotically degraded to CO2, chloride, and water. In other words, microorganisms derive energy from these intermediates by forming specific enzymes to degrade them. Figure 5-5 demonstrates the pathway of one of the intermediates. The ability of the bacteria to obtain energy from these compounds indicates that they might serve as substitutes for methane to drive the cometabolism of TCE. o Formate formate dehydrogenase NAD + CO2 NADH Figure 5-5 Heterotrophic Degradation of Intermediate as the Final Step in Cometabolic Degradation (University of Minnesota Biocatalysis/Biodegradation Database, 1997) Most of the work seen in literature and experiments to date has been devoted to the cometabolism of TCE using methanotrophs. However, in addition to methaneoxidizing bacteria and the intermediates described above, there are numerous other types of substrates that have been found to catalyze the cometabolic degradation of TCE and other chlorinated compounds, including phenol, propane, ethylene, toluene, cresol, ammonia, isoprene, and vinyl chloride. (Kerr, 1994) Several recent research projects have determined that phenol-utilizing bacteria are more effective than methanotrophic bacteria for the cometabolic transformation of TCE. (Kobus, 1996) In a similar manner to methane, phenol (hydroxybenzene) is oxidized to dihydroxybenzene, which is transformed to products used for cell synthesis and energy and, ultimately, to CO 2. For cometabolism to occur, an active population of microorganisms having the cometabolizing enzymes must be present. Therefore it is important to have the proper growth substrate available to the microorganisms in order for the enzymes to be produced. 5.3 PCE: Anaerobic Cometabolic Degradation Aerobic cometabolic degradation is not effective on saturated chlorinated aliphatic hydrocarbons like tetrachloroethylene (PCE, CC12=CC1 2 ), where the organic compound is completely chlorinated. The oxygenase enzyme produced for cometabolism reacts with unsaturated chlorinated compounds like TCE by adding oxygen to a C-H bond to form an epoxide, a highly unstable intermediate that further degrades to harmless products. With a saturated compound like PCE, the oxygenase will add the oxygen to the C-Cl bond, and substitute a chlorine group with a hydroxyl group, resulting in the formation of trichloroethanol, a chemically stable and toxic compound (CCIlCCIOH). In general, the less chlorinated the compound is, the more susceptible it is to aerobic degradation. Conversely, as Figure 5-6 shows, the more chlorinated compounds such as PCE are effectively degraded with another cometabolic process, known as reductive dechlorination. o 6. -0 Uo o Degree of Chlorination Monochlorinated ca--sa -- Polychlorinated -4 0.25 Figure 5-6 Relationship between Degree of Chlorination and Aerobic and Anaerobic Degradation Rates (Kerr, 1994) In an anaerobic environment, highly chlorinated aliphatic hydrocarbons, such as PCE, are degraded to less chlorinated compounds by reductive dechlorination. Reductive dechlorination occurs when anaerobic bacteria produce an enzyme to react with a primary growth substrate, which then fortuitously catalyzes the reductive dechlorination of PCE. In the reaction, PCE acts as the electron acceptor, whereby a chlorine atom is replaced with a hydrogen atom, and the primary substrate acts as the electron donor. Many different electron donors have been shown to be effective in reducing PCE, including lactate, methanol, hydrogen, formate, acetate, and glucose. (Kerr, 1994) One microbial system (DiStefano et al, 1991) which showed complete transformation of PCE to ethene (C2H4) involves the use of methanol as the primary electron donor and ammonium and phosphate as nutrients for the bacteria. The stoichiometric relationships for this system are as follows: C2C14 (PCE) + 1.33CH 3OH (methanol) + 1.33H,O 3CH OH + 2.25HCO0' - C2H4 (ethene) + 4HCI + 1.33CO, a 2.25CH 3COO (acetate) + 0.75CO, + 3.75H20 2.25CH 3C0 + 0.056NH 4+ + 2.02H20 + 0.083CO 2 -- 0.056C5 H,7 N (biomass) + 2.1 1CH 4+2.19HCO3- Although the phosphorus requirement is not shown in the above equations, phosphorus, in the amount of approximately one-sixth of the nitrogen requirement, was added to the system. This system produced high rates of PCE degradation to ethene, up to 275 jimol/(L*day). Further microbiological studies are necessary to better understand this system. Unlike the methanotrophic bacteria which help to degrade TCE, the bacteria and the enzyme chemistry which contribute to the reductive dechlorination of PCE have yet to be identified. 5.4 Combination of Anaerobic and Aerobic Processes Anaerobic microorganisms are effective in reducing highly chlorinated compounds to less chlorinated compounds. However, as the number of chlorine atoms on a compound decreases, reductive dechlorination reactions become more limiting, and aerobic oxidation reactions become more favorable. Reductive dechlorination alone would result in the accumulation of monochlorinated, dichlorinated, and other hydrocarbon compounds. The complementary capabilities of aerobic and anaerobic bacteria can be utilized to effectively degrade PCE and TCE to harmless products. As demonstrated in Figure 57, a design of sequential anaerobic and aerobic biozones would allow the plume contaminated with TCE and PCE to first travel through the anaerobic biozone, where the PCE is degraded to less chlorinated compounds, followed by the aerobic zone, where the products of the incomplete dechlorination and TCE are oxidized to CO2, H,O, and Cl'. In order for cometabolism to occur, the proper growth substrates, nutrients, and electron acceptors/donors must be present in each biozone. PCE TCE PCE ANAEROBIC BIOZONE TCE TCEAEROBIC BIOZONE Other Less Chlorinated Compounds CO, H,O Cl' Figure 5-7 Ideal Schematic for Sequential Anaerobic/ Aerobic Degradation of PCE and TCE 6. DEVELOPMENT OF CONCEPTUAL DESIGN 6.1 Basis for Conceptual Design Since bioremediation is still a new technology, there are very few standard requisites for the design of a bioremediation system. Therefore the conceptual design for the bioremediation of chlorinated solvents in the LF-1 plume will be constructed on the basis of results from different laboratory and field experiments which also apply to the conditions at LF-1. Once the conceptual design is completed, pilot tests based on the design can be implemented, and the results of the pilot tests will help to develop a more detailed bioremediation design. 6.2 Substrate Requirement There have been many experiments performed to determine the substrate concentration necessary for the degradation of contaminants. For the utilization of methane as a primary growth substrate, one study determined that approximately 2 mg/L of methane is required to treat 10 pjg/L of TCE, while 6.5 mg/L of methane is required to degrade 100 gg/L of TCE. (Chang and Alvarez-Cohen, 1995) This estimation will be used to specify the necessary injection concentration of methane at the wells. Since this concentration range is safely below the solubility of methane in water (22 mg/L at 200 C), the injection of methane solution is feasible for this design. Methanol is a substrate that has been demonstrated to successfully biotransform PCE with one of the highest reported reductive dechlorination rates. (DiStefano, 1991) According to the stoichiometry of the microbial system (section 5.3), 4.3 moles of methanol is required to degrade 1 mole of PCE completely to ethene. This molar requirement takes into account the whole microbial system referred to in section 5.3. A portion of the methanol requirement therefore corresponds to the methanol required by the bacteria to form cell material (biomass). This estimation will be used to specify the necessary injection concentration of methanol at the wells. Since methanol is completely soluble in water at 20*C, the injection of methanol is feasible for this design. 6.3 Oxygen Requirement A steady supply of oxygen is necessary to maintain the aerobic biozones in the conceptual design. In bioremediation systems for petroleum hydrocarbons, first approximations of oxygen requirements are typically based on a 3:1 weight ratio of oxygen to organic carbon, or approximately a 1:1 molar ratio. (Kerr, 1994) The sources of organic carbon include PCE, TCE, and the injected methane. Since TCE is present at concentrations significantly lower than the injected methane (see Table 7-1 for the injection concentrations of methane), the oxygen required to degrade TCE is negligible compared to the amount of oxygen needed to degrade methane. As a result, methane is considered to be the limiting organic carbon which will determine the oxygen requirement in this design: CH 4 +20 2 C02 + 2H 20 Even though the stoichiometry requires a 2:1 molar ratio of oxygen to methane, a 3:1 molar ratio of oxygen to organic carbon will be utilized in this conceptual design because experience with bioremediation has shown that some of the oxygen can be consumed by other reactions or is lost by inefficient distribution, which results in a required ratio that is usually higher than the stoichiometric requirement. (Kerr, 1994) Sources of oxygen that may be used include air, which is approximately 20% oxygen, pure oxygen, which is industrially produced oxygen with a typical purity of 90% or greater, and hydrogen peroxide, which is dissociated into oxygen and water. By aerating water, a dissolved oxygen concentration of approximately 8 mg/L can be achieved. Sparging pure oxygen into water can deliver approximately 40 mg/L of dissolved oxygen. Hydrogen peroxide can provide as much as 47 mg/L of dissolved oxygen. (National Research Council, 1993) 6.4 Bacteria The conceptual design will assume that the concentration of bacteria is not a limiting factor in the bioremediation. Since bacteria typically produce 10-20 pounds of bacteria per 100 pounds of organic carbon consumed, it is acceptable to assume that the source of bacteria for degradation will not be depleted as long as methane and methanol are injected into the plume. (Nyer, 1992) 6.5 Nutrient Requirements For the aerobic biozones, nutrient requirements are calculated on the basis of all the methane being converted to cell material, which is approximated by a weight ratio of carbon to nitrogen to phosphorus of 100:10:1. (Kerr, 1994) This is typically a conservatively high estimate for the nutrient requirements since some of the methane might be directly converted to carbon dioxide and water. For the anaerobic biozones, nutrient requirements will be calculated with the stoichiometry provided in the microbial system (Section 5.3). The weight ratio of methanol carbon to nitrogen to phosphorus is 1046:6:1. It is important to note that these nutrient requirements are specific to the microbial system discussed in Section 5.3, and might not be applicable to other anaerobic systems, especially since the type of microorganism(s) involved in these reactions are unknown. 6.6 AnalyticalModels Analytical equations were used to develop the design for the injection wells and determine the total time of bioremediation. It was decided that analytical equations are sufficient to create a preliminary, conceptual bioremediation design, which is the aim of this project. 6.6.1 Injection Wells Analytical equations derived by Welty and Gelhar (1994) quantify the behavior of a solute which is injected at a recharge well. Although the intent of these diverging flow equations was to evaluate longitudinal dispersivity from tracer tests, they are also useful in determining other unknown parameters, given a known longitudinal dispersivity. The equation assumes that the injected solute is conservative, and that the dispersivity, aquifer thickness, and injection rate are all constant. It also assumes that there is radial flow away from the well. The equation for solute concentration in diverging flow is expressed as: S- erfc , (6-1) r2 where = CR / Ciet , CR = the concentration of the injected solute at a radial distance R from the injection well, Cm,• = the injection concentration at the well, t=t/tm, t = time, tm= R2 / (2*A), A= Q / (2*x*rl*b), Q = injection flowrate, l = aquifer porosity , b = aquifer thickness, c = longitudinal dispersivity of aquifer. This equation will give the relationship between the desired radius for a biozone, R, the time it will take for that biozone to be established, t, the injection rate needed to establish and maintain that biozone, Q, and the injection concentration required (Ci.j) to attain a minimum concentration at the edge of the biozone, CR. The spreadsheet calculations of these variables are in Appendix C and the results of these calculations are in Table 7-2. The aim of this conceptual design will be to remediate the highest levels of contamination in the PCE and TCE plumes. The input parameters are R, CR, and t, the desired time for the system to reach steady state. As Figure 6-1 shows, the distance R coincides with the maximum local concentration of contaminant. Direction of Groundwater Flow x: injection point 0: high local contaminant concentration R,: Radius of influence of well 1 R,: Radius of influence of well 2 Figure 6-1 Injection Well Schematic using Parameters in Welty-Geihar Solution The variable CR will be determined by the requirements for remediating the area of high local contaminant concentration indicated by the dot in Figure 6-1. These requirements could include supplemental levels of substrate, oxygen, and/or nutrients, depending on the conditions of that location. Once R is given and the dispersivity is known, the value for tFat which steady state is reached can be determined by using the breakthrough curves shown in Figure 6-2, which were developed by Welty and Gelhar. 0.8 0.6 A C 0.4 0.2 0 0 1 2 3 4 Figure 6-2 Dimensionless Breakthrough Curve for Continuous Injection and Constant Dispersivity (Welty and Gelhar, 1994) The estimation of t can then be used to calculate C, which will provide the concentration needed to be injected at the recharge well to achieve a concentration of CR at a radial distance R from the well. The desired time to reach steady state can then be introduced, and the necessary injection flow rate is calculated from the expression for L. Once the radii of the biozones are determined, the well placement can be designed in a way that the radii of influence do not overlap, thereby allowing the formation of distinct anaerobic and aerobic biozones. 6.6.2 Biodegradation Estimates for the biodegradation rates of TCE and PCE will be used to determine the total time necessary to biodegrade the contaminants to 5 jig/L, the MCL for both compounds. The cometabolic biodegradation of TCE and PCE will be modeled using both zeroth order and first order kinetic expressions. Zeroth order and first order estimates of biodegradation will provide lower and upper bounds, respectively, for the total bioremediation time, which is sufficient for the purposes of a conceptual design. Table 6-1 shows the degradation rate estimates found in the literature. The zeroth order estimates are based on laboratory experiments and the first order estimates are based on field experiments in groundwater. These rate estimates are uncertain given the conditions surrounding the experimentation, however, due to the lack of rate estimates in the literature, they will be used in this design as a first approximation for degradation. Table 6-1 Degradation Rate Estimates for PCE and TCE A: DeBruip ward, 1991 The zeroth and first order reaction rate expressions for the degradation of PCE and TCE are: C = Co-(ko. to), and (6-2) C = Co* exp(- ki* ti), (6-3) where Co = the initial concentration of the contaminant, C = the final concentration of the contaminant, ko = the zeroth order rate constant from Table 6-1, k, = the first order rate constant from Table 6-1, to = the time it takes for the contaminant to degrade to C in a zeroth order reaction, and t, = the time it takes for the contaminant to degrade to C in a first order reaction. These equations will be applied at every injection well in the conceptual design. Since every injection well has a radius of influence which coincides with the highest local contaminant concentration, the biodegradation equations will estimate the time to remediate the contaminant at this concentration. Therefore the conceptual design will be constructed such that the final concentration of TCE and PCE will be the desired remedial level, which is 5 glg/L (the MCL value), and the initial contaminant concentration will vary depending on the conditions surrounding the specific injection well. 7. CONCEPTUAL DESIGN The purpose of this bioremediation system is to remediate the highest concentrations of TCE and PCE in the LF-1 plume to their respective MCL values. Therefore, the design will focus on utilizing injection wells to create aerobic and anaerobic biozones which contact the maximum contaminant concentration at the well's radius of influence, to ensure that mixing between the injected bioenhancers and the contaminant due to dispersion and diffusion will occur. 7.1 Placement of Injection Wells As Figure 7-1 shows, there are fourteen recommended injection points, half of which utilize existing well locations. There will be four alternating aerobic/ anaerobic biozones, followed by two final aerobic biozones. The four aerobic biozones will be created by injections at: wells 1 and 4 (Aerobic Biozone 1), wells 5 and 6 (Aerobic Biozone 2), wells 10, 11, and 12 (Aerobic Biozone 3), and at wells 13 and 14 (Aerobic Biozone 4). The two anaerobic biozones will be created by injections at: wells 2 and 3 (Anaerobic Biozone 1), and wells 7, 8, and 9 (Anaerobic Biozone 2). 3W000L "U. "INJECTIONwELL 9 ' Aerobic Biozone Q Anaerobic Biozone 32000SJI3IIO1 (S 20000 2MC00, 7 2,e0000 24000 100O !00 Well Well Well Well 1: MW701A 2: MW22 3: MW23 4: Near MW48 00009C00100 0 7ROOM Well 5: Near Edmunds Pond Well 6: Near WT23 Well 7: MW43 Well 8: Near MW36B oGoi?000130) I00s S000Ifl0GW700'Ji560~90Q0 TEET1 Well 9: WT26 Well 13: MW52 Well 10: MW44 Well 14: Near MW5 Well 11: Near MW45 Well 12: Near MW40 Figure 7-1 Injection Well Placement The injection wells are placed with the purpose of straddling the target of remediation, with equivalent but counteractive velocities acting on the point of high concentration, as Figure 7-2 illustrates. This will in turn form a radially stagnant zone, where the contaminant-containing groundwater will be restricted from moving laterally, but it will continue to move downgradient, thereby ensuring movement of the plume through the biozones. The biozones have been configured so that the radius of influence of each injection well is undisturbed by the surrounding injection wells, so that each biozone is well-defined. It is expected that the high concentration points of the contaminant plumes will travel through the alternating aerobic/ anaerobic biozones and undergo bioremediation, with a final aerobic biozone established to prevent any high levels of contamination from bypassing treatment and reaching the bay. Since there is a regional gradient, it is possible that the injected contents will also travel downstream, however it is assumed that mixing between the contaminants and the injected contents, which leads to bioremediation, will occur. Anaerobic * High Local Concentration Biozone .---. Velocity Induced by Injection Well Aerobic Biozone x Injection Well Figure 7-2 Conceptual Design of Injection Wells 7.2 InjectionParameters 7.2.1 Injection Contents The contents for injection will vary from well to well. The regulatory issues involved with the injection of the different substances will not be addressed in this conceptual design. At injection points where aerobic conditions need to be induced or maintained, water sparged with pure oxygen will be provided for the oxygen supply. Even though the majority of the plume is fairly aerobic, the dissolved oxygen concentrations are inadequate for the mineralization of the methane which is injected into the aerobic biozones. While the background oxygen concentration in the plume ranges between 0-10 mg/L (Figure 4-3, Appendix A), the total necessary oxygen concentration varies from 4-40 mg/L. Since high concentrations of dissolved oxygen are required, as shown in Table 7-1, the injection of pure oxygenated water is necessary. In addition, a primary growth substrate and nutrients will be added to stimulate the bacteria to high levels of cell production. For the anaerobic biozones, a primary growth substrate and nutrients will still be added, but the oxygen will no longer be injected and the heightened bacterial population will exert a higher BOD (biochemical oxygen demand), thereby creating anaerobic conditions. The site characterization determined that most of the plume had sufficient levels of nitrogen, however several places with adequate levels were nearby to place(s) which were depleted of nitrogen. This design assumes that nitrogen will reach these places by transport, so that additional nitrogen will not be injected. There is also the assumption that the entire plume contains no phosphorus, so that it will be added at all injection points. The injected contents for this conceptual design were determined from the literature sources that demonstrated the most successful results. For the aerobic biozones, the recommended primary substrate is methane (in dissolved form). (Semprini and McCarty, 1991b) For the anaerobic biozones, the recommended primary substrate is methanol, which has the highest demonstrated PCE degradation rate in the literature. (DiStefano et al, 1991) The recommended nutrients for all the biozones are ammonium (NH4+) and phosphate (P0 4"). (Nyer, 1992) This conceptual design does not address potential regulatory issues involved with the injection of the different substances. 7.2.2 Injection Flowrates and Concentrations The recommended flowrates and concentrations for methane, methanol, ammonium, phosphate, and oxygen are summarized in Table 7-1. The concentrations were calculated on the basis of the requirements addressed in sections 6.2- 6.5, and the flowrates were calculated on the basis of the analytical equation described in section 6.6.1. For the aerobic biozones, the relationship between the substrate (methane) concentration and TCE concentration is assumed to be linear and calculated with the following information: 2 mg/L methane is necessary to degrade 10 jLg/L TCE and 6.5 mg/L methane is required for 100 gg/L TCE. (Section 6.2) The linear relationship based on these two data points is: C.,h,,.e = (50* CTcE)+ 1500 (7-1) where Cm,., = the required concentration of methane, and CTCE = the concentration of TCE. The oxygen requirement in the aerobic biozones was calculated by utilizing the 3:1 molar ratio of oxygen to organic carbon (Section 6.3): Ca.n = Cme,,* ( MWan * 3 mol oxygen 1 mol methane MW.mehnme where Co.g. = the required concentration of oxygen, MWm,t. , = the molecular weight of methane, and MWoxys . = the molecular weight of oxygen. The nitrogen and phosphorus requirements in the aerobic biozone are expressed by the 100:10:1 weight ratio of organic carbon to nitrogen to phosphorus (Section 6.5), or: Cmethane Cniro.gen = C(7-3) 10 Cmehsane Cphosphorus = Cmed 100 (7-4) Once Cg,, and Cphosphor w are calculated, the necessary ammonium (NH4') and phosphate (P0 4-3) concentrations can be determined: Cammonium = Cnitogen * M) MWnitro.en (7-5) Cphosphate = Cphosphorus * MWphsphat -MWWphospho (7-6) (7-6) where C.moni. = the required concentration of ammonium, Cphosphae = the required concentration of phosphate, MWmo. = the molecular weight of ammonium, MW.gosn,= the molecular weight of nitrogen, MWpho.h = the molecular weight of phosphate, and MWpho.phom, = the molecular weight of phosphorus. For the anaerobic biozones, the substrate (methanol) requirement is calculated using the stoichiometric relationship described in Section 6.2, which states that 4.3 moles of methanol are needed to completely reduce 1 mole of PCE to ethene: C.,,ano = CPCE* = -MWmeMha• MWPCE * ( 43 mol methanol) I(7-7) 1 mol PCE = the required concentration of methanol, CPCE = the concentration of PCE, MW, = the molecular weight of PCE, and MW.mc.., = the molecular weight of methanol. where Cm,~e, The nutrient requirements are expressed by the 1046:6:1 weight ratio of organic carbon to nitrogen to phosphorus (Section 6.5), or: Cnir,•en = C.hao* 6gN 6g 1046g C lg P 1046gg C' Cphosphonr = Cmethanol* 1 (7-8) (7-9) Once CGgin and Cphosphor,, are calculated, the necessary ammonium (NH4) and phosphate (P04-') concentrations can be determined using equations 7-5 and 7-6. The calculated concentration values are then corrected for the initial concentrations which may be present. This conceptual design assumes that the "background" concentration is constant. Since the transport equation is linear with respect to concentrations, the required concentration is equal to the difference between the calculated concentration (from Equations 7-1 through 7-9) and the background concentration. Since all of the calculations in Equations 7-1 through 7-9 correspond to the required concentrations at the injection well's radius of influence (highest local contaminant concentration), it is important to calculate the injected concentrations which are necessary to achieve the concentrations at the radius of influence. Once C is determined from Equation 6-1, S erfc , (6-1) the necessary injection concentration can be calculated: Cinject - Ccalculated C where Cj,, = the necessary injected concentration and Ccaculat.d = the concentration calculated from Equations 7-1 through 7-9. (7-10) As described in Section 6.4.6.1, C is dependent on ?, which is dependent on the desired time to reach steady state, the desired radius of influence, the porosity of the medium, the aquifer thickness, and the injection flowrate. The required injection flow rate was calculated using Equation 6-1. Recalling that A = Q / (2*n*l*b), tm = R2 / (2*A), and t = t / t., these expressions are utilized to obtain an expression for flowrate: r* qr*b* R2 * t Q= t , (7-11) where Q = injection flow rate, q = average aquifer porosity = 0.39 (Alden et al, 1996), b = average aquifer thickness = 113 ft (Stone & Webster, 1996), R = radius of influence of injection well, and t = the time to reach steady state. The variable t represents the time that it will take for the concentration of the solute at the radius of influence to reach the desired concentration. Therefore the flowrate will change depending on the value of t. The more time that is designated for the system to reach steady state, the smaller the flow rate that is required. As Table 7-1 shows, this design utilizes a t value of five years because it resulted in velocities at the radius of influence which were on the same order of magnitude as the regional groundwater velocity. (See Appendix C) This ensures that the disturbance to the regional flow is minimized. Table 7-1 Summary of Injection Contents, Concentrations, and Flowrates Well Number Contaminant Concentration (g/L) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Injected Substrate (Ig/L) Injected Oxygen (mg/L) Injected Ammonium (Ig/L) Injected Phosphate (og/L) 64 4869.21 29.22 293.04 149.22 (years) 5 17069 65 65 64 10 10 30 30 48 26 26 10 0 0 57.58 56.75 4897.31 2072.01 2072.01 25.86 25.86 41.21 2900.81 2949.26 2090.82 1554.00 1562.97 0 0 23.67 12.43 12.43 0 0 0 17.40 9.57 4.35 9.32 9.38 0* 0* 294.73 0 266.40 0 0 0.30 0 0* 0 0 0 0.17 0.17 150.08 63.50 63.50 0.08 0.08 0.12 88.90 90.38 64.07 47.62 47.90 5 5 5 5 5 5 5 5 5 5 5 5 5 4916 8011 11835 17069 17069 13769 13769 22531 17069 8011 9955 17069 11835 S. Time to Reach Steady State Injection Flowrate (ft/ hr) Bold: PCE concentration, Plain: TCE concentration, *: Surrounding well(s) show very low nitrate levels. 7.3 Estimation of Bioremediation Time The total time for bioremediation of TCE and PCE to 5 gg/L must incorporate the time it takes for a biozone to reach steady state (i.e. the concentration of the injected bioenhancer at the radius of influence reaches the maximum intended concentration) and the actual time of biodegradation of the contaminants. Therefore equations 6-2 and 6-3 are amended to include the time needed to establish the biozone: C= Co - [ko*(to-tb)], and (7-12) C = Co *exp[- ki* (ti-tb)], (7-13) where C = the ultimate contaminant concentration = 5 Jtg/L, Co= the initial contaminant concentration, tb = the time needed to establish the biozone, to = the total time to bioremediate the contaminant to 5 jtg/L if it is a zeroth order reaction, and t, = the total time to bioremediate the contaminant to 5 jtg/L if it is a first order reaction. Note that tb is subtracted from the total time of bioremediation because the extra time needed for the biozone to reach steady state results in a larger value for the total bioremediation time. (See Appendix B) The total bioremediation time will vary depending on the desired time for the system to reach steady state. For this conceptual design, if the system reached steady state in five years, the total time for bioremediation (which includes the time to reach steady state and the time of biodegradation) would be in the range of five to fourteen years, as summarized in Table 7-2. Table 7-2 Summary of Bioremediation Time Estimates Well R (ft) a / R Number C.,,,, Time to Q Total Oth order (glg/L) Reach (ft/hr) bioremediation Steady time (yrs) State: t Total Ist order bioremediation time (yrs) (yrs) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 1500 720 960 1200 1500 1500 1320 1320 1800 1500 960 1080 1500 1200 0.06 0.125 0.094 0.075 0.06 0.06 0.068 0.068 0.05 0.06 0.094 0.083 0.06 0.075 2.4 3 2.8 2.6 2.4 2.4 2.5 2.5 2.2 2.4 2.8 2.7 2.4 2.6 0.9652 0.9357 0.9494 0.9597 0.9652 0.9652 0.9616 0.9616 0.9656 0.9652 0.9494 0.9566 0.9652 0.9597 64 65 65 64 10 10 30 30 48 26 26 10 0 0 5 5 5 5 5 5 5 5 5 5 5 5 5 5 17069 4916 8011 11835 17069 17069 13769 13769 22531 17069 8011 9955 17069 11835 Bold: PCE concentration, Plain: TCE concentration 5.00 5.00 5.00 5.00 5.00 5.00 5.00 5.00 5.00 5.00 5.00 5.00 n/a n/a 7.72 13.87 13.87 7.72 5.74 5.74 11.20 11.20 12.82 6.76 6.76 5.74 n/a n/a 8. CONCLUSION 8.1 Summary of Conceptual Design The basic components of bioremediation are the electron acceptor, electron donor, nutrients, and microorganisms. These components, along with other environmental conditions such as proper pH and temperature, are necessary for the successful bioremediation of TCE and PCE in the LF-1 plume. The conceptual design relies on the establishment of sequential anaerobic and aerobic biozones to degrade the high local PCE and TCE concentrations. Each biozone consists of the essential components required for bioremediation, as illustrated in Figure 8-1. ANAEROBIC Electron Acceptor: PCE Electron Donor: Methanol Nutrients: Ammonium, Phosphatc AEROBIC Electron Acceptor: Oxygen Electron Donor: TCE Nutrients: Ammonium, Phosphate Directionof ContaminantPlume Movement Figure 8-1 Bioremediation with Sequential Anaerobic/ Aerobic Biozones In order to reduce the TCE and PCE concentrations at the areas of highest measured concentration to 5 jtg/L by complete mineralization to carbon dioxide, water, and chloride, the following parameters of the conceptual design must be implemented. The required injection rates, which range from 4,916- 22,531 ft3/hr, should be examined when assessing the cost-effectiveness of this design. While most of the plume contains a sufficient background level of nitrogen, some spots require the injection of 0.30- 295 gg/L of additional ammonium. The phosphate requirement for the areas of highest measured TCE and PCE concentration ranges from 0.08- 150 pg/L. The substrate for the anaerobic biozones, methanol, will be injected at concentrations ranging from 25-58 jtg/L. The substrate for the aerobic biozones requires higher concentrations, with methane concentrations ranging from 1550-4900 gg/L. There is no oxygen injected at the anaerobic biozones, but the aerobic biozones require 4-30 mg/L of additional dissolved oxygen. The total time for bioremediation will range between 5 and 14 years, depending on the conditions at the specific spot in the plume. 8.2 PotentialProblems 8.2.1 Clogging The stimulation of bacteria is an integral part of the effective bioremediation of chlorinated solvents. The bacteria population is stimulated through the addition of necessary nutrients, substrate, electron donors, and electron acceptors. The resulting enlarged bacterial population can often have the counterproductive effect of clogging the aquifer, thereby reducing the hydraulic conductivity and porosity, which can then affect the flow of groundwater used to deliver the essential supplements to the bacteria in the contaminated region of the aquifer. Clogging may also occur due to inorganic reactions, such as the precipitation of metals. (Nyer, 1992) 8.2.2 Intermediate Toxicity When an intermediate product of the biodegradation process exerts toxic effects on bacterial cells, it can significantly affect the rate of biodegradation of PCE and TCE. For example, the TCE epoxide has been shown to react with cell protein. (Hinchee et al, 1995a) As the amount of the chlorinated compound degraded increases, the bacterial cell activity declines until the cell becomes completely inactive; this degraded amount per amount of cells inactivated has been termed the transformation capacity (Tj). Intermediate toxicity poses serious limitations in biodegradation with methane-utilizing bacteria. It has been demonstrated that TCE concentrations of 0.1 mg/L or greater are necessary to induce the toxicity produced by intermediate products. (Chang and AlvarezCohen, 1995) Since concentrations at LF-1 are significantly less than this value, intermediate toxicity is not likely to be a problem. 8.2.3 Competitive Inhibition Since chlorinated compounds and primary growth substrates are degraded by the same enzyme in cometabolic degradation, competition can occur between them for the enzyme's active sites. As a result, the degradation rates for both the growth and cometabolic substrates can be reduced. At the LF-1 plume, for example, where TCE is present at relatively low concentrations, the methane can more effectively compete for the active sites, thereby lowering the degradation rate of TCE. One set of experiments found that the optimal TCE degradation rate would occur when the methane concentration was between 1.2 and 2.4 mg/L, however this corresponded to a TCE concentration of 7.95 mg/L, which is significantly higher than the TCE concentrations at LF-1. (Chang and Alvarez- Cohen, 1995) Therefore it can be inferred that this conceptual design will need to be modified to account for inhibition effects, due to the high substrate levels calculated in section 7.2.2. 8.3 Recommendations for Future Work Given that the purpose of this thesis was to develop a conceptual design for bioremediation, there are many ways on which the design can be improved to make the process more cost-effective and efficient. These improvements, which were beyond the scope of this thesis, include the following: * Use of horizontal wells instead of vertical wells will enhance the distribution of the injected contents. (Lindsey, 1997) * Gas phase injection instead of aqueous phase injection will result in minimal displacement of groundwater and better mixing. * Injection of bioenhancers in alternating pulses, instead of continuous injection, will ensure that high concentrations of the growth-stimulating materials do not accumulate around the injection well. * Use of hydrogen peroxide as the oxygen source, instead of oxygenated water, will help to prevent clogging because of its strong disinfectant characteristics. (National Research Council, 1993) * Use of phenol as a primary growth substrate in aerobic conditions, instead of methane, may result in higher TCE degradation rates. Although the injection of phenol to the groundwater may raise some regulatory issues, there are studies which have shown that phenol is more effective than methane in the cometabolic degradation of TCE. Furthermore, the phenol-utilizing bacteria have not shown any intermediate toxicity effects in experiments, whereas intermediate toxicity effects on methane-oxidizing bacteria have been reported. (Hinchee et al, 1995a) Phenol also has a higher solubility in water and, unlike methane, has no explosive properties. * Methanol may be able to serve as the primary growth substrate for both aerobic and anaerobic degradation, which would simplify the treatment process, and probably reduce the total bioremediation cost. The ability of the aerobic bacteria to obtain energy from methanol (a methane oxidation intermediate) indicates that it might serve as a substitute for methane to drive the cometabolism of TCE, as discussed in section 5.2. * Use of detailed numerical groundwater modeling to refine design parameters. (Wagle, 1997) If the aforementioned enhancements were incorporated into this conceptual design, insitu bioremediation would be a more suitable and attractive remediation technology for the achievement of a cost-effective, thorough, and efficient solution to the contamination at LF-1. 8.4 Summary Chlorinated compounds represent the most prevalent organic groundwater contaminants in the country. (Kerr, 1994) Until recently, bioremediation had not been considered a viable alternative for the remediation of chlorinated solvents. However, when degradation products of the chlorinated contaminants started to be found in the groundwater, an intense effort to understand the responsible processes began. As a result, there is now a wealth of theoretical and experimental information suggesting that bioremediation can be utilized to effectively degrade chlorinated compounds. However, the lack of full-scale implementation to date leaves many questions to be answered, particularly regarding the cost-effectiveness of the process. Thus, there is much yet to be learned about this new, but promising, remediation technology. 9. REFERENCES ABB Environmental Services, Inc., "Record of Decision, Interim Remediation, Main Base Landfill (AOC LF-1) Source Operable Unit," Installation Restoration Program, Massachusetts Military Reservation, Cape Cod, MA, January 1993. Alden, Dan, Amarasekera, Kishan, Collins, Michael, Elias, Karl, Hines, Jim, Jordan, Benjamin R., Lee, Robert F., An Investigation of Environmental Impacts of the Main Base Landfill Groundwater Plume, Massachusetts Military Reservation, Cape Cod, MA, Master of Engineering Group Thesis, Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA, 1996. Anderson, James E., McCarty, Perry L., "Model for Treatment of Trichloroethylene by Methanotrophic Biofilms," JournalofEnvironmental Engineering,120(2), 379400, 1994. Bedient, Philip B., Rifai, Hanadi S., Newell, Charles J., Ground Water Contamination: Transport and Remediation, PTR Prentice Hall, Englewood Cliffs, NJ, 1994. Cape Cod Commission, http://www.vsa.cape.com/-cccom/trend.htm, 1996. CDM Federal Programs Corporation, "Remedial Investigation Report: Main Base Landfill and Hydrogeological Region I Study," Boston, MA, April 1995. Chang, Hsiao-Lung, Alvarez-Cohen, Lisa, "Model for the Cometabolic Biodegradation of Chlorinated Organics," EnvironmentalScience and Technology, 29(9), 23572367, 1995. DeBruin, Wil P., Kotterman, Michiel J.J., Posthumus, Maarten A., Schraa, Gosse, Zehnder, Alexander J.B., "Complete Biological Reductive Transformation of Tetrachloroethene to Ethane," Applied andEnvironmentalMicrobiology, 58(6), 1996-2000, 1992. DiStefano, Thomas D., Gossett, James M., Zinder, Stephen H., "Reductive Dechlorination of High Concentrations of Tetrachloroethene to Ethene by an Anaerobic Enrichment Culture in the Absence of Methanogenesis," Applied and EnvironmentalMicrobiology, 57(8), 2287-2292, 1991. Gorelick, Steven M., Freeze, R. Allan, Donohue, David, Keely, Joseph F., Groundwater Contamination: Optimal Capture and Containment, Lewis Publishers, Boca Raton, FL, 1993. Gupta, S.K., Djafari, S.H., Zhang, J., "Oxygen Transport in an In-Situ Bioremediation Application," Innovative Technologies for Site Remediation, Proceedings of the National Conference, American Society of Civil Engineers, New York, NY, 165172, 1995. Hazen, Terry C., Lombard, K.H., Looney, B.B., Fliermans, C.B., Eddy-Dilek, C.A., "In Situ Bioremediation of Chlorinated Solvent with Natural Gas," Savannah River Technology Center, http://www.srs.gov/general/sci-tech/expertise/bioreml.html. HAZWRAP, "Installation Restoration Program, Phase I - Records Search, ed. 2," Otis Air National Guard Base, Massachusetts, 1987. Hinchee, Robert E., Leeson, Andrea, Semprini, Lewis, Ong, Say Kee, eds., Bioremediation of Chlorinated and Polycyclic Aromatic Hydrocarbon Compounds, Lewis Publishers, Boca Raton, FL, 1994. Hinchee, Robert E., Leeson, Andrea, Semprini, Lewis, eds., Bioremediation of Chlorinated Solvents, Battelle Press, Columbus, OH, 1995a. Hinchee, Robert E., Wilson, John T., Downey, Douglas C., eds., Intrinsic Bioremediation, Battelle Press, Columbus, OH, 1995b. Howard, Philip, ed., Handbook of Environmental Degradation Rates, Lewis Publishers, Chelsea, MI, 1991. Kerr, Robert S., ed., Handbook of Bioremediation, Lewis Publishers, Boca Raton, FL, 1994. Kobus, Helmut, Barczewski, Baldur, Koschitzky, Hans-Peter, eds., Groundwater and Subsurface Remediation: Research Strategies for In-situ Technologies, Springer, Berlin, 1996. Kostek, Rebecca, "Identification of a Potential Contaminant Source to the Main Base Landfill Plume at the Massachusetts Military Reservation," Master of Engineering Thesis, Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA, 1997. Leon, Roberto, "Post-Closure Management of a Hazardous Waste Landfill at the Massachusetts Military Reservation Main Base Landfill," Master of Engineering Thesis, Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA, 1997. Levin, Morris A., Gealt, Michael A., eds., Biotreatment of Industrial and Hazardous Waste, McGraw-Hill, New York, NY, 1993. Lindsey, Mia, "The Use of Horizontal Wells for Environmental Remediation for the Landfill-i Plume at the Massachusetts Military Reservation in Cape Cod, MA," Master of Engineering Thesis, Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA, 1997. Massachusetts Department of Environmental Protection, http://www.magnet.state.ma.us/ dep/sero/mmr/mmr.htm, 1996. Masterson, John P., Barlow, Paul M., "Effects of Simulated Ground-Water Pumping and Recharge on Ground-Water Flow in Cape Cod, Martha's Vineyard, and Nantucket Island Basins, Massachusetts," Open-File Report 94-316, U.S. Geological Survey, Marlborough, MA, 1994. Masterson, John P., Stone, Byron D., Walter, Donald A., Savoie, Jennifer, "Hydrogeologic Framework of Western Cape Cod, Massachusetts," Open-File Report 96-46, U.S. Geological Survey, 1996. Metcalf & Eddy, Inc., "Installation Restoration Program, Phase I - Records Search," Otis Air National Guard Base, Massachusetts, January 1983. National Research Council, In Situ Bioremediation: When Does it Work?, National Academy Press, Washington, D.C., 1993. Nyer, Evan K., Groundwater Treatment Technology, Van Nostrand Reinhold, New York, NY, 1992. O'Brien and Gere Engineers, Inc., Innovative Engineering Technologies for Hazardous Waste Remediation, Van Nostrand Reinhold, New York, NY, 1995. OPTECH (Operational Technologies Corporation), "Plume Containment Design Data Gap Field Work Technical Memorandum, MMR, Cape Cod, MA," San Antonio, TX, 1996a. OPTECH (Operational Technologies Corporation), LF-1 60% Work Design Data, Cape Cod, MA, 1996b. Semprini, Lewis, Hopkins, Gary D., Roberts, Paul V., Grbic- Galic, Dunja, McCarty, Perry L., "A Field Evaluation of In-Situ Biodegradation of Chlorinated Ethenes: Part 3. Studies of Competitive Inhibition," Ground Water, 29(2), 239-250, 1991a. Semprini, Lewis, McCarty, Perry L., "Comparison between Model Simulations and Field Results for In-Situ Biorestoration of Chlorinated Aliphatics: Part 1. Biostimulation of Methanotrophic Bacteria," Ground Water, 29(3), 365-374, 1991b. Semprini, Lewis, McCarty, Perry L., "Comparison between Model Simulations and Field Results for In-Situ Biorestoration of Chlorinated Aliphatics: Part 2. Cometabolic Transformations," Ground Water, 30(1), 37-43, 1992. Skiadas, Panagiotis, "Design of an In Situ Bioremediation Scheme of Chlorinated Solvents by Reductive Dehalogenation Sequenced by Cometabolic Oxidation," Master of Engineering Thesis, Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA, 1996. Skipper, H.D., Turco, R.F., eds., Bioremediation: Science and Applications, Soil Science Society of America, Madison, WI, 1995. Stone and Webster Engineering Corporation, "Massachusetts Military Reservation, Data Gap Supplemental Remedial Investigation Draft Report, Area of Concern: LF- 1 NWOU," December 1996. University of Minnesota Biocatalysis/ Biodegradation Database, http://dragon.labmed. umn.edu/-~ynda/tce/reac/tce_mechAl.gif, 1997. Wagle, Mandeera, "A Simulation of Pumping Schemes for the Containment of the Groundwater Contaminant Plume under the Main Base Landfill on the Massachusetts Military Reservation in Cape Cod, MA," Master of Engineering Thesis, Department of Civil and Environmental Engineering, Massachusetts Institute of Technology, Cambridge, MA, 1997. Welty, Claire, Gelhar, Lynn, "Evaluation of Longitudinal Dispersivity from Nonuniform Flow Tracer Tests," Journalof Hydrology, 153, 71-102, 1994. 10. APPENDICES Appendix A: Dissolved Oxygen Concentration Data for LF-1 (OPTECH, 1996b) *m·r3\··l ~~. · · · rvr~vvv~rvv IU-JUI-o3|,IIlY 27MWX2A3XXA9XX X 72OMWX20XX95X 13-Jul-95 13-Jul-95 I3-Jul-95 L 27MWXs4XXX952X·-~---- Z7MWX5OXXA9SX)1I 27MWS67XXA9SXX 27MWXS4XXX9S2X 71.331 LF• LF-I LF-1 LF-I 27MWX45XXX95XX 27MW103XXA95XX 27MW103XXZ95XX 27PSXX2XXX95XX LF-I 271PSXXSXXX95XX -- LF-I LF-I 27MWX47XXX95XX 27MWX26XXA95XX LF-I 27MWX4IXXX95XX LF-I 27MWX35XXX95XX LIF-1 27MWX38XXZ94XX LF-I LF-I 27MWX3SXXA9SXX 27MWX52XXX95XX LF-I I.E-T I.E-I 27MWX46XXA95XX 27MWX3 IXXA95XX 27MWXS3XXX953X LF-I LF-I 27MWX39XXZ9SXX LF-I LF-I I-F,-I ICF-i IF-I IF-I IF-I X' 27MWX39XXA952X 27MWX40XXX952 LF-I 27MW567XX119527 27MW567XXX9523 27MWX-ISXXX9S2. LF-I 2tiiDX2X 9> 114qU 1136 0817 ~~""~""-~` '~~ WxTiiBbr>Ex ifiT LF-I . 19-Jul-95 x"`~~~"""^~~^^xI~x~'~'- 27MW IO3XXZ9S2X 2i7iWX26XXA952X 27MW103XXA952X 27MWX26XXB952X 27MWX53XXX952X 27MWX50XXA952X 27MWX47XXX952 X 27PSXX5XXA9SXX X ?7MWX- 7XXA952, 020 11006 1045 1029 NA 1123 112I ILNA gIqj4 [NA 1009 INA 1032 NA jAA I1IIU NA NA NA INA 1349 1336 11455 INA INA 11520 INA INA NA NA -NA 1450 11320 INA 11322 10802 836 10748 0824 INA NA 0915 0908 NA 10751 INA 0827 NA 0911 NA 11042 11448 INA INA ýA -- ~--1NA NA J 1NA 11326 NA NA A NA NA NA NA NA NA NA NA NA NA NA 4-27 9,"76 6.19 0757 1047 1254 1124 1538 NA NA 5.10 9i95 6.65 0800 1050 1257 1127 1542 NA NA 5.14 10.05 5.58 0851 NA I INA 0754 INA 0830 NA 0914NA f NA NA NA NA NA NA NA NA- NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA 151.91 111.77 120.35 T49.41 141.87 139.37 162.44 141.81 138.11 59.18 144.31 140.61 109.27 117.85 162.35 159.85 293.70 291.20 ------ L--- ""-· - -· - --- 21-Sep-95 --f--- liiW mr28 1625 i1i-- 0958 10421033700 0912 0952 0936 650 1036 _17.48 094-0 7.06 1458 1739 1734 I 39 L.91 il1S 1.96 NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA NA fix'~ - NA 10,46 .. 11025 3.51 1430 1517 ·2.52 · I I 1 ------ · c------· i- 1345 7.72 t-.-?-_~=-?-~t---~~·~-·---t---- -24 5liS 1159 0910 7.48 7.86 0915 8.40 0920 ...... ........ . ~-- .......... . ...... ....................... ~-x~·----·-~··Lr^rr~·rrr^ Irr~-r~*r~r~----~-----~-L-^_-^-r--- · - ----- -------4 - -II~I~^I 11243 ^--. -I 18.13 _I,- ... 4------4, ------ -4- · · 0928 3.95 11057 17.67 10397.51 10127,22 0945 7.71 1743 NA NA NA NNA NA NA NA NA NA NA NA NA _NA NA NA 1207 11247 11232 12.10 11237 12.31 1124012.21 1225 ~-~----7-~--7~------`111-------~111~--7---1--·1~1~-1·___1_11_1~·__1_·__·- ·3r---"~·-·----C---t--1-·~----~·11-- 13.83 11540 14.43 2osep95 --1510 11544 11531 13.02 11535 ~~(,~ · INA INA INA INA INA INA INA 20-Sep-95 NA 3.82 t 0937 14.16 0914 10943 0931 3.91 1.934 , -, 21-Sep-95 1045 11115 1101 7.94 1105 7.38 1109 7.45 1232 1257 1246 8.38 1249 8.61 1252 8.21 21-Sep-95 1339 1351 1356 8.17 6i)- 1403 1348 7.44 1.•-7.80 1632 1-30 .95 9 7.39 1543 1532 7.46 15387.84 22-Sep- 5 iaiii-" 7.99 ".4';- i36-7.85 1146 1214 1202 7,29 1206 7.26 1209 7.33 0937 0923 8.59 0928 6.90 0931 8.73 22-Sep-95 0855 NA NA NA NA NA 54 NA 1030 15.75 11033 5.67 .1036 [5.90 11435 15.03 11440 J5.22 11444 6.23 I NA NA 1128 INA ... ....... ... .. ~~ ~~I ---- ý.... l---C-NA I--·NA N.~ijNA NA •.• ýWA-ýý "E NA NA NA 1-*1111 LIIII~~I_ - · ~· -----·}NA INA I INA NA 1343 11444 NA NA NA IPAN INA 1140 1132 1525 1105 1159 1419 1007 0922 0830 NA iiq 1458 0934 NA NA NA 0837 0754 1100 1044 1309 1250 1135 1121 1549 1535 11453 NA NA NA NA NA NA NA NA NA NA NA 11026 4. 1~11 INA I I 1003 NA INA NA NA 1436 1413 1017 1001 0934 0916 82- 1352 1339 1136 1130 1522 1102 1156 1416 1004 0919 0827 NA NA NA NA NA 0824 NA 11035 NA IIJJ IA1 0931 0945 0928 1148 1132 1146 1128 1536 1519 1116 1059 446 0840 11012 1514 INA .~''"" c ^"l"- 1~5i ~ -~1""1*1- 11332 1209 1153 f14if IIAA 1055 NA 0846 INA --1-~-·-t-~---~··---r----c---A 0913 INA - 052i IANA 13-Jul-95 1435 Ji500 11447 13-Jul-95 1447 11508 11452 13-Jul-5 1510 11530 11517 ... .. , 19-Jul-95 LF-1 27GBX20XXX95XX IANA 1020 18-Jul-954 1316 18-Jul-95 0741 18-Jul-95 08 10 18-Jul-95 0900 18-Jul-95 0928 1117 18-Jul-95 1120 18-Jul-95 1500 1050 19-Jul-95 1407 19-Jul-95 0953 0912 19-Jul-95 0817 20-Jul-95 20-Jul-95 0740 20-Jul-95 1030 I2-Oct-95 1235 1026 1615 1O-Nov-951012 1418 F-1 i 27MWX26XXi9SXX nin 1340 140011346 13-Jul-95 1326 1134811333 68.81 61.68 ~~x"x"~r59.18 ~~c1~ 11£i1 13-Jul-95 6)84"0jiý---'• Oý49 N`A ·_·-_~-~-_~--t·~-_·-_~---~ 13-Jul-95 - 093-9 091 N 145.96 LF-I 143.46 ?--=-1--~T?---·~-~_~-11-1·~1_1~~ ^ZI~II_"=~X~·" ~*"l-T1*"~" LF-I 130.70 128.20 LF-I 27MWXI7XXB95XX 111.60 109.10 1.87 '39.37 LF- I 27MWXI7XXA95XX i 141.81 140.63 iS8.ii 7.93 4- --------- . -·--------*--~· -,-•-,,--- ~------- .. .. 711 p1-h- J.......... -------t------- · . . . ... .,I............. -,·-· ... a........ K ______I I _i Appendix A: Dissolved Oxygen Concentration Data for LF-1 (OPTECH, 1996b) 30-Nov-95 0931 30-Nov-95 1445 30-Nov-95 1306 30-Nov-95 1120 30-Nov-95 0930 29-Nov-95 1040 29-Nov-95 0845 30-Nov-95 0930 1105 1621 1440 1240 1113 27MWX62XXX96XX 02-Jan-96 1303 1606 1331 27MW568XXZ95XX 27-Dec-95 0830 1050 27MW568XXZ95ZZ 22-Dec-95 1049 27MWX63XXX96XX IF-I LF-1 LFLFLF-I LF- I LF- I LF- I 27GBX22XXA95XX 27MWS68XXA95XX 27MW568XXB95XX 27MW568XXC95XX 27MWX59XXX95XX 27MWX35XXZ95XX 27MWX59XXX95XX 27MW568XXD95XX LF- I LF- I 101.46-121.31 101.46 78.63 61.21 204.98 66.21 199.98 61.21 5.89 6.70 3.39 2.37 7.97 3.84 4.51 7.97 1101 1539 1355 1210 1029 1202 0948 1029 3.78 3.7 6.53 3.77 2.20 7.75 5.14 4.95 7.75 1506 1330 1142 1602 1152 0906 1002 5.56 3.90 1.50 7.89 2.09 5.46 7.89 19.79 1336 17.99 1341 17.71 1316 17.05 1321 1031 17.62 1036 18.40 1041 19.27 0929 15.44 1302 1232 13.04 1236 13,57 1242 14.53 1204 16.69 02-Jan-9( 0947 1040 1029 17.96 1033 18.66 17.98 1004 03-Jan-96 1130 07-Feb-94 1104 1219 1203 1135 8.14 19.43 1208 13.80 1213 114018.45 1145 07-Feb-9( 0927 23-Feb-9 1500 1028 1549 1005 17.88 1010 18.47 1539 1054 1531 1345 1202 4.83 6.37 3.88 1.60 1021 7.98 12041158 1.80 5.11I 1032 '0939 1113 1021 7.8 1058 1535 1350 1207 1025 1200 0944 1025 -----· -I1511 5.87 13353.33 1.02 1147 1007 7.56 1156 2.37 0911 5.91 7.56 1007 1521 1516 1340 1152 1012 6.41 5.95 3.90 1.91 1157 1.76 7.95 1016 8.10 0916 1012 7.95 19.12 1326 18.26 0934 19.99 0939 18.86 0944 1208 14.26 1213 14.75 17.75 1010 18.01 1015 7.64 1148 11.79 1153 18.34 18.65 16.42 0955 19.07 1000 19.39 18.58 1241 11.17 0942 10.33 0947 8.04 11 81 0921 1016 1526 ------- *~~~ r·------ 5.78 0926 8.10 5.96 LF- 19.55 1022 19.26 2227 13.95 1223 13.31 18.70 1020 16.23 1024 18.40 1143 12.64 12.97 27MWX61XXX96XX 27MW602XXC96XX LFLF- I 27MW602XXC96XX 27MWX66XXX96XX LF-I 27MWX61XXZ96XX 27MWX29XXX96XX 22-Feb-94 0909 20--Feb:9 l215 1014 1304 1000 1251 14.511004 27MWX69XXX96XX 04-Mar-9W 1340 1435 1425 17 05 1430 17.76 1435 15.06 1410 15.48 1415 15.68 1420 15.78 27MWX68XXX96XX 04-Mar-9 1000 1035 1025 155.7 7.6 157.4 7.6 153.2 1010 17.25 1015 17.57 1020 18.68 27MWX66XXX962X 27MWX64XXB96XX 27MWX 64XXA96XX 04-Mar-9f 12-Mar-96 12-Mar-9( 1--r----19-Mar-96 1135 1255 1425 1049 1220 1334 1210 1220 1334 1449 1117 18.13 1215 17.28 13.87 1454 17.86 1459 17.74 '-------* 11.38 1123 11.70 1128 17.99 13.39 17.25 11.53 1155 1459 1311 14.63 1200 14.94 17.74 1444liii11.25 16.10 1205 12.87 18.09 11.47 12.3 1267 11.97 1216 1514' 19 94 10 82 1356 i0.32401i 1636 IS48 ii8.30 39-.0 120 1200 1535 1.1.1 LF-I LF-I LF- I LF-1 LF-I I .I'- I 11-I IF-I MAi ~wX-----37X S(1 IUCKI SCIIUCK 2 ,-- ~~-- 300+ --- -- ··- - ·- 22-Mrar-9 120I 22-MN-I 22-Mar-9( 1330 150.) 1128 1242 1416 1015 19.02 0950 15.43 16.49 1531 18.44 1008 1606 0987 1323 '13.08 13281253 21232 2524 12 95 1406 17,79 1217 1529 1412 11295 18.08 17.42 10.28 LF- I 13.59 17 41 1215 16 59 12.47 12142 1416 1440 1106 19.711130 L---- .......... S8.62 0949 13.34 LF- I 4.54 i~~~~-~~~-~~-- 1218 LF1037 0932 1158 0951 ~~"~I~ '--~--I----- ~~---~-- 133.33 0956 17.32 i24•5 1221 18.50 ..... ....... ... Appendix A: Dissolved Oxygen Concentration Data for LF-1 (OPTECH, 1996b) ~h~i~ -t~T"1 155 i;8 220 255 YY I.F-1 1-F I LF-I LF-I LF-I LF-1 LF-1 LF- I LF-I L[-I LF-I LF- I F-I LFiLF-I LF-I LF- I LF-I LF-I LF-I LF-I 27MW567XXA952X 27MWX8 I XXX96XX 27MWX82XXX96XX 27MW568XXX96XX 27MW568XXX96XX 27MW568XXY96XX 27MW568XXX96XX SX9X 2M Z7MWS68XXT96XX 27MW56811YD96XX ISF-I 1k·-I 27 X66XXX9)63X 27MWXJOXXX962X LF- I 27MWX8XXX96XX LF-I 7MWXS4XXZ96XX LF-1L 27MWX71XXX 962X Lf-ij37MWwX-69XXX :962X TO-I 15-Apr-96 1430 23-A1221 23-Apr- ISIS 23-Apr-96 1410 15- 1430 15-Apr-6 1600 01-Ma- 101 195-200 177-182 154-159 148-153 181-186 146-151 163-168 149-154 •I27N1',.X86XXA96XX_148-1505 St- 11:-I 03MPX86XXB96XX 27MPX86XXC96XX 27MPXB6XXD96XX 27NII'XK.XS7(XX YXX 27KIWXS7XXX96XX iL,. 11140 16.16 IIJ.L05I IJ. '-~"~"' L--,J· ........... L 16.47 145 ' 15.41 11439 116.77 11444 115.14 ---~·---c----*---·r-----· --12.34 0929 61 .39 ( ~II~ 0934 7.22 1012 . 146.07 . 156.42 133.5-136 156.42 11-9-1215 156642 975-100 I1t42 1304 1604 1457 1543 1706 1533 111.1411538 1255 16.65 1258 19.69 1603 1601 1450 12.69 1454 1533 11.14 1538 1655 10.83 1701 1348 1203 1350 1104 1100 1259 1249 1336 1339 1429 1419 1626 1616 1622 1612 0948 0935 1150 1140 1617 1607 12.10 13.08 1139 7.31 3.83 9.11 9.19 1.57 8.62 1102 1254 1338 1424 1621 1617 0940 1145 1612 11.49 11543 15.50 1304 18.31 1605 14.89 1457 11.51 15.47 19.32 15.61 11.49 11.51 11523 1247 1556 1440 1342 19.99 1346 13.28 1104 1259 1339 1429 1626 1622 0948 1058 1239 1332 1349 1606 12.66 18.53 9.56 462 3.73 10.55 1244 1334 1354 1611 11.98 5.63 3.84 1359 6.33 1404 6 74 1150 8.63 1617 0920 1045 1537 9.51 400 8.65 0925 1050 1542 9.28 2.93 8.22 0930 1.58 1013 6.64 1018 6.489 ý-63-- 6.39 3.95 1305 6.96 1526 6.77 1531 6.64 102357 10.15 1053 10.16 1339 1.26 1.25 1.31 8.96 9.46 1602 9.22 4.54 5.89 3.01 7.02 1405 6.91 1410 6.86 11.98 12.77 11.49 745 3.82 9.07 8.24 1.68 8.588 1608 9.17 6.14 1008 6.58 9.91 .82 1516 7.15 1043 10.25 1329 1.48 8.39 155210.08 1557 6.53 6.65 1042 2.53 1355 7.17 17-inn- 1540 1653 1643 9.51 6.64 1032 1022 18-inn- 0905 18-Jun-9611215 11400 11350 1648 1027 9.50 6.58 1653 1032 9.48 6.58 7.05 1608 0942 1245 110251•-10 3.87-- 13303.88-- 7.95 9.28 2.59 8.17 -- 123 11107 11.39 11112 26-Jul-961 ns - LI 1.43 6.22 6-97 ........... 416 0Oo 1117 1205 1245 I1.14 1401 f= ......... 1.45 6.22 6.97 41% 403 11 1409 7 01 ....... ·"4" ....... ..... --,-.-4. ......--- 1521 1048 1334 1047 1400 7.17 8.11 1557 8.39 1028 6.32 1315 3.91 1536 6.74 1602 864 1607 862 1033 6.28 1038 6.27 1541 6.66 10.09 110310.07 110810.0511139.95 1349 1.10 1612 8.67 1102 6.02 1415 6.80 fi-52 10.53 6.41 14.14 .9541.178 1414 2.0711051.8211101.711115170 -1255 4.84- 1300 4.24 1613 10.34 1618 10.20 7]6.56 3.77 1250 3.81 ~~l~~'~~~~c-----·l-·------~----7..70 1530 17.55 jI.57 110141,58 10191.59 26-JnI-9610905 11123 11107 11.3911112 26-Jul-96 0920 1211 1155 6.23 1200 6.95 1240 26-Jul-96 441 11ii 1320 1146 09-Jul-90 8 14 1432 1444 1427 * -·· 4" .. lllxl1-~4 1050 12.55 'Z"f-1"7TTI~ 8.60 11600 ---.8--------E·------ ~~~xl~-r----r----t---r-·---- 9 26-i•utjO9S .. · 12.26 •-~~ •--•- +_---------13.34 1058 1-605 1555 1 c----~------t------r----·--·c·---- 11.98 12.05 12.92 7.40 3.81 9.04 8.85 1551 1154 1404 1637 1157 1440 i3ou ------ 13.68 135212.98 6.60 9.85 .80 8.40 6.54 6.67 18-un-1450 . · -" ~~~~^"" ~ ~7"---r"~" --------- ------~ ---- t-----~-----Jxxlx~---~t-----·l---------·I --------· (---------(--------· 1528 10.81 6.20 1048 1320 1541 1143 1354 1627 1147 1430 . ' 1523 11.26 1543 ....I 10.82 1706 11.06 1646 14.98 1649 13.15 1053 1325 1546 1148 1359 1632 1152 1435 1058 1330 1551 1154 1404 1637 1157 1440 ! ------I ----~ --" --- 111.26 16.95 1252 12.20 14.39 1558 18.91 18.02 1444 1605 6.24 3.88 6.66 9.85 .91 843 660 6.69 27-Jun- 0927 27-Jun- 1211 27-Jun- 1445 13-Jun-961010 13-Jun-96 1300 13-Jun- 961515 17-un- 61010 17-Jun-61325 ~----~ ,= : -7 -- IS-Ar-96 115 1340 1133015.39 11335 .57 11340 3.46 1320 9.9 --------- f--------- --- t--~-~-·(---i-··C----~---- 181 159-164 27MWX68XXX962X 27MWX83XXX96XX 2WXSOXXD962X ImO I .7 51 192-197 [f-I LF-I LF-I LF-I LF- I I.F-I V, 01-Apr-961110611151 11140 01-May 0955 102 27MW568XXB96X3 1219 01-May27MW568XXY9x3____ 108 157.96 27MWXSOXXD96XX 1306 17-Apr-96 291,2 288-293 II-Jun- 1306 2iMWX62XXX962X 220-225 12-Jun- 1500 27MWXXXX96XX 13-Jun- 1350 184-189 27MWXBIXXX962X 250-255 14-Jun-96 0825 27MWX4 IXXA962X 201-206 25-Jun- 1006 27MWX6XXX962X 26-Jun-96 1439 183-188 27MWXS8XXX962X 27MWX60XXX962X 27MWXXXX96XX I6.3I . _ 112 . ý_ !ý 3ýJO93 12.00 11130 115.39 11135 ~"^1"Cl~rxrrr~Errr--~ -----L-1-t-1-~-'·*-~XXIII~I^-1~-~~"I~'I"1~-~CT"=IIZ~-*I i150-7 1113 152 128 j15i7 1160f1-3 1---0"5529 14ý25 11,80 *---*---·C----~---OlApr-96--__#,------------------- -------------------------I I~----~-----*-------09 58 71.31 1039 68.81 .22:Se[95 P1042 1033 7..00 11036 7.48 I----~ Oý39 1l-·-----~ 1 1.4ý Lýý 12.15 1146 12.32 1149 12-Ar-96 1101 1204 11J54 .I1496 1159 14.05 1204 n ~I k -- 1150 1449 10.07 16.32 1200 14.66 16.90 15.22 -* 16.83 17.37 0943 ::.,-.,..-.--4 6 11.97 1i14 11.76 11151 1300 k- 9.94 7.03 3.99 1628 9.81 1002 6.92 1305 14.35 ... .... ... 8.08 1545 18.24 ;·----r·-··--·---r---·---E-I----- 1617 8.57 1107 6.39 1420 6.74 1633 1007 .... 9.71 6.82 4.82 ... 8.4. 1622 8.44 1112 590 1425 6.72 1638 1012 11320 ..... -T-" 9.56 6.73 5.61 • ...... 1.57 1004 1,56 1057 1052 1.51 1057 1.35 · 11150 16.36 1230 16.89 i nIof 109~ 1111% m 1943211fs- 1102 I 1.36 ---- -*-)-- iin-l·-·· -1·-·-··-·I 1121 112% 1407 HAIS 1412? 8.13 ........ I---- - 1ý117 jk30 j1422 [8-22 L ......... . Appendix A: Dissolved Oxygen Concentration Data for LF-1 (OPTECH, 1996b) LF-I LF-I LF-1 LF-I 27MW568XXZ963X 27MWXS4XXX96XX 27MWX59XXX96XX 27MWXS6XXX9XX 27 i 37x 1341 2 LF127MWX73XXX962X LF-I 27MWX65XXX962X LF-I 127MWX72XXX962X LF-I 27MWX69XXX962X ------ I--------~_~·I1-2·"---1----; LF-1 LF-I j 27MPX74XXA96XX 27MPX74XXB96XX LF-: 127Mi'X74XXC96XX LF-I J27MPX74XXD96XX -·---1·---------·---~ LF-I j27MPX74XXE96XX | ....... 325-330 149-154 11540 11~-~111. ·III~XII~l~ 1322 - I........... 28-Jun. 18-Jun 1215 i15s 15 15J1553 1443 IIII~-·XI*I^I~··~X^IIX ·IIXIII~X~-·I ii ..I - - 1540 8.08 h ·I~-·^·~X~*IIIIII_-X11IIXI Illlll^ill~-·IX IXI11~1I · 172.35-174 29-Jul-960855 1018 0955 3.93 11000 3.94 11005 3.96 0945 3.96 10950 3.94 29-Jul-96i 06-55 1032 1037 8.55 11042 8.55 1022 8 45 157-160 8.55 1027 3.47 143-145 29-Jul-96 5.91 s-K.4, 3_47._ 5.90 1149 5.90 I 129 15.911134 5.91 +-------1-------------(-----546o" 97ulk ·-·· 0855 123-126 5.27 I3340 I----C----C-----C---5.38 ~---------C--------S L L ·--------1-----·-I, • 4-~------- · ·--- ~----------- ~-----------~-------r~--------· li -104-106 8.27 11444 18.30 1422 18.31 11427 8.31 1432 18.28 ! 913 8.26 I'442 84-87 8.21 E--~------~--~------~--------11523 8.23 11528 18.19 8.20 Ii---_·-~-~-~-~-t----t----~~-z~-~-~--· 29-Jul-9610855 11540 11518 -------1150818.21 11513 ---------~-------i--------~7---~----(----*----------*-------· I~ 42-45 25-JuI-9610920 1409 1353 .54 1403I .54 1358 i.54 11348 11.42 3 ~--------i-------- t • ----& -4- ~ 33338 I 1425 1403 63 1413 .70 1408 11348 11.04 11353 .84 .59 1.77 I -1434- lu---- ·· f27MPX59XXE96XX 27MWX61XXZ962X 1407 6.27 8.15 3.77 219-223 ^."I^II*L~*X1X^.~.IX~··111 193-198 iiM27PX74XXF96XX LF-I F:-1 iN6i 1600 6.43 F 4 ~I ----·l----·C-··---6.20 1145 8.39 1125 3.03 1402 1412 3.80 4.33 1327 4.03 132 13.l96 1337 3,82 11342 3.74 1347 3 751 1352 13.77 8.32 11351 8.23 ---11246 111.23-·11251 ------· 10.38 11256 110.10 1301 9.91 E---4· 11306 9.74 1311-- .531 113161953 · -· E----~--*----- ~---4----· ---... .4-.... -. '4......1200 11210 .94 3.93 13.90 11330 1043 11l35 11.30 31140 1.13 11145 1.09 1125 1150 i1.03 1155 1.99 1125 1108 t·"---2.56 11118 !~"~X"LL"*~~^"~.~""I*~~ 2.87 1043 2.77 2.58 11113 '~"XI*I~L"t'~"~·I 2.55 11018 13.70 13023 2.99 1028 12.96 1033 2.92 11038 1048 2.73 -^~"~Z~^T~"I~* --- ~---fr------~~------~-----~--~I·--: ---I--I--.... .. 6.37 11453 6.44114586.46 11413 1.63 31418 .421423 1.66 1428 3 .04 1J4 3 3 4.96 1438 5.85 6.24 3505 13448 I .53 1133 .54 .26 11203 .22 13208 .24 1123 1.75 1128 .52 1138 .45 11143 .35 .28 .30 8.iS 8.60 600 8.67 3605 8.73 52517.50 1530 7.55 13535 17.76 3.29 8.43 · 111" Tg.................... f ........ 1................... •.................... i ...........g................. .................. ff ..................... 6.20 1555 7.95 1140 3.78 8.49 11346 11205 .95 - L L dW-if - -5- -- · S1-Jul-96j0900 i144 ijj5-XII 5.28 .54 1.320 ?04 38 1325- 11343 11.42 1-- i. .. Q I1358 F::--··-|...... .. ............ i, ......... A---- ----~ ·-.. . . . 4... ... "t, . ... ----- 10.2 Appendix B: Explanation for Equations 7-1 and 7-2 If the total time of bioremediation was only dependent on the time needed for the contaminant to biodegrade, the zeroth order and first order equations would be: C = Co - (ko*to), and (A-l) C = Co*exp(-kl*t1 ), (A-2) and the bioremediation time would be derived by rearranging the equation and isolating t: to = (C - C) / ko, (A-3) t, = (-1 / kj) * In (C / Co). (A-4) If the total time of bioremediation was also dependent on the time for the biozone to be established, tb, equations A-i and A-2 would be modified: C = Co - [ko*(to - tb)], (7-1) C = Co * exp[-k,*(t, - tb)]. (7-2) Now if the bioremediation time is derived by rearranging and isolating t: to= [(C - C)/ ko] + tb, (A-5) t, = [(-1 / k,) * In (C / Co)] + tb. (A-6) Equations A-5 and A-6 demonstrate that the bioremediation time is greater when the time to reach steady state is taken into account, as shown in Equations 7-1 and 7-2. Appendix B. Spreadsheet Calculations for Conceptual Design Location R (ft) al R of Well 1 2 3 4 5 6 7 8 9 10 11 12 13 14 1500 0.06 720 0.125 960 0.094 1200 0.075 1500 0.06 1500 0.06 1320 0.068 1320 0.068 1800 0.05 1500 0.06 960 0.094 1080 0.083 1500 0.06 1200 0.075 that erfc(-(1 2.4 2.5 2.2 2.4 2.8 2.7 2.4 2.6 6*a*(tha t 1.5)/3/R)) 1.930497262 1.871406366 -~---~ 1.898778508 3 2.8 2.6 2.4 2.4 2.5 that)/(SQRT(1 1.919419896 -- 1.930497262 1.930497262 1.923167114 -~~---~~ 1.923167114 1.93112827 1.930497262 1.898778508 1.913124088 1.930497262 1.919419896 Chat 0.9652 0.9357 0.9494 0.9597 0.9652 0.9652 0.9616 0.9616 0.9656 0.9652 0.9494 0.9566 0.9652 0.9597 Ccontam (pglL) 64 10 30 30 48 26 26 10 0 0 BOLD: PCE plain: TCE Required Substrate: Required Oxygen: substrate Oxygen Cinject Cinject (pg/L) (pg/L) (mg/L) (mg/L) 4700 53.87952 53.87952 4700 2000 2000 24.86747 24.86747 39.78795 2800 2800 2000 1500 1500 4869.2118 57.581848 56.751767 4897.313 2072.005 2072.005 25.860956 25.860956 41.206949 2900.807 2949.2645 2090.821 1554.0038 1562.9722 28.2 n/a n/a 22.72 12 29.2153 n/a n/a n/a 16.8 9.09 4.16 9 9 n/a n.a 23.6738 12.432 12.432 n/a n/a n/a 17.4048 9.57458 4.34891 9.32402 9.37783 Appendix B. Spreadsheet Calculations for Conceptual Design time to Required Phosphate: reach Required Required Required Ammonium: steady Q (ft3lhr) Phosphorus Phosphate Nitrogen Ammonium Cinject (pg/L) state: t (iglL) (pglL) Cnjct(glL) (pglL) (pglL) (yrs) 17069.2 5 144.032258 149.21778 47 282.857143 293.040709 220 5 4915.92 0.051510056 0.1578534 0.16870029 0 0 0 0.16626836 8011.13 5 0.1578534 0.051510056 0 0 0 11834.6 5 144.032258 150.078947 47 282.857143 294.731907 220 17069.2 5 61.2903226 63.4969277 20 0 0 0 17069.2 5 61.2903226 63.4969277 20 257.142857 266.400644 200 0.07576608 13769.1 5 0.07285541 0.023773872 0 0 0 13769.1 0.07576608 5 0.023773872 0.07285541 0 0 0 22531.3 5 0.228229 0.2934375 0.30390265 0.038038195 0.11656866 0.12072596 17069.2 5 85.8064516 88.8956988 28 0 0 0 8011.13 90.3806855 5 85.8064516 28 0 0 0 9954.74 5 61.2903226 64.0735465 20 0 0 0 5 17069.2 45.9677419 47.6226958 15 0 0 0 11834.6 45.9677419 47.8975362 ----------I I 0 I 0 45.9677419 I SS seepage drawup (ft) at d (ft) velocity (ft/day) distance d 0.986301 0.591781 0.723288 0.854795 0.986301 0.986301 0.90411 0.90411 1.084932 0.986301 0.723288 0.798904 0.986301 0.854795 2.023005 0.552555 0.919667 1.380608 2.023005 2.023005 1.617221 1.617221 2.704602 2.023005 0.919667 1.152563 2.023005 1.380608 assuming regional drawdown velocity = <<<aquife 0.86 r thicknes ft/day s 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 0.001 Appendix B. Spreadsheet Calculations for Conceptual Design Total Oth order bioremediation time (yrs) n/a n/a Total 1st order bioremediation time (yrs) 5.000010052 5.000011152 5.000011152 5.000010052 5.000000852 5.000000852 5.000004647 5.000004647 5.000007992 7.719930409 13.87280115 13.87280115 7.719930409 5.739498976 5.739498976 11.19814401 11.19814401 12.82400408 5.000003578 6.758906911 5.000003578 5.000000852 6.758906911 5.739498976 n/a n/a