Appendix: Some illustrative computations.
advertisement

Appendix: Some illustrative computations.
In this appendix we present some numerical computations to illustrate the model we discussed in the
Project Background.
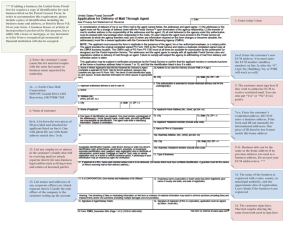
The relevant geometry is given in Figure I.
Figure I: Geometry
Tumor colony
y= l
Extracellular Matrix
(ECM)
y=0
Basement Lamina
x=0
Capillary
(BL)
x=L
In [18] we considered three cases for the angiostatin:
A.
B.
ar (x; t) = 0; no therapeutic agent present.
ar (x; t) = A0 > 0 agent uniformly distributed in the circulatory system and EC produce protease
inhibitors in response to angiostatin.
C. ar (x; t) = A0 > 0 and angiostatin acts directly as an inhibitor.
Below we present some of the simulations for cases A, B above. We have included only that part of the
computations that involve propagation in the ECM and none of the computations involving the breakdown
of the capillary wall in the interest of brevity.) We took 1 = 0: That is, we assumed in nite tumor capacity
to supply growth factor at a xed rate.
The model involves 67 biological and empirical parameters. Most of them (enzyme constants, cell movement constants, protein di usion constants) were found in the literature.(Table 1 below.) Others were
guesses or, in the absence of other information, set to zero or unity where appropriate. (For example, we
took 1 = 2 = 0 since we did not have an estimate for these rate constants for the EC induced growth
factor and angiostatin when [18] was being prepared.)
The curvature sensitivity constant , was adjusted to give tip proliferation. It is a surprising consequence
of this that the value for this parameter that gave realistic looking EC densities also gave realistic tip speeds
and crossing times. (Changing this constant also changes the tip speeds. Low curvature sensitivity slows
the tip speed while very high sensitivity causes singularities in the EC density.)
I. No angiostatin case:
1
2
Distance
from
capillary
to tumor
in microns
0:00
2:50
5:00
7:50
10:00
12:50
15:00
17:50
20:00
22:50
Extrap.
times
Time Mean
in days
in
velocity
for
hours (mm/day) 1 mm
3:49
5:817
3:74
0:242
6:817
3:88
0:436
7:317
3:99
0:545
7:650
4:09
0:580
7:900
4:18
0:703
8:100
4:25
0:831
8:267
4:32
0:914
8:410
4:38
1:015
8:535
4:43
1:015
8:646
( ` = 25mm, L = 50mm)
Extrap.
times
in days
for
2 mm
11:633
13:633
14:633
15:300
15:800
16:200
16:533
16:819
17:069
17:291
A. The onset of sprouting time and the onset of vascularization time, when scaled up to one or
two mm are in very good agreement with the experimental results of Folkman and his colleagues
[1, 4, 6, 9, 10] as well as with CAM assay experiments. (Table below.)
B. The channel widths are in agreement with the known (6-10 mm) widths for capillary diameters.
C. EC proliferation is a maximum a little behind the moving tip.
D. The tip speed increases as the forming capillary approaches the tumor source. (This was observed
in the rabbit cornea experiments also.)
E. The model predicts the onset of sprouting without EC
movement into the ECM when one allows for protease di usion in the ECM.
II. Angiostatin case:
A. The opening from the mother capillary closes.
B. The EC density in the daughter capillary drops.
C. The tip retreats and the channel closes.
D. It takes much longer for the channel to close completely than for the EC density to fall to negligible
values.
Travel times and tip speeds
In the gures below we plot time courses for ECM propagation of endothelial cell density, forbronectin
density and protease density in the absence of angiostatin (Figures 1-3) and in the presence of angiostatin
(Figures 4-6). Figure 7 is a time course for the active protease. The vertical axes are in relative units.
In the interests of brevity we have omitted time courses for the other variables. The notation Tx in the
gures below refers to the fraction of the ECM the sprout has crossed from the mother capillary to toward
the tumor. For example, T:25 = 3:90 means 25% of the ECM has has been crossed by the daughter capillary.
3
Figure 1: Time course for EC propagation in the ECM (no angiostatin)
T0.02 = 3.52 hrs
0.5
T0.25 = 3.90 hrs
Capillary
side
20
10
0
0
0.8
T
0.60
0.8
0.6
0.5
y
0
0
0.5
0.4
1
0.2
0.6
y
0.4
1
x Tumor side
0.2
x
T0.90 = 4.43 hrs
= 4.25 hrs
40
50
20
0
0
0.8
0
0
0.8
0.6
0.5
0.4
y
1
0.2
x
0.6
0.5
0.4
y
1
0.2
y axis scale: 0.1 = 2.5 microns, x axis scale: 0.1 = 5 microns
x
4
Figure 2: Time course for fibronectin propagation in the ECM (no angiostatin)
T0.25 = 3.90 hrs
T0.02 = 3.52 hrs
1
1
0.999
Tumor side
0.5
0.998
1
0.997
0.2
0.5
y
0.4
0.6
x capillary side
0.8
1
0
0.2
0.5
0.4
0
0.6
x
T0.60 = 4.25 hrs
0.8
y
0
T0.90 = 4.43 hrs
1
1
0.5
0.5
1
0
0.2
0.5
0.4
x
y
0.6
0.8
1
0
0.2
0.5
0.4
0
0.6
x
y axis scale: 0.1 = 2.5 microns, x axis scale: 0.1 = 5 microns
y
0.8
0
5
Figure 3: Time course for protease propagation in the ECM
T
0.02
= 3.52 hrs
T 0.25 = 3.90 hrs
0.02 Capillary
side
10
0.01
5
0
0
0.8
T
0.60
1
0.2
y
= 4.25 hrs
T
0.90
20
10
10
0.8
0.4
1
0.2
0.2
x
= 4.43 hrs
0
0
0.8
0.6
0.5
0.4
1
x Tumor side
0
0
0.6
0.5
0.4
20
y
0.8
0.6
0.5
y
0
0
0.6
0.5
0.4
y
1
0.2
x
y axis scale: 0.1 = 2.5 microns, x axis scale: 0.1 = 5 microns
x
6
Figure 4: Time course for EC propagation in the ECM (with angiostatin)
T = 4.45 hrs
T = 8.61 hrs
50
50
Capillary
side
0
0
0.8
y
0
0
0.8
0.6
0.5
0.5
0.4
1
0.2
0.6
0.4
y
1
x Tumor side
x
0.2
T = 16.7 days
T = 11.93 hrs
50
50
0
0
0.8
0.8
0.6
0.5
y
0
0
0.4
1
0.2
x
0.6
0.5
0.4
y
1
y axis scale: 0.1 = 2.5 microns, x axis scale: 0.1 = 5 microns
0.2
x
7
Figure 5: Time course for fibronectin in the ECM (with angiostatin)
T = 8.61 hrs
T = 4.45 hrs
1
1
Tumor
side
0.5
0.5
1
0
0.2
0.5
0.4
0.6
x Capillary side
0.8
1
y
0
0
0.2
0.5 y
0.4
0.6
0.8
0
x
T = 11.93 hrs
T = 16.7 days
1
1
0.5
0.5
1
0
0.2
0.5
0.4
0.6
0.8
0
y
1
0
0.2
0.5
0.4
x
y axis scale: 0.1 = 2.5 hrs, x axis scale: 0.1 = 5 microns
0.6
x
0.8
0
y
8
Figure 6. Time course for protease in the ECM (with angiotatin)
T = 4.45 hrs
T = 8.61 hrs
15
10
5
30
20
Capillary
side
10
0
0
0.8
1
0.6
0.5
0.4
y
0.2
0.4
1
x Tumor side
x
0.2
T = 16.7 days
T = 11.93 hrs
30
30
20
20
10
10
0
0
0.8
0
0
0.8
0.6
0.5
y
0.8
0.6
0.5
y
0
0
0.4
1
0.2
x
0.6
0.5
0.4
y
1
y axis scale: 0.1 = 2.5 microns, x axis scale: 0.1 = 5 microns
0.2
x
9
Figure 7: Time course for active protease in the ECM (with angiostatin)
T = 8.61 hrs
T= 4.45 hrs
−3
x 10
15
2
10
5
1
Capillary
side
0
0
0.8
0.8
0.6
0.5
y
0
0
0.5
0.4
1
0.6
y
0.2
0.4
1
x Tumor side
x
T = 16.7 days
T = 11.93 hrs
−3
−3
x 10
x 10
2
2
1
1
0
0
0.8
0
0
0.8
0.6
0.5
y
0.2
0.4
1
0.2
x
0.6
0.5
0.4
y
1
0.2
x
y axis scale: 0.1 = 2.5 microns, x axis scale: 0.1 = 5 microns
Notes on the tabular entries
0. Remark. The natural length scale for our computations is millimeters (mm) while the natural time
scale is hours. Therefore, before entering the values discussed below into Table 1, we converted, where
necessary, the literature values to these units.
1. Cell densities 0: In [13], the length of an endothelial cell was estimated to be in the range 94 ; 141
microns while in [19] an approximate width was given in the range of 10 ; 18microns. It is generally
known that an endothelial cell has a thickness of about 1 micron. Using these dimensions, we estimate
a volumetric density for endothelial cells to be about 1012 cells per liter. As we noted earlier, the
0 s
kinetic equations may be rewritten so that the 0 s, 0s can be expressed directly in terms of the Kcat
0
and Km s.
10
Table 1.
Physiological and kinetic constants
i =K i :)
(In the table below i = 1=Kmi and i = Kcat
m
;
1
;
1
;
1
capillary
1 = 73:0 M h
1 = 0:007 M
= 4:56h;1
chemistry f0 = 1:0 10;2 M
Tf = 18:0 h
2 = 146:0 M;1 h;1
2 = 0:014 M;1
e = 1:7 103M;1
;
1
e = 1:0 M
Trel = 1:0h
;
3
2
;
1
ECM
DV = 3:6 10 mm h 1 = 73:0 M;1 h;1 1 = 0:007 M;1
chemistry Vr (x; y; t) = 0:0 Mh;1
2 = 0:014 M;1
;
3
2
;
1
DA = 6:5 10 mm h 2 = 146:0 M;1h;1 e = 1:7 103M;1
e = 1:0 M;1
Trel = 1:0h
TF = 18:0 h
3 = 19:0 M;1 h;1 3 = 1:28 M;1
DF = 3:6 10;8 mm2h;1 F0 = 1:0 10;2 M 3 = 19:0 M;1h;1
capillary
D = 3:6 10;6 mm2 h;1 1 = 0:1 M
2 = 1:0 M
EC eqn
1 = 1:0 M
2 = 0:1 M
ECM EC
DN = 3:6 10;6 mm2h;1 1 = 0:1 M
2 = 1:0 M
equation
= 1:40
1 = 1:0 M
2 = 0:5 M
= 1:1 10;9M;2
m1 = 2
A2 = 44:13 M;1
;
1
0
;
4
1 = 0:005 h
Ca = 10 M
capillary
A1 = 0:0
B1 = 1:0=h
;
1
source
Ar = 10:0 Mh
Tiv = T1
f1 = 0:60 M
1 = 0:3
TGF, angio 1 = 2:0 mm/h
2 = 2:0 mm/h
TGF
0 = 0:0 mm/h
source
m0 = 12
= 0:0
v0 = 4:0 Mmm h;1
Trel = +1
= 4:56h;1
Trel = +1
3 = 1:28 M;1
1 = 4:0
2 = 4:0
1 = 2:0
2 = 1:5
= 0:056 h;1
The capillary EC equation is linear in . Therefore earlier, if we re-normalize , = 0^, we may
take 0 = 1, i. e., in the computations below, the capillary cell density is expressed as a fraction of the
equilibrium cell density in the capillary and is therefore a dimensionless quantity. Likewise, the EC
equation, can also be rescaled in a similar manner and the kinetic equations correspondingly rewritten
since the ratio N=0 appears in the logistic factor.
2. Length scales. In Figure II we have taken ` = 25 microns = 2:5 10;2mm and L = 50 microns =
5:0 10;2mm: Therefore, in the gures which illustrate our computations, along the capillary, the
scale is 0:1 = 10 microns while the scale from the capillary to the VEGF source, 0:1 = 2:0 microns:
3. Cell movement and di usion constants. We took the "porosity" power m = 1: It is well known that
the time of travel across the ECM (the time interval from tumor activation to the onset of sprouting)
will increase with m: (We took m = 1:5 in another simulation (not shown) to verify this and to test
the code in the case m > 1.)
In [21] the authors used values in the range 6:9 10;11cm2 s;1 ; 3:5 10;10cm2 s;1 for the cell movement constant DN : (The reader is cautioned that in Table 1, we have converted these cell movement
constants as well as the di usion constants DV ; DA in units of mm2 =h.)
They also used values for DV in the range 3:1 10;7cm2s;1 ; 5:9 10;6cm2s;1. However, these
values are not appropriate for growth factor di usion in the ECM since there the authors were modeling
wound healing and di usion was presumed to be taking place in the uids that ll the wound after
injury. Molecular di usion is presumably much slower in the ECM which can be viewed as a porous
medium. (The image of the ECM we have in mind can be found on page 973 of [2] for the cornea of a
rat or the cartoon on page 991 of [2] for the basel lamina.)
11
4.
5.
6.
7.
8.
In order to obtain growth factor di usion coecients, we argued as follows. If one assumes that a
protein is spherical, then its di usion coecient should be inversely proportional to the two-thirds power
of its volume and consequently of its molecular weight The molecular weight of VEGF is of the order of
1:65 105: In the literature, [12], the authors give the value DTr = 7:4 10;7cm2 s;1 for tirapazamine
(3-amino-1,2,4-benzotriazine-1,4-dioxide) which has a molecular weight of 168 daltons as one easily
calculates from its structure given in [7]. Thus DV 7:4 10;7 (168=165000)2=3 7:4 10;9cm2 s;1 :1
Because proteins are not spheres and the ECM is not a homogeneous uid, we have been somewhat
more conservative than this and used DV 1:0 10;9cm2 s;1:
In order to estimate the di usion coecient DA for angiostatin, note that the molecular weight
of some angiostatins may be taken to be of the order 3:8 104 daltons. Therefore, DA DV (16:5=3:8)2=3 10;8cm2s;1: In [28], the authors estimated the di usion coecient of bronectin, DF
to be smaller than 5 10;12cm2 s;1 .
Proliferation and death rate constants, ; 1 : The proliferation rate, , was given [21] as 0:04h;1 and
in [22] as 0:056h;1 . For the death rate, the value %0:5=day was given in [8] and as 0:12=day in [3]. We
took the latter value in the above table.
Proliferation response function, : We took this function to be of the form (C) = A2 Ce;C m1 where
we used the proliferation response data given in [26]. The data there gives the proliferation response
as a function of growth factor.
For the curvature sensitivity factor we took the function
Q() = p 2 2 :
1+ This choice was made not only because we wanted the curvature sensitivity to be dimensionless, but
also because we wanted to control the sensitivity to proliferation. (With this choice and with > 0
the maximum sensitivity is 1=: ) It was found that for small the solutions of the EC cell movement
equation attempted to blow up in nite time.2
;1 . We took = 4:56h;1 for illustrative purposes based on our
Enzyme and inhibitor decay rates ; Trel
reading of [5]. As remarked earlier we were not able to nd in vivo values for the relaxation times.
;1 = +1 when angiostatin generates an
Therefore, in order to test angiostatin ecacy we took Trel
;
1
inhibitor and Trel = 1 hour when angiostatin acts directly as an inhibitor. (In the latter case, a
decay term in angiostatin must be included in the model, otherwise the model will be computationally
unstable.)
Initial densities bronectin, f0 , F0 The density of bronectin has been estimated to be about 10;2M
[24]. We took the density of the lumen, f0 to be the same as the background density of bronectin, F0
in the ECM, for want of better information.
The number of angiogenic response receptors r0 ; ra0 . The number of receptors per endothelial cell, has been variously estimated as 150000 [27] and 175000 [25]. We took 105 as an order of magnitude
estimate based on these two numbers. Then r0 = 0 1M. We also took ra0 = 1:70 1:7M:
1 The ratio of di usion coecients for small molecules is inversely proportional to the one-thirds power of its volume and
consequently of its molecular weight. This follows from the Stokes formula for the drag on spheres moving through a uid and
the Einstein formula D = kT u where k is the Boltzman constant, T is the Kelvin temperature and u is the particle mobility.
[15]. However, proteins are large molecules and the ECM is not a uid. Hence our assumption seems more reasonable. If we
use the Stokes-Einstein relationship, the di usion constant would be larger by a factor of ten, i. e. DV
7:4 10;8cm2 s;1
which is at the lower limit of the range of values used in[21].
2 This is to be expected. We can show that for N takes the form, for large V; N ,
N Nt
DN
N ln
+ M N 2Q()
T (Ca ; F )
where M = M (V; C ) will be positiveif max G(C )1 =1 > : Thus Q() is large, we might expect nite time blow up. (Di erential
equations of the much simpler variety such as Nt = DN + N 2 are well known to possess solutions which form singularities in
nite time.)
r r
12
V , and K V re9. The kinetic parameters 1 ; 1 for VEGF. In [14], the following values are given for kcat
m
V
;
1
V
V =K V
spectively, namely kcat = 162min and Km = 130M. Using these, we easily determine 1 = kcat
m
and 1 = 1=KmV :
10. The kinetic parameters 2 ; 2 for angiostatin. The mechanism for the conversion of angiostatin to protease inhibitor which we propose here has yet to be documented in the literature. Therefore, the values
we have taken are for illustrative purposes only. We took 2 = 1 and 2 = 21:
11. Protease inhibitor equilibrium constant e. In [23], the authors give values for 1=e in the range 0:59nmol/L
to 2:4nmol/L. We took 1=e = 1:0nmol/L = 10;3M. The value used in the computations has to be
non-dimensionalized. This means e = 1000 ra0 = 1700:
In the case for which angiostatin is itself an inhibitor, we took e = 1(M);1: This is based on the
reported value for plasminogen derived angiostatin which is an inhibitor of tPA. See [24].
12. The kinetic parameters 3 ; 3 for bronectin. For " bronectin", we have taken values for 3 ; 3 from
[11]. We took, Kcat = 16 per hour and Km = 0:83 M for the hydrolysis of type I collagen (rat tendon)
by HFC (human broblast collagenase).
13. The bronectin production times Tf ; TF . The value Tf = 18 hours has been reported in [28, 20]. We
shall take TF = Tf = 18 hours and F0 = f0 .
14. The angiostatin source term ar (x; t). Here we took Ar as in the table. We took Tiv = T1 where T1 is
the time from the initiation of the tumor secretion into the ECM to the time the responding capillary
has crossed the ECM back to the tumor. Since we do not as yet have a good mathematical model for
the penetration of the capillary into the tumor region, we have made this choice for Tiv for illustrative
purposes only. We took Tiv = 4:5 hours.
16. Other constants.
i. Sensitivities, i ; i; i : These constants do not a ect the travel time across the ECM nor do they
a ect the width of the nascent capillary opening as was demonstrated numerically in [16]. However,they do control the distribution of endothelial cell density within the forming capillary. We
have taken them so that the endothelial cells are somewhat more responsive to protease and
bronectin changes in the capillary than they are in the ECM. The values in Table 1 are for
illustrative purposes only.
ii. Threshold constants f1 ; 1; Ca0; F1. These were selected for illustrative purposes only. The threshold f1 is a measure of the percentage of the lumen that must be destroyed before endothelial cells
can escape into the ECM while 1 represents the percentage of endothelial cells that are able to
cross this barrier into the ECM.
iii. Transport velocities ; 0 . These constants were also selected for illustrative purposes only.
References
[1] Ausprunk, D.H., Folkman, J., Migration and Proliferation of endothelial cells in performed and newly formed blood
vessels during tumor angiogenesis, Microvasc Re., 14, 73-65, (1977).
[2] Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K., Watson, J. D., Molecular Biology of the Cell, 3rd Ed.,
Garland Pub. Inc. N.Y. and London, 1994.
[3] Araki, S., Shimada Y., Kaji, K., Hayashi, H., Apoptosis of vascular endothelial cells by broblast growth factor
deprivation Biochem. Biophys. Res. Commun., 168(3), 1194-1200, (1990).
[4] Brem, H., Folkman, J., Inhibition of tumor angiogenesis mediated by cartilage,J. Exp. Med. , 141 ,427-439, (1975). an
[5] Boffa, M.B., Wang, W., Bajzar, L., Nesheim, M.E. Plasma and recombinant thrombin-activable brinolysis inhibitor
(TAFI) and activated TAFI compared with respect to glycosylation, thrombin/thrombomodulin-dependent activation,
thermal stability, and enzymatic properties J. Biol. Chem. , 273(4), 2127-35, (1998).
[6] Brem, S., Preis, B. A., Langer, ScD., Brem, B. A., Folkman, J., Inhibition of neovascularization by an extract
derived from vitreous Am. J. Opthalmology, 84,323-328, (1997).
[7] Cahill,A, Jenkins, T. C., White, I. N. H., Matabolism of 3-amino-1,2,4- benzotriazine-1,4-dioxide (SR 4233) by
puri ed DT-diaporase unde aerobic and anaerobic conditions Biochem. Pharmocao., 45, 321-329, (1993).
[8] Cho, A., Mitchell, L., Koopmans, D., Langille, B. L., E ects of changes in blood ow rate on cell death and cell
proliferation in carotid arteries of immature rabbits, Circ. Res. , 81(3) , 328{337, (1997).
13
[9] Folkman,J., Angiogenesis-Retrospect and outlook: in Angiogenesis: Key Principles-Science-TechnologyMedicine, Steiner, R. Weisz , P. B., Langer, R., Birkhauser, Basel, 1992.
[10] Folkman, J., The vascularization of tumors, Scienti c American, 234, 58-64, (1976).
[11] Fields, G., Netzewl-Arnett, S. J., Windsor, L.J., Engler, J. A., Berkedal-Hansen, H., van Wart, H. E.
Proteolytic activities of human broblast collagenase; Hydrolysis of a broad range of substrates at a single active site,
Biochemistry, 29,6600-6677, (1990).
[12] Hicks, K. O., Fleming, Y., Siim, B. G., Koch, C. J., Wilson, W. R., Extravascular di usion of tirapazamine: e ect of
metabolic consumption assessed using the multicellular layer model, Int. J. Radiat. Oncol. Biol. Phy. , 42(3), 641-649,
(1998).
[13] Haas, T. L., Duling, B. R., Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles.
Microvasc Res. , 53(2), 113-20, (1997).
[14] Kendall, R. L., Rutledge, R. Z., Mao X., Tebben, A. J., Hungate, R. W., Thomas, K. A., Vascular endothelial
growth factor receptor KDR tyrosine kinase activity is increased by autophosphorylation of two activation loop tyrosine
residues. J. Biol. Chem., 274(10), 6453-60, (1999).
[15] Landau, L.D., Lifschitz, E.M., Course of Theoretical Physics, Volume 6, Fluid Mechanics, Pergamon Press, Oxford,
UK, 1982.
[16] Levine, H.A., Sleeman, B.D., Nilsen-Hamilton, M., Mathematical modeling of the onset of capillary formation
initiating angiogenesis. J. Math. Biol., 42(3), 195-238, (2001).
[17] Levine, H.A., Sleeman, B.D., Nilsen-Hamilton, M., A Mathematical model for the roles of pericytes and macrophages
in the onset of angiogenesis: I. The role of protease inhibitors in preventing angiogenesis, Math Biosci., 168, 77-115,
(2000).
[18] Levine, H.A., Pamuk, S., Sleeman, B.D., Nilsen-Hamilton, M., Mathematical Modeling of Capillary Formation and
Development in Tumor Angiogenesis: Penetration into the Stroma, J. Math. Biology, (in press)
[19] Nerem, R. M, Levesque, M. J., Cornhill, J. F., Vasuclar endothelial cell morphology as an indicator of the pattern
of blood ow, J. Biomech. Eng., 103(3), 172-176, (1981).
[20] Orme, M.E., Chaplain, M. A. J., A mathematical model of the rst steps of tumour - related angiogenesis: Capillary
Sprout Formationand SecondaryBranching. I.M.A. Journal of Mathematics, Appl. in Med. and Biol., 13, 73-98, (1996).
[21] Sharratt, J. A., Murray, J. D., Models of epidermal wound healing, Proc. R. Soc. Lond. B., 241, 29-36, (1990).
[22] Stokes, C. L., Lauffenburger, D.A., Analysis of the roles of microvessel endothelial cell random motility and chemotaxis in angiogenesis, J. Theor. Biol., 152 ,377-403, (1991 ).
[23] Takahashi, K., Kwaan, H. C., Koh, E., Tanabe, M., Enzymatic properties of the phosphorylated urokinase-type
plasminogen activator isolated from a human carcinomatous cell line. Biochem. Biophys. Res. Commun., 182(3, 147381, (1992).
[24] Terranova, V.P., DiFlorio, R., Lyall, R.M., Hic, S., Friesel, R., Maciag, T. Endothelial Cells are Chemotactic
to Endothelial Cell Growth Factor and Heparin J. Cell. Biol. , 101, 2330-334, (1985).
[25] Terman, B. I., Dougher-Vermazen, M., Carrion, M.E., Dimitrov, D., Armellino, D. C., Gospodarowicz,
D., Bohlen, P., Identi cation of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor
Biochem. Biophys. Res. Commun., 187(3) , 1579-86, (1992).
[26] Unemori, E.N., Ferrara, N., Bauer, E.A., Amento, E.P., Vascular endothelial growth factor induces interstitial
collagenase expression in human endothelial cells. J. Cell Physiol. , 153(3), 557-62, (1992).
[27] Waltenberger, J., Claesson-Welsh, L., Siegbahn, A., Shibuya, M., Heldin, C. H. , Di erent signal transduction
properties of KDR and Flt1, two receptors for vascular endothelial growth factor, J. Biol. Chem., 269(43),26988-95,
(1994).
[28] Yamada, K. M., Olden, K., Fibronectins - adhesive glycoproteins of cell surface and blood, Nature, 275, 179-184,
(1978).