Three Year Follow-up of an Allograft Kidney from a Metachromatic... – Ultrastructural Study

Light microscopy
Three Year Follow-up of an Allograft Kidney from a Metachromatic Leukodystrophy Donor
– Ultrastructural Study
Shefali S. Ballal, MD
1
, Neal Sondheimer, MD
2
, Sirma Koutzaki, MS
1
, Alden Doyle, MD
3
, Suganthi Soundararajan, MD
1
1 - Department of Pathology, Drexel University College of Medicine, Philadelphia, PA, USA
2 - Department of Pediatrics, The Children’s Hospital of Philadelphia, PA, USA
3 - Department of Internal Medicine, Drexel University College of Medicine, Philadelphia, PA, USA
Introduction
Metachromatic leukodystrophy (MLD) is an autosomal recessive lysosomal storage disorder characterized by the deficiency of the enzyme arylsulfatase A 1 .
It is characterized by demyelination and deposition of metachromatic material in the central and peripheral nervous system and visceral organs 2 .
Ultrastructural analysis of the metachromatic granules shows that they are composed of different types of inclusions 2 .
A literature review did not reveal previous reports of viscera from MLD being used for organ donation.
Case Presentation
The donor presented at two years of age with psychomotor retardation and cognitive decline and was diagnosed with MLD.
He suffered an unexplained cardiac arrest at age ten and his parents requested that the organs be transplanted.
One of the kidneys was transplanted to a 61 year old female with diabetic nephropathy. The graft started functioning effectively immediately.
There was one episode of acute cellular rejection which was treated with steroids.
Currently, the patient is well with a creatinine of
.
1.1 mg/dl.
The renal surveillance biopsies were performed at time zero, 3, 12, 24, and 36 months and examined by light and electron microscopy.
12 months 24 months 36 months
Acute cellular rejection
No evidence of rejection with subcortical scar
No evidence of rejection
Thick sections
Pale targetoid vacuolated structures in less than 5% of the distal tubules
Dark brown intracytoplasmic targetoid inclusions in 20% of the distal tubules.
Previously described Inclusions are absent
EM
Membrane bound concentric whorled inclusions with alternating light and dark circular bands in the proximal and distal tubules
Prominent inclusions of varying sizes
The inclusions are fragmented and dispersed. There is focal disruption of the proximal tubular brush border with extrusion of the inclusions
A
A
B
C
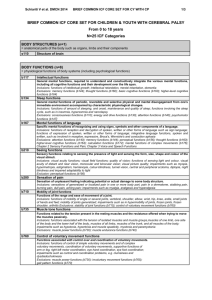
Figure 1: A. H&E: Intact proximal and distal tubules. B. PAS stain: normal glomerulus C. Toluidine blue stain: intracytoplasmic metachromatic granules D. Toluidine blue stain : dark brown intracytoplasmic targetoid inclusions in the distal tubules
D
B
C D
Figure 2: A. Electron microscopy reveals distal tubules with intracytoplasmic inclusions(x 4000) B. Higher power showing the membrane bound whorled concentric inclusions with alternating dark and light bands (x 30000) C. Proximal tubule with dispersed fragmented inclusions (x 4000) D. Focal disruption of the proximal tubular luminal brush border with extrusion of the inclusions into the lumen
Conclusion
.
1. In MLD patients metachromatic inclusions were observed in the distal convoluted tubules, thin limb of the loop of Henle, collecting tubules, tubular lumen and urine 3,4,5 . In our case, the inclusions were present in the cytoplasm of the distal and proximal tubules and tubular lumen.
2. The morphology of the inclusions in MLD may be quite variable. Large lamellae, herring bone, tuftstone and zebroid inclusions have been observed 3,4,5 .The
inclusions in our case resemble the zebroid type and type III inclusions described by Joosten et al 5 .
3. The inclusions are present in few tubular epithelial cells. Toludine blue stain highlights the targetoid vacuolated structures within the epithelial cells.
Extensive ultrastructural examination is often needed to identify and characterize the inclusions 6 .
4. During early post-transplant period, the inclusions are large and membrane bound within the cytoplasm.
5. Three years after transplantation, fragmentation of the inclusions and extrusion into the tubular lumen is observed.
6. Simultaneous expulsion probably indicates that the lysosomal pathology is being remedied, most likely due to uptake of host arylsulfatase A enzyme by the allograft 7
7. There are no reports of impaired renal function in patients with MLD, except for one case of proximal renal tubular acidosis 8 . The kidney function in the recipient has markedly improved due to transplantation.
8. The three year follow-up study, suggests the possibility that MLD patients can be potential renal donors, although further long term studies are required.
References
1.
Hess B, Saftig P, Hartmann D, Coenen R, Lullmann-Rauch R, Goebel HH, Evers M, Von Figura K, D’Hooge R, nagels G, De Deyn P, Perters C, Gieselmann V. Pheotype of arylsulfatase A-deficient mice: Relationship to human metachromatic leukodsytrophy. Proc Natl Acad Sci USA . 1996 Dec 10; 93(25): 14821-6
2.
Kolodny EH, Fluharty AL. Metachromatic leucodystrophy and multiple sulfatase deficiency: sulfatide lipidosis. In:
Scriver CR, Beaudet AL, Sly WS, Valle D, ed. The metabolic and molecular basis of inherited disease, New
York: McGraw – Hill; 7 th e. Vol 2, 1995; 2693-2740
3. Resibois A. Electron microscopic studies of metachromatic leukodystrophy. IV. Liver and kidney alterations .
Pathol Eur 1971. 6: 278-298
4.
Toga M, Berard-Bardier M, Pinasard N, Gambarelli D, Hassoun J, Tripier MF. Etude Clinique, histologique et ultra structural de quatre eas de leucodystrophie metachromatique infantile et juvenile. Acta Neuropathol 1972.
21:23-38
5.
Joosten E, Hoes M, Gabreels – Festen A, Hommes O, Schrumans Stlehoven H, Sloof JL. Electron microscopic investigation of inclusion material in a case of adult metachromatic leukodystrophy: observations on kidney biopsy, peripheral nerve and cerebral white matter. Actal Neuropathol 1975. 33: 165-171
6.
Rodriguez-Waitkus PM, Byrd R, Hicks J. Metachromatic leukodystrophy and its effects on the gallbladder:
A case report. Ultrastruct Pathol. 2011. Dec; 35(6); 271-6
7.
Kornfeld S. Trafficking of lysosomal enzymes in normal and disease states. J Clin Invest. 1986. Jan; 77(1):1-6
8. Rodriguez-Soraino J, Rivera JM, Vallo A, Prats-Vifias JM, Castillo G. Proximal renal tubular acidosis in metachromatic leukodystrophy. Helv Paediatr Acta. 1978 Apr; 33(1): 45-52