An algorithm to sample unobservable genotypes in complex pedigrees
advertisement

An algorithm to sample unobservable
genotypes in complex pedigrees
SOLEDAD A. FERNANDEZ, ROHAN L. FERNANDO and ALICIA L. CARRIQUIRY
Iowa State University, USA
SUMMARY
Probability functions such as likelihoods and genotype probabilities play an
important role in the analysis of genetic data. When genotype data are incomplete
Markov chain Monte Carlo (MCMC) methods, such as the Gibbs sampler, can
be used to sample genotypes at the marker and trait loci. The Markov chain
that corresponds to the scalar Gibbs sampler may not work due to slow mixing.
Further, the Gibbs chain may not be irreducible when sampling genotypes at
marker loci with more than two alleles. These problems do not arise if the
genotypes are sampled jointly from the entire pedigree. When the pedigree
does not have loops, a joint sample of the genotypes can be obtained efficiently
via modification of the Elston-Stewart algorithm. When the pedigree has many
loops, obtaining a joint sample can be time consuming. We propose a method
for sampling genotypes from a pedigree so modified as to make joint sampling
efficient. These samples, obtained from the modified pedigree, are used as
candidate draws in the Metropolis-Hastings algorithm.
Keywords:
METROPOLIS-HASTINGS; PEELING; ITERATIVE PEELING.
1. INTRODUCTION
Determining genotype probabilities is important in genetic counseling, linkage analysis
and in genetic evaluation programs. In genetic counseling, for example, it is important
to know which individuals in a population are probable carriers of a recessive disease
allele. The first methods for determining genotype probabilities were developed in human
genetics by Elston and Stewart (1971) and were reviewed by Elston and Rao (1978) and by
Elston (1987). Also, several human genetics computer packages are available to compute
genotype probabilities. In livestock, pedigrees are usually much larger because animals,
specially males, have multiple mates. Thus, the application of computer intensive methods
developed for humans will often be difficult or inappropriate in livestock data.
To obtain estimates of the genotype probabilities,
the likelihood of the pedigree is
P
needed. The likelihood L is obtained as L / g f (yjg)P (g), where y is the vector of
phenotypes and g is the vector of genotypes, f (yjg) are the conditional probabilities of
y given g, and P (g) are the genotype probabilities. The summation is over all possible
genotypes for all the individuals in the pedigree. The computations involved in the
likelihood are not feasible except in trivial examples. For example, assume that there are
1
two possible alleles for a locus, resulting in 3 possible genotypes for every individual in
the pedigree (AA, Aa or aA and aa). If the pedigree consists of 100 individuals then 3100
summations need to be performed in order to compute the likelihood as before.
For pedigrees without loops, the likelihood can be computed efficiently using the
Elston-Stewart algorithm (Elston and Stewart, 1971), which is also called peeling. Generalizations of this algorithm (Cannings et al., 1978; Lange et al., 1975, 1983) provide
strategies to compute the likelihood efficiently for general pedigrees with simple loops.
When a mixed model of inheritance is used, the likelihood is not easy to obtain. Under
this model, the phenotypic values of individuals in the pedigree cannot be assumed to be
conditionally independent given the pedigree members because they are also influenced
by the polygenic loci. Markov chain Monte Carlo (MCMC) methods, such as the Gibbs
sampler, have been proposed to overcome these problems.
When using the Gibbs sampler, however, mixing can be very slow due to the
dependence between genotypes of parents and progeny (Janss et al., 1995). The larger the
progeny groups, the stronger the dependence; thus the Gibbs chains do not move. When
this happens it is said that the chains are reducible ‘‘in practice’’.
In this paper we propose a method to sample genotypes from large and complex
pedigrees, and apply the proposed algorithm to estimate the genotype probabilities in a
Labrador Retriever pedigree under study. We use the Metropolis-Hastings algorithm and
sample genotypes (candidates) jointly from a proposal distribution that is close to the true
posterior distribution of interest. When there are no loops, our proposal is the ‘‘true’’
posterior distribution and the Metropolis-Hastings algorithm reduces to direct sampling.
When there are loops in the pedigree, we construct our proposal distribution by first
sampling genotypes using iterative peeling. A variation of this approach consists in peeling
the pedigree exactly up to the point where the complexity of loops makes it difficult
to continue, and then using Metropolis-Hastings to sample from a ‘‘partially’’ peeled
pedigree.
2. THE PEELING APPROACH FOR SAMPLING GENOTYPES
Before describing iterative peeling we discuss the Elston-Stewart algorithm (Elston and
Stewart, 1971) known as peeling.
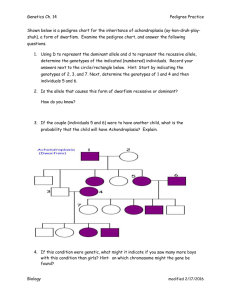
Consider the simple pedigree shown in Figure 1. To introduce the principles involved
in peeling we show how to sample genotypes from f (gjy) for a monogenic trait in
pedigrees without loops.
To obtain a random sample from f (gjy), we can use a rejection sampler based on
f (gjy), but this may be very inefficient. Instead, we sample individuals sequentially as
m m
m m
m
1
2
3
m
m
4
5
6
7
Figure 1. Simple two-generational pedigree.
described below. Thus, to obtain a sample from
2
f (g1 g2 g3 g4 g5 g6 g7jy) in Figure
1, we first sample the genotype for individual 1 from f (g1 jy). Next we sample g2 from
f (g2 jg1 y), g3 from f (g3 jg1 g2 y), and so on. To compute f (g1 jy) we use peeling
(Elston and Stewart, 1971; Cannings et al., 1978). The first step in computing f (g1 jy) is
to compute the likelihood.
The likelihood for the pedigree in Figure 1 can be written as
L/
XX
g1 g2
::
X
g7
h(g1)h(g2 )h(g1 g2 g3)h(g1 g2 g4)h(g5 )h(g4 g5 g6)h(g4 g5 g7)
(1)
where h(gj ) = P (gj )f (yj jgj ) is the probability that an individual with genotype gj has
phenotype yj (penetrance function), P (gj ) is the marginal probability that an individual has
genotype gj (founder probability), h(gm gf gj ) = P (gj jgm gf )f (yj jgj ), gm and gf are
the genotypes for the mother and father of individual j , P (gj jgm gf ), is the probability that
an individual has genotype gj given parental genotypes gm and gf (transition probability).
Computing the likelihood as given by (1) is feasible only for small pedigrees. The
Elston-Stewart algorithm can be thought of as providing an efficient reordering of the
additions and multiplications in computing the likelihood. Thus, L in (1) is rearranged as
XX
g1 g2
h(g1 )h(g2)
X
g3
h(g1 g2 g3)
X
g4
h(g1 g2 g4)
X
g5
h(g5)
X
g6
h(g4 g5 g6) X
g7
h(g4 g5 g7):
(2)
Note that (2) is identical in value to (1) but is computationally more efficient. For example,
consider the summation over g7 . In (1) this summation is done over all combinations of
values of g1, g2 , g3, g4 , g5 and g6. However, the only function involving g7, is h(g4 g5 g7),
which depends only on two other individual genotypes (g4 and g5 ). In (2), the summation
over g7 is done only for all combinations of values of g4 and g5. An expression involving g5
and g4 is obtained after summing out g7. These expressions must be stored to be used in the
final computation of the likelihood. The result, from peeling an individual
Pis called a cutset.
For example, after peeling g7 we obtain a cutset of size 2, c7 (g4 g5) = g7 h(g4 g5 g7).
To compute L efficiently, the order of peeling is critical. For example, consider
peeling g1 as the first step, the result is a cutset of size 3, c1(g2 g3 g4) =
P
h
g1 (g1)h(g1 g2 g3)h(g1 g2 g4). The computation of c1 (g2 g3 g4) involves summing
over g1 for all genotype combinations of g2 , g3 and g4 . Computing c7 (g4 g5) has lower
storage and computational requirements than computing c1(g2 g3 g4).
Thus, to evaluate the likelihood for this pedigree we first need to define the peeling
order. The peeling order is determined as follows. First we list all the individuals that need
to be peeled and then sort them according to the size of the cutset that is generated if that
individual is peeled. We always start peeling the individual with the smallest cutset in each
step.
In this case, the peeling order could be: 7, 6, 5, 4, 3, 2 and 1. Once all individuals
have been peeled we sample individual’s genotypes in the reverse order to which they
were peeled (reverse peeling, Heath, 1998). In this example, after peeling individual 1 we
compute the marginal probability for 1 as f (g1 jy) = P (g1)f (y1 jg1)c1 (g1 )=L.
Once f (g1 jy) has been obtained, we sample g1 using the inverse cumulative function.
Next, we compute
3
hP
i
f (g2 jg1 y) = f (y2 jg2)c4 (g1 g2)c3(g1 g2)= g2 (y2 jg2)c4 (g1 g2)c3(g1 g2) ,
and then we sample g2 from f (g2 jg1 y). Repeatedly applying this procedure, we eventually
generate a sample from the joint distribution of all genotypes for the entire pedigree. The
sampling sequence in this case is: sample g1 from f (g1 jy), g2 from f (g2 jy g1), g3 from
f (g3 jy g1 g2) and so on.
In pedigrees with complex loops, peeling methods as described above are not feasible.
The reason is that the cutsets generated after peeling some individuals are larger when there
are loops in the pedigree.
3. ITERATIVE PEELING TO SAMPLE GENOTYPES
Peeling methods cannot be applied when pedigrees are large and have complex loops.
Iterative peeling (Van Arendonk et al., 1989;Janss et al., 1992;Wang et al.,1996), however
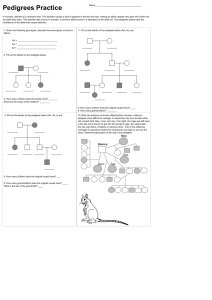
can be used to get approximate results. To describe iterative peeling, it is convenient to
present the pedigree as a directed graph (Figure 2 (a)). Before peeling, the graph contains
1
2
1
S11
2
S21
1
3
3
4
S 31
S41
S
S42
32
4
2
5
5
6
(a) Graph with 12 edges
S52
S62
6
(b) Graph with 8 edges
Figure 2. Graph representation of a two-generational pedigree with loops.
individual nodes and mating nodes. Each individual node is indicated by the individual
identification number; they correspond to the penetrance functions, and in the case of
founders, also include the founder probability function. Each mating node is indicated by
an oval, which corresponds to the transition probability function. The edges in the graph
connect the mating nodes with the parents and with the offspring.
Before proceeding with iterative peeling we modify the graph by merging mating
nodes into nuclear family nodes. The resulting graph with the merged mating nodes is
shown in Figure 2 (b). Here, the nuclear-family nodes are represented by rectangles. There
are 8 edges: S11, S21, S31 , S32 , S41, S42 , S52 , S62 in this graph. The first subindex of
S indicates the individual number, and the second subindex indicates the nuclear-family
node number. The edges Sij can be interpreted as either conditional or joint probabilities,
depending on their location in the graph. We use this small example to explain iterative
peeling. The general expressions and more details on the computations are presented in
Fernandez et al., (2000).
Suppose we want to sample the genotype for individual 1 from f (g1 jy). We first
obtain an estimate for the edge probability S11 , connecting individual 1 to the rest of the
pedigree through nuclear family 1. Once S11 is computed, the genotype probabilities can
be obtained from the normalized values of f (y1 jg1)P (g1)S11. Below we describe how to
4
iteratively compute S11 .
We first initialize all the edge probabilities. Typically, all edge probabilities are
initialized to 1. For this example, however it is convenient to set S41 to be equal to
the founder genotype probabilities. Once the edges are initialized we iteratively update
edge probabilities using the phenotypes and the current values of the appropriate edges
(explained below) of all the individuals in the corresponding nuclear family. Thus, we
update S11 as
S11 =
XXX
g2 g3 g4
f (y2 jg2)P (g2)f (y3 jg3)P (g3jg1 g2)f (y4 jg4)P (g4jg1 g2)S32 S42 :
At this stage, S11 is the conditional probability f (y2 y3 y4jg1). Note that the edges that
contributed to updating S11 are those that connect the members of nuclear family 1 to other
nuclear families.
Similarly S21 is updated as
S21 =
XXX
g1 g3 g4
f (y1 jg1)P (g1)f (y3 jg3)P (g3jg1 g2)f (y4 jg4)P (g4jg1 g2)S32 S42 and is the conditional probability f (y1 y3 y4jg2). Next, we update S31 as
S31 =
XXX
g1 g2 g4
f (y1 jg1)P (g1)f (y2 jg2)P (g2)P (g3jg1 g2)f (y4 jg4)P (g4jg1 g2)S42 which is the joint probability f (y1 y2 y4 g3). Next, we update S32 as,
S32 =
XXX
g4 g5 g6
f (y5 jg5)P (g5jg3 g4)f (y6 jg6)P (g6jg3 g4)f (y4 jg4) |{z}
S41 P (g4 )
which is the conditional probability f (y4 y5 y6jg3). Note that in these three cases, when
we multiplied by an edge probability we used the initial values.
Next, we update S41 as
S41 =
XXX
g1 g2 g3
f (y1 jg1)P (g1)f (y2 jg2)P (g2)f (y3 jg3)P (g3jg1 g2)P (g4jg1 g2)|{z}
S32 :
f (y4 y5 y6 jg3 )
In this case, the edge probability S32 was already updated once. Thus, the value of
S41 = f (y1 y2 y3 y4 y5 y6 g4) is the joint probability of the genotype of individual 4
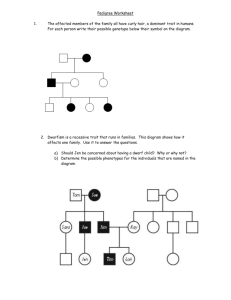
and of all the phenotypic values connected to 4 through nuclear family 1 in the cut-extended
pedigree shown in Figure 3 (a). Next, we update S42 as
S42 =
XXXX
g1 g2 g5 g6
f (y5 jg5)P (g5jg3 g4)f (y6 jg6)P (g6jg3 g4)f (y3 jg3)P (g3jg1 g2)|{z}
S31 :
f (y1 y2 y4 g3 )
Again, in this case we use an edge probability that was already updated, and thus
S42 = f (y1 y2 y3 y4 y5 y6jg3), which is the conditional probability of all the phenotypic
values connected to 4 through nuclear family 2 in the cut-extended pedigree shown in
Figure 3 (b), given the genotype of individual 4.
5
1
S11
2
S21
1
S11
1
1
3
S31
S41
S32
4*
3
S31
1*
4
3*
S52
S41
2*
4
S42
4*
2
2
5
2
S21
S62
S52
6
5
S62
6
(a)
(b)
Figure 3. Cut and extended graphs
Each subsequent iteration results in further extensions to a cut pedigree. After a
sufficient number of iterations we sample genotypes as follows from the iteratively peeled
pedigree. First we sample the genotype of individual 1 from f (g1 jy), which is computed
using S11 as described above. Next, to sample the genotype of 2 we update S21 to reflect
the sampled value for the genotype of 1 as
S21 =
XX
g3 g4
f (y1 jg1)P (g1)f (y3 jg3)P (g3jg1 g2)f (y4 jg4)P (g4jg1 g2)S32 S42 (3)
where g1 is the sampled value for the genotype of 1. Using this updated value for S21 ,
f (g2 jy g1) is computed as
f (g2 jy g1) = Pf (yf2(jyg2j)gP)(Pg2()gS21
:
)S
g2
2
2
2
21
(4)
This process is continued until all individuals are sampled. We propose to use these
sampled genotypes as the proposal distribution in Metropolis-Hastings algorithm to accept
or reject the candidate draws.
The general algorithm is the following. First, all edge probabilities are iteratively
updated. After a sufficient number of iterations, we sample genotypes for all individuals
in the pedigree. We start from an arbitrary individual, and sample its genotype using
the marginal probability function, f (gj jy). Then we sample a neighbor conditional on
the sampled genotypes as follows. A neighbor is defined as any individual who is also
a member of those nuclear-family nodes to which the sampled individual belongs to. To
sample a neighbor, we first update all its edges to reflect the already sampled genotypes. To
update edges, we use expressions like (3), but the summations are only over the unsampled
genotypes. Now to sample the genotype conditional on the already sampled genotypes we
use expressions like (4) with the edges that were updated for the sampled genotypes.
3.1 Improving efficiency of sampler
The efficiency of the sampler can be improved by combining exact peeling with iterative
peeling. Exact peeling (Section 2.) is used until the size of cutsets gets large enough to
make computations unfeasible. Then, iterative peeling is used, which as discussed above is
equivalent to cutting and extending the remaining loops.
After iteratively updating all the edge probabilities, we sample genotypes for all
individuals in the pedigree. First we sample genotypes for the individuals that were not
6
peeled out. Once all remaining individuals are sampled, we sample genotypes of the
‘‘peeled’’ individuals in the inverse order of peeling (see Section 2).
3.2 Metropolis-Hastings algorithm
We consider the special case of independence sampling. Thus, the acceptance probability
(Gilks et al., 1996) is
)
(5)
= min 1 ((ggc )q()gqprev
prev (gc )
where is the target distribution and q is the proposal distribution, gprev is the accepted
draw from the previous round and gc is the sampled candidate from the present round. The
chain moves from gprev to gc with probability , and it stays at gprev with probability 1 ; .
We sample genotypes from the iteratively peeled pedigree (proposal) and use them in the
Metropolis-Hastings
Qn1 step to be
Q rejected or accepted. To obtain (:) on the true pedigree,
we use (g) = j =1
P (gj ) nj=n1 +1 P (gj jgfj gmj ), where gfj gmj are the genotypes
of the parents of individual j and n1 is the number of founders. In this example,
(g) = P (g1)P (g2)P (g3jg1 g2)P (g4jg1 g2)P (g5jg3 g4)P (g6jg3 g4).
To compute q(:) we multiply the probabilities that were used in the sampling process
described above. For example, for this pedigree
q(g) = f (g1 jy)f (g2 jy g1)::f (g6 jg1 g2 g3 g4 g5 y).
4. ESTIMATION OF GENOTYPE PROBABILITIES
The proposed sampling method can be used to estimate the genotype probabilities by
sampling from a proposal distribution generated by iterative peeling for the entire pedigree
or by peeling exactly up to a certain point and then perform iterative peeling.
4.1 Assessing the performance of the algorithm
To asses the performance of the algorithm we used a small pedigree with loops. We
considered the inheritance at a single biallelic disease locus. This small pedigree consists
of 77 individuals, and two of them are affected. There are four generations in this pedigree
and large families (more than five offspring per family). This pedigree also has a few loops.
We sampled genotypes for all the individuals and computed the genotype probabilities.
In this small pedigree we can perform exact calculations by exact peeling. These exact
calculations were verified with the results from package for pedigree analyses (Hasstedt,
1994; PAP). The probabilities obtained by PAP can be thought as the true results. The range,
means and standard deviations of the absolute differences between genotype probabilities
from our algorithm and PAP for the 77 individuals are shown in Table 1.
Table 1. Absolute differences between probabilities computed by PAP and exact peeling.
Range
P (AA)
P (Aa)
P (aa)
0 to 4.810;5
0 to 4.910;5
0 to 4.910;5
7
Mean
St. Dev
2.510;5
2.310;5
2.210;5
1.610;5
1.410;5
1.410;5
In Table 1 we observe that the genotype probabilities computed by the two methods do not
differ. The small differences are due to rounding errors.
We then compare the results from PAP with estimates from the proposed sampling
method where no exact peeling was done (Table 2) and also with the estimates from the
proposed method where exact peeling was done until the cutset size was 4 (C4) and then
iterative peeling was done for the rest of the pedigree (Table 3). The length of the chain
was 10,000 iterations and we discarded the first half, thus the genotype probabilities are
obtained based on the second half of the chain. Tables 2 and 3 show the ranges, means and
standard deviations for the absolute differences between PAP and the proposed method
with no partial peeling and with partial peeling, respectively. We observe that the results
are similar, indicating that for this small pedigree there is no advantage in partially peeling
the pedigree prior to sampling.
Table 2. Absolute differences between PAP and proposed method with no exact peeling.
P (AA)
P (Aa)
P (aa)
Range
Mean
St. Dev
0 to 2.110;2
0 to 2.510;2
0 to 2.210;2
5.610;3
6.910;3
6.310;3
5.410;3
5.810;3
5.310;3
Table 3. Absolute differences between PAP and proposed method with exact peeling (C4).
P (AA)
P (Aa)
P (aa)
Range
Mean
St. Dev
0 to 2.010;2
0 to 1.910;2
0 to 1.610;2
3.910;3
5.610;3
5.610;3
3.910;3
3.910;3
3.810;3
The rejection rates were 29% and 5% for the proposed method with no exact peeling and
with exact peeling up to cutset size=4, respectively. Thus, it seems that it is more efficient
to peel exactly as much as possible and then perform iterative peeling to the remaining core
pedigree.
Results from Tables 2 and 3 show that the proposed method yields accurate estimates.
If we increase the number of samples, the estimates improve even more.
4.2 Applications of the methods in a real pedigree
A real dog pedigree was used to test the performance of the sampling algorithm that we
just described. The trait of interest in this pedigree is a disease called progressive retinal
atrophy (PRA). This disease is transmitted by a recessive allele and the dog is affected
when it has the recessive homozygous genotype (aa). The pedigree consists of 3,052 dogs
(Labrador Retrievers) from ‘‘The Seeing Eye, Inc’’, and 33 of them are known to have the
disease. That is, for these 33 dogs we know the genotype and phenotype. For the rest of
the dogs we are interested in obtaining estimates of the genotype probabilities to determine
8
which dogs are at highest risk of transmitting the PRA gene to their offspring and which
dogs are at lower risk of both transmitting the gene and of being PRA affected.
Exact peeling methods cannot be used in this pedigree because there are 679 loops that
need to be cut. Thus, we used the proposed method. We peeled exactly the pedigree up to
cutsets of size 4 (C4). We then iteratively peeled the remaining core pedigree and sampled
genotypes according to Metropolis-Hastings algorithm as described above. The rejection
rate was 53% and the length of the chain was 20,000 (first 5,000 were discarded) . This
rejection rate is dramatically reduced when more genotypes in the pedigree are known.
We also tried running the program for cutset size=0, that is using the proposal
generated by performing iterative peeling for the entire pedigree (with no exact peeling).
After three days the program was at iteration 568 and we decided to stop the execution.
In general, it seems that it is more efficient to peel exactly the pedigree as much as
possible and then perform iterative peeling to the remaining core pedigree to obtain the
proposal distributionto be used in Metropolis-Hastingsalgorithm. We cannot however, peel
too deeply because then the cutsets become large increasing the expense in computation
time and memory.
4.3 Results: proportion of dogs carrying the PRA allele
The estimated numbers of affected and carrier animals are presented in Table 4. We observe
that the estimated number of affected dogs is 41 (including the 33 dogs known to have the
disease).
Table 4. Estimated number of affected and carrier animals.
Genotype probability No. of animals
: P (aa) < 0:6
: P (aa) < 0:7
0:7 P (aa) < 0:8
0:8 P (aa) < 0:9
0:9 P (aa) < 1
P (aa) = 1
0:5 P (Aa) < 0:6
0:6 P (Aa) < 0:7
0:7 P (Aa) < 0:8
05
06
526
547
284
115
161
41
482
218
56
5. SUMMARY AND CONCLUSIONS
Estimating the genotype probabilities at biallelic loci is non-trivial when pedigrees are large
and contain loops. In this case, a standard scalar Gibbs sampling approach cannot be used,
as the chains are reducible in practice. We propose a more general Metropolis-Hastings
approach to sampling genotypes jointly from complex pedigrees, in which candidate
draws are obtained from modified pedigrees. These modified pedigrees are obtained by
applying extensions of traditional peeling methods, and are used as candidate draws in the
9
Metropolis-Hastings step. The resulting Markov chains satisfy the assumptions that are
required for good performance of MCMC methods.
The method for sampling genotypes was developed to address the problem of
estimating genotype probabilities in Labrador Retrievers.‘‘The Seeing Eye, Inc.’’ provided
the pedigree data of interest that included over 3,000 animals, and had over 600 closed
loops created by inbreeding and multiple matings. A summary of the results of this pedigree
analysis is given in Table 4.
Another important application of this method is in linkage analyses where the
genotypes at the marker loci must be sampled when data are incomplete. The proposed
method can be applied to sample genotypes at marker loci with more than two alleles. It
can be shown that the resulting chains are irreducible.
REFERENCES
Cannings, C., Thompson, E.A. and Skolnick, E.H. (1978). Probability functions on complex
pedigrees. Adv. Appl. Prod. 10, 26-61.
Elston, R.C. and Stewart, J. (1971). A general model for the genetic analysis of pedigree
data. Hum Hered 21, 523-542.
Elston, R.C. and Rao, D.C. (1978).Statistical modeling and analysis in human genetics.
Annu. Rev. Biophys. Bioeng. 7, 253-286.
Elston, R.C. (1987). Human quantitative genetics.Proc. 2nd. Int. Conf. Quant. Genet.,p.281.
Fernandez, S.A., Fernando, R.L., Carriquiry, A.L. and Gulbrandtsen, B. (2000). Estimating
genotype probabilities in complex pedigrees. Case Studies in Bayesian Statistics. V.
Berlin: Springer. (In Press).
Gilks, W.R., Richardson, S. and Spiegelhalter, D.J. (1996). Markov Chain Monte Carlo in
Practice. London: Chapman and Hall.
Hasstedt, S.J. (1994). Pedigree Analysis Package. Department of Human Genetics, University of Utah. Revision 4.0.
Heath, S.C. (1998). Generating consistent genotypic configurations for multi-allelic loci
and large complex pedigrees.Hum. Hered. 48, 1-11.
Janss, L.L.G. and Thompson, R. and Van Arendonk, J.A.M.. (1995) Application of Gibbs
sampling for inference in a mixed major gene-polygenic inheritance model in animal
populations. Theor. Appl. Genet. 91, 1137-1147.
Janss, L.L.G. and Van der Werf J.H.J and Van Arendonk J.A.M. (1992). Detection of
a major gene using segregation analysis in data from generations. Proc. Eur. Assoc.
Anim. Prod..
Lange, K. and Boehnke, M. (1983). Extensions to pedigree analysis. V. Optimal calculation
of Mendelian likelihoods.Hum. Hered. 33, 291-301.
Lange, K. and Elston, R.C. (1975). Extensions to pedigree analysis. I. Likelihood calculations for simple and complex pedigrees.Hum. Hered. 25, 95-105.
Van Arendonk, J.A.M. and Smith, C. and Kennedy, B.W. (1989). Method to estimate
genotype probabilities at individual loci in farm livestock.Theor. Appl. Genet. 78,
735-740.
Wang, T. and Fernando, R.L. and Stricker, C. and Elston, R.C. (1996). An approximation
to the likelihood for a pedigree with loops. Theor. Appl. Genet. 93,1299-1309.
10