Open-file Report 212 and Mineral Resources
advertisement

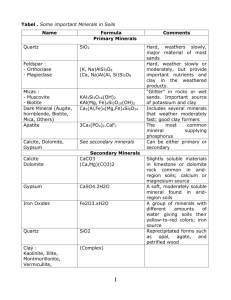
Open-file Report 212 New Mexico Bureau of Mines and Mineral Resources Origin of the Riley travertine as constrained by the clay mineralogy of acid- and EDTA-insoluble residues by James M. Barker, Industrial Minerals Geologist New Mexico Bureau of Mines and Mineral Resources Socorro, NM 87801 January 31, 1984 Introduction The Riley limestone County, travertine deposit present travertine its 1). of the varied deposition formation Barker (1983), in Table Barker Kottlowski supported is environments possible Riley origins. Aft.er unpublished references states the that sepiolite clay clay Table 2, the along with environments minerals. their over others. covered is area this report of calcite in summarized include Massingill The origin (1977), most (pedogenesis). depositional can be used to constrain over 300 published and caliche, fraction, (attapulgite) relative (caliche), work, of proposed reviewing or absence and paper deposit Based on this presence widely on this 1941). travertine mineral clay because secondary (1982), (1940, on calcrete and palygorskite other and others assemblages of the vary Previous in the and Denny The clay-mineral the is controversial and pervasive studies of caliche Socorro Riley pedogenesis directly Chamberlin that of the calcrete). bears (1962), residues Opinions (travertine), Earlier (1983), investigates deposition, which 1. paper travertine morphology. (nonpedogenic economic origin. Riley playa-lacustrine spring This its and potentially age in northwestern insoluble to constrain highly include (Fig. in the The origin of an unusual of Plio-Pleistocene New Mexico minerals is most but and other of attapulgite abundance, favors and Goudie frequently (1972) contains may also work the contain summarized and sepiolite some depositional in M AUPN I T S QUATERNARY Piedmont slope deposits(ps) Fanglomerate deposits ( f ) QUATERNARY-TERTIARY Sonta Fe Group(QTsf1 upper(u)inpartcorrelative with Sierra Lodrones Fm. and including Riley travertine ( t ) Popotosa Fm.(p) MESOZOIC Mesaverde Group undifferentiated; shole.siltstone,sandstone c Figure I . Generalized geologic map of 2 lhe Riley lravenine and adjacent area. 3 _.... G.C. Mssingill (1977) Age 1 - 3 my. Plio-Pleistocene 1-3 m.y. < 3.5 million years < late Pliocene F.E. Kottlovski (1952) C.S. Cenozoic Quaternary Denny (1940, 1941) t e I S, A I I I ! A A, S Geographicandgeologicsetting masses ( n o r t h mesa The R i l e y t r a v e r t i n e o c c u r s i n t w o m a i n a n ds o u t h mesa) c e n t e r e da p p r o x i m a t e l y a p p r o x i m a t e l y1 7 km s o u t h o f t h e 6.5 km west and s u m m i t o fL a d r o nP e a ka n de a s t - R i t a ) , N e w Mexico(Fig.2).TheRiley southeaso t fR i l e y( S a n t a t r a v e r t i n e is i n t e r m i t t e n t l y e x p o s e d by l o c a l e r o s i o n o f o v e r l y i n gS a n t aF eG r o u ps e d i m e n t sa l o n gm e s ae d g e sf a c i n gt h e Rio Salado. of n o r t h m e s a u n d e r l i e s a p p r o x i m a t e l y The R i l e y t r a v e r t i n e 46km2 i n T. 2 N., R. 3 W. a n d T. 3 N. R. a p p r o x i m a t e l y2 1 S., R. 2 W., k m 2 i n T. 1 N., R. T. 1 S., it underlies A t s o u t hm e s a , o ft h eL a d r o nM o u n t a i n s . R. 3 W., S e v i l l e t a Game R e f u g eo nt h e 2 west f l a n k 3 W. o n t h e w., T. w., T. 1 N., R. 3 1 and a s m a l l p o r t i o n of t h e west s i d e o f t h e S i l v e r Creek drainage. i s v i ag r a d e dr o a d sf r o mM a g d a l e n a Access t o t h e s t u d y a r e a (US-60) o rf r o mB e r n a r d 0 ( e x i t 175on1-25). When d r y ,t h eR i o S a l a d op r o v i d e ss e c o n d a r yf o u r - w h e e l - d r i v ea c c e s st ob o t hn o r t h a n ds o u t h mesas. N o r t h mesa is a p p r o a c h e dm o s te a s i l yf r o mt h e n o r t h o nr a n c hr o a d sb e a r i n gs o u t h e a s t w a r dt o w a r d st h ew e s t e r n s l o p eo ft h eL a d r o nM o u n t a i n sf r o mt h ec o u n t yr 0 a . dt oR i l e y .T h e s o u t hm e s a i s r e a c h e dm o s te a s i l yf r o mt h eH u d g i n sR a n c hr o a d w h i c hi n t e r s e c t s m o s to fs o u t h US-60 e a s t ofMagdalena. A l l o fn o r t h mesaand mesa a r e o nt h eR i l e y1 5 - m i nq u a d r a n g l ew i t ht h e r e m a i n d e ro nt h eM a g d a l e n a1 5 - m i nq u a d r a n g l e .P r e l i m i n a r y m i nq u a d r a n g l e s 7.5- are available. T h eR i l e yt r a v e r t i n e t h eS a n t aF eG r o u p( B a r k e r , is a r e l a t i v e l yu n d e f o r m e d 1983) d e p o s i t e db e t w e e n member o f 1 and 3 m.y. 6 . . ago (Chamberlin and others, 1982). It descends in elevation from north mesa to south mesa and overlaps progressively younger (Paleozoic, Mesozoic, and Cenozoic) units to the south. The Riley travertine rangesfrom massive to laminar with locally significant reworked, vuggy, algae-like, and fragmented portions. This varied morphology is interpreted by Barker (1983) as follows. The limestone is the result of a nonpedogenic process producing proximal (surface and subsurface) and distal (subsurface) secondary carbonate deposits related primarily to lateral groundwaterflow. The carbonate-charged water is interpreted to havebeen generated in Paleozoic limestones on the west flank of the Ladron Mountains and the southeast flank of Sierra Lucero in the late Cenozoic. These waters flowed over and through alluvial fansand other sediments into and down the axis of an elongatebasinor valleydraining southward. This drainage merged with an east-trending valley which drained eastward near the present latitude of San Lorenzo Canyon and emptied into the ancestral Rio Grande. Proximal spring, lacustrine, and reworked carbonate deposits were formed at thesurface. They are contemporaneous with pervasive and expansive subsurface secondary calcite cementation preof existing host sediments. This depositional system is analogous in part to the ground-water calcrete (nonpedogenic) described in Western Australia by several authors (see for example Carlisle and others, 1978). This model should yield clay-mineral assemblages that result from the depositional environmentof the host sedimentary rocks or sediments and, therefore, that are varied. In contrast, one mode of formation wouldtend towards a single clay-mineralassemblage. If the Riley travertine is a caliche, attapulgite should be dominant over other clays. If it is an alkaline lacustrine-playa deposit, sepiolite should be dominant. A spring, fresh-water lake, or pervasive secondary origin shouldyield detrital clays -- such as illite, smectite, and kaolinite, typical for an arid climate acting on a Paleozoic limestone substrate. Sampling and experimental techniques Seventeen samples of the Riley travertinewere collected in March 1983. Preliminary data, including clay mineralogy of HC1 (10%) insoluble fractions, are published in Barker (1983). Additional work was done in gently derived insoluble fractions. the fall of 1983 togenerate These samples werepartially digested using very dilute acetic acid followed by warm EDTA solution. The clay-size fractions of the insoluble residues were then analyzed by X-ray diffraction (XRD) for clay and detrital mineralogy. Samples of the Riley travertinewere treated initially with dilute (0.25 M) acetic acid as described by Ostrom (1961). Because of the samples' high calcium carbonate content, this p r o v e d t o o slow. The samples werepurgedof washing and decanting with deionized water. E D T A w a s t h e n used Fernalld (1973). for digestion as acetic by acid A 0.2 M solution of described by Bodine and This greatly sped up carbonate solution but at much greater cost in reagents. The EDTA procedure was modified slightly as describedbelow. Carbonate-solution procedures Acetic-acid method--Samples of the Riley travertine (approximately 150-gr) were scrubbed and washed thoroughly in deionized water. Then, they were crushed for 5 min in a Buehler concentric-ring vibratory.crusher yielding avery fine carbonate 8 more powder.Such a f i n eg r i n du l t i m a t e l yc a u s e dp r o b l e m si nb o t h d i s s o l v i n gm e t h o d su s e d ,b e c a u s e much o f t h e c a r b o n a t e m a t e r i a l b e g i n sa sc l a y - s i z ep a r t i c l e s .T h e s e ,p l u st h el a r g e rp a r t i c l e s j u s t b e f o r ep a r t i c l e d i s s o l v e di n t ot h ec l a y - s i z er a n g e e x t i n c t i o n ,y i e l d i n gp e r s i s t e n tc l a y - s i z ec a r b o n a t er e s i d u e s t h e X R D slides. (1961) p r o c e d u r e is T h i s c h a n g ef r o mO s t r o m ' s is n o t p o s s i b l e n o tr e c o m m e n d e ds i n c ec l a y - c a r b o n a t es e p a r a t i o n is c o m p l e t e l yd i s s o l v e d . u n t i la l lt h ec a r b o n a t e on I l e f tc o a r s e r p a r t i c l e s ( l c m + ) t o i n s u r e t h a t c a r b o n a t e w a s a l w a y s p r e s e nst o t h ea c i dw o u l db el e s sl i k e l yt oa t t a c k t h e c l a y s .O s t r o m ' s a -60 mesh g r i n ds h o u l db eu s e d ,b e c a u s e (1961)recommendationof t h er e l a t i v e l yc o a r s ec a r b o n a t ec a nb es e p a r a t e de a s i l yf r o mt h e time d u r i n g t h e d i s s o l v i n g liberatedclay-sizematerialatany process. A c e t i ca c i d ,m i x e dt o 0.25 M c o n c e n t r a t i o n ,w a sa d d e dt ot h e samples,whichwerestirredperiodicallyuntilreaction complete. The s p e n ta c i d was d e c a n t e d ,a n d new a was c h a r g e of 0.25 M a c e t i c a c i d a d d e d f o r a s many i t e r a t i o n s a s n e e d e d . m a t e r i a l sa sr i c h With i n c a r b o n a t ea st h eR i l e yt r a v e r t i n e ,d o z e n so f c h a r g e so fa c i dw o u l db en e e d e da tt h e0 . 9 - l i t e rc h a r g es i z e utilized. T h u s , a n EDTA-based p r o c e d u r ew a si n i t i a t e dp a r t way t h r o u g ht h es a m p l e - d i s s o l v i n gs t a g e . EDTA m e t h o d - - C h e l a t i o n o f c a r b o n a t e e t h y l e n e d i a m i n et e t r a c e t i ca c i d by t e t r a s o d i u m (EDTA) a f f o r d s a methodof c a r b o n a t es o l u t i o nt h a td o e sn o ta f f e c to r i g i n a lc l a ym i n e r a l o g y . T h em e t h o dd e s c r i b e d Basedon t h e i rc h a r t s , byBodineandFernalld(1973)wasused. a 0.2 M EDTA s o l u t i o n( 7 5 9 gm t e c h n i c a l - grade disodium EDTA in 1 liter deionized water at pH 12.5) was applied to the ground carbonate partially digested by acetic The solution was heated to90° C for 6 0 to 9 0 min using a acid. continuous magnetic stirrer. After settling and decanting, a new charge of EDTA was added until the carbonatewas entirely chelated. The problems caused by the very fine grind as described above were also present in this procedure. After dissolution, decanting, and deflocculation, using deionized water only, the clay fraction was separated from the coarser insoluble particles. Standard clay-mineralogy techniques were then used as follows. Following deflocculation, the dispersed clay was left undisturbed for 10 min. Then, eyedropper charges of the suspension were collected carefully from the topmostsurface and deposited on glass slides until they were fully covered. Four slides of each sample were prepared as described above and then allowed to air dry overnight. The oriented slides thus derived are not useable for quantitative work because of size fractionation in the suspension columnduring drying. The larger kaolinites settle out first and then are masked somewhat by later mantling by the slower-settling smaller clayssuch as srnectite or illite. The slides 38O to 2O02 and a second . were runon a diffractometer a t 2 O 28/minfrom The slide was thenglycolated at 60° C for 3 hrs run was made as describedabove. then heated to approximately300-330° C Theslides were (some to 390° C) for 1 hr and run hot as described above, except thespan was shortened t o 15O-Zo 20. Controlled-humidity and cation-saturation runs were 10 not done. Clay-mineral reactions during carbonate solution Two problems possibly developed using the acetic-acid or EDTA digestion techniques. as These are destruction of clays, such illite or smectite, by excess acidity and the alteration of attapulgite-sepiolite by either excessively acid or basic conditions. The work by Ostrom (1961) in proving his technique showed.no alteration in acidic solutions (if less than 0.3 M) for randomly interstratified illite-montmorillonite, chlorite, illite, or kaolinite. He did not include attapulgite or sepiolite. Carroll (1970, pp. 43-44) states that attapulgite and sepiolite require alkaline conditions for survival and that they are decomposed by acid and won't survive below pH 7 . the threshold. Other workers suggest pH 3 as In addition, Khoury, et al. (1982) predict that " chlorite will forminstead of attapulgite-sepiolite at high pH and high Mg and/or Al. This prediction is based on their interpretation of the work of Siffert (1962, in Khoury et al., " 1982), who found that sepiolite precipitates at pH8.5, trioctohedral smectite (mixed-layer kerolite-stevensite of Khoury et al., -~ 1982) at pH 8.5-9, at pH above 9. and talc plus trioctohedral smectite The EDTA solution used has a pH of approximately 12.5, which is possibly high enough to alter sepioliteattapulgite to mixed-layer kerolite or stevensite (chlorite) plus talc (Ebrl,et al., 1982). The XRD data suggest that no " conversion occurred during digestion of the Riley travertine since neither kerolite, stevensite,or talc were detected. 11 such Bodine and Fernalld (1973) evaluated the effects of EDTA treatment on clayminerals. They found no significant alteration occurred with treatment times under 4 hrs. They examined chlorite and illite (mixture), montmorillonite, and kaolinite. They did not determine effects on attapulgite-sepiolite. Glover (1961) implied that EDTA treatment was less destructive than acid treatment where clays wereinvolved, but he studied no clays individually. Hill and Runnels (1960) made a similar Suggestion. The original digestion using 10% HC1 (Barker, 1983) produced clay-size residues containing kaolinite, illite, and smectite in order of decreasing abundance (Table 3). Treatment with this concentrated a solutionof HC1 probably biased the clay data so a less chemically harsh dissolution technique was used for this study. The presence 01: absence of attapulgite or sepiolite is significant in the identification of depositional environment, and the HC1 technique might have removed or altered them. Clay-mineral analysis The clay size fraction of the insoluble residues from eight samples ofRiley travertine were analyzed by X-ray diffraction techniques. The samples were deflocculated by repeated rinsings with deionized water and 10-min centrifugation cycles. No deflocculating chemicals were used. Standard oriented slides were prepared by sedimentation (Stokes Law) techniques with their inherent bias (Stokke and Carson, 1973; Gibbs,,1965, 1968). This bias precluded meaningful measurement of clay-mineral percentages, and many of the illite curves are masked by mixed-layer clays. 12 Thus, the following analysis is based on the presence or absence of significant clays rather than on relative abundance. The XRDanalysis was done a Rigaku on D-Max. copper fine focus at40 KV and 25 ma. Settings Slits were lo divergence, lo scatter, 0.3O receiving, and 0.3O monochromator. was run untreated were followed by aglycolated run Each sample and heated (300° C and/or 390° C) runs. Results The mineralogy of the clay fraction, based on number of occurrences in the samples and in decreasing order is as follows: - quartz feldspar (Na-Ca-K combined) kaolinite - illite - mixed-layer - illite smectite (randomand ordered) rhodochrosite amphiboles zeolite (clinoptilolite) vermiculite (masked) chlorite (masked) smectite (difficult to identifyas mashed) superlattice clay or ordered mixed-layer (vermiculite-illite) This is the mineralogy derived from acetic-acid/EDTA insoluble residues. In contrast, the harsher HC1-derived' insoluble residues have a much simpler mineralogy in their claysize fraction, as shown below: 13 Sample 1 Quartz Plagioclase K-spar Hb Kaolinite Illite Calcium Smctite ~~~~~ 4 + 0 0 0 + 0 tr RTB 12 0 0 tr tr 0 0 0 RTB 13 + + + + + tr 0 0 0 0 - 0 + tr 0 0 0 0 0 RTB RTB 14 RTB 15 Symbol Relative abundance + - major minor absent trace 0 tr Source: Barker, Table 3. Cu f i n e f o c u s KV = 40, ma = 25 K-spar = potassium feldspar Hb = hornblende 1983 Q u a l i t a t i v e X-ray d i f f r a c t i o n a n a l y s i s of t h e f i n e f r a c t i o n (-230 mesh) of selected samples of t h e R i l e y t r a v e r t i n e t r e a t e d w i t h 10% HC1. The clay peaks were very small except for kaolinite. 14 - quartz feldspar kaolinite illite - amphibole - smectite The minerals described below are from the EDTA/acetic-acid procedure. Each mineral is described by characteristic and common peaks seen in the samples. Quartz Quartz is identified by its characteristic peak at approximately 26.7O 2 9. A common peak also occurs at approximately 21° 2 9 with some variability. Feldspar The feldspars occur from 27.5O 2 8 to 28.1° 29 with some variation depending on potassium, calcium, and sodium content. The various feldspars were not differentiated. I Kaolinite Kaolinite is found in 85% of the samplesexamined. around 12.4O 2 9 , 20.5O(+) 28 , and 24.9O 2 9 Peaks are typical. Kaolinite is a very stable clay so it is least affected by acid or EDTA techniques and,thus, is dominant in both suites of analyses. Illite Illite is as common as kaolinte(85% of samples), but its main peak at 9 is masked by the approximately 28-80 illite-smectite mixed-layer "bump". broad Illite in untreated slides is typically represented by a "shoulder" on the mixed-layer 15 Sample Clay Number K I S C V M + ? +? +? 5 + - - ? 0 I + + ? 8 + + 9 + 11 + L SL Remarks minerals Q F A Z R +(nr) ? 0 + + - 0 + +? +(nr)? 0 + + + o n 0 0 +(r) 0 + + - 0 + ? 0 0 +(r) 0 + + 0 + 0 + ? + 0 +(r) 0 + + + 0 0 0 0 + - tr + 0 tr venniculiteillite superlatice 12 t r 0 0 0 0 0 0 + tr 0 0 tr virtually no clay minerals 13 0 tr ? 0 0 +(r) 0 + + 0 + 4 I Other minerals I I 0 + 0 t no heated run r 1 ? cannot be differentiated K = kaolinite + present 1 = illite s = smectite - trace C = chlorite 0 absent V = vermiculite ML = mixed-layer illite-smectite (random = r, ordered = nr) SL = superlattice vermiculite-illite(?) Q = quartz A = amphibole R = rhodochrosite F = feldspar Z = zeolite (probably clinoptilolite) Table 4. Qualitative X-ray diffraction analysis of the acetic-acid/ EDTA insoluble residues of selected of samples the Riley travertine. curve, which separates somewhat upon glycolation. The illite peak is greatly sharpened and enhanced by heating of slides because of recrystallization of the mixed-layer clay. Mixed-layer Illite-Smectite Mixed-layer bumpbetween clays % ofthesamples occur 7 0in approximately3 O 2 8 and go 28. This aas broad rangevaries and usually includes a masked illitepeak and often a masked vermiculite-chlorite one as well. The mixed-layer clays are frequently random, but one possible instance of an ordered mixedlayer clay was observed in sample 4. However, this could also be interpreted as a vermiculite o r chlorite peak. Sample 4 has no heated run because of slide disintegration; consequently, a definitive answer is not possible. The broad mixed-layer peak shifts to a lower 2 8 upon glycolation (lattice expands). Rhodochrosite Rhodochrosite (MnC03) is found in 85% of the slides, usually in trace amounts. It is recognized by a sharp peak at approximately 31.4O 2 8. The Riley area is noted for high manganese values including aonce-active mine at north mesa. The probable source rocks in the Paleozoic carbonatesalso have appreciable amounts of manganese (Table 5). Four samples with peaks at 31.4O 2 8 were analyzed for MnC03 (Table 6). Measurements range from a trace to 0.25% manganese. Given the abundance of manganese and the close relationship between calcium and manganese carbonates, the presenceof rhodochrosite is not unusual. Rhodochrosite is soluble in HC1, so it did not appear in the hydrochloric-acid treated samples (addendum 1). 17 . ... +sal, 1*t€d . w 2.20 0.47 14.47 16.27 ,1.14 0.96 2.03 5.74 .4.11 6.32 8.26 97.80 99.53 85.53 83.73 98.86 99.04 97.97 94.26 95.89 93.68 91.74 M 10.01 12 mssive, recrystell. mssive, saw 13 recrystal. M 2.43 calrrete not Riley traverine 21.07 RTB 1 2 3 4 ,: mss. to la. M mssive 91 5 . laminated 5a l&ted w M 6 7 laminatel. ??4 mssive missive N4 . 8 9 10 14 15 16 18 LAD 7 9 12c 12d M crulely1aminatEd travertine facies 11 MATB ' upper mdSs1ve middle. ... .. EM -. . . - 92.15 97.89 87.66 79.16 36.9 39.2 35.1 31.7 0.26 0.23 0.27 0.39 1300 639 583 278 92.40 95.65 37.0 38.3 0.23 0.22 528 635 91.64 39.1 0.30 757 1483 775 e57 490 1087 1099 933 710 ' 857 1083 1099 89.99 96.89 38.8 0.33 462 97.57 78.93 72.66 70.96 95.42 87.61 99.a 39.9 0.22 416 64.93 83.66 93.40 26.0 33.6 37.4 0.38 0.38 0.34 34 81 53 35 6 7 6 6 <30 117 23 5 5 (30 t30 150 7 <30 608 78 8 (30 555 1123 5 32 488 114 4 331 373 233 e4 6 56 7 28 35 <30 6 30 ' ~~ -I ' mssive 91 mssive, r e v s t . mssive 91 mssive SM % 27.34 29.04 4.58 12.39 569 G-mkerlin et al.1982 M alalhrlin e t al.1982 w Qlambeelin et al.1982 M chanberlin et al.1982 travertine, Lucero >20 .20 20 >20 w 0.91 99.09 98.39 39.4 3% 318 (300 0.15 0.3 0.2 0.5 1500 0.27 37 471 500 1000 700 617 759 1,339 759 99.46 40.04 0.13 ' +23.2 ffi.8 ffi.2 +7.0 i0.6 Amphibole I did not differentiate the various peaks from 1l0 210 8 to but rather assigned them to the general class of amphiboles. Depending on composition, a range of peaks is possible, and the varied lithologies of provenance areas open possibilities beyond the scope of this paper. Zeolites A strong peak at 9.8O indicates clinoptilol'ite. 2e with a secondary peak at 22.3O 2 8 The presence of a zeolite in 25% o f the samples is not unexpected, since these are very common sedimentary minerals in arid terranes. Zeolites form generally in alkaline ground-water conditions or in igneous rocks. Clinoptilolite is more indicative of a sedimentary origin but may be diagenetic on a small scale rather than representing largescale alkaline conditions. Vermiculite-Chlorite Vermiculite (sample 5) or chlorite (sample 9) is indicated by a peak at 6.3-6.5O 2 8. Neither was affected by glycolation, but, in one instance, the peak disappeared upon heating (vermiculite) but did not in the other (chlorite). Smectite Smectite may be present aas distinct phase is m a s k e d b y but the broad mixed-layer illite-smectite peak. - Super lattice Clay One sample showed a broad peak at 3.8O 2 8 and a multiple at 7.6O 2 6 , which, when converted to d-spacing, yielded a clay most likely made up of vermiculite-illite. This clay did not expand much upon glycolation nor contract much upon heating (from 23O 2 8 19 Sample Number Remarks 4 5 11 12 I Table 6. Manganese Percent 0.025-0.125 trace trace 0.05-0.25 massive facies, south mesa laminated facies, north mesa massive facies, some recrystallization, north mesa Semiquantitative X-ray fluorescence analysis of manganese content of Riley travertine samples with a 31.5O 2 8 (rhodochrosite). to 20.50 2 e to 2 2 0 2 8 1. Discussion The data for the HC1 digestion are in Table 3, and the data for the acetic-acid/EDTA digestion are in Table 4. The XRD plots are in addendum 1 (HC1) and addendum 2 (acetic acid-EDTA). X-ray fluorescence (XRF) plots of selected samples for their manganese content are in addendum 3. Glass and others (1973) and Frye and others (1974, 1978) studied the Ogallala Formation in eastern New Mexico. The general regional geology is similar to that of the Riley travertine area. Both are underlain by Permian and Triassic rocks, often with identical units. The clay minerals they assigned to a "normal" detrital assemblage from such basement rocks were smectite, illite, and kaolinite. This assemblage is basically the same as that found in the Riley travertine insoluble residues. Based on this association, the Riley travertine has only a detrital claycomponent. Attapulgite and sepiolite were not detected in the Riley travertine. The data in Table 2 can be used to differentiate the occurrence of attapulgite and sepiolite. to be mutually exclusive. These two minerals tend However, they often occur together during a transition, and this mixture represents slightly varying conditions at the interface between the stability fields for each. Universal requirements for both attapulgite and sepiolite are relatively dry or dessicating conditions. The chemistry of these minerals requireshigh silica, high Mg, and high (but 21 restricted) pH. Table 5 shows the high Mg content of the regional carbonates. Ishphording (1973) has shown that attapulgite additionally requires high aluminum. sepiolite occurs under drier conditions. Climatically, Attapulgite has much greater resistance to weathering than sepiolite. Attapulgite is usually sedimentary, whereas sepioliteis sedimentary but also is common in igneous association. Since both require high magnesium and alkalinity, conditions may not havebeen correct. However, Glass and others (1973) and Frye and others (1974, 1978) found abundant attapulgiteand sepiolite in certain facies dependent on environment. So attapulgite and sepiolite do occur in eastern New Mexico where source rocks and climate are very similar to northwestern Socorro County. Thus, the lack of attapulgite and sepiolite in the Riley traverinte must be related to factors other than lack of magnesium, low alkalinity, etc. since they formed in eastern New Mexico under similar conditions. The variable seems to be the environment ofdeposition. is Attapulgite is primarily associated with soils while sepiolite more common in alkaline lakes or playas (Table 2). Apparently, these environments were not present during formation of the Riley travertine, which is thus neither asoil nor an alkaline lacustrine deposit. This conclusion is in harmony with recent work (Barker, 1983). The main 2-8 peaks for attapulgite (A) and sepiolite ( S ) are as follows: A 8.4*(10) 13.75(3) S 7.3*(10) 11.91(2)** 19.86(4)** 27.61(3)** 35.19(3) 19.77(3)** 20.60(2)** 26.52 22 (2)** * ** key line usually masked in Riley samples (relative intensity) The 10-intensity peaks are maskedby the broad mixed-layer illite zone. In addition, many of the secondary peaks are maskedby I I various other clay and nonclay minerals. Thus, a small amount of I attapulgite i I or sepiolite could be present and missed. the basis for assigning a pedogenic OK However, alkaline lacustrine environment to the Riley travertine is based on attapulgite or sepiolite dominating the claymineralogy. Minor amounts of attapulgite and sepiolite overwhelmedby illite, smectite, kaolinite, etc. is nondiagnostic. that the Riley For these reasons, I conclude travertine was not formed a soil or as as an alkaline lake-playa deposit. The illite-smectite-kaolinite suite does not define other environments conclusively. Therefore, the Riley travertine could be a spring deposit or a pervasive secondary carbonatedeposit. The detrital clays couldbe deposited in a travertine during its deposition either by wind or, as circumstances permit, by fluvial activity. In contrast, during pervasive carbonate deposition, the clays are an artifact of the depositional environmentthe host sediments represent. Carbonate crystallization can destroy some silicates (including clays), so the initial host clay mineralogy cannot be known with certainty. Summary The origin of the Riley travertineis constrained by the absence of attapulgite and sepiolite. These clay minerals are associated with soils (caliche-calcrete) and alkaline lakes, 23 respectively, so the probability that these are the depositional environments for the Riley travertine is low. The clay-mineral assemblage present is a typical detrital one for areas of New Mexico with Permian-Triassic bedrock. The vermiculite, chlorite, illite, mixed-layer illite-smectite, and kaolinite are not diagnostic and may represent either syngenetic minerals in a spring depositor minerals representative of the host-rock depositional environment if a pervasive calcite origin is correct. The presence of vermiculite and chlorite is uncertain pending more detailed sampling. Because of the preliminary natureof this report, some additional work mustbe done to confirm the conclusions reached. Additional work includes, but may not be limited to, the following: Dissolve by EDTA a calcareousrock with attapulgite and sepiolite as a control for the above experiments. Redissolve all the Riley travertine samples using EDTA in the alone to eliminate possible problems inherent acetic-acid procedure. 3 The clay-size fraction should be further sized and scanning-electron micrographs should be made of a fraction appropriate to detect acicular attapulgite and/or sepiolite. 4) A recent calichefrom the Riley area should be analyzed for attapulgite and sepiolite to see if they are forming under present conditions. This could include secondary carbonate material fromcase-hardened portions of the 24 Riley travertine. Drill cores of the Riley travertineare in hand, and deeper portions shouldbe analyzed as described earlier to eliminate any overprint in clay mineralogy from collecting near-surface samples. Undertake additional detailed geology including dating, mapping (geologic and facies), thin-section analysis, and detailed sampling. Acknowledgments All the ideasexpressed and the conclusions reached in this report are own. my My colleagues at the New Mexico Bureau Mines and Mineral Resources were a great help in various portions of this study. In particular,I thank G. S. Austin, J. Renault, J. Love, R. M. North, K. B. Faris, and contributions. andP. Cooksey for their time Technical assistance was supplied by M. Bowie, B. Casselberry, and J. Hintgen. References Barker, J. M., 1983, Preliminary investigationof the origin of the Riley travertine, Socorro County, New Mexico: New Mexico Geological Society Guidebook to 34th Field Conference, pp. 269-276. Bodine, M. W., Jr., and Fernalld,T. H., 1973, EDTA dissolution of gypsum, anhydrite, and Ca-Mg carbonates: Journal of Sedimentary Petrology, v. 43, no. 4, pp. 1152-1156. Carlisle, D., and others, 1978, The distribution o f calcretes and gypcretes in southwestern United States and their uranium favorability based on deposits in western Australia and southwest Africa (Namibia): U.S. Department of Energy, Open-file Report GJBX-29(78), 274 pp. Carroll, D., 1970, Clay minerals--a guide to their x-ray identification: Geological Society of America, Special 25 of P a p e r1 2 6 ,7 9p p . C h a m b e r l i n , R. M., a n do t h e r s ,1 9 8 2 ,P r e l i m i n a r ye v a l u a t i o no ft h e m i n e r a lr e s o u r c ep o t e n t i a lo ft h eS i e r r aL a d r o n e sW i l d e r n e s s N e w Mexico: N e w MexicoBureauof s t u d ya r e a ,S o c o r r oC o u n t y , Mineral R e s o u r c e s ,O p e n - f i l eR e p o r t1 7 9 ,1 9 3p p . Minesand t h eS a nA c a c i aa r e a Denny, C. S., 1 9 4 0 , T e r t i a r yg e o l o g yo f M e x i c o :J o u r n a l of Geology, V . 4 8 , no. 1, pp. 73-106. Denny, C. S . , 1 9 4 1 ,Q u a t e r n a r yg e o l o g yo ft h eS a n M e x i c o :J o u r n a lo fG e o l o g y v ,4 . 9n , o3 .p , p. New Acacia a r e a , N e w 225-260. H. N., 1 9 8 2 , M i x e d - l a y e r E b e r l , D. D., J o n e s , B. F., a n dK h o u r y , Desert, Nevada: C l a y s k e r o l i t e / s t e v e n s i t ef r o mt h eA m a r g o s a Minerals, v.30,no.5,pp.321-326. a n dC l a y , 978L , a t eC e n o z o i cs e d i m e n t s m , olluscan F r y e , J. C., a n do t h e r s 1 New i n n o r t h e a s t e r n New Mexico: a n d c l a y m i n e r a l s faunas, C i r c u l a r 160, Mexico Bureauof Minesand M i n e r a lR e s o u r c e s , 32 PP. F r y e , J. C., a n do t h e r s ,1 9 7 4 ,C a l i c h ea n dc l a ym i n e r a lz o n a t i o n c e n t r a l - e a s t e r n N e w Mexico: o fO g a l l a l aF o r m a t i o n , Mexico B u r e a uo fM i n e sa n dM i n e r a lR e s o u r c e s ,C i r c u l a r 1 6 PP. New 144, Galan, E., a n dF e r r e r o , A., 1 9 8 2 ,P a l y g o r s k i t e - s e p i o l i t ec l a y so f V. 30, no. L e b r y a s, o u t h e r nS p a i n C : l a y sa n dC l a yM i n e r a l s , 3,pp.191-199. Gibbs, R. J., 1 9 6 8 ,C l a ym i n e r a lm o u n t i n gt e c h n i q u e sf o rx - r a y A d i s c u s s i o n J: o u r n a l of Sedimentary d i f f r a c t i o na n a l y s i s : P e t r o l o g y , V . 38,pp.242-244. G i b b s , R. J., 1 9 6 5 ,E r r o rd u et os e g r e g a t i o ni nq u a n t i t a t i v ec l a y m i n e r a lx - r a yd i f f r a c t i o nm o u n t i n gt e c h n i q u e s :A m e r i c a n pp. 741-751. M i n e r a l o g i s t ,v .5 0 , G l a s s , H. D., a n do t h e r s ,1 9 7 3 ,C l a ym i n e r a l si ne a s t - c e n t r a l Mexico: N e w Mexico Bureau o fM i n e sa n dM i n e r a lR e s o u r c e s , C i r c u l a r1 3 9 ,1 4p p . New G l o v e r , E. D., 1 9 6 1 ,M e t h o do fs o l u t i o no fc a l c a r e o u sm a t e r i a l EDTA: J o u r n a lo fS e d i m e n t a r y u s i n gt h ec o m p l e x i n ga g e n t , no. 4, pp. 622-626. P e t r o l o g y , v. 31, , hemistry Goudie, A., 1 9 7 2 C v. 80,no. o fG e o l o g y , Hill, of w o r l d c a l c r e t e d e p o s i t s :J o u r n a l 4 , pp.449-463. W. R., J r . , a n dR u n n e l s , R. T., 1 9 6 0 ,V e r s e n e , a ndw t o o l f o rs t u d yo fc a r b o n a t er o c k s :A m e r i c a nA s s o c i a t i o no f P e t r o l e u mG e o l o g i s t sB u l l e t i n , v. 44, pp., 631-632. Isphording, W. C., 1 9 7 3 ,D i s c u s s i o n 26 of t h eo c c u r r e n c ea n do r i g i n o fs e d i m e n t a r yp a l y g o r s k i t e - s e p i o l i t ed e p o s i t s :C l a y sa n d C l a yM i n e r a l s , v. 21,no.5,pp.391-401. K h o u r y , H. N., E b e r l , D. D., a n d J o n e s , magnesium c l a y sf r o mt h eA m a r g o s a C l a y Minerals, v.30,no.5,pp.327-336. B. F., 1 9 8 2 , O r i g i n o f Desert, Nevada:Claysand K o t t l o w s k i , F. E., 1 9 6 2 ,R e c o n n a i s s a n c eo fc o m m e r c i a lh i g h - c a l c i u m N e w MexicoBureauof Mines and l i m e s t o n e s i n N e w Mexico: Mineral R e s o u r c e s ,C i r c u l a r6 0 ,p p . 19-21,28. map o ft h eS o c o r r o M a c h e t t e , M. N., 1 9 7 8 a ,P r e l i m i n a r yg e o l o g i c lo x 2O q u a d r a n g l e ,c e n t r a l N e w Mexico: US. Geological S u r v e y ,O p e n - f i l eR e p o r t7 8 - 6 0 7 , map, s c a l e 1:250.000. M a s s i n g i l l , G. L., 1 9 7 7 ,G e o l o g yo ft h eR i l e y - P u e r t e c i t oa r e a , s o u t h e a s t e r nm a r g i no fC o l o r a d oP l a t e a u ,S o c o r r oC o u n t y , Mexico: N e w MexicoBureauofMinesandMineralResources, Open-fileReport107,pp.127-128. McLean, S. A , , a n do t h e r s ,1 9 7 2 , a t t a p u l g i t eo nt h es o u t h e r n Minerals, v.20,pp.143-149. New The o c c u r r e n c eo fs e p i o l i t ea n d H i g hP l a i n s :C l a y sa n dC l a y m i n e r a l s f r o mc a r b o n a t e Ostrom, M. E., 1 9 6 1 ,S e p a r a t i o no fc l a y r o c k sb yu s i n ga c i d :J o u r n a lo fS e d i m e n t a r yP e t r o l o g y , v. 1, pp.123-129. 31,no. Papke, K. G., 1972, A s e p i o l i t e - r i c hp l a y ad e p o s i ti ns o u t h e r n v. 20,pp.211-215. N e v a d aC : l a y sa n dC l a yM i n e r a l s , P o s t , J. L., 1 9 7 8 ,S e p i o l i t ed e p o s i t so ft h e a r e a :C l a y sa n dC l a yM i n e r a l s , v.26,no. L a s Vegas,Nevada 1, pp. 58-64. S t o k k e , P. R., a n d C a r s o n , B., 1 9 7 3 , V a r i a t i o n i n c l a y m i n e r a l r a y d i f f r a c t i o n r e s u l t s w i t ht h eq u a n t i t yo fs a m p l em o u n t e d : J o u r n a lo fS e d i m e n t a r yP e t r o l o g y , v. 3,no. 4 , pp.957-964. 27 x- Addendum 1 XRD Data on HC1-derived Insoluble Residues of Riley Travertine Samples a a a 29 30 e e e e e e 34 a e a 35 36 38 Addendum 2 XRD Data on Acetic Acid/EDTA Derived Insoluble Residues of Riley Travertine Samples 39 40 41 ! I 0 0 44 0 0 45 49 I .t - ? :> 52 53 54 e e e 57 a a e 62 63 e e a 69 70 Addendum 3 XRF DataonManganese C o n t e n t of some R i l e y T r a v e r t i n e Samples a ' 72 '., . .. , I r: 4. .~. - -. - " - Rigaku Part No. KC-01 e , e . . e - 75