(1)
advertisement

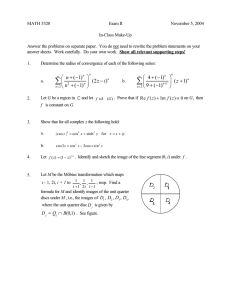
PHYSICS 210A : STATISTICAL PHYSICS HW ASSIGNMENT #3 SOLUTIONS P (1) Show that the Boltzmann entropy S = −kB n Pn ln Pn agrees with the statistical entropy S(E) = kB ln D(E, V, N ) in the thermodynamic limit. Solution : Let’s first examine the canonical partition function, Z = R∞ dE D(E) e−βE . We compute this 0 integral via the saddle point method, extremizing the exponent, ln D(E)−βE , with respect ∂S to E. The resulting maximum lies at Ē such that T1 = ∂E , where S(E) = kB ln D(E) is Ē the statistical entropy computed in the microcanonical ensemble. The ordinary canonical partition function is then Z∞ 2 2 Z ≈ D(Ē) e−β Ē d δE e−(δE) /2kB T CV −∞ = (2πkB T CV )1/2 D(Ē) e−β Ē . 2 Taking the logarithm, we obtain the Helmholtz free energy, Now SOCE F = −kB T ln Z = −kB ln D(Ē) + Ē − 12 kB T ln 2πkB T 2 CV . P = −kB n Pn ln Pn , with Pn = Z1 e−βEn . Therefore Z∞ kB SOCE (T ) = dE D(E) e−βE ln Z + βE Z 0 R∞ dE E D(E) e−βE 1 . = kB ln Z + · 0R ∞ −βE T 0 dE D(E) e The denominator of the second term is Z, which we have already evaluated. We evaluate the numerator using the same expansion about Ē. The only difference is the additional factor of E = Ē + δE in the integrand. The δE term integrates to zero, since the remaining 2 2 factors in the integrand yield D(Ē) e−β Ē e−(δE) /2kB T CV , which is even in δE. Thus, the second term in the above equation is simply Ē/T , and we obtain SOCE = kB ln D(Ē) + 12 kB ln 2πkB T 2 CV ) . The RHS here is dominated by the first term, which is extensive, whereas the second term is of order ln V . Thus, we conclude that SOCE (T, V, N ) = SµCE (Ē, V, N ), where Ē and T are ∂S . related by T1 = ∂E Ē (2) [Kardar 4.12] Consider rod-shaped molecules with moment of inertia I, and a dipole moment µ. The contribution of the rotational degrees of freedom to the Hamiltonian is 2 Ĥrot = pφ p2θ + − µE cos θ , 2I 2I sin2 θ 1 where E is the external electric field, and (θ, φ) are polar and azimuthal angles describing the molecular orientation. (a) Calculate the contribution of the rotational degrees of freedom of each dipole to the classical partition function. (b) Obtain the mean polarization P = hµ cos θi of each dipole. ∂P (c) Find the zero-field isothermal polarizability, χ(T ) = ∂E . E=0 (d) Calculate the rotational energy per particle at finite field E, and comment on its high and low-temperature limits. (e) Sketch the rotational heat capacity per dipole as a function of temperature. Solution : (a) The rotational contribution to the single particle partition function is ξrot Z∞ Z∞ Zπ Z2π 2 2 2 = dpθ dpφ dθ dφ e−pθ /2IkB T e−pφ /2IkB T sin θ eµE cos θ/kB T −∞ −∞ 0 0 1/2 = 2π · (2πIkB T ) Z∞ Zπ 2 2 µE cos θ/kB T dpθ e−pφ /2IkB T sin θ dθ e −∞ 0 Zπ µE 8π 2 I(kB T )2 2 µE cos θ/kB T sinh . = 4π IkB T dθ sin θ e = µE kB T 0 The translational contribution is ξtr = V λ−3 T . The single particle free energy is then µE 2 2 2 − kB T ln V /λ3T . f = −kB T ln 8π IkB T + kB T ln(µE) − kB T ln sinh kB T (b) The mean polarization of each dipole is µE ∂f kB T P =− =− + µ ctnh . ∂E E kB T (c) We expand ctnh (x) = x1 + x3 +O(x3 ) in a Laurent series, whence P = µ2 E/3kB T +O(E 3 ). Then χ(T ) = µ2 /3kB T , which is of the Curie form familiar from magnetic systems. (d) We have ξrot = Tr e−β ĥrot , hence o ∂ n ∂ ln ξrot − 2 ln β + ln sinh(βµE) =− ∂β ∂β µE = 2kB T − µE ctnh . kB T εrot = hĥrot i = − 2 Figure 1: Rotational heat capacity crot (T ) for problem 3e. At high temperatures T ≫ µE/kB , the argument of ctnh x is very small, and using the Laurent expansion we find εrot = kB T . This comports with our understanding from equipartition, since there are only two quadratic degrees of freedom present (pθ and pφ ). The orientational degree of freedom θ does not enter because µE cos θ ≪ kB T in this regime. Unlike the rotational kinetic energy, the rotational potential energy is bounded. In the limit T ≪ µE/kB , we have that the argument of ctnh x is very large, hence εrot ≈ 2kB T − µE. This can be understood as follows. If we change variables to p̃φ ≡ pφ / sin θ, then we have ξrot Z∞ Z∞ Zπ Z2π 2 2 = dpθ dp̃φ dθ sin θ dφ e−pθ /2IkB T e−p̃φ /2IkB T eµE cos θ/kB T −∞ −∞ 0 0 Z∞ Z∞ Z1 Z2π 2 2 = dpθ dp̃φ dx dφ e−pθ /2IkB T e−p̃φ /2IkB T eµEx/kB T , −∞ −∞ −1 0 where x = cos θ. We see that x appears linearly in the energy, and simple dimensional analysis reveals that any degree of freedom ζ which appears homogeneously as U (ζ) ∝ ζ r contributes kB T /r to the average energy. In our case, we have quadratic contributions to the Hamiltonian from pθ and p̃φ , a linear contribution from x = cos θ, and φ itself does not appear. Hence ε = −µE + 2 × 12 kB T + kB T = −µE + 2kB T . The −µE term is the minimum value of the potential energy. (e) The rotational heat capacity per molecule, sketched in Fig. 1, is given by crot 2 µE/kB T ∂εrot . = 2kB − kB = ∂T sinh(µE/kB T ) (3) A surface consisting of Ns adsorption sites is in thermal and particle equilibrium with an ideal monatomic gas. Each adsorption site can accommodate either zero particles (energy 0), one particle (two states, each with energy ε), or two particles (energy 2ε + U ). 3 (a) Find the grant partition function of the surface, Ξsurf (T, Ns , µ). and the surface grand potential Ωsurf (T, Ns , µ). (b) Find the fraction of adsorption sites with are empty, singly occupied, and double occupied. Express your answer in terms of the temperature, the density of the gas, and other constants. Solution : (a) The grand partition function is Ns Ξsurf (T, Ns , µ) = 1 + 2 eβ(µ+ε) + eβ(2µ+2ε+U ) , hence Ωsurf (T, Ns , µ) = −kB T ln Ξsurf = −Ns kB T ln 1 + 2 eβ(µ+ε) + eβ(2µ+2ε+U ) . (b) Thermal and particle equilibrium with the gas means that the fugacities of the gas and surface are identical, and for the gas we have z = nλ3T . Thus, ν0 = 1+ 2nλ3T eε/kB T 1 + n2 λ6T e(2ε+U )/kB T ν1 = 2nλ3T eε/kB T 1 + 2nλ3T eε/kB T + n2 λ6T e(2ε+U )/kB T ν2 = n2 λ6T e(2ε+U )/kB T . 1 + 2nλ3T eε/kB T + n2 λ6T e(2ε+U )/kB T (4) Consider the Hamiltonian below with N kinetic DOF, N2 quadratic potential DOF, and N4 quartic DOF, with N ≥ N2 + N4 . Ĥ = 1 2 N X m−1 ij pi pj i,j + N2 X N2 +N4 2 1 2 Ki qi + i=1 X 4 1 4 Aj qj . j=N2 +1 Find the free energy F and the internal energy E in terms of T , V , N , N2 , and N4 . Assume the matrix mij is nondegenerate. Solution : If mij is nondegenerate, its rank is N . By transforming to an eigenbasis, one finds Z Y N dp l=1 h l −m−1 ij pi pj /2kB T e 4 = kB T 2π~2 N /2 √ det m . As for the potential energy, we have Z∞ 2πkB T 1/2 −Kq 2 /2kB T dq e = K −∞ and Z∞ Z∞ 4kB T 1/4 4 −Aq 4 /4kB T dq e = du e−u = Γ A −∞ where Γ 5 4 5 4 −∞ 4kB T 1/4 , A ≈ 0.906402. Thus, the partition function is 1 Z = C V N −N2 −N4 (kB T ) 2 where C≡ √ 2Γ ~ 5 4 (N +N2 )+ 14 N4 √ !N Q The free energy is det m Q 1/2 i Ki j F = −kB T ln Z = −kB T ln C − (N − N2 − N4 ) kB T ln V − The energy follows from E= ∂(βF ) = ∂β 1 2N 1/4 Aj 1 2N . . + 21 N2 + 14 N4 kB T ln(kB T ) . + 21 N2 + 14 N4 kB T . 5