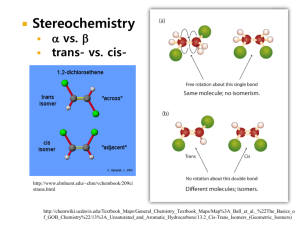
THE ROLE OF LIGNIN IRVING A. B.S.,
advertisement

THE ROLE OF LIGNIN IN COAL GENESIS By IRVING A. BREGER B.S., Worcester Polytechnic Institute (1941) Massachusetts Institute of Technology S.M., (1947) SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY (1950) Signature of Author. . . . . . , . ,,,, . ./ I. Department of Geolo y, May 12, 1950 Certified by . . . 0 0 0 0 . . S . . . . . . . Thesis Supervisor . . . . 0 Chairman, Department Committee on Graduate Students k 4/ TABLE OF CONTENTS Page . FIGURES . . . . . . . ABSTRACT . INTRODUCTION . of Statement . . ... . 'viii . . . . . . . . . Problems . . . . . . . . . . *... . 1 . .. . . . . . . . . . . . . Historical . vi - - - - - - - - - - - - - - . . .. . . . . .. - - - - - -. . . -. . . . . . . ACKNOWLEDGEMENTS. -. - - - - - - - - -. . . . . . . . . . . . . . . TABLES . 7 ..... .. 13 LIGNIN - REVIEW OF THE LITERATUR:E ............ ...... . . Introduction . . 1 13 ........ Relationship of Lignin and Cellulose in . . . 14 . Plant Structure Isolation The of LIGNIN - of Structure Summary . .......... . Lignin . . . . . EXPERIMENTAL WORK. Introduction . Brauns' . Lignin . . . . .. ..... . . . . . . . . . . . . . Native Lignin ........ . . . Periodate Lignin .......... Russell Lignin . Summary . . a.. . . . . . . . . . . . . . . . 25 . . . 34 . 35 ...... ....... . . . . . ... . .. . 15 .... . . 35 .. .. .. .. . 36 ........ 39 . . 44 . . . . . . . . . . . . . . .. ... .. .. . 46 ii. Page LIGNIN - CHEMICAL AND STRUCTURAL RELATIONSHIP TO HUMUS . Introduction . . . . . . . . . . . . . . . . . . . 47 . 47 48 Microbial Decomposition of Plant Components Conclusions . . Summary . . . LIGNIN - .. . . .. . . .. .. .. . . . . .. .. .. . ........ . . . . . . . . . . . . . Vacuum Differential Thermal Analysis . . Discussion of Thermograms . Summary and Conclusions . HIGH PRESSURE STUDIES . ... . . . . . .. Analytical . Experimental . . Summary of Results CONCLUSIONS . Techniques . . . . . .. . .. . . . . . ... . . . . . . . . . . . . . AND PROPOSED WORE . . Conclusions . . ..... ... . ... Proposed Work . . . . . APPENDIX - . . . Punched Card Bibliography BIBLIOGRAPHY . . . BIOGRAPHICAL SKETCH . . . . . OF AUTHOR. . . . . . . . . . . . . . . . . . . . . . . . 79 . 94 . . 97 . . . . . . . . . . . . . . .. . . 62 94 . . . . 61 . .. . . . 101 . 106 112 . . . 115 .. .. .. . 115 . . . . . 117 121 .......... ......... . . 70 . . . . . . . . . . . 61 . . . .. ...... . . . . . Introduction and Review of the Literature Description of Equipment . . . ......... . . . . . . 60 .. BASIC STRUCTURES IN THE COAL SERIES . . . Introduction 51 . . . . . . . . 133 . 159 iii. FIGURES No. 1 Title Page Infrared Absorption Curves for Humic . . . 57 . . Acid and Lignin 2 Furnace for Vacuum Differential Thermal . . . . 64 Analysis 3 Details of Furnace Construction......... 4 Details of Furnace Construction . 5 Sample Block for Vacuum Differential Thermal Analysis 6 Photographs of Thermographic Equipment 7 Photographs of Thermographic Equipment . 8 Thermograms for Wood, Cellulose, and Lignin 81 9 Thermographic Comparison of Various Lignins 82 10 Thermograms of Peats . 83 11 Thermograms of Peats . . . . . . . . . . . . . 84 12 Thermograms of Lignites. ......... 85 13 Thermograms of Bituminous Coals o........ 86 14 Thermograms of Bituminous Coals . . . . . . . . 87 15 Thermograms of Anthracite Coals . . . . . .. 88 16 Thermograms of Vitrain, Durain, Clarain, and Fusain . 89 17 Thermograms ... of Unclassified 65 . . .. 66 . . 67 . . . . 71 . . 72 . . . . . . . ......... . . Coals . . ... . . . . . . 90 iv. Page . . . Thermograms of Unclassified Coals 19 Thermograms of Pyritized Coal and Pyrite . 20 Thermograms of Shales . . 21 Details of Construction of Bomb and Piston . . . . . . . . . . 91 . . . . 18 . . . 92 *..93 . . . 99 for High Pressure Studies 22 Photograph of Equipment for High Pressure . . 100 23 Apparatus for the Determination of Methoxyl . . . 104 . . Groups 24 Photograph of Golden-Brown Component and . . . 110 . . 124 . Radial Fractures in Compressed Lignin 25 SubdIvision of Subject Matter and Code for . Punched Cards 26 Samole Pumched Card . . . . . . . . . . . . . . . 127 TABLES Page Title No. Treatment of Spruce Chips with Alcohol. . . . 36 to Obtain Native Lignin II Comparative Data for Various Isolated. . 43 Correlation of Infrared Absorption,. ..... 58 . Spruce Lignins III Peaks for Humic Acid and Brauns' Native Lignin IV Summary of Pressure Experiments and. Blanks .... 112a ACKNOWLEDGEMENTS The studies described in this thesis serve ,s an excellent example of research which can be done in borderline fields. In this particular instance the geological advice, suggestions, and inspiration of Professor Walter L. Whitehead deserve infinitely more appreciation than words could possibly convey. Without the unlimited assistance and coo-eration of Professor Harold Fairbairn, the high pressure studies would have been difficult to carry out. Professor Fairbairn has been extremely generous with his time and equipment. The author wishes to acknowledge the inspirational comments of Professor Robert R. Shrock who has demonstrated keen insight, along with Professor Whitehead, in the problems of organic geochemistry. To Profs. Clark Goodman and Ernest H. Huntress, who also served on the committee governing the graduate activities of the author, Mr. are extended gratitude and appreciation. B. C. Parks of the U. S. Bureau of Mines was extremely helpful in used in this work. supplying many of the coal samples Dr. E. E. Harris of the U. S. Forest Product Laboratory supplied a large quantity of sulphuricacid lignin, and Dr. C. A. Sankey of the Ontario Paper Company vii. assisted the author in obtaining spruce wood chips for the isolation of periodate lignin. Particular acknowledgement is made of the cooperation of Miss Virginia Burton and Miss Geraldine Sullivan who assisted the author in every possible manner. The reference librarians of the Massachusetts Institute of Technology Libraries were particularly helpful during the bibliographic phase of this work. To my wife, Ruth, and my parents and friends who encouraged me at every possible opportunity, I dedicate this work. viii. ABSTRACT Many theories have been proposed in the past to account for the formation of coal from plant materials. present it At is generally accepted that two distinct stages are recognizable in the coal-forming process: (1) the biochemical stage, and (2) the dynamochemical stage. In the first of these two steps plant components are biochemically degraded to humic substances, and in the second step the humic materials are metamorphosed into high grade coals. Considerable controversy has raged on the proposals on one hand that cellulose is the chief progenitor of coal, and on the other hand that lignin is the most probable source naterial for coal formation. A very thorough and critical survey of the literature has been made and the evidence in favor of and against these proposals has been weighed. On the basis of numerous biochemical studies it appears likely that cellulose is rapidly degraded and removed from organic sediments leaving behind the lignin which is microbially transformed but not completely decomposed. Degradation of the lignin molecule appears to result in a complex mixture of products which is collectively called humus. On the basis of a study of the structure of lignin, a proposal has been made for the chemical changes which take place in the lignin molecule during its conversion to humic materials. ix. Apparatus has been designed and constructed for the differential thermal analysis of complex organic substances under vacuum. Using this equipment, a study of several plant components and of various members of the coal series has been carried'out. The decomposition pattern of lignin appears in the lignite and bituminous members of the coal series, and suggestions are made for the reasons for its absence in peats and anthracite coals. The thermogram for cellulose is distinctive and aoes not appear in bituminous or anthracite coals. Thermographic analyses of peats and lignites, however, provide evidence for the presence in some of these materials of small quantities of residual cellulose. The thermographic evidence points strongly towards lignin as the progenitor of coal. A number of preliminary studies have been carried out to test the possibility that shear stresses are of importance in the dynamochemical devolatilization of humic materials into high grade coals. Lignin, humic acid isolated from peat, and peat have been sheared while under pressures of up to 2280 atmospheres. These experiments have been carried out at temperatures as high as 1500 C. and for durations as long as 32 days. humic acid appears to undergo Under these conditions a definite change in structure that has not yet been explained. Lignin, which contains 11.09% of methoxyl groups, is demethylated only to the extent of 0.4% under these conditions. These preliminary results, although not conclusive, do appear to indicate that a piezochemical devolatilization of coal-forming materials is possible under the proper conditions. Geological evidence points to shear forces as being important in the coalification process, but further studies will be required before this fact can be confirmed on a laboratory scale. CHAPTER I INTRODUCTION HISTORICAL Theories concerning the origin of coal have been many and varied. As early as 1778, Franz von Beroldingen (278) proposed the well known peat-lignite-bituminous-anthracite series which has withstood the test of nearly two centuries of continuous study. The apparent simplicity of this coalification scheme was perhaps responsible for attracting many early workers to the study of naturally-occurring solid fuels. Microscopic examinationsof coal were first carried out by Witham in 1833 (307). Improvements in both instruments and technique, especially within the last fifty years, have made it possible to distinguish between and to identify the plant components which have contributed to the various types of coals. As a result of these studies, carried out by such eminent microscopists as David White (300,303), Reinhardt Thiessen (259, 260), Marie Stopes (245, 246, 248, 249),C. E. Marshall (127, 128, 129, 179, 180, 209), and others (125, 126, 143, 144, 205, 206, 232-5, 253), it is now agreed by geologists that most coals originate from woody materials. 2. Although microscopic examination has demonstrated the plant origin of coal, the chemical nature of the material has not yet been clearly established, Extraction studies were carried out prior to 1909 (66), but critical work was not reported until 1911 when Pictet and his collaborators undertook the isolation and identification of low molecular weight components of coal using procedures familiar in organic chemistry (194-8). Low temperature extractions of large quantities (5.5 tons) of various coals led to the separation of toluene, m-xylene, mesitylene and other identifiable organic compounds. Vacuum distillation of the original coal to 4500 C. led to a mixture of hydrocarbons which was found to be somewhat optically active. Upon heating the distillate to 350 0C., the optically active components were destroyed and an upper limit was established for the temperature to which the coal had been exposed during its formation. The purely chemical studies which had been carried out by Pictet were amplified in England where Marie C. Stopew, a petrologist, and R. V. Wheeler, a chemist, together engaged in a study of coal (247, 250, 251, 263). Wheeler's early work eventually led to a series of studies in which various coals were fractionated empirically by means of solvent extraction procedures (11, 60, 61). These investigations gave rise to the conclusion (297) that coal is primarily a mixture of three principal types of materials: (a) extractable resins and hydrocarbons, (b) resistant plant skins, and (c) the ulmins, produced by decay and subject to progressive alteration. In coals of similar rank, as well as in the banded constituents of coal, differences were thought to exist as a result of varying proportions of the three major components. Although coal chemistry had made rapid progress between 1900 and 1920, the geochemical aspects of coal genesis had been comparatively neglected.'-In 1907, H. Potonie (203) summarized his earlier work with the conclusion that coal formation is a multifold process having the following steps: (a) Decay of plants under aerobic conditions to produce gaseous products. (b) Mouldering to yield a small amount of solid organic residue. (c) Conversion to peat. With thickening of the peat bed the lower strata become devoid of oxygen. (d) Faulnis, In this step the lower peat strata undergo anaerobic decomposition. (e) Incoalation,. Conversion of the product from (d) into coal without the formation of free carbon. Although this scheme still serves to describe the general process of peat formation, no effort was made by Potoni6 to establish the relative contributions of the various plant constituents to the peat. In 1912 Bergius, in the course of experiments on the origin of coal, heated wood, cellulose, and peat in water under high pressure to a temperature of 3500 C. and reported that under these conditions peat led to a product which was "precisely 4. similar to natural bituminous coal" (12). In later papers Bergius reported that by heating peat in water at 34000 ,f or 24 hours a product was obtained which closely resembled anthracite and which was composed of 84% carbon, 10.4% oxygen, 4.6% hydrogen, and 0.96% nitrogen (13, 14). This analysis must be compared with that for an everage anthracite coal containing 96% carbon, 2% hydrogen, and a total of 2% of oxygen, nitrogen, and sulphur (154). When cellulose wassimilarly heated in water to 340?0. for 64 hours, 4 was again reported (13). a product similar to anthr&cite coal The experiments with cellulose marked an early critical point in geochemical studies of the origin of coal since Bergius soon became convinced that all coals originated from the same parent, namely, cellulose. To explain variations in naturally-occurring coals, it was postulated that peats are generated at the surface of the earth whereas carbonization takes place only under the influence of the "enormous pressure of overlying strata" (15). With the continuation of these studies, sufficient evidence in the new and exciting field of coal geochemistry was presented to cause wide international adherence to the cellulose theory of coal formation. Among the proponents of this theory were Donath (67), Schwalbe (226-8), Marcusson (170, 172, 173, 175, 176, 178), Berl (16-30), and many others (31-3, 52, 109, 111, 167, 204, 211, 239, 256, 257, 296). When these studies are carefully considered, it becomes evident that the geological limitations of hydrogen ion concentration and temperature were 4 neglected during most of the experimental work. Although a number of early investigators in the field of coal chemistry had suggested approximately 2500C. as a maximum temperature for the formation of coal, the advocates of the cellulose theory consistently employed temperatures of 3000 to 35000. Reactions were admittedly carried out at elevated temperatures in an effort to speed up geological processes which normally proceed slowly with less heat, but the important fact was completely neglected that most complex organic compounds will decompose rapidly above 3000C. to yield carbonized residues. In recent years conclusive evidence for the low-temperature origin of coal (lower than 20000.) has been published by Treibs (265-8) who isolated acid porphyrins from many coals. Bearing this major consideration in mind, the conclusions developed from laboratory experiments with cellulose lose their importance and the entire theory of the cellulosic origin of coals, as proposed, appears to rest on an unstable foundation. In 1921 Fischer and Schrader (79, 80) critically examined the theories of coal genesis and concluded that lignin, not cellulose, was the parent material from which coal had been formed. Their reasons were as follows: 1. Lignin has an aromatic structure with acetyl and methoxyl groups. 2. Cellulose is largely destroyed by bacterial activity in the early stages of peat formation. 3. The methoxyl content of peat increases and the portion soluble in concentrated hydrochloric acid decreases with increasing age. 4. The methoxyl content eventually decreases owing to saponification, reduction, or replacement by hydroxyl; but, in any case, a phenol results which is taken to be identical with humic acid. 5. Oxidation or polymerization of humic acid forms alkali-insoluble humin. 6. Further splitting off of water and carbon dioxide, and perhaps methane, at ordinary temperature leads to the formation of lignite and coal. The six points presented by Fischer and Schrader were intended to show an extension of the basic aromatic structure of lignin into coal. Fischer and his collaborators have supported this theory with a large number of studies dealing with the oxidation, reduction, and decomposition characteristics of a variety of substances as lignin, cellulose, coals, etc. (73-8, 81-8, 222). The conclusions of Fischer and Schrader with regard to the extensive and rapid biochemical decomposition of cellulose in the early stages of coal formation have also been substantiated by numerous workers during the past thirty years (44, 218, 221, 261, ?79-83, 286-8, 293). Similarities in chemical structure between lignin and various coals have, moreover, been established by X-ray studies (120, 186, 229, 230). In recent years (since 1935), disclosures bearing directly on the origin of coal have been relatively few. Waksman, who contributed greatly to knowledge regarding peat formation and soil organic matter (284), has concentrated his efforts on the development of antibiotics, while Fischer, prior to his death in 1949, was concerned chiefly with the FischerTropsch Process for the synthesis of liquid fuels. For modern evidence for or against the lignin theory of coal formation it has been necessary to resort to an extensive literature survey of publications in the field of microbiology, high pressure physics, analytical chemistry, and geology. The entire bibliography for this thesis consists of the abstracts of approximately 2000 papers dating mainly from 1907 to the present. Since any effort to summarize all or even most of these articles in this dissertation would lead to an improperly balanced product, only the most important references will be cited. The entire bibliography, which is assembled on McBee Keysort Punched Cards, will be deposited in the Headquarters Office of the M.I.T. Department of Geology, and the Appendix to this work will include a description of the code and procedures for its use. STATEMENT OF PROBLEMS Having reached the conclusion from a critical survey of the literature that lignin is the most probable progenitor of the principal components of coal, several questions remained I I,=-- -- Z_ to be answered when the studies described in the following chapters were undertaken. At the very first stage of coal formation, plant components apparently undergo severe biochemical decomposition resulting in the concentration of an organic sediment which has been extensively studied under the name "humus" (284). This substance has been fractionated on the basis of solvent extraction and chemical properties (102, 106, 159, 171, 174, 212, 241, 242, 258, 264, 274, 275), and other studies have led to the isolation of degradation products which are similar to those obtained from lignin (100, 103). Although the chemical evidence for the structural similarities between lignin and humus (also humic acids, ulmic, acids, ulmin, hymatomelanic acid, etc.) (9, 99) has been good, the colloidal characteristics of the humus (89, 148, 187, 310) have made a complementary physical correlation impossible. For this and other reasons, there exists nowhere in the literature today a satisfactory explanation of the changes which occur in the basic lignin structure during its conversion to humus. To interpret the chemical changes which take place in lignin between the death of the contributing plant and the peat stage, it is necessary to know the structure of lignin. Continuous and concerted studies by lignin chemists for 75 years have failed to produce a non-controversial structural formula for the material. A review of the most reliable information in this field was, therefore, considered to be of 9. fundamental importance in order to establish points of general agreement with respect to the structure of lignin. With this background, a theoretical interpretation of the lignin-humus conversion was thought possible. Regardless of its origin, there is a general agreement among chemists and geologists that the principal constituents of most coals are derived from humus or humic acids. This opinion is based on the well-known fact that peats and lignites contain large percentages of acidic organic complexes which are easily soluble in dilute aqueous alkaline solutions. With bituminous coals this type of extraction yields no significant quantity of extract, and the humic acids are considered to be in a "coalified stage" (156). A number of investigators have reported the regeneration of humic acids from bituminous coals by careful oxidation in air or with mineral acids (65, 105, 149, 238, 309) thus providing further evidence for the humic acid-bituminous coal relationship. Many theories have been developed to account for the manner in which humic substances have been converted into hard coals. Among the various causal factors which have been cited to account for the formation of different kinds of coals are the following (181, 254): 1. Different types of vegetation and differences in 2. Duration of exposure of vegetation before burial climates. by sediments. 10. 3. Duration since burial of sediments. 4. Depth of burial of vegetation. 5. Action of heat from compression or from intrusions of igneous rocks. 6. Possibility of escape of volatile constituents after burial beneath sediments because of fractures or pores in the overlying rocks and jointage in the coal seams. 7. Pressure resulting from compression of the seam during dynamic changes in the enclosing rocks. Although different types of vegetation and different modes of accumulation have undoubtedly led to variations among coals, these factors can hardly be considered as having contributed to the energy necessary to devolatilize humus material during coalification. This same argument is applicable to most of the above suggestions, and in the final analysis heat and pressure must be considered as two of the most reasonable sources of energy for the coal-forming process. Bacteria cannot be considered effective agents for coalification in the post-peat stage since lignin, upon which bacteria do not thrive, has been shown to accumulate in the peat bog (255). Moreover, radioactivity, which has recently been found to convert naturally-occurring organic acids into hydrocarbons, (45, 46, 236, 237) is almost completely absent in coals (53, 54, 72, 182). 11. Petrascheck (191) has recently shown conclusively that igneous activity affects coal seams only locally and that depth of burial and accompanying temperature effects are hardly important in the coalification process (147, 272). This work, coupled with that of Treibs (265-8) has effectively eliminated temperature and hydrostatic pressure as sources of energy for the formation of hard coals. It has long been recognized that bituminous and anthracite coals are common in areas where geological folding has taken place. David White realized this relationship early in this century (298, 299, 301, 302, 304) and his opinions have been substantiated by the observations of other investigators (155, 252). To investigate the possibility that shear forces might be effective in the coal-forming process, a literature survey was proposed to review the effects of high pressure and shear on organic compounds, and an experimental program was initiated to study the effects of high pressure and shear on lignin and related materials. To summarize, the major sections of this study are as follows: 1. A survey of the literature on lignin, its structure and its relationship to the origin of coal. 2. An interpretation of the chemical and structural relationships between lignin and humic material. 3. An investigation of the basic structures of lignin and coals. 12. 4. A survey of the literature on the effects of high pressures on organic compounds. 5. Studies of the effect of high pressure and shear on lignin and related materials. 13. CHAPTER II LIGNIN - REVIEW OF THE LITERATURE INTRODUCTION Lignin, which was considered a botanical curiosity for many years after its discovery by Payen in 1838 (190), has become within the last fifty years a subject of widespread chemical investigation. With the development of the paper pulp industry, lignin has become a major problem, both as to its removal from wood and as to means for its disposal. In recent years, especially the last two decades, these problems have been reflected in an enormous growth of the technical literature on lignin. It is almost unbelievable that all of the studies since 1838 have failed to establish with absolute certainty either the manner of formation of lignin in wood or the exact structure of the isolated material. As outlined in Chapter I, a survey of the literature was considered necessary for a theoretical interpretation of the lignin-humus relationship. The literature on many phases of lignin research has been examined, and the major contributions are summarized in the following pages. 14. RELATIONSHIP OF LIGNIN AND CELLULOSE IN PLANT STRUCTURE Wood is composed of ash-forming minerals (1.0%), lignin (18-27%), and holocellulose (70-80%). These three components make up the wood structure which is infiltrated with water-soluble materials and ether-soluble resins and The lignin and cellulose are so uniformly waxes (189). dispersed throughout the tree that either one alone forms a complete structural pattern of the wood. this effect is Recent evidence to conclusive (3, 107, 161, 189) and refutes with finality the older view that lignin is not an original wood component but rather a material produced during isolation procedures (130-4, 224). The exact association between the cellulose and the lignin is unknown. Centola (57) recently suggested that some rings of the macromolecule of cellulose may, a.lteration, by chemical take part in the formation of the lignin molecule in the plant cell. The view that lignin and cellulose are chemically united in wood by primary valence forces has been presented by Staudinger (244), and by Freudenberg and Ploetz ( 94, 199-202). As further evidence of chemical union between the two major constituents of wood, it should be pointed out that the greater portion of the lignin (about 90%) can be isolated only by chemical destruction of the cellulose or 15. alteration of the lignin itself. and Yirak (43) In a recent paper, Brauns proposed twelve possible lignin-cellulose linkages which may exist in spruce wood. As is often the case with studies in this field, their efforts to establish the actual bond type led to inconclusive evidence. Evidence against the theory of chemical combination between lignin and cellulose in wood has been presented by Bailey (4) who assumed that his isolation procedures, carried out in buffered solutions, would prevent hydrolysis of any existing bonds. The results of this study appear to be controversial (40). wood by thermophilia Experiments on the fermentability of bacteria have also been interpreted to indicate the absence of a chemical bond between lignin and cellulose in wood (277). ISOLATION OF LIGNIN It should be well understood that there is no such material as pure lignin. The substance called lignin is known to vary in amount in different parts of the plant, being concentrated in the middle lamella of the cell (162, 216). The constitution of lignin is known to vary with the seasons in a particular species, and the isolated product from coniferous trees differs from that of deciduous trees. These peculiarities alone would be sufficient to render the isolation of a pure substance improbable. To add to these difficulties, however, the previously-described intergrowth of cellulose and 16. lignin is such as to require drastic chemical treatment for the separation of these two components. Consequently, it is hardly to be expected that a product isolated from a material of variable composition by vigorous chemical procedures can This point cannot be stressed be called chemically pure. too strongly. Many procedures have been developed for the isolation of lignin from wood, but most methods follow two general approaches: (1) Isolation of lignin by destruction of the cellulose, or (2) Isolation of lignin by extraction with or without chemical alteration. The most important procedures falling in these two categories are described below. One of the earliest procedures for the isolation of lignin from wood was that developed by Willstatter and In this method 40 - 42% hydro- Zechmeister (306) in 1913. chloric acid, that is, concentrated hydrochloric acid saturated with hydrogen chloride gas, is used to hydrolyze Willst~tter and Zechmeister and dissolve the cellulose. demonstrated that pine wood is rapidly attacked by 42% acid to leave a residue of lignin. It appearsthat the cellulose is actually dissolved first and then, on standing, is hydrolyzed to glucose which leads to the formation of a dark yellow solution. The obvious disadvantage in this procedure lies in the use of strong acid and its effect on the residual lignin. Brauns (39) has shown that the acid leads to partial demethylation of the lignin: R - 0 - CH + HC1 -----> R - OH R = lignin structure + CH 3 01. 17. The Willst~tter isolation procedure is summarized below (68, 157): Wood shavings are extracted with a 1:1 benzene- alcohol mixture to remove fats, resins, and waxes. The shavings are then dried and treated with ten parts by weight of 40% hydrochloric acid (d. 1.42) which is prepared by passing hydrogen chloride gas into ordinary concentrated hydrochloric acid maintained at 00 C. for fifteen minutes, After being shaken the mixture is poured into an excess of hot water and then rapidly diluted with a large excess of cold water. The lignin settles out first and the lighter cellulose precipitate is removed by decantation. The lignin is filtered and then subjected to two further ten-minute fuming hydrochloric acid treatments, first with five parts of acid and finally with two and one-half parts of acid. The product is thoroughly washed with water and dried, The lignin obtained by this procedure still contains approximately 8% of pentosans. The product is insoluble in most solvents and only slightly soluble in boiling phenol. It dissolves readily in trichloroacetic acid, ;iving a violet-brown solution, and on addition of water the lignin is reprecipitated in a form soluble in alkalies and acetone. Sulphite solutions do not dissolve this lignin readily, but ferric chloride is reduced and Schiff's reagent is colored. Fehling's solution is not affected by the lignin which contains several per cent of chlorine. 18. A second common acid hydrolysis procedure for the isolation of lignin is one which employs 72% sulphuric acid (153). As is the case with other procedures, so here also the isolation technique has certain disadvantages and leads to a degree of conversion of the lignin (69). Since 72% sulphuric acid acts both as a hydrolyzing agent and a condensing agent, insoluble products are formed from pentosans, hexosans, proteins, and tannins. Theseproducts are isolated with the lignin. Demethylation of lignin produces phenolic hydroxyl groups which are able to react with furfuralsformed by the action of the sulphuric acid on the pentosans of the wood. The insoluble products from these reactions are also isolated with the lignin. To minimize these and other difficulties inherent in this procedure, a method employing low reaction temperature and a short period of exposure to the acid reagent has been developed (217). given below, is This procedure, as for the estimation of lignin in wood, but can serve as well for the isolation of lignin. Two grams of wood (80 mesh) are dried at 10500. and then Soxhlet-extracted with a 2:1 alcohol-benzene mixture to remove fats, waxes, and resins. The dried wood is then slowly mixed, at room temperature, with 25 ml. of 72% sulphuric acid by weight. Care must be taken that the temperature does not rise during this step. At the end of two hours sufficient water is slowly added to decrease the acidity to 3% and the mixture is then boiled under reflux for three hours to complete 19. the hydrolysis of the cellulose. The solution is filtered and the lignin, after thorough washing, is dried under vacuum. Several modifications of this procedure have been published (153, 169, 220, 225) as well as a series of investigations with regard to the difficulties and limitations of the method (56, 62, 108, 183, 184). In recent years a number of attempts have been made to isolate lignin from wood by the oxidative degradation of the cellulose. Purves and his collaborators have reported the use of sodium paraperiodate as an oxidant (214, 294), and Leavitt has reported the unsuccessful use of lead tetraacetate (163). Since periodate lignin was isolated in the course of studies related to this thesis, a description of the isolation procedure will be reserved for Chapter III. Extensive studies have been carried out on the effects of enzymes on wood and on the possibility of enzymatic destruction of cellulose for the isolation of lignin (94, 164, 199-202, 243, 277). Since all results in this field appear to be inconclusive and point to a fairly extensive conversion of lignin, it does not seem necessary to describe pertinent isolation procedures. Extractive procedures for the isolation of lignin are of three types: (a) Partial extraction of lignin by organic solvents with no chemical interaction; (b) Chemical reaction of inorganic salt and lignin to form a water-soluble compound; or (0) Acetal formation between lignin and an alcohol under acidic conditions to produce a soluble product. 20 Brauns has reported the isolation of approximately 3% of the total lignin in spruce wood by means of extraction, under neutral conditions, with alcohol (35, 37). The light brown product, called "native lignin", has been isolated in the course of the present studies and discussion of this procedure will, therefore, be reserved for Chapter III. In commercial paper pulp preparation, wood chips are digested at elevated temperatures with calcium bisulphite which reacts with the lignin to form a water-soluble lignosulphonic acid. The reaction involved in the formation of the soluble product has been thought to proceed by means of the addition of sulphurous acid to an enolic double bond in the lignin molecule (36, 41, 116). H R - C = 0 - R OH H OH + H2 S03 ---- R - - RI IH H S03 H Lignin Lignosulphonic Acid The lignosulphonic acid becomes soluble only with increase in the digestion temperature. Brauns and Brown (41) have suggested that this solubility results from the reaction of the lignosulphonic acid with excess calcium bisulphite: 21. 2 R - H OH H .- - Rt + Ca (HSO 3 ) 2 --- R - OH - R' .--- I H- Ca + 2H2so3 H- S@H SO H _02 Lignosulphonic acid is unstable and can be handled only-:in the form of a salt. The acid contains approximately 3.5% sulphur or approximately one sulphonic acid unit per building block (assuming a molecular weight of 840) of lignin (36). Other commercial processes for the extraction and isolation of lignin from wood by means of soluble salt formation yield highly converted products of more or less unknown constitution. For this reason it seems unnecessary to discuss the sulphate or soda processes with respect to the present work. The isolation of lignin from wood by means of acid catalysts and compounds containing hydroxyl groups has long As early as 1920, Grutss demonstrated the isolation been known. of lignin from wood by means of ethyl alcohol and hydrochloric acid (112, 113). Since that time studies have been carried out using methanol (42, 117), ethanol (47, 97, 98, 118, 135, 138, 139, 140, 168, 208), butanol (4-7, 58, 188), isobutanol (118, 119), amyl alcohol (118, 119), ethylene glycol (110, 213), and many other solvents (101, 104, 107, 110, 123). lignin has been studied extensively, preparation, Butanol- and a procedure for its taken from Charbonnier (58) is given below. w J9 22. Air-dry wood meal (107.5g.), corresponding to 100g. of moisture-free material, is covered with 1200 ml. of anhydrous butanol. Four hundred ml. of the butanol are distilled off under reduced pressure to remove the moisture in the wood meal. Then 90 ml. of dry butanol containing 5.1%hydrogen chloride by weight are added to make the total 0.5% in hydrochloric acid. The butanol-hydrochloric acid solution is prepared by passing hyarogen chloride gas (dried over phosphorous pentoxide) into a flask containing anhydrous butanol. After the acid-alcohol solution is thoroughly mixed with the wood meal, the mixture is refluxed for two hours and then allowed to stand overnight. The following day, 90 ml. of the 5.1% acid-alcohol solution are added, the mixture is thoroughly stirred and again refluxed for two hours. When the material has cooled to room temperature, 200 ml. of butanol are distilled off under reduced pressure to remove the hydrochloric acid, the butanol solution is separated by filtration on a BUchner funnel, and the residue is washed twice with 500-mi. portions of purified dioxane. filtrate. The wash liquors are added to the original Several portions of ethyl alcohol are then used for washing the residue until no color appears in the filtrate, after which the extracted wood meal is spread out to dry for several days. it When the residue (pulp) has become air-dried, is weighed and sampled for moisture and lignin determinations. The residue from the butanol treatment is reported to contain 12.9% lignin. 23. The butanol-lignin is recovered from its dioxane solution by precipitation with anhydrous ethyl ether. To accomplish this step, 4-ml. portions of the dioxane solution are added dropwise to 80-ml. portions of ether with vigorous stirring. After centrifugation, the supernatant liquid is replaced with fresh ether, and the preoipitat ion cycle is Following precipitation, the lignin ip repeated. washed twice in ethyl ether and twice in petroleum ether and then allowed to stand overnight in the latter solvent. Finally the product is centrifuged, decanted, and dried in a vacuum desicocator over sulphuric acid. is The purification procedure repeated until the methoxyl content becomes constant. Since Charbonnier has been able to isolate hydro- lytically and to identify butanol from the butanol-lignin product, there can be no question regarding the existence of a chemical bond between the alcohol and the lignin. Chemical evidence points to the formation of acetal-type linkages by which carbonyl groups in the lignin are converted to hydroxyl groups. This has been proved by the fact that methanol-lignin can be methylated with diazomethane (42). (Lig) C= 0 (Lig) C + CH OH - - -) 3OOCH OH + CH N 00H 2 2 ------ (Lig) NC ,OH (Lig) C ,OCH3 3 + N OCH 2 24. The preparation of methanol-lignin and similar products can be carried out by using modifications, where necessary, of the general procedure outlined above. Formic and acetic acids have been known for several years to extract lignin from wood. In a paper on the isolation and structure of formic acid lignin (308), Wright and Hibbert indicated that the product has lost methyl groups. In spite of some loss of methyl groups, however, the ease of preparation makes the procedure extremely valuable. There can be no doubt that the native lignin has been changed chemically and/or physically by formic acid treatment, is soluble in many organic solvents. since the final product A procedure for the preparation of formic acid lignin, as taken from the literature, is given below. A suspension of 40 g. of spruce wood meal (75 mesh) (previously extracted with 1:1 alcohol-benzene, then with water, and dried at 550 C. under a pressure of 30 mm.) in 300 ml. of 95% formic acid is refluxed for six hours. After cooling, the mixture is filtered by suction and washed with 100 ml. of cold 90% formic acid. During the reaction period, the reflux condenser is maintained at 400 C. to allow methyl formate (b. p. 31.50 C.) to boil off. The formic acid solution is evaporated almost to dryness at 25 mm. pressure, and the residue is then thoroughly washed with water to remove soluble carbohydrates. After filtering by suction the final lignin is dried under vacuum (25 mm.). 25. THE STRUCTURE OF LIGNIN The structure of lignin has been extensively studied by means of the chemical compounds which are formed when the lignin is destructively decomposed. Attempts to produce small fragments by drastic oxidative procedures have led only to the formation of oxalic, formic, and acetic acids, and carbon dioxide (95). For this reason, it has been necessary to employ rather mild conditions during the process of decomposition. Phillips and Goss (192) have reported the use of ozone for a structural study of lignin. From among the products of their decompositions of methylated alkali lignin obtained from corn cobs, these authors have reported the isolation of anisic acid (I). Similarly, vanillin (II) has been obtained In studies of from ozotized'birch formic acid lignin (70). the action of selenium on alkali lignin from corn cobs, guaiacol (III) and 1 - propyl - 3 - methoxy - 4 - hydroxy- benzene (IV) have been isolated (193). Alkali fusion of spruce lignin has led to the formation of pyrocatechol (V) 26, 014 CHO Anisic Acid Vanillin (II) (I) / ocH o 04C. /;0 Guaiacol 1-propyl-3 -met hoxy-4hydroxybenzene (IV) (III) 00' onI 01 go COOH Pyrocat echol (V) oc Pyrocatechuic acid (VI) (VII) 14 HOOC Veratric acid 0C i I OC14 3 Coo1 (VIII) Gallic acid Isohempinic Acid (IX) C. Ko Syringaldehyde (X) 27. and pyrocatechuic acid (VI) (91, 122). Beech lignin, under the same conditions, decomposed to yield pyrocatechuic (VI). and Treatment of spruce wood with sodium gallic (VII) acids (96). hydroxide at 165-1700C., methylation with dimethylsuiphate, and oxidation by potassium permanganate has led to the formation of veratric (VIII) and isohempinic acids (IX). The oxidation of lignin in the presence of nitrobenzene is used to produce commercial quantities of vanillin. With beech lignin this type of treatment produces syringaldehyde (X) (63, 93). The catalytic reduction of lignin at 250 atmospheres and 25000. has led to the isolation of a large number of similar compounds of which only three are shown (XI, XII, XIII) (1, 121, 124)0. H OH H OH OH H SS H CH 2 H CH2 CH2CH (XII) (XI) 0 H H - CH 4-n-Propyl-1,2-cyclohexa~diol 4-n.-Propy.1--l-cyclohexanol 4 2 OH CH2CH2H OH (0-Hydroxy-n-propyl) 1-cyclohexanol (XIII). - 28. The characteristic building block derived from the hydrogenation and degradation studies is the phenylpropane nucleus upon which many structural interpretations of lignin have been based. Evidence for the aromaticity of lignin, as 90 CH2CH 2CH3 Phenylpropane (XIV) indicated from the derived benzene products, is confirmed by the ultraviolet data previously cited (3, 161) and also by the knowledge that aromatically bound methoxyl groups are present in the original material (90). Acetyl groups, which were originally thought to be a part of the lignin structure (79, 80), are now definitely known to be absent (160). On the basis of further chemical evidence, it has been estimated that each building block unit of lignin, with a molecular weight of 840 (35), contains four hydroxyl and four methoxyl groups. The presence of a carbonyl group in lignin has been demonstrated by acetal formation, and carboxyl groups are known to be absent (115). Attempts to incorporate the various data regarding the composition of lignin into a molecular structure have led to fair agreement among chemists only within the last decade. Earlier efforts in this direction resulted in the formulation of a compound by Schrauth (223) which may today 29. be regarded as nothing more than a curiosity (XV). 0 "1 0$i " ' 0 0 Schrauth's Lignin Structure (XV) In 1917 Klason, the father of modern lignin chemistry, proposed a structure made up of one molecule of coniferyl alcohol (XVI) and three molecules of hydroxyconiferyl alcohol (XVII) (151). Color reagents tests, OH OCH CH HO CHCH 2 OH Coniferyl alcohol (XVI) 0 OCH CH = CHCH20H Hydroxyconiferyl alcohol (XVII) when applied to lignin, usually indicate the presence of coniferyl alcohol and this information undoubtedly played an important role in Klason's reasoning (64). By 1922, Klason was able to propose a structural formula for lignin (XVIII) (152). 30. OCC. cii cHCH8N Klason Lignin (1922,) (XVIII) As more information was obtained with regard to the structural components of lignin, this formula underwent considerable revision. In 1948 Brauns proposed a possible structure composed of five phenylpropane building blocks (38). These units are condensed in the formula to demonstrate the "known" presence in lignin of simple ether linkages (A), furan rings (B), pyran rings (C), and a carbonyl group (D). Under- neath the proposed structure (XIX) are shown various isolated degradation products from lignin and their relationship to the parent molecule. Since 1947 several interesting papers have been published in which lignin has been pictured as a molecule composed of repeating 8-methoxydihydrobenzopyrone units (XX). (215, 219). 31. Lignin Structure Proposed by Brauns (XIX) CO-CH 3 B C ?3 (N C A CM CH 13 H(C 2H 5 ) E COOH COO 003 C==0, Vanillin Cyclohemyl, propane derivatives CHO0 OCH 0H) ON09) 0 OH Aceto guaiacone le -vanilloyl ethyl alcohol Figure OR Acetyl vanilloyl, OR p-hydroiy benzoic acid OCH3 Isoher ipinic acid -- I 32. The late Alfred Russell pictured CA3 I I 0 8-4ethoxydihydrobenzopyrone (XX) gymnosperm lignin as ply-8methoxydihydrobenzopyrone (XXI). Ritter and his associates, working independently, derived the same formula and indicated the reaction between this lignin and sulphite solution (XXII). Although these formulae 0 CR 1 C. C H c. t~ 0 C., 0 0oe6ti 3 Poly-8-methoxydihydrobenzopyrone (XXI) U 0 'Ii ~ 33. Ritter's Sulphite Lignin (XXII) have been subjected to rather harsh criticism by many lignin chemists, they do have a number of attractive features. In agreement with formula (XIX), it is obvious that Russell's lignin has the same basic flavanone structure. Moreover, Russell's lignin is made up from a simple repeating structure, whereas Brauns' lignin formula (XIX) is heterogeneousin its parts. It has long been known by those chemists working on natural products that natural synthetic processes lead to simple rather than to complex polymeric structures. Undoubtedly the work of Russell and Ritter will open the entire question of lignin structure to further and more careful scrutiny, and it seems probable at this time, that a generally acceptable structure may be proposed in the near future. is In any case, the polyflavanone structure simple and easy to discuss and can be used for illus- trative demonstrations. A sample of Russell lignin was synthesized in the course of the studies reported in the following Chapters. in Chapter III. Details of the synthesis are given 34. SUMMARY The historical background of lignin has been reviewed and its association with cellulose in plants discussed. Several laboratory procedures have been cited for the separation and isolation of lignin from wood. A short account has been given of the most reliable -information regarding the structure of lignin. 35. CHAPTER III LIGNIN - EXPERIMENTAL WORK INTRODUCTION To examine the effects produced when lignin is sheared while under pressure (Chapter VI), it was necessary to have available a fairly large supply of a relatively unaltered lignin. After an examination of the literature on preparative procedures, those methods of isolation were considered best which led to light-colored products. For this reason the lignins isolated from wood by the acid hydrolysis of cellulose were eliminated from consideration. Butanol-lignin and similar materials are chemically converted into new compounds and, therefore, could not be used for the proposed studies. The two best lignins for high pressure studies were thought to be periodate lignin(?1 4 , 294) and Braunb' native lignin (35). Braunb' native lignin is extremely difficult to isolate in quantity because of the large volumes of wood and solvents which must be used. For this reason only a small quantity of the native lignin was isolated, whereas the periodate lignin was prepared in an amount sufficient for the pressure studies. A small quantity of Russell's lignin was synthesized (219) for comparison with the naturallyoccurring material (Chapter V). 36. BRAUNS' NATIVE LIGNIN It had been known for some years before Braunst work that treatment of wood with neutral alcohol or hot alcoholbenzene led to the extraction of minute quantities of a ligninlike substance (92, 150). It remained for Brauns, however, to apply this information on a sufficiently large scale to isolate enough lignin to characterize. The procedure for the isolation of native lignin that was used in the present research is given below and is followed by a discussion of differences between this and the procedure recommended by Brauns. Canadian spruce wood (27 board feet) was obtained from a local lumber company. After some experimentation, it was found that small chips could be obtained by running the wood through a joiner. The air-dried chips were treated in three batches with 95% ethyl alcohol (see Table I). Table I Treatment of Spruce Chips with Alcohol to Obtain Native Lignin Batch Bottle or Flask capacity Chips Alcohol Liters Liters No. Grams 1 1200 12 11.25 2 1410 12 11.25 3 1850 22 19. 37. Each batch was set aside for at least a week after which the alcohol was recovered by decantation. The alcohol was removed under vacuum at room temperature to leave a dark brown oil. This oil was washed several times with benzene to remove terpenes and resins, and then was repeatedly washed with ether. After several ether treatments the oil finally solidified into a light yellow flocculent precipitate which was washed further with ether and then centrifuged. The precipitate was then dissolved in anhydrous dioxane and this solution was poured into water. The precipitate from this step was again centrifuged and redissolved in dioxane, the final solution being poured dropwise into anhydrous ether with vigorous stirring. There was a tendency at this point for the lignin to oil out but, with care, the oil could be made to solidify to a light yellow powder. Precipitation of the lignin from the dioxane solution was carried out three more times, and the final product was washed with petroleum ether and dried under vacuum below 500 C. Yield, 3.8 g. (0.08%). In Brauns' procedure for the isolation of native lignin, the spruce wood is first treated with cold water and ether before it is subjected to digestion with alcohol. Since large scale ether treatment involves certain hazards, this step and the water washing were reserved for the end phase of the revised isolation procedure. Benzene was used in the washing of the final lignin, a step which Brauns did not report. The native lignin was not purified as carefully as 38. that reported in the literature, since with only a small quantity desired for thermographic studies (Chapter V), it was felt that extreme purity was not necessary. One of the most unusual properties of native lignin is its ability to dissolve in dioxane, methanol, ethanol, 4% sodium hydroxide solution, and pyridine. In this respect the material differs so greatly from other lignins that questions were originally raised regarding the identity of this lignin and the natural product in the wood. At present there is no dispute, however, and it has generally been agreed that the product of this type of alcohol extraction represents a low molecular weight form or an uncombined form of lignin in wood. The great advantage of native lignin preparations over other isolated lignins is the fact that the product has not been allowed to react with the solvent and has not suffered the degradation which is caused by strong acids. For these reasons, native lignin has been particularly useful for structural studies. On the basis of empirical analyses of various ligning,Brauns concluded that the fundamental building block of lignin has a molecular weight of 840. This infor- mation, and data derived from acetylation, methylation, and other experiments led Brauns to propose the following formula for lignin (XXII): 39. CH3O CH 30 CH O OH 0 H C 4 n CH O OH 6 OH CH30 0 H __CH3*C OH CH3 0 OH CH3O :3 41 3L. OH OH OH :3 Keto form Enol form Brauns' Lignin Formula (XXII) Braunstcame to the conclusion that the enolic form of this formula is already present in wood or that the keto form is slowly enolized during isolation procedures. with a molecular weight of 838.3, The formula, corresponds remarkably well with that derived from empirical analyses (840). PERIODATE LIGNIN In 1947 Ritchie and Purves (214, 294) reported a novel procedure for the isolation of lignin from wood. Their procedure, utilizing sodium paraperiodate as a celluloseoxidant, was reported to lead to a light-colored product (golden brown) in good yield and was, therefore, chosen for the isolation of the lignin necessary for the studies described in Chapter VI of this report. The paraperiodate procedure is based on the well-known fact that periodic acid causes the oxidation of cellulose with its conversion into water-soluble products (142) (XXIII). 40 0 -C0 -C 1 I..co C-C=0 Ia o-C- Periodic acid Soluble t =. * Pro duct Periodic Acid Degradation of Cellulose (XXIII) In the course of studies reported in the literature it was demonstrated that xylans, starch, and other plant polysaccharides are also oxidized to soluble products, of side reactions, at pH 4 and 200C. It with a minimum was, therefore, considered possible to isolate lignin from the carbohydrate components of wood by selectively oxidizing the polysaccharides to compounds soluble in boiling water. Through the kind cooperation of Prof. Purves, an unpublished procedure was obtained for the large scale isolation of periodate lignin. It was this communication which made the following work possible. Approximately 25 pounds of black spruce wood chips were obtained from the Ontario Paper Company through the courtesy of Dr. C. A. Sankey. After passing the wood through a hammer mill slowly to prevent the chips from overheating, the resultant meal was spread out on paper to dry overnight. The air-dried meal was screened with a Tyler Ro-Tap sieve shaker and the (-80 + 100) fraction was collected and treated, in a 41. specially built Soxhlet extractor, with a 2'.1 alcohol-benzene mixture. The terpene- and resin-free meal was air-dried, vacuum-dried at 600C. until constant weight was attained, and bottled. To prepare trisodium paraperiodate (Na3H2I0 6 ), 198. (1 mol) of sodium iodate was dissolved in the smallest necessary volume of water and the solution was vigorously stirred while 200g. (5 mols) of sodium hydroxide was slowly added. Chlorine gas was passed into the solution until the first odor of hypochlorite was observed. flow of chlorine was stopped and 4 0g. At this point the (2 mols) of sodium hydroxide was added followed by vigorous stirring for three hours. The temperature of the solution was maintained at 10000. throughout the preparation. When cool, the solution was filtered through a fritted glass funnel and the crystals were washed with cold water and dried at 11000. crystals was 235g. (80%). The yield of white Analysis of the product by the arsenate procedure recommended by Purves (214) showed the trisodium paraperiodate to be 95-99% pure. For the oxidation of the spruce meal, a trisodium paraperiodate solution was prepared by dissolving 20 4 g. of the crystals in 5.5 liters of water containing 250 ml. of glacial acetic acid. The mixture was adjusted to pH 4.1 with glacial acetic acid and, after being left overnight, was filtered through a fritted glass funnel to yield six liters of a 3.4% paraperiodate solution. 42. Oxidation of the spruce wood was carried out by adding 510g. of the meal (-80 + 100) to the six liters of 3.4% paraperiodate solution. for 24 hours at 2000. The mixture was stirred vigorously after which the supernatant liquid was siphoned off and the remaining suspension was filtered through a Buchner funnel, This procedure was necessary in order to effect the best recovery of unused oxidant. .The oxidized wood meal was thoroughly washed with water until the washings were free of iodate ions. The washing was carried out for 24 hours in a continuous liquid-solid extractor built, with minor modifications, according to the suggestions of Ritchie and Purves. Upon completion of the washing, the wood meal was suspended in 10 liters of distilled water and refluxed at 10000. for three hours to hydrolyze and dissolve oxycelluloses. The meal was next filtered on a Buchner funnel, washed with water, and reoxidized as before in a 3.4% solution of trisodium paraperiodate. By the end of three oxidation-extraction cycles the volume of wood meal had markedly decreased; after five cycles, the wood particles showed almost imperceptible birefringence in polarized light indicating that most of the cellulose had been removed. The final product was air-dried, washed with methanal and benzene, and dried to constant weight under vacuum at 6000. The yield of lignin was 125g. having a composition of 61.82% C., 6.07%H, 0.92% ash, and 10.88% 00H . Literature: 62.1%C., 5.8%H, and 10.7% OCH 3 ' 430 To recover the unused oxidant from the decantate following each oxidation, sufficient sodium hydroxide was dissolved in the solution to cause precipitation of the trisodium paraperiodate. After filtration, 40 g. of sodium hydroxide was added to the filtrate (containing sodium iodate), chlorine was passed into the solution at 1000 C., arid the preparation of paraperiodate was carried out as before. There can be no doubt that the periodate spruce lignin underwent some degradation during the isolation process since the methoxyl content dropped from the normal 14.9 - 15.4% to approximately 10.9%. With this exception, periodate lignin has properties which are comparable to those of lignin in situ in wood. Both native lignin and periodate lignin are isolated only by the use of long, tedious procedures. however, lead to light-colored products, These procedures, indicating that the lie nin undergoes minimum degradation during isolation. Comparative elementary data for the two lignins are shown in Table II. Table 1I Comparative Data for Various Isolated Spruce Lignins (294) Lignin 72% H204 C 65.7-67.4 H 5.4-5.8 OCH -- Brauns native 63.9 6.15 14.9 Peri odat e 62.1 5.8 10.7 44.0 RUSSELL LIGNIN As discussed in Chapter II, Alfred Russell in 1947 proposed that lignin might be a variant of 8-methoxydihydrobenzopyrone Because the analytical and chemical data (XXI). for this material match closely those for other lignins, it was considered of interest to synthesize a small quantity of Russell lignin and to obtain a thermographic curve for the material (Chapter V). The synthesis was carried out as recommended by Russell (219) using 4X quantities. Vanillin (6 0.8g., 4 mols) was dissolved in 160 ml. of dry pyridine and acetyl chloride (4 0g., 5 mols) was added The hot dropwise over a period of approximately 20 minutes. orange-colored mixture was heated on a steam bath for 30 minutes and, after cooling, the solution was poured into a mixture of ice and water. The precipitate was washed with water, recrystallized from alcohol to which charcoal had been added, and dried. (XXIV) The final product of vanillin monoacetate melted at 78-79.5 0 C. on a microscope hot stage (literature, 7?-78 0 C.), and weighed 35.2g. (45.5% theoretical). o+rH f~jocHy CAo Vanillin ooc-c C 1 -I ?few /ji ~I:JoCCIAO Acetyl Chloride Vanillin Monoacetate Preparation of Vanillin Monoacetate (XXIV) 13 +' MCI 45. A Fries rearrangement was carried out on the vanillin monoacetate (XXV) by dissolving 30g. of that material in 127 ml. of dry, redistilled nitrobenzene and slowly adding of aluminum chloride. 4 1.2g. With the addition of the aluminum chloride the solution became very dark and viscous and the temperature rose steadily. When the addition was complete, the mixture was heated at 1000C. for two hours, cooled, acidified with dilute hydrochloric acid, and steam distilled to remove the nitrobenzene. The residue, a black, tarry mass, was filtered and dissolved in alcohol from which it was precipitated by the addition of 1:5 hydrochloric acid. This purification was repeated twice, and the final material was The brittle, black, amorphous dried under vacuum for two days. product was highly electrostatic (yield, 8.8g). ooC--CH 3 ocM3 g oI+ e screo cI4o oH 0C4 o C oil 0 OC043 0 0I Preparation of Russell Lignin by Fries Rearrangement of Vanillin Monoacetate (XXV) Wk A A major objection to this synthesis has been that the condensation of acetyl vanillin with itself is the type of reaction which may lead to a mixture of materials rather than to a single product. At the time of his death, Russell was investigating new synthetic routes by which to eliminate this difficulty, SUMMARY Brauns' native spruce lignin and periodate lignin were isolated for the work described in later chapters of this report. Procedures for the isolation of these two products have been outlined. Details are given for the synthesis of Russell's hypothetical lignin structure. CHAPTER IV LIGNIN - CHEMICAL AND STRUCTURAL RELATIONSHIP TO HUMUS INTRODUCTION As outlined in Chapter I, the origin and nature of humus and related materials is still open to discussion. Several disputed theories have been evolved to account for the conversion of cellulose into humic substances (10, 52, 71, 103, 145, 174, 177), and several possible structures for these products have been proposed. Since the principal constituents of most coals are of humic origin, it was felt necessary to examine carefully the fate of cellulose, lignin and other plant substances in the formation of soil humus or- peat. From this study it was considered possible to relate humus to its parent material and, perhaps, to propose a skeletal structure for humus and its associated substances. 48. MICROBIAL DECOMPOSITION OF PLANT COMPONENTS Sedimentologists examine rocks to establish the mineral components and then attempt to explain how various weathering processes affect the disposition of these particles. By analogous reasoning an attempt was made to determine the history of the organic components of sediment.~ The preliminary exposure of plants is to atmospheric gases. In temperate and sub-tropical areas of average humidity and rainfall, the combination of moisture and oxidizing conditions may lead to destruction of the plant by presenting favorable conditions for microbial growth. Fungi are particu- larly active in the destruction and conversion of organic material under damp, aerobic conditions. These organisms, which are devoid of chlorophyll, cannot synthesize a significant amount of cell matter from atmospheric carbon but must depend upon their enzymatic processes for the production of assimilable food. The presence of fungi, therefore, indicates that a process is taking place in which the host material is being modified with the elimination of energy required for the growth of the organism. As previously noted, cellulose is of plant materials. the major constituent Cellulose is hardly ever found in the free state, but is generally associated with lignin in complexes of unknown character which have been termed "lignocelluloses". 49. Deposition of leaves, bark, needles, and wood exposes the "lignocelluloses" to extensive decomposition by a large variety of fungi that normally inhabit the soil and are capable of destroying cellulose (158, 295). The development of fungi in the upper few centimeters of forest soil is so great that, even under careful microscopic examination, it has been reported difficult to determine whether the residues from plants or the fungi predominate (290). Fungi require nitrogen for growth in the ratio of one part of nitrogen for every thirty to fifty parts of cellulose decomposed. Waksman and Starkey (289) have demonstrated that addition of sodium nitrate and 1% of cellulose to a raw soil will raise the fungus count per gram from 160,000 to 4,800,000. In an area covered by substantial vegetation, this nitrogen demand can undoubtedly be met by the proteins of previous generations of plants, bacteria, and other soil microbes. Relatively little is known concerning the metabolic products which are formed during the destruction of cellulose by funi. It has generally been assumed that the poly- saccharides are hydrolyzed to component sugar units which are destroyed so rapidly by other soil microorganisms as to be undetectable (285). Besides being cellulose-destroyers, several species of fungi are able to attack the lignin of plants to produce 50. "white rot". This "corrosion", as the process is termed, has not been studied in sufficient detail to lead to information regarding the transformation products. It should be emphasized, however, that the lignin-destroyers are of far less abundance and importance than are the cellulose-destroyers. Many species of bacteria are capable of attacking and destroying cellulose under aerobic conditions. A number of these organisms employ cellulose as their exclusive source of energy and are unable to multiply on simple sugars such as glucose. Aerobic bacteria decompose cellulose to yield a "mucilaginous mass of bacterial cells imbedded in gum-like compounds". also formed. can exist in Small amounts of organic acids and pigments are It is particularly important to note that fungi soil media which are acid to a pH of 4.0 or less, while bacteria will multiply only if the medium has a pH between 6.0 and 8.5 (291). During the process of plant growth, needles, leaves, twigs, bark, and pieces of wood are shed and buried beneath successive layers of organic and inorganic sediments. Should this burial become sufficiently deep or complete so that atmospheric oxygen is excluded from the organic material, then anaerobic bacteria must be considered as agents of decomposition. Fungi can no longer attack the organic debris. Anaerobic bacteria decompose sulphates or nitrates and remove the oxygen for their metabolic processes. Many 51. species of anaerobes are found in places where fresh organic matter is abundant, that is, rivers, and marsh soils. in peat bogs, manure piles, Of particular importance are the anaerobic thermophilic bacteria which grow best at temperatures to be present between 550 and 650 0. These organisms, which have been reported to be present in the uppermost strata of a peat bog (262), are known to decompose cellulose very rapidly with the production of organic acids, ethyl alcohol, carbon dioxide, methane, and hydrogen. In this respect it has been reported that 3.3 g. of cellulose can be decomposed to produce 2.2 g. of fatty acids, 1.0 g. of carbon dioxide, and 0.014 g. of hydrogen. In a different experiment 42 g, of cellulose yielded 21.6 g of acetic acid, 10.3 g. of ethyl alcohol, and 11.9 g. of carbon dioxide, in addition to considerable hydrogen (292). CONCLUSIONS In summing up this discussion several points stand out clearly. Most important, it appears that cellulose can be destroyed rapidly by soil organisms under a fairly wide range of hydrogen ion concentrations and temperatures (34, 55). Secondly, with the exception of a few relatively unimportant fungi, no organisms are known which can directly destroy lignin (158, 295). It is well known that a considerable portion of the humus complex is acidic and can be extracted with alkalies, A chemical degradation of the extractable humic acids has been reported to lead to the isolation of fragments identical with or similar to those obtained by the decomposition of lignin. On the basis of biochemical and chemical evidence, therefore, it appears probable that lignin, during its accumulation in the soil or in a peat bog, undergoes chemical transformations which lead to the series of products known collectively as humus. The interpretation of the lignin-humus relationship has been attempted using, as an example, for Russell lignin (XXV). the simple formula The major difference between this Cc.14 3 M3 Russell Lignin (XXV) compound and a substancesuch as cellulose (XXVI) is that cellulose can be hydrolytically split to simple structural units (glucose molecules) while the former cannot. In the case of lignin, however, it may be possible, at the proper pH, 53. -C C C ct H0-c- w-C- -c-oNo HO C -0o I I. I II4- M 0'16 Vt C H4,0o Ik ~ CH1 OH Hydrolysis of Cellulose to Glucose (XXVI) to open the heterocyclic ring and thus to produce a compound having a structure similar to that of (XXVII) . This new OC0 3 ~~CN .. C ~CH 0 oH 0 G-C4UcSR r~t:K%~y0H C-'cH:~H OR II 0 *W Cc- " CH= CJ40 0)4 Possible Degradation of Russell Lignin (XXVII) 54. structure, it will be noted, has phenyl rings, acidic phenolic hydroxyl groups, double bonds, and a structure based on phenylpropane. These chemical features have all been reported in studies of the structure of lignin. The demethylation of organic residues under natural conditions has also been reported, and peat is known to have a methoxyl content of only about 1%. This conversion has been attributed in the literature to the biochemical removal of methyl groups from lignin, a process which probably leads to phenolic hydroxyl groups (XXVII). It is obvious that the lignin molecule, even if it does not possess a structure as simple as that proposed by Russell, has groupings which are rather easily changed without complete molecular destruction. Only one transformation in a single building block of the lignln molecule need occur to produce acidic properties. Multiple conversions along the molecular chain, coupled, perhaps, with occasional chain cleavage through the formation of carboxyl groups, which are known to exist in humic materials (103, 273), may lead to products called humic acid, hymatomelanic acid, ulmic acid, apocenic acid, melanin, fulvic acid, etc. Waxes and resins account for only a small percentage of material of vegetable origin and are highly resistant to decomposition. These materials are concentrated into particular types of deposits or finely distributed throughout a sediment such as peat. The resinous components of plants 55. apparently play no important part in the formation of humus. To examine experimentally the structural relationship between lignin and humic acid, specimens of these two substances were submitted for infrared absorption analysis performed with a Baird Spectrophotometer owned by the Massachusetts Institute of Technology Department of Chemistry. The spectra are shown in Fig. 1 and are discussed below. Following the recommendations of Puri (207), the humic acid for this work was isolated from peat collected in Wisconsin by Reinhardt Thiessen in 1937. The peat (405 g.) was crushed in a porcelain mortar and then refluxed for two hours with three liters of 0.1 N hydrochloric acid. After standing overnight, the mixture was filtered to yield a darkbrown (almost opaque) filtrate which was discarded. The precipitate was thoroughly washed with water and then divided into two equal portions. Each portion of the material was placed in a 5-liter flask along with 4 liters of a solution 0.2 N in sodium carbonate (10.6 g./liter) and 0.2 N in sodium hydroxide (8.0 g./liter). The suspensions were heated to 700 C., stirred very vigorously for two hours, and then allowed to cool overnight. The cool solutions, smelling faintly of ammonia, were filtered and then adjusted to a pH of 1.0 by the slow addition, with vigorous stirring, of 175 ml. of concentrated hydrochloric acid. The humic acid precipitate was centrifuged free of supernatant liquid, mixed with fresh water, and 56. recentrifuged. As the pH of the suspension rose, centrifugation became progressively difficult making it necessary to complete the washing procedure on a Buchner funnel. The washed material was a dark brown gel which was dried in a vacuum desiccator over calcium chloride for 48 hours. During the drying process the gel contracted enormously in volume. The final humic acid was pitch black when massive, showed concoidal fracture, and was dark brown when powdered. A small quantity of the material was finely ground and redried under vacuum at 550C. Analysis: C, 52.34%; H, 4.93%; N, 2.09%; Ash, 9.80%. On an ash-free basis the analysis becomes: C, 58.02%; H, 5.46%; and N, 2.32%. A small quantity of the dry humic acid was pulverized as well as possible in a mortar and the powder was then suspended in Nujol for infrared absorption analysis. The center and top curves of Fig. 1 refer to mulls having high ("20 mg./80 mg. Nujol) and low (~20 mg./150 mg. Nujol) concentrations of powder in the oil. The bottom curve is mull of native spruce lignin in Nujol. for a The peaks which appear on all three curves at 3.4, 6.8, 7.25, and 13.8 microns arise from the absorption of the infrared radiation by the Nujol. Other peaks, some of which are very weak and subject to reconsideration with further work, are listed in Table III. By analyzing the humic acid and lignin curves according to published data on infrared spectra-structure Figure 1 Infrared Absorption Curves for Humic Acid and Lignin Top curve: Low concentration mull of humic acid (-20 mg. sample/150 mg. Nujol). Center curve: High concentration mull of humic acid (~20 mg. sample/80 mg. Nujol) . Bottom curve: Nujol mull of Brauns' native spruce lignin. Wave Numbers in cm soo 4000 3000 2 00 2000 40 50 1500 1400 1300 1200 1000 1100 900 B00 NO.g DATE INDEX SAMPLE H, Fro-m'8 me r Semp.CON: MmT ems Comp. Coil: mm ems mg Vol. F.S. mg cc c.c Sd Gas mm BAIRD ASSOCIATES 20 30 80 70 60 NA CL Pism I1:0 91 130 120 Wave Length in Microns Wave Numbers in cm 5000 BAIRD ASSOCIATES NAIR ASSOCIES NA CL 4000 3000 30 20 200 2000 40 50 1500 60 1300 1400 70 1200 1000 90 80 PossM 11001 00 800 900 11 0 120 Wave Numbers in cm 5000 to00__ 4000 3000 200 2000 1500 1400 1300 _ _- 1200 ' 1000 1100 -- 8oo 900 . 9 a 770 a I 120O SPECTROPNOTMETER L NA cL P...M 130 Wave Length in Microns Wave Length in Microns 58. Table III Correlation of Infrared Absorption Peaks for Humic Acid and Brauns Native Lignin Humic Acid (Low conc.)microns Humic Acid (High conc.) microns Brauns Lignin microns 3.0 3.0 3.8 4.3 4.3 *5-9 5.8 4.9 5.3 *5 .9 6.1 *6.2 *6.2 *6.25 *6.6 *7.9 8.1 8.2 8.8 8.8 9.1 9.2 9.7 9.7 9.7 10.3 10.8 11.7 *Large peaks 59. correlations (2, 8), it has been possible to obtain infor- mation regarding the structural components of these two substances. The absorption maxima at 3.0, 7.9, 8.1, and microns appear to be caused, in part at least, by the presence of phenolic hydroxyl groups. The 5.9-micron peak indicates a carbonyl stretching frequency. Maxima at 8.1 or 8.2 microns may be caused by aromatic ketone groups, and those at 8.8 microns can be correlated with aliphatic ketone groups. The absorption peaks at 6.2 microns may indicate an amide linkage in the humic acid. This point in particular requires further clarification for it may be extremely important in understanding the manner by which nitrogen is combined in coal. Other peaks in the curves of Fig. 1, especially those at 9.7 microns, may indicate the presence of multiple substitutions on benzene rings. The exact type of substitution cannot be established with certainty from these spectra. A procedure which is occasionally used in attempting the identification of complex organic compounds from their infrared spectra, is to superimpose the curve of an unknown substance upon that of a known material. When this is done with the humic acid and lignin curves of Fig. 1, a clear correlation of peaks is evident. With further experimentation on methods of preparation of humic acid for analysis, it may be possible to show more conclusively that the skeletal structures of humic acid and lignin are related or even identical. 6o. SUMMARY A critical analysis of the literature has shown that cellulose is probably removed from sediments by biochemical processes soon after deposition of plant fragments. Lignin, on the other hand, is apparently not destroyed completely, but is instead transformed into humus by microbial action. Using the structure for Russell lignin as a model, proposals have been advanced for the chemical changes which may occur in the lignin molecule to convert it to a series of related compounds known as humic acid, fulvic acid, melanin, etc. Infrared absorption spectra have been obtained for humic acid and for native spruce lignin. Structural simularities between these two substances are indicated by their spectra. It is proposed that further infrared studies may completely clarify the lignin-humus relationship, 61. CHAPTER V LIGNIN - BASIC STRUCTURES IN THE COAL SERIES INTRODUCTION The formation of coal is generally thought to take place in two distinct stages. In the first or biochemical phase, the plant components are degraded and concentrated to produce the humic substances of peat and lignite. In the dynamochemical or metamorphic stage, the components of the low grade coals are devolatilized to produce fuels having higher percentages of fixed carbon (166). Following the second phase of coalification, the humic components have lost their identity and can only be recovered by oxidative degradation of the coal. For this reason it has been difficult to relate the structure of coal to that of lignin by chemical processes. Because of its physical and chemical properties, coal can be compared to a three-dimensional polymer which probably formed by the condensation of humic molecules. If this is true, then the basic structure of coal would be expected to be similar to that of humic substances and probably similar to that of lignin. To examine this relationship, differential thermal analysis has been used as a basic technique for the determination and comparison of the decomposition characteristics of lignin, cellulose, and coals. 62. VACUUM DIFFERENTIAL THERMAL ANALYSIS Differential thermal analysis is carried out by heating a material under investigation at a constant rate to about 100000. and recording the temperatures at which exothermic and endothermic reactions occur. In practice this is most readily accomplished by employing a metallic block with two holes, one of which is packed with the unknown substance, while the other is packed with an inert powder such as gamma alumina. A two-headed thermocouple, one head of which is imbedded in each powder, is then used to determine the direction and extent of the differential changes as the block is heated at a constant rate in a furnace. During recent years, differential thermal analysis has become a standard analytical procedure for the study of complex mixtures of minerals when resolution is difficult by conventional microscopic techniques or X-ray methods. It has been especially useful in the analysis of clays and other colloidal materials. The use of differential thermal analyses for the study of complex organic substances and shales has been reported (231, 271, 305). Conventional equipment, however, leads to the combustion of the specimen and makes it difficult to obtain reproduceable results with certain materials (271). For this reason a new instrument for differential thermal 63. analysis has been designed to operate under vacuum or inert atmosphere. Previous differential thermal analytical studies have been carried out with magnesia-packed furnaces, but the high thermal capacity of the packing made it difficult to attain heating rates exceeding 100 or 120 C. per minute. For this reason, and because of the impracticability of attempting to evacuate a packed furnace, a vertical heating unit was designed with radiation shielding rather than with conventional packing. The furnace (Figs. 2, 3, and 4) is constructed with a specially-cast alundum core 9" long, 20 1. d., and having an 1/8" wall. Sufficient 16 gauge Chromel-A wire is wound onto the core to make the power input approximately 2 kilowatts at 110 Volts A.C. The core is shielded by four cylinders constructed of 0.01811 sheet nickel. On top and bottom shielding is by four discs made of the same material. Elec- trical connections within the furnace are made with stainless steel lugs and 1 mm. platinum wire. For electrical insulation ceramic fish-spine beads are strung over the leads. All steel parts of the furnace were machined from material obtained from the Rustless Iron and Steel Corporation, Baltimore, Maryland. Steel type 446, containing 23.00 to 30.00% chromium and a maximum of 1% nickel, and type 309, containing 22.00 to 26.00% chromium and 12.00 to 14.00% nickel, 64. Figure 2 Furnace for Vacuum Differential Thermal Analysis STAND-OFF INSULATOR - -PLATE C - ALUNDUM INSULATOR - PLATE D NICKEL SHIELDS 0.018" T - D. 3" - D. 3.5" D. 5.75 - -D. 6.5" - SUPPORT RODS I/4" O.D. SIX, EQUALLY SPACED ALUNDUM CORE 2" I.D. 1/8" WALL -PLATE B ALUNDUM SPACERS NICKEL BAFFLES 0.018"T BEARING -PLATE A BEARINGS FOR SUPPORT OF FURNACE THREE, EQUALLY SPACED 65. Figure 3 Details of Furnace Design D. 1/2" THREE HOLES *D.1/4" SIX HOLES D. PLAT E A 1/4'" -D. 1/4" I THREE HOLES I4 1I/4" A 8 I/4" 1 I 1/4" PLATE B THREE HOLES 4111 I 5 1/2"1 -- l" 21/A" V"8- 66. Figure 4 Details of Furnace Design 1/4" PLATE C 1/4 SIX HOLES THREE HOLES PLATE D THREE HOLES 67. Figure 5 Sample Block for Vacuum Differential Thermal Analysis 31 DRILL 41 ~ 1I 1/16- SAMPLE BLOCK 1/16 68. are used interchangeably depending upon availability. These steels were chosen because of their resistance to scaling and to sulphur at high temperatures. The furnace is mounted on three posts, 18" long, which are screwed into an 18" x 18" x 1/2" brass plate. A groove 3/16" deep and of width to accomodate an inverted 12" x 24" Pyrex jar is machined into the plate. To prevent failure of the glass jar, its flat bottom is rounded and then the entire jar is carefully annealed. By setting the plate on 2" adjustable legs and screwing and silver soldering a brass line into a hole through the plate, a means is afforded for complete evacuation of jar and furnace or for replacement of the air by an inert atmosphere such as helium. Electrical leads to the furnace and all thermocouple leads are insulated from the base p'ate by means of Kovar-to-glass seals obtained from the Stupakoff Ceramics and Manufacturing Company, Latrobe, Penn. The block (Fig. 5) in which analysis is carried out is machined from type 446 or type 309 steel, has a diameter of 1 and has a length.of 3/40. Its lower end has a groove 1/32" deep to enable the block to set firmly in place on a ceramic 1" o.d. x 12" furnace core which serves as a support post. This post is firmly fixed onto the base plate by means of a tightly fitting brass sleeve. The furnace bearings are of such length that when they rest on the brass plate, the heating block is exactly centered in the furnace. 69. The heating rate of the furnace is controlled from a Chromel-Alumel thermocouple junction placed between the center two windings of the spiral heating element. The output of the thermocouple is fed into a Leeds and Northrop Micromax controller equipped with an auxiliary device which enables variation of the heating rate of the furnace from 10 to 500 C. per minute. A heating rate of 120 C. per minute was chosen for all comparative work. Additional control devices make possible a constant cooling rate and "soak" of a specimen at a predetermined temperature. Block temperature is measured by means of a Chromel-Alumel junction placed in the center hole of the block. The output of the thermocouple is recorded on a Brown recorder. The differential thermocouple is made from two leads of a 28 gauge Chromel wire connected by a short length of 28 gauge Alumel wire. The fine wire is used in order to eliminate conduction of heat away from the specimen and the short length of connecting Alumel is used for the same reason. The output of the differential thermocouple is fed through a resistance box into a galvanometer having a short time constant. The position of a light beam reflected from the mirror of the galvanometer suspension is recorded on a Beckman Photocell Recorder (National Technical Laboratories, Pasadena, California). For automatic operation of the installation, the control unit, recorders, and galvanometer light are wired 70. through a Type T-27 General Electric time switch which may be adjusted to start the run and then to turn off the installation after a pre-set time interval. Samples of 100 mgs. of material ground to pass a 200 gauge screen are used for the analysis. For maximum effectiveness the samples are packed immediately around the thermocouple junction. Using specially-designed tools, it is possible to pack the material consistently into the sample hole at 530 pounds per square inch, while at the same time assuring horizontal and vertical centering of the thermocouple junction. The sensitivity of the differential recording installation is approximately ± 0.15 millivolt. During operation with organic materials, the bell jar is evacuated to a pressure of less than 1 mm. of Hg and pump operation is continued throughout the experiment. Figs. 6*and ?*are photographs showing the general arrangement of the installation. To distinguish the vacuum instrument from conventional installations, the former has been named a thermograph and its differential records are called thermograms. DISCUSSION OF THERMOGRAMS Figures 8 to 20 show the thermograms obtained from a number of substances ranging from wood and lignin to coals and shales. The small peak at 5750 0, in each curve 71. Figure 6 Photographs of Thermographic Equipment Upper photograph: Furnace in raised position for loading of specimen. Lower photograph: Close-up of interior of furnace. *Note: The apparatus illustrated and described was constructed under joint sponsorship of the Nova Scotia Research Foundation and the American Petroleum Institute, Research Project 43C, to whom acknowledgement is made for its use in this research. Furnace in Raised Position for Loading of Specimen Close-Up of Interior of Furnace 72. Figure 7 Photographs of Thermographic Equipment Upper photograph: Apparatus for thermographic (vacuum differential thermal) analysis showing furnace, controller, recorders, galvanometer, time-switch, vacuum gauge, and pump. Lower photograph: Close-up of furnace showing vacuum leads through base plate. Apparatus for Thermographic Analysis showing Furnace, Controller, Recorders, Galvanometer, Time-Switch, Vacuum Gige and Pump. Close-Up of Furnace Showing Vacuum Leads Through Base Plate 73. arises from the quartz which is added to the inert material in the sample block to serve as an internal standard. Not only does the low-to-high endothermic quartz transformation at 575 0.- serve as a temperature standard, but the peak also points in an exothermic direction because the material is in the thermocouple recess opposed to that which contains the material under study. The temperature coordinates of all the curves of Figs. 8 to 20 vary slightly from linearity over their heating ranges. For this reason an average scale for the absdissahas been adopted. The discrepancy between actual and scale temperatures above 6000 C. in no case exceeds 150 C. In Fig. 8 thermograms are shown for A, finelyground spruce wood extracted with a mixture of benzene and alcohol; B, highly purified spruce cellulose obtained from the Brown Company of New Hampshire; and C, Brauns' native spruce lignin. The spruce lignin undergoes a very large exothermic decomposition at about 4400 C., while the cellulose decomposes endothermally at about 4000 C. The thermal differences arising from lignin and cellulose are unmistakably reflected in the thermogram for the spruce wood. The low temperature peaks in the spruce wood curve at about 1500 and 2800 C. may be caused by the decomposition of compounds such as hemicelluloses or pentosans. 740 Lebeau (165) in a series of careful studies on the gases evolved on pyrolysis of various substances, showed that the maximum formation of gas from cellulose takes place at 4000. At this temperature, carbon dioxide and carbon monoxide are The formation of hydrogen from cellulose is produced. negligible at 4000 C., but becomes large at 7000 C. Dehy- drogenation of many of the substances shown in Figs. 8 to 20 may cause the broad exothermic reaction which occurs between . 6800 and 7100 C. From the sharpness of the endothermic peak shown by cellulose at 4000 C., it is probable that this material, if present in any appreeiable quantity in organic sediments, is detectable by the thermographic technique. The curves of Fig. 9 are for various lignins as follows: D Brauns' native spruce lignin (same as curve _); E, periodate lignin; _F, Russell's synthetic lignin; and G, lignin obtained from the Forest Wisconsin. Products Laboratory, Madison, This material was prepared by the treatment of wood with 72% sulphuric acid. The most important feature of the curves of Fig. 9 is that they all have large 4400 C. exothermic peaks between 4000 and The curve for the Russell lignin is very similar to the other thermograms indicating a relationship of fundamental structures between the synthetic and natural material. The periodate lignin curve has a small shoulder at about 3800 C. which probably indicates the presence of a small amount of 75. residual cellulose in the material. obtained with 50 mg. These records were all specimens. From Lebeau's data (165) it appears likely that the large peak at 4000 - 4500 C. shown by all the lignins of Fig. 9 is caused by an exothermic decomposition of the material during which carbon dioxide and carbon monoxide are released. As before, the peaks at about 7000 C. may be caused by decompositions during which hydrogen is evolved. In Fig. 10 the thermograms for five peat specimens are shown. The maximum exothermic decompositions appear at about 3000 C. on these curves. Shoulders close to 4000 0. on curves I and K and on curve N of Fig. 11 may indicate the presence of residual cellulose in these peats. Below 5000 C., each specimen appears to have a decomposition curve which varies from those of the other samples. Endothermic decom- positions of unknown origin appear in curve H at 675* C. and in curve I at 7?50 C. Curves M and P are for specimens of Wisconsin peat taken from the same swamp. Minor differences between the curves may reflect variations in plant components and sedimentation, but in general the plots below 5000 C. are characteristic for this particular material. Curve 0 was obtained from a small quantity of lustrous black material which was picked by hand from the Wisconsin peat. This material, which still retained its original woody structure, led to a thermogram having the characteristics of neither fusain nor vitrain (see Fig. 16). From a consideration of 76. Figs. 10 and 11 and those which follow, it appears that peats are mixtures of plant components in intermediary stages of decomposition. The variety of partially decomposed substances results in a number of minor peaks below 4000 C. The thermograms of lignites, shown in Fig. 12, all have exothermic maxima between 4000 and 4500 0. correspond extremely well to those for lignin. These peaks In the case of Gurve T, a shoulder at 3500 0. may indicate a small amount of residual cellulose in the sample. The similarity between lignin A and lignite curves below 6000 C. cannot be overlooked. Lignites do not show broad exothermic peaks at about 7000 C. as do the lignins. On the other hand, two of the lignites do have strong exothermic peaks at about 9000 C. as in the case of the peats shown in Fig. 11. The reappearance of strong exothermic peaks at about 4000 C. in the thermograms for the lignites may indicate that plant products that probably mask this portion of the curve in peat, have been completely degraded and removed. The thermograms of Figs. 13 and 14 are for a number of bituminous coals ranging from those with high volatile content, Ourve .U., to those with low volatile content, Curve BB. In all specimens, with the exception of BB, strong exothermic peaks appear between 4000 and 4500 C. In Curve B, the related exothermic peak appears to have been displaced to about 5250 C. The exothermic peaks of Curves W, X, and AA at about 900* 0. cannot be explained at this time. 77. Thermograms for anthracite coals (Curves CC and DD of Fig. 15) show no clear decomposition peaks below 6000 C. On the other hand, semi-anthracite coal, Curve EE, still exhibits a small exothermic peak at about 5000 C. From an examination of Figs. 8 through 15 it appears that the low temperature characteristics of the lignin thermogram (below 6000 C.) disappear or are masked in peat and then reappear strongly and unmistakably in lignite. With increasing rank of coal, the important exothermic peak at about 4000 C. becomes smaller and is displaced to higher temperatures. By the time anthracite coal is formed, the devolatilization process is complete in the coal and there is no significant peak in the thermogram below 6000 C. There is some evidence that small cellulose residues may be present in peat and lignite. In Fig. 16 thermograms are shown for vitrain, FF; durain, GG; clarain, HH; and fusain, II. Of these particular curves, which were obtained by Mr. Charles Welby using Nova Scotian coals, those for vitrain, clarain, and durain show exothermic peaks at about 4750 C. If the thermograms are representative, then the coals of the area would appear to be of a rather high grade bituminous variety. Since fusain is mineral charcoal, the Curve II would not be expected to show the same characteristics as do the other curves. Low temperature endothermic peaks on the fusain thermogram may indicate the removal of adsorbed gases from the material. A broad but exothermic peak of low intensity at about 5000 C. 78. may indicate incomplete devolatilization or carbonization of the fusain. The two top curves of Fig. 17 are for a gas coal, JJ, and a bright block coal, BK. oth specimens exhibit thermal characteristics similar to those of bituminous coals at 4000 to 4500 C. Curve LL was obtained with a cannel coal from Indiana and has decomposition characteristics which differ from those of the gas and bright block coals. It is difficult to establish whether the curve LL is truly exothermic at about 4250 If this were the case, then the thermogram might point to the origin of the coal from mixed humic and, perhaps, algal materials. Petrographic study of the coal could very well clarify this point. Curves MM and NN of Fig. 18 were obtained with metamorphosed and weathered coals. It is apparent that the coal material has undergone considerable change- in the process of decomposition (see Curves KK and LL, Fig. 17). The sharp endothermic peak at 7250 C. in Curve MM may be caused by the presence of a mineral (carbonate?) component of the coal. Thermogram 00 was obtained with an unclassified coal from Oklahoma. From the position of the exothermic peak at 4250 C., the coal would appear to be a bituminous coal with a fairly high volatile content. In Fig. 19 the top Curve, PP, is for a pyritiferous coal from Indiana (100 mg.) and the bottom curve is for a small specimen of pyrite (25 mg.). The pyrite in the coal 79. can easily be identified by the large endothermic peak at about 6500 C. In Fig. 20, Curve RR is the thermal decomposition pattern for a marine shale, and Curve record for the Green River shale. SS is the Both materials have small exothermic peaks between 4000 and 4500 C. indicating the presence of organic components which probably formed from lignin. The sharp endothermic peak at about 5250 C. in the marine shale.thermogram is distinctive and has appeared in other shales. The Green.River shale is distinguished by its enormous endothermic decomposition at 8000 C. SUMMARY AND CONCLUSIONS Vacuum differential thermal analytical apparatus has been designed and constructed for the study of the structural relationships existing between cellulose, coals, and other substances. lignins, Thermographic analyses obtained by this technique indicate a sharp exothermic peak for lignin at about 4250 C. and a sharp endothermic peak for cellulose at 400* C. peat, where it is The lignin peak can be traced through thought to be masked by a large variety of partially degraded plant components, into lignite where it shows up strongly again. The humic acid component of peat shows a very strong exothermic decomposition at 4350 C. The thermogram for this material has not been included in this 80. report because the large evolution of heat from humic acid causes a severe distortion of the heating rate of the sample. Low percentages of cellulose may be present in some peats and lignites, but not in any coals of higher rank. The lignin curve is visible in bituminous coals where the 4250 C. peak is somewhat smaller and is displaced to a slightly higher temperature. These two effects probably result from the devolatilization of the humic materials and from their polymerization into a more stable structure which decomposes at a higher temperature. Although a semi-anthracite coal shows a small exothermic decomposition at 5250 0., two anthracite coals which were examined had no significant decomposition peaks below 6000 C. The curves for the anthracite coals are interpreted to indicate that devolatilization is nearly complete at this stage of ~Ghe coalification process. The difference in constitution of a cannel coal from other coals is clearly demonstrated as are the changes in coal structure upon weathering or metamorphosis. 81. Figure 8 Thermograms for Wood, Cellulose, and Lignin Curve A: Spruce wood, 50 mg. alcohol and benzene. Curve B: Pretreated with Record No. 248. Spruce cellulose, 50 mg. Highly purified material obtained from the Brown Co., New Hampshire. Curve C: Record No. 240. Brauns' native spruce lignin, 50 mg. Record No. 229. ------------- I O LUJ x LUI 0 OLUI 0 I 1 200 I1 1I I 400 600 TEMPERATURE, C. 1 800 82. Figure 9 Thermographic Comparison of Various Lignins Curve D: Brauns' native spruce lignin, 50 mg. Record No. 229. Curve E: Spruce periodate lignin, 50 mg. Record No. 226. Curve F: Russell's synthetic lignin, 50 mg. Record No. 230 Curve G: Sulphuric acid lignin obtained from the Forest Products Laboratory, Madison, Wisconsin, 50 mg. Record No. 233. (U) 0 x D E 2 F Zw G 0 0 200 400 600 TEMPERATURE, - -- -- - Ii1I1III11 iii1111 IIII11111111 III1111 1, ,, . 'C. 800 83. Figure 10 Thermograms of Peats Curve H: Peat from Gresshartmanndorf, 100 mg. M.I.T. Sedimentology Collection No. 814 - 100; Curve I: Record No. LT - 76. Peat (recent) from South Boston, 100 mg. M.I.T. Sedimentology Collection No. 814 - 34; Record No. 258. Curve J: Bog peat (recent) from New Jersey, 50 mg. M.I.T. Sedimentology Collection No. 814 - 34; Record No. 254. Curve K: Peat from Boston, 50 mg. M.I.T. Sedimentology Collection No. 314 - 26; Curve L: Record No. 253. Peat fuel from Sweden, 50 mg. Collection No. S14 - 32: Sedimentology Record No. 265. LLJ 0 x L16 K o L 0 200 400 TEMPERATURE, 600 'C. 800 84. Figure 11 Thermograms of Peats Curve X: Peat from Hawk Island Swamp, Manitowoc County, Wisconsin, 50 mg. M.I.T. Sedimentology Collection No. 314-22; Curve N: Record No. 264. Sphagnum moss peat obtained from the U. S. Bureau of Mines, 50 mg. Curve 0: Record No. 235. Hand-picked lustrous black component of Peat from Hawk Island Swamp, Manitowoc County, Wisconsin, 50 mg. This sample of peat is described in Chapter IV. Record No. 249. Curve P: Peat from Hawk Island Swamp, Manitowoc County, Wisconsin, 50 mg. This specimen was collected by Reinhardt Thiessen in 1937 and was obtained from the U. S. of Mines. Record No. 237. Bureau UA LU~ 0 x M U z Lii 0 200 400 600 TEMPERATURES ,C. 800 85. Figure 12 Thermograms of Lignites Curve Q: Lignite from Yukon, 100 mg. M.I.T. Sedimentology Record No. LT - 84. Collection No. 814 - 96; Curve R: Lignite from Noonan Bed, Divide County, North Dakota, 100 mg. Specimen obtained from U. S. Bureau of Mines. 15250; Curve S: Record No. Bur. of Mines. No. D - LT - 129. Lignite (Eocene) from Flathead River Area, British Columbia, 100 mg. M.I.T. tology Collection No. 814-31; Curve T: Sedimen- Record No. LT - 85. Lignite (Miocene) from Queen Charlotte area, British Columbia, 100 mg. Collection No. 814 - 28; M.I.T. Sedimentology Record No. LT-86. cf. w x S uR w z 0 I 200 | 400 600 TEMPERATURE) I 800 C. | 1000 86. Figure 13 Thermograms of Bituminous Coals Curve U: High volatile C bituminous coal from Sweetwater County, Wyoming, 50 mg. U. S. Bureau of Mines No. D-15782; Record No. Curve V: Subbituminous A coal from Moffat County, Colorado, No. Curve W: LT-131. 100 mg. D - 11551; U. S. Bureau of Mines Record No. Subbituminous coal, 100 mg. LT - 132. M.I.T. Sedimen- tology Collection No. S14 - 50; Record No. LT - 107. Curve X: Subbituminous C coal from El Paso County, Colorado, 100 mg. U. S. Bureau of Mines No. C-89738; Record No. LT - 149. UU c. 0 w xW m z 0 200 400 600 TEMPERATURE, 800 'C. 1000 87. Figure 14 Thermograms of Bituminous Coals Curve Y: Low bituminous coal from Stanton, Mo., 100 mg. No. Curve Z: M.I.T. Sedimentology Collection S14 - 33; Record No. LT - 103. Bituminous coal from the mines of the Colorado Fuel and Iron Company, Colorado, 100 Mg. No. Curve AA: M.I.T. Sedimentology Collection 314 - 107; Record No. LT-38. Bituminous coal from the mines of the Colorado Fuel and Iron Company, Colorado, 100 Mg. No. Curve BB: M.I.T. Sedimentology Collection S14 - 129; Record No. LT - 82. Low volatile bituminous coal from Somerset County, Pennsylvania, 100 mg. Bureau of Mines No. No. LT - 144. D-15855; U. S. Record x xwi Lii BB I I -III 400 20uu 600 TEMPERATURE, ii I I I .. Ial - I 11 1 ---- - -- I 800 I 1000 *C. -- ,, - -- - - - - - 88. Figure 15 Thermograms of Anthracite Coals Curve CC: Anthracite from Scranton, Penna., 100 mg. Record No. LT - 39. Curve DD: Anthracite from Wales, 100 mg. M.I.T. Sedimentology Collection No. S14 - 131; Record No. LT - 81. Curve EE: Semianthracite from Kayak District, Alaska, 100 mg. Collection No. No. LT - 83. M.I.T. Sedimentology Sl4 - 69; Record x acc TEPRTUEwc di' 0 EE 200 400 600 800 1C0 89. Figure 16 Thermograms of Vitrain, Durain, Clarain, and Fusain Curve FF: Vitrain isolated from coal from Sydney Coal block area, Nova Scotia, 100 mg. IV-6, B 19; Curve GG: Record No. 238. Durain isolated from coal from Sydney area, Nova Scotia, 50 mg. IV-4-36; Curve HH: Record No. 280. Clarain isolated from coal from Sydney area, Nova Scotia, 50 mg. IV-5-6; Curve II: Coal block Coal block Record No. 267. Fusain isolated from coal from Sydney area, Nova Scotia, 100 mg. Record No. 203. 0 x III GG HH II 200 400 600 TEMPERATURE, 800 'C. 1000 90. Figure 17 Thermograms of Unclassified Coals Curve JJ: Gas coal from Pennsylvania, 100 mg. M.I.T. Sedimentology Collection No. S14-47; Curve KK: Record No. LT - 93. Bright block coal from Greene County, Indiana, 100 mg. M.I.T. Sedimentology Collection No. Sl4 - 88; Curve LL: Record No. LT - 79. Cannel coal from Greene County, Indiana, 100 mg. No. M.I.T. Sedimentology Collection 14 - 90; Record No. LT - 80. K O z L y (- 200 400 600 TEMPERATURE) 800 C 10-0 91. Figure 18 Thermograms of Unclassified Coals Curve MM: Metamorphosed fossiliferous coal (Carboniferous) from Portsmouth, Rhode Island, 100 mg. M.I.T. Sedi- mentology Collection No. S14 - 64; Record No. LT - 92. Curve NN: Weathered coal from Greene County, Indiana, 100 mg. M.I.T. Sedimentology Collection No. S14 - 79; Record No. LT - 99. Curve 00: Coal (Pennsylvanian) from Henryetta, Oklahoma, 100 mg. M.I.T. Sedimentology Collection No. S14 - 40; LT - 100. Record No. O 0 x Li NN 0 Li I- 00 200 400 800 600 TEMPERATURE) 'C. 1000 92. Figure 19 Thermograms of Pyritized Coal and Pyrite Curve PP: Pyritized coal from Greene County, Indiana, 100 mg. Collection No. M.I.T. Sedimentology S14 - 86; Record No. LT - 90. Cur ve QQ: Pyrite, 25 mg. Record No. LT - 128. LI 200 400 600 TEMPERATURE) 800 'C. 1000 93. Figure 20 Thermograms of Shales Curve RR: Miocene marine nodular shale from California (Inglewood oil field), 100 mg. Curve SS: Record No. LT - 44. Green River shale from Fossil, Wyoming, 100 mg. M.I.T. No. S14 - 62; Sedimentology Collection Record No. LT - 105. Lii RR 0 0 ISS z Lii 0 200 400 600 TEMPERATURE, 800 *C. 1000 94, CHAPTER VI HIGH PRESSURE STUDIES INTRODUCTION AND REVIEW OF THE LITERATURE Relatively few studies have been reported on the effects of high pressure on organic substances. Fortunately, the most significant work in this field has been carried out by outstanding investigators leaving no doubt as to the reliability of their results. As early as 1914 Bridgman reported that egg albumin is coagulated by the application of a pressure of 5000 to 7000 atmospheres for thirty minutes (48). It was not until 1929 that Bridgman and Conant (51) extended the original study with a series of investigations using many types of organic compounds. A pressure of 10,000 atmospheres exerted over a period of 24 hours was found to have no effect on amylene, pinacone, tertiary amyl alcohol, diacetone alcohol, and other substances; but almost the same conditions led to the partial polymerization of isoprene, 2, 3-dimethyl-1, 3-butadiene, styrene, and indene, Isobutyraldehyde and n-butyraldehyde were converted to soft, waxy solids by the action of 12,000 atmospheres for 40 hours. In this and a later paper (50) polymerization was attributed to the presence of peroxides in the compounds under investigation. These are the first reported examples of irreversible chemical 95. transformations caused by hydrostatic pressure. In 1939, Raistrick and coworkers (210) found that cyclopentadiene polymerizes in three stages under pressures up to 5000 atmospheres and at temperatures of 00 to 400C. It was specifically found that dimerization takes place first and is then followed by the production of higher polymers. Within definite pressure limits at each temperature, an explosive disruptive reaction takes place resulting in the formation of a carbonaceous residue and much gas (92% methane and 81 hydrogen). An important observation by Raistrick was that the carbonaceous residue has an X-ray structure similar to that of low-temperature coke obtained from coal at 5500 - 6000 c. Further polymerization studies were carried out in 1945 by Vereschlagin and cowerkers (276) who found that cyclohexadiene polymerizes at 3000 to 3600 under 4000 atmospheres, but that the polymerization is independent of pressure. The polymers which were produced were found to be identical with those formed thermally. The polymerization of vinylcyclohexene is reported to be pressure-dependent at 2500 C. It is interesting to note the results of experiments by Johnson and Lewin (146) in which B. escherichia coli were subjected to pressures up to 8000 pounds per square inch. These investigators found that a pressure of 1000 pounds per square inch retards the growth of the bacterium, while 5000 pounds 96. per square inch causes disinfection or bacteriostasis. At temperatures above the optimum for the growth of the bacteria, a pressure of 1000 pounds per square inch has an opposite effect and actually accelerates growth. This work may offer a clue as to the importance of bacteria in compressed sediments.. Much work has been reported with respect to the effects of high pressures on proteins and enzymes (50). The results of these experiments are difficult to interpret. Several Bulgarian workers recently described experiments in which they subjected coal samples to hydrostatic pressures in the range of 10,000 atmospheres (269, 270). Analysis of coke yields and of tar fractions before and after the application of pressure indicated that a small but significant structural change in the coal material must have taken place as a result of the compression. A very significant series of experiments was reported by Bridgman in 1935 (49). In this work samples of organic and inorganic materials were compressed to pressures up to approximately 50,000 atmospheres and then sheared to the point of plastic flow. Many substances such as graphite, sugar, rosaniline, or Rochelle s4lt underwent no change as a result of this treatment. Rubber, Neoprene, wood, paper, and linen cloth, however, were changed to horn-like substances which were, in some cases, translucent. When celluloid or iodoform 97. was placed under a hydrostatic load of up to 50,000 atmospheres, no chemical changes were observed. When sheared under this pressure, however, both substances decomposed explosively. These experiments emphasize the fact that it may be possible by shearing complex substances, under the proper conditions, to cause the cleavage and re-formation of chemical bonds thereby producing a new type of compound without the use of high temperatures. This observation is extremely important to an analysis of the factors influencing the formation of high rank coals from humic substances under conditions of geological folding (see Chapter I). A series of experiments was carried out in which periodate lignin, sulphuric acid lignin, and humic acid were subjected to shear under high pressures. The results of these experiments are described below. DESCRIPTION OF EQUIPMENT The equipment for this work was simple, consisting only of a small bomb to hold the specimen (Fig. 21),a piston to bear on the specimen, a yoke to bear on the piston, and a lever arm with a 30 to 1 ratio to bear on the yoke. lead weights were hung onto the end of the lever. Suitable For experiments in which the piston was rotated, a small motor, geared to produce one revolution in 48 hours, was used. 98. To heat the specimen, a small furnace was constructed to fit snugly around the bomb. Before it was used., the furnace was calibrated to determine the voltage necessary to produce any desired temperature up to 2500 0. in the sample recess of the bomb. equipment, This calibration was carried out with the including the lever arm, assembled as if for a run. In preliminary experiments, the surface of the piston bearing on the sample was flat. In later work, this surface was ground to the shape of a 450 cone pointing into the sample (Fig. 21). This construction was chosen in order to obtain maximum shear stress in the material being compressed. The bomb and piston were constructed from No. 309 stainless steel. With the rotating piston arrangment, weights totaling more than 70 pounds on the end of the lever arm caused the motor gears to slip. Consequently, an upper limit was set for the pressure in these experiments using the available equipment. An experimental set-up with 150 pounds on the lever arm is shown in the photograph of Fig. 22. 99. Figure 21 Details of 6onstruction of Bomb and Piston for High Pressure Studies 7WORM- 0.253" GEAR 7/8" 45 D. 3/16" I 1/211 0 ~ I I I I ii hA' F~i 11/16" I I i 7/16" THREADS 4' I I I! 1 1/2" i Figure 22 Phot ograph of Equipment for High Pressure Studies 101. ANALYTICAL TECHNIQUES It was considered possible to follow piezochemical changes in the lignin molecule by the determination of the methoxyl content before and after compression of the material. Since the methoxyl content of coals is low (about 1%), it seemed reasonable to expect demethylation of the lignin as a possible effect induced by shear stresses. The best features of several procedures which have been suggested for the determination of methoxyl groups in organic substances have been incorporated in the method In this technique, the organic proposed by Clark (59). compound is dissolved in boiling phenol and treated with 48% hydriodic acid in order to split the methoxyl groups: + R -OCH HI --- > R OH + CH1. The methyl iodide which is formed is swept through the apparatus by a slow current of carbon dioxide and is collected in an acetic acid solution off potassium acetate Under these conditions the methyl containing bromine. iodide is oxidized to iodate: CH 1 IBr + 2 Br2 + Br 2 + --- * 3H2 0 --- CHRBr HIO + IBr + 5H Br. 102. The iodate is determined as follows: The reaction mixture is diluted with water, a sodium acetate buffer is added, and the excess bromine is destroyed with formic acid. The liquid is then acidified with sulphuric acid, potassium iodide is added, and the liberated iodine is titrated with standard sodium thiosulphate solution. Addition of the equations for all the reactions of this procedure leads to the following overall equation: ROCH ROH + + 3 Br + CH3 Br + 6 Na2S203 + 5HBr From this equation it % Methoxyl is = + 6 KI + 3H2O 3 Na2S40 6 + 6 Nal --- + 3 K2 S04 easy to show that (31) (Vol. Na2 S 2 03 ) (Norm. Na2S20 3 ) (60) (Sample in grams) Many attempts were made to analyze a carefully purified standard sample of vanillin by the procedure recommended by Clark. In each instance very poor precision and accuracy were obtained. After much experimentation a number of improvements were made in the method for determining methoxyl content. The revised procedure is outlined below and a photograph of the apparatus is shown in Fig. 23. (1) mg. Samples of 10 to 13 mg. are weighed to 0.01 on a Berman torsion microbalance. The weighing is best 103, accomplished in a piece of cigarette paper folded into the form of a small boat and suspended on the balance arm by the shortest necessary length of the finest platinum wire available. (2) The sample and paper are very carefully transferred into the 100-ml. flask with side-arm shown at the bottom of Fig. 23. Approximately 2 to 3 ml. of molten pheno.l are then added to the flask along with a small wad of glass wool to prevent bumping. Hydriodic acid (2 ml.) of the highest available grade is added to the flask which is quickly connected with the air condenser, trap, and collection tubes. The dimensions of these parts follow those recommended by Clark. The arrangement, however, which proved to be very satisfactory, evolved from discussions with Leavitt (163). A Glas-Col heating element is turned on (optimum boiling at 28 volts) and the flow of sulphuric-acid washed carbon dioxide is started immediately after the sample flask is connected to the air condenser. (Silicone grease proved to be the best lubricant for the stopcock and joints). (3) To assure complete demethylation the sample is allowed to boil for two hours, contrary to the 4 5-minute period suggested by Clark. (4) After two hours the bromine solution is thoroughly washed from the collection tubes into a 250 ml. 1o4. Figure 23 Apparatus for the Determination of Methoxyl Groups I 105. flask, excess bromine is decolorized with 3 to 5 drops of formic acid, 5 ml. of 10% sulphuric acid and a pinch of potassium iodide crystals are added, and the iodine thus released is titrated to a sterch end-point with standard 0. 01 N sodium thiosulphate solution. The thiosulphate solution is standardized on alternate days against a solution of approximately 0.6000 g. of potassium iodate in one liter of aqueous solution. (6) Na2 2 20 a 3 (Grams K103/liter) (214.02) (Vol. (Vol. KIO Sol.) Na2 S2 03 Sol.) The procedure was at first standardized with vanillin (20.39% OCH3 ), but anisic acid was later chosen as a more J desirable standard (20.39% OCH3 ). The anisic acid was re- crystallized from alcohol and then dried at 1050 C. for three days. In a series of twelve analyses by two operators the methoxyl content of anisic acid was found to be 20.09 ± 0.21%. The precision of the method was + 0.2%. Carbon and hydrogen analyses which are reported were obtained by the conventional dry combustion technique. The analyses were made by the Massachusetts Institute of Technology Department of Chemistry on 5-mg. samples. 1o6. EXPERIMENTAL Several preliminary experiments were carried out in which the sulphuric-acid lignin obtained from the Forest Products Laboratory was compressed. Using a Buehler hand pump and temperature of 1150 C., an estimated pressure varying from 11,000 to 14,000 atmospheres caused the material to turn black and to shatter into flaky fragments upon removal from the bomb. In similar experiments which were carried out at 2000 to 6000 atmospheres under more carefully controlled conditions, the lignin was recovered in the form of a solid cylinder, dark at the center and still retaining the brown color of the original material around the sides. Most studies were carried out with the piston having a conical end. The results of the most significant of these experiments are described below. Experiment L 33-1: Periodate lignin was packed into the bomb and compressed to 1655 atmospheres by hanging 49.25 pounds on the end of the lever arm. After two days at 1000 C. (one revolution of the piston) the bomb was allowed to cool and the pellet of lignin was removed. Maximum shearing stress was probably applied to the lignin on that surface of the pellet which was in contact with the rotating piston. For this reason the pellet was carefully centered in a lathe, 107. with a specially-constructed chuck, and a layer of material 0.050" thick was ground from its upper surface. Extreme care was taken to prevent contamination of the pellet or powder by grease. The collected powder was analyzed and was found to have a methoxyl content of 10.30 + 0.2%. The periodate lignin used for this experiment had a methoxyl value of 11.09 ± 0.2%. Experiment L 41-1: To establish the effect of temperature on the decomposition of periodate lignin by pressure, a sample of the lignin was compressed as in Experiment L 33-1, but at 150* C. instead of 1000 C. After onerevolution of the piston,the pellet was removed from the bomb and a layer of material 0.050" thick was ground from its upper surface for analysis. The methoxyl content of this material was found to be 11.07 i 0.2%; the original lignin, 11.09 + 0.2%. A powdered specimen of the residual pellet was thermographically analyzed (Record LT-152). Experiment L 42-1: To determine the effect of increased pressure on the piezochemical decomposition of periodate lignin, a sample of the material was compressed at 2280 atmospheres and 1500 C. After one revolution of the piston (48 hours), the pellet was removed from the bomb and a sample was ground from it on the lathe as in previous experiments. The powder thus obtained had a methoxyl content of 10.67 + 0.2%; original lignin, 11.09 + 0.2%. The residual lignin 108. pellet was struck a sharp blow and split longitudinally into two equivalent fragments. One fragment was ground to a brown powder which was thermographically analyzed (Record LT-153). Experiment L 43-1: Using the conditions of experiment L 42-1 (2280 atmospheres and 1500 C.), a sample of periodate lignin was compressed for four days (two revolutions of the piston) to establish the effects of increased piston rotation. When the base of the bomb was unscrewed, a small amount of material was found to be very tightly compressed in the conicallyshaped cavity. Microscopic examination of this material showed it to be nearly pitch black and to have a pattern of radial shear cracks. The material in each crack was golden-brown in color and had a resinous appearance. When an attempt was made to force the remainder of the sample out of the upper section of the bomb, the material shattered. The shattered material (50 mg.) was thermographically analyzed. The entire experiment was repeated (Experiment L 44-1) with the same results. When the shattered fragments of the pellet were analyzed, a methoxyl content of 10.91 + 0.2% was found; original periodate lignin, 11.09 + 0.2%. Experiment L 46-1: The conditions employed in experiments L 43-1 and L 44-1 (2280 atmospheres and 1500 C.) were main- tained, but the duration of the experiment was extended to 32 days (16 revolutions of the piston). As in the two 109. previously-described experiments, a small segment of the lignin pellet broke off and remained in the base secion of the bomb. The cross-section of the pellet which was exposed by the break (Fig. 24) showed a radial fracture pattern with golden-brown resinous material following each fracture as in other experiments. Careful microscopic examination of the surface of the cross-section showedthe structure of the black material to be pitted and to resemble charcoal. A small flake of the golden..brown resinous substance was removed from the broken pellet and was transferred to a microscope slide. The material was heated slowly on a micro- scope hot stage and was found to darken at about 2500 C. At 2900 C. the materialhad become opaque, and by 3500 C. it appeared to be completely charred. No signs of softening or melting were observed. The main portion of the sample pellet was removed from the upper section of the bomb and was found to have a methoxyl content of 11.36 + 0.2%; original periodate lignin, 11.09 + 0.2%. Experiment L 48-1: To determine the effects of temperature on the periodate lignin during the course of the pressure studies, a small amount of the material was placed in a thermostatically-controlled oven at 1500 C. At the end of 21 days the lignin had turned quite dark, had lost 27.5% of its original weight, and had a methoxyl content of only 9.01 + 0.2%; 110. Figure 24 Photograph of Golden-Brown Component and Radial Fractures in Compressed Lignin. --- +- - -- anew -r',,------- --- ---------- ------- - -- ---- - - - - original lignin, 11.09 ± 0,2%. By the end of four days the lignin had already lost approximately 10% of its weight but still had a methoxyl content of 11.01 + 0.2% (Experiment L 54-2). Experiment L 53-1: A sample of peat (Hawk Island Swamp, Manitowoc County, Wisconsin - see Fig. 11) was packed into the bomb as before and compressed at 1500 C. and 2280 atmospheres for four days (two revolutions of the piston). The sample pellet was removed from the bomb intact and specimens were ground from off its upper surface on a lathe. The first layer removed from the pellet was 0.005" thick and had a methoxyl content of 1.32%. The second layer (0.100") had a methoxyl content of 1.32%, and the third layer (0.050"), 1.16 + 0.02%. When the original peat was heated for five days at 1500 C., the final material had a methoxyl content of 1.18 + 0.09%. Experiment L 60-1: Humic acid which had been isolated from the Wisconsin peat (Chapter IV) was packed into a bomb and compressed at 1000 C. and 2280 atmospheres for three days (1.5 revolutions of the piston). At the end of this time the sample pellet was removed from the bomb and a layer of humic material 0.025" thick was stripped from the top surface of the pellet. Analysis of the powder gave the following data: C, 48.27%; H, 4.04%; Ash, 14.03%. On an ash-free basis: C, 56.10%; H, 4.70%. 112. A sample of original humic acid was heated for three days at 1000 C. At the end of this period the material had the following composition: C, 53.43%; H, 4.06%; Ash, 8.48%. On an ash-free basis: 0, 58.2%; H, 4.43%. The humic acid lost 3.5% of its weight during the period it was heated. SUMMARY OF RESULTS Table IV is a tabulation of the results of all the pressure experiments that have been cited. It is interesting that compression of the lignin at only 1655 atmospheres and at 1000 C. for only two days has produced the largest decrease in the methoxyl content of the material. Taking the precision of the analytical procedure into account, these relatively mild conditions appear to have caused a decrease of 0.4% of the methoxyl content of the lignin. All other experiments which were carried out at 1500 0. and 2280 atmospheres led to no appreciable change in the methoxyl content of- the lignin with the exception of an experiment which lasted only two days. In this case a decrease of 0.2% in the methoxyl content of the lignin was indicated. When lignin was heated for 21 days at 1500 C., the methoxyl content of the material dropped from 11.09% to 9.01%. After 32 days at 1500 C. and under a pressure of 2280 atmospheres, the lignin still had a methoxyl content of 11.36%. This might indicate that, whereas thermal decomposition alone Table IV Summary of Pressure Experiments and Blanks Material Temp.. 0C. Pressure, Atmos. Time or a Revolutions Analytical Data 11.09 + 0.2% OCH 3 Periodate lignin Periodate lignin 100 655 1 Rev. Periodat e lignin 150 1655 1 Rev. Periodate lignin 150 2280 1 Rev. 10.30 + 0.2% OCR 3 11.07 + 0.2% OCH3 10.67 + 0.2% OCH3 Periodate lignin 150 2280 2 Rev. 10.91 + 0.2% OCH 3 Periodate lignin 150 2280 16 Rev. 11.36 + 0.2% OCH 3 Periodate lignin 150 21 Days 9.01 + 0.2% OCH 3 Periodate lignin 150 4 Days 11.02 + 0.2% OCH3 Wisconsin peat 150 2280 2 Rev. 1.32% OCH3 1.32% 0CO 1.16 + -0.o2% OCH Wisconsin peat 150 Humic acid Humic acid 100 2280 100 aOne revolution = 2 days bTop layer, 0.005" thick cMiddle layer, 0.100" thick 5 Days 1.18 + 0.09% OCH 3 1.5 Rev. 3 Days 56.10% C, 4.70% He 58.2% C, 4.43% He dLower layer, 0 .050" thick eAsh-free basis 113. causes demethylation, heat treatment under pressure results in other structural degradations in the lignin molecule. This point should be checked in future studies by means of carbon-hydrogen ratios. Thermographic analyses of sheared products proved to be of little value in determining structural changes in lignin. In future studies, however, the use of micro thermographic equipment now under construction may make it possible to examine specimens of as little as 5 mg. taken from the top surface of the sample pellet. When peat was sheared under a compression of 2280 atmospheres, little or no change was observed in the methoxyl content of the material. The most highly sheared peat may have a slightly higher methoxyl content than the original material. This might again indicate structural degradations in the peat molecules. When humic acid is heated at 1000 C. for three days the carbon-hydrogen ratio of the final material is 13.15. When heated to 1000 C. for three days at 2280 atmospheres, however, the carbon-hydrogen ratio of the final material drops to 11.95. This result is difficult to explain and the experi- ment warrants careful repetition. In most studies, the material being compressed underwent continuous decrease in volume throughout the duration of the experiment. The decrease in volume was rather rapid at first and then quickly settled down to a gradual change. In the case 114. of the humic acid, repeat experiments indicated the usual gradual decrease in volume during the first part of the run, By the end of the first half revolution of the piston, however, a sharp increase in the volume of the material was noted. Following this sudden change, there was a gradual increase in the volume of the sample. This observation may indicate a structural decomposition of the humic material. Further work should be carried out to determine the changes taking place in the humic material. 115. CHAPTER VII CONCLUSIONS AND PROPOSED WORK CONCLUSIONS It was the stated purpose of these studies to obtain further information regarding: lignin and humic materials, (a) the relationship between (b) the possibility that the basic structure of lignin occurs in the various members of the coal series, and (c), the possible effects of high pressure and shear on the conversion of lignin and other materials into high grade coals. On the basis of a critical analysis of the literature it appears that cellulose is probably removed from sediments by biochemical processes soon af-ter deposition of plant fragments. Lignin, on the other hand, is resistant to microbial decomposition, but is degraded into humic substances. Using several structural models for the lignin molecule, suggestions have been made for the chemical or biochemical changes which lead to the transformation of lignin into humic compounds. Preliminary infrared absorption studies appear to indicate similar basic structures in lignin and in a sample of humic acid isolated from peat. 116. The study of several plant components and of various members of the coal series by vacuum differential thermal analysis seems to indicate that the fundamental structure of lignin occurs in lignites and bituminous coals. The large number of possible incompletely degraded plant materials is thought to mask the appearance of the -major thermographic peak for lignin in peats. By the time anthracite coal is formed, the lignin structure has probably been devolatilized to the point where the thermographic peak for lignin can no longer be recognized. There is no evidence from the thermographic studies that cellulose structure is present in any high grade coals. In some peats and lignites the thermographic studies do indicate the possible presence of small percentages of residual cellulose. . Several preliminary studies have indicated that pressures up to 2280 atmospheres, temperatures to 1500 C., and the application of shear stress produce only slight changes in the chemical structure of lignin, peat, and humic acid. Further experiments at lower, as well as higher, temperatures and pressures appear to be warranted from an examination of the experimental data. An effort was made to apply the model theory developed by M. King Hubbert (136, 137) to a solution of the dynamochemical phase of coalification. On calculation, it was found that a hydrostatic pressure of over 1020 atmospheres would be required to produce coal under laboratory conditions. This result could indicate that hydrostatic pressure plays little or no part in the formation of coal, or that the equations used for the calculation are not adaptable to this type of problem. Dr. Hubbert, who was consulted on this matter, felt that the latter conclusion was correct and suggested that attempts to investigate the problem from the scale-model point of view be abandoned. PROPOSED WORK It is proposed that the thermographic studies of coals be continued and amplified using specimens carefully selected from a number of seams on the basis of field data. In a small section of a single seam it may be possible to demonstrate thermally the heterogeneity or homogeneity of a petrographically homogeneous substance such as vitrain. If petrographic components can be recognized across lateral distances in the same seam, then it may be possible to carry out stratigraphic correlation studies. A particular advantage of thermographic analysis is that, if successful, it may supplant long and tedious microscopic studies based on thin or polished sections. 118. Studies should be carried out in which the thermal behaviour of the macro or micro constituents of the major petrographic subdivisions of coal are separated and thermographically analyzed. This work will require the development of procedures for the isolation of significant components. Thermal analyses may be made using a newly designed micro furnace which is under construction. This phase of the work should make it possible to establish the sources of certain characteristic thermal peaks which occur on the thermograms. Thermal decomposition studies of coal should be carried out in which each characteristic peak of the thermogram is correlated with the composition and quantity of the evolved gas. This study of gases evolved on heating coals should be based on the exact data furnished by the thermographic analyses. The results of this work will provide information regarding the type of chemical degradation taking place in the organic structure of the coal, and can be a part of the fundamental evidence used to determine chemical structure of the constituents of coal. Infrared analysis has become one of the most powerful tools for the study of organic substances. This method should now be extended to the study of coals and related materials. There is good evidence that coal has been formed from lignin. Infrared studies of the following series will be of primary importance in understanding the nature of the organic 119. components in coal: 1. Lignins isolated by various procedures. 2. Humic materials. These substances can be isolated from peat by certain procedures of extraction reported in the literature for the separation of humic acid, ulmic acid, hymatomelanic acid, etc. Infrared studies should indicate the similarities and differences among these materials and help to eliminate existing confusion regarding these substances. 3. Peats of various types. 4. Lignites. 5. Bituminous coals. Petrographic entities and macro and micro constituents should be separately examined. It will be necessary to develop new, and undoubtedly novel, techniques for some of this work. Since coals are probably aromatic in structure, ultraviolet absorption studies should prove helpful in the determination of structural characteristics. Emission spectroscopy should be applied to the study of the inorganic constituents of coals. Metallic elements such as vanadium, nickel, or silver may provide clues to the origin of the coal, its structure, and possible catalytic activity during the coalification process. Since lignin appears to be considerably altered soon after it is deposited in sediments, the piezochemical 120. studies should be repeated using peat, lignite, or humic materials. Studies at pressures and temperatures both below and above those already used are indicated from the work already carried out. Thermographic analysis and ultraviolet or infrared spectroscopy should be useful for analysis of the products and to establish any increase in rank during the experiments. 121. APPENDIX PUNCHED-CARD BIBLIOGRAPHY Punched-card bibliographies have been used for some time to answer specific questions in limitedfields. In such cases this method is an excellent aid to the literature and to identification procedures (114, 141). In the course of the research covered by this thesis, it has been necessary to compile an extensive bibliography on the origin of coal. A complete survey of this subject requires an understanding of the following fields: I. Wood chemistry A. Cellulose chemistry 1. Physical properties 2. Chemical properties 3. B. C. Analytical techniques Lignin chemistry 1. Physical properties 2. Chemical properties 3. Analytical techniques 4. Isolation procedures Chtemistry of wood resins and waxes, tannins, proteins, polysacchorideB other than cellulose, etc. 122. II. Biological agents for the destruction or alteration of wood components. A. Fungi 1. Physical and chemical effects B. Actinomyces 1. Physical and chemical effects C. Bacteria 1. Aerobic a. Physical and chemical effects 2. Anaerobic a. III. IV. Humus, Physical and chemical effects. Humic Acids, Humates. A. Origin B. Analytical and physical chemistry C. Isolation Coal A. B. C. Peat 1. Origin 2. Chemical analysis and physical properties Lignite 1. Origin 2. Chemical analysis and physidal properties Bituminous Coal 1. Origin 2. Chemical analysis and physical properties 123. D. Anthracite 1. Origin 2. Chemical analysis and physical properties V. VI. VII. VIII. General Geological Data of Importance Petrography of Coal Inorganic Constituents of Coal Colloidal Chemistry of Humus, Coal, and IX. Associated Materials. IX, Temperature and High Pressure Effects. Only a casual glance at this outline is sufficient to indicate a number of fields in which sub-classifications overlap. For instance, analytical chemistry shows up under cellulose, lignin, humus, peat, lignite, bituminous coal, and anthracite. If It is once understood that analytical chemistry covers manifold procedures ranging from electron microscopy to dialysis, then the futility of classifying analytical chemistry under each of the above major headings quickly becomes evident. Not only would such aprocedure require the use of all the holes on a card, but the duplication of recorded information would be such as to almost degrade the punched card index to the status of a conventional card file. For these reasons, only a single section of the punched card has been used to cover the field of analysis. Inasmuch as analysis presented a major interest in the study of the origin of coal, a major portion of the card was allotted to this subject (Fig. 25). 124. Figure 25 Subdivision of Subject Matter and Code for Punched Cards O 0 z B 0 0 I.I 0 g0 z 00 w AND DIFFRACTIONN ELECTRONMICROSCOPY VISIBLE LIGHT- ,,,REVIEW 'BOOK -- OWN WORK EMISSION- 00 W 0 INFARAREDR AMAN--' ULTRAVIOLETOR FLUORESCENCEOPTICAL ACTIVITY-/ EROBIC *5 ANAEROBIC O BACTERIA .- UNGI SPORESOR ENZYMES 0 @INERT_ THERMOGRAM\ VACUUM ENDOTHERMIC' ----- EXOTHERMIC GAS ANALYSIS- is-- YS COLOR ANAL 0- /GEOLOGY BOILING POINT- PETROLOGY OSSILS MELTINGPOINT-0 CHROMATOGRAPHY OR ADSORPTION- GEOCHEMISTRY REFRACTIVE INDEX---FRACTIONAlION. * CRYSTALLIZATIONMOLECULAR WEIGHT--" EXTRACTION+ MICROSC OP Y COKE-. TAR- ' @0 @ PYROLYSISSOXYGEN @ ISOLATION '--...NITROGEN NADIUM SULPHUR ST:UCTUREN GENERAL ANALYSIS ,,ICKEL "- STRONTIUM ,,-POTASSIUM -HYDRO GEN LOW TEMPERATURE 100-199 0 - 99- 200 -299\ 300-399 4 00 - 499.BG0-599-\ 700 000-099 -79 -SYNTHESIS 900 -99 1000 - 1099i 00089 OVER 200 11 00 -1199\ HIGH TEMPERATUREI 0 0 QV 2 12 5. In using this decentralization procedure, several points become obvious. First, it becomes necessary to employ two passes of the needle to establish the results of a study such as the chromatographic analysis of peat; and second, the single grouping under analysis saves space which in turn makes possible a more complete classification and allows more room for future expansion in original procedures which might be developed and incorporated into the cards during the course of the research. Once the break-down of subject matter has been decided upon, the process of codification is greatly simplified. With the easily available McBee Keysort card used in this work, the sides of the card were reserved for major subjects and the top and bottom were used for the entry of information pertaining to the major subjects. By employing this system, a single pass of the needle produces a set of cards referring to a single unambiguous topic. It is particularly important to eliminate the extraction of undesired cards at this point since later operations generally lead to the selection of a proportion of rejects as shown below. By eliminating unwanted categories at the very beginning, the number of unwanted cards which are isolated at the end of a series of passes is very greatly reduced. The code for the punched cards used in the present work is shown in Fig. 25. Where lettering extends to the edge of the diagram, an outside punch is indicated. Similarly, lettering in an intermediate position represents a center 126. punch, and deep lettering represents a deep punch. These three punches are represented on the sample card of Fig. 26. When entering information on the cards, it is of great assistance to know or to be able to predict the relative frequency with which the various subjects will occur. Since center punches do not automatically separate the required cards but necessitate manual isolation, it is especially desirable to use center punches only for those topics which occur infrequently. Similarly, the use of deep punches is permissible only where the subjects so coded occur rarely. It must be remembered that each time a card with a deep punch is removed, those cards with margin or center punches on the same two-hole series also are removed. Deep punches are mainly valuable where an undesignated category is to be indicated as illustrated in the case of the "bacteria" punch. (Fig. 25). As the accompanying figures show, information is dispersed on a card and considerable space is allowed between subjects. This room is available for later expansion or for the introduction of new fields which may become of interest to the research. The use of the cards and the extraction of particular information from them offers no problem if it is remembered that fundamentals rather than specific ideas have been coded. As an example, if all the cards bearing information on peat 127. Figure 26 Sample Punched Card 1 7 4 2 1 7 4 2 1 7-SF 4-0 2-SF 1-0 7 4 2 1 29 28 27 26 25 24 23 22 21 20 19 18 17 16 15 14 13 Lieser, 12 4 2 1 7-SF 4-0 2-SF 1-0 7 4 2 1 11 10 9 8 7 6 5 4 3 2 1 C. A., 21, 8213 (1927) Cellulosechemie, 1, 15676o (1926) . N Proposes isolation of lignin by cellulose-decomposing bacteria, fungi, etc. possibilities. Thermophilic bacteria offer good -4 A summary of important observations with N references. N FORMI MCBEE KEYSORT U. S. PAT. NO. 2,213,607 -KS-152 U 128. are removed from the file, then a second pass of the needle can yield cards with information on peat and ultra-violet light. Since the ultra-violet entry is under the broad heading of analytical chemistry, it is to be expected that all the cards will be removed which bear upon ultra-violet light as a tool in the analysis of peat. Since this light is a source of energy for certain reactions, however, it should be understood that cards bearing on the effects of ultra-violet light on peat will also be separated. This explanation is intended to show both how an unusual entry can be made, and how the desired cards can be removed. Where broad interests are to be coded, maximum use must be made of every item on a card to eliminate the necessity for repetition under specific headings, Taking a more difficult search for information as an example, it is proposed that the index be used to determine the role that anaerobic bacteria play in the origin of humus, peat, and lignite. Since peat, lignite, and humus are major interests in this file, it is only natural that all cards pertaining to these subjects be removed first. Having isolated and stacked these cards, a new file of diminished proportions is presented for further separations. The next major item in the request for infor- mation centers about the word "origin". It will be noted - 129. in Fig. 25 that this word is the turning point of the whole file and has been accorded the place of honor on the code. By removing all cards dealing with "origin", the following subjects have now been isolated: 1. Origin of peat 2. Origin of lignite 3. Origin of humus If the reverse procedure had been used and all cards dealing with "origin" were removed first, then an unnecesarily large group of cards would have been separated to form a bulky and only partially useful sub-file. In the third step of this illustrative search, the needle is passed through the hole reserved for anaerobic bacteria. it Since this is a center punch, is necessary to remove the displaced cards manually. During this pass of the needle, cards dealing with both aerobic and anaerobic bacteria will drop out along with references to undesignated bacteria. The series of three passes has, therefore, isolated all references to the effects of anaerobic bacteria on the origin of peat, lignite, and humus along with a small number of undesired cards. To emphasize a difficulty which may be encountered with the use of center punches, consider a possible search for information regarding the use of color reactions for the determination of the structure of lignin. By successively isolating those cards pertaining first to lignin and then to structure, only one further step remains. When the needle is U ~ 130. passed through the color analysis hole a number of cards drop out and a number are displaced. unambiguous, The displaced cards are and those which drop are concerned not only with "color reactions", but also with "gas analysis". If there were a deep punch designation on this two-hole series, then a number of those cards which fell during this operation might have no relation to the desired subject. This would be an example of an over-extended code where a large number of items were placed on the card at the expense of lost time in manual separations during the isolation procedure. It is helpful on occasion to be able to subdivide the zotal index and to remove a particular section for further study. purpose. Thus the "coal" punch in this index serves dual On one hand, it offers a means of entering infor- mation on experimental work on coal where the type of coal is not designated. On the other hand, by punching the "coal" code each time peat, brown coal, lignite, bituminous, or anthracite is entered, it later becomes possible to isolate all the cards of the index which are related to coal. In this fashion only a limited number of cards need be handled instead of the entire file. A similar procedure has been applied in the case of "lignin", "cellulose", and "other wood constituents", all of which have been reentered under the general "wood" punch. 131. To conserve space in a number of instances, several minor related subjects have been simultaneously designated Thus the "brown coal" entry also contains on the cards. references to cannel coal, boghead coal, Torbanites, etc. Similarly, hydrogen, hydrogenation and dehydrogenat ion, and oxygen and oxidation are entered under the same code designations. The entry of highly specialized information in an index of this type can be accomplished with relatively little difficulty. As an example, the results from differential thermal analyses can be entered on the cards under the following code headings: I. II. Thermographic analysis A. Vacuum B. Inert atmosphere Results A. Exothermic reaction 1. B. Temperature Endothermic reaction 1. Temperature In a typical analysis two significant decompositions take place when an organic substance is heated at 12 degrees per minute under vacuum. The fir-st, an endothermic reaction, reaches its peak at 2250, and the second, an exothermic 132. decomposition, attains its maximum at 4450 C. this information on the card, it To enter is necessary to designate a vacuum thermogram by the proper deep punch, and then to employ both edge and center punches (which add up to a deep punch) in the adjacent two-hole series to indicate that both exothermic and endothermic reactions have taken place. Proper entries would next be made on the temperature scale to indicate that reactions had taken place between 2000 and 3000, and between 4000 and 5000 C. It will be noted that deep punches have been used in the temperature code. In this case, there is a definite need for contraction of space and there is no fear of loss of information through the overlapping of data. The thermographic analysis entries pre-sent a difficulty since it is not possible to directly determine from the code which temperature corresponds to the exothermic or endothermic reaction. This is a relatively minor point, however, since in actual use there would be no loss of information through incomplete coding, but rather, a number of extra and undesired cards would fall during a search for information. Convenience is therefore sacrificed in order to have e highly useful sub-code which will enable the identification of unknown substances by comparing their thermal decomposition peaks. U ~ 133. BIBLIOGRAPHY 1. Adkins, H., Frank, R. L., and Bloom, E. S., "The Products of the Hydrogenation of Lignin", J. Am. Chem. Soc., .. , 549-55 (1941). 2. American Cyanamid Co., Stamford Research Laboratories, "Spectra-Structure Correlations", a chart obtained through R. C. Gore and dated Feb. 1, 1950. 3. Aulin-Erdtman, G., "Ultraviolet Spectroscopy of Lignin and Lignin Derivaties", Tappi, 160-6 (1949). 4. Bailey, A. J., "Chemistry of Lignin. I. 2, The Existence of a Chemical Bond Between Lignin and Cellulose", Paper Trade J., 110, No. 1, 29-34 (1940). 5. Bailey, A. J., "The Preparation and Properties of ButanolLignin", Paper Trade J., 111, No. 6, 27-30 (1940). 6. Bailey, A. J., "The Heterogeneity of Lignin", J., 111, No. 7, 27-30 (1940). 7. Bailey, A. J., "The Chemistry of Butanol-Lignin", Paper Trade J., 111, No. 9, 86-9 (1940). 8. Barnes, R. B., Paper Trade Gore, R. C., Stafford, R. W., and Williams, V. Z., "Qualitative Organic Analysis and Infrared Spectrometry", Anal. Chem., 402-10 9. Beckley, V. A., 20, (1948). "The Preparation and Fractionation of Humic Acid", J. Agri. Sci., ll, 66-8 (1921). 10. Beckley, V. A., "The Formation of Humus", J. Agri. Sci., 11, 69-77 (1921). 11. Belcher, R., and Wheeler, R. V., "Composition of Coal: Extraction with Quinoline", J. Chem. Soc. 866-9 (1940). 12. Bergius, F., "Artifical Formation of Coal", J. Gas. Lighting, 119, 571 (1912). 134, 13. Bergius, F., "The Use of High Pressures in Chemical and Technical Chemical Processes", Z. Elektrochem., 18, 660-2 (1912). 14. Bergius, F., "High Temperature High Pressure Reactions", Engineering, 96, 262-3 (1913). 15. Bergius, F., "Formation of Anthracite", 12, 858-60 (1913). 16. Berl, E., "Formation of Bituminous Coal", Colliery Guardian, 144, 914.-6, 961-2 (1932). 17. Berl, E., et al., "The Origin of Petroleum, Asphalt, and Coal", Naturwiss., 20, 652-5 (1932). 18. Berl, E., "The Origin of Coal, Oil, and Asphalt", Montan. Rundschau, 24, No. 19, 1-10 (1932). 19. Berl, E., "The Artificial Formation of Substances Similar to Bituminous Coal and Petroleum", Prod. 3rd Intern. 20. Z. Elektrochem., Conf. Bituminous Coal, 1, 820-30 (1932). Berl, E., "Cellulose as the Precursor of Coal and Petroleum", Papier-Fabr., 31, Tech.-wiss. Teil, 141-9 (1933). 21. Berl, E., "Thg Origin of Coal, Asphalt, and Mineral Oil", Osterr. Chem.-Ztg., _4, 385-7 (1937). 22. Berl, E., "Role of Carbohydrates in Formation of Oil and Bituminous Coals", Bull. Am. Assoc. Pet. Geologists, 24, 1865-90 (1940). 23. Berl, E., 24. Berl, E., and Koerber, W., "Fermentation of Cellulose and Cellulose Humic Acid and Lignin and Lignin Humic Acid", J. Am. Chem. Soc., 60, 1596-8 (1938). 25. Berl, E., and Koerber, W., "Partly Aromatic Constitution of Artificial Carbohydrate Coals", Ind. Eng. and Keller, H., "Petrographical and Chemical Researches on the Formation of Coals", Ann., 501, 84-106 (1933). Chem., 26. 2, 676-7 (19 Berl, E., and Schmidt, A., "Behavior of Cellulose on Heating under Pressure with Water", Ann., 461, 192-220 (1928) . 135. 27. Berl, E., II. "Genesis of Coals. Coalification of Cellulose and Lignin and Schmidt, A., in Neutral Medium", Ann., 493, 97-123 (1932). 28. Berl, E., and Schmidt, A., "Genesis of Coals. IV. Carbonization of Artificial Coals", 91, 135-52 (1932). Ann., 29. Berl, E., and Schmidt, A., "Genesis of Coals. V. Coalification of Cellulose and Lignin in Alkaline Medium", Ann., 426., 283-303 (1932). 30. Berl, E., Schmidt, A., and Koch, H., "The Origin of Coal" Z. angew. Chem., 4, (1930$; 44, 329-30 (1931); (1932). 31. Binaghi, R., "Present Knowledge of the Phenomena of Huminization of Fossil Fuels", Atti V. cong. nazl. chim. pura. applicata, Rome, 1932i, P . 1, 231-51 (1936); Chem. Abst., 22, 327 32. (1938). Bode, H., "Cellulose in Brown Coal and its Bearing on the Origin of Coal", (5), 33. 1018-9 517-9 4., Bone, W. A., Z. prakt. Geol., 38, 70-74 (1930). et al., "Researches on Coal. II. The Resinic Constituents and Coking Propensities of Coals", Proc. Roy. Soc. (London), 100A, 582-98 (1922). 34. Brandl, A., "Examination of Decayed Oak. Remarks on the Fischer-Schrader Coalification Theory", Brennstoff-Chem., F. 89-94 (1928). Isolation and E., "Native Lignin. I. Methylation" J. Am. Chem. Soc., 61, 2120-7 (1939 . 35. Brauns, 36. Brauns, F. E., 37. Brauns, F. 2, E., "Recent Advances in the Chemistry of Lignin", Paper Trade J., 111, 33-39 Oct. 3, (1940). "The Nature of Lignin from Western Hemlock (Tsuga heterophylla)". J. Org. Chem., 1, 211-15 (1945). 136. 38. Brauns, F. E., "The Proven Chemistry of Lignin", Lignin Chemistry and Utilization, Report on Conference at New Haven, Conn., September 10, 1947, Northeastern Wood Utilization Council, P. 0. Box 1577, New Haven. 39. Brauns, F. 40. Brauns, 41. Brauns, F. E., E., "The Proven Chemistry of Lignin", "Lignin Chemistry and Utilization", Bull. 19, Northeastern Wood Utilization Council, F. 0. Box 1577, New Haven, Conn., page 8 (January, 1948). F. E., "Lignin", in Progress in the Chemistry of Organic Natural Products, Vol. V, L. Zechmeister, Ed., Springer Press, Vienna, 1948. and Brown, D.8., "Sulphite Cooking Process.Effect of Pretreatment of Spruce Wood on the Reaction", Ind. Eng. Chem., 30, 779-81 (1938). 42. Brauns, F. E., and Hibbert, H., "Studies on Lignin and Related Compounds. XII. Methanol Lignin", Can. J. Research, l3B, 28-34 (1935). 43. Brauns, F. E., and Yirak, J. J., "The Decompostion of Methylated Spruce Wood", Paper Trade J., 125, No. 24, 55-60 (Sept. 18, 1947). 44. Bray, M. W., and Andrews, T. M., "Chemical Changes of Ground Wood During Decay", Ind. Eng. Chem., 16, 137-9 (1923). 45. Breger, I. A., and Burton, V. L., "The Effects of Radioactivity on a Naphthenic Acid", J. Am. Chem. Soc., 68, 1639-42 (1946), 46. Breger, I. A., "Transformation of Organic Substances by Alpha Particles and Deuterons", J. Phys. & Colloid Chem., .. 2, 47. Brickman, L., Pyle, J. J., McCarthy, J. L., and Hibbert, H., "Studies on Lignin and Related Compounds. The Ethanolysis of Spruce and Maple XXXIX. Woods", 48. 551-63 (1948). J. Am. Chem. Soc., 61, 868-9 (1939) Bridgman, P. W., "The Coagulation of Albumen by Pressure", J. Biol. Chem., 13, 511-12 (1914). - - - 137. 49. Bridgman, P. W., "Effects of High Shearing Strees Combined with With Hydrostatic Pressurb", Phys. Rev., 48, 825-47 (1935). 50. Bridgman, P. W., "Recent Work in the Field of High Pressures: Addendum", Rev. Mod. Phys., 18, 1-93 (1946). 51. Bridgman, P. W., and Conant, J. B., "Irreversible Transformations of Organic Compounds under High Pressures", Proc. Nat. Acad. Sci., 15, 680-3 (1929). 52. Burian, 0., "The Constitution of Natural Humic Acids", Brennstoff.-Chem., 6, 52-4 (1925). 53. Bursker, E. S., et al., "Radioactivity of the Coal and Anthracite of the Doneta Basin", Biochem. Z., gZ, 276-81 (1931). 54. Bursker, E. S., et al., "Radioactivity of Kuznetgk Coal", Ukrain. Khem. Zhur., ., 441-5 (1934): Chem. Abst., ?., 6138 (1935). 55. Buswell, A.M., and Boruff, C.S., "Decomposition of Cellulose and Cellulose Material by Bacteria. II.", Cellulose, 1, 162-5 (1930): Chem. Ast., ?I, 49042 (1931), 56. The DeterCampbell, W. G., and Bamford, K. F., "LXVII. mination of Lignin in the Analysis of Woods", Biochem. J., l0, 419-28 (1936). 57. Centola, G., "Transverse Bonds Between Macromolecules of Cellulose", Chimica e industria, 27, 1-4 (1945). H. Y., "Lignins Isolated in the Presence of Butanol", Paper Trade J., 114, No. 11, 31-36 (1942). 58. Charbonnier, 59. Clark, E. P., "Semimicro Quantitative Organic Analysis", Academic Press, N. Y., pp. 68-72 (1943). 60. Cockram, C., and Wheeler, R. V., "Studies in the Composition of Coal. The Resolution of Coal by Means of Solvents". J. Chem. Soc., 700-18 (1927). 138. 61. Cockram, C., and Wheeler, R. V., "Studies in the Composition of Coal. The Soluble Constituents of Coal and their Degree of Coalification", J. Chem. Soc., 854-60 (1931). 62. Cohen, W. E., and Harris, E., "Pretreatment of Wood With Hot Dilute Acid. Effect on Lignin Values", Ind. Eng. Chem., Anal. Ed., 2, 234-5 (1937). 63. Creighton, R. H. J., McCarthy, J. L., and Hibbert, H., "Aromatic Aldehydes from Spruce and Maple Woods", J. Am. 64. Chem. Soc., 6, 312 (1941). Crocker, E. C., "Significance of 'Lignint Color Reactions", Ind. Eng. Chem., 12, 625-7 (1921). 65. Dennstedt, M., and Schaper, L., "Dangerous Aspects of Hard Coals", Z. angew. Chem., 25, 2625-29 (1912). 66. Donath, E., "Contribution to the Knowledge of Fossil Coals", Chem. Ztg., )2, 1271 (1909). 67. Donath, E., 68. Dore, Charles, "The Methods of Cellulose Chemistry", "Petroleum and Coal", Chem. Ztg., (1919). Chapman and Hall, Ltd., London, 43, 497-9 p. 494 (1947). 69. Ibid., p. 370. 70. Dorland, R. M., Hawkins, W. L., and Hibbert, H., "Studies on Lignin and Related Compounds. XLVI. Action of Ozone on Isolated Lignins". The J. Am. Chem. Soc., 61, 2698-2701 (1939). 71. Enders, C., and Sigurdsson, S., "The Chemistry of Humic Acid Formation under Physiological Conditions. V. The Early Phase of Humic Acid Formation: An Aldol Condensation of Methylglyoxal", Ber., 76B, 560-3 (1943). 72. Evans, R. B., and Goodman, C., "Radioactivity of Rocks", Geol. Soc. Am., Bull., 2, 459-90 (1941). 73. Fischer, F., "Formation and Chemical Structure of Coal", Naturwiss., 2, 958-65 (1921); J. Soc. Chem. Ind., 41, 207A. 139. 74. Fischer, F., "The Decay of Cellulose and Lignin", Brennstoff -Chem., 3, 132-3 (1924). 75. Fischer, F., "Origin of Coal" Z. deut. 77A. 534-50 (19251. 76. Fischer, F., "Defense of the Lignin Theory of Coal geol. Ges., Formation", Brennstoff-Chem., 14, 147-9 (1933). 77. Fischer, F., and Horn, O., "Formation of Mineral Coal" Qes. Abhandl. 682-4' (1934). Kenntn. Kohle, 11, 78. Fischer, F., and Lieske, R., "Studies on the Behavior of Lignin in the Natural Decomiposition of Plants", Biochem. Z., 203, 351-62 (1928). 79. Fischer, F., and Schrader, H., "The Origin and Chemical Structure of Coal", Chem., Brennstoff- 2, 37-45 (1921). 80. Fischer, F., and Schrader, H., "The Origin and Chemical Structure of Coal", Brennstoff-Chem., 2, 213-19 (1921). 81. Fisher, F., 82. Fischer, F., and Schrader, H., "New Contributions to the Origin and Chemical Constitutianof Coal", Brennstof'f-Chem., 2, 65-72 (1922). 83. Fischer, F., and Schrader, H., "Notes on the Lignin Origin of Coal", Brennstoff -Chem., 2, 341-3 (1922). 84. Fischer, F., 85. Fischer, F., Schrader, H., and Treibs, W., "Chemical Breakdown of Cellulose by Pressure Oxidation", Ges. Abhandl. Kenntn. Kohle, 3, 211-20 (1921). and Schrader, H., "Effect of Heating Cellulose and Lignin under Pressure in the Presence of Water and Aqueous Alkalies", Ges. Abhandl. Kenntn. Kohle, 1, 332-59 (1921). and Schrader, H., "The Constitution of Coal", Festschrift Kaiser Wilhelm Ges. Forderung Wiss. Zehnjnhrigen Jubilaum, 59-63 (1921); Chem. Abst., 16, 4792 (1922) - - 140. 86. Fischer, F., Schrader, H., and Treibs, W. "Effect of Heating Under Pressure the Alkaline Solutions obtained in the Pressure Oxidation of Cellulose and Lignin", Ges. Abhandl. Kenntn. Kohle, 3, 311-18 (1921). and Tropsch, H., "Comparative Investigations 2418-28 on Lignin and Cellulose", Ber. 56B, (1923). 87. Fischer, F., 88. Fischer, F., and Tropsch, H., "The Comparative Vacuum Distilation of Cellulose, Lignin, and Deresinated Wood.", Chem. Zentr., 1923, II, 1400-1. 89. Fischer, G., "Acids and Colloids of Humus", Ku*hn-Archiv., 4, 1-136 (1914); Chem. Abst., 2, 2119 (1915). 90. Freudenberg, K., Belz, W., and Niemann, C., "The Aromatic Nature of Lignin. X", Ber., 62B, 1554-61 (1929). 91. Freudenberg, K., Harder, M., and Markert, L., "Observations on the Chemistry of Lignin ,Ber., 61B, 1760-65 (1928). 92. Freudenberg, K., Janson, A., Knopf, E., and Hoag A.," B 1415-25 (1936). Lignin. XV", Ber., 93. Freudenberg, 94. Freudenberg, K., and Ploetz, T.," Research on the Enzymatic Decomposition of Wood", Holz Rohu. Werkstoff, 1, 105-9 (1940). 95. Freudenberg, K., and Sohns, F., 96. Freudenberg, K., Zocher, H., and DUrr, W., "Further Researches on Lignin.XI", Ber., 62B., 1814-23 (1929). 97. Friedrich, A., and BrUda, B., "Lignin. II. The Structure of Primary Lignin", Monatsh., K., Lautsch, W., and Engler, K., "The Preparation of Vanillin from Spruce Lignin", Ber., 12B, 167-71 (1940). 66B, 262-9 (1933). 46, 600-10 (1925). "Lignin. XXI", Ber., 141. 98. Friedrich, A., and Diwald, J., "Lignin. I. Spruce Lignin", Monatsch., 46, 31-46 (1925). 99. Fr'mel, W., "Fulvic Acid. II.", Bodenkunde u. Pflanzenernhr., 27, 247-55 (1942). 100. Fuchs, W., "The Relation Between Humic Acids and Lignin", Brennstoff-Chem., 2, 298-302 (1928). 101. Fuchs, W., "Extraction of Lignin with Hydrochloric Acid in Methylglycol", Ber., 62B, 2125-32 (1929). 102. Fuchs, W., "Our Present Knowledge of the Chemical Nature of Humic Acid, the Chief Constituent of Lignite", Ges. Abhandl. Kenntn. Kohle, 9, 176-81 (1930). 103. Fuchs, W., and Daur, R., "New Investigations Regarding the Constitution of the Pine Lignins, Humic Acids, and Humins", Brennstoff-Chem., 12, 266-8 (1931). 104. Fuchs, W., and Daur, R., "Methylglycol Lignin", Cellulosechem., 12, 103-10 (1931). 105. Fuchs, W., Polansky, T. S., and Sandhoff, A. G., "Coal Oxidation", Ind. Eng. Chem., 3, 343-5 (1943). 106. Fuchs, W., and Stengel, W., "Hydroxyl and Carboxyl Groups in Humic Acids", Brennstoff-Chem., 10, 303-7 (1929). 107. Fuller, W. H., and Norman, A. G., "Cellulose Decomposition by Aerobic Mesophilic Bacteria from Soil. III. The Effect of Lignin", J. Bact., 46, 291-7 (1943). 108. Goss, M. J., and Phillips, M., "Studies on the Quantitative Estimation of Lignin. I. Factors Influencing the Determination by the Fuming Hydrochloric Acid Method", J. Assoc. Official Agric. Chem., 19, 341-50 (1936). 109. Gothan, W., "New Methods of Investigation of Brown , Coal", Braunkohle, 21, 400-1 (1922). , ilg!911110 ANZQ __ ___ 142. 110. Gray, K. R., King, E. G., Brauns, F. E., and Hibbert, H., "Studies on Lignin and Related Compounds. XII. The structure and Properties of Glycol Lignin" Car.J.Research, B 35-47 (1935). 111. Gropp, W., and Bode, H., "The Metamorphosis of Coals and the Problem of Synthetic Coalification", Braunkohle, 3l, 277-84, 299-302, 309-13 (1932). 112. Gruss, J."Lignin" (923), 113. GrUss, 114. Guy, A. 115. Hagglund, E., "Holzchemie", Akademische Verlagsgesellschaft m. b. H., Leipzig, 200 (1939). 116. Hggglund, E., and Carlsson, G. E., "Sulphonation of Spruce Li nin. II.", Biochem. Z., 212, 467-77 (1933). 117. Hagglund, E., and Rosenquist Ber. dent, botan Ges., 41, 48-52 J. "A New Reagent for Wood and Vanillin", dent. botan. Ges., 38, 361-68 (1920). Ber. G., "A Punch Card Filing System for Metallurgical Literature", Metal Progress, 993-1000 (December, 1947). Biochem. Z., !.2, T "Spruce Lignin", 376-83't1926) 118. Hagglund 119. Hagglund, E., and Urban, H.", Lignin Acetals. II", Cellulosechem., 9, 49-53 (1928). 120. Hamdi, H., "The Colloidal Chemical Properties of Humus. Dispersoid Chemical Observations with Graphite-Oxide", Kolloid-Beihefte, 54, 554-634 (1943). 121. Harris, E. E., D'Ianni, J., and Adkins, H., "Reaction of Hardwood Lignin with Hydrogen", J. Am. Chem. Soc., 60, 1467-70 (1938). 122. Heuser, E., and Winsvold A., "Formation of Oxalic Acid From Lignin, Cellulosechem., 2, 113 (1921); Ber., 56B, 902-9 (1923). 123. Hibbert, H., and Phillips, J. B., "Lignin and Related Compounds. III. Glycerol-Chlorohydrin-Lignin", Can. J. Research, 2, 65-9 (1930). 6 E., and Urban, H., "Lignin Acetals. I," ellulosechem. , 69-71 (1927). 143. 124. Hibbert, H., et al., "Studies on Lignin and Related Compounds. LX, LXI LXII", J. Am. Chem. Soc., 62, 3052-56, 3056-61, 3061-66 (1941). 125. Hickling, H. G. A., "The Microscopic Constitution of J. Coal" Soc. Dyers and Colourists, 2, 9-11 (1916 . 126. Hickling, H. G. A., "Properties of Coals as Determined by their Mode of Origin", Colliery Guardian, _4 401-4 (1932). 127. Hickling, H. G. A., and Marshall, C. E., "The Microstructure of the Coal in Certain Fossil Trees", Trans. Inst. Min.'Eng., 84 (2), 13-23 (1932). 128. Hickling, H. G. A., and Marshall, C. E., "The Microstructure of Coal in Certain Fossil Tree Barks", Trans. Inst. Min. Eng., 86 (2), 56-75 (1933). 129. Hickling, H. G. A., and Marshall, C. E., "The Origin of Vitrain, Chemical Constitution and Further Iron and Coal Trades Rev., 127, Research", 637 (Oct. 27, 1933). 130. Hilpert, R. S., and Pfutsenreuter, J., "The Influence of Ethylenediamine Copper Oxide Solution on Wood and Straw", Ber., 72B, 607-10 (1939). 131. Hilpert, R. S., and Hellwage, H., "Beech-Wood Lignin, a Reaction Product of Carbohydrates in Lignin Determination", Ber., 68B 380-3 (1935). 132. Hilpert, R. S., Kruger, W., and Hechler, G., "Chemistry of Lignin. The Action of Nitric Acid on Woods", Ber., a2, 1075-82 (1939). 133. Hilpert, R. S., and Meybier, H., "Relationships Between the Determinations of Pentosans and Lignins", Ber., 71B, 1962-5 (1938). 134. Hilpert, R. S., and Pfutzenreuter, J., "Characterization of the Plant Cell Wall by Treatment with Copper Oxide-Ammonia Solution", Ber., 71B, 2120-2 (1938). 135. Holmberg, B. Woodi, and Runius, S., Svensk Kem. "Alcoholate Digestion of Tid., E, 189-97 (1925). 144. M. K., "Theory of Scale Models as Applied to the Study of Geologic Structures", Bull. Geol. Soc. Am., 48, 1459-1520 (1937). 136. Hubber, 137. Hubbert, M. K., "Strength of the Earth", Bull. Am. Assoc. Pet. Geologists, 29, 1630-53 (1945). 138. Hunter, M. J., Cramer, A. B., "Structure of Lignin", 60, 2815-6 (1938). and Hibbert, H., J. Am. Chem. Soc., 139. Hunter, M. J., Cramer, A. B., and Hibbert, ,H. "Studies on Lignin and Related Compounds. XXXVI. Ethanolysis of Maple Wood", J. Am. Chem. Soc., 61, 516-20 (1939). 140. Hunter, 141. Hurlbut, C. S., M. J., and Hibber, H., "Studies on Lignin and Related Compounds. XLIII. The Absence of the Piperonyl Group in the Lignin Structure", J. Am. Chem. Soc., 61, 2196-8 (1939). "Mineral Determination by Means of Mineralogist, ll, 508-12 Punched Cards", Am. (1948). 142. Jackson, E. L., and Hudson, C. S., Application of the Cleavage Type of Oxidation by Periodic Acid to Starch and Cellulose", J. Am. Chem. Soc., 52, 2049-50 (1937). 143. Jeffrey, E. C., "Nature of Some Supposed Algal Coals", Proc. Am. Acad., 46, 273-90 (1910), 144. Jeffrey, E. C., "The Mode of Origin of Coal", J. Geol., 29, 145. 218-31 (1915). Jodl, R., "Carbohydrate Humic Acids and their Relations to Natural and Phenol Humic Acids", BrennstoffChem., 22, 217-20 (1941). 146. Johnson, F. H., and Lewin, I., "The Influence of Pressure, Temperature, and Quinine on the Rates of Growth and Disinfection of Escherichia Coli in the Logarithmic Growth Phase" J. Cellular Comp. Physiol., 28, 77-97 (1946S. 147. Jones, 0. T., "Hilt's Law and the Volatile Contents of Coal Seams", Geol. Mag., 86, 303-312, 346-364 (1949). 145. 148. Junker, E., "Colloid Chemical Properties of Humus. Dispersion Chemistry of Lignin", Kolloid(1941). 213-50 Z., 2, 149. Kinney, C. R., Polansky, T. S., and Gauger, A. W. "Preparation of Humic Acids from Bituminous Coals and Their Separation from Mineral Matter and Fusain by Solvent Treatment", Penna. State College Min. Ind. Exp. Station, Bull. 44 (1946) 150. Klason, P. 151. Klason, P.,"The Chemical Structure of Pine-Wood Lignin", Svensk, Kem. Tidskrift, 5-16, 47-52 (1917). 152. Klason, P., "Constitution of Pine-Wood Lignin. II.", Ber., B 448-55 (1922). 153. Klason, F., "Lignin Content of Spruce Wood", Cellulosechem., 4 81-4 (1923). 154. Kreulen 155. Ibid, 38-41. 156. Ibid 157. KUrschner, K. 158. "Beitr~dge Zur Kenntnis der Chemische usammensetzung des Fichtenholzes", B8erlin, 34-36 (1911). D. J. W., "Elements of Coal Chemistry Nijgh & van Ditmar, Rotterdam, p. 16 (1948). 47. Brennetoff-Chem., 158162 177-80, 188-94 208, 304-11 (1925). Kti*rschner, K., "The Humification of Wood by Merulius Lacrymans", Z angew. Chem. 40O, 224-32 (1927). "Lignins", 159. Ktrschner, K., "Humic Acid from Cassel Brown Coal. Ni/2"h, Cellulosechem., e, 59-67 (1941). 16o. Kurth, E. F., and Ritter, G. J., "Spruce Holocellulose and the Composition ofits Easily Hydrolyzable Fraction", J. Am. Chem. Soc., 16, 2720-23 (1934). 161. Lange, P. W., "Nature and Distribution of Lignin in 262-5 Spruce Wood", Svensk Papperstidn., 4', (1944). 146. 162. Lange, P. W., "Ultraviolet Absorption of Solid Lignin", Svensk. Papperstidn., 48, 241-5 (1945). 163. Leavitt, W. Z., "A Study of the Possibility of Isolating Lignin by the Selective Oxidation of Cellulose in Black Spruce Wood with Lead Tetracetate", S.B. Thesis, Dept. of Chemistry, Mass. Inst. of Tech., 1949. 164. Lieser, T., "Lignin", Cellulosechem., Z, 156-60 (1926). 165. Lebeau, P., "The Gaseous Products of Carbonization", Chimie et industrie, -1, Spec. No., 73-90 (April, 1928). 166. Lowry, H. H., "Chemistry of Coal Utilization", John Wiley and Sons, New York, Vol. I (1945). 167. Luft, F., "The Fiber Structure of Lignite", PapierFabr., Tech.-Wiss. Teil, 28, 787-91 (1930). 168. MacInnes, A. S., West, E., McCarthy J. L,, and Hibbert, H., "Studies on Lignin and Related Compounds XLIX. Occurrence of the Guaiacyl and~Syringyl Groupings in the Ethanolysis Products from Various Plants", J. Am. Chem. Soc., 62, 2803-2806 (1940). S.A., and Cable, D.E., "The Chemistry of Wood. IV. The Analysis of the Wood of Eucalyptus Globulus and Pinus monticola", Ind. Eng. Chem., 14, 933-4 (1922). 169. Mahood, 170. Marcusson, J., "Petroleum Oil and Coal", Chem. Z+g., 42, 437-9 (1918). 171 MacusonJ.,"Humic Acids", Z. angew. Chem., l I, 237-8 (1919). 172. Marcusson, J., "Origin of Asphalt and Coal", Chem. Z+g. 44, 43-4 (1920). 173. Marcusson, J., "The Structure and Formation of Humic Acids and Coals", Z. angew. Chem., 21., C.A.16, 21228 (1922). 165-6 (1922). 174. Marcusson, J., "Structure of the Humic Acids and Coals" Ber., .8B, 869-72 (1925). 147. 175. Marcusson, J., "The Composition of Peat and the Lignin Theory", Z. angew. Chem., 38, 339-41 (1925). 176. Marcusson, J., "Lignin and the Oxycellulose Theory of the Origin of Coal", Z. angew. Chem., 12, 898-900 (1926); 40, 48-50 (1927); 40, 1233.-4 (1927). 177. Marcusson, J., "Chemical Constituents of Lignites", Z. angew. Chem., 40, 1104-6 (1927). 178. Marcusson, J., and Wisbar, G., "The Composition of Lignite", Z. angew. Chem., 179. ll, 917-8 (1924). Marshall, C. E., "Contribution to the Comparative Petrology of British and American Coals of Carboniferous Age", Fuel, 20 (3), 52-59 (1941); 2Q (4), 89-91 (1941). 180. Marshall, C. E., "The Constitution of Anthraxylon (Vitrain or Vitrainite) and its Relation to Type and Rank Variation in Coal Seams" Fuel, 22, 140-55 (1943). 181. Moore, E. S., "Coal", John Wiley and Sons, New York (1922). 182. Moureau, C., and Lepape, A., "Helium from Five-Damp and Radioactivity of Coals, Compt. rend., 158, 598-603 (1914). 183. Norman, A. G., and Jenkins, S. H., "The Determination of Lignin. I. Errors Introduced by the Presence of Certain Carbohydrates", Biochem. J., 28, 2147-59 (1934). 184. Norman, A. G., "CXCVI. The Determination of Lignin. III. Acid Pretreatment and the Effect of the Presence of Nitrogenous Substances", Biochem, J., 21, 1567-74 (1937). 185. Norton, F. H., "A Critical Study of the Differential Thermal Method for the Identification of the Clay Minerals", J. Am. Ceram. Soc., 22, 54-63 186. (1939). Odintsov, P. N., "The Effect of Methods of Preparation of Lignin on its Chemical Nature", Lesokhim. Prom No 1, 10-17 (1939); Chem. Abst., l4, 54176 (1940). 148. 187. Ostwald, Wo., and Steiner, A., "Colloid Chemistry of Humus and Peat", Kolloid-Chem. Beihefte, 21, 97-170 (1925). 188. Ott, E., "Cellulose and Cellulose Derivatives", Interscience, New York, 103 (1946). 189. Ibid., p. 287-8. 190. Payen, M., "Memoir on the Composition of Plant Tissue and of Lignins", Compt. rend., 7, 1052 (1838). 191. Petrascheck, W., "The Metamorphosis of Coal and its ;nfluence on the Visible Components", Sitz. Osterr. Akad. Wissen., Mathem.-naturw. Kl. Abt I, 156, Nos. 7 and 8, 375-444 (1947). 192. Phillips, M., and Goss, M. J., "Chemistry of Lignin. VIII. The Oxidation of Alkali Lignin", J. Am. Chem. Soc., jj, 3466-70 (1933). 193. Phillips, M., and Goss, M.J., "The Dehydration of Alkali Lignin From Corn Cobs with Selenium", J. Assoc. Off. Agr. Chem., 21, 632-5 (1938). 194. Pictet, 195. Pictet, A., 196. Pictet, A., and Ramseyer, L., "Constituents of Coal", Ber. 44 2486-97 (1911). 197. Pictet, A., 198. Pictet, A., Rayseyer, L., and Kaiser, O., "Hydrocarbons 358-61 Present in Coal", Compt. rend., 16 (1916). 199. Ploetz, T., "The Enzymatic Decomposition of Polymerized Carbohydrates. IV. Comparative Enzymatic Decompositions of Several Isolated Wood Components by means of Snail Enzyme", Ber., A. "The Chemistry of Coal", Gas J., 142, (1918). 333 "Researches on Coal (Certain Constituents of Coal)", Ann. Chim. (9), 10, 249-330 (1918). ardRamseyer, L., "Hydrocarbon from Coal", Arch. sci. phys. nat., lis, 57-60 (1940). 4, 234-49 (1913). 149 200. Ploetz, T., "The Enzymatic Decomposition of Polymerized Carbohydrates. V. Enzymatic Decomposition of of some native Lignin-Containing Materials", Ber., 73.B, 61-73 (1940). "The Enzymatic Decomposition of Polymerized Carbohydrates. VI. The State of Combination of Lignin in Wood," Ber., 72B, 74-8 (1940). 201. Ploetz, T., 202. Ploetz, T., "The Enzymatic Decomposition of Polymerized Carbohydrates. VII. Further Fractionation Research on Linden Wood and Enzymatic Decomposition of the Fractions", Ber., 73B, 790-4 (1940). 203. Potonie, H., "The Origin of Coal and Related Products Including Petroleum", Ber. deut. pharm. Ges., 12, 204. Potonie, 180-223 (1907). R., "The Lignin Origin of Coal, A Geologic and Paleontologic Impossibility", Braunkohle, 21, 365-9 (1922). 205. Potonie, R., "Microscopy of Brown Coals. Pollen Forms", Braunkohle, 30, Tertiary 325-33 (1931). 206. Potonie, R., and Bosenick, G., "On the Origin of Coal: Results of A Petrographic-Microchemical Comparison of the Physical Constituents of Low and Medium-Rank Bituminous Coal", Preuss. Geol. Landesanst. Laborat., Mitt. 12, 75-110 (1933); Abst. Brennetoff-Chem., 15, 195-6 (May 15, 1934S. 207. Puri, A. 208. Pyle, J. J., Brickman, L., and Hibbert, H., "Studies on Lignin and Related Compounds. XLIV. The Ethanolysis of Maple Wood; Separation and Identification of the Water-Soluble Aldehyde Constituents," J. Am. Chem. Soc., 61, 21982203 (1939). 209. Raistrick, A., and Marshall, C. E., "The Nature and Origin of Coal and Coal Seams", English Universities Press, London (1939). N., "Soils, Their Physics and Chemistry, "Reinhold Publishing Corp., New York, pp. 222-25 (1949). 150. 210. Raistrick, B., Shapiro, R. H., and Newitt, D. M., "Liquid-Phase Reactions at High Pressures. V. The Polymerization of Cyclopentadiene and (-Dicyclopentadiene", J. Chem. Soc., 1761-69 (1939). 211. Rakovskl4,E. V., "The Formation of Humus Substances and Li gnin" Khim. Tverdogo Topliva 1 13-22 (1936; Chem. Abst., 31, 15844 T1937). 212. Randall, R. B., et al., "The Alkaline Permanganate Oxidation of Organic Substances Selected for their Bearing on the Chemical Constitution of Coal", Proc. Roy. Soc. (London), A165, 432-52 (1938). 213. Rassow, B., and Gabriel, H.," Pinewood Lignin", Cellulosechem., 12, 227-35, 290-95, 318-20 (1931). 214. Ritchie, P. F., and Purves, C. B., "Periodate Lignins; Their Preparation and Properties", Pulp Paper Mag. Canada, 48, No. 12, 74-82 (1947). 215. Ritter, 216. Ritter, G. J., D. M., et al., "Constitution of Gymnosperm Lignin," Science, 107, No. 2766, 20-22 (1948). "Distribution of Lignin in Wood", Ind. Eng. Chem., 12, 1194-7 (1925). "Factors of Method", (1932). 217. Ritter, G. J., Seborg, R. M., Mitchell, R. L., Affecting Quantitative Determination Lignin by 72 Per Cent Sulphuric Acid Ind. Eng. Chem., Anal. Ed., 4, 202-4 218. Rose, 219. Russell, A., "Interpretation of Lignin. I. The Synthesis of Gymnosperm Lignin", J. Am. Chern. Soc., 20, 1o60-64 (1948). 220. Schorger, A. W., "Chemistry of Cellulose and Wood", McGraw-Hill, New York, p. 524 (1926). 221. Schrader, H., "The Behavior of Cellulose, Lignin, Wood, and Peat Toward Bacteria", Ges. Abhandl. Kenntn. Kohle, 6, 173-6 (1921). "The Chemistry of Wood R. E., and Lisse, X. W., Decay. I. Introductory", Ind. Eng. Chem., 2, 284-7 (1917). 151. 222. Schrader, H., "Recent English Work on the Constitution of Coal", Brennstoff-Chem., 1, 155-7 (1926). 223. Schrauth, W., "Lignin", Z. angew. Chem., 36, 149-52 (1922). 224. Schuitz, F. and Sarten, P., "Wood Chemistry. The Pormation of Lignin from Wood and Wood Extracts and the Oxidation of Sugars, Cellulose, Wood, and Wood Extracts with Diazo Compounds to the Acids of the Sugar Group", Cellulosechem., 21, 225. 35-48 (1943). Schwalbe, C. G., "A New Method for the Determination of Lignin", Tech.-Wiss. Teil, Papierfabr., 2, 174-7 (1925); Chem. Abst., 12, 2127 (1925). 226. Schwalbe, C. G., and Schepp, R., "Transformation of Ligneous Plant Substances into Coal. I. Production of Coal-Like Products from Cellulose", Ber. 57B, 319-22 (1924). 227. Schwalbe, C. G.., and Schepp, R., "Transformation of Ligneous Plant Substances into Coal. II. Chemical Composition of Coaly Substances from Cellulose", Ber. 57B, 881-3 (1924). 228. Schwalbe, C. G., and Schepp, R., "Conversion of Lignified Plant Matter into Coal. III. Sugar Formation as an Intermediate Stage of Coal Formation", Ber. 58B, 2500-2 (1925). 229. Sedletskj4, I. D., and Brunooski., B., "The Structure of Humic Acid and Its Relation to Lignin and Coal", Kolloid-Z+g, 12, 90-1 (1935) 230. Sedletskil,I. D., "X-Ray Investigations of Coals", Soviet Geol., 26 No. 6 Abst., 4, 6793 48-63 (1939); Chem. (1940S. 231. Sedletski$, I. D., and Shmakova, G. V., "Thermal Characteristics of Humic Acid" Compt. rend. acad. sci. URSS, )1, 255-7 (1942). 232. Seyler, C. A., "The Microstructure and Banded Constituents of Anthracite", Fuel, 2, 217-8 (1923). 233. Seyler, C. A., "The Microstructure of Coal", Fuel in Science and Practice, 4, 56-66 (1925). 152. C. A., "The Nomenclature of the Banded Constituents of Coal", J. Roy. Soc. Arts, 14, 609-10 (1926). 234. Seyler, 235. Seyler, C. A., "Microstructure of Coal", Gas J. 173, 419-20 (1926). 236. Sheppard, C. W., and Burton, V. L., "The Effects of Radioactivity on Fatty Acids", J. Am. Chem. Soc., 237. 68, 1636-39 (1946). Sheppard, C. W., and Whitehead, W. L., "Formation of Hydrocarbons from Fatty Acids by AlphaParticle Bombardment", Bull. Am. Assoc. Pet. Geologists, 30, 32-51 (1946). 238. Smith, R. C., and Howard, H. C., "Equivalent and Molecular Weights of Humic Acids from a Bituminous Coal", J. Am. Chem. Soc., 52, 512-16 (1935). 239. Smith, R. C., and Howard, H. C., Cellulose by Heat", J. 234-6 (1937). 240. Spiel, S., Berkhamer,: L. H., Pask, J. A., and Davis, B., "Differential Thermal Analysis" U. S. Bur. of Mines, Tech. Paper 664 (1945 . 241. Stach, H., 242. Stach, H., "Moscow Brown Coals. IV. Humic Coalsu, Braunkohle, 22, 617-21 (1933). 243. Staudinger 244. Staudinger, M., "The Constitution and Chemical Structure of Bright Brown Coals. III. Humus Coals", Brennstoff-Chem, 14, 201-7 (1933). H., Staudinger, M., and Schmidt, H., Macromolecular Compounds. CCXXXVII. The Destruction of Cellulose by Microorganisms" Zellwolle, Kunstseide, Seide, 4J, 2-4 (19404. "Chemical Anatomy of Wood", Holz Roh-u. Werkstoff, 245. "Aromatization of Am. Chem. Soc., 3-, , 193-201 (1942). Stopes, M. C., "Studies on the Composition of Coal. I. The Four Visible Ingredients in Banded Bituminous Coal", Proc. Roy. Soc. London, 90B, 470-87 (1919). 153. M. C., and Wheeler, R. V., "The Structure of (1916). 4641 Coal", Colliery Guardian, 246. Stopes, 247. Stopes, M. C., and Wheeler, R. V., "The Constitution of Coal", Dept. Sci. Ind. Research, 1-58 (1918); J. Chem. Soc. 114A II, 270-1 (1918); Chem. Abs., 12, 2426 (1918). 248. Stopes, M. C., and Wheeler, R.V., "The Constitution of Coal. III.", Fuel in Science and Practice, 2, 196-204 (1924), 249. Stopes, M. C., and Wheeler, R. V., "The Constitution of Coal. IV., "Fuel in Science and Practice, -, 254-61 (1924). 250. Stopes, M. C., and Wheeler, R. V., "The Constitution Fuel in of Coal. VII. Artificial Coal" Science and Practice, 251. 2, Stopes, M. C., and Wheeler, R. V Thories;, of Coal. VIII. and Practice, 2, 393-5 (1924). "The Constitution Fuel in Science 395-9 (1924). 252. Strahan, A., and Pollard., W., "The Coals of South Wales With Special Reference to the Origin and Distribution of Anthracite", Mem. Geol. Survey, England and Wales, 2nd Ed., pp. 73-74 (1915). 253. Stutzer, 0., "Microscopic Coal Examination", Mikrokosmos, 132-4 (1919-1920). 254. Stutzer, 0., and Nob, A. C., "Geology of Coal" University of Chicago Press, Chicago (1940 . 255. Tenney, F. G., and Waksman, S. A., "Composition of Natural Organic Materials and their Decomposition in the Soil. IV. The Nature and Rapidity of the Decomposition of the Various Organic Complexes in Different Plant Materials Under Aerobic Conditions", Soil Sci., 256. 28, 55-84 (1929). Thaysen, A., C., et al., "Studies on the Bacterial Decomposition of Textile Fibers. III. The Occurrence of Humus Compounds in Deteriorated Fabrics and the Bearing of Their #ormation on bie Origin of Peat and Coal", Biochem. J., 20, 210-16 (1926), ~ir 154. 257. Thaysen, A. C., "The Microbiological Aspect of Peat Formation", Fuel in Science and Practice, 2, 560-3 (1930). 258. Thiessen, G., and Engelder, C. J., "Isolation of the Humic Acids", Ind. Eng. Chem., 22, 1131-33 (1930). 259. Thiessen, R., "Structure in Paleozoic Bituminous Coals", U. S. Bureau of Mines Bull, 117, 292 pp. (1920). 260. Thiessen, R., "What is Coal?", U. S. Bureau of Mines Information CirculaRr 7397, 53 pp. (1947). 261. Thiessen, R., and Strickler, H. S., "Distribution of Microorganisms in Four Peat Deposits", Mining and Met. Investigations, U. S. Bureau of Mines, Carnegie Inst., Tech. and Mining and Met. Advisory Boards, Coop. Bugl., 61 1-18 (1934); Chem. Abst., 34, 7398 (193M. 262. Thiessen, R., and Strickler, H. S., "Distribution of Microorganisms in Four Peat Deposits", Mining and Met. Investigations, U. S. Bur. Mines, Carnegie Inst., Tech. and Mining and Met. Advisory Boards, Coop. Bull., 61, 1-18 (1934). F. V., and Wheeler, R. V., "Studies on the Composition of Coal. II. A Chemical Investigation of Banded bituminous Coal", J. Chem. Soc., 115, 619-36 (1919). 263. Tideswell 264. Tideswell, V., and Wheeler, R. V., "Dopplerite. The Composition of Coal", J. Chem. Soc., 121, 2345-62 (1922). 265. Treibs, A., "Organic Minerals. III. Chlorophyll and Hemin Derivatives in Bituminous Rocks, Petroleum, Mineral Waxes, and Asphalts. Origin of Petroleum", Ann., 5j0, 42-62 (1934). 266. Treibs, A., "Organic Mineral Substances. TV. Chlorophyll and Hemin Derivatives in Bituminous Rocks, Petroleums, Coals, and Phosphorites", Ann., 11, 172-96 (1935). 155. 267. Treibs, A., "Organic Mineral Substances V. Porphyrins inCoals", Ann., 32, 144-50 (1935). 268. Treibs, A., "Chlorophyll and Hemin Derivatives in Organic Mineral Substances", Angew. Chem., 42, 682-6 (1936). 269. Trifonow, I., and Toschew, G, "Alteration of the Properties of Coals by Compression under High Pressures, Brennstoff-Chem., 20, 128 (1939); 21, 85-7 (1940). 270. Trifonow, I., and Phillipow, A., "Changes in Coals upon Compression at Very High Pressures", Brennstoff-Chem., 22, 193-5 (1941). 271. Tripp, R. M., "Classification of Organic Shales Based gThermographic Analysis" D. Sc. Thesis, Dept. of Geology, M.I.T. (1948). 272. Trotter, F. M., "The Devolatilization of Coal Seams in South Wales", Quart. J. Geol. Soc., 104, 387- 437 (1949). 273. Ubaldini, I., "Humic Acids", Brennstoff-Chem., 18, 273-9 (1937). 274. Ubaldini, I., and Magaldi, F., "The Constitution of Italian Fuels. II." Ann. chim. epplicata, 27, 146-56 (1937). 275. "Humic Acids. III.. Ubaldini, I., and Siniramed C Thermal Decompositionf , Ann. chim. applicata, 27, 94-102 (1937). 276. Vereshchlagin, L. F., and Polyakova, A.M., "The Explosive Decomposition of Cyclopentadiene at High Pressure", Compt. rend. acad. sci. U.R.S.S., 47, No. 3, 197-8 (1945). 277. Virtanen, A. I., "The Fermentation of Native Cellulose in Wood", Rept. Proc. 3rd Intern. Congr. Micr biol., 747-8 (1939); Chem. Abst., .4, 5246' (1940). 278. von Beroldingen, F., "Beobachtungen, Zweiful, and Fragen, die Mineralogie uilberhaupt, and inbesondere ein natUrliches Mineral-System betreffend", Hannover, First Edition, 1778; Second Edition, 1792, Volume 1. 156. 279. Waksman, S. A., "Cellulose and its Decomposition in the Soil by Microorganisms", Proc. Intern. Soc. Soil Sci., (N.S.), 2, 293-304 (1926); Chem. Abst., 21, 3099 (1927). 280. Waksman, 281. Waksman, S. A. "The Chemical Composition of Peat and the Aole of Microorganisms in its Formation", Am. J. Sci., (5), 12, 32-54 (1930). 282. Waksman, S. A., "The Chemical Composition and Methods of Analysis of Peat-Forming Plants and Peats", S. A., "The Origin and Nature of Soil Organic Matter or Soil "Humus", III. Nature of Substances Contributing to the Formation of Humus", Soil Science, 22, 323-33 (1926). Brennstoff-Chem., 11, 277-81 (1930). 283. Waksman, S A., "Decomposition of the Various Chemical Constituents of Complex Plant Materials by Pure Cultures of Fungi and Bacteria", Arch. Mikrobiol., 2, 136-54 (1931). 284. Waksman, 285. Ibid.., p. 96. 286. Waksman, S. A., and Dubos, R. J., "The Nature of Organisms which Decompose Cellulose in Arable Soils", Compt, rend., 185, 1226-8 (1927) 287. Waksman, S. A., and Gerretsen, F. C., "Influence of Temperature and Moisture on the Nature and Extent of Decomposition of Plant Residues by Microorganisms", Ecology, 12, 33-60 (1931). 288. Waksman, S. A., and Purves, E. R., "The Microbiological S. A., "Humus", Williams and Wilkins Co., Baltimore (1936). Population of Peat", Soil Science, 34, 95-113 (1932). 289. Waksman, S.A., and Starkey, R. L., "Microbiological Analysis of Soil as an Index of Soil Fertility. VII. Carbon Dioxide Evolution", Soil Sci., 17, 141-61 (1924). 290. Waksman, S.A., and Starkey, R. L., "Soil and the Microbe", John Wiley and Sons, New York, p. 82 (1931). 157. 85-6. 291. Ibid., pp. 292. Ibid., pp. 86-7. 293. Waksman, S. A., and Stevens, K. R., "Decomposition of Wood with Reference to the Chemical Composition of Fossilized Wood", J. Am. Chem. Soc., j1, 1187-96 (1929). 294. Wald, W. J., Ritchie, P. F., and Purves, C. B., "The Elementary Composition of Lignin in Northern Pine and Black Spruce Woods and of the Isolated Klason and Periodate Lignins", J. Am. Chem. Soc., 12, 1371-7 (1947). 295. Wehmer, C., "Experiments on the Conversion of Cellulose, Lignin, and Wood into Humins by Fungi", Brennstoff-Chem., 6, 101-6 (1925). 296. Weyland, H., "Chemical Processes in the Formation of Coal", Naturwiss., ., 327-35 (1927). 297. Wheeler, R.V., "The Composition of Coal" Chemistry and Industry, 10, 335-44 (19315. 298. White, 299. White, D., D., "Some Problems in the Formation of Coal", Econ. Geol., 2, 293-318 (1908). "The Origin of Coal", U. S. Bureau of Mines, Bull. 300. 38, 1-4 (1913). White, D., "Analyses of Coal Samples Studied under the Microscope" U. S. Bureau of Mines Bull., 38, 46-51 (19135. D., "Some Relations in Origin Between Coal and Petroleum", J. Wash. Acad. Sci., 5, 189-212 (1915). 301. White, 302. White, D., "Progressive Regional Carbonization of Coals", Trans. Am. Inst. Mining Met. Engrs., -1, 253-81 (1925). 303. White, D., "The Role of Water Conditions in the Formation and Differentiation of Common Banded Coals", Econ. Geol., 28, 556-70 (1933). 158. 304. White, D., "Metamorphism of Organic Sediments and Derived Oils", Bull. Am. Assoc. Pet. Geologists, 2., 589-617 (1935). 305. Widell, T., "Thermal Analysis of Peat", IVA, 18, 178-80 (1947). 306. Willstatter, R., and Zechmeister, L., "Hydrolysis of Cellulose. I.".Ber., 46, 2401-12 (1913). 307. Witham, H., "On the Internal Structure of Fossil Vegetables found in the Carboniferous and Oolitic Deposits of Great Britain, 1833; Thiessen, R., U. S. Mines Bureau, Bull. 38, p. 189 (1913). 308. Wright, G. F., and Hibbert, H., "Studies on Lignin and Related Compounds. XXVI. The Properties of Spruce Lignin Extracted with Formic Acid", J. Am. Chem. Soc., 32, 125-130 (1937). 309. Yohe, G. R., and Blodgett, E. 0., "Reaction of Coal with Oxygen in the Presence of Aqueous Sodium Hydroxide. Effect of Methylation with Dimethyl Sulfate", J. Am. Chem. Soc., 69, 2644-48 (1947). 310. Zadmard, H., "The Colloid Chemical Properties of Humus", Koll6id-Belhefte,. 42, 315-64 (1939). 159. BIOGRAPHICAL SKETCH OF AUTHOR BORN: January 28, 1920 in Boston, Mass. ELEMENTARY EDUCATION: Frank V. Thompson Junior High School, Boston, Mass, Boston Public Latin School, Boston, Mass. COLLEGES ATTENDED: Worcester Polytechnic Institute, Worcester, Mass., 1937-1941, B.S. in Chemistry. Boston University, Boston, Mass. 1942. Massachusetts Institute of Technology, Cambridge, Mass., 1945-1950, S.M. in Chemistry, 1947 EMPLOYMENT: Inspector of Powder and Explosives, Boston Ordnance District, War Department, 1941-1942. Assistant Senior Chemist Kop ers United Co., Kobuta, Penna., 1942-1943. Research Chemist, Publicker Commercial Alcohol Co., Philadelphia, Penna., 1943-1945. Research Assistant in Physics, Mass. Inst. Tech., Cambridge, Mass., 1945-1946. of Research Assistant in Geology, Mass. Inst. of Tech., Cambridge, Mass., 1946-1947. Research Associate in Geology, Mass. Inst. of Tech., Cambridge, Mass., 1947 to present. 160. PUBLICATIONS: Breger, I. A., and Burton, V. L., "The Effects of Radioactivity on a Naphthenic Acid", J. Am. Chem. Soc., 68, 1639-42 (1946). Breger, I. A., "Transformation of Organic Substances by Alpha Particles and Deutrons", J. Phys. Colloid Chem., E_, 551-63 (1948) Breger, I. A., "Semimicro Molecular Still", Anal. Chem., 20, 980-2 (1948). Breger, I. A., "Effects of Electrical Discharge and Radiation on Organic Compounds", Report of Progress - Funda- mental Research on Occurrence and Recovery of Petroleum, 1946-1947, American Petroleum Institute, N.Y., 214- 33 (1949). Whitehead, W. L., and Breger, I. A., "Vacuum Differential Thermal Analysis", Science, 1ll, 279-81 (1950). Whitehead, W. L., and Breger, I. A., "The Origin of Petroleum: Effects of Low Temperature Pyrolysis on the Organic Extract of A Recent Marine Sediment", Science, 1ll, 335,- 7, (1950) Breger, I. A., "Simple Applications of Punched Card Techniquesi, a chapter in a book entitled "Punched Cards -- Their Application to Science and Industry", Reinhold Publishing Corp., in press. SCIENTIFIC SOCIETIES: American Chemical Society Society of the Sigma Xi