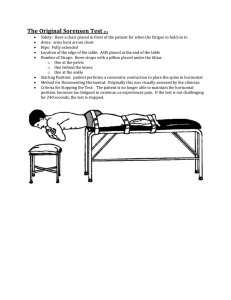
Hengwei Lin, Ravuri Kishore and Michael Schmittel
advertisement

Universität Siegen, Organische Chemie I, Adolf-Reichwein-Str. 2, D-57068 Siegen, Tel: +49 271 740 4340 e-mail: lin@chemie.uni-siegen.de Hengwei Lin, Ravuri Kishore and Michael Schmittel Introduction: Surface functionalization has, in recent years, grown into an ever expanding area of research.1 This has fueled a constant need for universal, efficient, and robust strategies to fabricate various substances on the surface. Herein, we present a simple but effective strategy to achieve surface functionalization by metal complexation as a post-electropolymerization step. This method opens an exciting possibility of exploring a whole gamut of metal coordinated species on the surface and extends their solution state applications to the surface.2 II Functionalization of 2 by crown ether-containing ruthenium (II) phenanthroline complex Monomer synthesis HO-(CH2)12-OSi Br N NaH, DMF N N N MeOH/H2O N N O-(CH2)12-OH N N Ru O O O O N N N O-(CH2)12-Br N N O N N O 1 Cl 2 N O S O O Ru N (a) O O Cl N O O O N O O DMF, 81% O N O S S S n-BuLi,THF, 62% O N N 55% PBr3 O O KOH O-(CH2)12-OSi O O 2 -0,4 -0,2 DMF, 120 °C, 24h S S 0,0 0,2 0,4 0,6 0,8 0,6 0,8 Potential (VFc) n n S S Pt or ITO The steps of surface functionalization Pt or ITO 2 4 f f N N Step 1 N N Step 2 N f O Electropolymerization on Pt or ITO electrodes in 1.0 mM CH2Cl2 solution O M O M = Ru(II), m = 2 or Cu(I), m = 1 f n S S S S m f Functionalization via a coordination reaction S Pt or ITO 1 S = Desired functionality 2 I Functionalization of 2 by ruthenium (II) phenanthroline complex N N N 450. 550. 0Wavelength0(nm) 650. 0 -0,2 0,0 0,2 0,4 Potential (VFc) 750. 0 Cyclic voltammograms of electropolymer 4 on ITO before (a) and after (b) overoxidation of electropolymer 2 in monomer-free acetonitrile solution. III Functionalization of 2 by a porphyrin appended phenanthroline copper (I) complex N Ru N N Zn N N 2 O -0,4 350. 0 Comparison of UV-Vis spectra: Electropolymer 2 before complexation (blue line), Ru (II) complex 4 on ITO (red line), and Ru (II) complex 4 monomer in dichloromethane (brown line). n Pt or ITO N (b) 0. 6 0. 5 0. Abs 4 0. 3 0. 2 0. 1 0. 0 250. 0 N N N O N (a) (a) Br Cl N N Ru 2 N N Cu Cl O N N Zn N S 0,0 n S S Pt or ITO n 0,4 0,8 N N 1,2 N Br -0,2 Potential (VFc) Pt or ITO 2 N N N O DMF, 120 °C, 24h S N 0,0 S S 0,4 0,6 0,8 0,6 0,8 n Pt or ITO Pt or ITO 5 2 (b) 2.0 S S n 0,2 Potential (VFc) DMF, 120 °C, 24h 3 1,8 (b) 1,5 1.5 1,2 Ab s 1.0 Abs 0,9 0.5 0,3 0,0 0.0 0,6 300.0 400.0 500.0 600.0 700.0 800.0 0,4 0,8 1,2 Potential (VFc) Comparison of UV-Vis spectra: Electropolymer 2 before complexation (blue line), Ru (II) complex 3 on ITO (brown line), and Ru (II) complex 3 monomer in dichloromethane (green line). 0,0 -0,2 300 Wavelength (nm) Cyclic voltammograms of electropolymer 2 on ITO before (a) and after (b) overoxidation of electropolymer 2 in monomer-free acetonitrile solution. Acknowledgments: We are greatly indebted to Deutsche Forschungsgemeinschaft for financial support. 400 500 600 700 Wavelength (nm) 800 Comparison of UV-Vis spectra: Electropolymer 2 before complexation (red line) and porphyrin appended Cu (I) complex 5 on ITO (brown line). 0,0 0,2 0,4 Potential (VFc) Cyclic voltammograms of electropolymer 5 on ITO before (a) and after (b) overoxidation of electropolymer 2 in monomerfree acetonitrile solution. Conclusions: A simple 2-step surface functionalization procedure has been developed. This method could be utilized effectively in modifying coordination complexes on surfaces and exploring their properites. References: 1 (a) Deronzier, A.; Moutet, J.-C. Coord. Chem. Rev. 1996, 147, 339-371. (b) Roncali, J. J. Mater. Chem. 1999, 9, 1875. 2 (a) Ng, P. K.; Gong, X.; Chan, S. H.; Lam, L. S. M.; Chan, W. K. Chem. Eur. J. 2001, 7, 4358-4367. (b)McQuade, D. T.; Pullen, A. E.; Swager, T. M. Chem. Rev. 2000, 100, 2537-2574. (c) Schmittel, M.; Kishore, R. S. K. Org. Lett. 2004, 6, 1923-1926. (d) Gust, D.; Moore, A. L. Acc. Chem. Res. 2001, 34, 40-48.