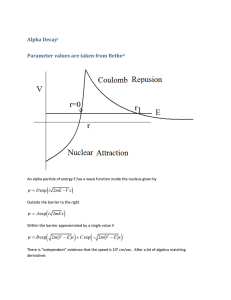
Physical Chemistry Particle in a finite 1-D box Lecture 16
advertisement

Particle in a finite 1-D box For E > V0 Wave functions are oscillatory functions of x Sums of exponential functions with imaginary arguments Amplitudes are related by boundary conditions Physical Chemistry For E , it approaches the behavior of the free particle in one dimension Lecture 16 Using the particle in a box to understand special problems Oscillatory behavior Deviation from the free particle depends on energy relative to the potential For a / 2 x a / 2 ( x) Very different from predictions of classical mechanics Equations in the three regions look similar Solutions are sums of exponential functions Different kinds of solutions for E < V0 and E > V0 Quantum conditions are determined by boundary conditions k ik ' x e k' 2mE 2 2m( E V0 ) 2 Compelx matching conditions For x a / 2 and x a / 2 ( x) Be k '|x| Classically the “external regions are “forbidden” Quantum mechanically the particle is allowed to be in this classically forbidden region The amount of penetration depends on the energy Tunneling – appearance in regions where classically not allowed For a / 2 x a / 2 ( x) De ikx Ee ikx where k for x a / 2 and x a / 2 For " external " regions E V0 2m(V0 E ) 2 In the low-energy regime (E < V0) d 2m dx 2 2mE 2 Particle in a finite 1-D box E k' for a / 2 x a / 2 2 d 2 2m dx 2 2 The solutions in the box are oscillatory The solutions in the “external” regions decay exponentially Matching at boundaries gives limits on possible values of E For a / 2 x a / 2 2 B Different from classical picture Particle in a finite 1-D box k ik ' x e k' Particle in a finite 1-D box For E < V0 Schroedinger’s equation in the three regions looks similar With a finite potential in the “external” regions, the solutions – even for E < V0 include the possibility of the particle being in those regions A where k' With a finite potential in the “external” regions, the wave function can be nonzero in those regions Must solve Schroedinger’s equation piecewise in three regions Match the solutions at the boundaries between regions Match derivatives at the boundaries Uses symmetry to simplify problem Puts origin at the center of the box, rather than one V ( x) V0 edge Solutions can be classified 0 as even or odd under inversion through zero Be ikx For " external " regions ( x) k Particle in a finite 1-D box Ae ikx Only certain allowed energies Quantum nature apparent for small boxes Tunneling into the nearby external space for finite V0 As the box becomes large, the spacing between states becomes small Model of translation of an ideal gas molecule In the high-energy regime (E > V0) Essentially a free particle with a large number of available energy states Often called “the continuum” 1 Metals, semiconductors, insulators Two adjacent potential wells Electrical conductivity Solve Schroedinger’s equation subject to boundary conditions at each discontinuity Qualitative picture Solutions depend on the width of the barrier Metals conduct electricity when subject to a voltage Insulators do not conduct electricity, even under a voltage This phenomenon can be understood by a simple model based on the particle in a box There are many quantum states closely spaced in energy that form bands of energy levels For a low, narrow barrier, the solution must go over to that of a single box with a small perturbation For wide enough (or high enough) barrier, solutions go over to a wave function that has separate lobes in each box Divided between core and valence states Some states from different configurations are separated in energy Whether bands are close in energy determines the ease of conduction If the valence band is only partially filled, the material is a metal If the valence band is filled AND there are no bands at nearby energies, the material is an insulator If the lowest valence band is filled but there are states nearby, the material is a semiconductor Depends on the band gap of the material Wave functions are approximately sums and differences of such lobal structures Wave functions classified as Symmetric Antisymmetric electrons Two adjacent potential wells Solution to Schroedinger’s equation for E < V0 approximated as a sum or difference of wave functions for the two separate wells Qualitative picture In the barrier region and nearby, the wave functions overlap Amount of overlap depends on the barrier width and height Overlap provides “spreading” of the wave function in space to span both wells Procedure provides a means of thinking about the manner in which electron wave functions on atoms delocalize to form molecular orbitals when the two atoms are brought into contact Atomic centers at lattice points Particle-in-box potential with a periodic corrugation For states at energies above the corrugation limit Considered as a 1 D box E E f Ei h2 n 2f ni2 8ma 2 Linear combination of atomic orbitals (LCAO-MO) Solid structures Delocalized over a large range in, e.g., aromatic molecules Particle-in-a-box model approximately represents the situation of electrons Estimate “size” of the box from spectroscopy of the * transition Wave functions similar to those of a particle in a very large box Wave functions extend over the whole box A huge number of states close in energy because the box is large Virtual continuum of states Tunneling through a barrier Earlier model shows that a finite potential gives the possibility of finding the particle in the classically forbidden region Narrow finite potential: a barrier Particle entering from left with energy less than V0 Encounters barrier Probability in barrier region finite Probability of finding particle on right side of barrier is finite Probability of transmission depends on Potential amplitude Energy of particle Width of barrier Characterized by the decay length, barrier d 0 e x 1 2 2m(V0 E ) 2 Tunneling junction Use of the concept of tunneling through a barrier Involves two adjacent metals that are separated by a small distance (< 1 micron) Applied voltage allows tunneling of electrons through the barrier between the two metals Summary Models can be used to approximate many situations Particle-in-a-box model useful for understanding Conjugated systems Metallic conduction Quantum dots Tunneling into or through a barrier useful for Junctions Scanning tunneling microscopy Certain chemical reactions Scanning tunneling microscopy Use tunneling to determine surface structure Run in the constanttunneling-current mode Height adjusted to give constant tunneling current and recorded as a function of position Display of height versus position gives an appearance of the “surface” Can “see” atoms in the surface Scanning tunneling microscopy Adsorption of palladium phthalocyanine on graphite Shows regular array structure Resolution to the atomic level 3