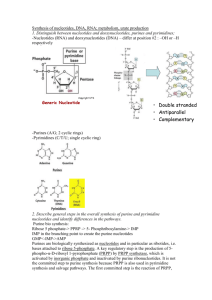
Document 10816850
advertisement

1 STUDIES OF THE PURINE BIOSYNTHETIC PATHWAYS IN THE GASTROINTESTINAL TRACT by Neal Simon LeLeiko B.S., Brooklyn College of the City University of New York (1967) M.D., New York Medical College (1971) SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY at the MASSACHUSETTS INSTITUTE OF TECHNOLOGY (June, 1979) i I - . /7 / 1 . . .. . . .. . . Signature of Author Department of Nutrition and Food Science, - - - -- - - - - - Certified by . .......... /11 Accepted by .. .. V... - Z- ... .. ARCHIVES MASSACHUSE S OF TECHNOLOGY OCT 1. 8 1979 LIBRARIES V0... ,.. . . . . . ... hesis Supervisor a 2 STUDIES OF THE PURINE BIOSYNTHETIC PATHWAYS IN THE GASTROINTESTINAL TRACT by Neal Simon LeLeiko Submitted to the Department of Nutrition and Food Science on June 29, 1979 in partial fulfillment of the requirements for the Degree of Doctor of Philosophy. ABSTRACT A series of experiments was performed on rats in order to determine the origin of dietary purines (nucleic acids, nucleosides or bases) for the metabolism of the mucosal cells of the gastrointestinal tract. The relative importance of the synthesis of purine nucleotides by the de novo and by the salvage pathways was assessed both by incorporation of isotopic precursors in each pathway (glycine for de novo synthesis, adenine for salvage), and also by measuring the activities of the rate-limiting enzymes in each pathway (phosphoribosyl amidotransferase for the de novo pathway, hypoxanthine-guanine phosphoribosyl transferase for the salvage route). De novo purine synthesis by small intestinal mucosal cells incubated in vitro with 14 C-glycine was shown to be very slight in contrast to the very active de novo pathway demonstrable in colonic mucosal cells. In contrast a salvage pathway in the mucosa of the small intestine was demonstrated in vivo by oral administration of 3 H-adenine and especially 3 H-adenosine, which 3 resulted in restoration of some in vitro labeling of mucosal RNA adenine from 4C-glycine by the de novo route. The small intestine was found to contain significantly less amidotransferase activity (first enzyme of the de novo pathway) than either the colon or the liver. This was observed whether the animals were on a purine-free diet or on a purinecontaining diet. Measurements of the hypoxanthine-guanine phosphoribosyl transferase of the salvage pathway demonstrated considerable activity in the mucosal cells of the small intestine. Significantly more HGPRT activity was found in the small intestine than in either the liver or colon when the animals were receiving a diet containing purines. The data thus demonstrates that the diet is a major source of purines for the small intestine, whereas the de novo pathway is not a major source of purine bases on diets containing purine bases as such or as nucleic acids. However, a modest increase in the de novo pathway (without enzyme induction) was achieved by eliminating dietary sources of purines. This allowed the existing low activity of amido- transferase to act more efficiently, probably through having more substrate (PRPP) because less is diverted to salvage. An additional mechanism could be that, on a purine-containing diet, more free nucleotides may accumulate and result in allosteric inhibition of the enzyme. The significance of these findings is discussed in relation to the participation of dietary purines in the maintenance and repair of the intestinal mucosa. Hamish N. Munro, M.B., D.Sc. Professor of Physiological Chemistry (Thesis Supervisor) 4 Acknowledgments I wish to thank Dr. Hamish Munro for granting me the privilege of working in his laboratory. His patience and interest made this work possible. I wish to thank Dr. Richard Grand, Dr. Paul Newberne, Dr. George Wolf and Dr. Vernon Young for their advice and encouragement. I wish to especially express my appreciation to Dr. Richard Hamilton of Toronto for his crucial early assistance, and to Dr. Richard Grand of Boston, not only for his contin uous advice and encouragement, but also for the tremendous fairness and decency that he has shown in our relationship. I wish to thank my many new friends who helped lighten my burdens. They provided many hours of stimulating debate, heartening camaraderie and personal friendship. I could ask nothing more of Andy Bronstein, David P. Katz, Samuel AdenyiiJones and Kathleen Motil. The lessons taught to me by Surendra Baliga will remain with me throughout my life. When I think of those whose help and friendship were always available I will always think most warmly of Suren. I would be remiss if I did not acknowledge the financial support supplied initially by the National Institutes of Health for a joint nutrition training program at the Children's Hospital Medical Center and M.I.T., and for the last two years by the Nutrition Support Service of the Children's Hospital Medical Center. 5 Finally, but certainly most importantly, I want to thank my entire family, especially my wife Marilyn. I wish that I could adequately express how I feel about the help and understanding that she has provided. I cannot do that, however, and if I could she would probably edit it out of the text. 6 TABLE OF CONTENTS TPage Title Page ............................................... 1 Abstract................................................. 2 4 Acknowledgments .,........................................ Table of Contents .. ...................................... 6 List of Figures ...... ................................... 8 List of Tables ...... ................................... 13 I. II. Introduction: The Nature of the Problem ........ 17 Survey of Literature . ........................... 24 A. The effect of nutrition on metabolism in 24 the intestinal mucosa ................... 1 - Metabolism in the intestinal mucosa and enteral nutrition - ............. .24 2 - The effect of parenteral nutrition upon the intestinal mucosa .... - 3 - A trophic effect of pancreatic ... 26 secretions upon the intestinal mucosa-----.-.. -..--...--. B. C. D. E. F. G. III. IV. Purine metabolism and diet .......... .... Intestinal RNA metabolism .............. Adenine metabolism ............ .........Glutamine Amidophosphoribosyl transferase and de novo synthesis -- -o Hypoxanthine-guanine, phosphoribosyl transferase; purine salvage; and LeschNyhan syndrome .......................... Intraperitoneal parenteral alimentation... 30 32 38 40 42 45 47 Plan of Research ... .............................. 52 Materials and Methods ... ..... A. B. C. D. E. ................. In vitro studies of the de novo and salvage pathways ........................ The fate of absorbed versus injected adenine and adenosine .................. The influence of protein and purine content of the diet ............. .......... The measurement of amidotransferase activity in the small intestine, colon and liver ........... .................... Additional studies related to or influencing intestinal purine metabolism 55 55 58 64 6 69 7 Page V. Results .. ........................................... 75 A. B. C. D. E. VI. In vitro studies of the de novo and salvage pathways .................................. 75 The fate of oral versus parenteral adenine and adenosine .. ............................. 88 Influence of-the protein afid purin-e content of the diet........................ The activity of the glutamine amidophosphoribosyl transferase in the small intestine, colon and liver and the effect of dietary 130 purines ...... ............................. The activity of the hypoxanthine-guanine phosphoribosyl transferase in the small intestine, colon and liver and the effect of dietary purines ........................ Results of Preliminary Studies ................... 169 A. B. C. The effect of a self-emptying blind loop of intestine on the activity of the de novo pathway ................................... 169 Studies of the different intestinal cell populations . ............................... 169 The effect of catheter placement and normal saline infusion ............................ 171 VII. Discussion and Conclusions ....................... 192 VIII. Suggestions for Future Research ................... 202 References ...........--................................... 203 Biographical Note ......................... 214 8 LIST OF FIGURES Figure No. 1 2 3 4 5 6 7 8 9 10 11 12 Title Page A general representation of the major synthetic and catabolic pathways of purine metabolism in mammalian cells... 18 The de novo biosynthetic pathway for purine nucleotides...................... 19 The sources of the atoms in the purine ring...................................... 20 The role of PRPP in purine nucleotide biosynthesis,........................... 21 The incorporation of 4C-Glycine into the RNA of small intestinal mucosa (everted loops) and into liver (finely diced )............................. 77 The incorporation of 1C-Glycine into small and large intestinal mucosal protein (everted loops) after pancreatic duct ligation or sham surgery..... 80 The incorporation of 3 H-adenine into mucosal RNA of everted loops of small and large intestine with pancreatic duct ligation and sham surgery........ 83 The incorporation of 1C-Glycine into the RNA in mucosal scrapings of small and large intestines.................... 85 The incorporation of 1 4 C-Glycine into mucosal protein of small and large intestinal mucosal scrapings.......... 87 The design of the experiment to determine the fate of absorbed versus injected adenine and adenosine................ 90 The incorporation of injected 3 H-adenine into the gastrointestinal tract of animals on a regular chow diet..... 91 The incorporation of injected 3 H-adenosine into the gastrointestinal tract of animals on a regular chow diet........ 94 9 Figure No. 13 14 15 16 17 18 19 20 21 22 23 24 25 Title Page The incorporation of injected 3 H-adenine into the gastrointestinal tract o'fanimals on a regular chow diet......... 96 The incorporation of injected 3 H-adenosine into the gastrointestinal tract of animals on a regular chow diet...... 97 The incorporation of gavaged 3 H-adenine into the gastrointestinal tract of animals on a 0% protein diet................ 99 The incorporation of gavaged 3 H-adenosine into the gastrointestinal tract of animals on a 0% protein diet........... 100 The incorporation of injected 3 H-adenosine into the gastrointestinal tract of animals on a 0% protein diet........... 102 The incorporation of injected 3H adenine into the gastrointestinal tract of animals on a 0% protein diet.............. 104 into The incorporation of 3 H-adenine the mucosal RNA of the stomach......... 105 The incorporation of 3 H-adenosine into the mucosal RNA of the stomach......... 106 The incorporation of 3 H-adenine into the mucosal RNA of the proximal small intestine.............................. 108 The incorporation of 3 H-adenosine into the mucosal RNA of the proximal small intestine................................ 109 The incorporation of 3H-adenine into the mucosal RNA of the middle small ... intestine............................... 110 The incorporation of 3H-adenosine into the mucosal RNA of the middle small intestine,.............................. 111 The incorporation of 3 H-adenine into the mucosal RNA of the distal small intestine.,............................. 112 10 Figure No. 26 Title The incorporation of 3H-adenosine inr to the ucosal RNA of the distal small intestine.,,, 27 28 29 30 31 32 33 34 35 36 Page 9,,,. 113 9.,...,........,., The incorporation of 3H-adenine into the mucosal RNA of the caecum........... 114 The incorporation of 3H-adenosine into the mucosal RNA of the caecumn........... The incorporation of 3H-adenine into 115 the mucosal RNA of the proximal colon.. 116 The incorporation of 3H-adenosine into the mucosal RNA of the proximal colon.. 117 The incorporation of 3 H-adenine.into the mucosal RNA of the distal colon........ 118 The incorporation of 3H-adenosine into the mucosal RNA of the distal colon.... 119 The incorporation of 3H-adenine into the mucosal RNA of the liver............. 120 The incorporation of 3H-adenosine into the mucosal RNA of the liver........ 121 The correlation of the amount of glutamate formed per mole glutamine used as substrate at different PRPP concentrations in the liver....................... 133 The correlation of the amount of glutamate formed as a function of PRPP concentrations in the reaction at different glutamine concentrations in the liver...,.,..,...........--....-.-.-135 37 38 The correlation of the amount of glutamate formed per mole glutamine used as substrate at a PRPP concentration of .3mM in the liver...................... 136 The correlation of the amount of glutamate formed as a function of PRPP concentration in the reaction at a glutamine concentration of .044mM in the live ...,,, .... ,,.. ... .... ... .. , 137 11 Figure No, 39 Title Page The amount of glutamate formed as a fuction of the PRPP concentration and glutamine concentration in the reaction mixture with liver amidotrans- ferase,.,.,,.....................139 40 41 42 43 44 45 46 47 48 The correlation of the amount of glutamate formed per mole glutamine used as substrate at different PRPP concentrations in the small intestine.. 141 The correclation of the amount of glutamate formed as a function of the PRPP concentration in the reaction at different glutamine concentrations in the small intestine................. 143 The correlation of the amount of glutamate formed per mole glutamine used as substrate at a PRPP concentration of 0.3mM'in'the small'intestine........... 144 The amount of glutamate formed as a function of the PRPP concentration and glutamine concentration in the reaction mixture with small intestinal amidotransferase....................... .. 146 The amidotransferase activity (total activity) in crude tissue extracts from liver and small intestine......... 148 The amidotransferase activity (PRPP dependent) in different tissues........ 150 The amidotransferase activity in different tissues as a function of the purine content of the diet............. 154 The liver HGPRT activity (using a crude tissue extract) determined by spotting 10 lambda, 20 lambda or 40 lambda of reaction-enzyme mixture on DEAE Cellulose Discs..,.......................... 156 The liver HGPRT activity............... 158 12 Figure No. 49 50 51 52 53 54 55 56 57 58 59 Title Page The HQPRT reaction in different tissues using five lambda of enzyme source.. 161 The HGPRT reaction in different tissues using ten lambda of enzyme source......... 162 The HGPRT reaction in different tissue using twenty lambda of enzyme source..... 163 The effect of dietary purines on HGPRT activity in different tissues............ 167 The incorporation of 1 4 C-glycine into the protein and the nucleic acids in different intestinal cell populations (expressed as counts/min/mg protein.).... 172 The incorporation of 1C-glycine into the protein and the nucleic acids in different intestinal cell populations (expressed as counts/mm/mg DNA).......... 174 3 The incorporation of H-adenine and C-glycine into the RNA of different intestinal cell populations............. 176 The average weight loss per day in animals receiving either intraperitoneal amino acids or normal saline............ 183 The average weight loss per day (expressed as percentage of initial weight) in animals receiving either intraperitoneal amino acids or normal saline............ 184 The serum albumin of animals receiving either intraperitoneal amino acids or normal saline.............................. 185 The liver (wet) weight of animals receiving either intraperitoneal amino acids or normal saline.................... 186 13 LIST QT TABLES Table No. 1 Title Page The 0% protein and 0% purine diet for the study of the fate of absorbed versus in)ected adenine and adenosine.. 60 2 The composition of Roger and Harpers salt mixture.,......................... 61 3 The composition of vitamin mix employed in experimental diets............... 62 4 The composition of diets for diet studies,,., .. 5 6 7 8 9 The incorporation of 1 4 C-Glycine into the RNA of liver (finely diced) and small intestine (everted loops)...... 76 The incorporation of 1 4 C-Glycine into small and large intestinal mucosal protein (everted loops) after pancreatic duct ligation or sham surgery...... 79 Incorporation of 1 4 C-glycine into small and large intestinal mucosal RNA (everted loops) after pancreatic duct ligation or sham surgery.......................... The incorporation of 3 H-adenine into small and large intestinal mucosal RNA (everted loops) after pancreatic duct ligation or sham surgery................ 81 82 The incorporation of 1C-glycine into small and large intestinal mucosal RNA (mucosal scrapings).......... 10 65 ,....................... .. ...... The incorporation of 1 4 C-glycine into mucosal proteins of the small and large intestines(mucosal scrapings).......... 84 86 11 The fate of absorbed ve sus injected adenine and adenosine ( H-adenine incorporation cpm/mg RNA)...................92 12 The fate of absorbed velsus injected adenine and adenosine ( H-adenosine incorporation cpm/mg RNA)............... 95 14 Table No. 13 Title Page The influence of protjin and purine C-glycine incontent of diet upon corporation into small intestinal mucosal protein and mucosal RNA..... 125 14 The influence of protTin and purine C-glycine incontent of diet upon corporation into small intestinal mucosal protein and mucosal RNA during refeeding.....................-.........127 15 The influence of protgin and purine content of diet upon H-adenine incorporation into colonic mucosal RNA. 16 17 18 19 20 21 22 The influence of protgin and purine content of diet upon H-adenine incorporation into colonic mucosal RNA during refeeding..................- - The correlation mate formed per as substrate at trations in the of amount of glutamole glutamine used different PRPP concenliver................. 128 . 129 132 The correlation of amount of glutamate formed as a function of PRPP concentration in reaction at different glutamine concentrations in the liver............ 134 The glutamate formed as a function of PRPP concentration and glutamine concentration in reaction mixture with liver amidotransferase................... 138 The correlation of amount of glutamate formed per mole glutamine used as substrate at different PRPP concentrations in the small intestine................... 140 The correlation of amount of glutamate formed as a function of PRPP concentration in reaction at different glutamine concentrations in the small intestine................................ 142 The glutamate formed as a function of PRPP concentration and glutamine concentration in reaction mixture with small intestinal amidotransferase...... 145 15 Table No. Title Page PRPP amidotransferase activity (total activity) in crude tissue extracts.....* 149 PRPP amidotransferase activity (PRPP dependent) in different tissues...... 151 Amidotransferase activity in different tissues as a function of the purine content of the diet........... 153 The liver HGPRT activity (using crude enzyme preparation) and spottinglO pil, 20pIl, or 40 jil of reaction-enzyme 'mixture on DEAE cellulose discs.......... 157 27 The Liver HGPRT activity............... 159 28 The effect of concentration of HGPRT enzyme source on enzyme reaction in different tissues......................... 164 HGPRT activity as a function of the purine content of the diet in different tissues............................... 166 The ratio of HGPRT activity/amidotransferase activity in different tissue as a function of the purine content of the diet............................... 168 The incorporation of 1 4 C-glycine into mucosal RNA-adenine and into mucosal protein in a self-emptying blind loop of small intestine............. 170 The incorporation of 1 4 C-glycine into the protein and nucleic acids in different intestinal cell population-5expressed as count/min/mg protein)............. 173 23 24 25 26 29 30 31 32 33 34 The incorporation of 1C-glycine into the protein and nucleic acids in different intestinal cell populations (expressed as counts/min/mg DNA)....... 14. C-glycine into The incorporation of the RNA of different intestinal mucosal cell populations..................... 175 177 16 Table No, 35 'Title The incorporation of 3Headenine into the RNA of different intestinal mucosal cell populations............. 36 37 38 39 40 Page 178 The effect of intraperitoneal catheter placement and normal saline infusion (5.5 ml BI.D.)................... 18,0 The effect of intraperitoneal catheter placement and normal saline infusion (19 ml B,ID.)................ 181 The effect of intraperitoneal amino acid infusions versus intraperitoneal glucose infusions...................... 188 The effect of control diets upon intraperitoneal study control animals....... 189 The incorporation of 1 4 C-glycine into small intestine mucosal RNA and protein in parenterally alimented, and control fed animals..................... 190 17 I. INTRODUCTION: THE NATURE OF THE PROBLEM Adenine and guanine, the purine bases, are utilized in the synthesis of nucleotides, nucleic acids, nucleoproteins and co-enzymes. The purine nucleotides can be synthesized in most tissues by two routes: cursors; (1) de novo synthesis from pre- (2) re-utilization of the free purine bases liber- ated through catabolism of nucleic acids via the so-called "salvage" pathway. The identification of the individual enzymes and metabolites of these pathways has provided a picture of the major synthetic and catabolic pathways of the purine nucleotides in mammalian cells (figure 1). The recycling or "salvage" of the purine bases is catalyzed by the enzymes hypoxanthine-guanine phosphoribosyltransferase (HGPRT)(E.C.2.4.2.8 ) and by adenine phosphoribosyltransferase (APRT)(E.C.2.4.2.7 ). The de novo formation of the purine ring is the result of a pathway (figure 2) involving glutamine, aspartic acid, glycine,formate and CO2, while figure 3 concisely illustrates the final location of these precursors in the purine ring. The first committed step requires the rate-controlling enzyme glutamine amidophosphoribosyltransferase (amidotransferase)(E.C.2.4.2.14). The competition between the salvage and de novo pathways for phosphoribosyl pyrophosphate (PRPP) (figure 4) represents a regulatory element in purine metabolism within the cell. APRT has the highest affinity for PRPP, HGPRT has the next highest, and the amidotransferase has the lowest (Wood and Seegmiller, 1973). Amidotransfexase as. well as PRPP appear to regulate ATP PRPP ADP i t 5 nucleotidose ADENINE ADENOSINE AMP .0 adenosine uodeny I succ inate deammnase GTP glutamine PR PP PRPP synthetase Ribose 5-P s s I omido PRPP tronsferase s glutommne ATP uspartote o PRA + HYPOXANTHINE INOSINE IMP gI ycne nHGPRT x ilthine I XMP oxicdose PR PP XANTHINE -4URIC No XANTHOSINE- ACID GMP GUANOSINE - GUANINE HGPRT GDP PRPP GTP A GENERAL REPRESENTATION OF THE MAJOR SYNTHETIC AND CATABOLIC PATHWAYS OF PURINE METABOLISM IN MAMMALIAN CELLS Figure 1 lw (P)-0-CH, 5 HC s5 (P)-o-CHI H NH 3 5 OH OH H HCs N (P)-O-C " Ribose NHH 5 o~gc AMP ATP 0 PiO-CHg SYMITU"TA,7 5-P Am I s iH (3) H OH OH ON PPriboseIP 5-1Phosphuoribosylaiminc ATP HM\ ADF HC 4 Mg HA P, H/ [" a u L 0 OH OH puo. 5-P Formylglyrinamide ribnsyl-S-P Glycinanide ribosyl-5.-P A -00c I t 0 *. A] l ATP Not+ + Aspartale HC-NH* CH -OC -oOC0 N 0 SN -O - H -00C -OOC COHO's v 0 C N C 0 H CH CN CN H I--, 0Avo ra fmt -CH' CH n H1 0 N RISOSE H3N 5-P rihosyl-5-P N RISOSE P carbhxylale ribosyI-5- I mm4 CLO* M S-P Aminsimidazole ribosyl-S-P Aminoiimsdaole RM301K U-P Formylglycinamidine ribosyl-S-P 0 700 0o1r 4 11 H \0) T01-s1Oi1LAst ATP. Mg + H3 N Uccinyl I O= C , N N N HNH RIBOSE 5-P -N Ht, C -------- aH AlSOSE Aminmtiiid1d, minoimidazic carbnxamde ocaas1 09 - -- N HIN REOSE 5-P A CH CH 11 (7) N H0 N SC H 0 Fumarale 0 5-P tle carbsxamide () 'IIC HN C I I HN WMS CMCI C N RMIE riblliyl-5-P I 5-P IMP THE DE Novo BIOSYNTHETIC PATHWAY FOR PURINE NUCLEOTIDES Figure 2 20 cO : C ASPARTIC GLYCINE / N FORMATE ACDN FORMATE ' -N / NN:N / /GLUTAMINE /I I GLUTAMINE THE SOURCES OF THE ATOMS IN THE PURINE RING Figure 3 \, 21 RNA BREAKDOWN G LY C INE DE NOVO < feedback PR pP AMI DO .---------TRANS FERASE AMP IMP GMP PRPP po ols APRT GLUTA MINE ADENINE HGPRT GUANINE HYPOXANTHINE THE ROLE OF PRPP IN PURINE [NUCLEOTIDE BIOSYNTHESIS Figure 4 s a Iv ag e 22 the de novo pathway within the cell. In some systems high levels of adenosine monophosphate (AMP) and guanosine monophosphate (GMP) tend to shut down the de novo pathway via allosteric inhibition (Holmes et al.,1973). The apparent result of these various interactions is the preferential use of PRPP for the recycling or "salvage" of purine bases over the de novo synthetic pathway. The de novo pathway uses six high energy phosphate molecules to produce either AMP or GMP whereas the salvage pathway requires only one. Thus intracellular conservation of energy favors salvage over de novo synthesis. The ability of various tissues to utilize each of the synthetic pathways has not been systematically studied A few studies or, isolated tissues suggested limited capacity for the de novo synthesis of purines in cells of the bone marrow (Lajtha and Vane, 1958), leukocytes (Williams,1962), and erythrocytes (Scott,1962; Smellie et al.,1958). Reem (1977) reported, however, that circulating leukocytes are capable of de novo synthesis. McKeran and Watt (1976) recently suggested that peripheral human granulocytes are not capable of purine synthesis de novo. Brosh et al,(1976) found that mixed mature peripheral human leukocytes synthesize nucleotides de novo, It is interesting to note that they also reported a ten-fold increase in the rate of synthesis in patients with Lesch.-Nyhan Syndrome (HGPRT deficiency), MacKinnon and Deller C1973) have presented evidence to suggest that mucosa of the small intestine lacks the capacity to synthesize AMP and GMP via the de novo pathway. If the small intestine is incapable of pro- 23 viding purine bases via the de novo synthetic pathway, then the source of the preformed purines becomes a significant question. The salvage pathway could use either exogenous purines provided *as a result of digestion or endogenous purines provided by the bloodstream. If the dietary source is a significant or nec- essary source of purines for the mucosal cell, then there would be a role for dietary purines in the nutrition of this cell population. This concept can be extended to the question of whether a change in the amounts of purine available from the diet affects mucosal function in health and disease, Intriguing questions such as the relationship of dietary purines to mucosal cell turnover and the role of purine lack in the mucosal atrophy of parenteral nutrition are practical outcomes of the exploration of this problem. Accordingly, before defining the experi- mental protocols used, it is necessary to review the current understanding of the response of the small intestinal mucosa to dietary change, and also the details of purine metabolism. 24 II. SURVEY OF LITERATURE A. The Effect of Nutrition on Metabolism in the Intestinal Mucosa 1. Metabolism in the Intestinal Mucosa and Enteral Nutrition The concept that diet affects intestinal mucosal metabolism is not new. The full extent of the dietary effect is, however, unknown. The effects of starvation and protein deprivation upon the small intestine have been studied by many investigators. Both have been found to decrease cell turnover, but their effect upon intestinal cell size is not the same. Protein deprivation does not affect intestinal cell size, while starvation results in decreased cell size. purine-deficient as well. Protein-deficient diets are usually The role of purines in diet studies of intestinal metabolism has been ignored, and therefore no specific metabolic effect has been attributed to them. Ju and Nasset (1959) demonstrated that diet could be a major factor in the regulation of intestinal mucosal structure and cell metabolism. They subjected rats to eight days of fasting and found that the small intestine, pancreas, and liver lost almost 50% of their protein content. The stomach lost only 10% of its protein content, Upon re-feeding, the intestinal mucosa was rapidly restored. Hooper and Blair (1958) demonstrated that starvation decreases intestinal cell division. Munro and Goldberg (1964) found that a protein- deficient diet also resulted in decreased cellular division. Similar findings were reported by Durand et al. (1965) when 25 they fed rats a protein of low biological quality. Hopper et al.(1968) found that protein deficiency if allowed to continue resulted in increased transit time up the intestinal villi and Platt et al. (1964) observed that eventually protein restriction led to a flattening of the mucosal surface. Although Munro and Goldberg (1964) and Hill et al.(1968) found that mucosal cell size is maintained in animals fed a protein-deficient diet, Thaysen and Thaysen (1944) as well as Steiner et al.(1968) have shown that starvation tends to reduce the size of the average mucosal cell. The rapidity with which the small intestine responds to starvation has been emphasized in the studies of McMannus and Isselbacher (1970). They found that that the small intestines of a group of rats that were fasted overnight contained 13% less mucosal DNA than did fed controls. They described a decreased number of mucosal cells, decreased size of individual cells, and a decrease in proliferative activity. They suggested that their observations were the combined result of an inhibitory effect of the fast and a stimulatory effect of feeding. At the cellular level Hagemann and Stragand (1977) found during a two-to-three day fast the small intestinal crypt proliferative compartment size and total cellularity declined significantly. This was accomplished by an increase in total cycle time as a result of the lengthening of the G1 and S phases. Following re-feeding there was a reduction in the cycle time and a gradual return to the control values for the proliferative compartment size and cellularity. During a three-day fast 26 the small intestine lost about 40% of its cells. In the colon, Hagemann and Stragand (1977) found that fasting had no effect on the proliferative compartment size or total crypt cellularity. There was an approximate doubling of the cycle time, however, due to an increase in G . When the fast ended, and a normal diet was begun, there was a hyperplastic burst of DNA synthesis in colonic crypts due to the entry of the large number of cells blocked in Gi into the S phase. Peak levels of S cellularity exceeded four times the fasting and two times the normal-fed control value. It is of interest to note that when the authors re-fed a "lowresidue" diet consisting of soluble casein, glucose, and corn oil, there was a return to control values but no hyperplasia. In the small intestine re-feeding was followed by return to near control values with only a slight overshoot. Thus it is clear that both starvation and protein deprivation adversely affect cell turnover and that protein deprivation affects cell size. There is, however, no convincing data on any effect of dietary purines on the intestine. In the studies performed, endogenous purines available from shed mucosal cells would be expected to negate any effect of dietary purine deprivation. (Furthermore the actual purine content of the diets utilized in all studies above is unknown.) 2. The Effect of Parenteral Nutrition Upon the Intestinal Mucosa Johnson et al.(1975) reported that parenterally-fed rats underwent significant losses in small intestinal and pancreatic 27 mass. In the small intestine there was a significant diminut- ion of both RNA and DNA although the RNA:DNA ratio was nearly double in the parenterally-fed group. The authors attribute much of the effect to "hormonal factors". Eastwood (1977) studied small bowel morphology and epithelial cell proliferation in intravenously-fed rabbits, He found that proximal small intestinal mucosa was thinner in the parenterally-fed animals. In addition the parenterally-fed group showed significantly decreased incorporation of 3 H-thymidine into crypt cells. Eastwood (1977) also demonstrated increased goblet cell number in the parenteral group. All of the effects were most definitive proximally and not present in the distal small intestine. Electron microscopy of the mucosa of parenterally fed rabbits (Eastwood, 1977) demonstrated an increased number of multivesicular bodies in the tip cells along with longer microvilli. These changes, which were most manifest in the jejunum, may be interpreted as indicating an older cell population and therefore decreased shedding and decreased migration in addition to decreased proliferation. The speed of change in intestinal and pancreatic structue and function with the elimination of enteral nutrition was assessed in parenterally-alimented rats by Hughes et al. (1977). They found that jejunal villus height fell quickly from 432 Mm to 342 pm at three days, to 313 pm at six days, and 177 pm at fifteen days. Similarly, ileal villus height fell from 237 pm to 204 pm at three days, 197 pm at six days, and 177 pm at fifteen days. There were corresponding changes in crypt 28 depth and mucosal protein, DNA content, md wet weight. In vivo absorption studies in both jejunum and ileum disclosed significantly diminished galactose absorption after ten days. Pancreatic mass fell from 500 to 307 mgm/100 gms body weight after fifteen days. Dowling and Gleeson (1973) and McDermott et al. (1976) have demonstrated that in rats resection of the proximal small intestine produces mucosal cell hyperplasia and an increase in the proliferative zone of crypt cells in the residual distal intestine. There is also villous enlargement and an increase of the muscular wall. Weser and Hernandez (1974) and Urban and Pena (1974) demonstrated, after intestinal resection, that stimulated cell proliferation occurs within five to six weeks, Obertop et al. (1977) have shown that within two days of removal of the proximal one-third of the small bowel in the rat, there was a marked increase in the nucleic acid content of the remaining small intestine. This increase became progressively less marked in more distal sections. Feldman et al. (1976) showed that in dogs which were alimented parenterally after jejunectomy, the adaptive mucosal hyperplasia in the remaining small bowel was blunted, The villus height in residual ileum of parenterally-alimented animals was lower than orally-fed control animals after six weeks. In addition, the villus height reduction was even more prominent in intravenously-fed dogs whose bowels were left intact (Feldman, et al., 1973). Other studies after experimental intestinal resection (Altman, 1974; Dowling, 1974; 29 Johnson, et al., 1975; Johnson, 1976) all similarly demonstrated that return of morphology and function is clearly related in intraluminal nutrition. Levine et al. (1974) fed an identical liquid diet to two -groups of rats for one week. One group received the diet parenterally, the other via the enteral route. Body weight gains for the two groups were comparable, yet the group on total parenteral nutrition had a 22% decrease in small intestinal weight, 28% decrease in mucosal weight, 35% decrease in mucosal protein, and a 25 % decrease in total DNA. Of note is that these effects were minimal in the distal small intestine and maximal at the proximal end. The net effect was a loss of the proximal-to-distal morphological gradient. This would support the premise that this gradient was the result of the preferential utilization of the luminal contents. In addition to the studies of Levine et al. (1974) cited above, Dworkin (1976) compared intestinal mass between rats receiving a formula orally and a group receiving the same formula parenterally. He found that the gut and mucosal weights, DNA and protein contents and sucrose activity were all significantly greater in the animals receiving enteral nutrition. Since there were similar though smaller differences in sections of intestine that were defunctionalized, it is possible that there is both a topical effect from food and an effect that is mediated through the vascular system. It is clear that the deprivation of enteral feeding has an adverse effect upon the intestinal mucosa. The exact mechanism of this effect is unknown. An effect of dietary purine content is an intriguing possibility. 30 3. A Trophic Effect of Pancreatic Secretions Upon the Intestinal Mucosa The primary cause of the loss of small intestinal mass which is consistently reported during starvation and during total parenteral nutrition continues to be debated. Ecknauer et al. (1977) created self-emptying blind loops of intestine which although defunctionalized contained the pancreatico-biliary ducts. They re- ported that after thirty-two days the defunctionalized loops did not lose any mucosal mass when compared to control sections of intestine. When compared to the controls the defunctionalized loops had similar protein and DNA content per dry weight, and similar protein/DNA ratios. The loops in vivo had normal galactose ab- sorption but showed low sucrase and normal maltase activity compared to the control. This would suggest a trophic effect of either the pancreatico-biliary secretions or a trophic effect of a hormonal factor released secondarily to eating. Altmann (1971) transplanted a small segment of duodenum along with the duodenal papilla to various parts of the small intestine. He found that the presence of pancreatico-biliary secretion resulted in significant villous enlargement. In addition, he found that the major portion of the villous-enlarging influence was from the pancreatic secretions rather than from the bile. Hughes et al. (1978) report that they were able to prevent the small intestinal atrophy caused by total parenteral alimentation by infusing daily CCK and secretin. They were, however, unable to dis- tinguish an intrinsic hormonal effect upon the intestine from a secondary effect of either or both hormones acting primarily to stimulate pancreatic secretions. Thus, one might expect that any adverse effect from the exclusion 31 of intraluminal nutrition might be at least partially offset as a result of pancreatic enzymic digestion of sloughed intestinal epithelial cells. It is, however, well established that intravenous hyperalimentation has an adverse effect upon pancreatic mass and results in decreased pancreatic secretions Hughes et al., ton et al., (Kotler and Levine, 1979; 1978; Hughes et al., 1977; Towne et al., 1973; Hamil- 1971; Dudrick et al., 1970; Johnson et al., 1977). Accordingly, it is plausible that the loss of intestinal mass as a result of dietary deprivation may in large part be due to a deficiency of dietary purines or more specifically to a deficiency of intraluminally-available purines. In protein-free diets where cell size remains normal (Munro and Goldberg, 1964; Hill et al., 1968) the dietary intake may provide enough pancreatic stimulus to allow utilization of endogenous purines (and protein) available from the sloughed mucosal cells. On hyperalimentation the decreased pancre- atic protein secretion is inadequate to allow for the necessary reutilization of the endogenous purines (and proteins). Whether the deprivation of the intraluminal supply of nutrients via total parenteral nutrition has its adverse effect upon intestinal mucosal integrity as a primary effect or as a secondary effect through failure to stimulate hormonal secretion or as a combination of these is unclear. It seems, however, well established that intra- luminal nutritional factors have a distinct role to play in the adequate maintenance of the gastrointestinal tract. It is possible that under certain circumstances intestinal mucosal cells are able to utilize endogenous proteins and purines, The degree to which the intraluminal protein and purines are available might ultimately affect cell turnover. Hershfield and Kern (1969) have shown that in the protein-depleted rat the mucosa made better use of orally- 32 administered amino acids than did the mucosa of normal controls. Thus, one must conclude that amino acids from protein digested in the gut are preferentially used for mucosal cell renewal and -that a similar situation may exist for the endogenous purines. In summary, it is established that pancreatic secretions have a trophic effect upon the intestine. effect is unknown. The mechanism of that That the endogenous purines liberated as a result of the action of pancreatic ribonucleases are responsible for the trophic effect of the pancreatic secretions is another intriguing possibility. B. Purine Metabolism and Diet If the small intestine is incapable of providing purines via the de novo synthetic pathway (MacKinnon and Deller, 1973), then the actual source of the preformed purines becomes a very significant question. There is evidence that purines are transported from one tissue to another. Studies by Pritchard et al. (1970) and by Lerner and Lowy (1974) suggest that adenosine is exported from the liver. Adenosine levels in hepatic venous blood were reported (Pritchard et al., 1970) to be ten times greater than those in the portal and arterial blood. The work of Pritchard appears to establish adenosine as the major utilizable purine compound exported from the liver. None of these studies contain data concerning the quantitative aspects of purine supply. Burnstock (1977) states that rat lung takes up large amounts of adenosine. This finding raises the possibility that the lung is an active site of purine metabolism. Should this be so, the importance of hepatic venous adenosine will have to be reassessed. 33 The small intestine is ideally suited to make use of ingested purines aRd to reuse digested desquamated mucosal cell purines. It may be that under certain conditions endogenous purines as well as protein might be made available to the -mucosa in significant amounts. Ju and Nasset (1959) estimate total endogenous protein in man to be of the order of 64 - 263 grams per day. Specifically, they estimate 71 - 91 grams per day of protein from desquamated cells. Even at this lower level there would be approximately 2 grams of available purine bases potentially liberated from the shed mucosal cells. The avail- able pancreatic ribonucleases adsorbed to the small intestinal membranes could, therefore, (even on a purine-free diet) make a significant amount of purines available to the intestine. It is well established (Clifford et al.,1976; Zollner and Griebsch,1974; Zollner and Griebsch,1973; Rauch-Janssen et al.,1977; Zollner, 1973; Griebsch and Zollner,1974) that increased dietary intake of purine bases, purine nucleotides or nucleic acids results in increased uric acid output. Grobner and Zollner (1977) found that after ten days of an iso-energetid, purine-free diet, serum uric acid and urinary uric acid excretion reached a minimum and then remained constant. Addition of purines yielded a linear relationship between dietary purines and serum uric acid level and urinary uric acid. They concluded that their results were "consistent with a theory that purines or purine containing compounds absorbed from the gut exert little or no influence on purine biosynthesis...". 34 This "nitrogen balance" type of approach to purine metabolism seems inadequate with regard to the actual metabolic events that occur as a result of dietary intake of purines. et al. Clifford (1976) clearly demonstrated that, despite the great biochemical similarity of the various purines, they produce different alterations in uric acid metabolism when administered Oral hypoxanthine, AMP, GMP, IMP, and to human subjects. adenine elevated serum uric acid levels while guanine and xanthine did not. Hypoxanthine, AMP, GMP, and IMP produced a greater hyperurecemic effect in subjects with gout compared with hyperureceicanfidnormoureceiic controls. Urinary uric acid levels were increased equally by all purines except for guanine which did not alter urine uric acid levels. Bowering et al. (1969) studied the influence of different dietary levels of protein and yeast RNA on uric acid metabolism in humans. They found that urinary urea, uric acid, alpha-amino nitrogen, and ammonia varied directly with protein intake, but the percentage contribution of individual compounds to total nitrogen excretion differed at each dietary nitrogen level. The high protein and RNA diets produced identical urinary uric acid excretion but only RNA increased serum urate levels. used 1 5N The authors labeled uric acid with their different dietary regimens. The increased uric adid excretion with the high protein diet appeared to be the result of a two-fold increase in the uric adid turnover rate without an alteration in urate pool size. The similarly-increased uric acid excretion with the RNA feeding was the result of a very slight increase in the turnover rate 35 and a 100% expansion of the miscible pool size. Their data do not appear to support a concept of inhibition of purine synthesis by exogenous nucleic acid. It does not, however, demonstrate that the regulation of purine metabolism is strongly interrelated with the nutrition of the organism. This is consistent with the findings of Clifford et al. (1972). They found that de novo purine synthesis in the livers of male rats was considerably stimulated by proteincontaining meals and conversely underwent a decrease following a twelve-hour period of fasting. In addition protein-con- taining meals caused a small decrease in the concentration of free adenine and guanine mucleotides in the liver and fasting resulted in a transient increase in these nucleotides. These nucleotides, however, were eliminated as factors regulating activity in the de novo pathway because they occurred some hours after alteration in purine biosynthesis was evident. A close correlation between alterations in purine biosynthesis and changes in the state of polysome aggregation was observed. They suggest that amino-acid-dependdnt alterations in poly- some aggregation determine the rate of RNA breakdown and that an unidentified product of RNA degradation regulates purine biosynthesis. Sonoda and Tatibana (1978) studied the meta- bolic fate of pyrimidines and purines in dietary nucleic acids ingested by mice. labeled diet. Animals were fed a 14 C-adenine RNA- The adenine was rapidly absorbed with 85% excretion in eight hours. Most of the exogenous nucleic acid bases appeared to have been removed by both degradation 36 and utilization by the intestine and liver. With increase in dose of dietary nucleic acids, the amount of labeled purine was increased in all tissues examined, but it was always greatest in the intestines. Not only do the authors document the active utilization of the ingested bases by the intestine, but in addition, they strongly suggest a limited capacity of the liver and gastrointestinal tract to remove exogenous nucleic acid precursors. This would mean that with a large dose of purine or pyrimidines the contribution to tissue synthesis of dietary nucleotides and nucleic acids might be significant. The question of the actual extent of the utilization of purines derived from the diet continues to be debated. et al. Roll (1949) reported that approximately 1% of the nucleic acids of the combined viscera were derived from fed to rats. Burridge et al. 1 5 N-labeled RNA (1976) fed labeled yeast RNA to mice and found 0.26% of the administered radioactivity in the body tissues at six hours. They noted that dietary nucleic acid adenine appeared to be utilized somewhat more efficiently than was dietary nucleic acid guanine. Berlin et al. (1968) found a large amount of xanthine oxidase activity in the intestinal epithelial cell layer. Furthermore, they found that hypoxanthine, xanthine and uric acid were actively secreted in vitro into the lumen. offered an extra-renal excretory mechanism for urates. This They utilized isolated sacs of hamster small intestine in their experiments and warn that in vivo, in the presence of an active blood circulation, absorption may be significantly increased. The net balance of these factors in vivo needs experimental 37 evaluation. Pritchard et al. (1970) documented that the liver supplies purines to other tissues. In the introduction to their paper they state, "the source of preformed purines has not been conclusively established. They are not derived from the diet, since mammals may be maintained indefinitely on purine-free diets. Moreover, dietary studies and in vitro examination of the intestinal transport mechanism indicate that purines may, in fact, be secreted rather than absorbed into the gut." They cited Berlin (1968) as their reference regarding purine secretion in the gut. Five years later Pritchard et al. (1975) again referred to the poor absorption of purines from the intestine and also referred to the possibility of purines being secreted as purine wastes by the intestine. Wilson and Wilson (1962) studied absorption of purine ribonucleotides by everted rat and hamster intestinal sacs. They concluded that ribonucleotides do not appear to be absorbed as such. Rather the mucosa was capable of metabolizing them along the following pathways: >guanine 2'(3') GMP & 5'GMP -->guanosine --. 2'(3') AMP & 5'AMP -o adenosine -- inosine -* xanthine ->uric hypoxanthine acid, -4 xanthine --- uric acid. They further reported that the absorption of the nucleosides and bases appeared to occur via passive diffusion. Khan et al. (1975) could demonstrate no active transport mechanism for uric acid, hypoxanthine or xanthine in rat or hamster jejunum. Berlin and Oliver (1975) in an excellent 38 and comprehensive review of membrane transport of purine pyrimidine and bases and nucleosides in animal cells conclude that purine bases and nucleotides seem to have their transport mechanisms well established, and appear to enter animal cells by facilitated diffusion. Transport carriers are clearly separate from the enzymes responsible for subsequent intracellular metabolism. Harms and Stirling (1977) studied the transport of purine nucleotides and nucleosides by in vitro rabbit ileum. Their data indicate that at least 85% of the They terminal phosphate of ATP is hydrolyzed during transport. postulate hydrolysis of ATP followed by a preferred uptake of the released adenosine at the membrane surface as explanation of their data. They provide support for a specific transport path for the hydrolyzed nucleotides and for the nucleosides. In summary, the literature on the role of dietary purines No in purine metabolism has stated that they are unimportant. attention however has been given to the specific effect of purines on the intestine. In addition no consideration has been made of situations where increased utilization or preceding depletion of purines have been a factor. This latter point is extremely relevant to the study of disease states. C. Intestinal RNA Metabolism Amano et al. (1965) concluded that significant RNA synthesis occurs only in the crypts. They base this determination upon auto-radiographic analysis after injection of adult male rats. 3 H-cytosine This is consistent with the findings of in 39 Fontin-Magana et al. (1970) and Herbst et al. (1970) who report that the enzymes of the pyrimidine biosynthetic pathways are located primarily in the crypts. Zaharko et al. (1977) noted that in mouse small intestine there is an anti-purine effect upon DNA synthesis at high methotrexate plasma concentrations. Altmann (1974) injected 3H-thymidine prior to injection of high dose methotrexate in normal adult male rats. He then maintained the high levels of methotrexate while following the labeled DNA in the small intestine over several days. He observed that while the crypts became partially emptied the labeled cells continued to move up the villi at a At 48 hours the entire villus contained only near normal rate. the older labeled cells. He concluded that since the metho- trexate inhibited RNA synthesis the surviving cells must have stored the RNA necessary for migration, differentiation, and protein synthesis. He felt that the length of cell survival was linked to the amount of stored RNA. Of related significance is the clinical observation of Craft et al. (1977) who reported on eighteen children with acute lymphoblastic leukemia who were on various treatment regimens, all of which included methotrexate. They noted that a progressive and significant increase in malabsorption (as determined by xylose absorption test) directly correlated with the cumulative methotrexate dose. Nakayama and Weser (1972) measured pyrimidine biosynthetic enzyme activity after proximal intestinal resection. They found an increase in total enzyme activity which appeared to correlate with increased crypt length and cellularity and with 40 a general increase in the total proliferative population of cells (as measured by methyl 3 H-thymidine incorporation). The increase in the enzyme activity was taken to reflect an increase in total nucleic acid synthesis. In support of this conclusion Nakayama and Weser (1972) reported findings in the small intestine showing an increase in the percent of glucose metabolized via the pentose phosphate pathway at three and at seven months after a 50% resection. Studies (Beaconsfield and Reading, 1964; Beaconsfield and Rainsbury, 1964; and Beaconsfield et al. 1965) have shown a definite relationship between the rate of the pentose phosphate shunt pathway activity and RNA synthesis. The literature suggests that RNA synthesis occurs entirely (or at least mostly) in the crypt cells. No studies to determine if isolated intestinal cells or cell nuclei are capable of RNA synthesis have been performed. Thus, the ability of the enterocytes to continue to synthesize RNA (and new proteins) as they age and migrate to the villus tip has not been adequately assessed. The role of purines (and dietary purines in particular) in RNA synthesis in the non-crypt enterocyte thus cannot be adequately evaluated. D. Adenine Metabolism Ely and Ross (1950) studied the effect of adenine on rats. They found that a dietary level of 0.17% adenine caused some enlargement of the kidneys and a reduction in the size of the liver and thymus. The maximum level that had no influ6nce on growth was 0.33% adenine. Higher levels reduced growth in addition to causing enlargement of the kidneys and reduction of the size of the liver and thymus. They noted crystalline deposits in the 41 rats' renal tubules at the doses they used. Clifford and Story (1976) studied the metabolic effects of several purines on rats. Male rats were fed purified amino acid diets supplemented with adenine, guanine, hypoxanthine, and xanthine at a level of 0.75gm/100 gm diet. Guanine, hypoxanthine, and xanthine did not affect the growth rate or the patterns of purine excreted in the urine. Adenin& suplem6ntation at that level resulted in reduced growth rate, altered hepatic purine enzyme activities and changes in purine excretion patterns in the urine. Excretion of 2,8, dioxyadenine was first evident at 0.1% adenine Increased urine volume, plasma urea nitrogen and in the diet. kidney 2,8, dioxyadenine were evident at 0.3% dietary adenine. Under the conditions of their work Clifford and Story (1976) concluded that the maximum safe level of adenine in rat diets should not exceed 0.1%. Bendich et al. (1950) showed that the crystalline deposits in the kidney tubules of the rats which were fed high doses of adenine were 2,8, dioxyadenine. They presented evidence to suggest that 2,8,~dioxyadenine was the direct oxidation product of adenine. Philips et al. (1952) studied adenine intoxication in relation to in vivo formation and deposition of 2,8, dioxyadenine in renal tubules. They found that adenine was nephrotoxic as the result of its in vivo oxidation to 2,8, dioxyadenine and the subsequent deposition of the latter as crystalline occlusions in renal tubules. They showed that a threshold amount of adenine had to be reached before deposition began. All other effects previously ascribed to adenine intoxication were probably the result of 42 complications secondary to uremia. Bennett (1953) examined incorporation of adenine-4,6 1 4 C into nucleotides and nucleic acids in C-57 mice. He found that intraperitoneally-administered isotope is rapidly and extensively incorporated into the nucleotides and nucleic acids of the mouse liver, stomach and intestines. Saviano and Clifford (1978) found when they administered various free purine bases that they were extensively absorbed. Approximately 5% was incorporated into tissues and the rest was excreted in the urine. Adenine metabolism in man has been studied by deVerdier et al. (1972). They found that the basal plasma level of adenine varied about a mean of 70 nmole/l. rate was around 30 nmole/minute. The renal excretion During intravenous infusion of adenine about 2% of the dose appeared in urine as adenine and less than 2% as 2,8, dioxyadenine. The authors conclude that adenine was quickly metabolized, primarily to the nucleotides. The results of their oral loading test show that adenine is readily absorbed and appears in the plasma. Following the oral loading test 3.3% of the adenine appeared in the urine as adenine and 4.5% as 2,8, dioxyadenine. Clifford and Story (1976), extrapolating from their rat data, estimated that if 0.1% adenine is the safe intake for a rat then the maximum safe intake for a 70 kg. adult human would correspond to about 3 grams of RNA per day. E. Glutamine Amidophosphoribosyltransferase and De Novio Synthesis Amidophosphoribosyltransferase (E.C.2.4.2.14) catalyzes 43 the reaction whereby phosphoribosylamine (PRA) is formed. There is some evidence that a sub-unit of this enzyme may utilize NH3as a substrate in mammalian cells (Reem, 1974; Sperling et al., 1973). Glutamine, however, is generally considered as the usual nitrogen source (Wyngaarden, 1972) and the major substrate along with phosphoribosyl-pyrophosphate (PRPP) in the reaction: Glutamine + PRPP + H 2 0 Mg++ ) PRA + Glutamate + PP. This is the first reaction that is unique to purine nucleotide biosynthesis and it has been the main focus of attention as far as end-point inhibition of purine biosynthesis de novo is concerned. Various purine nucleotides have been found to act as allosteric inhibitors of amidophosphoribosyltransferase from avian (Caskey et al., et al., 1964), mammalian (Holmes 1973) and microbial (Nierlich and Magasink, 1965) sources. Only limited success with purification has been obtained (Lewis and Hartman, 1978). In spite of a great deal of work, the kinetic and catalytic mechanisms of reaction are still unclear. This only contributes to experimental difficulties that have been encountered in the study of the enzyme. The inhibition of amidotransferase by ribonucleotides appears competitive with respect to PRPP. Caskey et al. Hartman (1963) and (1964) have demonstrated that the enzyme may be totally desensitized to the action of nucleotide inhibitors while retaining catalytic activity. Thus it is clear that the inhibitors bind at regulatory sites which are distinct from 44 sites for substrates. The work of Holmes et al. (1973) estab- lished that in the human there are two forms of enzymes, a 270,000 molecular weight form and a 133,000 molecular weight Glutamine does not appear to influence the change from form. one form to another but PRPP converts the large form to the smaller active form. LaLanne and Henderson (1975) found that the PRPP concentration in mouse liver varies in response to diurnal influences as well as to hormones and drugs that influence carbohydrate metabolism. Clifford et al. cant variation using rats. (1972) did not find such signifiBagnara et al. (1974) studied Ehrlich ascites tumor cells incubated with purine bases. They observed that the inhibition of purine bi6synthesis de novo in that system may be due largely to the decreased availability of PRPP that resulted from its utilization by the purine phosThis would be consistent with the phoribosyltransferases. observed Michaelis constants in that system which are 5pM for adenine phosphoribosyltransferase (E.C.2.4.2.7) (Ellis and Scholefield, 1962), 20-4OpM for hypoxanthine-guanine phosphoribo- syltransferase (E.C.2.4.2.8) (Hori and Henderson, 1966), and 1mM for amidophosphoribosyltransferase (E.C.2.4.2.17) (Bagnara et al., 1.974). LaLanne and Henderson caution that the consequences of the observed elevations of PRPP in mouse liver are uncertain and that they may not lead to marked increases in the rates of any PRPP-dependent reactions. This is because studies in other systems show that the rate of PRPP synthesis readily increases when the concentration of purine bases is increased. Thus the 45 amount of PRPP that is actually available for nucleotide synthesis can be considerably greater than that measured as "free" PRPP (Henderson and Khoo, 1965; Bagnara et al., 1974). While the regulation of nucleotide biosynthesis de novo can be assessed in terms of influences upon the amidophosphoribosyltransferase reaction, it must be remembered that the actual activity of this enzyme in vivo may be regulated by: (1) metabolic effects, which may be remote or proximate, which affect the concentration of either or both substrates (PRPP or glutamine); (2) changes in the concentration of any of the nucleotides which may act as allosteric inhibitors; and (3) factors that actually affect the amount of the enzyme (synthesis and breakdown). F. Hypoxanthine-Guanine Phosphoribosyltransferase; Purine Salvage; Lesch-Nyhan Syndrome Hypoxanthine-guanine phosphoribosyltransferase (IMP: pyro- phosphate phosphoribosyltransferase; E.C.2.4.2.8) (HGPRT) catalyzes the reaction: Hypoxanthine or Guanine + PRPP Mg y IMP or GMP + PP Adenine phosphoribosyltransferase (APRT) catalyzes the reaction: Adenine + PRPP MgJ++ AMP + PP Together they are responsible for the salvage of purine bases. Although the phosphoribosyltransferases are generally referred to as "salvage enzymes" it is increasingly clear that this term is too restrictive. regulatory function as well. They appear to serve an important HGPRT deficiency results in massive 46 overproduction of uric acid. APRT deficiency allows adenine to be increasingly oxidized to its less soluble product 2,8,dioxyadenine. Three possible functions of HGPRT have been suggested (Rosenbloom et al., One is to utilize exogenously 1968). A second role is to act in supplied hypoxanthine and guanine. the re-utilization of purine bases formed during turnover of endogenous ribonucleic acid. A third specific role is to function in a cycle in which adenine ribonucleotides are regenerated via the series of reactions: AMP - IMP - INOSINE - HYPOXANTHINE HGPRT P> AMP Inherited variation in HGPRT first became evident through clinical observations of the Lesch-Nyhan syndrome. In the most severe form of this disease HGPRT activity is almost zero. The clinical syndrome then comprises mental retardation, choreoathetosis, compulsive self-mutilation, aggressiveness, hyperurecemia, hyperuric-aciduria, gout, and uric acid urolithiasis. There are, in addition to partial and complete deficiencies, a range of genetically-heterogenous states with varying traces of residual enzyme activity (McKeran et al., 1975). In the cours-e of studies on patients with Lesch-Nyhan syndrome, Arnold and Kelly (1973) noted that with time each patient seemed to display a wide range of HGPRT activity determinations. They considered that environmental factors might be important and studied the effect of diet upon HGPRT activity in three patients. During dietary purine restriction erythrocyte HGPRT activity increased in all three patients. With resump- 47 tion of either normal purine intake or the addition of only normal The changes adenine intake there was a fall of HGPRT activity. were of the order of sixty to three hundred percent. The changes appeared to be the result of an activation or inactivation of the mutant enzyme, despite a constant amount of immunoreactive HGPRT protein concentration. McKeran et al. (1975), in their studies on the diagnosis of the carrier state for the Lesch-Nyhan syndrome, examined HGPRT activities for several tissues including the jejunum in seven patients with variations in their genotype. Unfortunately, while they comment upon the mucosal morphology of their controls, they make no mention of the mucosal morphology of their subjects. It is, however, clear that the intestinal tissue lacked HGPRT in the severe forms of the disease. G. Intraperitoneal Parenteral Alimentation Since Dudrick's study on total parenteral nutrition (Dudrick et al., 1969), parenteral nutrition has assumed an ever-increasing role in the management of the extremely sick and debilitated patient. Problems remain, however, which inhibit the full development of this therapy. Among these problems are (1) the absence of well-controlled laboratory experiments which could provide a firm metabolic basis for parenteral nutrition and (.2) the absence of methods which would allow widespread pediatric out-patient use of the therapy. The absence of well-controlled laboratory experiments is caused, in part, by the difficulty and high cost of maintaining 48 significant numbers of animals on intravenous parenteral nutrition. Intraperitoneal alimentation is a simpler and less costly technique which would provide the well-controlled experiments required. The use of this pathway for the administration of nutrients has never been studied in a systematic way. The use of intravenous parenteral nutrition at home has proved to be a costly and difficult technique. failed to gain widespread acceptance. As such it has By contrast, with the development of a bacteriologically safe peritoneal access device (Tenchoff and Schecter, 1968) maintenance home peritoneal dialysis is already feasible (Counts, 1974). This indicates that the development of a similar home peritoneal alimentation program would also be feasible. Using the bacteriologically safe access device developed by Tenchoff and Schecter (1968), Counts (1974) carried out chronic home peritoneal dialysis in 12 children ages 2 10/12 years to 15 10/12 years. During 179 dialysis months, peritonitis occurred fourteen times in eight patients for an incidence of less than one episode per twelve dialysis months: four times after a documented break in sterility; three times associated with an exit-site infection; once in a piece of intracath free in the peritoneal cavity; twice during a hospital Herella epidemic; and four times for unknown causes. One patient accounted for more than one-third of all infections. These infections were milder and of a shorter duration than classical peritonitis and treatment was sometimes carried out at home. Petrie et al. (1976) reported on the use of this technique 49 in 37 adult patients with end-stage renal disease. The mean duration of treatment was 14.4 weeks; the longest was 78 weeks. During this time, 27 patients had one catheter, nine had two, and one had three catheters. Peritonitis occurred fourteen times in ten of the 37 patients (27%). Generally, the course of those infections seems to have been benign, as dialysis was continued in all infected patients. is not necessarily contraindicated That peritoneal dialysis in peritonitis is confirmed by Merrill (1963) who further mentions that it has been used within one week of the performance of an appendectomy. These studies suggest that while infection would be a factor in peritoneal alimentation, there is reason to believe that it may occur at an acceptable rate when compared to other techniques of alimentation. Overall, apart from metabolic complications, one must consider the expense of maintaining a patient on a central venous line or even with a peripheral line. Skilled nursing, extensive laboratory facilities, complicated infusing equipment and full-time hospitalization (for infants and children) all contribute to the cost, and this does not include the expense of placing a central line. Although successful home hyper- alimentation is readily carried out in adults (Broviac and Scribner, 1974; et al., Shils, 1975; Jeejeebhoy et al., 1973; Langer 1973) with good results, it is unlikely that with present techniques this can ever become feasible on a large scale for infants and children. It is expected that absorption of nutrients from the 50 peritoneal cavity might be ultimately controlled not by an external infusion pump, but rather by the inherent absorptive characteristics of the tissues. This in turn might make home hyperalimentation for infants and children a realistic alternative to prolonged hospitalization. A reasonable model might follow the one employed in chronic home peritoneal dialysis. Furthermore, a peritoneal catheter would be less "fragile" and subject to displacement than a catheter or needle placed in a vein. Because of the requirements for central venous alimentation, it is clear that only the tertiary care center is able to undertake the challenge and expense of this treatment in children. The flexibility inherent in a system of peritoneal ali- mentation might lessen geographic problems by allowing for adequate follow-up in less specialized secondary care centers. In addition, the economics of this method would likely bring hyperalimentation technology to nations unable to afford the sophisticated technology of central venous alimentation. Furthermore, metabolic considerations may suggest another advantage of the peritoneal route. In central or peripheral intravenous alimentation, large amounts of amino acids and carbohydrates (and possibly fat emulsion) are directly intro- duced into the systemic circulation, the liver being initially by-passed. Whether this has an adverse effect is unknown, but metabolic derangements such as abnormal liver function are very common in the hyperalimentation experience. It is likely, how- ever, that the bulk of the fluid infused intraperitoneally 51 will be absorbed via the portal system, thus placing the liver back in its physiologic position. Most studies of the peritoneal transport have concentrated upon alimentation of substances from the body. been documented about peritoneal uptake. Very little has In 1877, Wegner first discussed the peritoneal surface as a membrane across which The first report of peritoneal dialysis solute could be removed. was by Ganter in 1923. Boen (1961) discussed the kinetics of peritoneal dialysis and briefly mentioned absorption of bicarbonate and glucose. He commented on the use of glucose as a source of energy, but since he carried out only five investigations in four subjects, his data are very limited. Cunningham (1920) studied the effects of intraperitoneal dextrose upon the peritoneal lining of rats. His studies were also very limited, but it appeared to him that none of his rats suffered any ill effects. There was a suggestion that the absorptive capacity of the peritoneum and its contents might have increased with time. Unfortunately, there is no further elaboration of his work. While there are a great many unanswered questions about the use of the trans-peritoneal route, there is no reason to doubt that the peritoneal cavity and its contents are capable of absorbing enough nutrients to sustain life. In the laboratory this may well provide a relatively simple and efficient method of studying parenteral nutrition. value for the human patient. Conceivably it may also be of 52 III. PLAN OF RESEARCH A series of experiments have been performed on rats to determine the importance of dietary purine sources (nucleic -acids) for the metabolism of the mucosal cells of the gastrointestinal tract. Purine nucleotides can be provided within cells by de novo synthesis or by salvaging free purine bases, the key enzymes being phosphoribosylamidotransferase and adenine or hypoxanthine-guanine phosphoribosyltransferase (figure 1). As the diagram shows, both pathways use phosphoribosylpyrophosphate (PRPP) as a major substrate. The relative importance of the synthesis of purine nucleotides by the de novo and the salvage pathways was assessed both by measuring the incorporation of isotopic precursors in each pathway (glycine for de novo synthesis, adenine for salvage), and also by measuring the activities of the rate-limiting enzymes in each pathway (phosphoribosyl amidotransferase for the de novo pathway, hypoxanthine-guanine phosphoribosyl transferase for the salvage route). Section I: (A) In vitro studies of the de novo and salvage pathways. The activity of the de novo pathway was assessed by examining the incorporation of 1C-glycine into the mucosal RNA of the small and of the large intestines. (B) The viability of the excised mucosa was checked in part by examining the incorporation of into the mucosal proteins. 1 4 C-glycine This also served to show 53 that 14 C-glycine pool differences were not the cause of differences in labeling of mucosal RNA. The assumption is made in these experiments that the glycine pool available for protein synthesis is either essentially the same pool as that available for purine nucleotide synthesis or at least varies quickly and proportionately with it. The salvage pathway was assessed by examining (C) the incorporation of 3 H-adenine into RNA utilizing the same techniques as for the de novo pathway. Section II: The fate of oral versus parenteral adenine and adenosine. (A) The intestinal absorption of labeled adenine and adenosine were studied in vivo in rats. (B) The results obtained by gavage feeding (oral) of isotope were compared to those obtained by intraperitoneal injection (parenteral) Animals received either a Purina Rat Chow diet, or a synthetic, agar-based diet which contained no protein and no purine source. Section III: The influence of protein and purine content of the diet. (A) The effect of dietary protein and purines on the de novo pathway in the small intestine was assessed. (B) The effect of dietary protein and purines on the salvage pathway in the colon was assessed. The actual incorporation experiments employed are 54 essentially the same techniques as those performed in Section I. Section IV: The measurement of amidotransferase and salvage activities in the small intestine, colon and liver. (A) Verification of the above incorporation studies was sought by assaying the amount of amidotransferase activity in the small intestine, colon and liver. (B) Modification of an assay for HGPRT activity and use of this assay to assess the effect of dietary purines on HGPRT activity in the small intestine, colon and liver. Section V: Other conditions influencing purine utilization. This group of experiments comprises a heterogenous series of studies which are preliminary in nature but helpful in understanding the data at hand and in suggesting avenues for future research. (A) Studies of a self-emptying blind loop of intestine free of pancreatic secretions. (B) Development of a technique to separate viable enterocytes from villus tips as well as villus crypts, along with results of incorporation studies utilizing this technique. (C) A preliminary assessment of the technique of alimenting laboratory animals via an indwelling intraperitoneal catheter. 55 - A. IV. MATERIALS AND METHODS In Vitro Studies of the De Novo and Salvage Pathways Animals utilized in these studies were male 200-gram white CD rats obtained from Charles River Breeding Laboratories (Wilmington, Massachusetts). On arrival the animals were individually housed in standard screen-bottomed galvanized cages measuring 25 cm X 18 cm X 18 cm. The rats were main- tained on standard Purina Rat Chow diets. with fresh tap water daily. was maintained at 22*C. They were provided The environmental temperature Room lighting was automatically controlled to provide fourteen hours of light and ten hours of darkness. Animals which were operated upon had their food removed approximately six hours before surgery. Animals were fasted for the 24 hours following surgery but were allowed water ad libitum. Ether anesthesia was employed. All animals were sacrificed in the cold room at 4*C by decapitation. Sections of small and large intestine were rapidly dissected and flushed until clear with ice cold KrebsRinger bicarbonate buffer. glass sheet. The tissue was placed on an iced Sections of intestine were everted over a glass rod and then cut into 5 cm lengths. The everted intestinal segments were placed in iced beakers with 10 mls of KrebsRinger bicarbonate buffer in 95% 02/5% C02 atmosphere, and 50 microcuries of either 3 H-adenine or 14 C-glycine. For experi- ments involving mucosal scrapings the intestine was cut longi- 56 tudinally and scraped with a glass slide. The scrapings were placed into a tared flask and weighed. An equal weight of buffer was added and the scrapings were thoroughly mixed by swirling the flask. Isotope was added, microcuries per gram of tissue, or per gram of tissue. either 3 H-adenine, 1 4 C-glycine, 10 25 microcuries Each flask contained either everted sections of tissue from four to six different animals or mucosal scrapings from four to six different animals. In experiments utilizing everted loops, separate flasks were used for each time point. In experiments utilizing mucosal scrapings samples were withdrawn from the same flask at each time point. All samples were incubated at 37*C with gentle agitation for ten, thirty or sixty minutes. At the end of the incubation period samples were precipitated with ice cold 0.6N~~PCA> homogenized and centrifuged. The precipitate was first washed with 95% EtOH, then washed twice with a mixture of EtOH-chloroform (3:1), then with EtOH-ether (2:1) and finally with ether. The residue was dissolved in 0.3N KOH brought to 37*C for one hour to render the RNA acid non-precipitable (Fleck and Munro, 1962). A sample was then placed aside for later protein determination by the method of Lowry (1951). was performed by the method of Burton (1956). DNA determination Incorporation of isotope into protein and purines was determined by neutralizing with glacial acetic acid and counting an aliquot in 10 mls of ACS (Amersham, Arlington HeightsIllinQis}, Then 0.2 ml of 1ON PCA was added to the solution to precipitate the protein and DNA (leaving the hydrolyzed RNA). The pH of the remaining solution was adjusted to pH 7 with PCA and KOH. 57 The RNA content was determined by the method of Fleck and Munro (1962). The solution was then made to 1N HC1 and heated for one hour at 90'C to hydrolyze the RNA to purine bases and pyrimidine nucleotides (Markham and Smith, 1951). An aliquot was evaporated from an evaporating dish on a sand bath. The crystalline purine bases were redissolved in water and spotted on Whatman 3mm chromatography paper. The chromatography was run using a methanol: water (50:25:6:19) solvent. ethanol: HC1: The position of the purine bases was determined using an ultra violet light source. The respective spots were cut into uniformly small pieces and placed in a scintillation vial. To the vial was added 0.5ml of Protosol (New England Nuclear, 549 Albany Street, Boston, Massachusetts 02118) and it was then incubated at 50*C for two hours. The vials were then allowed to cool thoroughly in the cold room, one to two drops of water were added and then 10 ml of toluene PPO-POPOP (12.2 gms PPO, 0.7 gms POPOP, 1 gal. toluene) was added. The vials were placed in tie cold room Tabulations were overnight and then counted the next morning. made of 14 C-glycine incorporation into protein, incorporation into RNA purines, and 3 H-adenine 1 4 C-glycine incorporation into RNA purines. Isotope was obtained from New England Nuclear. was: The adenine NET-350 Adenine (2-3 H), Lot Number, 1014-180, specific activity 23 Curies/mmole. The glycine was: NEC-276 Glycine, Lot Number, 1046-084, specific activity 98.7 millicuries/mmole. Animals who underwent pancreatic duct ligation had this 1 4 C(U)] 58 procedure performed under ether anesthesia. sterile technique was employed. Clean but not After shaving of the animals' abdomens tincture of merthiolate was used to clean the surgical A three to four cm midline abdominal incision was made area. with a sharp-pointed scissors, was readily identified. The pancreatico-biliary duct Two 4/0 silk sutures were placed tightly around the duct just proximal to its entrance into the duodenum. The duct was cleanly transected between the ligatures, just proximal to the distal ligature, with a sharppointed scissors. The intestine was carefully placed back in proper position and the abdomen was closed with a single layer of interrupted 3/0 silk sutures. The entire procedure rarely took more than 20 minutes. Control animals underwent "sham" surgery involving an identical procedure including identification of the pancreatic ducts and manipulation of the area. B. The Fate of Absorbed Versus Injected Adenine and Adenosine Animals utilized in this experiment were 200-gram white male CD rats obtained from Charles River Breeding Laboratories, On arrival the animals were individually housed in standard screen-bottomed galvanized cages measuring 25 cm X 18 cm X 18 cm. The rats were initially placed on Purina Rat Chow diets and tap water ad libitum. Room lighting was automatically controlled to provide fourteen hours of light and ten hours of darkness. The environmental temperature was maintained at 224C. Forty animals were divided into two groups of twenty, one group for each isotope. The animals were further divided 59 into one of two diet groups. The "regular" diet was Purina Rat Chow, the "0% protein" diet was an agar-based synthetic diet mixed as follows: The agar and water were brought to a full boil in a large stainless steel pot. The mixture was allowed to cool in a large stainless steel mixing bowl. When sufficiently cool to allow comfortable holding the salt mixture and carbohydrates were added. This was followed by the addition of corn oil and then the protein source (if any). vitamin mix and choline were added. while electric mixing was proceeding. Finally the All additions were made After all additions were made the entire diet was mixed for an additional thirty minutes. The contents of the diet utilized in this study are tabulated in table 1. Isotope for this experiment was obtained from ICN (Irvine, California). One ml of 3 H-adenine (ICN cat #27001, lot # 853976) containing 1 mC/ml with a specific activity of 55 Ci/mM was diluted to a total volume of 22 cc's with sterile normal saline. One ml of the final solution containing 45 microcuries was either injected intraperitoneally or infused intragastrically via a 5 french polyethylene gavage tube. One ml of 3 H-adenosine (ICN cat #24008, lot # 889572) con- taining 1 mC/ml and with a specific activity of 50.2 Ci/mM was diluted to 22 cc's and one ml of this solution containing 45 microcuries was either injected intraperitoneally or infused intragastrically. 60 Table 1 0% Protein and 0% Purine Diet for Study of the Fate of Absorbed versus Injected Adenine and adenosine Components Casein 267.4 Sucrose 225.4 Dextrin 275.0 Corn oil 150.0 Salt Mix* 40.0 Vitamin Mix** 10.0 Agar Water * 0.0 Dextrose Choline ** Grams/1000 grams dry wt. See table 2. See table 3. 4.0 35.0 1000.0 61 Table 2 Composition of Roger-Harper's Salt Mixture Constituent Gm. % CaCO 3 29.29 CaHPO4 . 2H20 0.43 KH 2PO 34.31 NaCl 25.06 MgSO 4 . 7H20 Fe(C 6 H 5 ) 7 ).6H 9.98 20 0.623 CuSO 4 0.153 MnSO4 H 2 0 0.121 ZnCl 2 0.020 KI 0.0005 (NH4 )6 Mo 7 0 2 4 .4H 2 0 0.0025 Na 2 SeO 3. 5H20 0.0015 62 Table 3 Contents of Vitamin Mixture Constituent Amount (g/Kg and IU/Kg) Thiamine hydrochloride 1.0 Riboflavin 2.0 Niacin 5.0 Vitamin C 20 Pyridoxine 1.0 PABA 10 Biotin 0.05 Ca Pantothenate 5.0 Folic Acid 0.2 Inositol 20 Vitamin B1 2 Vitamin A 5.0 (As acetate) 500,000 IU. Vitamin D 2 50,000 IU. Vitamin E 10,000 IU. Vitamin K Sucrose .005 910.25 63 After receiving the isotope the animals were kept overnight in a special isolation room to prevent radioactive contamination of the facility. The animals were fasted overnight but allowed water ad libitum. Fourteen to twenty hours after they received the isotope they were anesthesized with ether and bled by cardiac puncture. Sera and red blood cells were separated by centrifugation. The liver was rapidly removed and placed on an iced glass sheet. The entire gastro- intentinal tract was removed rapidly and flushed with iced saline until clear and then also placed on an iced glass sheet. The gastrointestinal tract was divided into stomach, proximal small intestine (the 10 cm of intestine just distal to the ligament of treitz), middle 10 cm of small intestine, distal 10 cm of intestine (the 10 cm just proximal to the caecum), caecum, proximal half of the colon and the distal half of the colon. All sections were slit longitudinally and scraped with a glass slide to remove the mucosa. A section of the median lobe of the liver was diced with a sharp-pointed scissors. Mucosal scrapings and liver were placed in 10 X 75 mm glass test tubes and then 5 ml of ice cold 0.6N PCA was added. Each test tube was thoroughly mixed on a vortex mixer and placed on ice. Subsequently the test tubes were spun down and washed in 0.2N PCA. The precipitates were then hydrolyzed in 5 ml of 0.3N KOH at 37*C for 60 minutes. Then 0.2 ml of 10 N PCA was added. The RNA in the supernatant was determined by the method of Fleck and Munro (1962). An aliquot of the supernatant was counted in 64 10 mls of ACS scintillation fluid. CPM/mgm RNA for each sample was determined. C. The Influence of Protein and Purine Content of the Diet Male 200-gram white CD rats were obtained from Charles River Breeding Laboratories. On arrival they were individually housed in standard, screen-bottomed galvanized cages measuring 25 cm X 18 cm X 18 cm. For one week they were fed a standard Purina Rat Chow diet. After this stabilization period they were The animals had access to weighed and assigned to diet groups. tap water which was changed daily. Their food cups were weighed and changed every other day. ature was maintained at 22*C. The environmental temper- Room lighting was controlled automatically to provide 14 hours of light and ten hours of darkness. The diets were mixed three days before the start of the experiment and stored at 4*C in tightly-sealed plastic bags. The diets were mixed as per the procedure detailed in Section B of Materials and Methods (page # 59). listed in table 4. The diet contents are After seven days the animals were weighed and killed by decapitation. Incorporation studies were per- formed as per the procedure in Section A of Materials and Methods, with the following modifications: 1. For each dietary group, proximal and distal sections of both the small intestine and the colon were studied. Mucosal scrapings from four animals were pooled for each such organ section. Thus for each dietary group two incorporation studies 65 Table 4 Composition of Diets Components Diet 1 Diet 2 Diet 3 Diet 4 Diet 5 Diet 6 g/1000 g dry wt. Dextrin 442 442 442 562 562 562 Sucrose 221 221 221 281 281 281 Lactalbumin 180 180 180 0 0 0 50 50 50 50 50 50 Vitamin Mix** 5 5 5 5 5 5 Choline 2 2 2 2 2 2 100 100 100 100 100 100 Adenine 0 1 0 0 1 0 Guanine 0 1 0 0 1 0 Cytosine 0 1 0 0 1 0 Uracil 0 1 0 0 1 0 Yeast RNA 0 0 1 0 0 1 30 30 30 30 30 30 1000 1004 1005 1000 1000 1000 Mineral Mix* Corn oil (wesson) Agar Water * ** See table 2. See table 3. 66 were performed on each organ. Each incorporation study con- sisted of four or five individual samples at different time points. For each of these samples RNA, DNA and protein were determined in addition to isotope incorporation. 2. Animals were killed in the laboratory instead of the cold room. 3. Ten microcuries of 14 C-glycine/gram of mucosa were added to the flasks containing small intestinal mucosa. 4. Twenty-five microcuries of 3 H-adenine/gm mucosa were added to the flasks containing colonic mucosa. 5. After identification of the purine spots on chroma- tography paper the spots were cut out and eluted with 0.1N HCl. An aliquot was taken and O.D. 2 6 3 was determined for adenine, and O.D. 2 7 5 for guanine. Molar extinction coefficients for those bases in O.1N HCl were calculated and micromoles of base per ml were determined. 6. Aliquots were removed directly from'the spectrophoto- metric curvettes and placed in scintillation vials with 10 cc's of ACS and counted. 7. Incorporation was expressed as cpm/micromole base. 8. In the re-feeding experiments incorporation was ex- pressed at cpm/mg RNA. 9. In the re-feeding experiments the mucosa from eight animals was pooled for each incorporation experiment. 67 D. The Measurement of Amidotransferase Activity in the Small Intestine, Colon and Liver Animals utilized in these studies were male 200 to 300 gram white CD rats obtained from Charles River Breeding Laboratories. On arrival the animals were individually housed in standard screen-bottomed galvanized cages measuring 25 cm X 18 cm X 18 cm. They were maintained on standard Purina Rat Chow or on the special diets described below. with fresh tap water daily. maintained at 220 C. They were provided The environmental temperature was Room lighting was automatically controlled to provide fourteen hours of light and ten hours of darkness. Animals were decapitated in the cold room. Their liver and intestines were immediately removed to an iced-glass sheet. The intestinal tract was flushed clear with ice cold saline. The proximal 15 cm of the jejunum and the entire colon were separated. They were slit longitudinally and then mucosal scrapings were obtained with a glass slide. Approximately 1 ml of scrapings was placed in a 10 X 75 mm test tube. A portion of the median lobe of the liver was minced with a sharp-pointed scissors and approximately 1 ml of this material was similarly placed into a test tube. To the tissue in the test tubes was added 2 ml of homogenization buffer (10 mM Tris-HCl pH 7.8, 0.05 mM DTT). The cells and buffer were homogenized with a Potter-Elvehem homogenizer (while still in the iced homogenizer flask) with four strokes for the intestine and approximately twenty strokes for the liver. 27,000 X g for thirty minutes. The mixture was then spun at The supernatant was poured off 68 and kept on ice. The pellet was resuspended in 1 ml of homo- genization buffer and again spun at 27,000 X g for thirty minutes. The supernatant was added to the first fraction. One ml of the combined supernatant was passed through a column containing Sephadex G-25 to remove nucleotides and other small molecular weight compounds. Upon collection from the column the enzyme source was ready for assay. Final conc. in reactionenzyme mixture Reaction mixture: Tris-HC1 pH 8.0 (100mM) 12.5 Il DTT (100mM) 2.5 pl (10 0mM) MgCl 2 PRPP L-Glutamine* Sp.Ac. 45 mCi/mM 25 mM 0.50 mM 2.75 p1 5.5 mM (10mM) 1.5 p1 0.30 mM (0.1 Pc) 0.5 P1l 0.044 mM 0.25 V1 DD H 2 0 20.00 pl 30.00 p1 Enzyme source 50.00 p1 *Glutamine as L-Glutamine (U-1 4 C) Lot # 784273, cat. # 10068, ICN Pharmaceuticals, 2727 Campus Drive, Irvine, California 91664 The amidophosphoribosyltransferase activity was determined. (The general method of Tay et al. (1969) was employed.) The reaction was initiated by the addition of 30 p1 of the enzyme source. The 50 pl were vortexed and placed in a water bath at 30C for 20 minutes. The reaction was terminated by adding to the test tube 0.2 ml of ice cold 2 mM glutamate in 69 80% (v/v) propan-2-ol at O'C. placed on ice. The mixture was vortexed and 100 pl was spotted on a 1 inch wide strip of Whatman 3 mm chromatography paper along with 10 pl of 10 mM glutamine (to serve as a marker). The chromatography paper was run in an ascending system using isopropranol: water (40:2:10) as a solvent. with 0.3% ninhydrin in acetone. 99% formic acid: The strips were dried and sprayed The glutamate spots were cut 1 ml out so as to have an equal amount of paper in each sample. of 0.1N HC1 was added to the cut strips in scintillation vials to elute the glutamate. Fifteen ml of scintillation fluid (ACS) was added and the samples were counted. The counting efficiency was 60% as determined by the channels-ratio method. Control assays were carried out without PRPP and the control rate was subtracted to give the net "PRPP dependent" rate. All assays were carried out in triplicate. E. Additional Studies Related to, or Influencing Intestinal Purine Metabolism 1. Hypoxanthine-guanine phosphoribosyltransferase was assayed by the method of Olsen and Milman (1978) modified as detailed below. 70 Conc. in reaction- 'enzyme., mix Reaction mixture Assay buffer: Tris-HC1 pH 7.8 MgC1 2 DTT *PRPP Hypoxanthine **Hypoxanthine-1 4 C 500 mM 5 mM 20 mM 10 mM 10 Al .6mM 10 pl 10 Pl .luCi DD H 2 0 50 mM 0.5 mM 2.0 mM 1.0 mM .060mM .024mM 10 ;J1 40 il 60 pl Enzyme source: 100 pl *PRPP (Sigma Chemical Company, St. Louis, Mo., cat. # P-9758) **Hypoxanthine (8-1 4 C), Lot # 1025-198, New England Nuclear The reaction is initiated when the reaction mix and the enzyme source are mixed together, vortexed and placed in a 37 C water bath. The reaction is terminated by placing the test tubes in boiling water for one minute. Thirty lambda is then spotted on DEAE discs (Whatman DE-81) and allowed to dry. The discs are then washed three times in 1 mM ammonium formate for fifteen minutes each wash, then washed two times in distilled and deionized water for fifteen minutes each wash, and finally in 95% ethanol for fifteen minutes and allowed to dry. When dry the discs are placed in scintillation vials to which 10 ml of scintillation fluid is then added (Toluene PPO-POPOP; 12.2 gm PPO, 71 .9 gm POPOP, 1 gal. toluene) and counted. Counting efficienty was 82% as determined by the channel-ratio method. All deter- minations were made in triplicate. The effect of a self-emptying blind loop of intestine 2. upon the activity of the de novo pathway was assessed. Animals were prepared as in Section B of Materials and Methods. On the day of surgery animals received 40 mgm/kg of Nembuton i.p. for anesthesia. The animals' abdomens were shaved and washed with tincture of merthiolate and EtOH. Surgery was performed using clean but not sterile technique. A midline longitudinal incision was made three to four cm long with a pointed scissors. The abdominal organs were displayed. The ligament of Treitz was located as a landmark. Approximately 10 cm distal to the ligament of Treitz the intestine was transected with a sharp scissors. The distal cut intestine was sewn with a purse-string invaginating suture. Distal to the transection, 15 cm on the anti-mesenteric side of the intestine, a three to five mm incision was made along the longitudinal axis. The proximal stump was affixed so that its mesenteric and anti-mesenteric borders were transfixed precisely at the angles of the longitudinal incision with guiding sutures. The two suture lines created were then closed with invaginating seromuscular stitches using 6/0 silk suture. The position of the viscera and the respective blood supply was examined and the abdomen was closed with a single layer of interrupted 3/0 silk sutures. 72 Following recovery from surgery the animals were placed back in their individual cages with rat chow and tap water. Two weeks later incorporation studies were performed as outlined in Section A of Methods and Materials. 3. The placement of an indwelling intraperitoneal catheter Most experiments have employed CD male rats weighing 200 to 300 grams. The rats were housed in galvanized suspension cages, one per cage. The animals were anesthetized with ether. A two cm longitudinal incision through the skin was made in the dorsal midline of the neck. A second two cm incision was made through the skin in the midline of the ventral abdominal wall. A 20 cm length of silastic tubing (Dow-Corning o.d. 0.085 inch and i.d. 0.040 inch) which had been fit with a 1 X 1.5 cm rectangular silastic "butterfly" at the proximal end and glued with silastic cement one cm from the end of the tubing was used as the catheter. The distal end was fitted with a silastic grommet to which a silk suture had been sewn. The tubing was grasped firmly in the jaws of a goose-neck forceps which had been tunneled subcutaneously through the abdominal incision until it protruded through the neck incision. The forceps were then withdrawn pulling the catheter through the subcutaneous tunnel. The peritoneum was entered and the catheter tip (with grommet) was placed into the peritoneum. The peritoneum was sewn over the grommet using the attached silk suture as an anchor. abdominal wound was closed. The The incision in the neck was then closed over the butterfly apparatus on the proximal end. One centimeter of tubing was left protruding from the dorsal aspect 73 Two milliliters of normal saline were infused of the neck. through the line into the peritoneal cavity to make certain that the line was patent and a small plug was then inserted in the protruding open end of the catheter. 4. Studies upon different intestinal cell populations Because the crypt cell compartment of the small intestine represents the proliferative zone it was clear that the optimal tissue to use for incorporation studies would be a crypt cell population. Several techniques to obtain differential separa- tion of crypt and villus tip cells have been reported (Weiser, 1973; Culling, 1973; Kimmich, 1970; Harrison and Webster, 1969; Iemhoff, et al., 1970; Imondi, et al., Cornell and Meisters, 1969; Gall, et al., 1971; 1976). The major criteria for the work at hand was to develop a method of reliably obtaining viable cells. Gall, et al. (1977) was utilized. The method of This method was a modifica- tion of the work of Harrison and Webster (1969) and Iemhoff, et al. (1970). The technique was further modified as follows: (1) The isolation solution employed was the Krebs-Ringer bicarbonate solution used in all incorporation studies. (2) During the cell separation the buffer was continuously gased with 95% 02/5% C02. (3) After centrifugation the cells were re-suspended in the same buffer with help of a vortex mixer. (4) An aliquot was treated with Trypan Blue to check for viability. (5) The remainder was used for incorporation experiments. 74 (6) Isotope was added for incorporation studies, glycine 10 pCi/ml of cell suspension and for 1 4 C- 3 H-adenine 25 pCi/ml of cell suspension. (7) The rest of the incorporation protocol was the same as that outlined in Section A of Methods and Materials with the only other exception being that because of the small amount of tissue the DNA assay had to be done by the method of Cerrioti (1955). (8) Cell viability by Trypan Blue exclusion on repeated assays showed that after re-suspension of the pellet approximately 50% of the villus tip cells were viable. approximately 92% of the crypt cells were viable. However, 75 V. RESULTS A series of experiments has been performed on rats to determine the influence of dietary purines (nucleic acids, nucleosides or bases) on the metabolism of the mucosal cells of the gastrointestinal tract. The relative importance of the synthesis of purine nucleotides by the de novo and salvage pathways was examined. 3 H-adenine In order to evaluate the two pathways, (or adenosine) uptake into mucosal RNA was used to measure salvage and 1 4 C-glycine incorporation into RNA was used to measure de novo synthesis. A. In Vitro Studies of the De Novo and Salvage Pathways In the first studies, the procedure of MacKinnon and Deller (1973) was followed. (1) The incorporation of 1 4 C-glycine into the mucosal RNA of everted small intestinal loops was compared to 1 4 C-glycine incorporation into the RNA of finely diced liver tissue. During the sixty minutes of incubation there was a linear seven-fold increase in incorporation into liver RNA. In the small intestine the increase was less than one and one-half times and not statistically different from the baseline reading (table 5, figure 5). (2) The incorporation of 1 4 C-glycine into mucosal RNA was compared between everted loops of jejunum and everted loops of colon. To enhance the specific activity of the isotopic marker and to minimize the possible role that pancreatic proteases and ribonucleases might play, pancreatic duct ligations were performed 76 Table 5 Incorporation of 1 C-Glycine into the RNA of Liver (finely diced) and Small Intestine (everted loops) Time Tissue Liver* 10 148+40 243+60 Small Intestine* 30 (min) 45 60 438+220 757+264 1018+381 335+103 323+85 363+64 Significance p4.01 not sig. w L3 0 0 I '1400 z I RAT LIVER CHOW DIET g1200 E EIOQO CL I I I II I 1 w I I SMALL INTESTINE CHOW DIET z 0800 C- 600 0 z w z 200 A00 0 0 10 I I 30 40 20 MIN UTES INCORPORATION OF I 50 60 0 I I 10 20 I I 30 40 MINUTES 50 I C GLYCINE INTO THE RNA OF SMALL INTESTINAL IUCOSA (EVERTED LOOPS) AND INTO LIVER (FINELY DICED) Figure 5 ij 60 mw 78 24 hours prior to the experiment. In addition some animals underwent sham surgery and served as controls. Although the pancreatic duct ligation resulted in an apparent increase in incorporation into mucosal protein (figure 6, table 6) in both tissues (when compared to the controls), the measurable incorporation into the mucosal RNA was nil (table 7). (3) Using the identical system of everted loops of intestine, the salvage pathway was assessed in the small bowel and in the colon. Again the experiment was carried out with ligation of the pancreatic which underwent sham surgery. duct and there were controls The results (table 8 and figure 7) show that an active salvage pathway is demonstrable in both tissues with the techniques employed. It appears that the demonstrable incorporation can be enhanced by performing pancreatic duct ligation 24 hours prior to the experiment. (4)Because of the relatively low specific activity of the 1 4 C-glycine (98.7mC/mM) and because the incorporation into the protein fraction was so low, the everted loop preparation was dispensed with and mucosal scrapings were utilized for the next set of experiments. When mucosal scrapings were used incorporation of labeled glycine into large intestinal mucosal RNA was readily demonstrable. However, significant incorporation into small intestinal RNA could not be found (table 9 and figure 8). (5) When incorporation into the protein fraction of the mucosal scrapings preparation was assessed, it was evident that incorporation into both the small and the large intestine was significant and similar (table 10 and figure 9). 79 Table 6 Incorporation of 14C-Glycine into Small and Large Intestinal Mucosal Protein (everted loops) after Pancreatic Duct Ligation or Sham Surgery Time of incubation 10 Tissue 30 (min) 60 counts/min/mg RNA Small* Large* Sham 76 94 118 Lign. 66 88 186 Sham 72 132 262 114 204 300 Lign. *n=1 80 300 LIGAT ED z Ld 0 0r CL CONTROL NTESTIN 200 LIGATED SMALL INTESTINE CONTROL p J00 al 0) 10 20 30 40 50 60 MINUTES INCORPORATION OF C GLYCINE INTO SMALL AND LARGE INTESTINAL MIUCOSAL PROTEIN (EVERTED LOOPS) AFTER PANCREATIC DUCT LIGATION OR SHAM SURGERY Figure 6 81 Table 7 Incorporation of 1 4 C-Glycine into Small and Large Intestinal Mucosal RNA (everted loops) after Pancreatic Duct Ligation or Sham Surgery Time of incubation (min) 30 10 Tissue 60 counts/min/mg RNA Small (n=3) Large (n=2) Sham 430+245 855+491 828+309 Lign. 446+187 400+28 414+176 Sham 709+144 1154+171 1105+290 742+109 884+254 Lign. 1035+74 82 Table 8 Incorporation of 3 H-Adenine into Small and Large Intestinal Mucosal RNA (.everted loops) after Pancreatic Duct Ligation or Sham Surgery Time of incubation 10 Tissue 30 (min) 60 counts/min/mg RNA Small* Large* Sham 3836 10825 18525 Lign. 9762 50670 32756 Sham 15934 14649 6321 8665 38075 24954 Lign. *n=1 0 9 qP I SMALL INTESTINE LARGE INTESTINE 15,000 - 16,000 4 zE' z LIGATED LIGATED 1000 10,000 0 N a- a5,000 5,000 60 30 CONTROL 4a CONTROL 10 0~ 30 10 MINUTES MINUTES THE INCORPORATION OF 31 ADENINE INTO rUCOSAL RNA OF EVERTED Loops OF SMALL AND LARGE INTESTINE WITH PANCREATIC DUCT LIGATION AND SHAM SURGERY Figure 7 60 84 Table 9 Incorporation of 1 4 C-Glycine into Small and Large Intestinal Mucosal RNA (mucosal scrapings) Time of incubation 10 Tissue 30 60 Significance (CPM/mg RNA) Small * 1564+684 1209+190 4026+20 6020+85 2750+71 Intestine* Large Intestine* *n = 2 - - 15805+2553 Not sig. pL.01 85 20000 I I I I I I INCORPORATION OF 14C GLYCINE INTO PURINE BASES OF MUCOSAL RNACHOW DIET (mucosal scrapings) z E E Q. U z0 LARGE 0 a. 10000 INTESTINE z z 0j 400 - ISMALL INTESTINE -J -I 2000 10 SI 20 I I 30 40 MINUTES THE INCORPORATION OF RNJA I 50 I 60 C-GLYCINE INTO THE IN MUCOSAL SCRAPINGS OF SMALL AND LARGE INTESTINES Figure 8 86 I Table 10 Incorporation of 14C-Glycine into Mucosal Protein of the Small and Large Intestine (mucosal scrapings) Time of incubation (min) Tissue 10 30 60 counts/min/mg protein Small Intestine* 426 88,720 78,534 Large Intestine* 20,852 27,640 69,504 87 90000 SMALL INTESTINE 83000-- 7000000 400003 0000- 2~0000-. 10000- 10 30 MINUTE S THE INCORPORATION 60 OF lC-GLYCINE INTO I'UCOSAL PROTEIN OF SMALL AND LARGE INTESTINAL UCOSAL SCRAPINGS Figure 9 88 In view of the success in achieving satisfactory levels of 1 4 C-glycine incorporation into mucosal protein when using mucosal scrapings, the failure to accomplish this with the everted loop preparation was attributed to the failure to expose adequate numbers of cells, especially crypt cells, to the isotope in the incubation media. CONCLUSIONS The final conclusion from this series of studies is provided in experiments (4) and (5), the results of which are shown in tables 9 and 10 and in figures 8 and 9. (figure 8) that only a small uptake of 1 4 C-glycine It is apparent occurs into the RNA of mucosal scraping from the small intestine, contrasted with a vigorous and prolonged uptake into the RNA of the large intestinal mucosa. In contrast, figure 9 shows no difference in uptake into the protein of these two mucosal samples. This latter finding implies that differences in labeling of the free glycine pool are unlikely to account for the low uptake of 1 4 C-glycine into RNA. This finding thus confirms and extends the observations of MacKinnon and Deller (1973). B. The Fate of Oral versus Parenteral Adenine and Adenosine Without an active pathway of de novo biosynthesis in the small intestine, the possible use of dietary purine by the salvage pathway was considered. Absorption of adenine and adenosine was examined in two groups of twenty animals each. Two dietary regimens were 89 a regular Purina Rat Chow diet and a synthetic agar- employed: based 0% protein and 0% purine diet. The design of the experiments is illustrated in figure 10. The twenty rats were divided into two groups of ten and then assigned to one of the diets. were given 45 microcuries of After thirty-one days, the animals 3 H-adenine (or 3 H-adenosine) by nasogastric gavage or by intraperitoneal injection. either The animals were fasted overnight and then sacrificed fourteen to twenty hours after receiving isotope. Incorporation of label into the RNA of the entire gastrointestinal tract and liver was measured. activity was expressed as cpm/mg RNA. The tissue radio- Within each tissue diff- erences in incorporation were evaluated by one-way analysis of Between different tissues graphic analysis was em- variance. ployed to illustrate differences as well as two-way analysis of variance and testing for multiple comparisons (Tukey test). Oral isotope - regular diet In animals that were maintained on the chow diet but received 3 H-adenine by gavage the incorporation into the liver was 2600 cpm/mgm RNA. The distal small intestine (2700 cpm/mgm RNA), caecum (2600 cpm/mgm RNA), proximal colon (1500 cpm/mgm RNA), and distal colon (2100 cpm/mgm RNA) all incorporated label to a similar extent. The proximal small intestine (14,000 cpm/mgm RNA), and the mid small intestine (15,000 cpm/mgm RNA) both incorporated the label to a significantly (P( .05) greater extent than did the distal small intestine, caecum, colon or liver (figure 11 and table 11). 90 Study Design for Investigation of the Fate of Absorbed versus Injected Adenine and Adenosine 40 - 200 Gram White Charles River CD rats 3 20 20e H-Adenosine) (H-Ad enine ) 10 10 Regular Chow x 31 days 0% protein synthetic agar based diet x 31 days 5 i.p. isotope 5 p.o. isotope fasted overnight 14-20 hours sacrificed sto ach 1 2 3 - dist. p ox. mid. small intestine caecum dist. pr Ix. colon liver assay RNA assay CPMs/mg. RNA analyze within each tissue by one-way analysis of variance THE DESIGN OF THE EXPERIMENT TO DETERMINE THE FATE OF ABSORBED VERSUS INJECTED ADENINE AND ADENOSINE Figure 10 0 0 0 45000 I I I 40000- I I I I P D REGULAR DIET Po. 3 H ADENINE 35000- z<r 3000025000- E E 20000- 15000 1000050001I S I I P . M D C L THE INCORPORATION OF GAVAGED 31-ADENINE INTO THE GASTROINTESTINAL TRACT OF ANIMALS ON A REGULAR CHOW DIET Figure 11 92 Table 11 3 H-Adenine Incorporation cp/mg 1NA Stan. Small Intestine Prox. Mid. Dist. 1 17421 39941 2 3 4 5 37757 77836 9386 5829 34866 63784 x 30014 +11002 6 7 8 9 10 Caecum Prox. Colon Dist. Colon Liver 6067 3097 1826 3002 2704 3478 2178 1623 1732 2482 30666 5312 7137 7157 2116 2467 2642 2828 1976 3592 1001 3233 60520 +19157 12798 +12049 5864 +1914 2353 +506 2913 +385 2342 +864 2112 +961 35887 43291 20319 14956 16184 17314 5921 16364 16601 28145 4051 24138 10328 9050 1634 1251 1652 7582 1509 2099 1580 5489 3004 784 1114 1343 493 4152 384 2113 1573 2315 2372 2250 2019 3841 2656 1480 2727 x 26128 +12719 14051 +5435 15142 +10408 2726 +2719 2591 +1810 1498 +1538 2125 +323 2544 +885 11 12 0% 13 Prot. 14 Diet 15 287321 41978 152971 25664 17236 11499 14557 9279 9121 11777 9786 9283 12723 10813 47505 12224 7066 9510 12498 5711 29564 56561 16056 8109 69313 32430 21779 53562 6243 14871 7448 8363 - - 126834 +121102 13143 +3483 Oral 0% Prot. Diet Oral Beg. Diet I.P. x I.P. Reg. Diet - 9992 +1224 - 20816 +17811 - 8696 +2982 - 27573 +21258 - 44271 +21288 - - 9231 +3854 - 16 - - - - - 17 18 19 20 8451 25754 43028 87919 4039 4794 6649 3290 1291 1500 7098 6525 1181 1817 5567 9730 4221 1173 1868 1530 1488 2859 1635 1739 841 837 4877 11034 795 863 5244 7805 x 41288 +34142 4693 +1441 4163 +3137 4574 +3945 2198 +1378 1430 +628 4397 +4817 3677 +3451 - 93 A second group of animals (figure 12 and table 12) which were also maintained on a chow diet but which received 3 H-adenosine by gavage had incorporation into the liver of 3200 cpm/mgm RNA. The proximal small intestine (3400 cpm/mgm RNA) -and the mid small intestine (4400 cpm/mgm RNA) both had greater incorporation than did the rest of the gastrointestinal tract but the differences were not statistically significant. (The stomach incorporation was 44,000 cpm/mgm RNA. Parenteral isotope - regular diet A third group of animals maintained on the chow diet and injected intraperitoneally with 3 H-adenine demonstrated incorporation into the liver of 3700 cpm/mgm RNA. exception of the stomach (41,000 cpm/mgm RNA), With the all other tissues had similar levels of incorporation (table 11, figure 13). The average incorporation into the liver in a fourth identical group of animals injected with adenosine was 2000 cpm/ mgm RNA, and similarly the other tissues (again with the exception of the stomach, which was 22,000 cpm/mgm RNA) all incorporated isotope essentially to the same extent (table 12, figure 14). Thus, animals fed a chow diet and injected with 3 H-adenine (or adenosine) demonstrated uniform incorporation in all gastrointestinal tissues except the stomach (see below for discussion of the stomach). The route of incorporation was clearly via the blood stream, after absorption of the intraperitoneal in- jection. Animals on similar chow diets but who received isotope via gavage had similar findings. There was, however, significantly v v 0 0 0 45000 ±38000 40000 REGULAR DIET Po. 3 H ADENOSINE 35000 4 z 30000 0' : 25000 E L E 20000 15000 10000 5000 S P M P D THE INCORPORATION OF GAVAGED 3 H-ADENOSINE D INTO THE GASTROINTESTINAL TRACT OF ANIMALS ON A 0% PROTEIN DIET Figure 12 L 95 Table 12 3 H-Adenosine Incorporation cpmVmg RNA Prox. Dist. Colon Colon Liver 3092 2086 3020 1452 847 2850 2392 1605 3533 1124 5366 2032 1778 1345 2652 4170 4395 5140 2213 2444 9565 +2091 2094 +977 2301 +961 2635 +1599 3673 +1280 6075 2581 3879 3798 7947 995 909 1275 2406 2540 1021 1651 779 792 5645 912 563 706 886 1790 696 1070 860 1071 4477 2232 1721 906 4089 6857 3352 +776 4856 +2138 1625 +787 1978 +2081 971 +979 1735 +1819 3161 +2374 31 50448 32 24795 33 19247 34 152648 35 216835 6266 10817 5021 6851 14313 7620 15324 11766 10558 8593 11584 8668 9818 5815 6273 3947 7333 8230 11505 5730 12299 22181 23706 14322 16227 46753 34081 45677 34408 3755 6852 5925 2569 4008 x 93794 +86797 8654 +3836 10772 +3018 8432 +2420 7754 +3100 16648 +7512 35429 +12297 4618 +1727 36 37 38 39 40 15435 13701 22885 23725 32091 2170 3747 2573 2952 1411 2740 2030 2263 1489 1796 5871 1966 2265 4072 1834 1460 2611 2579 1670 4176 1762 1651 3847 3884 817 3393 7257 2230 5173 1107 2056 2107 2758 2057 1389 x 21567 +7359 2071 +720 2164 +363 3202 +1743 2449 +1072 2393 +1395 3832 +2435 2073 +484 Small Intestine Oral 0% Diet Oral Reg. Diet Mid. Dist. Caecum 66287 126885 117848 84036 48000 32867 17587 73911 6485 5994 10654 7855 12609 7584 9122 10585 +2749 88611 +33497 27369 +2818 26 18462 27 108000 28 47188 29 13365 30 34750 2338 4372 2983 3795 3274 44353 +38019 Stcrn. Prox. 21 22 23 24 25 8872 10202 4628 8826 15396 x x I.P. 0% Diet I.P. Reg. Diet 9 0 4500C I 40000 I I I 0 9 I i I +34000 REGULAR CHOW DIET 35000 z<r I P INJECTED 3H ADENINE 3000025000- E E 20000- 150001000050001- -I s P M D THE INCORPORATION OF INJECTED C D P 3 H-ADENINE INTO THE GASTROINTESTINAL TRACT OF ANIMALS ON A REGULAR CHOW DIET Figure 13 L 0 9 45000 -r 11 -- 40000 -- I I REGULAR DIET I. P INJECTION OF 3 H ADENOSINE 35000 z 0 0 30000 <r- E 25000 E '.0 E 20000 CL U 15000 10000 5000 S P M C D THE INCORPORATION OF INJECTED P 3 H-ADENOSINE D INTO THE GASTROINTESTINAL TRACT OF ANIMALS ON A REGULAR CHOW DIET Figure 14 L 98 (P/ 0.05) greater incorporation of 3 H-adenosine) 3 H-adenine (but not into the proximal and middle small intestine than into the rest of the gastrointestinal tract. Thus, under the conditions of this experiment, adenine was significantly incorporated into proximal small intestinal RNA after absorption from the intestinal lumen. Oral isotope - protein-free diet A fifth group of animals which were maintained on the synthetic agar-based protein-free and purine-free diet but which were treated with 3 H-adenine per os demonstrated a pattern of incorporation that differed markedly from the earlier patterns described (table 11, figure 15). RNA was 2100 cpm/mgm RNA. mgm RNA), Incorporation into the liver Incorporation into the caecum (2400 cpm/ the proximal colon (2900 cpm/mgm RNA), and the distal colon (2400 cpm/mgm RNA) did not significantly differ from the liver. Incorporation into the proximal small intestine (61,000 cpm/ mgm RNA), the middle small intestine (13,000 cpm/mgm RNA) and the distal small intestine (5900 cpm/mgm RNA) were all strikingly (P<0.05) increased above the rest of the gastrointestinal tract. Incorporation into the gastric mucosal RNA was 30,000 cpm/ mgm RNA. A sixth group of animals was maintained on an agarbased, protein-free and purine-free diet but was treated with 3 H-adenosine (instead of 3H-adenine as was the fifth 6 9 0 50000 I i 64000 ±17000 I 0 --- T 0% 45000- PO. 0 I '4 D L PROTEIN DIET 3 H ADENINE 40000- 35000- z 30000 _ E 25000E 0. 0 2000015000IOOOO- 5000II-- 1- S P D M THE INCORPORATION OF GAVAGED I C 3 H-ADENINE P INTO THE GASTROINTESTINAL TRACT OF ANIMALS ON A 0% PROTEIN DIET Figure 15 0 U-1 S SS 50000 I 45000- I i 89000 33000 I I I 0% PROTEIN DIET Po. 3 H ADENOSINE 40000- 35000- z 30000- E 25000E 2000015000OOO5000ki S i I P . M I D I C P I D L THE INCORPORATION OF GAVAGED 3H-ADENOSINE INTO THE GASTROINTESTINAL TRACT OF ANIMALS ON A 0% PROTEIN DIET Figure 16 101 group discussed immediately above). the 3 H-adenosine The group received per os and demonstrated a pattern of incorporation that was essentially identical to that of the fifth group. Specifically, the liver incorporation was 3700 cpm/mgm RNA, the caecum was 2100 cpm/mgm RNA, the proximal colon was 2300 cpm/mgm RNA and the distal colon was 2600 cpm/mgm RNA. corporated extent. 3 H-adenosine All of these tissues in- into mucosal RNA to the same The incorporation into the proximal small in- testine was 89,000 cpm/mgm RNA, into the middle small intestine 27,000 cpm/mgm RNA and into the distal small intestine 9600 cpm/mgm RNA. Parenteral isotope - protein-free diet A seventh group of animals maintained on the synthetic protein-free and purine-free diet received adenosine intraperitoneally. 3H- The pattern of incorpor- ation into the RNA was completely different from any of the earlier experiments (table 12, figure 17). The incorporation into proximal small intestine 0 6 0 50000 I I 6 w w w Tf I 99000 45000- 87000 00% PROTEIN DIET I P INJECTION OF 40000- 3 H ADENOSINE 35000- z 30000- E 25000E CL 0 20000150001oooo 5000[ I S I P I I M D THE INCORPORATION OF INJECTED C 3 H-ADENOSINE I I P D INTO THE GASTROINTESTINAL TRACT OF ANIMALS ON A 0% PROTEIN DIET Figure 17 L w 103 (8700 cpm/mgm RNA), middle small intestine (10,800 cpm/mgm RNA), distal small intestine (8400 cpm/mgm RNA) and caecum (7800 cpm/ mgm RNA) were somewhat greater than the incorporation into the liver (4600 cpm/mgm RNA). The proximal colon (17,000 cpm/mgm RNA) and the distal colon (35,000 cpm/mgm RNA) however both demonstrated markedly greater incorporation than did the rest of the gastrointestinal tract. This unexpectedly high level of in vivo incorporation of nucleoside into the colonic mucosal RNA was essentially mimicked in an eighth group of animals identical to the seventh, except that they received 3H-adenine instead of 3 H-adenosine (table 11, figure 18). COMPARISON OF GROUPS The results of these experiments have been expressed in a series of tables (tables 11 to 12) and graphs (figures 19 to 34) which compare the incorporation of isotope in various tissues within a particular dietary and treatment group. An examination of this data utilizing the four groups of diets within a particular organ was performed, comparing the results by one-way analysis of variance. Stomach In the stomach there is no statistically significant difference in incorporation into mucosal RNA among all groups (figures 19 and 20). Proximal Small Intestine In the proximal small intestine there was a highly significant variance component among the groups for both adenini Rw w qw 50000 w w I I 45000~ 130000 ± 12 1000 40000- v w w I I 0 % PROTEIN DIET I P INJECTION OF 3 H ADENINE I I w I Ii 2 35000- z I 30000- E 25000E 20000- 1500010000 5000[S I P M THE INCORPORATION OF INJECTED D I C 3 1-ADENINE |I P 18 I L INTO THE GASTROINTESTINAL TRACT OF ANIMALS ON A 0% PROTEIN DIET Figure |I D w 105 0 H3 ADENINE 150,000 z< 100,000 50,000 00 8 o 0 00 P.O. O%PROTEIN P.O. REGULAR DIET DIET I.P IP. STOMACH ( MUCOSA) THE INCORPORATION OF 3 H-ADENINE INTO THE MIUCOSAL RNA OF THE STOMACH Figure 19 106 200000 - 0 H3 ADENOSINE - 150.1000 0 z 100,000 - o - 50,000 I. P.O. OT.PROTEIN DIET STOMACH I.P. Po. REGULAR DIET CMUCOSA ) THE INCORPORATION OF 3 1I-ADENOSINE THE MUCOSAL RNA OF THE STOMACH Figure 20 INTO 107 and adenosine. Examination of figures 21 and 22 clearly shows the striking elevation of incorporation when the isotopes are ingested by animals on the experimental diet. Again the results are similar whether the base or the nucleoside is employed. The animals who received adenine even though on the regular diet also had significantly increased incorporation of isotope when the oral route was compared to the parenteral route. Middle Small Intestine The results in the middle small intestine were essentially the same as those for the proximal small intestine but to a lesser degree, being statistically significant only for the adenosine group (PLC0.05) (figures 23 and 24). Distal Small Intestine, Caecum, Proximal Colon, Distal Colon and Liver The distal small intestine (figures 25 and 26), caecum (figures 27 and 28), proximal colon (figures 29 and 30), and distal colon (figures 31 and 32) all have a similar pattern of incorporation to that found in the liver (figures 33 and 34). That is, greater incorporation when label was injected parenterally as opposed to when it was administered per os. CONCLUSIONS The presence of a salvage pathway in mucosa of the small intestine was demonstrated in vivo istration of 3 H-adenine and following the oral admin- 3 H-adenosine, which resulted in uptake of the label by the RNA of the small intestinal mucosa. The finding that this was much greater on a diet devoid of purines suggests that exclusion of a normal dietary source of 108 15000 - -1 0 - H 3 ADENINE 100,000 z cr 0 T 504000 H C 1.P. P.O. 0% PROTEIN DIET PROXIMAL THE RNA I.P. P.O. REGULA R DIET SMALL INTESTINE INCORPORATION OF MUCOSAL t 3 H-ADENINE INTO THE OF THE PROXIMAL SMALL INTESTINE Figure 21 109 150,000 H3 ADENOSINE 0 0 z 100,00 502001 0 I.P. 1.2 RP0. 0% PROTEIN DIET PROXIMAL REGULAR DIET SMALL THE INCORPORATION OF P.O. INTESTINE 3 H-ADENOSINE INTO THE MUCOSAL RNA OE THE PROXIMAL SMALL INTESTINE Figure 22 110 r 60400 ADENINE H3 - 0- - z 40,00 0 - 20000 IP. P.O. REGULAR DIET I.P. P.O. C / PROTEIN DIET MID SMALL INTESTINE THE INCORPORATION OF rIUCOSAL RIJA 3 11-ADENINE OF THE MIDDLE SMALL INTESTINE Figure 23 INTO THE 111 H3 - ADENOSINE 60.00 z 40,000 -0 - 0 - 20O0DO I.P P 0. 0/ PROTEIN DIET I.p P0 REGULAR DIET MID SMALL INTESTINE THE INCORPORATION OF 31-ADENOSINE INTO THE MUCOSAL RNA OF THE MIDDLE SMALL INTESTINE Figure 24 112 H3 ADENINE .. . 20D00 0 0 o - 10,000 CL 0. 0 U - P 0. I.P 0% PROTEIN I.P. P.O. REGULAR DIET DIET DISTAL SMALL INTESTINE THE INCORPORATION OF 3 H-ADENINE THE r'UCOSAL INTO RINA OF THE DISTAL SMALL INTESTINE Figure 25 113 H3 ADENOSINE <20,000 cr 00 o 10,000C-b P0. I.P REGULAR DIET P.O. I.P. 0% PROTEIN DIET DISTAL SMALL INTESTINE THE INCORPORATION OF 3H-ADENOSINE INTO THE MIUCOSAL RNA OF THE DISTAL SMALL INTESTINE Figure 26 114 H3 ADENINE 10,000 5,000 - 1 0) p P.O. 0%PROTEIN DIE T . PO. REGULAR DIET CAECUM THE INCORPORATION OF THE MUCOSAL RNA 3 H-ADENINE INTO OF THE CAECUM Figure 27 115 0 H3 ADENOSINE < 10,0 z 0 0DO 0 -- 00 .P 0/ P 0. 0PROTEIN DIET I.P. P 0. REGULAR DIET CAECUM THE INCORPORATION OF MUCOSAL RNA 3 H-ADENOSINE OF THE CAECUM Figure 28 INTO THE 116 30,000 - 0 H3 ADENINE 20Z0 CL 10,000 -C) .P P.O. 0% PROTEIN DIET PROXIMAL THE INCORPORATION OF PC. I.P REGULAR DIET COLON 3 H-ADENINE THE MUCOSAL RNA OF THE PROXIMAL COLON Figure 29 INTO 117 30000 H3 ADENOSINE <20,00 -8 z 0,00 CL I.P. PO. o % PROTEIN DIET PROXIMAL THE INCORPORATION OF I.P P. O. REGULAR DIET COLON 3 H-ADENOSINE INTO THE MUCOSAL RNA OF THE PROXIMAL COLON Figure 30 118 H3 ADENINE 60,000 - Z 40,000 0 _ 20,000 4,000 I.P P.O. 0%PROTEIN DIET DISTAL THE INCORPORATION OF JIUCOSAL RNA I.P P.O. REGULAR DIET COLON 3 H-ADENINE INTO THE OF THE DISTAL DIET Figure 31 119 H3 ADENOSINE 60000 z cr 40,000 20,000 0 4,000 .p P.O. O/ PROTEIN DIET DISTAL THE INCORPORATION OF MUCOSAL RNJA 0 0 I.P P.O REGULAR DIET COLON 3 H-ADENOSINE INTO THE OF THE DISTAL COLON Figure 32 120 1500 H3 ADENINE - z 10,000 0 0 5200 CL 0 0 I.p 0% PROTEIN T7 T P0. DIET 1.RP - o. REGULAR DIET LIVER THE INCORPORATION OF 3 H-ADENINE INTO THE MUCOSAL RNA OF THE LIVER Figure 33 121 H3 ADENOSINE 0,000 Iz c) 5,000 0 F 18 mTtt LP. I.P. PO. 0% PROTEIN DIET P. 0. REGULAR DIET LIVER THE INCORPORATION OF 3 H-ADENOSINE RNJA OF THE LIVER MUCOSAL Figure 34 INTO THE 122 purines resulted in less dilution of the labeled base. This is accordingly evidence that dietary purines are normally an important source of purines for the mucosa. When the protein and purine depleted animals were studied, there was significant (P L 0.05) utilization of gavaged isotope by the proximal portion of the small intestine. The caecum, colon, and liver demonstrated an amount of incorporation clearly attributable to the vascular supply. The high level of small intestinal incorporation was not the result of an increased extraction by that tissue of vascular purines. This is demon- strated in the studies of identical animals who received isotope by intraperitoneal injection. In these latter studies, the small intestine demonstrated incorporation essentially at the same level as the liver. Hence, the small intestine has no unusual ability to extract vascular purines. Striking, however, is the extraordinary incorporation of vascular purines via the colon. It is concluded that the small intestine has no unusual ability to utilize vascular purines. When compared to the colon, the small intestine even appears defective in its ability to utilize purines from the vascular supply. The unexpectedly large amount of isotope incorporated into the mucosal RNA of the stomach is evidence of an extremely active salvage pathway. The stomach's ability to utilize purines for the vascular supply appears to be the greatest of any gastrointestinal tissue. 123 C. Influence of the Protein and Purine Content of the Diet As a result of the above data, the role of dietary-purine sources in relation to mucosal supply was further explored in mucosa of the small intestine. (1) The effect of dietary protein and purine content upon the activity of the de novo pathway of purine nucleotide biosynthesis in the small intestine was investigated. Groups of four rats were placed on one of six experimental diets (table 4). Diet # 1 was an 18% lactalbumin diet, free of purines; diet # 2 was identical to # 1 except that it contained 0.1% adenine, 0.1% guanine, 0.1% uracil and 0.1% cytosine; diet # 3 differed from diet # 1 in that it contained 0.5% yeast RNA; diets # 4, # 5, and # 6 were analogous to # 1, # 2, and # 3 respectively except that they contained no protein. After seven days on their respective diets, the animals were sacrificed and intestinal mucosal scrapings were utilized 1 4 C-glycine to determine in vitro incorporation of protein and mucosal RNA. into mucosal RNA/DNA and protein/DNA ratios were also measured. The assumption is made that the intracellular pool of 14 C-glycine available for RNA synthesis via the de novo pathway is essentially the same pool from which glycine is incorporated into protein. Furthermore, to the extent that the pools might be different it is assumed that they tionately. vary. quickly and propor- Thus any factor that tiight affect 1 4 C-glycine uptake by the cell will similarly affect incorporation into both RNA and protein. Therefore any effect upon 1 4 C-glycine uptake and 124 resultant labeling of RNA can be eliminated by expressing 1 4 C-glycine incorporation into RNA as a ratio of incorporation of label into RNA/protein. Thus a ratio of 1 4 C-glycine incor- porated into RNA (or RNA purine) to isotope incorporated into protein was used to assess activity of the nucleotide synthesis pathway in the various experiments. Animals on the complete diet lacking only purines demonstrated significantly higher (P L 0.002) incorporation of 1 4 C-glycine into mucosal RNA than did any of the groups of animals on the other diets. The ratio of incorporation into adenine (isolated from mucosal RNA) to protein was similarly significantly higher (P 4 0.01) for the group of animals of diet # 1 (table 13). Omission of purines from the synthetic diets used in these studies resulted in restoration of some in vitro labeling of mucosal RNA adenine from 1 4 C-glycine changes in protein labeling. without corresponding Additionally the RNA/DNA ratio is significantly reduced by adding free purine bases to the diet whereas protein/DNA is reduced on the protein-free diet irrespective of purine sources. smaller. This latter data implies that the cells are The reduction in RNA/DNA on adding purines to the diet could conceivably be the result of stimulation of mucosal cell multiplication by the dietary purines with resulting increased DNA. (2) In another experiment the effect of dietary purine content upon recovery from a protein-free diet was assessed. Three groups of eight rats each were placed on diet # 4, 125 Table 13 Influqoe of Protein and Purine Content of the Diet upon -''C-Glycine Incorporation into Smadl Intestinal Mucosal Protein and Mucosal RNA 1 4 C-Glycine Diet Incorporation RNA-Adenine (cpVumloe) (n=2) Protein (Cxng/n) (n=2) Aden. Incorp. Prot. Incorp. 114750+15900 3200+ 170 35.5+ 3.5 2.30+ .20 26.29+ 3.76 BNA/DNA Prot/DNA (n=8) (n=8) Protein-containing 1. No purines 2. + purine bases 11500+16300 1900+1100 4.4+ 6.2 1.51+ .42 24.86+ 3.98 3. + RNA 21700+13500 3700+ 250 6.5+ 5.2 2.01+ .28 27.65+ 9.94 26300+15800 2500+1100 13.9+11.1 2.12+ .19 23.92+ 3.41 14600+ 4400 5000+1600 3.9+ 0.3 1.48+ .13 16.68+ 6.18 37300+26000 8000+1440 5.0+ 4.2 2.68+1.02 17.28+12.06 p,0.002 not sig. p4.01 pO0.001 Protein-free 4. No purinas 5. + purine ba 6. + RNA Significance s p<O. 05 126 a purine-free and protein-free synthetic diet, for seven days. They were then switched to either diet # 1, @ 2, or # 3 for seven days. Overall The same analyses were carried out as in (1) above. incorporation was much lower in this experiment. When incorporation of isotope into total mucosal RNA was assessed and expressed as the ratio, incorporation into RNA: incorporation into protein, the results were similar to those obtained in (1). Specifically, the incorporation ratio was greater (P = 0.09) in the group that was re-fed the purinefree diet (table 14). (3) The effect of purines and protein in the diet upon the salvage pathway of the colon was assessed. Diets # 1 through # 6 as above were employed and after seven days the animals were sacrificed. Incorporation studies employing 3H-adenine and measuring its appearance as either RNA adenine or RNA guanine were performed. No significant difference in incorporation into either adenine or guanine or their sum could be demonstrated (table 15). (4) The effect of dietary purines and protein upon the refeeding of a protein-depleted animal was assessed. seven days of a 0% protein diet (diet # 4), Following the animals were placed on either diet # 1, # 2, or # 3 for seven days and then had repeat incorporation studies. The incorporation of 3 H-adenine into RNA guanine was not significantly different in the three groups studied (table 16). 127 Table 14 Influence upon Protein and Purine Content of the Diet C-Glycine Incorporation into Small Intestinal Mucosal Protein and Mucosal RNA during Refeeding 1 4 C-Glycine RNA Incorporation Protein Incorporation Protein cnVmg RNA Incorporation RNA cpm/g RNA (n=2) (n=2) 1. Protein diet alone 362+ 16 6076+1774 16.7+0.4 2. Sane + purine bases 588+220 3181+2950 4.8+3.2 3. Same + IqA 409+ 93 2397+1670 5.5+2.8 Not sig. Not sig. Protein-free diet followed by * Significance *7-day refeeding period Not sig. (p=0. 0 9 ) 128 Table 15 Influence of Protein and Purine Content of Diet upon 3 H-Adenine Incorporation into Colonic Mucosal RNA 3 H-Adenine Diet Incorporation into RNA-Guanine RNA/DNA Prot/DNA cpn/umole (n = 8) (n = 8) (n = 2) Protein-containing 1. No purines 13254+ 5741 1.0 +0.4 26+ 9 2. + purine bases 15796+ 3384 1.2 +0.5 27+16 3. + RNA 25721+18344 2.0 +0.9 25+30 8268+ 2740 1.2 +0.2 20+ 6 22749+17673 1.3 +0.5 35+20 9251+ 2210 0.83+0.4 20+10 not sig. not sig. not sig. Protein-free 4. No purines 5. + purine bases 6. + RNA Significance 129 Table 16 Influence of Protein and Purine Content of Diet upon 3 H-Adenine Incorporation into Colonic Mucosal MA during Refeeding* 3 H-Adenine Protein-free diet followed by Incorporation into RNA-Guanine cpm/unnle RNA/DNA (n = 10) Prot/DNA (n = 10) (n = 2) 1. Protein diet alone 3321+3420 0.50+0.16 10.5+2.2 2. Same + purine bases 8951+9044 0.61+0.25 10.4+2.1 12391+4580 0.73+0.32 9.3+3.7 not sig. not. sig. not sig. 3. Same + RNA Significance *7-day refeeding period 130 CONCLUSIONS Animals receiving diets containing purine bases or RNA had low de novo synthesis as judged by 14 C-glycine incorporation into RNA. However, with diets deficient in purines, labeling by the de novo pathway was much increased, especially when the diet contained protein. This is apparently independent of labeling changes in the precursor pool of free glycine, since protein labeling did not show these changes. This suggests that some stimulation of the de novo pathway in the small intestinal mucosa occurs when purine sources are withdrawn from the diet. Free purines may have an additional effect on cell division in the crypt, since the RNA/DNA ratio and the protein/DNA ratio both fall for ahiials fed purines as con'pared to those fed no purines. D. The Activity of the Glutamine Amidophosphoribosyl Transferase in the Small Intestine, Colon and Liver and the Effect of Dietary Purines (1) Validation of the glutamine-amidophosphoribosyl-transferase assay The method of Tay et al. and Methods (page 68) Experiment # 1: (1969) as detailed in Materials was utilized. To test the effects of substrate concentra- tions upon demonstrable liver enzyme activity the concentrations of PRPP and 1 4 C-glutamine were altered. A grid pattern was con- structed varying the amount of the PRPP in the reaction mixture as follows: 0 mM, 0.3 mM, 0.6 mM, 1.2 mM, and 2.4 mM. The gluta- mine was varied utilizing 0.022 mM (0.05 pcurie), 0.044 mM (0.1 131 pcurie), 0.088 mM (0.2 pcurie) and 0.17 mM (0.4 pcurie). The amount of glutamate formed was directly proportional to the amount of glutamine used as substrate at PRPP concentrations (table 17, figure 35) over the entire range of glutamine concentrations employed. The amount of glutamate formed was also directly proportional to the amount of PRPP used up to the concentration of 0.6 mM PRPP. With greater amounts of PRPP the amount of glutamate formed reached a plateau (table 18, figure 36). For the experiments performed 0.3 mM PRPP (figure 37) and 0.044 mM employed. 1 4 C-glutamine (0.1 pcurie) (figure 38) were (Table 19 contains all the data in this experiment and figure 39 represents that data on a single plot.) Experiment # 2: To test the effects of substrate con- centrations upon demonstrable small intestinal enzyme activity, the identical procedure was followed as in Experiment # 1. The amount of glutamate formed was directly proportional to the amount of 1 4 C-glutamate used as substrate at all PRPP concen- trations (table 20, figure 40). The amount of 1 4 C-glutamate formed was proportional to the concentration of PRPP when greater than 0.044 mM (0.1 pcurie) 1 3 C-glutamine was used, and then only when the concentration of PRPP did not exceed 0.6 mM (table 21, figure 41). For the experiments performed 0.3 mM PRPP (figure 42) and 0.044 mM 14C-glutamine (0.1 pcurie) (figure 41) were employed. (Table 22 contains all the data in this experiment and figure 43 represents that data in a single plot.) 132 Table 17 Correlation of Amount of Glutamate Formed per mole Glutamine Used as Substrate at Different PRPP Concentrations in the Liver PRPP conc in reaction mix. r 0.0 mM 0.96 0.3 mM 1.00 0.6 mM 0.98 1.2 mM 0.97 2.4 mM 0.99 L w v w w w uJ 0 40,0001 [PRPP] 2.4 M LIJ 30,0001 M 0.6 0 U- - 20,000 1. 2 m (A (A Iii UOmnM I00001 0.022 mM 0.044 0.088 ELUTAMINE] IN 0.176 REACTION MIXTURE THE CORRELATION OF THE AMOUNT OF GLUTAMATE FORMED PER MOLE GLUTAMINE USED AS SUBSTRATE AT DIFFERENT PRPP Figure 35 CONCENTRATIONS IN THE LIVER 134 Table 18 Correlation of Amount of Glutamate Formed as a Function of PRPP Concentration in Reaction at Different Glutamine Concentrations in the Liver Glutamine Conc. Correlation coef. (r) PRPP concentration 0-2.4 mM 0-1.2 mM 0-0.6 mM 0.022 mM 0.98 0.30 0.56 0.044 mM 0.99 0.95 0.89 0.088 mM 0.78 0.62 0.89 0.176 mM 0.97 0.34 0.46 135 S40,000 -7 GLUTAM IN E z 7 30,000 Li Ce S20,00-- Io,000:- 4 4 pMy- I- 22 PM 2,100 0.3 1.2 0.6 2.4 [PRPP] rnM THE CORRELATION OF THE AMOUNT OF GLUTAMATE FORMED AS A FUNCTION OF PRPP CONCENTRATIONS IN THE REACTION AT DIFFERENT GLUTAMINE CONCENTRATIONS IN THE LIVER Figure 36 136 I I 30,000 LiJ 20,000 -=- r 1.00 L) 22 44 88 176 y M GLUTAMINE IN REACTION MIXTURE THE CORRELATION OF THE AMOUNT OF GLUTAMATE FORMED PER IOLE GLUTAMINE USED AS SUBSTRATE AT A OF ,3mMIN THE LIVER Figure 37 PRPP CONCENTRATION 9 9 8 a-12,500- 0 GL 10,000 U 7,500 - (A-4 0 L 0.3 I 0.6 I 2.4 1.2 mM LPRPP] THE CORRELATION OF THE AMOUNT OF GLUTAMATE FORMED AS A FUNCTION OF PRPP CONCENTRATION IN THE REACTION AT A GLUTAMINE CONCENTRATION OF .014 Figure MW IN THE LIVER 38 138 Table 19 Glutamate Formed as a Function of PRPP Concentration and Glutamine Concentration in Reaction Mixture with Liver Amidotransferase Time (min) Glutamine (mM) PRPP Concentration (mM) in Reaction Mixture 0.0 0.3 0.6 2.4 1.2 counts/min 14C-Glutamate formed 0 15 0.022 1511 2948 1903 2045 2158 0.044 3363 3842 3948 4034 4607 0.088 6511 6895 7500 7034 8767 0.176 12500 12925 14543 13681 12001 0.022 2509 4852 6080 3933 5976 0.044 3189 6983 9024 2509 12584 0.088 7789 15334 14163 14466 22536 0.176 10560 31316 40211 23765 35548 139 40,000- 30,000 - U-j U---- uj 2a,00 M176M 0,010,000 - 0 44 p.M 6000 4,000 2,000 2 M 15 MINUTES 0 [PRPP] 0 0 15 0 PRPP [PRPP] 0.3 1 0.6 0 1 [PRPP 1.2 0 1 [PRPP 2.4 THE AMOUNT OF GLUTAMATE FORMED AS A FUNCTION OF THE PRPP CONCENTRATION AND GLUTAMINE CONCENTRATION IN THE REACTION MIXTURE WITH LIVER AMIDOTRANSFERASE Figure 39 140 Table 20 Correlation of Amount of Glutamate formed per mole Glutamine Used as Substrate at Different PRPP Concentrations in the Small Intestine PRPP conc. in reaction mix. r 0.0 .98 0.3 mM .98 0.6 mM 1.00 1.2 mM 1.00 2.4 mM .99 141 20,000 LPR PP .6mM 0.3m 15,000 1.2m 0 LU A 10,000 0 0mM 0 aL0i 5,000 0.022 0.044 mM 0.088 0.176 GLUTAMINE THE CORRELATION OF THE AMOUNT OF GLUTAMATE FORMED PER MOLE GLUTAMINE USED AS SUBSTRATE AT DIFFERENT PRPP CONCENTRATIONS IN THE SMALL INTESTINE Figure 40 142 Table 21 Correlation of Amount of Glutamate formed as a Function of PRPP Concentration in Reaction at Different Glutamine Concentrations in the Small Intestine Glutamine Conc. Correlation coef (r) PRPP concentration 0-0.6 mM 0-1.2 mM 0-2.4 mM .022 mM 0.21 0.15 0.26 .044 mM 0.99 0.37 0.11 .088 mM 0.91 -0.31 -0.63 .176 mM 1.00 0.02 0.06 143 Li 00 0 LlU GLN] 176 pL M G 20OO =2,000 -j 8 8 p. M- 310,00 4 4 y M ,0 C, 2 2M - 0.3 1.2 0.6 mM 2.4 LPRPP] THE CORRELATION OF THE AMOUNT OF GLUTAMATE FORMED AS A FUNCTION OF THE PRPP CONCENTRATION IN THE REACTION AT DIFFERENT GLUTAMINE CONCENTRATIONS IN THE SMALL INTESTINE Figure 41 144 15,000 U- -LJ r= 100 -S 00 - -. 1000 0.022 0.044 0.088 0.176 GLUTAM INE CONCENTRATION ( m M ) THE CORRELATION OF THE AMOUNT OF GLUTAMATE FORMED PER MOLE GLUTAMINE USED AS SUBSTRATE AT A CONCENTRATION OF .3Mt IN THE SMALL INTESTINE Figure 42 PRPP 145 Table 22 Glutamate Formed as a Function of PRPP Concentration and Glutamine Concentration in Reaction Mixture with Small Intestinal Amidotransferase Time (min) PRPP Concentration (mM) in Reaction Mixture Glutamine (iM) 0.0 0.3 0.6 2.4 1.2 counts/min 14C-Glutamate formed 0 15 0.022 889 1094 1222 983 1171 0.044 1947 1716 2221 2807 2088 0.088 4491 3840 4644 4301 4730 0.176 9207 8607 9434 1027 8615 0.022 1426 2930 1755 2118 2330 0.044 2892 3819 5163 3739 3768 0.088 7599 7767 9313 6692 6339 0.176 11375 16639 20423 12287 15679 146 020,000 0 176 w. LD M -88 6,000 4,000 -44 0 M 2,000 0 15 0 15 0 I5 0 is 0 IS MINUTES EPRPG o [PRPP]O.3 [PRPPj.6 [PRPpl 1.2 [PRPP] 2.4 THE AMOUNT OF GLUTAMATE FORMED AS A FUNCTION OF THE CONCENTRATION AND GLUTAMINE CONCENTRATION IN THE REACTION MIXTURE WITH SMALL INTESTINAL AMIDOTRANSFERASE Figure 43 P RPP 147 Experiment # 3: The PRPP amidotransferase activity was measured utilizing a crude enzyme extract (that had not been passed through the Sephadex G-25 column). Only total enzyme activity (PRPP dependent and independent) activity was measured in both the small intestine and in the liver. Over thirty minutes the reaction was linear (figure 44, table 23). No significant amount of amidotransferase activity was demonstrable in the small intestine. Experiment # 4: The assay was next performed utilizing an enzyme source prepared as in Materials and Methods (page 68). Here the high speed supernatant was passed through a Sephadex G-25 column. PRPP dependent activity was determined and is plotted in figure 45. Data for total activity as well as PRPP independent activity are tabulated in table 24. 14 C-glutamate The amount of formed was linear over the sixty minutes for both liver and colon. There was no significant demonstrable activity in the small intestine. (2) The effect of dietary purines upon amidotransferase activity Experiment # 5: Glutamine amidophosphoribosyltransferase activity was measured in four animals who were on a synthetic agar-based diet free of purines and in four animals on an identical diet with 0.1% of each purine and pyrimidine base added (diets # 1 and # 2 from table 4, respectively). Regard- less of the diet the enzyme activity in the small intestine was found to be significantly lower than in either the liver or the colon (P < 0.025 in the purine-added group and P < 0.005 in the purine-free group by one-way analysis of variance). The colon 148 II I I I I LIVER i wMIT000- C 30 ,00 -0 INTESTINE 0SMALL 10 20 6 4-0 30 40 50 60 MINUTES THE AMIDOTRANSFERASE ACTIVITY (TOTAL ACTIVITY) IN CRUDE TISSUE EXTRACTS FROM LIVER AND SMALL INTESTINE Figure 44 149 Table 23 PRPP Amidotransferase Activity (total activity) in Crude Tissue Extracts Time of incubation (min) 60 30 15 5 cpm 1C-glutamate formed Small Intestine 348 351 510 463 Liver 428 856 1669 1572 150 4,000 X LIVER i3,000 - COLON < 2,000 CD l- 0~0 1000-00 0 SMALL INTESTINE 10 20 30 40 MINUTES 50 60 THE AMIDOTRANSFERASE ACTIVITY (PRPP DEPENDENT) IN DIFFERENT TISSUES Figure 45 151 Table 24 PRPP Amidotransferase Activity (PRPP Dependent) in Different Tissues Tissue Min. Total PRPP PRPP Correlation Indep. Depend. Coefficient counts/min of Small Intestine 0 5 15 30 1464 1612 1602 1713 1361 1429 1491 1509 102 182 111 203 1811 1597 213 0 5 15 30 60 1470 2248 2754 3445 5961 1441 1535 1484 1740 2302 29 713 1265 1705 3658 r =0.99 0 5 15 30 60 1586 2106 2392 3601 5653 1420 1588 1650 1851 2769 166 518 742 1750 2883 r =0.99 60 Liver Colon 4C-Glutamate formed ~ r =0.71 152 and the liver had essentially similar amounts of enzyme activity. The purine-free diet resulted in a decrease in the enzyme activity of the liver from 2.11±0.52 micromoles glutamate formed/gram protein/minute (diet with bases) to 1.27±0.50 micromoles glutamate formed/gram protein/minute (diet without bases). This difference had a p value of 0.06 by t-test. The small intestine had a small (but not statistically significant) increase in activity when switched to the purine-free diet. The colon had a slight (but not statistically significant) decrease in activity when on the purine-free diet. This data is summarized in table 25 and figure 46. Thus, the small intestine contains significantly less amidotransferase than either the colon or the liver. This was found to be true on a purine-free diet (P Z 0.05) and on a purine-added diet (P 4 .025). There was no significant diff- erence in the amount of small intestinal enzyme with the two diets. Similarly, the colon showed no significant change in activity with the diet. On the purine-free diet, however, there was a 66% increase in the liver amidotransferase activity which was significant at the P = 0.06 level. concluded that the increased 1 4 C-glycine Therefore, it is incorporation into RNA observed in the experiment described above is not due to more enzyme molecules but must be due to a change of some factor influencing enzyme activity, i.e., the supply of substrate. As figure 4 shows, this could well be PRPP, the level of which is regulated by utilization in the competing salvage pathway. Thus, the presence of purine in the diet could rapidly reduce 153 Table 25 Amidotransferase Activity Diet Small Intestine Liver Colon Significance umoles glutamate formed/g Protein/min Purine Added 0.064+.025 0.019+.020 0.105+.032 p<0.0 2 5 Purine 0.106+.026 0.020+.014 0.086+.032 p<0.005 not sig. not sig. Free (t test) (n=4) p=.06 9 v 0 0 0 0 0 0.140 LUJ 0.120 0.100 0 N. S. N =4 0.080 PURINE FREE ci: 0.060 0 DIET U- DIET DIETDIET WITH BASES LUJ 0.040 0.020 PURINE FREE DIET DIET WITH BASES WITHt BASES PURINE FREE DIET N.S. N =4 N.NS. N 4 LIVER SMALL INTESTINE COLON THE AMIDOTRANSFERASE ACTIVITY IN DIFFERENT TISSUES AS A FUNCTION OF THE PURINE CONTENT OF THE DIET Figure 46 0 155 the availability of PRPP for the de novo pathway and regulate it as is shown in figure 4. E. The Activity of the Hypoxanthine-Guanine Phosphoribosyl Transferase in the Small Intestine, Colon and Liver and the Effect of Dietary Purines (1) Validation of the HGPRT assay The HGPRT assay was performed as per Materials and Methods (page 70). Experiment # 1: To test the capacity of the DEAE cellu- lose (Whatman DE-81) discs to hold the reaction-enzyme mixture, HGPRT activity was assayed in a crude liver enzyme mix. Ten pl; 20 pl and 40 pl of the original reaction-enzyme mixture were spotted on the discs. The results are illustrated in figure 47 and tabulated along with the relevant correlation coefficients in table 26. The correlation coefficients confirm the linear relationships expected and demonstrate that the capacity of the discs was not exceeded. As a result of this experiment it was decided to spot 30 pl of enzyme-reaction mixture for future experiments. Experiment # 2: Liver HGPRT-activity was assayed utilizing the reaction mixture and enzyme source detailed above. The enzyme source was a high-speed supernatant, passed through a Sephadex G-25 column. The reaction was linear over the sixty minutes (figure 48, table 27). Experiment # 3: The effect of concentration of enzyme in enzyme preparation was assessed in liver, colon, and small 156 11,000 I0000 HGPRT LIVER 8,000 7,000 CPM - 4O r 1.00 6,030 - 4~O0O 4,00=\.0 r =1.00 10 A 3,000 - _ 2,000 1,000, I 20 I 10 30 I 40 I 50 60 MINUTES THE LIVER HGPRT ACTIVITY (USING A CRUDE TISSUE EXTRACT) DETERMINED BY SPOTTING 10 LAMBDA, 20 LArBDA OR 110 LAMBDA OF REACTION-ENZYME MIXTURE OR DEAE CELLULOSE DISCS Figure 47 157 Table 26 Liver HGPRT Activity (Using Crude Enzyme Preparation) and Spotting 10 Lambda, 20 Lambda, and 40 Lambda of of Reaction-Enzyme Mix on on DEAE Cellulose Discs Minutes 10 Lambda Spotted 20 40 r 0 623 800 1035 0.99 5 834 1190 2130 1.00 15 1371 2026 3415 1.00 30 2193 3171 5565 1.00 60 3417 5763 11032 1.00 1.00 1.00 1.00 2794 4964 9998 (60-0) 1.00 158 5O0C0 400 3.000 HGPRT LIVER = 1.00 -r CPM IWOO 400 10 20 30 50 40 MINUTES THE LIVER HGPRT 60 ACTIVITY Figure 48 70 159 Table 27 Liver Hypoxanthine-Guanine Phosphoribosyltransferase Activity Time (min) Total Minus Blank Minus Zero counts/min 14C-Hypoxanthine converted to nucleotide Blank 617 (0) - 0 800 183 (0) 5 1190 573 390 15 2026 1409 1226 30 3171 2554 2371 60 5763 5146 4963 160 intestine. The assay was performed using five ul, ten ul, and twenty ul of enzyme preparation. The time points chosen were 0, 2, 5, 10, 15, 20, and 30 minutes. The results are illustrated in figures 49, 50, and 51, and tabulated in table 28. The reaction is linear over 30 minutes for the liver, small intestine and colon when 5 ul of the enzyme source is used (figure 49). When 10 ul of the small intes- tinal enzyme source is used the reaction reaches a plateau after 15 minutes. When 10 ul of the liver enzyme source is used a plateau is reached by 5 minutes (figure 50). When 20 ul of the small intestinal enzyme source is used the reaction reaches a plateau after approximately 10 minutes. When 20 ul of liver enzyme source is used the reaction reaches a plateau in less than 5 minutes (figure 51). (2) The effect of dietary purines upon HGPRT activity Experiment # 4: Based upon the foregoing data, the assay was applied to the same animals and tissues that were studied in experiment # 5 of the amidotransferase group. Specifically, a group of animals that had been on a purinefree diet for seven days and a group that had been on an identical agar-based synthetic diet with 0.1% of the purine and pyrimidine bases added were studied. The small intestine was found to have significantly (P < 0.01) more HGPRT activity than either the liver or colon when the animals were on a purine-containing diet. By comparison, when the animals were on a purine-free diet their intestinal HGPRT activity was less while their liver HGPRT activity was increased. The 161 LU LU o 15,000 LIVER 0 0 0 r =0.96 LU Zi 0,000 * SMALL INTESTINE r =1.00 o 5,000 COLON . 2 5 10 x r Is 20 =0.95 MINUTES THE HGPRT REACTION IN DIFFERENT TISSUES USING FIVE LAMBDA OF ENZYME SOURCE Figure 49 30 162 M t-- 0 i 5,-00- LIVER z u-j z SMALL INTESTINE 0 5,000 COLON 0 2 5 10 15 20 30 MINUTES THE HGPRT REACTION IN DIFFERENT TISSUES USING TEN LAMBDA OF ENZYME SOURCE Figure 50 163 9- UJ20,000 L0IVER SMALL INTESTINE 0 0 <I0,000-- I0...00 | S5,000-- COLON 2 5 10 15 20 30 MINUTES THE HGPRT REACTION IN DIFFERENT TISSUE USING TWENTY LAMBDA OF ENZYME SOURCE Figure 51 164 Table 28 The Effect of Concentration of HGPRT Enzyme on Enzyme Reaction in Different Tissues Amt. of Enzyme Time of Incbn. Small Intestine cpm (min) 14 Liver Colon C Hypoxanthine converted to nucleotides 5 pl 0 2 5 10 15 20 30 543+0 1210+71 2246+93 4283+28 6624+20 9060+282 12802+764 r 10 Pl 20 j1 0 2 5 10 15 20 30 *0 0 2 5 10 15 20 30 =1.00 546+39 804+65 1162+240 1633+147 1661+117 2084+234 2287F50 r = .95 1168+80 2869+346 4668+0 8187+326 9991+363 15620+1085 15573F56 r = .96 1083+114 2508+20 4490+187 8307+704 11755+342 16899+725 15397F860 524+106 1178+277 1608F95 2323+45 2986+173 3326+58 36377271 3594+342 7016+76 12221+149 13207+345 14137+620 16412+163 16712F2976 r 3 0 = .94 r 2 0 =1.00 r 3 0 = .95 r20= .90 r 1 5 = .99 r 5 =1.00 r 10= .92 r 1 5 = .89 r 2 0 = .91 r30= .87 552+66 1737+221 5495+139 9571+586 13057+864 15023+554 14529+1894 15061+1328 555+174 848+18 2030+3 3266+649 3888+23 3839+200 4567+102 5145+13 570+3 10423+3748 14206+578 12342F869 13008F822 11400+2454 14770+202 11395~404 r r 1 5= r20= r 3 0= .98 .0= .96 .91 .84 r5 = r1 0 = r 1 5= r 2 0= r 3 0= .99 .94 .88 .90 .90 r5 * Enzyme boiled before addition to reaction mixture. .41 165 data are tabulated in table 29 and illustrated in figure 52. In each organ for each animal a ratio of HGPRT;amidotransferase activity was computed. For the liver (n = 2) the ratio was 46 ± 1 in the group fed the purine-containing diet. In the group fed the purine-free diet the ratio was 130 t 69. In the small intestine (n = 2) the purine-containing group had a ratio of 2413 ± 1326 versus 1081 ± 1326 for the purinefree group. These were not statistically significant at the P = 0.05 level. There was only a minimal change in the colon. On the purine-free diet (n = 4) the ratio was 62 t 31 and on the purine-containing diet it was 52 ± 26. The data are tabulated in table 30. Thus in the liver the purine-free diet appears to be associated with a shift towards increased salvage as compared to de novo synthesis. In the small intestine the purine-free diet is associated with either a shift toward de novo synthesis or at least a shift away from salvage. These results are completely consistent with the results obtained in the incorporation studies reported above. 166 Table 29 The HGPIRI Activity in Different Tissues as a Function of the Purine Content of the Diet Diet Liver Small Colon Significance Intestine pmole glutamate formed/g protein/min (anova) Purine added 4.06+ .47 9.45+0.85 5.59+0.54 p(.01 Purine free 5.62+2.43 7.90+1.4 5.06+1.82 not sig. (t-test) not sig. not sig. not sig. qp 0 Vw w w Vw z N.S. N=2 Uj N.S. N=2 -- 0 cr 0 w N.S. N4 - 0~ IT 0 BASES 9-j _ t z PURINE FREE DIET 0 - DIET WITH ~ BASES PURINE FREE DIET LIVER DIET WITH BASES SMALL INTESTINE THE EFFECT OF DIETARY PURINES ON IN DIFFERENT TISSUES Figure 52 PURINE FREE DIET COLON HGPRT ACTIVITY 168 Table 30 Ratio of HGPRT Activity/Amidotransferase Activity in Different Tissues Diet Purine Free Diet Purine Added Diet Liver S.I. Colon (n=2) (n=2) (n=4) 130+69 46+1 Not sig. 1091+986 - 62+31 2413+1326 - 52+26 - Not sig. Not sig. 169 THE RESULTS OF PRELIMINARY STUDIES VI. A. The Effect of a Self-Emptying Blind Loop of Intestine on the Activity of the De Novo Pathway A self-emptying blind loop of intestine that excluded pancreatic secretions was studied two weeks after surgery. An adult male 300 gm rat that had been on a regular Purina Chow diet was operated upon and continued to gain weight normally after surgery. In this single experiment there appears to have been unequivocal incorporation of 3 H-adenine 1 4 C-glycine into RNA purines. incorporation was not unequivocally demonstrated, the actual values in fact showing a high initial value with decreased values over the time course of the experiments (table 31). B. Studies of the Different Intestinal Cell Populations Experiment # 1: Animals in this study were 250 gm white male CD rats obtained from Charles River Breeders and were maintained precisely as in the other studies above. mals were on Purina Rat Chow diets. The ani- They were not fasted be- fore killing. On the day of the experiment following decapitation the small intestine was rapidly dissected and flushed until clear with normal saline at 370 C. The entire small intestine be- ginning approximately 10 cm distal to the ligament of Treitz was utilized. It was cut into four sections approximately 15 cm long each. The segments were everted and shaken as 170 Table 31 14 C-Glycine into Mucosal Incorporation of RNA-adenine and into Mucosal Protein in a Self-emptying Blind Loop of Intestine Time (min) 10 30 60 90 RNA (cpm/)imole adenine) 5345 15206 68125 35882 Protein (cpm/mg 1094 1046 2042 2835 Incorporation protein) 171 described by Gall et al. (1977). The results are illustrated in figure 53. It is evident that labeling in the acid precipitable pool (which is mainly protein and nucleic acids) is much greater in the fraction of cells obtained from close to the crypts. Histologic examin- ation revealed that after shaking there was still an appreciable amount of cells left in the crypts although many crypts were empty. The data are expressed as cpm (in acid insoluble fraction) per mg protein in figure 53 and table 32 and as cpm per mg DNA in figure 54 and table 33. Experiment # 2: This study was carried out the same way as Experiment # 1 except that only the specific incorporation of both 1 4 C-glycine mined. The results demonstrate that there is a distinct and 3 H-adenine into RNA purines was deter- gradient of purine salvage activity increasing in the cells closer to the crypts (figure 55 and tables 34 and 35). Examin- ation of the data on de novo activity shows that there is an approximate doubling of incorporation into the cells of the the intermediate and crypt fractions as compared to the villous fraction (table 34). It is not certain that this represents meaningful incorporation via the de novo Dathway. C. Effect of Catheter Placement and Normal Saline Infusion Experiment # 1: Two groups of animals were used. The test group was prepared as per Materials and Methods, section E. The other group served as an unoperated control. The test group received two bolus injections of 5..5 cc's each of normal saline 172 200,000 CRYPT CELLS z S100,000 INTERMEDIATE 60.00040,000- TIP CELLS 20,000- 10 20 30 40 MINUTES 50 60 THE INCORPORATION OF LC-GLYCINE INTO THE PROTEIN AND THE NUCLEIC ACIDS IN DIFFERENT INTESTINAL CELL POPULATIONS (EXPRESSED AS COUNTS/ MIN/MG PROTEIN) Figure 53 173 Table 32 14 C-glycine into The incorporation of the protein and nucleic acids in different intestinal cell populations Cell Population Time of incubation 10 30 (min) 60 counts/min/mg Protein Villus tip cells 14530 13490 14386 Intermediate cells 82010 76780 75030 Crypt cells 81000 178210 190830 174 3co0o0 00- ~20,000 CRYPT CELLS x 10000 - x 2,000 TIP .ELeS 10 THE INCORPORATION OF 30 MINUTES 1 4 C-GLYCINE 60 INTO THE PROTEIN AND THE NUCLEIC ACIDS IN DIFFERENT INTESTINAL CELL POPULATIONS (EXPRESSED AS COUNTS/MIN/MG Figure 54 DNA) 175 Table 33 The incorporation of 14 C-glycine into the protein and nucleic acids in different intestinal cell populations Cell Population Time of incubation (min) 10 30 60 counts/min/mg DNA Villus tip cells Crypt cells 480 1000 1300 10000 12700 19000 176 3 H-ADEN INE ' 4 C-GLYCINE o CRYPT CELLS a INTERMEDIATE CELLS * TIP CELLS 1,500 <\,000 z 500 a_ 4 00 300 300 200 100 200 100 10 20 30 4050 60 MINUTES THE INCORPORATION OF THE RNA 3 H-ADENINE 10203040 50 60 MINUTES AND 1 4C-GLYCINE INTO OF DIFFERENT INTESTINAL CELL POPULATIONS Figure 55 177 Table 34 Incorporation of 14C-Glycine into the RNA of Different Intestinal Mucosal Cell Populations Cell Population 10 Time of incubation 30 (min) 60 counts/min/mg RNA Villous tip 54 48 74 Villous intermediate 60 75 185 Villous crypt 55 - 164 178 Table 35 3 Incorporation of H-Adenine into the RNA of Different Intestinal Mucosal Cell Populations Cell Population Time of incubation 10 30 (min) 60 counts/min/mg RNA Villous tip cells 105 255 340 Intermediate cells 160 710 850 Crypt cells 440 1655 1750 179 per day. Both groups were allowed a complete synthetic agarover 18 days. based diet ad libitum There was no significant difference in the average daily food intake of the animals, or Histopathology as well as gross path- in their daily weights. ology was normal. There was no significant difference in their serum albumin levels (table 36). The Two groups of animals were used. Experiment # 2: test group was prepared as above. an unoperated control. The other group served as The test group received two bolus injections of 19 cc's each of normal saline for fourteen days. (Diets were as above in Experiment # 1.) the animals were sacrificed. After fourteen days Weight gain expressed as weight gained per day, liver weight and spleen weight at autopsy and serum albumin on day fourteen all showed no significant differences between the groups. Histopathology as well as gross pathology was normal (table 37). Experiment # 3: Both Two groups of animals were used. were prepared as per Materials and Methods, section E. Both were placed on similar synthetic agar-based 0% protein diets. The groups were pair-fed so that their oral intakes were comparable 2). (34 kcal/day for group 1; and 32 kcal/day for group In addition animals in group 1 received 3 grams of crystalline amino acids injected as two bolus injections of 19 cc each per day. Animals in group 2 received the same volume of normal saline. Animals in group 1 lost 10% of their initial weight compared to 18% for group 2 (P < 0.05). The actual daily 186 Table 36 The Effect of Intraperitoneal Catheter Placement and Normal Saline Infusion (5.5cc twice a day) Init. Wt. Final Wt. Change in wt/day Average Daily Food Intake Serum Albumin Day 18 (gm) Control 369+48 469+112 5.56 47+10 3.5+.24 362+54 465+60 5.72 45+10 3.3+.17 not sig not sig not sig not sig (n = 4) Injected (n = 3) Sig.A not sig 11 Table 37 The Effect of Intraperitoneal Catheter Placement and Normal Saline Infusion (19cc twice a c-y) Init. Wt. Final Wt. Change in Wt/day Wt. Liver Wt Spleen Wt. Serum Day 14 Day 14 Albumin (gm) Day 14 (gn) Control (n = Injected Sig. 394+42 465.5+54 62.5+12.9 4.46 15.19+2.26 .81+.12 3.36+.21 383+59 463 79.8+14 5.70 16.92+1.72 .91+.13 3.33+.19 not sig. not sig. 8) +49 not sig. not sig. not sig. not sig. not sig. 182 weight losses averaged 2.59 gms for group 1 and 5.49 gms for group 2 (P < 0.01) (figures 56 and 57). When sacrificed the serum albumin of group 1 averaged 2.89 gms versus 2.63 gms for group 2 (P < 0.02) (figure 58). The liver weights for the two groups were 13.1 gm (group 1) and 10.2 gm (group 2) (P < 0.05) (figure 59). Despite the hypertonicity of the amino acid solution (approximately 780 mosm/1) the only consistent adverse tissue reaction observed was some focal fibrosis directed to the silk suture that was placed to anchor the catheter grommet in the peritoneum. Experiment # 4: Two groups of animals were used. Both groups had intraperitoneal catheters placed at least one week prior to the beginning of the experiment. Both groups re- ceived an agar-based synthetic diet that consisted only of water, agar, anhydrous dextrose, and NaCl. Group "AA" (amino acid infused) received approximately 1.7 grams of amino acids as crystalline amino acid solution, divided into two bolus injections. The other group "GLU" (glucose infused) received an equivalent amount of glucose in two daily intraperitoneal injections. After fourteen days the animals were matched for total caloric intake. The animals thus had the same total caloric intake, but group AA received 1.7 grams of intraperitoneal amino acids per day and group GLU received approximately the same amount of intraperitoneal glucose. Both groups selected an amount of food that was hypocaloric and therefore their weight loss 183 AVERAGE WEIGHT LOSS PER DAY 5.4 5p <.01 4- 0 -J* 2.59 Il c/) 2- IPAA IPN S THE AVERAGE WEIGHT Loss PER DAY IN ANIMALS RECEIVING EITHER INTRAPERITONEAL AMINO ACIDS OR INORMAL Figure 56 SALINE 184 WEIGHT LOSS 20 18% C!, 10% 101-J 0L THE AVERAGE WEIGHT IPAA P NORMAL SALINE Loss PER DAY (EXPRESSED AS PERCENTAGE OF INITIAL WEIGHT) IN ANIMALS RECEIVING EITHER AMINO INTRAPERITONEAL ACIDS OR NORMAL SALINE Figure 57 185 SERUM ALBUMIN N.S. Oak 4 3 ap1 c 3.57 H1 p <.02 2.89 3.28 - IPAA PNS 2f 2. C, 1 0 S Ills', 2 0 CONTROL 4 6 8 DAYS 10 12 14 THE SERUM ALBUMIN OF ANIMALS RECEIVING EITHER INTRAPERITONEAL AMINO ACIDS OR NlORMAL SALINE 186 LIVER WEIGHT 20 p <.05 15 13.1 0% 0 10.2 10 I 5 0LNO PROT DIET IPAA NO PROT DIET IPNS THE LIVER (WET) WEIGHT OF ANIMALS RECEIVING EITHER INTRAPERITONEAL AMINO ACIDS OR NORMAL SALINE Figure 59 187 was expected. Group AA (amino acid infused), however, lost only 56% as much weight as group GLU (glucose infused). was significant at the P < 0.01 level. This All parameters are listed in table 38. Experiment # 5: Concurrent with the running of groups AA and GLU in Experiment # 4 two additional groups were studied: group "C" and group "S". Group C received a diet of NaCl, glucose, and amino acids and never had any surgery. Group S received the same diet as group C except that NaCl was omitted. In addition group S received normal saline via intraperitoneal injection twice a day. The original goal was for all four groups to have matched intakes of NaCl and glucose with the key variable being intraperitoneal amino acids. The animals in groups C and S consistently ate more than those in groups AA and GLU. Therefore the pair feeding aspect of The data for group C and the entire study was invalid. group S are listed in table 39. After completion of the above study incorporation experiments were performed on groups C, AA, and GLU to assess 1 4 C-glycine uptake into RNA. Table 40 summarizes the data. Biologically significant incorporation via the de novo pathway is present in the group receiving the agar-based, amino acid, glucose diet. The level of incorporation in the other groups was essentially at baseline. A one-way analysis of variance utilizing the three groups of data has an F value of 7.96 which is significant at approximately the P = 0.08 level. 188 Table 38 The Effect of Intraperitoneal Amino Acid Infusion versus Intraperitoneal Glucose Infusion Amino Acid Infused Effect Initial wt. Final wt. (gms) (gms) Change in wt. (gms) Oral calories Sig. Glucose Infused 371 +28 393 +21 Not Sig. 295 +18 264 +44 Not Sig. 75 +36 128.8 +37 37 +11 36 p<.01 +12 Not Sig. Not Sig. Intraperitoneal Protein calories/day 6.8 + 0.6 0 Intraperitoneal Glucose calories/day 0 8.7 + 0.8 Total calories 44 +10 44 +11 - Not Sig. Average daily wt. loss 5.36+ 2.56 9.20+ 2.67 p<.01 Serum albumin (Day 14) 3.1 + 0.2 3.0 + 0.3 Not Sig. BUN (Day 14) 7.5 + 2.7 6.0 + 2.2 Not Sig. 9.4 + 1.1 10.7 + 0.6 Not Sig. Liver weight (Day 14) (gm) Spleen weight (Day 14) (gm) 7.0 + .23 .84+ .05 Not Sig. 189 Table 39 The Effect of Control Diets upon Intraperitoneal Study Control Animals Effects Unoperated Controls (C) Operated & Saline Infused Controls (S) 63.0 + 5.0 54.0 +4.0 Average wt loss/day 2.1 + 0.8 2.6 +1.0 Serum albumin 3.72+ 0.38 2.73+0.29 Total Calories BUN 11.9 + 1.2 11.5 +1.9 Liver weight 11.3 + 1.4 10.4 +1.4 Spleen weight Interperitoneal saline infused/day/gm .64+ 0.07 - .73+0.13 21.9 + 1.9 * 9 9 qP 10 4P w Table 40 Incorporation of 1C-Glycine into Small Intestinal Mucosal RNA and Protein in Parenterally Alimented, and Control Fed Animals Total Protein Average wt. loss per day (gms) Serum albumin day 14 Incorp into Protein day 14 cpm/mg Prot Oral Diet I.P. Infusion Total Calories Unoperated Controls Amino Acids Glucose NaCl (purine free) none 63 2.2 Amino Acid Infused Glucose NaCl (purine free) amino acids 44+10 ~ 1.7+0.1 ~ 5.4+2.6 ~ 3.1 +0.2 Glucose Infused Glucose NaCl (purine free) glucose 44+11 0 9.2+2.6 3.0 + 0.3 Sig. 2 3.72 BUN Incorp into RNI Purines cpn'mg IA 11.9 6639+54 43520+14730 7.5 2162+653 3450+ 4879 1300+285 8468+10800 +2.7 6.0 +2.2 p<.-08 '~0 0 191 This data shows incorporation of 1 4 C-glycine into RNA when the animals were on an amino-acid-containing but proteinfree diet and is consistent with the previous data. 192 VII. DISCUSSION AND CONCLUSIONS A series of experiments has been performed on rats to determine the influence of dietary purines (nucleic acids, nucleosides or bases) on the metabolism of the mucosal cells of the gastrointestinal tract. Purine nucleotides can be provided within cells by de novo synthesis or by salvaging free purine bases, the key enzymes being phosphoribosyl amidotransferase and adenine or hypoxanthineguanine phosphoribosyl transferase. Both pathways use phosphoribosyl pyrophosphate (PRPP) as a major substrate. In order to evaluate these two pathways for purine nucleotide formation, 3 H-adenine (or adenosine) uptake into mu- cosal RNA was used to measure salvage and 1 4 C-glycine in- corporation into RNA was used to measure de novo synthesis. Experiments were conducted when the animals were on an unrestricted chow diet. Initially, everted loops of intes- Pancreatic duct ligation was performed tine were utilized. with the expectation that this would decrease the available pool of endogenous glycine and adenine. poration of 4C-glycine and 3 H-adenine The actual incorinto the mucosal cells was enhanced, but no significant incorporation of 14C-glycine into small intestinal RNA was demonstrable. Therefore, the pancreatic duct ligation was not performed in other experiments. The everted loops of intestine were replaced by mucosal scrapings. The use of scrapings was found to offer 193 the most reliable system for obtaining adequate incorporation of isotope into cells. This was probably because with the use of scrapings an increased concentration of cells, especThe mucosal ially crypt cells, was exposed to the isotopes. scrapings were employed for the incorporation studies reported. De novo purine synthesis by small intestinal mucosal cells incubated in vitro with 1C-glycine was shown to be very slight in contrast to the brisk de novo incorporation by colonic mucosal cells. In contrast, uptake of 1 4 C-glycine by the mucosal protein was equally active for the small and large intestine and continued throughout 30 to 60 minutes of incubation, an indication of continuing viability. The experiments thus show the absence of an active de novo pathway in the small intestine of rats on a normal diet and its presence in the colonic mucosa. The final conclusion from this series of studies is shown in tables 9 and 10 and in figures 8 and 9. apparent (figure 8) that only a small uptake of It is 14 C-glycine occurs into the RNA of mucosal scrapings from the small intestine, contrasted with a vigorous and prolonged uptake into the RNA of the large intestinal mucosa. In contrast, figure 9 shows no difference in uptake into the protein of these two mucosal samples. This latter finding implies that differences in labeling of the free glycine pool are unlikely to account for the low uptake of 1C-glycine into RNA. This finding thus confirms and extends the observa- tions of MacKinnon and Deller (1973). 194 Since there was no active pathway of de novo biosynthesis, the use of dietary purines by the salvage pathway was considered. Animals on chow diets who received isotope via gavage provided evidence of salvage of dietary purines. There was significantly (P < 0.05) greater incorporation of 3 H-adenine (but not 3 H-adenosine) into the proximal small intestine than into the rest of the gastrointestinal tract. Thus adenine was incorporated into proximal small intestinal RNA after absorption from the intestinal lumen. When animals deprived of dietary protein and purine were studied, there was significantly (P K 0.05) greater utilization of both 3 H-adenine and 3 H-adenosine by the proximal and middle portions of the small intestine when compared to the rest of the gastrointestinal tract. The incorporation of label into the caecum, colon and liver was attributable to salvage of purines derived from the vascular system. The high level of small intestinal incorporation when isotope was fed was not the result of an increased extraction by that tissue of vascular purines. This is demon- strated in the studies of identical animals who received isotope by intraperitoneal injection. In these studies, the small intestine demonstrated incorporation essentially at the same level as the liver. Thus the finding that the uptake of label by the RNA of the small intestinal mucosa was more extensive on a diet devoid of purines suggests that exclusion of a normal dietary source of purines resulted in less dilution of the labeled base. This is accord- 195 ingly evidence that dietary purines are normally an important source of purines for the mucosa. Animals fed a chow diet and injected with 3 H-adenine (or adenosine) demonstrated uniform incorporation in all gastrointestinal tissues except the stomach (see below for The route of incorporation discussion of the stomach). following absorption of the intraperitoneal injection was clearly via the blood stream. Animals fed a purine-free and protein-free diet and injected with isotope demonstrated markedly elevated incorporation into the RNA of the colon. Thus, intraluminal purines are utilized by the small intestine for RNA synthesis. On a protein and purine deficient diet, the small intestine is capable of deriving a highly significant amount of purines for RNA synthesis from the intestinal lumen. The small intestine extracts less purines for RNA synthesis from the vascular compartment than does the colon, and indeed the colon has a special ability to extract vascular purines for RNA synthesis. As a result of the above data, the role of dietary purine sources in relation to mucosal supply was further explored in the mucosa of the small intestine. The effects of six diets, all identical except for protein and purine contents, were studied with regard to the activity of the de novo pathway, and the data was analyzed by two-way analysis of variance and the Tukey test. When animals were fed a complete synthetic diet without purines 196 there was a restoration of some in vitro labeling of 14 C-glycine without corresmucosal RNA adenine from ponding changes in protein labeling. This change in purine labeling through de novo synthesis could be due to less dilution by a reduced pool of free adenine nucleotides when purines are absent from the diet, but such a large change seems unlikely. A more probable cause is an increase in de novo biosynthesis when purines are excluded from the diet and a near obliteration in the activity of this pathway when they are available from the diet. The supply of purines thus regulates de novo synthesis. Specifically, 14 C-glycine (via the de novo pathway) the incorporation of in the group fed the adequate protein but no purine diet was significantly (P ( 0.05) greater than the incorporation in any of the other groups. Detailed analysis showed that this was the result of three significant effects: (1) the effect of the protein content of the diet (P < 0.001); (2) the effect of the purine content of the diet (P( 0.01); and (3) the interaction effect between the two (PK(0.01). The exa'mination of the RNA/DNA ratio by the same methods as above reveals that there is a demonstrable purine effect (P 4, 0.001) but no significant protein or interaction effect. Multiple comparison testing, however, shows that the only significant (P ( 0.05) differences were between the animals fed the protein-free and purine-free diet on the one hand and both groups (with and without protein) receiving the purine bases on the other hand. If this represents a true 197 biological phenomenon rather than chance causing an elevation of the RNA/DNA ratio in one group, then an explanation will demand further work. Similar analysis of the protein/DNA ratios revealed a significant (P < 0.05) variance component as a result of the dietary-protein content. Multiple comparison testing utilizing the Tukey test and the Student-Newman-Kuel's test, however, revealed no significant differences among the group means. The lower ratios in the protein-free diets containing purine bases or RNA are suggestive of a possible biological effect. It is however only speculation that they represent a true decrease in cell size. This speculation is not entirely unwarranted if based upon the premise that the dietary purines have a role to play in the regulation of cell turnover. The increased cell turnover in the presence of protein deprivation would result in a reduction in cell size. This decrease in cell size however would be offset, at least in part, by the increased number of young, large cells. (Intestinal cells decrease in size as they age and migrate up the villus.) After animals had been fed a protein-free and purinefree diet for seven days, the effect of re-feeding with a protein-containing diet, varying in its purine content, was evaluated. The de novo activity was demonstrated only in the group re-fed on a purine-free diet (P = 0.09). The lower level of statistical significance is attributed to the small number of experiments performed and the resultant decreased degrees of freedom available in statistical analysis. 198 No significant effects of the various diets on the salvage pathway in the colon was demonstrated in either the feeding or re-feeding studies. The demonstration of the presence of a weak de novo pathway in the small intestine when purines were excluded from the diet 5uggested that the key enzyme in de novo purine biosynthesis, amidophosphoribosyl pyrophosphate transferase, might be present in low concentrations but inhibited under normal dietary conditions. In an effort to verify this and to try to determine possible mechanisms by which this would be explained, a method for the assessment of the enzyme in liver and small intestine was developed. of Tay et al. (1969) was employed. assay was confirmed. The general method The validity of the The small intestine was found to con- tain significantly less amidotransferase than either the colon or the liver. This was true on a purine-free diet (P / 0.005) and on a purine-added diet (P 4 0.025). There was no significant difference in the amount of small intestinal enzyme between the two diets. Similarly, the colon showed no significant change in activity related to the diet. On the purine-free diet, however, there was a 66% increase in the liver amidotransferase activity which was significant at the P = 0.06 level. cluded that the increased Therefore, it is con- 1 4 C-glycine incorporation into RNA observed in the experiment described above is not due to more enzyme molecules but must be due to a change of some factor influencing enzyme activity, i.e., the supply of sub- 199 strate. As figure 4 shows, this could well be PRPP, the level of which is regulated by utilization in the competing salvage pathway. Thus, the presence of purines in the diet could rapidly reduce the availability of PRPP for the de novo pathway and regulate it as is shown in figure 4. Measurements of the hypoxanthine-guanine phosphoribosyl transferase demonstrated a very active salvage pathway in the small intestine. Significantly more (P4( 0.01) HGPRT activity was found in the small intestine than in either the liver or the colon when the animals were being fed a diet containing purines. Animals on a purine-free diet showed opposite enzyme -activity changes in the small intestine and liver. The liver HGPRT activity increased on the purine-free diet, while the small intestinal activity decreased. These latter studies just failed to reach statistical significance probably because of the limited number of animals involved. Preliminary studies have been done to explore certain aspects of purine metabolism in the mucosa of the small intestine. The de novo pathway appears to be more active when diet and digestive juices are excluded from a selfemptying blind loop of small intestine, In contrast, in- corporation by this pathway was inhibited in the mucosal atrophy caused by parenteral feeding. Of considerable im- portance was the finding of inactivity of the de novo pathway in all cell types (crypt as well as villus), indicating that possible selection of villous cells in the mucosal preparation is not the reason for the failure to observe an 200 active de novo pathway. It is thus shown: (1) Under normal dietary con- ditions, no de novo pathway for purine biosynthesis is demonstrable; (2) The small intestine utilizes dietary purines for RNA synthesis; (3) The colon utilizes intravascular purines to a much greater extent than does the small intestine; (4) The purine content of the diet has an effect upon the ability to demonstrate an active de novo pathway of purine biosynthesis, the pathway being demonstrable only on a purinefree diet; (5) There is low amidotransferase activity in the small intestine compared to either the colon or liver and it does not significantly change with alterations in the purine content of the diet; (6) There is a very active salvage path- way in the small intestine. In conclusion, the data reported here show that the diet is a major source of purines for the mucosa of the small intestine, and that the de novo pathway is not a major source of purines on diets containing purine bases as such or as nucleic acids. However, a modest increase in this pathway (without enzyme induction) can be achieved by eliminating dietary sources of purines. This allows the existing low-level of PRPP amidotransferase to act more efficiently, probably through having more available substrate (PRPP) because less is used for salvage. It is also possible that on the purine-containing diet there may be more free nucleotides present resulting in a greater level of allosteric inhibition. 201 This work confirms that of MacKinnon and Deller (1973) and goes significantly beyond their study. A new area for the study of intestinal metabolism has been opened. The results presented suggest many new questions for further work. 202 VIII. 1. SUGGESTIONS FOR FUTURE RESEARCH The essential findings of this work should be confirmed in the human gastrointestinal tract. 2. Studies of intestinal cell turnover should be done on diets of varied purine content. 3. The effect of dietary purine content upon the mucosal hyperplasia that occurs after intestinal resection must be examined. 4. The relationship of dietary purine content to the development of carcinoma in the stomach, small intestine and colon should be examined after feeding carcinogens. 5. The relationship between dietary purine content and purine biosynthetic enzyme activities in the liver and gastrointestinal tract should be further studied. 6. During total parenteral nutrition the effect upon the intestinal mucosa of both feeding and infusing purines should be studied. 7. The clinical effects of defined formula diets devoid of purines should be studied. 8. The use of purines in tissue culture media should be studied in relation to efforts to culture mature enterocytes in vitro. 203 REFERENCES Altmann, G.G. 1971. Influence of bile and pancreatic secretions on the size of the intestinal villi in the rat. Am. J. Anat. 132:167-178. Altmann, G.G. 1974. Changes in the mucosa of the small intestine following methotrexate administration or abdominal x-irradiation. Am. J. Anat. 140:263-279. Amano M., Leblond CP., Nadler, NJ. 1965. Radio-autographic analysis of nuclear RNA in mouse cells revealing three pools with different turnover times. Exp Cell Res. 38:314-340 Arnold, W.J. and W.N. Kelley. 1973. Dietary-induced variation of hypoxanthine-guanine phosphoribosyl transferase activity in patients with the Lesch-Nyhan syndrome. J. Clin. Invest. 52:970-973. Bagnara, A.S., A.A. Letter and J.F. Henderson. 1974. Multiple mechanisms of regulation of purine biosynthesis de novo in intact tumor cells. Biochim. Biophys. Acta. 374:259270. Beaconsfield P. Grinsburg, J. Kosinki Z. 1965. Glucose metabolism via the pentose phosphate pathway relative to cell replication and immunological response. Nature. 205:50-51. Beaconsfield, P. and Rainsburg, R. 1964. Reduction de la synthese des acides nucleques par inhibition de la metabolique de pentose phosphate. CR Acad Sui (Paris) 259:2729-2732 Beaconsfield P., and Reading HW. 1964-. Pathways of glucose metabolism and nucleic acid synthesis Nature. 202:464466. Bendich, A., Brown, G.B., Philips, F.S. and Thiersch, J.B. 1950. The direct oxidation of adenine in vivo. J. Biol. Chem. 183:267-277. Bennett, E.L. 1953. Incorporation of adenine into nucleotides and nucleic acids of C57 mice. Biochim. Biophys. Acta. 11:487-496. Berlin, R.D., and R.A. Hawkins. 1968. Secretion of purines general characteristics. Amer. by the small intestine: J. Physiol. 215:932-941. Berlin, R.D. and R.A. Hawkins. 1968. Secretion of purines transport mechanism. Amer. by the small intestine: J. Physiol. 215:942-950. Berlin, R.D. and J.M. Oliver. 1975. Membrane transport of 204 purine and pyrimidine bases and nucleosides in animal cells. Int. Rev. of cytol. 42:287-336. Boen, S.T. 1961. 40:243-287. Kinetics of Peritoneal Dialysis. Medicine Bowering, J., D.H. Calloway, S. Margen and N.A. Kaufmann. 1969. Dietary protein level and uric acid metabolism in normal man. J. Nutrition. 100:249-261. Brosh, A., Buer, P., Kupfer, B., DeVries, A. and Sperling. 1976. 0. De Novo Synthesis of Purine Nucleotides in Human Per!pheral Leukocytes. Excessive Activity of the Pathway in Hypoxanthine-Guanine Phosphoribosyltransferase Deficiency. J. Clin. Invest. 58:289-297. Broviac, J.W. and Scribner, B.H. 1974. Prolonged Parentheral Nutrition in the Home. Surg. Gynecol. Obstet. 139:2428. Burnstock, G. 1977. In Purine and Pyrimidine Metabolism. CIBA Foundation Symposium 48 (New Series), Elsevier, North Holland. p. 178. Burridge, P.W., R.A. Woods and J.F. Henderson. 1976. Utilization of dietary nucleic acid purines for nucleotide and nucleic acid synthesis in the mouse. Can. J. Biochem. 54:500-506. Burton, K. 1956. Study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. 62:315-323. Cerrioti, G. 1955. Determination of Nucleic acids in animal tissues. J. Biol. Chem. 214:59-70. Clifford, A.J., J.A. Riumallo, B.S. Baliga, H.N. Munro and P.R. Brown. 1972. Liver nucleotide metabolism in relation to amino acid supply. Biochim. Biophys. Acta. 277:443-458. Clifford, A.J., J.A. Riumallo, V.R. Young and N.S. Scrimshaw. 1976. Effect of oral purines on serum and urinary uric acid of normal, hyperuricemic and gouty humans. J. Nutrition. 106:428-434. Levels of purines in Clifford, A.J. and D.L. Story. 1976. foods and their metabolic effects in rats. J. Nutr. 106:435-442. Glutathione and Cornell, J.S. and A. Meister. 1976. y-glutamyl cycle enzymes in crypt and villus tip cells 73:420of rat jejunal mucosa. Proc. Nat. Acad. Sci. 422. 205 Counts, S. 1973. Chronic Home Peritoneal Dialysis in Children. Vol. Trans. Amer. Soc. Artif. Int. Organs, pp: 157-163. Counts, S., Hickman, R., Garbaccio, A., and Tenckhoff, H. 1973. Chronic Home Peritoneal Dialysis in children. Trans. Amer. Soc. Artif. Int. Organs 19:157-163. Craft, A.W., H.E.M. Kay, D.N. Lawson and T.J. MCElwain. 1977. Methotrexate-induced malabsorption in children with acute lymphoblastic leukaemia. Br. Med. J. 2:1511-1512. Culling, C.F.A., P.E. Reid, L.S. Trueman and W.L. Dunn. 1973. A simple method for the isolation of viable epithelial cells of the gastrointestinal (GI) tract. P.S.F.B.M., 142:434-438. Cunningham, R.S. 1920. Studies on Absorption from Serious Cavities. III. The Effect of Dextrose upon the Peritoneal Mesothelium. Amer. J. Physiol. 53:488-494. Dowling, RH. 1974. The influence of luminal nutrition on intestinal adaptation after small bowel resection and bypass in Intestinal Adaptation Ed. by R.H. Dowling, E.O. Riecker, Stuttgart, Schottauer Verlag. pp: 35-46. Dowling, R.H., Gleeson, M.H. Cell Turnover Following Small Bowel Resection and Bypass. Digestion 8:176-190, 1973. Dudrick, S.J., Wilmore, D.W., and Steiger, B. 1970. Spontaneous Closure of Traumatic Pancreatoduodenal Fistulae with Total Intravenous Nutrition. J. Trauma 10:542-553. Dudrick, S.J., Wilmore, D.W., Vars, H.M., Rhoads, J.E. 1969. Intravenous Feeding as the Sole Means of Nutrition Support Growth in the Child and Restore Weight Loss in an Adult? Affirmative answer. Annals of Surg. 169: 974-984. Durand, G., Fauconneau, G., and Penot, E. 1965. E. Croissance des tissues du rat et Qualite des Proteines Alimentares: Influence sur le Nombre et al Tuille des Cellules. Ann. Biol. Anim. Bioch. Biophys. 6(3):389-409. Dworkin, L.D., Levine, G.M., Farber, N.J., and M.H. Spector. 1976. Small intestinal mass of the rat is partially determined by indirect effects of intraluminal nutrition. Gastroenterology 71:626-630. Eastwood, G.L. 1977. Small Bowel Morphology and Epithelial Proeration in Intravenously Alimented Rabbits. Surgery 82 (5):613-620. Ecknauer, R., R.M. Clarke, G. Feyerabend. 1977. An experi- 206 mental model for studies on the effects of food and digestive secretions on the digestive absorption capacity of rat small intestine. J. Clin. Chem. Clin. Biochem., 15:361-366. Ellis, D.B., Scholefield, PG. 1962. The effects of adenine and glucose on synthesis of nucleotides by Ehrlich asites carcinoma cells in vitro. Canad. J. Biochem. 40:343-52. Ely, J.O. and M.H. Ross. 1950. Arch. Path. 50:359-365. Effect of adenine on rats. Feldman, E.J., Dowling, R.H., MacNaughton, J., and Peters, T.J. 1976. Effects of Oral Versus Intravenous Nutrition on Intestinal Adaptation After small Bowel Resection in the dog. Gastroenterology 70:712-719. Feldman, E.J., MacNaughton, J., Dowling, R.H. October, 1973. Proceedings: Comparative Effect of Intravenous and Oral Nutrition of Intestinal Adaptation. Gut 14:831. Fleck, A. and H.N. Munro. 1962. The precision of ultaviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim. Biophys. Acta. 55:571-583. Fortin-Magana, R., Hurwitz, R., Herbst, J.J. and Kretchmer, N. 1970. Intestinal enzymes: indicators of proliferation and differentiation in the jejunum. Science 167:16271628. Gall, D.G., D. Chapman, M. Kelly, and J.R. Hamilton. 1977. Na+ transport in jejunal crypt cells. Gastro. 72:452456. Griebsch, A., and Zollner, N. 1974. Effect of ribomononucleotides given orally on uric acid production in man. Adv. Exp. Med. Biol. B. 41:443-449. Grobner, W. and Zollner, N. 1977. The influence of dietary purines and pyrimidines on purine and pyrimidine biosynthesis in man. Second European Nutrition Conference, Munich 1976, Nutr. Metab. 21:26-32. Hagemann, R.F. and J.J. Stragand. 1977. Fasting and refeedcell kinetic response of jejunum, ileum and colon. ing: Cell Tissue Kinet. 10:3-14. Hamilton, R.F., Davis, W.C., Stephenson, D.V., Magee, D.F. April 1971. Effects of Parenteral Hyperalimentation of Upper Gastro-Intestinal Tract Secretions. Arch. Surg. 102:348-352. 207 Harms, V. and C.E. Stirling. 1977. Transport of purine nucleotides and nucleosides by in vitro rabbit ileum. Am. J. Physiol. 233:E47-E55, or Am. J. Physiol. Endocrinol. Metab. Gastrointest. Physiol., 2:E47-E55. Harrison, D.D. and H.L. Webster. 1969. The preparation of isolated intestinal crypt cells. Exp. Cell Res. 55:257-260. Hartman, S.C. 1963. Phosphoribosyl pyrophosphate amidotransterase purification and general catalbic properties J. Biol Chem 238:3024-3035. Henderson, J.F. and Khou, M.K.Y. 1965. Synthesis of 5Phosphoribosyl 1-Pyrophosphate from Glucose in Ehrlich Ascites. Tumor cells in Vitro. J. Biol. Chem. 240: 2349-2357. Herbst, J.J., Fortin-Magana R., Sunshine P. 1970. Relationship of pyrimidine biosynthetic enzymes to cellular proliferation in rat intestine during development. Gastroenterology. 59:240-246. Hill, R.B., Jr., Prosper, J., Hirschfield, J.S. and Kern, F. 1968. Protein Starvation and the Small Intestine I. The Growth and Morphology of the Small Intestine in Weaning Rats. Exp. Molec. Path. 8:66-74. Hirschfield, J.S.. Kern, F., Jr. 1969. Protein Starvation and the Small Intestine. 3. Incorporation of Orally and Intraperitoneally Administered 1-leucine 4,5-3H into Intestinal Mucosal Protein of Protein Deprived Rats. J. Clin. Invest. 48:1224-1229. Holmes, E.W., J.A. McDonald, J.M. McCord, J.B. Wyngaarden, and W.N. Kelley. 1973. Human glutamine phosphoribosylpyrophosphate amido-transferase. Kinetic and regulatory properties. J. Biol. Chem. 248:144-150. E.W. Holmes, J.B. Wyngaarden and W.N. Kelley. 1973. Human glutamine phosphoribosylpyrophosphate amidotransferase: two molecular forms interconvertible by purine ribenucleotides and phosphoribosylpyrophosphate, J. Biol. Chem. 248:6035-6040. Hooper, C.S. and Blair, M. 1958. The effect of starvation on Epithelial Renewal in the Rat Duodenum. Exptl. Cell Res. 14:175-181. Hopper, A.F., Wannemacher, R.W., Jr., McGovern, P.A. 1968. Cell Population changes with Intestinal Epethelium of the Rat following starvation and protein depletion. Proc. Soc. Exp. Biol. Med. 128:695-698. 208 Hughes, C.A., Bates, T., Dowling, H. 1978. Cholecystokinin and Secretion prevent the intestinal Mucosal Hypoplasia of total parenteral nutrition in the dog. Gastro 75:3441. Hughes, C.A, Sabin E., Dowling, R.M. 1977. Speed of change in intestinal and pancreatic structure and function during exclusive parenteral nutrition. (abstr). EVR J. Clin Invest 7:230-231, Iemhoff, W.G.J., J.W.O. van den Berg, A.M. de Pijper, and W.C. Hulsmann. 1970. Metabolic aspects of isolated cells from rat small intestinal epithelium. Biochim. Biophys. Acta. 215:229-241. Imondi AR, Balis E. Lipkin M: 1969. Changes in enzyme levels accompanying differentiation of intestinal epithelial cells. Exp Cell Res. 58:323-330. Jeejeebhoy, K.N., B. Langer, G. Tsallas, R.C. Chu, A. Kuksis and G.H. Anderson. Total Parenteral Nutrition at Home: Studies in Patients Surviving 4 months to 5 years. Gastroenterology 71 (6):943-953. Jeejeebhoy, K.N., Zohrab, W.J., Langer, B., Phillips, M.J., Kuksis, A., and Anderson, G.H. 1973. Total parenteral nutrition at home for 23 months, without complications and with good rehabilitation. A study of technical and Metabolic features. Gastrenterology 65:811-820. Johnson, L.R. 1976. The trophic action of gastrointestinal hormones. Gastro 70:278-288. Johnson, L.R., E.M. Copeland, S.J. Dudrick, L.M. Lichtenberger, and G.A. Castro. 1975. Structural and hormonal alterations in the gastrointestinal tract of parenterally fed rats. 68:1177-1183. Johnson, L.R., Schanbacher, L.M., Dudrick, S.J. and Copeland, E.M. 1977. Effect of Long-Term Parenteral Feeding on Pancreatic Secretion and Serum Secretion. Amer. J. of Physiol. 233(6):524-529. Ju, J.S. and Nasset, E.S. 1959. Changes in Total Nitrogen Content of some abdominal viscera in fasting and realimentation J. Nutr. 68(3):633-645. Khan, A.H., S. Wilson and J.C. Crawhall. 1975. The influx of uric acid and other purines into everted jejunal sacs of the rat and hamster. Can. J. Physiol. Pharmacol., 53:113-119. 209 Kimmich, G.A. 1970. Preparation and properties of mucosal epithelial cells isolated from small intestine of the chicken. Biochemistry 9:3659-3668. Kotler, D.P., Levine, G.M. 1979. Reversible gastric and pancreatic hyposecretion after long-term total parenteral nutrition. NEJM 300:241-242 Lajtha, L.G., Vane, J.R. 1958. Dependence of Bone Marrow Cells the Liver for Purine Supply. Nature 182:191-192. LaLanne, M. and J.F. Henderson. 1975. Effects of hormones and drugs on phosphoribosyl pyrophosphate concentrations in mouse liver. Can. J. Biochem. 53:394-399. Langer, B., McHattie, J.D., Zohrab, W.J.., Jeejeebhoy, K.N. 1973. Prolonged Survival after complete small bowel resection using intravenous alimentation at home. J. Surg. Res. 15:226-233. LeBlond, C.P. and Walker, B.E. 1976. Renewal of Cell Population. Physiol. Reviews 36(2):255-276. Levine, G.M., J.J. Deren, E. Steiger and R. Zinno. Gastro. 67:975-982. 1974. Lewis, J.M. and S.C. Hartman. 1978. Ajidophosphoribosyltransferase (Chicken Liver). IN: Methods in Enzymology, Vol. LI, pp. 171-178. New York. Academic Press. Lowry, B.A., Williams, M.K. 1960. The Presence of. a Limited Portion of the Pathway De Novo of Purine Biosynthesis in the Rabbit Erythrocyte in Vitro. J. Biol. chem. 235(10): 2924-2927. Lowry, O.H., N.J. Rosebrough, A.L. Farr, and R. Randall. 1951. Protein measurement with the folin phenol reagent. Biol. Chem. 193:265-275. MacKinnon, A.M. and Deller, D.J. 1973. Purine Nucleotides Brosynthesis in Gastrointestinal Mucosa. B.B.A. 319:1-4. Markham R. and Smith, J.D. 1951. Chromatographic studies of Nucleic acids Biochem. J. 491:401-406. McDermott, F.T., Roudnew, B. 1976. Ileal Crypt Cell Population Kinetics after 40% small bowel resection: Autoradiographic studies in the rat. Gastro. 70:707-711. McKeran, R.O.., T.M. Andrews,A. Howell, D.A. Gibbs, S. Chinn and R.W.E. Watts. 1975. The diagnosis of the carrier state for the Lesch-Nyhan syndrome. Quart J. of Med. XLIV:189-205. 210 McKeran, R.O.., Watts, R.N. 1976. Use of Phytohemagglutin stimulated lymphocytes to study effects of hypoxanthineguanine phosphoribosyltransferase (HGPRT) Deficiency on polynucleotide and protein synthesis in the Lesch-Nyhan syndrome. J. Med. Genet. 13(2):91-95. McManus, J.P.A., and K.J. Isselbacher, 1970. Effect of fasting versus feeding on the rat small intestine. Gastro. 59: 214-221. 1963. Dialytic Methods of Treatment. In Merrill, J.P.. Diseases of the Kidney by Strauss, M.B. and Welt, E17G., Chapter 6, pp. 218-232, Little Brown & Co., 1st ed. Munro, H.N., Goldberg, d.M. 1964. In The Role of the Gastrointestinal Tract in Protein Meta5olism, H.N. Munro(Ed.) p. 189, 197 Blackwell, Oxford. Nakayama, H. and E. Weser, 1972. Adaptation of small bowel increase in the pentose after intestinal resection: phosphate pathway. Biochim. et Biophys. Acta. 279:416423. D. P. Nierlich and B. Magasanik. 1965. Regulation of purine ribonucleotide synthesis by end product inhibition. J. Biol. Chem. 240:358-374. Olsen, A.S. and G. Milman. 1978. Hypoxanthine Phosphoribosyltransferase from Chinese Hamster Brain and Human Erythrocytes. IN: Methods in Enzymology, Vol.LI, pp. 543-549. New York, Academic Press. Obertop, H., Nundy, S., Malamud, D., and Malt, R.A. 1977. Onset of Cell Proliferation in the shortened gut: Rapid hyperplasia after jejunal resection. Gastro. 72:267-270. Petrie, J.J.B., Jones, E.O.P., Hartley, L.C.J., Olive, K.P., and Clunie, G.J.A. 1976. The use of an indwelling peritoneal catheter in the treatment of chronic renal failure. Med. J. Aust. 2:119-122. Philips, F.S.., Thiersch, J.B. and Bendich, A. 1952. Adenine intoxication in relation to in vivo formation and deposition of 2,8 dioxyadenine~Tn renal tubules. J. Pharm. Exp. Therap. 104:20-30. Platt, B.S., Heard, C.R.C. and Stewart, R.J.G. 1964. In The Rose of the Gastrointestinal Tract in Protein Metagolism, H.N. Munro (Ed.) p. 224, Blackwell, Oxford. Pritchard, J.B.., F. Chavez-Peon, and R.D. Berlin. 1970. Purines: supply by liver to tissues. Amer. J. Physiol. 219:1263-1267. 211 Pritchard, J.B., N. O'Connor, J.M. Oliver and R.D. Berlin. 1975. Uptake and supply of purine compounds by the liver. Amer. J. Physiol. 229:967-972. Rausch-Janssen, A., W. Grobner, and N. Zollner. 1977. Comparison of serum uric acid and urinary uric acid excretion under a soy protein diet low in purines and under a formula diet virtually free of purines. Second European Nutrition Conference, Munich, 1976, Nutr. Metab. 21 (Suppl. 1), 94-97. Reem, G.H., 1974. Enzymatic synthesis of phosphoribosylamine in human cells. J. Biol. Chem. 249:1696-1703. Reem, G.H. 1977. Discussion of Purine and Pyrimidine MetabolPathways, Pitfalls and Pertubations. In Purine and ism: Pyrimidine Metabolism. CIBA Foundation Symposium 48(New Series), Elsevier, p. 18. Roll, P.M., Brown, G.B. DiCarlo, F.J. and Schultz, A.S. 1949. Metabolism of Yeast Nucleic Acid in the Rat. J. Biol. Chem. 180:333-340. Rosenbloom, F.M., J.F. Henderson, I.C. Caldwell, W.N. Kelley 1968. Biochemical bases of and J.E. Seegmiller. accelerated purine biosynthesis de novo in human fibroblasts lacking hypoxanthineguanine phosphoribosyltranferase. J. Biol. Chem. 243:1166-1173. Saviano, D.A. and Clifford, A.J. 1978. Absorption, tissue incerperation and.excretion of free purine bases in the rat. Nut. Report. Int. Vol 17 P551-556. Scott, J.L. 1962. Human Leukocyte Metabolism in Vitro. I. Incorporation of Adenine-8-C-14 and Formate-C-14 into Nucleic Acids of Leukemic Leukocytes. J. Clin. Invest. 41:67-79. Shils, M.E. 1975. A Program for Total Parenteral Nutrition in the Home. Amer. J. Clin. Nutr. 28:1429-1435. Smellie, R.M., Thomson, R.Y., Davidson, J.N. 1958. The Nucleic Acid Metabolism of Animal Cells in Vitro. I. The Incorporation of 14C-Formate. B.B.A. 29:59-74. Sonoda, T,and M. Tatibana. 1978. Metabolic fate of pyrimidines and purines in dietary nucleic acids ingested by mice. Biochim. et Biophys. Acta. 521:55-66. The 1973. Sperling, 0, J.B. Wyngaarden and C.F. Starmer. kinetics of Intramolecular distribution of 15 N in uric acid after administration of (1 5 N) glycine. A reappraisal of the significance of prefeientiaLl labeling of N-*(3+9) of uric acid in primary gout, J. Clin. Invest. 52:24682485. 212 Steiner, M.,Bourges, H.R.,Freedman, L.S., Gray, SJ. 1968. Effect of Starvation on the Tissue Composition of the Small Intestine in the Rat. Amer. J. of Physiol. 215: (1)75-77. Tay, B.S., McC.Lilley, R., Murray, A.W., Atkinson, M.R. 1969. Inhibition of phosphoribosyl pyrophosphate amidotransferase from Ehrlich ascites-tumour cells by thiopurine nucleotides. Biochem. Pharma. 18:936-938. Tenckhoff, H. and Schecter, H. 1968. A Bacteriologically Safe Peritoneal Access Device. Vol. XIV, Trans. Amer. Soc. Artif. Int. Organs, pp. 181-186. Thaysen, E.M. and Thaysen, J.H. 1944. Morphological changes in the Gastrointestinal Tract of the White Rat Following Inanition. Acta Pathologica et Microbiologica Scandinavica. 26:370-379. Towne, J.B., Hamilton, R.F., Stephenson, D.V. 1973. Mechanism of Hyperalimentation in the Suppression of Upper Gastrointestinal Secretions. Amer. J. of Surg. 126: 714-716. Urban, E., Pena, M. 1974. In Vivo Calcium Transport by Rat Small Intestine After Massive Small Bowel Resection. Amer. J. Physiol. 226:1304-1308. de Verdier, C.H., Ericson, A., Niklasson, F. and Westman, M. 1977. Adenine metabolism in man. 1. After intravenous and peroral administration. Scand. J. Clin. Lab. Invest. 37:567-575. Wegner, G. 1877. Chirurgische Bemerkungen uber die Peritonealho hle, mit besonder Berucksichtigung der Ovariotomie. Arch. F. Klin. Chir. 20:51. Intestinal epithelial cell surface memWeiser, M.M. 1973. brane glycoprotein synthesis. J. Biol. Chem. 248:25362541. Weser, E., Hernandez, M.H. 1974. Studies of Small Bowel Adaptation After Intestinal Resection in the Rat. Gastro. 60:69-75. Williams, A.M. 1962. Nucleic Acid Metabolism in Leukemic I. In Vitro Incorporation by Human Leukocytes. Leukocytes from Chronic Granulocytic Leukemia. Can. Res. 22:314-321. Wilson, D.W. and Wilson, H.C. 1962. Studies in vitro of the digestion and absorption of purine ribonucleotides by the intestine. J. Biol. Chem. 237:1643-1647. 213 Wood, A.W. and Seegmiller, J.E. 1973. Properties of 5Phosophoribosyl-l-pyrophosphate Amidotransferase from Human Lymphocytes. J. Biol. Chem. 248:138-143. Wyngaarden, J.B. 1972. Glutamine phosphoribosylpyrophosphate amidotransferase, pp. 135-136 in Cellular Regulation Academic Press, (B. HORECKER and E. STADTMAN, eds). New York. Zaharko, D.S., W.P. Fung, and K.H. Yang. 1977. Relative biochemical aspects of low and high doses of methotrex37:1602-1607. ate in mice. Can. Res. Zollner, N. 1973. Influence of various purines on uric acid metabolism. Bibl. Nutr. Diet 19, Karger, Basel, Madridp. 34. Zollner, N. and Grierbsch, A. 1973. Proceedings of the International Symposium on Renal Stone Research, Madrid, Karger, Basel-p. 84. Zollner, N. and Griebsch, A. 1974. Diet and gout. In: Purine metabolism in man, Plenum Publishing, New Yorkp. 435. 214 BIOGRAPHICAL NOTE Neal Simon LeLeiko was born in Brooklyn, New York on October 26, 1946. He attended Brooklyn College of the City University of New York and received his Bachelor of Science Degree (Chemistry) in June, 1967. He received his M.D. degree from New York Medical College in June, 1971. He did his internship, residency and chief residency in Pediatrics at The Mount Sinai Hospital in New York City from July, 1971 to June, 1974. From July, 1974 through June, 1976 he served in the United States Air Force as Chief of Pediatrics at the Rickenbacker Air Force Base Hospital. From July, 1976 to June, 1979 he served as a Fellow in Medicine (Gastroenterology and Nutrition) at the Children's Hospital Medical Center. He married Marilyn Bush on August 20, 1967. They have two daughters, Sarah (four years old) and Rebecca (eight months old).