Name ________________________ Date ___________________ Per________ Chapter 16 reading Guide Key
advertisement

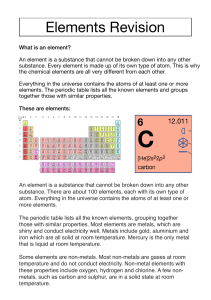
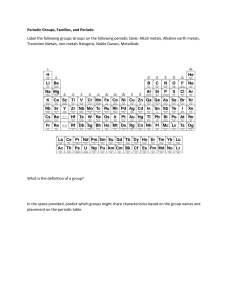
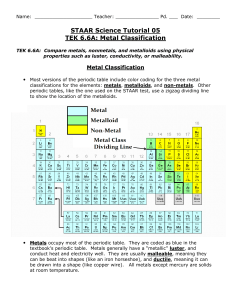
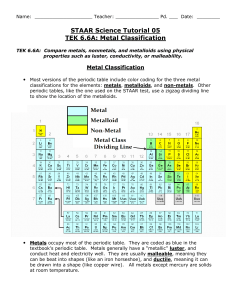
Name ________________________ Date ___________________ Per________ Chapter 16 reading Guide Key 1. What physical or chemical properties are used to organize the periodic table? (3) Atomic number Atomic radius (size) Ionization energy Metals Non-metals Metalloids 2. Rows in the periodic table are referred to as ___periods__ (1) 3. All the elements in the periodic table are sorted into three main categories. List them. (3) Metals, non-metals, metalloids 4. What are the main properties of these three classes? (3) Metals : shiny, conduct electricity, opaque, malleable, ductile Non-metals : poor conductors, transparent, shatter when hammered (brittle), non-malleable, non-ductile Metalloids: mixture of metallic & non-metallic properties 5. Columns in the periodic table are referred to as __ groups___. (1) 6. Fill in the group names in the following blank periodic table. (3) 1 2 3 – 12 13 – 16 17 A M l e k t a a l l i s A l k a l i E a r t h M e t a l s T r a n s i t i o n M e t a l s N o C o m m o n N a m e H a l o g e n s 18 NG o a b s l e e s 7. Give a description of each group from the previous question. (3) 1. used in soaps, found in ashes 2. resistant to fire 3 – 12 harder than alkali metals, less reactive with water, used for structural purposes 13 – 16 None 17. salt – forming 18. unreactive gases 8. What is a valence electron? (2) Electrons in the outermost shell 9. What is electro-negativity? Electron affinity – how much an atom is able to attract electrons from another atom 10. What is ionization energy? (1) amount of energy its takes to remove an electron from an atom