Single molecule techniques to probe decision-making processes in developmental biology
by
Annalisa M. Pawlosky
B.S. Physics
Virginia Polytechnic Institute and State University, 2006
SUBMITTED TO THE HARVARD-MIT DIVISION OF HEALTH SCIENCES AND TECHNOLOGY
IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF
DOCTOR OF PHILOSOPHY IN HEALTH SCIENCES AND TECHNOLOGY
AT THE MASSACHUSETTS INSTITUTE OF TECHNOLOGY
FEBRUARY 2014
© 2014 Annalisa Pawlosky. All rights reserved.
The author hereby grants to MIT permission to reproduce
and to distribute publicly paper and electronic
copies of this thesis document in whole or in part
in any medium now known or hereafter created.
ARCH VE4;
MA
SACHUSETTSiN T
OF TECH-NOLOGy
FEB 20 2j014
.RARIES
Signature of Author:
-
14
-
--
- - - -
-
4
- - - -
:
1
6
/1
Certified by
-
0
4 ision of Health Sciences and Technology
October 31, 2013
1/'
%'
Alexander van Oudenaarden, PhD
Professor of Physics and Biology at Massachusetts Institute of Technology
Director of Hubrecht Institute for Developmental Biology and Stem Cell Research at the Royal
Netherlands Academy of Arts and Sciences and University Medical Center Utrecht
Thesis Supervisor
Accepted by:
Emery Br
n, D, PhD/Director, Harvard-MIT Division of Health Sciences and Technology
Krofessor of Computational Neuroscience and Health Sciences and Technology
Chairman, Committee for Graduate Students
1
TE
2
Single molecule techniques to probe decision-making processes in developmental biology
by
Annalisa M. Pawlosky
Submitted to Harvard-MIT Division of Health Sciences and Technology
On October 31, 2013, in partial fulfillment of the requirements for the degree of
Doctor of Philosophy in Health Sciences and Technology
Abstract
This work investigates the fundamental processes used by mammalian cells and organisms to
Current technologies that evaluate biological
make decisions during embryonic development.
phenomenon often force a compromise between quantification of gene expression via bulk assays and
qualitative imaging of cell and tissue heterogeneity. There are few options that allow for quantitative,
high-resolution, single-cell analysis that is robust but not associated with a high degree of technical
difficulty or obscured by amplification. Here, we address these issues using two model systems, the
developing mammalian inner ear and single mouse embryonic stem cells (mESCs) during the process of
X inactivation, to demonstrate our ability to perform single-cell, single-molecule assays that reveal both
highly quantitative and spatial information. Accordingly, we adapted a high resolution, single-molecule
RNA fluorescent in situ hybridization technique (smFISH) to study gene expression in the inner ear and
perform allele-specific detection of the X chromosome in mESCs.
We used previously-published smFISH procedures as our initial template for investigating
biological signaling phenomena in these two systems. To study gene expression in the mouse inner ear,
we developed a modified smFISH strategy to investigate mRNA transcript expression patterns in the
cochlea during auditory hair cell development. The mammalian cochlea, a highly specialized and
complex organ, beautifully demonstrates both the depth and breadth of the smFISH technique. To
assay signaling behavior and topological changes of the X chromosome prior to X inactivation, we
incorporated a novel allele-specific modification into the smFISH technique. We investigate the allelespecific expression patterns of eight genes that tile the X chromosome, which were chosen for their
varied putative roles before, during and after X chromosome inactivation. Taken together, these two
its
adaptations.
strength of the smFISH technique and
recapitulate the
systems
The goals of this thesis were twofold: (1) expand the smFISH technique to work in specialized
mammalian systems such as the cochlea and (2) demonstrate allele-specific DNA topological changes
and expression patterns in mESCs. Elucidating high-resolution, single-molecule quantifiable imaging
methods for application to complex tissues or allele-specific probing will have profound impacts on
future investigations and promote a deeper comprehension of these systems.
Thesis Supervisor: Alexander van Oudenaarden, PhD
Title: Professor of Physics and Biology, Massachusetts Institute of Technology
Director of Hubrecht Institute for Developmental Biology and Stem Cell Research at the Royal
Netherlands Academy of Arts and Sciences and University Medical Center Utrecht
Thesis Chairman: M. Charles Liberman, PhD
Title: Professor of Otology and Laryngology, Harvard Medical School
Thesis Reader: William F. Sewell, PhD
3
Title: Professor of Otology and Laryngology, Harvard Medical School
Thesis Reader: Matthew W. Kelley, PhD
Title: Chief Section on Developmental Neuroscience, National Institutes of Health, National Institute on
Deafness and Other Communication Disorders
4
5
Acknowledgments
First and foremost, I would like to thank my advisor Alexander van Oudenaarden. I remember
our first meeting when I approached him to study the mammalian auditory system in his laboratory.
Not only did he allow me to enter his lab, and work with the tremendous team of talent that he had
assembled, but he also let me pursue my passions. I realize this experience is rare, and I will forever be
grateful for this opportunity and for the experiences I had while working in the van Oudenaarden
Laboratory. I am grateful for the imprinting that I received while working until Alexander's tutelage.
I would like to thank the members of my committee, M. Charles Liberman, Matthew Kelley and
William Sewell. During the dynamic latter stages of my PhD, my committee offered additional scientific
guidance and support needed to reach the finish line. Dr. Liberman led this charge, and I appreciated his
critical perspective, requests for additional controls and determination to declare precisely what had
been found. Dr. Liberman taught me how to be a better scientist, which is a priceless gift that I was
lucky to receive. I learned much during our interactions and will use these tools as I continue on my
path in science. Matthew Kelley provided many resources above and beyond what could be expected
from a member of a PhD thesis committee. Matt opened up his lab, and made available the tools and
techniques needed to study the developing auditory system. Matt continued to answer questions and
offer resources throughout the entire time I worked in the van Oudenaarden Laboratory, and any
success that I may have achieved is a direct result of the help from him and his gifted team. Finally, I
would like to thank William Sewell, for whom one could not speak enough praise. I am indebted to Bill
for the gift of a career in science. During the tough moments when I could not see a clear path forward,
Bill offered endless support and advice on how to proceed. I do not know what I did to have the good
fortune to interact with such a wonderful scientist and human being, but I will spend the rest of my life
paying back what I have received.
Science is rarely done in a vacuum. I have had such a fantastic experience working at MIT and
interacting with the members of the Eaton-Peabody Laboratory at Massachusetts Eye and Ear Infirmary.
Thank you for the many conversations, answered questions, shared protocols and support. I especially
want to thank Connie Miller and Leslie Liberman for their help. Also, I would like to thank Konstantina
Stankovic for helping to set the stage for my PhD thesis research and for the support over the years. I
want to thank the members of the van Oudenaarden Laboratory, both past and present. Thank you so
much to Anna Lyubimova for her training on smFISH, and to Magda Bienko and Nicola Crosetto for
sharing their expertise on DNA FISH. I especially want to thank Clinton Hansen, Sandy Klemm, Ya Lin and
Kay Wiebrands not only for their scientific and technical aid, but also for their friendship. Thank you to
Monica Wolf for your support and making sure things always worked out. Thank you so much to my
friends, both at MIT, within Boston and around the world. A special thanks to my dear friends who
always went above and beyond to support me - Blanche Staton, Monica Orta, Deborah Fernandez, and
Yee Lok Wong. Thank you to Marytheresa Ifediba, for your strength and encouragement to apply to
HST, helping me get through it and to graduate.
Most importantly, thank you to my family. Thank you to my younger brother Steffen who
always pushes me to think differently and pursue what I love. Thank you to my sister who is so
thoughtful, so beautiful, so loving and so kind. Thank you to my older brother, who only sees the good
in others, whom I will always look up to and respect. Thank you to my mother, for whom is there is not
enough thanks. Thank you for pushing me to try new things, to never give up, to reach higher and to
continue to explore in life. Your passion for learning will always inspire and impress me.
And finally, I dedicate this thesis to my father. Dad, you are one who continues to dedicate his
life's work, both professionally and personally, to helping others. Your strength and intelligence
continue to amaze me. Thank you -
6
Contents
Abstract.............................................................................................................................................3
Acknowledgm ents .............................................................................................................................
6
Chapter 1 Introduction.....................................................................................................................13
1.1 Thesis Contributions ........................................................................................................................
13
1.2 Thesis Outline....................................................................................................................................14
1.3 References ........................................................................................................................................
15
Chapter 2 Investigation of mammalian tissue with single molecule RNA fluorescent in situ
hybridization (sm FISH).....................................................................................................................16
2.1 Introduction ......................................................................................................................................
16
2.1.1 Review of conventional techniques to study RNA expression in cells and tissue ..................
16
2.1.2 Introduction to single molecule RNA FISH (sm FISH).............................................................
17
2.1.3 Current limitations of smFISH technology and its application to cochlear gene expression
analysis................................................................................................................................................18
2.2 M aterials and m ethods ....................................................................................................................
21
2.2.1 Reagents.....................................................................................................................................21
2.2.2 M ouse m odel system for sm FISH ..........................................................................................
22
2.2.3 Sm FISH probe design .................................................................................................................
22
2.2.4 Cochlear fixation ........................................................................................................................
22
2.2.5 Tissue em bedding and sectioning..........................................................................................
22
2.2.6 Hybridization..............................................................................................................................23
2.2.7 SmFISH Im aging..........................................................................................................................23
2.3 Results...............................................................................................................................................23
2.3.1 Modifications to smFISH technique for improved tissue adhesion and morphology ............ 24
2.3.2 Sm FISH in a transgenic mouse m odel...................................................................................
27
2.3.3 Validation of sm FISH technique in the m am m alian cochlea .................................................
30
2.3.4 SmFISH in the developing mammalian cochlea during hair cell development......................33
2.4 Discussion..........................................................................................................................................36
2.5 Conclusions .......................................................................................................................................
36
2.6 References ........................................................................................................................................
37
Chapter 3 Graded Notch signaling robustly specifies fate decisions during mammalian hair cell
developm ent ...................................................................................................................................
40
7
3.1 Introduction ......................................................................................................................................
40
3.1.1 M am m alian Inner Ear Developm ent......................................................................................
40
3.1.2 M urine Inner Ear Anatom y ..................................................................................................
42
3.1.3 Hair Cell Developm ent ...............................................................................................................
44
3.1.4 Notch Pathway Signaling............................................................................................................45
3.1.5 Models of Notch Pathway Signaling .....................................................................................
3.2 M ethods ............................................................................................................................................
46
46
3.3 Results...............................................................................................................................................47
3.3.1 Expression of Atohi during hair cell developm ent...............................................................
48
3.3.2 Notch signaling during hair cell developm ent ........................................................................
49
3.3.3 M odeling Notch signaling in the organ of Corti......................................................................
59
3.3.4 M odeling of cochlear convergence and extension ...............................................................
64
3.4 Discussion..........................................................................................................................................66
3.5 Conclusions .......................................................................................................................................
67
3.6 References ........................................................................................................................................
67
Chapter 4 Expression patterns of Wnt ligands during mammalian hair cell development ................
4.1 Introduction ......................................................................................................................................
71
71
4.2 M ethods............................................................................................................................................71
4.2.1 M ouse m odel system s and PCR analysis ..............................................................................
71
4.2.2 Frozen tissue section preparation for single m olecule RNA FISH ..........................................
71
4.2.3 Single m olecule RNA FISH probes ..........................................................................................
72
4.3 Results...............................................................................................................................................72
4.4 Discussion..........................................................................................................................................76
4.5 Conclusions .......................................................................................................................................
77
4.6 References ........................................................................................................................................
77
Chapter 5 Allele-specific detection of molecules during X Chromosome Inactivation......................79
5.1 Introduction ......................................................................................................................................
79
5.2 M ethods ............................................................................................................................................
80
5.2.2 BD Cell-Tak coating ....................................................................................................................
80
5.2.3 Cell Culture for High Definition 3-D DNA FISH ......................................................................
80
5.2.4 Purification of DNA from m ESCs ............................................................................................
81
5.2.5 High definition 3-D DNA FISH probe design, generation, labeling and purification..............81
8
5.2.6 Allele-specific RNA FISH ............................................................................................................. 82
5.2.7 M ouse ESCs ................................................................................................................................ 83
5.3 Results ............................................................................................................................................... 83
5.4 Discussion .......................................................................................................................................... 88
5.5 References ........................................................................................................................................ 89
Chapter 6 Final Conclusions .............................................................................................................. 91
6.1 Future Directions .............................................................................................................................. 91
6.2 References ........................................................................................................................................ 93
9
List of Figures
Figure 2.1 Schematic of smFISH probes targeting an mRNA transcript ..............................................
18
Figure 2.2 Alterations to smFISH protocol demonstrate improved tissue morphology......................19
Figure 2.3 Cochlear spiral demonstrates the complex geometry of the mammalian inner ear ......... 20
Figure 2.4 Mid-modular cross-section of embryonic 16.5 cochlea depicts base to apex morphological
differences..................................................................................................................................................21
Figure 2.5 Example of smFISH for Jagi in E16.5 cochlea with confocal and wide-field microscopy ...... 25
Figure 2.6 Example of Beta actin expression with smFISH in HCI treated P19 cochlea....................... 26
Figure 2.7 Transgenic mouse allows for the visualization of cell boundaries with smFISH ................
Figure 2.8 GFP-labeled cell boundaries are evident in a fixed cochlea section ...................................
29
30
Figure 2.9 Comparison between conventional ISH and smFISH in the developing cochlea................ 31
Figure 2.10 Expression of Atohl in E16.5 and PO organ of Corti tissue............................................ 32
Figure 2.11 Expression patterns of Atohl and Sox2 in the E16.5 cochlea....................................... 35
Figure 3.1 Gross morphology of murine inner ear during development displays rapid changes .....
41
Figure 3.2 Cross-section of adult mammalian cochlea demonstrates precise patterning .................. 42
Figure 3.3 Hair cell lineage is derived from a prosensory cell ..............................................................
43
Figure 3.4 Loss of Atohl results in the loss of hair cells and support cells ..........................................
44
Figure 3.5 Upregulation of Atohl results in additional hair cells outside the region of conventional hair
cell specification .........................................................................................................................................
45
Figure 3.6 Expression of Atohl during hair cell development............................................................
48
Figure 3.7 Expression of Atohl in a stripe of cells during hair cell specification................................. 49
Figure 3.8 Single molecule RNA FISH demonstrates that Notch ligands Jag2 and Jagi are mutually
exclusive in the basal region of the E16.5 mouse cochlea...................................................................
50
Figure 3.9 Single molecule RNA FISH demonstrates the expression patterns of the Notch ligands Dill,
D113 and D114 ...............................................................................................................................................
51
Figure 3.10 Expression of Jagi and Jag2 along the E16.5 mouse cochlea ..........................................
52
Figure 3.11 Atohl and Jag2 expression.................................................................................................
53
Figure 3.12 Atohl and Jag2 correlation ................................................................................................
54
Figure 3.13 Atohl expression during E16.5 illustrating expression on the bottom of the cochlea ........ 55
Figure 3.14 Color maps of Atohl and Jag2 expression ..........................................................................
56
Figure 3.15 Notch effectors, Hesi and HesS, have distinct expression patterns in the developing
coch lea ........................................................................................................................................................
57
Figure 3.16 Hesi and HesS expression rise from the apex to base in the developing mouse cochlea... 58
Figure 3.17 Notch signaling inhibitor Numb is found to be expressed all throughout the developing
co ch lea ........................................................................................................................................................
59
Figure 3.18 M odel of the stripe of Atohl expression ............................................................................
60
Figure 3.19 Jag2/DIl deletions and Notch signaling schematic..........................................................
61
Figure 3.20 Comparison of three possible scenarios of notch signaling ..............................................
63
Figure 3.21 Two different patterns of Atohl expression ......................................................................
64
Figure 3.22 Convergent extension by removing prosensory cells in the stripe of Atohl expression..... 65
Figure 3.23 Plasticity of prosensory cells...............................................................................................
66
Figure 4.1 Expression of W ntll .................................................................................................................
73
Figure 4.2 Wnt ligands Wnt4 and Wnt11 are expressed in mutually exclusive regions in the developing
co ch lea ........................................................................................................................................................
73
Figure 4.3 Expression of Wnt ligand Wnt5a is found in regions neighboring developing hair cells....... 74
Figure 4.4 Expression of Wnt receptor Fzd6 is localized to cells within the developing mouse E16.5
co ch le a ........................................................................................................................................................
75
10
75
Figure 4.5 Wnt receptor Fzd4 is found to be expressed in the mouse E16.5 cochlea .........................
Figure 4.6 Axin2, a putative universal Wnt target, is expressed in the mouse E16.5 cochlea ............ 76
Figure 5.1 Dynamics of X chromosome inactivation............................................................................
79
Figure 5.2 3D DNA FISH for HER2 in human epithelia cells....................................................................84
Figure 5.3 RNA FISH for m ESCs ..................................................................................................................
85
Figure 5.4 Schematic of allele-specific smFISH intronic probes tiling X chromosome.........................86
Figure 5.5 Allele-specific sm FISH in m ESCs.............................................................................................
86
Figure 5.6 Filter shift alignment can be calculated with fluorescent beads.........................................87
Figure 5.7 Measurement between two positions.................................................................................88
11
List of Tables
Table 2.1 Comparison of fold change of Atohl with three different methods....................................33
Table 5.1 PCR conditions for creating DNA FISH probes......................................................................82
12
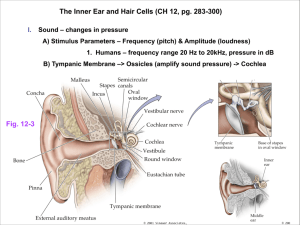
Chapter 1 Introduction
We study the way mammalian cells and organisms make decisions during embryonic
development. Biological decisions range from stochastic, or random, choices to highly robust, ordered
outcomes of a mosaic of cell types. We have chosen two model systems, one to represent each end of
this spectrum, to investigate the correlated molecular changes that occur during decision-making
processes. The first model system involves one of the most highly patterned structures in mammals, the
inner ear. The rigorous patterning and cell specification within the cochlea, the auditory sensory organ,
incorporates the integration of several different developmental pathways, which is indicative of a highly
complex genetic network underlying decision-making processes (Fritzsch et al. 2011). It has been shown
when the patterning of the organ is disrupted, either during development or induced in adulthood, so
too is its function (Eisen and Ryugo 2007). As these cells are not replicated beyond their cell
specification stage, the auditory system has a short window during which precise, robust decisionmaking processes can be made. Our second model system involves one of the first major decisions
made in mammalian female embryonic development - X chromosome inactivation. Female cells receive
both paternal and maternal X chromosomes (Nora and Heard 2010). One of these chromosomes must
transform its chromatin structure and silence its gene activity so that the female organism may develop
normally (Nora and Heard 2010). The decision of which X chromosome, maternal or paternal, will
silence itself is stochastic (Lyon 1961). The stochastic choice results in different patches of either
paternal or maternal X chromosome activation throughout the female organism (Migeon 2007).
Investigation of these two complementary systems will shed light on the numerous avenues by which
cells and organisms incorporate external and internal cues to make decisions during development.
To study the processes underlying the development of a hair cell, we investigated the
expression of ligands, receptors and putative downstream targets of both the Wnt and Notch signaling
pathways in the developing mouse inner ear. We gathered data across different regions of the cochlea
to observe the expression pattern changes throughout hair cell development. Finally, we integrated this
expression data into a model for hair cell specification. To study X chromosome inactivation, we
visualized the changes in the topology of the inactivated chromosome with a combination of allelespecific high definition 3-D DNA FISH and allele-specific single-molecule RNA intron FISH assays. We
conducted these experiments in a hybrid mouse embryonic stem cell model system. We demonstrated
the ability to detect changes in ncRNA and mRNA transcription with topology changes of the inactivated
chromosome in individual cells.
Although these two systems use different mechanisms to make decisions, they contain areas of
overlap. They both comprise highly structured biological decisions that result in cellular mosaics. When
operating normally, both X chromosome inactivation and hair cell specification lead to a concrete
decision of fate. When an X chromosome is inactivated, it remains inactive within that cell and its
descendants. Similarly, once a hair cell is specified, it remains a hair cell until its death. Thus, biological
decisions that are made during short time interval endure throughout the lifetime of the organism.
1.1 Thesis Contributions
The overall objective of this research was to apply a novel single molecule technique to gain a
deeper understanding of the signaling processes used by cells to make decisions. The study of the
fundamental mechanisms behind cell specification, differentiation and patterning leads to a deeper
understanding of the regulation of the heterogeneous patterns within healthy tissue and of the putative
mechanisms involved in disruption and disease. To achieve this objective, we utilized novel tools from
molecular biology in combination with a single-molecule RNA FISH technique that allows for the
13
quantification of individual molecules within cells. The aims of this research that are described in the
chapters of this thesis are as follows:
1.
2.
3.
4.
Adapt, validate and implement high resolution smFISH technology for application to the
developing murine cochlea (Chapter 2).
Investigate the expression of key components of the Notch signaling pathway during
mammalian hair cell development (Chapter 3).
Map the expression patterns of the Wnt ligands during mammalian hair cell development
(Chapter 4).
Expand allele-specific smFISH technology to probe signaling and topological changes in
hybrid mESCs (Chapter 5).
1.2 Thesis Outline
The thesis is structured into five chapters, which includes one brief introduction chapter, three
chapters that focus on the development and implementation of single molecule mRNA FISH for studies
in the mammalian inner ear and one chapter describing the application of allele-specific single-molecule
RNA FISH to study X chromosome inactivation. Chapter 2 presents a review of the current techniques
used to study RNA in cells and tissue alongside an introduction to single-molecule RNA FISH. The
primary focus of the chapter is the modifications and alterations made to the tradition single-molecule
RNA FISH (smFISH) technique in order to apply it to studies of the mammalian inner ear. This chapter
details the mouse model system used to observe the boundaries of individual cells, as well as the
materials and reagents needed for the applications of smFISH. Chapter 2 also details the probe design
implemented for the probes tested and used throughout this thesis and the fixation and hybridization
protocols applied for visualizing individual mRNA transcripts within individual cells. This chapter
concludes with example smFISH images and a discussion of the software used to analyze the gathered
data.
Chapter 3 focuses on the realization of using smFISH to study molecules of the Notch signaling
pathway during mammalian hair cell development. It begins with a brief literature review of the
development of the mammalian inner ear, specifically the development of the hair cell. This review
highlights the importance of the expression of Atohl, a molecule reported to be necessary and sufficient
for the production of hair cells (Bermingham et al. 1999, Chen et al. 2002). This is followed by an
overview of Notch pathway signaling and a few recent models that describe how Notch signaling can be
used to obtain certain phenotypic results in other biological systems. The results of this work show that
a graded expression pattern of key molecules, Atohl and Jag2, exists within the developing cochlea
precisely at the time that hair cells are being specified. This quantitative data is then used in a Notch
signaling model that recapitulates the known phenotype of specification and patterning for hair cells.
The model is robust to conversion and extension parameters and provides a potential perspective of
how Notch signaling, coupled with the onset of a graded pattern of Atohl, can produce robust
patterning of hair cells in the mammalian cochlea.
Chapter 4 focuses on one pathway that might be responsible for the onset and initial patterning
of Atohl. Recent work has highlighted the potential of the Wnt signaling pathway to influence hair cell
specification and patterning (Jacques et al. 2012). This chapter briefly reviews the current literature,
and touches on the impact of the disruption of Wnt signaling on the planar cell polarity within the inner
ear. Results show the expression patterns of all of the Wnt ligands within the developing mouse cochlea
at embryonic day 16.5 (E16.5). The results are summarized in a schematic of how the Wnt pathway may
be used to influence the initial staging of Atohl in the developing cochlea.
14
Chapter 5 transitions from studying a highly patterned mosaic composed of many cell types to the
two-cell mosaic fashioned by X chromosome inactivation. Chapter 5 highlights the use of smFISH to
study a classic question in biology - the mechanics and expression patterns underlying X chromosome
inactivation. SmFISH is expanded to include allele-specific probes, and to target RNA intron sites and
ncRNA. This study provides an example of tools developed using smFISH technology to investigate
several sites along the Central Dogma pathway. Experiments incorporate genetic tools, including a
hybrid mouse embryonic stem cell line, to demonstrate the feasibility of targeting individual alleles.
Taken together, these studies use quantitative tools to investigate the signaling patterns within cells
during important developmental decision-making processes.
Chapter 6 describes final conclusion and future directions for these projects.
1.3 References
Bermingham NA, et al. (1999) Mathi: an essential gene for the generation of inner ear hair cells. Science
284(5421):1837-1841.
Chen P, Johnson JE, Zoghbi HY, Segil N (2002) The role of Mathi in inner ear development: Uncoupling
the establishment of the sensory primordium from hair cell fate determination. Development
129(10):2495-2505.
Eisen MD, Ryugo DK (2007) Hearing molecules: contributions from genetic deafness. Cell Mol Life Sci
64(5):566-580.
Fritzsch B, et al. (2011) Dissecting the molecular basis of organ of Corti development: Where are we
now? Hear Res 276(1-2):16-26.
Jacques BE, et al. (2012) A dual function for canonical Wnt/0-catenin signaling in the developing
mammalian cochlea. Development 139(23):4395-4404.
Lyon MF (1961) Gene action in the X chromosome of the mouse (Mus musculus L.). Nature 190:327373.
Migeon BR (2007) Why females are mosaics, X-chromosome inactivation, and sex differences in disease.
Gend Med 4(2):97-105.
Nora EP, Heard E (2010) Chromatin structure and nuclear organization dynamics during X-chromosome
inactivation. Cold Spring Harb Symp Quant Biol 75:333-344.
15
Chapter 2 Investigation of mammalian tissue with single molecule RNA fluorescent in situ
hybridization (smFISH)
2.1 Introduction
2.1.1 Review of conventionaltechniques to study RNA expression in cells and tissue
Within an organism, almost all cells start with the same initial conditions; each one of these cells
contains the same deoxyribonucleic acid (DNA) content and presumably have the same developmental
potential. However, mature multi-cellular organisms are comprise of many diverse cell types. One of
the first steps in the differentiation of these cells is the expression of messenger ribonucleic acid
(mRNA). The expression of a particular mRNA transcript within a cell is a measure of the activity of the
gene in that cell. There are several conventional ways to measure the mRNA content within cells,
including northern blotting, reverse transcription polymerase chain reaction (RT-PCR) and microarrays.
Northern blotting is a standard technique for measuring gene expression that assesses the size,
quality and relative amount of RNA expression (Alwine et al. 1977). RNA is extracted and isolated from a
cell or tissue sample and separated according to size via electrophoresis. For this, the RNA sample is
mixed with a loading buffer, denatured and loaded onto an agarose gel where an applied current
induces the separation of the RNA into bands based on their size. The bands can subsequently be
stained to ascertain the RNA quality and quantity. The samples are then transferred to a nylon
membrane for hybridization to labeled antisense probes, which bind to the RNA samples by
complementary base pairing. The sample is exposed on x-ray film for imaging, and the amount of RNA
can be quantified by the densitometry of the resulting bands.
The northern blotting technique is known to have low sensitivity (ability to accurately detect low
levels of RNA) but high specificity (ability to identify the correct RNA molecule) when compared to other
RNA expression analysis techniques (Streit et al. 2009). The separation of RNA molecules by size is a
distinct advantage of the northern blotting technique, because a single probe can be used to evaluate
whether alternative splice products, deletions or errors are present in the sample (Killick and Richardson
1997). The main disadvantages of the northern blotting technique are that a relatively small number of
genes that can be visualized at one time, the need for abundant quantities of RNA for analysis, and the
use of relative optical density scale to quantify RNA amounts (Streit et al. 2009). On additional limitation
is the loss of spatial information for RNA expression within a heterogeneous sample required by the
northern blotting procedure.
Another common technique used in molecular biology to investigate mRNA expression is
reverse transcription polymerase chain reaction (RT-PCR). RT-PCR is a variant of polymerase chain
reaction (PCR) used to demonstrate both the presence and amount of RNA in a biological sample (Bustin
2002). RT-PCR is similar to northern blotting in that the RNA is extracted and isolated from cells or
tissue. RT-PCR relies on the use of target-specific primers to bind to a region of interest on an RNA
transcript to create complementary DNA, or cDNA (Bartlett and Stirling 2003; Hurteau and Spivack
2002). The RNA is reverse transcribed into cDNA in one cycle that anneals, extends and inactivates the
reverse transcriptase. The resulting cDNA fragments are amplified exponentially in cycles, and
quantitative measurements can be computed through comparisons of RNA transcripts of interests with
housekeeping genes (Vandesompele et al. 2002).
The RT-PCR technique has many advantages compared with northern blotting techniques,
including requiring only very little starting RNA, accurate quantification of the level of RNA within a
sample and high sensitivity. However, RT-PCR is also associated with significant limitations. Like
northern blotting analysis, RT-PCR causes the loss of spatial information of RNA expression within a
sample. It also is limited in the number of gene expression profiles that can be gathered simultaneously
16
and the difficulty in robustly replicating results due to the quality of quantitative PCR data analysis
(Shiao 2003).
A technique that is capable of identifying RNA expression levels on a large scale is microarray
analysis (Murphy 2002) and more recently RNA-seq, which uses next-generation sequencing to quantify
RNA levels (Marioni et al. 2008). As in RT-PCR and northern blotting, RNA is isolated from a bulk
biological sample. The RNA is reversed transcribed into cDNA, which in turn is converted into RNA in the
presence of labeled nucleotides. The result is a fluorescently labeled cRNA such that each sample is
labeled with a fluorescent dye. The labeled RNA is then hybridized to a chip of spotted DNA targets.
The chips are washed and scanned to determine the expression of many RNA molecules simultaneously.
The strength of microarray analysis is in their ability to observe RNA expression on a large scale.
However, disadvantages of microarrays include the fact that homology between sequences can make it
difficult for microarrays to distinguish between similar sequences. Additionally, microarrays do not span
the entire genome, and probe saturation exists at high expression levels.
The most crucial disadvantage shared by northern blotting, RT-PCR and microarray analysis that
is important for the investigations presented in this thesis is the tradeoff between acquiring highly
quantitative gene expression data and the loss of information describing where an RNA transcript is
specifically located within a sample, cell or complex tissue
2.1.2 Introductionto single molecule RNA FISH (smFISH)
Most tissues are composed of an assortment of parenchymal cells with distinct functions.
Differences in structure and function are reflected in a cell's transcriptome, or the unique RNA
expression profile of the cell that leads to distinct cellular phenotypes. Current gene expression profiling
methods, such as microarrays, northern blotting and RT-PCR, typically measure the number of
oligonucleotide transcripts in a tissue specimen after bulk processing (Itzkovitz and van Oudenaarden
2011). Accordingly, these methods quantify the average expression of genes in a tissue aggregate. In
tissues composed of cells with homogenous expression patterns and distributions, these bulk assays
provide an accurate measure of the transcriptome. However, these assays are not effective in tissues
with heterogeneous cell populations, in which high gene expression in a small cohort of cells is
indistinguishable from low uniform gene expression. For these tissues, a method capable of quantifying
the dynamic expression patterns of individual cells is needed.
Conventional fluorescence in situ hybridization (FISH) is a procedure that allows for
simultaneous monitoring of the expression level and spatial distribution of oligonucleotide transcripts
(Levsky and Singer 2003). FISH utilizes fluorescent nucleotide probes that base pair with complementary
mRNA molecules and thereby enable sequence- and site-specific identification of target gene
expression. The main limitations of conventional FISH are that it is semi-quantitative and is unable to
detect short transcripts due to the length of probes (Itzkovitz and van Oudenaarden 2011). A novel FISH
variant developed by the Tyagi laboratory, in collaboration with our lab, single molecule RNA FISH
(smFISH), overcomes these limitations by applying several short probes for mRNA detection (Raj et al.
2008). Probe design considers three main criteria: (1) probe length, (2) inter-probe spacing, and (3)
probe binding energy (Raj A and van Oudenaarden 2009). Probes are typically 17 - 20 nucleotides in
length to control for unspecific probe binding, optimize the tiling of transcripts and enable detection of
short transcripts (Raj et al. 2008). Each probe is coupled to a single fluorophore at a modified 3' end.
Hybridization of several tiled probes along the target mRNA molecule results in a diffraction-limited spot
detected by fluorescence microscopy (Figure 2.1). The binding energy of each probe is controlled by
selecting guanine-cytosine (GC) content in the range of 45 - 65%. Consequently, smFISH is capable of
resolving RNA transcript data with quantitative and spatial accuracy (Femino et al. 1998; Thompson et
al. 2002).
17
oligonucleotide probes
dft4%Mne
k
d mesArtSOM
Figure 2.1 Schematic of smFISH probes targeting an mRNA transcript.
For smFISH, 48-96 short flurophore labeled oligonucleotide probes, normally 20
nucleotides in length, are hybridized to a target mRNA molecule. SmFISH has been
applied to several organisms robustly such as yeast, C. elegans and Drosophila (Raj et al.
2008). When these samples are imaged with a wide-field microscopy setup, the
summation of the optical spectrums from each mRNA molecule produces a diffractedlimited fluorescent spot. These spots can be detected with algorithms thresholding for
size and intensity, and allow for the quantification of individual mRNA molecules.
2.1.3 Current limitations of smFISH technology and its application to cochlear gene expression analysis
SmFISH has primarily been applied to investigate mRNA expression in single cell systems, such as
yeast and cell lines (Raj et al. 2006; Zenklusen, Larson et al. 2008; Trcek et al. 2012), in simple organisms
such as Caenorhabditis elegans (C. elegans) or in robust tissues, like the gut (Itzkovitz, Lyubimova et al.
2012). Systems such as individual cells, D. melanogaster, and C. elegans larvae and embryos (Raj et al.
2006; Zenklusen et al. 2008; Raj et al. 2010; Trcek et al. 2012) are advantageous because they provide
the ability to resolve single transcripts without significant manipulation of the biological samples. More
recently, smFISH has been used to investigate gene expression in robust mammalian tissues, such as the
gut, that can withstand snap-freezing without cryo-protection and processing into ultra-thin sections
(Itzkovitz et al. 2012; Itzkovitz et al. 2012; Van Es et al. 2012). When this strategy was applied to more
fragile tissue samples, the morphology of the tissue was dramatically altered (Figure 2.2). In the present
work, we describe a method of adapting smFISH to monitor and quantify mRNA expression while
preserving the complex architecture of heterogeneous mammalian tissues. We have chosen the organ
of Corti of the cochlea as our sample mammalian tissue.
18
Figure 2.2 Alterations to smFISH protocol demonstrate improved tissue morphology. (A-F)
E16.5 mus musculus Sox2Cre;mT/mG cochleae. (A-C) Tissue processed with conventional
smFISH protocol, (A) phase image where arrow indicates the outer hair cell in the third row, (B)
DAPI staining, and (c) unsuccessful smFISH staining for Sox2 (green) and DAPI (blue). (D-F)
Tissue processed with adapted smFISH protocol, (D) phase image where arrow indicates the
outer hair cell in the third row, (E) DAPI staining, and (F) smFISH staining of Atohi (red), Sox2
(green) and DAPI (blue).
The organ of Corti of the cochlea contains the auditory sensory cells of the inner ear responsible
for transducing sound waves into chemical signals that activate auditory nerves (Dallos 1992). At
approximately day 14.5 of embryonic development, auditory prosensory cells begin to differentiate into
more than 12 different cell types, including inner hair cells, outer hair cells, pillar cells, Hensen cells, and
Dieter cells. Development occurs in a wave from base to apex of the cochlea, and at embryonic day 16.5
(E16.5) there are regions of the cochlea, which are both differentiated and undifferentiated (Figure 2.3
and 2.4). Each cell type has a precise spatial pattern and distinct function that is vital for normal hearing
(Groves and Fekete 2012). The specification of cell fate, number and patterning is influenced by many
genetic factors during cochlear development (Steel and Kros 2001). Here we describe the process used
to adapt the smFISH technique to image the cochlea. The cochlea is an ideal tissue to demonstrate the
power of the smFISH technique due to its fragile nature, complex geometry and composition of multiple
cell types of different functions. Furthermore, the developing cochlea is important because there is a
precise gradient of transcription factor expression extending from its base to apex.
19
IUll IMyOVI
Figure 2.3 Cochlear spiral demonstrates the complex geometry of the mammalian inner ear.
Figure adapted from Coate and Kelley 2013. (A) Shows the fluorescently labeled cochlea with the
spiral ganglion neurons (SGN) in green and hair cells (HC) in blue. The spiral of inner and outer
hair cells curves around the structure displaying the complex geometry of the cochlear duct. Midmodular sections are cross-sections taken through the middle of the organ and allow the
visualization of several frequency regions simultaneously. Peripherial axons (PA) and Central
axons (CA) are also stained in green. (B) A cross-sectional view of the peripherial axon (PA)
connection from the spiral neurons to the hair cells. This image displays the patterns of one inner
hair cell to three outer hair cells and connections of the auditory neuron fibers to the hair cells.
Hair cells (HCs), spiral ganglion neurons (SGNs), PAs, CAs.
20
Figure 2.4 Mid-modular cross-section of embryonic 16.5 cochlea depicts base
to apex morphological differences. The cross-sections of the organ of Corti are
seen in this figure as regions of cells surrounding the open circular spaces. The
base (high frequency) in located on the lower right-hand side and organ spirals
through to the apex (low frequency) in the upper left-hand corner. Dapi is used
to stain the cell nuclei and the spiral neurons can be visualized connecting the
hair cells to the nervous system. The patterning of one inner to three outer hair
cells is seen in the base. The scale bar is 125 microns.
2.2 Materials and methods
2.2.1 Reagents
Phosphate-buffered saline (lOx PBS, Ambion), formaldehyde 27% solution (Baker Analyzed
A.C.S.), paraformaldehyde 16% solution (EMS), 200 proof ethanol (VWR / Pharmco-AAPER
VWR),
nuclease-free water (Ambion), Vectabond (Vector Labs), dextran sulfate (Sigma), 20x SSC (Ambion),
formamide (Ambion), E.coil tRNA (Sigma), BSA (Ambion), vanadyl-ribonucleoside complex (NEB), TRIS
(Ambion), glucose (Sigma), 3M sodium acetate (Ambion), TE (Ambion), DMSO (Sigma), DAPI (Sigma),
glucose oxidase (Sigma), catalase (Sigma), sucrose (VWR) and Trolox (Sigma). All solutions were
prepared using RNAse-free reagents.
21
2.2.2 Mouse model system for smFISH
To acquire quantitative gene expression information as a function of cell type and location, it
was necessary to enable fluorescent detection of individual cells. Cre-Lox recombinant technology was
used to create transgenic mice expressing green fluorescent protein in cell membranes of cochlear cells.
For our studies, female homozygous mT/mG mice (Jackson Laboratory Stock# 007576) were bred with
Sox2cre heterozygous male mice mT/mG mice (Jackson Laboratory Stock# 004783) to produce
transgenic embryos.
2.2.3 SmFISH probe design
SmFISH probes were designed using a custom algorithm [now publicly available at
https://www.biosearchtech.com/stellarisdesigner/] to locate twenty oligonucleotide regions with 45 65% GC content in the cDNA of the open reading frame of a gene of interest. The restrictions on the GC
content are to ensure that all probes have a relatively uniform binding energy. The 5' and 3' regions of
the gene were included if the open reading frame search did not result in sufficient probe candidates.
For optimum performance, probes were separated by 2 nucleotides to ensure approximate spacing for
the fluorophore molecule. Selected probes were then subjected to BLAST (Basic Local Alignment Search
Tool) analysis of the entire genome, and those with significant alternative targets were removed from
the selection process. Forty-eight to 96 oligonucleotide probes with 3' amine end modification for
coupling to fluorophores were synthesized by Biosearch Technologies (Novato, CA). For coupling of
probes to fluorophores, 300 ng of Alexa594 (Invitrogen), TMR (Invitrogen) or Cy5 was combined with
48-96 nmol probes in 0.1 M sodium bicarbonate buffer and incubated at 37'C overnight. To each
sample, 0.1X volume of 3M sodium acetate and 2.5X volume of 100% ethanol was added. The solution
was vortexed thoroughly and incubated at -80'C overnight. The solution was centrifuged for 14000xg at
40 C for 20 minutes, and the supernatant was removed and discarded. Probes were then HPLC purified
using triethylamine acetate (0.1 M) and acetonitrile as buffers. The final probe concentration was
measured using Nanodrop.
2.2.4 Cochlear fixation
Female mice were placed in male mouse cages one day a week for precise timing and to
encourage mating. Pregnant female mice from our Sox2cre and mT/mG matings were euthanized at
select time points via CO 2 asphyxiation, and embryos were removed and dissected immediately. Right
inner ears were dissected out from GFP-positive embryos and incubated in 4% paraformaldehyde for 2-3
hours for fixation.
2.2.5 Tissue embedding and sectioning
Tissue was then subjected to a five-step sucrose gradient and placed in 30% sucrose in 1x PBS
overnight at 4C. The following morning, the inner ears were positioned in Optimal Cutting
Temperature (OCT; Sakura Finetechnical Co, Ltd, Tokyo, Japan) compound and immediately frozen with
dry ice. Normally, the sectioning of the block occurred within several days of embedding, however
blocks are stable if stored at either -80'C or -20*C for months.
Tissue was sectioned onto Vectabond-treated #1 coverslips (Electron Microscopy Sciences). In
preparation for Vectabond treament, coverslips were cleaned with 2% RBS soap for 15 minutes with
22
sonication and then rinsed well with nuclease-free water and with 70% ethanol for 15 minutes. The
coverslips are covered and air-dried for up to 2 days. Vectabond treatment was performed according to
the manufacturer's instructions. This involved immersion in acetone for 5 minutes, then in a 2%
Vectabond/98% acetone solution for 5 minutes followed by a gentle dip in RNAse-free water twice. The
coverslips were then dried at 30 0 C for 2-3 days
For sectioning, the blocks were mounted on a cryostat chuck using OCT mounting medium. All
cochleae were oriented in the same manner, with the modular of the spiral ganglion parallel to the
knife, for robust 3D reconstruction and consistency across cochleae. The chuck and tissue were
equilibrated to -20 0C before sectioning. The cut sections were placed on Vectabond-treated #1
0
0
coverslips and transferred onto dry ice and then stored at stored at either -80 C or -20 C, up to several
months.
As an alternative to OCT, we also embedded some tissue samples in gelatin. Porcine gelatin
powder (15 g) was mixed with 70 ml cold ddH20. The mixture was heated and stirred until the gelatin
was completely dissolved. Next, 2.5 ml glycerin was added to ddH20 to a total volume of 100 ml. The
0
prepared solution was stored at 40 C until use at which point it was remelted in a 60 C oven or water
bath. The solution was then cooled to 37 0C and used immediately. The warmed gelatin was poured into
a clean, RNAse-free 5-ml plastic beaker. Cochleae were transferred after cryoprotection into warmed
0
PBS at 37 0 C for 15 minutes. The cochlea were placed in the warmed gelatin and incubated in a 35 C 40 0 C oven for 30-45 minutes. The gelatin was transferred into a Peel-A-Way mold, and the cochlea was
0
oriented properly. The block was allowed to cool at room temperature and then chilled to 4 C until
sectioning. After sectioning, gelatin coverslips required additional time to dry before being storied at 80 0 C.
2.2.6 Hybridization
Frozen tissue sections were allowed to warm to room temperature for approximately five
minutes and then fixed to the treated coverslips with 4% paraformaldehyde for 15 minutes at room
temperature in a fume hood and washed with PBS. Sections were stored in 70% ethanol for 24 hours or
for up to three weeks at 4'C and then washed in a 10% formamide wash buffer solution for 5 minutes.
To hybridize tissue sections to probes, each section was incubated with 5 - 50 ng/50 - 100 ml probes at
30'C for 16-20 hours. Coverslips are then washed with a 10% formamide solution in preparation for
imaging.
2.2.7 SmFISH Imaging
SmFISH imaging of sections was performed on a Nikon Ti-E invetered fluorescence microscope
equipped with a 100x oil-immersion objective and a Photometricx Pixis 1024B charge-coupled device
(CCD) camera using MetaMorph software (Molecular Devices). Acquired images were then analyzed
with a custom-designed algorithm, ImageM, in MatLab. ImageM allows for the localization of individual
mRNA transcripts and segmentation of individual cells.
2.3 Results
Previous work has indicated that smFISH is a useful tool for elucidating gene expression patterns
in single cells and certain robust mammalian tissues (Buganim et al. 2012; Itzkovitz et al. 2012). In order
to apply this tool to the developing auditory system, we first had to modify the existing protocol to
ensure high quality tissue morphology and robust gene expression profiles. We obtained training on
23
traditional manipulation of embryonic cochlear tissues from the Kelley laboratory at NIH and adult
tissues from the Stankovic, Sewell and Liberman laboratories at Massachusetts Eye and Ear Infirmary.
We introduced these approaches to the smFISH technique in a systematic manner, thereby developing a
new approach to working with highly delicate cochlear tissues.
We used the previously-published smFISH technology as our initial template for investigating
biological signaling phenomena in these systems. To study gene expression in the mouse inner ear, we
adapted the smFISH strategy to investigate mRNA transcript expression patterns in the cochlea during
auditory hair cell development. Our first step was to make modifications to the smFISH protocol to
improve cochlear tissue morphology. Next, we applied the smFISH technique to a transgenic mouse
model to observe single transcripts within individual cells. We then investigated whether the adaptation
of smFISH to mammalian cochlear tissue results in an accurate assessment of target gene expression.
Finally, we expanded the use of this technique to study specific transcripts during hair cell development.
2.3.1 Modifications to smFISH techniquefor improved tissue adhesion and morphology
To study the transcriptional properties of hair cells in a single-cell, quantifiable fashion using
smFISH, we first considered the technical aspects of working with such a delicate tissue as the cochlea.
When previously published smFISH processing protocols were initially applied to the cochlea, they
resulted in the loss of structural integrity of the fragile tissue structures that impedes cell-specific
localization of mRNA transcripts (Figure 2.2). It was obvious that many additional adaptations would be
needed for successful implementation. First, we developed a successful working smFISH protocol for
use on developing cochlear tissue, which can be generalized to other tissue types of a similar sensitive
nature.
One of the first considerations was how to preserve the morphology of the fragile cochlear
tissue during smFISH processing. The body protects the mammalian cochlea by encasing it in the very
hard petrous portion of the temporal bone (Dallos and Fay 1996). Imaging tissue structures that are
embedded in bone can significantly complicate the imaging process. Bone often has a strong autofluorescent signal, which can obscure the smFISH signal. Additionally, the conventional smFISH protocol
for use on wide-field microscopy requires thin sections of tissue to reduce the noise from out-of-focus
light. Achieving thin sections of tissue embedded in bone is a difficult and complicated process. To
address the additional noise from increased autofluoresence, we attempted to perform smFISH on
cochlear tissue sections using confocal microscopy, which depends on an optical sectioning technique
that bypasses the noise from out-of-focus light. However we found that most confocal imaging set-ups
were not equipped with a detector with sufficiently high sensitivity; thus, either we were unable to see a
signal above the noise or the signal bleached during the process of finding the focal point of the tissue
(Figure 2.5).
24
Figure 2.5 Example of smFISH for Jagi in E16.5 cochlea with
confocal and wide-field microscopy. Confocal microscopy
with a Zeiss 780 instrument was used to obtain the top image
and wide-field microscopy with a Nikon instrument was used
to obtain the lower image. Both images were obtained with
Jagi staining of an E16.5 cochlea section. The confocal image
does not include any averaging, however with improved antibleaching techniques and additional averaging it is hopeful
that the resolution of these images would be very similar.
Images are from different sections from the same cochlea.
25
We then attempted single molecule fish on a specialized sectioning set up that could cut
through bone and tissue that had been decalcified. To do this, there are three options that we explored:
(1) decalcifying the bone using strong acids, (2) sectioning the bone using a Leica Cryostat and the
Cryojane tape transfer system (Leica Microsystems) and (3) targeting tissue before the petrous bone has
hardened. We found that smFISH works with all three avenues. However, in the first two cases the
tissue morphology was not as well preserved (Figure 2.6). Therefore, we focused on working with tissue
from the embryonic and early postnatal stages of the mouse before hardening of the petrous bone.
Figure 2.6 Example of Beta actin expression with smFISH in HCI treated P19 cochlea. The tissue was dissected
out after perfusion, fixed at 4*C for several hours and then treated with HCI for ten minutes. The cochlea
undergoes a sucrose gradient and embedded in OCT. The black box represents a zoomed-in-view of this region of
tissue. The images above are from a region near the hair cells, however the portions of the sections containing the
hair cells was torn away. It is possible that the high-acid treatment altered the tissue's fragility and additional
steps might be needed to perform smFISH robustly on juvenile cochlea tissue. DAPI - blue, Beta actin - green.
26
We found that embryonic cochleae were easily damaged by the snap-freezing step of the
conventional smFISH protocol. SmFISH was previously used on samples, which did not require cryoprotectant steps, so it was uncertain how a cryo-protectant would influence the smFISH signal since it
requires a very low auto-fluorescent background. However, if cochlear tissue was not exposed to a
cryo-protectant, then the snap-freezing process would create scattered holes in the tissue and the tissue
would tear during the sectioning process. The holes and the tears made it difficult to determine the
precise morphology. We then introduced a standard cryo-protectant technique, a graded concentration
of sucrose steps, which we will refer to from now on as a sucrose gradient, so that developing cochlea
could be protected during the snap-freezing process. We then performed a systematic analysis of
applying a sucrose gradient prior to snap-freezing the tissue and measuring the smFISH signal-to-noise
ratio. We found that a sucrose gradient mixture of 30% sucrose/1xPBS and 1/xPBS applied in steps of
5%, 10%, 15%, 20%, 25% and 30% produced the best tissue morphology, while preserving the RNA for
smFISH imaging. For each one of the steps from 5% through 25%, the tissue was transitioned to the
next stage as soon as it sank in the fluid. Once the tissue completed the 30% stage, it was placed in 30%
sucrose/1xPBS overnight at 40C. The tissue was placed in OCT, oriented and snap-frozen in CO 2 as
quickly as possible. We found that it was essential to move through the steps as quickly as possible, use
RNAse free reagents and freeze the tissue quickly to preserve the RNA.
Additionally, we wished to reduce the interval between tissue dissection and freezing to better
preserve the RNA within the tissue. Accordingly, we dissected out the cochleae from the temporal bone
and reduced the fixation time. We found that at embryonic day 16.5 (E16.5), a fixation time of 3 hours
in freshly prepared 4% paraformaldehyde (pfa) performed best.
In addition to improving the tissue morphology, we found that the method used to adhere the
tissue to the coverslips before imaging resulted in significant loss of tissue during the washing steps. As
much as 90% of the tissue would be lost before imaging. The fully-developed, adult organ of Corti is a
fluid-filled structure. When the tissue is fixed and sectioned, the natural morphology of the structure
contains several open spaces, which increase the fragility of the tissue and decrease the amount of
surface area that can adhere to the coverslip seen in Figure 2.4 (cross section of E16.5 tissue). To
mitigate this issue, we looked for an alternative method than the poly-l-lysine for adhering tissue. We
found that using Vectabond drastically improves adhesion of the cochlear tissue to the coverslip and can
be used with smFISH. Consequently, almost the entire tissue would adhere to the coverslips through all
of the washing steps. The addition of these steps allowed us to robustly apply the smFISH technique to
the developing mammalian inner ear.
2.3.2 SmFISH in a transgenic mouse model
For our studies, we wished to observe the RNA transcripts within individual cells as well as the
tissue structure. SmFISH had often been used with cell lines or simple organisms for which the cell fates
are precisely mapped out (e.g., C. elegans); consequently, there was previously no need to observe
cellular boundary lines (Raj et al. 2006; Zenklusen et al. 2008; Raj et al. 2010; Trcek et al. 2012).
Antibody staining for cell boundaries (e.g., CellMask or e-cadherin staining) can be used as an alternative
to transgenic technologies since immunofluorescence can be used in combination with smFISH. When
performing smFISH on the gut of adult mouse tissue, an antibody for e-cadherin was used to highlight
the boundaries between cells (Itzkovitz et al. 2012). There is some controversy regarding the expression
of e-cadherin in the organ of Corti during late embryogenesis and early postnatal development (Whitlon
1993; Whitlon et al. 1999; Simonneau et al. 2003). However, it is clear that e-cadherin is not universally
expressed throughout the ear during these periods. Therefore, we could not use the e-cadherin
antibody to observe the boundaries between cells during the development of mouse auditory hair cells
between embryonic day 13.5 until 18.5 (E13.5 - E18.5).
27
To observe cellular boundaries, we used Cre-Lox recombinant technology was used to create
transgenic mice expressing green fluorescent protein in cell membranes of cochlear cells. Cre-Lox
technology involved mating a mouse strain expressing Cre recombinase with a stain containing a
flanking loxP sites (Araki et al. 1997; Hayashi et al. 2002; Sauer and Henderson 1988; Sternberg and
Hamilton 1981). In the progeny, the Cre enzyme and the loxp sites undergo DNA recombination (Nagy
2000). For our study, mice with a Sox2 promoter driving Cre recombinase expression were mated to
mT/mG reporter mice (Figure 2.7A). The mT/mG mice express tdTomato in cell membranes before
recombination and GFP afterwards (Muzumdar et al. 2007). Approximately half of the embryos will
express GFP. The other half of the embryos will express a red fluorescent protein (RFP) and are not used
for experimentation because the bright signal in the red channel bleeds into channels containing smFISH
probes. When imaged, the GFP signal distinctly highlights the boundary of cells, as the GFP is modified
with an N-terminal membrane tag. We observed that all cells with nuclei contained GFP as a cell
boundary marker in the cochleae of Sox2cre;mT/mG progeny by age E6.5. Figure 2.7B shows the GFPlabeled boundaries (arrow) of cells in the cochlea in a Sox2cre;mT/mg mouse. Figure 2.7C shows the cell
boundary marker in conjunction with the smFISH signal (Fig. 2.7C). The GFP outline is used as a tool for
single cell analysis for all cochlear cells (Figure 2.8).
28
Transgenic vector
A
pCA
X
nhancer
kb
I kb
Cre
pCA
Figure 2.7 Transgenic mouse allows for the visualization of cell boundaries with smFISH (A)
Schematic and example of Cre-Lox technology used for generating fluorescent cell boundaries.
Sox2cre male mice with Sox2 transgenic construct (Hayashi, Lewis et al. 2002) are crossed with
female mT/mG mice (Muzumdar, Tasic et al. 2007). Mating produces progeny with Cre
recombination on loxp sites (pA) to convert expression of cellularly bound tdTomato (mT)
fluorescent to GFP (mG) driven by a Sox2 promoter. (B) An example of E16.5 tissue from a
Sox2;mT/mG embryo. Arrow points towards inner hair cell (IHC). GFP bound to cellular membranes
allows for the visualization of individual cell boundaries. (C) SmFISH with GFP tissue demonstrates
that smFISH can be used in conjunction with the transgenic mouse model. IHC with large arrow and
arrow heads point towards the three outer hair cells. Zoomed in image of (B). GFP staining is shown
in red and Atohl mRNA is in green.
29
.M
..
GFP
Figure 2.8 GFP-labeled cell boundaries are evident in a fixed cochlea section. The cross-section of
this E16.5 cochlea demonstrates that the GFP fluorescent cell boundaries are seen in cells all
throughout the cochlea. The scale bar 125 microns.
2.3.3 Validation of smFISH technique in the mammalian cochlea
To test our modified version of smFISH, we performed several validation experiments to confirm
that the integrity of the technique is maintained with the changes in tissue processing and to illustrate
the power of the applied signal-molecule technique. To first validate the smFISH procedure applied to
the mammalian cochlea, we chose to investigate Atohl, a well-characterized transcription factor that
has been shown to be a sufficient and necessary molecule for mammalian auditory hair cell production
(Bermingham et al. 1999; Chen et al. 2002). We compared Atohl transcriptional level changes during
hair cell development in embryonic mice using smFISH to that acquired using conventional FISH
techniques (Figure 2.9). We found that qualitatively the expression patterns for Atohl at E16.5 and PO
with smFISH match those reported in the literature with conventional in situ hybridization (Figure 2.9).
We proceeded to test other known molecules that are expressed throughout embryonic development.
We found that smFISH robustly replicated reported expression patterns in the literature, and in some
cases enhanced the expression pattern previously found (Figure 2.10).
30
C
Figure 2.9 Comparison between conventional ISH and smFISH in the developing cochlea. (A) Conventional ISH
for Atohl in E17 cochlea, tailed arrow indicates inner hair cell (IHC) and blunt arrows indicate outer hair cells
(OHC), (Hartman, Hayashi et al. 2007) (B) smFISH for E16.5 cochlea for Atohl (red), Sox2 (green) and DAPI (blue)
with same arrow pattern. (A) and (B) demonstrate similar qualitative results. (C) Conventional ISH for Wnt5a in
middle turn of E16.5 cochlea with IHC indicated by blunt arrow and OHCs indicated by tailed arrows (Qian et al.
2007), and (D) smFISH for Wnt5a in middle turn of E16.5 cochlea with similar arrow pattern.(C) and (D) indicate
similar expression patterns for regions neighboring hair cells, however smFISH demonstrates additional gene
expression in cells surrounding the entire cochlear duct.
31
Figure 2.10 Expression of Atohl in E16.5 and PO organ of Corti tissue. SmFISH was performed on two
different time points E16.5 and PO to demonstrate the quantitative and qualitative qualities of smFISH. In
the E16 apex there is no Atohl expression, but there is expression in the middle turn. At PO there is a high
level of expression in the apex and the expression is lower in the middle turn. This data was used in
additional serial sections from this and other cochlea to determine the fold change of Atohl from E16 to PO.
Atohl was found to increase over five-fold from E16 to PO in the three techniques explored. The scale bar is
10 microns.
We next validated the use of the smFISH technique by conducting a comparison assay with
established RNA-Seq and RT-PCR techniques, probing Atohl expression at two different time points
throughout hair cell development. The fold change expression profiles were evaluated for both Atohl
and Gapdh in cochlear tissue using RNA-Seq, RT-PCR and smFISH. For smFISH, six 8-im mid-modular
sections were taken from each cochlea for evaluation. Automated image processing was used to detect
the total number of transcripts from each cochlea. The results were then extrapolated and estimated
from base to apex. To do this we first estimated the length of the cochlea at the two different time
32
points. We then took our results from the 48 sections and calculated the average expression level in the
organ of corti for each of the 4 developmental stages, and multiplied by the approximately length of
each developmental stage to get total gene expression. Fold expression profiles from each method
were computed and compared, as shown in Table 2.1.
Quantitative
RT-PCR
SwissWebster
E16 E18
Increases by
5.66
Bermingham-McDonogh,
Univ of Washington
(Munnamalai et al. 2012)
Single
molecule
RNA FISH
Table 2.1 Comparison of fold change of Atohl with three different methods.
The smFISH-based quantification of Atohl gene expression shown in Table 1, which was
comparable to the results of RT-PCR and RNA-Seq obtained by other investigators, indicates that
identification of transcription factors is feasible using this method. It is important to note that
established methods such as RT-PCR do not measure the amount of mRNA transcripts in a sample
directly, but represent a comparison of a target gene and a control or housekeeping gene to produce a
relative expression ratio (Pfaffl et al. 2002). Also, RNA-Seq was performed on cells that were sorted
during flow cytometric analysis and represents the evaluation of a subset cells outside of their natural
environment. Consequently, both RNA-seq and RT-PCR are subject to RNA degredation and represent
only an estimate of the fold change gene expression rather than an absolute count of transcript levels.
In contrast, smFISH has the advantage of providing information about both the quantitative gene
expression and the spatial pattern of this expression in an intact cochlea
2.3.4 SmFISH in the developingmammalian cochlea during hair cell development
We chose to investigate two transcription factors known to play an important role in hair cell
development, Sox2 and Atohl (Kelberman et al. 2006; Kiernan et al. 2005). These genes were chosen to
demonstrate the power of smFISH with genes of dramatically different expression patterns that have
been thoroughly characterized with immunofluorescence (Bermingham et al. 1999; Chen et al. 2002;
Kelberman et al. 2006; Kiernan et al. 2005). Sox2 was chosen because its transcriptional expression
profile has well-defined increasing and decreasing stages during hair cell development (Mak et al. 2009).
Sox2 is expressed in the region of developing hair cells, and models have suggested that it has significant
interactions with Atohl during hair cell development (Hume et al. 2007; Mak et al. 2009; Dabdoub et al.
33
2008; Neves et al. 2012). Atohl encodes a transcription factor essential for hair cell differentiation and
has a well-characterized spatial and temporal pattern of expression during development (Bermingham
et al. 1999; Chen et al. 2002). In Figure 2.11 we show the expression patterns of both Sox2 and Atohl in
the E16.5 cochlea. Our data qualitatively matches that which has been previously published, and also
demonstrates the ability to use smFISH to detect 2 transcripts, in this case transcription factors,
simultaneously. Further investigation of the nature of Atohl and Sox2 expression will be presented in
Chapters 3.
34
Figure 2.11 Expression patterns of Atohi and Sox2 in the E16.5 cochlea. Here we show
the expression patterns of Atohi and Sox2 in the base of the E16.5 cochlea. (A) A crosssectional view of the whole cochlea using a 10x objective. GFP labels the cell boundaries
(green) and DAPI stains the cell nuclei. (B) smFISH expression pattern for both Sox2 (red)
and Atohl (green). (C) A zoomed-in view shows that both transcripts types are seen in
the hair cells. Transcripts are more localized to the apical ends of the hair cells because
they are more highly concentrated in the cytoplasm, as opposed to the nucleus. DAPI is
used to stain the cell nuclei in blue.
35
2.4 Discussion
This chapter reported the changes made to the conventional smFISH protocol to enable its
application to fragile tissues and illustrated its successful use in a transgenic mouse model. It also
showed that the smFISH modifications we made could be applied to embryonic and postnatal cochleae.
Although the development of smFISH for juvenile cochlea analysis is in the early stages, it is very likley
that with continued improvements, the tissue morphology of juvenile and eventually adult cochleae
processed with smFISH will advance. Some routes that have proven promising in preliminary
experiments include optimizing the gelatin embedding techniques and adding DMSO to the sucrose
gradient. As anti-bleaching techniques improve, the use of smFISH on confocal microscope set-ups will
also continue to advance.
Direct comparison of mRNA expression levels from smFISH and alternative techniques require
that some caveats be mentioned. There are some limitations using smFISH on tissue sections using
wide-field microscopy. Tissue sections used for smFISH must balance enough thickness to preserve
morphology with thinness because of the limited penetration depth of the microscope. We determined
that the penetration depth of our setup was approximately 2.5 microns. Additionally, we found with our
methods that sections thinner than 6 microns had compromised morphology, such as holes and tearing.
Therefore, we found that frozen tissue sections of 6.8 microns were optimal for our technique to
preserve morphology, even though we were not able to image through the whole section. Therefore we
can only estimate the overall levels of Sox2 and Atoh2, as we cannot measure every transcript. One
possible route to improve tissue morphology for thinner sections is using paraffin embedding, which
was tested with smFISH for other tissue types (Crosetta N, personal communication).
Although RT-PCR offers quantification over a wide numerical range, in addition to high
sensitivity and robust reproducibility, it too has its limitations (Pfaffl et al. 2002; Bustin 2002). RT-PCR is
dependent on the use of primers, and different primer sets can amplify transcripts at different
efficiencies. Discrepancies in amplication processes can skew quantitive results. Despite these
limitations, the comparison of data from mRNA levels between smFISH and RT-PCR has provided
promising findings in A549 cells (Raj et al. 2008) and the present study. RNA-Seq data is associated with
technical noise and read errors that may also obscure the quantitative gene expression results. In
addition to the potential limitations of the smFISH technique and the established techniques, different
mouse models were used in the validation experiments, and the experiments were conducted in
different laboratories. Despite all of these differences, the three techniques produced similar fold
changes at the time points tested.
2.5 Conclusions
In summary, the work presented here focuses on adapting smFISH technology to investigate
genes regulating hair cell developing in the cochlea. We demonstrated the application of smFISH to
postnatal and embryonic inner ear tissue. We applied the smFISH technique to the developing cochlea
in a transgenic mouse model and demonstrated the localization and quantification of mRNA transcripts
in individual cells. We validated the use of smFISH to study transcriptional profiles during hair cell
development by comparing the fold change of Atohl at two different type points with three separate
methods, including smFISH, RT-PCR and RNA-Seq. We found that the fold changes reported for each
method to be extremely similar, making us confident that we could use smFISH to study the
development of the mammalian hair cell. We detail these studies in the next two chapters.
36
2.6 References
Alwine JC, Kemp DJ, Stark GR (1977) Method for detection of specific RNAs in agarose gels by transfer to
diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc NatlAcad Sci USA 74(12):5350-4.
Araki K, Imaizumi T, Okuyama K, Oike Y, Yamamura K (1997) Efficiency of recombination by Cre transient
expression in embryonic stem cells: comparison of various promoters. J Biochem 122(5):977-982.
Bartlett J M, Stirling D (2003) A short history of the polymerase chain reaction. Methods Mol Biol 226:36.
Buganim Y, et al. (2012) Single-cell expression analyses during cellular reprogramming reveal an early
stochastic and a late hierarchic phase. Cell 150(6):1209-1222.
Bustin SA (2002) Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and
problems. J Mol Endocrinol 29(1):23-39.
Coate T, Kelley MW (2013) Making connections in the inner ear: recent insights into the development of
spiral ganglion neurons and their connectivity with sensory hair cells. Semin Cell Dev Bio 24:460-469.
Dabdoub AC, et el. (2008) Sox2 signaling in prosensory domain specification and subsequent hair cell
differentiation in the developing cochlea. Proc Natl Acad Sci USA 105(47):18396-18401.
Dallos P (1992) The active cochlea. J Neurosci 12(12):4575-4585.
Dallos P, Popper AN, Fay RR (1997) The Cochlea (Springer-Verlag, New York City).
Femino AM, Fay FS, Fogarty K, Singer RH (1998) Visualization of single RNA transcripts in situ. Science
280(5363):585-590.
Groves AK, Fekete DM (2012) Shaping sound in space: the regulation of inner ear patterning.
Development 139(2):245-257.
Hayashi S, Lewis P, Pevny L, McMahon AP (2002) Efficient gene modulation in mouse epiblast using a
Sox2Cre transgenic mouse strain. Mech Dev 119 Suppl 1:S97-S101.Hume CR, Bratt DL, Oesterle EC
(2007) Expression of LXH3 and SOX2 during mouse inner ear development. Gene Expr Patterns 7(7):798807.
Hurteau GJ, Spivack SD (2002) mRNA-specific reverse transcription-polymerase chain reaction from
human tissue extracts. Anal Biochem 307(2):304-315.
Itzkovitz S, Blat IC, Jacks T, Clevers H, van Oudenaarden A (2012) Optimality in the development of
intestinal crypts. Cell 148(3):608-619.
Itzkovitz S, et al. (2012) Single-molecule transcript counting of stem-cell markers in the mouse intestine.
Nat Cell Biol 14(1):106-114.
Itzkovitz S, van Oudenaarden A (2011) Validating transcripts with probes and imaging technology. Nat
Methods 8(4 Suppl):S12-19.
Kelberman D, et al. (2006) Mutations within Sox2/SOX2 are associated with abnormalities in the
hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Invest 116(9):2442-2455.
37
Kiernan AE, et al. (2005) Sox2 is required for sensory organ development in the mammalian inner ear.
Nature 434(7036):1031-1035.
Killick R, Richardson G (1997) Isolation of chicken alpha ENaC splice variants from a cochlear cDNA
library. Biochim Biophys Acta 1350(1):33-37.
Levsky JM, Singer RH (2003) Fluorescence in situ hybridization: past, present and future. J Cell Sci 116(Pt
14):2833-2838.
Mak AC, Szeto IY, Fritzsch B, Cheah KS (2009) Differential and overlapping expression pattern of SOX2
and SOX9 in inner ear development. Gene Expr Patterns 9(6):444-453.
Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y (2008) RNA-seq: an assessment of technical
reproducibility and comparison with gene expression arrays. Genome Res 18(9):1509-1517.
Munnamalai V, Hayashi T, Bermingham-McDonogh 0 (2012) Notch prosensory effects in the
Mammalian cochlea are partially mediated by Fgf20. J Neurosci 32(37):12876-12884
Murphy D (2002) Gene expression studies using microarrays: principles, problems, and prospects. Adv
Physiol Educ 26(1-4):256-270.
Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L (2007) A global double-fluorescent Cre reporter mouse.
Genesis 45(9):593-605.
Nagy A (2000) Cre recombinase: the universal reagent for genome tailoring. Genesis 26(2):99-109.
Neves J, Vachkov 1, Giraldez F (2013) Sox2 regulation of hair cell development: incoherence makes
sense. Hear Res 297:20-29.
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST@) for group-wise
comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res
30(9):e36.
Raj A, Peskin CS, Tranchina D, Vargas DY, Tyagi S (2006) Stochastic mRNA synthesis in mammalian cells.
PLoS Biol 4(10):e309.
Raj A, Rifkin SA, Andersen E, van Oudenaarden A (2010) Variability in gene expression underlies
incomplete penetrance. Nature 463(7283):913-918.
Raj A, van den Bogaard P, Rifkin SA, van Oudenaarden A, Tyagi S (2008) Imaging individual mRNA
molecules using multiple singly labeled probes. Nat Methods 5(10):877-869.
Raj A, van Oudenaarden A (2009) Single-molecule approaches to stochastic gene expression. Annu Rev
Biophys 38:255-270.
Sauer B, Henderson N (1988) Site-specific DNA recombination in mammalian cells by the Cre
recombinase of bacteriophage P1. Proc NatlAcad Sci USA 85(14):5166-5170.
Shiao YH (2003) A new reverse transcription-polymerase chain reaction method for accurate
quantification. BMC Biotechnol 3:22.
38
Simonneau L, Gallego M, Pujol R (2003) Comparative expression patterns of T-, N-, E-cadherins, betacatenin, and polysialic acid neural cell adhesion molecule in rat cochlea during development:
Implications for the nature of Kolliker's organ. J Comp Neurol 459(2): 113-126.
Steel KP, Kros CJ (2001) A genetic approach to understanding auditory function. Nat Genet 27(2):143149.
Sternberg N, Hamilton D (1981) Bacteriophage P1 site-specific recombination. 1. Recombination
between loxP sites. J Mol Biol 150(4):467-486.
Streit S, Michalski CW, Erkan M, Kleeff J, Friess H (2009) Northern blot analysis for detection of RNA in
pancreatic cancer cells and tissues. Nat Protoc (1) 37-43.
Thompson RE, Larson DR, Webb WW (2002) Precise nanometer localization analysis for individual
fluorescent probes. Biophys J 82(5):2775-2783.
Trcek T, Chao JA, Larson DR, Park HY, Zenklusen D, Shenoy SM, Singer RH (2012) Single-mRNA counting
using fluorescent in situ hybridization in budding yeast. Nat Protoc 7(2):408-419.
Van Es JH, et al. (2012) DI1+ secretory progenitor cells revert to stem cells upon crypt damage. Nature
Cell Biology 14(10):1099-1104.
Vandesompele J, et al. (2002). Accurate normalization of real-time quantitative RT-PCR data by
geometric averaging of multiple internal control genes. Genome Biol 3(7):RESEARCH0034.
Whitlon DS (1993) E-cadherin in the mature and developing organ of Corti of the mouse. J Neurocytol
22(12):1030-1038.
Whitlon DS, Zhang X, Pecelunas K, Greiner MA (1999) A temporospatial map of adhesive molecules in
the organ of Corti of the mouse cochlea. J Neurocyto. 28(10-11):955-968.
Zenklusen D, Larson DR, Singer RH (2008) Single-RNA counting reveals alternative modes of gene
expression in yeast. Nat Struct Mol Biol 15(12):1263-1271.
39
Chapter 3 Graded Notch signaling robustly specifies fate decisions during mammalian hair cell
development
3.1 Introduction
The mammalian inner ear connects the sounds of the outside world to the brain. Within the
inner ear, the cochlea converts physical sounds waves into electrical signals that are sent to the brain.
The mammalian cochlea houses the sensory cells of the auditory system (Kelley, Driver et al. 2009).
Study of the cochlea is made difficult by its location deep within the temporal bone and the structural
complexity of its main component, the organ of Corti (Gray and Carter 1858; Driver and Kelley 2009). It
is known that gene expression patterns during development are strictly controlled and vary across the
length of the organ of Corti from base to apex, leading to a highly stereotyped arrangement of
specialized hair cells and supporting cells (Kelley, Driver et al. 2009; Groves and Fekete 2012).
Additionally, it has been shown that transcription factors, such as Atohl, Neurodi and Neurogl, play
significant roles in determining cell fate (Bermingham, Hassan et al. 1999; Fritzsch, Eberl et al. 2010;
Jahan, Pan et al. 2010; Jahan, Pan et al. 2012). SmFISH is a suitable technique to investigate the genes
involved in this specification of cellular identity and distribution during cochlear development. In
particular, smFISH provides sufficient spatial resolution to detect mRNA transcripts within individual
cells, the sensitivity to visualize transcripts with low expression levels, and the quantitative power
necessary to refine the current theories about the molecular players involved in auditory hair cell
development.
The ordered distribution and specialization of hair cells is vital for proper sensory transduction in
the auditory system (Ashmore 2008). Investigating differential gene expression in the cochlea during
development using smFISH provides valuable insight into the molecular processes underlying hair cell
dynamics. In order to study the transcriptional properties of hair cells, we systematically modified our
established smFISH technique to preserve the morphology of the delicate tissues (Chapter 2). Results
indicated that these modifications preserved cochlear morphology while enabling effective hybridization
of smFISH probes to the specialized sensory structures of the developing murine inner ear. Here, we will
describe the expression patterns of gene expressed during hair cell development, and focus our
investigations on molecules linked to hair cell fate and the Notch pathway.
3.1.1 Mammalian Inner Ear Development
The inner ear forms from the surface of the ectoderm (reviewed in Wu 2012). The surface of
the ectoderm thickens and folds in to form the otic placode. The dorsal component of the placode
forms the vestibular organs of the inner ear, and the ventral portion of the otic placode continues on to
become the auditory structures of the inner ear (Figure 3.1) (Morsli 1998). There are five vestibular
organs that make up the inner ear: three semi-circular canals, responsible for the detection of angular
head movements, and two linear acceleration sensory organs, the utricle and the saccule. The auditory
component of the otic placode expands and forms into the mammalian cochlea over the course of seven
days in the mouse. The sensory epithelium of the auditory portion of the inner ear is responsible for
converting the physical sound waves collected by the external ear into the neurological signals for the
brain.
40
Figure 3.1 Gross morphology of murine inner ear during development displays rapid changes (adapted from
Morsli et al. 1998). Inner ear is derived from the otic placode, arising from the ectoderm and located next to the
hindbrain. The inner ear houses six sensory organs - five vestibular and one auditory cochlea. These paint fills
demonstrate the gross morphological changes that occur in the mouse inner ear from E10.75 to E17. Around E13
the cochlear duct begins to spiral out from the inner ear (arrows), a process which continues until E17. This spiral
contains the organ of Corti, home to the hair cells and support cells.
After formation, invagination and closure of the otic placode, the organ enters the otocyst stage
where cells are presumed to fall within three categories: neural, sensory or nonsensory. Cells that are
determined to fall within the neural category will proceed on to become the nerve fibers of the
cochleovestibular ganglion. Nerve fibers of the cochlear-type will branch out to the sensory hair cells
within the cochlea. Cells that fall into the nonsensory category will continue on to make up the support
structures of the organ, cells of secretory and absorptive functions, and cells of the endolymphatic duct
and semicircular canals. Finally, the cells categorized as sensory, referred to as prosensory cells,
continue to develop into the sensory hair cells and the support cells of the organ of Corti.
The transition of the otocyst into the five vestibular organs and the formation of the cochlear
duct involve many stages, signaling pathways and complex steps (Wu 2012). Decisions are made
concerning the anterior-posterior, dorsal-ventral and medial-lateral axes of the otic placode, and to
direct cells into the three distinct precursory cell types. These stages are outside the main focus of the
thesis and will not be reviewed here. The cochlear duct begins its formation at the ventral tip of the
otocyst and extends out into a spiral. This spiral shape gives the cochlea its name, since it resembles a
snail's shell (Dallos 1992). There are several molecular factors that play a role in the extension of this
organ, including molecules from the Hedgehog pathway, transcription factors Tbxl and Pou3f4 and
potentially influences from retinoic acid signaling (Riccomanor 2002, Bok 2005, Phippard 1999 and
Braunstein 2008, 2009).
At the adult stage, the cochlear duct consists of a bony and membranous labyrinth, where the
membranous portion of the structure is protected by the rigid wall of the bony labyrinth (Dallos 1992).
The membranous labyrinth contains both the sensory and nonsensory cells of the cochlea, in addition to
a system of fluid-filled cavities surrounding fragile precisely patterned tissue structures (Figure 3.2)
(Dallos 1992). The duct consists of three compartments, the scala vestibuli (SV), the scala media (SM)
and the scala tympani (ST). The fluids within these compartments help to make the mammalian cochlea
unique. The SV and ST are filled with perilymph, which is similar in ionic concentration to cerebrospinal
fluid and consists of predominantly sodium ions, however endolymphatic fluid is bound within the SM
(Bosher 1968). Endolymph contains a high concentration of potassium ions, and an unusually high,
positive potential of 80 to 90 mV. Specialized gap junctions and pumps exist within the cochlear duct to
supply the endolymph with the potassium ions it needs to maintain such a high potential. This high
41
extracellular potential is vital in maintaining a high intracellular-to-extracellular difference between the
fluids in the endolymph and the mammalian sensory hair cells that are housed in the organ of Corti.
3.1.2 Murine Inner Ear Anatomy
The spiral of the cochlear duct contains one of the most impressive and beautifully patterned
organs, the organ of Corti (Figure 3.2). The organ of Corti contains the basilar membrane, on which the
supporting cells sit. This membrane changes in its width and stiffness from base to apex, which
contributes.to the cochlea's ability to detect sound over a 100-fold frequency range. Active processes
that increase the sensitivity of sound detection are performed by specialized hair cells that are
surrounded by several supporting cell types. For each cross-section of the organ of Corti there are two
rows of pillar cells (inner and outer pillar cells), three rows of Dieter cells, a row of Hensen cells, several
rows of Claudius cells in addition the sensory cells - one row of inner hair cells (IHCs) and three rows of
outer hair cells (OHCs). There are several differences between the two types of hair cells. The
prominent stereocilia, which sit on top of their apical surface are in different formations, the innervation
patterns between the cells and the auditory nerve fibers differ, the number of cells in each cross-section
are different, there is a precise ratio of one inner hair cell to three outer hair cells in the mammalian
cochlea, and they have drastically different roles to play to converting the sounds of the outside world
to the brain. Additionally, there is a non-cellular structure called the tectorial membrane (TM) which sits
directly above the hair cells. OHCs have their stereocilia embedded in the gelatinous structure of the
TM, while the stereocilia of the IHCs are not embedded in the TM.
Figure 3.2 Cross-section of adult mammalian cochlea
demonstrates precise patterning (adapted from Dallos
1992). This drawing demonstrates the three separate regions
in the adult mammalian cochlea. The scala vestibule (SV) and
scala tympani (ST) are filled with perilymph. The scala media
is filled with a special fluid, endolymph, which contributes to
the active processes of the auditory hair cells. The outer hair
cells stereocilia are embedded in the tectorial membrane,
whereas the stereocilia for the inner hair cells is not
embedded. The adult mammalian cochlea demonstrates the
numerous cell types in the cochlea and the fragile tissue
structure.
42
The organ of Corti is formed over several stages during development. During the extension of
the cochlear duct, three domains begin to become apparent: the stria vascularis, Reissner's membrane
and the organ of Corti. Within the organ of Corti, prosensory cells begin to express markers which
designate different cell fates. These prosensory cells have an important choice to make - whether to
become a sensory hair cell or a non-sensory support cell (Figure 3.3). Both roles are vital for a properly
functioning peripheral auditory system, however each cell type is dramatically different. Adult human
hair cells allow for frequency discrimination between two sounds of only 0.2% apart, and contribute to
discriminating a timing difference of 6 - 10 microseconds between signals. The stereocilia which sit on
top of the hair cells can detect subatomic displacements (Sellick 1982). But most importantly, the ability
to hear is lost with the death of these cells. They are the direct connection of the sounds of the outside
world to the brain.
Otocyst cell
Neuroblast
Non-sensory
epithelial cell
Pro ensory
ell
Auditory or
Vestibular
neuron
All other
otocyst
derived cells
Support eli
Figure 3.3 Hair cell lineage is derived from a prosensory cell. This schematic showing the
potential differentiation pathways of cochlear epithelia cells. For this study, we focus on the
determination of hair cell versus support cell, where both cell types arise from a prosensory cell.
At the early stages of hair cell development Atohl is expressed and separates the group of
equally-potential cells into cells which may become hair cells and support cells. The Atohl
expressed is refined and the cells which do not pass the threshold towards a hair cell fate become
supporting cells. Auditory neurons and other otocyst derived cells split off from the hair cell
specification pathway prior to the prosensory cell stage.
In the mouse, as the cochlear duct begins to grow outwards around embryonic day 11 (Eli), the
prosensory cells begin to separate themselves into groups. Although their morphology is the same, a set
of cells begin to express particular markers, Jagi, Sox2 and Lfng, It is not fully understood which factors
limit the expression pattern of these initial markers. Approximately two days later, the prosensory
43
.
.
.......................
marker p 2 7kip is expressed in the cells within the Jagl, Sox2 and Lfng region (Chen 1999). The
expression of the cell cycle inhibitor p 2 7kpl at E13 begins in the apex and continues to the base over a 24
hour time window. This expression cycle marks the point at which these sensory cells enter into
terminal mitosis, and these cells will not continue to undergo cell division and replicate. As the
expression pattern of prosensory marker p2 7kiP1 begins to fade, the onset of the first pro-hair cell marker
Atohl begins in the base and over the course of a few days moves up towards the apex.
3.1.3 Hair Cell Development
Atonal homolog 1 (Atohl), which encodes a transcription factor essential for hair cell
differentiation, has a well-characterized spatial and temporal pattern of expression during development
(Bermingham, Hassan et al. 1999; Chen, Johnson et al. 2002). It has been shown that the loss of the
Atohl results in a complete loss of hair cells and support cells (Figure 3.4) (Bermingham, Hassan et al.
1999; Chen, Johnson et al. 2002). Additionally, previous work has shown that the forced upregulation of
Atohl in non-hair cells results in ectopic hair cells, or hair cells in regions that do not normally produce
hair cells (Figure 3.5) (Zheng and Gao 2000; Woods et al., 2004; Gubbles et al., 2008; Yang 2013). It was
through these studies that researchers concluded that Atohl is necessary for the production of hair
cells, and exogeneous expression in the cochlea results in additional hair cells. After the initial
expression of Atohl in cells, hair cells will proceed to express additional factors such as the proteins
Myosin VI and Myosin VII. The prosensory cells that do not become hair cells will express pro-support
cell markers, such as Jagi and Sox2, and become supporting cells.
Figure 3.4 Loss of Atohi results in the loss of hair cells and support cells
(adapted from Bermingham et al. 1999). When Bermingham et al. removed
Atohi both hair cells and support cells did not develop. These electron
microscopy images of cochlear tissue demonstrate the morphology of normal
(C) and Atohi-knocked out (D) cochlear tissue. These results indicated that the
expression of Atohi was necessary for hair cell production.
44
A
B
Figure 3.5 Upregulation of Atohi results in additional hair cells outside the region of
conventional hair cell specification (adapted from Yang et al. 2013). (A) Upregulation of
Atohl leads to the production of ectopic hair cells outside of the conventional location of
hair cells. The white bar shows the rows of inner and outer hair cells and the additional
The onset and regulation of both the pro-hair cell and pro-support cell markers is not fully
understood, however previous research has made significant gains in this direction. Recent work has
also implicated the Wnt signaling pathway, inhibitors of differentiation (Ids) and the transcription factor
Sox2 as candidates that regulate the expression of Atohl in the developing mammalian inner ear (Shi
2010, Norton 2000, Jones 2006, and Dabdoub 2008). However the source controlling Atohl's initial
onset is still unknown.
The path to become a hair cell is complex, and the lineage of Atohl positive cells gives rise to
both hair cells and support cells in the inner ear (Driver, Sillers et al. 2013). It is currently unknown how
many cells express Atohl and in what pattern. Is there a threshold of Atohi concentration that a cell
must reach in order to commit to becoming a hair cell? Perhaps there is a competition effect and some
of the cells that express Atohl compete with additional basic-helix-loop-helix (bHLH) factors to continue
its expression. Sox2 is expressed in a region of developing hair cells and models have implicated
significant interactions with Atohl during hair cell development (Hume, Bratt 2007; Mak, Szeto 2009;
Dabdoub, Puligilla 2008; Neves, Uchikawa et al. 2012). Do cell signaling pathways, such as the Notch
pathway, influence the expression pattern of Atohl in neighboring cells? Many of these questions are
currently being pursued and their answers should contribute significantly to our understanding the
production of mammalian hair cells.
3.1.4 Notch Pathway Signaling
The development, number and pattern of auditory hair cells is vital to normal hearing (Groves
2012). The Notch signaling pathway's role in the refinement of the number of hair cells is well
characterized (Landford 1999, Zine 2001 and Kiernan 2005). The Notch signaling pathway is a cell-to-cell
signaling pathway where transmembrane ligands and receptors interact to alter gene expression
(Artavanis-Tsakonas 1999). When one of the five ligands bind to one of the four receptors, a portion of
the receptor is cleaved and enters the cell's nucleus to bind to DNA and alter gene expression. When
45
the Notch pathway is disrupted in the developing inner ear, it can lead to effects on the number of hair
cells. Previous work has shown that when either the Notch receptor 1 or both Notch ligands, Delta-like
1 and Jagged 2, are knocked out, a phenotype of supernumerary hair cells and a loss of patterning
occurs (Landford 1999, Zine 2001 and Kiernan 2005).
3.1.5 Models of Notch Pathway Signaling
Previous work has shown that the Notch signaling pathway can be used to pattern a group of
identical cells into multiple cell types. The Notch signaling pathway is most commonly thought of as a
neighboring signaling pathway, meaning that a cell can only influence its touching neighbors, since the
ligand and receptor are located along cellular membranes and need to physically interact. Only rarely
have some specific Notch ligands been shown to diffuse (Chen and Greenwald 2004). Work done in
non-mammalian systems has shown that the expression of a bHLH can induce a Notch ligand (Fiuza and
Arias 2007), which then binds receptors in neighboring cells to downregulate the ligand-inducing bHLH
and upregulate inhibitory bHLHs. Thus, Notch ligand expression activates a downregulatory loop in
neighboring cells and decreases the chance that these cell differentiate into a similar cell type, a
mechanism known as lateral inhibition. This type of patterning produces salt-and-pepper, or every
other, patterns that are seen in C. elegan vulva development and Drosophila wing development.
Groups have used mathematical modeling to show how the Notch signaling pathway can
influence biological decision-making processes during development. The condition that cells must touch
in order to signal using the Notch pathway allows simple models to predict complex biological
patterning. Previous work has incorporated the concepts of transactivation and cis-inhibition (when a
ligand and receptor in the same cell bind to each other), mutual inactivation and coupled signal
amplification as potential concepts that are driven by Notch signaling pathway interactions (Sprinzak et
al. 2011; Sprinzak et al. 2010; Giurumescu et al. 2005). These putative mechanisms then are able to
explain or predict particular phenotypes that can be observed using transgenics or organisms with
mutated genes. Additionally, modeling can guide further experiments by predicting specific outcomes
to pathway perturbations.
To study the auditory mammalian hair cells, we used mouse cochleae, a standard model system
in inner ear development, and we begin our work with studying the bHLH transcription factor Atohl and
the Notch signaling pathway. This chapter focuses on incorporating the tool of smFISH to derive
quantitative data for the expression of these molecules during hair cell development, and to build a
model that would test a variety of phenotypes as a result of putative Notch signaling mechanisms
influencing the cell fate decision and patterning of auditory mammalian hair cells.
3.2 Methods
Many of the methods for the following chapter have been detailed above in the methods
section for Chapter 2. Some additional methods were incorporated for the analysis of our data. For this
chapter we compared results across several cochleae. This required careful attention to the embedding
and 3D reconstruction of each cochleae for similar comparison. To do this, we only evaluated the right
ear from each embryo and embedded it at approximately the same position within the mold each time.
Then, we gathered each serial section from the cochlea and labeled them appropriately. If a section was
lost, it was noted so that the 3D reconstruction of each cochlea was accurate. We hybridized and
imaged each section, and for each section gathered an image at 10x. We mapped each 10x image and
lined up each cochlear spiral to reconstruct each cochlea.
46
Since the cochleae could vary in size, we created a frequency map for each cochlea to compare
signaling patterns in similar frequency regions. Each position was mapped back to the cochlear spiral
and assigned a distance 'd' from the base, normalized to the length of each cochlea (ie base = 0 and
apex = 1), which assumes a logarithmic scale of frequencies to position. The range of hearing can vary
with each mouse strain, however in general mice can hear from approximately 3 - 75kHZ. The
frequency map was generated with the assistance of the following formula (Ho Bing, personal
communication):
F(kHz) = (10(1d)*0.92 - 0.68) * 9.8
3.3 Results
The molecular players determining cell specification and patterning of auditory hair cells have
highly robust and redundant networks to deliver the cellular organization required for normal hearing
(Kelley et al. 2009). Several putative genetic players are suggested to impact the foundational network
for hair cell patterning and specification (Pujades et al. 2006; Pan et al. 2010), yet a complete network of
this system has not been determined. Our goal is to advance the conventional network by mapping out
mRNA expression of molecular players during mammalian auditory hair cell development using a novel,
high resolution single molecule mRNA detection technique, single molecule RNA FISH. Single molecule
RNA FISH provides the spatial resolution of mRNA transcripts within individual cells, the ability to
visualize lowly expressed transcripts, and the quantitative power necessary to refine the current
theories on auditory hair cell development. To study the development of auditory mammalian hair cells,
we used mouse embryonic cochleae, a standard model system in inner ear development. We
investigated the expression of select ligands, receptors and downstream reporters of the Notch pathway
at specific developmental stages of inner ear formation, and developed a mathematical model to
demonstrate how these patterns influence Atohi expression in the mammalian inner ear.
We have adapted our single molecule RNA FISH method to work in the developing mammalian
inner ear. We can use the quantitative nature of the single molecule RNA FISH technique to support or
refute current theories of hair cell development. For example, one hypothesis was that Atohi and Sox2
mutually inhibit each other in a genetic network (Dabdoub et al. 2008). This network was thought to
play a role in the refinement of Atohl expression. We observed that recently developed hair cells
contain Sox2 transcripts (red) at similar levels to developing hair cells. Thus, the idea that the expression
of Sox2 and Atohl competed within individual cells for cell type specification does not appear to be true
at the transcriptional level.
This work investigates the fundamental processes used by auditory prosensory cells to make
decisions during hair cell development. The prosensory cell has two choices: a sensory hair cell fate, or a
non-sensory support cell fate. Currently, the expression of Atohl is accepted as the most predictive
determinant of prosensory cell fate (Bermingham et al. 1999). However, since Atohl is expressed in
cells in addition to hair cells, there is additional refinement that must occur to obtain the correct hair
cell numbering and pattern. We also investigate and model Notch signaling as a putative tool of
refinement of Atohl and for hair cell patterning. Additionally, immunofluorescence for MyoVI protein is
often used as a further predictor of hair cell fate (Kelly et al. 2012). Thus, we examined the expression
pattern of an additional hair cell marker MyoV in combination with Atohl during hair cell development.
Our goal is to determine if there is threshold level of Atohi, which is associated with initiation of MyoVi
in putative hair cells, and if a linear relationship between the two transcripts exist as hair cells are
specified.
47
3.3.1 Expression of Atohi during hair cell development
We were interested in examining the detailed aspects of both cell specification and the
patterning of cells during hair cell development. For this, we determined the transcriptional profile
underlying the development of a hair cell as well as the dynamics of cellular position. To qualitatively
confirm that our method replicates previously published results, we first observed Atohl in mid-modular
sections at E16.5 cochlea, followed by evaluating its expression in PO tissue (Figure 3.6). Qualitatively,
the expression patterns of Atohl in these cases were consistent with previously published reports
(Figure 2.10). We made the following key observations. Atohl is not restricted to cells only adjacent to
the endolymphatic space, referred to as the "top". Instead, Atohi is expressed across the whole width
of the organ of corti, including cells adjacent to the basilar membrane, referred to as the "bottom". This
occurs even though in the fully developed organ of corti, hair cells are only found adjacent to the
endolymphatic space at the top. We noticed a second feature of Atohl expression, which is that on a
gross level when Atohi turns on, it does so in a very robust pattern in a line of cells that routinely spans
the cochlear epithelium. This line forms a gradient of Atohl expression (Figure 3.7). This observation
that only one row of cells turn on is very interesting and is something we investigate more fully in the
modeling section below.
Atoh1
-.,HC
-- OHC
so
-&a
0 40
0
30
CD,
C=
20
10
-
--
0
Apex
(low)
Frequency RegionBase
(high)
Figure 3.6 Expression of Atohl during hair cell development. Expression of Atohi in inner (IHC)
and outer (OHC) hair cells for different development stages along the E16.5 cochlea. Here we
grouped sections into 8 different developmental regions and averaged the expression of Atohi.
Atohi transcription turns on in Inner hairs cells at an earlier developmental time point than outer
hair cells, as shown in the boxed region.
48
Figure 3.7 Expression of Atohi in a stripe of cells during hair cell specification. (A) Atohi turns on
in a graded stripe during hair cell development, as shown in this representative image. (B) Cell
segmentation (black outline) of the stripe of expression and computationally identified Atohi (red)
and Jag 2(green) dots. Data is shown for a section from an E16.5 cochlea from approximately the
25 kHz frequency region.
3.3.2 Notch signalingduring hair cell development
Several studies have demonstrated the importance of Notch signaling in the developing cochlea;
however, there is no current work on single molecule RNA FISH Notch signaling in the inner ear (Kelley
et al. 2009; Pan et al. 2010). Hair cells use lateral inhibition to prevent over induction of hair cell fate
(Kiernan et al. 2005; Yamamoto et al. 2006). Qualitative expression patterns for the Notch ligands Jagi,
Jag2, D1, and D113, as well as for downstream signaling reporters Hesi and Hes5 have been previously
established (Hartman et al. 2007; Brooker et al. 2006). We chose to examine quantitative expression
patterns for Notch ligands, receptors and downstream effectors in an effort to directly observe the
changes in Notch ligand transcription and Notch signaling during hair cell specification.
First, we performed an analysis of the expression of all the ligands in the Notch pathway (Figure
3.8 and Figure 3.9). Qualitatively our results were roughly consistent with those that have been
previously published. For Jag2, Dill, D113, and D114, we observed expression in cells differentiating into
hair cells, and in cells beneath these cells. At later development time points, this expression pattern is
refined to only hair cells. D114 has not previously been shown to be expressed in the inner ear. For Jagi
we saw a complimentary expression pattern to Jag2. Expression of Jagi was upregulated in cells
for
neighboring cells expressing Jag2. We decided to focus on Jag2 and Jagi as representative ligands
cochlea
E16.5
the
of
base
the
in
complimentary
these two expression patterns, as their patterns are
(Figure 3.8). Both Jag2 and Jagi have high expression levels and knock-outs cause changes in the
number of hair cells that are developed (Brooker 2006; Kiernan 2005).
49
2
3
JagI
Jag2
4
Figure 3.8 Single molecule RNA FISH demonstrates that Notch ligands Jag2 and Jagi are
mutually exclusive in the basal region of the E16.5 mouse cochlea. Expression of Jagi
(green) and Jag2 (red) mRNA transcripts detected using our image processing algorithm for
four different development stages. There are five Notch ligands, four of which have similar
expression patterns to Jag2 (data not shown). The expression of Jag2 in the developing
mammalian cochlea is well correlated with the expression of Atohl. Jagi has a different
expression pattern than the other four Notch ligands. Jagi is present in the prosensory
region and by final stages of hair cell specification is restricted to support cells. Single
molecule RNA FISH demonstrates that there are Jagl mRNA transcripts in the processes of
the support cells that separate the hair cells. Panels 1-4 are increasing in developmental
stage from apex to base.
50
Dill
D113
D114
Hes5
HesS
Hes5
Figure 3.9 Single molecule RNA FISH demonstrates the expression patterns of the Dill, D113 and D114 in the E16.5
cochlea. Expression of D1 (red, left panel), D113 (red, middle panel), and D114 (red, right panel), in conjunction
with Notch signaling transcriptional reporter Hes5. Transcripts were detected using our image processing
algorithm.
To obtain the dynamics of expression patterns of Jag2 and Jagi, we cut serial sections of
cochleae to map expression as a function of position. We found that Jagl maintains a high expression
level from apex to base, whereas the level of Jag2 increases (Figure 3.10). The large dips and peaks that
occur in the graph are due to portions of the tissue in which tangential sections were are not orthogonal
to the cochlear spiral, and do not reflect real gene expression changes. For Jag2, analysis in single cells
shows that expression roughly mimics Atohl, but in a slightly delayed manner (Figure 3.11). This is
consistent with earlier studies that have reported that Atohl is expressed before Jag2 (Fiuza and Arias
2007). We found that Atohl and Jag2 were well correlated along the whole E16.5 cochlea (Figure 3.12).
51
5000
C
0
4000-
0 30000
C,)
C
CU
2000-
I-
1000-
z
1**>
O[
E
Jagi
Jag2
I
-10005
UO
I
I
I
I
50
100
150
200
250
Position along cochlea (apex to base)
300
~ Time
120010000-
800-
C)
600CD
WU
400-
2'
131
.1
200-
~::'*'
*.J
I
~
*
*
* *.*
-,
1%
-
I
I
W-F
200
250
50
100
150
Position along cochlea (apex to base)
300
Figure 3.10 Expression of Jagi and Jag2 along the E16.5 mouse cochlea. The upper
panel shows the expression of Jagl (blue) and Jag2 (pink) for sections taken form
positions along the cochlea from apex to base. The extreme peaks and valleys
correspond to tangential sections that are not orthogonal to the cochlea duct, and do
not represent real changes in gene expression. The curves show smoothed data
calculated in Matlab. The lower panel shows expression of only Jag2.
52
Atohi
Jag2
Figure 3.11 Atohl and Jag2 expression. This image illustrates that Atohl turns on at an earlier developmental
time point than Jag2 in the E16.5 cochlea. Atohl also turns on in graded stripe across the cochlea. These two
images are taken at the same position in a section using two different fluorophore channels.
53
120
>
100
+O.797
-J
+
40
c 0
20
0
40
20
60
80
120
100
Atohl Transcript Level
Inner Hair Cells
Outer Hair Cells
120
>
ci)
100
... J
4-J
80
L
V)
-
60
+,.
.
0-
(N
a
20-
*
0
+
+"
20
40
60
+
80
.
100
120
Atohl Transcript Level
Figure 3.12 Atohl and Jag2 correlation. In the upper graph, we show the correlation for all prosensory cells with a
correlation coefficient of 0.797. In the bottom graph, we only show the correlation of cells at are destined to
become inner or outer hair cells. We can determine this because the cells are positioned at the top of the cochlea,
and contain Atohi.
54
We next focused on the precise Atohl and Jag2 patterns at the onset of expression.
Interestingly, we first observed a line of cells with expression, which we associated with inner hair cell
specification. Several sections towards the base, a patch of cells in a region 1-2 cells away from this
stripe turned on expression, with the cell layer in between having no expression. We associated this
region with outer hair cell specification. This pattern was then refined to only putative hair cells
adjacent to the endolymphatic space, adjoined by a patch cells that were generally refined to hair cells.
On occasion, a few cells appeared near the bottom of the cochlea (Figure 3.13). More careful
examination of the expression patterns revealed the presence of a gradient of expression. A gradient of
expression exists for both Atohl and Jag2. This expression is subsequently downregulated such that it
develops a biomodal pattern, in which cells express either a high level of Atohl or almost no Atohl
(Figure 3.14).
E16.5 Lower middle turn
E16.5 Base
Atohi
Atohi
Figure 3.13 Atohl expression during E16.5 illustrating expression on the bottom of the cochlea. In these two
example images of Atohl expression, we see that in addition to high Atohl expression in putative hair cells at the
top of the cochlea, cells at the bottom of the cochlea also express low levels of Atohl, but at a higher level than
cells in-between. The arrowheads indicate outer hair cells and the arrows indicate inner hair cells.
55
..........
..
.....
.......
. .......
....
....................................
-............
......
......
- - -............
. ....
Atohi
Jag2
1
1
4
Apex
3
Base
Apex
Base
Figure 3.14 Color maps of Atohl and Jag2 expression. Here we show averaged Atohl and Jag2 expression levels
in cells along the stripe of expression that turns on during hair cell development. As the number-thickness varies
slightly from section to section, we only included sections that exhibit the most prevalent cell-layer number for the
developmental time point.
We then chose to look at two downstream Notch signaling effectors, Hes5 and Hesi (Figure
3.15). We found that the expression of Hes5 and Jagi was well correlated in cells that neighbor hair
cells. We were interested to see if the levels of Hesi and Hes5 transcription increased from apex to
base in a similar pattern to Jag2 transcription. We repeated another serial section experiment for Hesi
and Hes5 expression (Figure 3.16). We found that indeed Notch signaling seen through downstream
effectors Hesi and Hes5 did increase from apex to base in the developing E16.5 cochlea. We also
noticed that Jag2 transcription rises above a basal level at approximately a quarter of the way from apex
to base, and so too does both Hesi and Hes5 increase at a quarter of the way from apex to base.
56
Hesi
Hes5
Hes5
Jag2
Figure 3.15 Notch effectors, Hesi and Hes5, have distinct expression patterns in the developing cochlea.
ImageM processed images from single molecule RNA FISH show that downstream targets of the Notch pathway,
Hesi (red right panel) and Hes5 (red left panel, green right panel) have distinct expression patterns. Hes5 is highly
expressed in supporting cells directly neighboring hair cells, and present at a low levels in other areas of the
cochlea. The left panel shows Jag2, a supporting cell marker, expression overlaid with Hes5 expression to
demonstrate the location of the inner (arrow) and outer hair cells (arrow heads).
57
900 800 -
0 700
Ow
U
600
E
500
4200
*
3
300
Lfl
*
200
0 0
50
100
150
200
250
Position along cochlea (apex to base)
Figure 3.16 Hesi and HesS expression rise from the apex to base in the developing mouse cochlea. An E16.5
mouse cochlea was serial sectioned and single molecule RNA FISH was used to evaluate the expression of Hesi and
Hes5 mRNA transcripts for a region of cells. As with Jag2 transcript expression, Hesi and Hes5 expression are seen
to increase after the first quarter of the developing cochlea.
We also chose to look at the Notch signaling inhibitor Numb, a promoter of neural
differentiation, to see if the level or pattern of its transcription changed as the presence of Notch ligands
increased. We found that the Numb transcripts do not gradually increase from apex to base in the E16.5
mouse cochlea, but are located at high levels all through the cochlear epithelium (Figure 3.17).
58
Numb
Atohi
Figure 3.17 Notch signaling inhibitor Numb is found to be expressed all throughout the developing cochlea. We
used single molecule RNA FISH to observe the expression patterns of Atohl and Numb in the developing cochlea.
The expression pattern of Numb transcripts does not appear to explain the unique patterning and cell specification
of hair cells in the cochlea.
3.3.3 Modeling Notch signalingin the organ of Corti
To help understand the expression patterns of Atohl and Jag2 during mammalian hair cell
development, we developed a mathematical model of Atohl/Jag2 mediated signaling in the prosensory
cell array. We first model the 1D initial stripe of Atohi positive prosensory cells, including the future
inner hair cells and prosensory cells underneath (Figure 3.18). Our model is derived from equations
described by Sprinzak et al. (2010), but is modified to include feedback onto the transcription factor
Atohl and the Notch ligand, Delta (Jag2).
59
ID
Figure 3.18 Model of the stripe of Atohi expression. We create a simple model of the
stripe Atohi expression. We presume a gradient of activate or inactivating ligand sets
up the initial Atohl pattern, which is then refined by notch signaling.
The model describes how a Delta ligand signals to a Notch receptor in a neighboring cell, causing
cleavage of the receptor to create a signaling domain. This signaling domain can move into the nucleus
and up-regulate or down-regulate gene transcription. The model also includes cis-inhibition, in which
the Delta ligand binds to a Notch receptor within the same cell and both proteins are degraded. We also
include negative feedback from Notch signaling onto Atohl and Delta (Jag2) transcription. The negative
feedback loop on to Atohl was included based on the work for Notch signaling in Drosophila, where a
signaling loop between Notch signaling and a proneural bHLH, such as Atohi, has been previously
described (Fiuza and Arias 2007). This loop, depicted in Figure 3.19, couples the onset of a Delta ligand
and the downregulation of a proneural bHLH to Notch signaling.
60
I
A
Figure 3.19 Jag2/1D1 deletions and Notch signaling schematic. The left panel shows the increase in hair cell
number with Jag2 and Dii deletion, adapted form Kiernan et al (2005) with license. The right panel shows a
traditional view of how the notch signaling pathway operates in the ear. Jag2 is induces in hair cells and
signals to neighboring support cells to inhibit the expression of Atohl, and repressing thereby hair-cell fate.
After a proneural bHLH is initially expressed, expression of a Delta ligand follows, which induces
Notch signaling in the neighboring cells by binding to a Notch ligand. This binding results in the signaling
domain entering the nucleus and activating the transcription of an inhibitory bHLH, which would
downregulate the expression of the proneural bHLH in the neighboring cell. Thus, Notch signaling active
in a cell leads to the downregulation of the proneural bHLH. Additionally, we included a negative
feedback loop to account the reduction of Delta producted by cells receiving Notch signaling (ArtavanisTsakonas 1999). We do not include any transcriptional feedback on the Notch receptor, as smFISH
shows transcript counts stay approximately constant over all the developmental time points that we
assayed. The equations describing our model are:
dNj
dt
DiN
kc
DtransNi
kt
61
[1]
dDi
_
fljD,i
dt
1+Si/kD
dSi
DtransNi
dt
kt
dA_
flAi
dt
1+SL/kA
DiN
_
DiNtrans
c
[2]
t
[3]
[4]
0A
Here, N is the full-length Notch receptor, D is to the Notch ligand, S is the truncated Notch receptor
signaling domain, and A is the transcriptional output of the system, Atohl. Cells are indexed in a row
from top to bottom increasing from i = 1 to i = 2, 3, or 4, depending on the number of layers in portion
of the cochlea being modeled. A significant portion of the gradient formation and refinement occurs in
a region of the cochlea in which the organ of corti has a thickness of approximately 4 cell layers, so for
most of our parameter sets, we use i = 1 to i = 4. The basal transcription of the Notch ligand and Atohi
both decrease with increasing i, representing a gradient of activation that decreases from the top cell to
the bottom cell. We assume that this gradient comes from an activating signal diffusing from the scala
media into prosensory cells, or an inhibitory signal diffusing from the basilar membrane. We set our
initial conditions of N = 1 and D = S = A = 0 to represent the pattern of expression at the apex, before
Atohl turns on. We have chosen parameters so our model yields a similar gradient of Atohl and Jag2
expression as what is experimentally observed (Figure 3.17). Unless otherwise specified, we have set kt
to 10, kA to 20, D,i = D,i to {1.00,0.86,0.33,0.12}, and all other parameters to 1. The exact
transcriptional gradients and notch signaling strength can be determined by comparing a mutant that
lacks Notch signaling to the wild-type system. We used Matlab's ode45 function to solve these firstorder equations.
In Figure 3.20 we compare three scenarios: (A) without Notch signaling, (B) with Delta
expression in all prosensory cells, and (C) with Delta expression only in the top prosensory cell. Previous
studies have suggested that the Delta ligand Jag2 is only expressed in cells that are destined to become
hair cells, while our data indicates that Jag2 is expressed across a broader region. Within our model,
Jag2 transcription could be restricted to the top cell if the response of Jag2 to Notch is highly
cooperative, or Jag2 has a high threshold of gene activation. A comparison of Fig. 3.20A and B illustrates
how Notch signaling amplifies the differences due to the initial gradient, increasing the difference in
expression of A from 0.86 to 0.68, between the top two cells. This is similar to the gradient
enhancement described by Giurumescu et al. (2006). We see that when only the top cell can produce
Jag2 (Fig. 3.20C), the gradient enhancement between the top cell and the neighbor actually increases.
This occurs because in this case, the second-most top cell cannot signal back onto the top cell to repress
Atohl transcription. Cells that do not neighbor the top cell produce more Atohl though, as they are not
Notch inhibited. Therefore allowing Jag2 expression in all cells in the strip may be helpful in repressing
Atohl expression in multiple cells when the initial gradient is very shallow. The optimal Notch signaling
that minimizes the probability of another cell besides the top cell expressing large amounts of Atohl
depends on the gradient shape and also the number of cell layers.
62
A
No Notch Inhibition
No enhancement of
gradient
B
C
Jag2 graded
Jag2 only in hair cell
Enhancement along
whole gradient
Enhancement of
gradient only
between top 2 cells
Figure 3.20 Comparison of three possible scenarios of Notch signaling. The plots are of steady
state values of A, the signaling output of the model as explained in. 3.3.3. Patterns are for
scenarios (A) without Notch signaling, (B) with Delta expression in all prosensory cells, and (C)
with Delta expression only in the top prosensory cell. For Delta expression only in the top
prosensory cell, we see only gradient enhancement across the two cells. For Delta expression in
all prosensory cells, we see enhancement along the whole gradient.
Our model predicts two different modes of Atohl/Jag2 expression, depending on how strong
the Notch signaling is compared to the steepness of the gradient (Figure 3.21). In the case of a strong
gradient and weaker Notch coupling, A; decrease monotonically from top to bottom. In the case of a
weak gradient and strong Notch coupling, the bottom-most cell is found to have the second-highest
level, only behind the top-most cell. This occurs because the bottom-most cell has only a single
neighbor signaling the strongly repressing Delta ligand, and it sees only slightly lower levels of the
activating gradient, due to the shallow gradient. We see both types of gene expression patterns in the
cochlea (Figure 3.21). Since the exact coupling between cells depends on the morphology of the
membrane attachments, and the gradient steepness depends on the thickness of cells, we do expect
some variation in both the gradient steepness and Notch signaling strength across the cochlea, leading
to the observation of both patterns.
63
Steep Gradient
Weak Notch Signaling
Shallow Gradient
Strong Notch Signaling
Figure 3.21 Two different patterns of Atohl expression. The pattern on the left
comes from a system with a step gradient and weak notch signaling, while the pattern
of the right comes from a system with a shallow gradient and strong notch signaling.
3.3.4 Modeling of cochlear convergence and extension
The documentation of the anatomical changes of the cochlea during development, and the
patterned rows of cells, have been well studied, however what is happening within tissues housed in the
cochlear duct is less well known (Morsli 1998). The gross anatomy spirals out from base to apex, and
the cells go from a cross-sectional view of a structure that is several cell-layers thick to one that one has
two cell layers. We can see this in the E16.5 cochlea, where there exist a different number of cells in the
cross sections from base to apex. In particular, the extreme apex is approximately 2-3 cells thick, the
middle turns range from 4 - 6 cells thick and the base is 2 cells thick. This particular pattern, where the
cells are thicker in the middle of the organ, as opposed to either the base or apex, proposes that cells
may be building up along one axis and then narrowing along a perpendicular axis. This phenomenon is
called convergent extension (CE) (Keller 2000; Keller 2002). Recently, researchers have suggested that
the mechanism of convergence and extension may play a role during cochlear development (Chen 2002,
Montcouqiuol 2003, McKenzie 2004, Wang 2005, Qian 2007 and Yamamoto 2009).
To incorporate convergent extension into our model, we systematically deleted one cell from
each position and determined the new patterns for Atohl expression (Figure 3.22). The deletion of a
cell is representative of what happens when a cell moves out of the plane along the orthogonal axis.
When a cell is removed, the remaining cells quickly formed a gradient of Atohi, similar to the gradient
before cell movement. The new top cell expresses a high level of Atohi, even if the original top cell
moves. This occurs because the new top cell is no longer inhibited by a cell above it expressing Notch
ligand, and also receives a higher graded signal.
64
0
*
Time
Time
0
Tirme
Time
Figure 3.22 Convergent extension by removing prosensory cells in the stripe of Atohl expression. To
model convergent extension, we remove hair cells along the stripe, and then track how our model
predicts Atohi expression changes in the three cells that are left. For deletion of any cell, including the
top cell, a similar gradient of Atohl forms, with a high level of expression in the top cell. Here the green
ovals represent Atohi, while the green circles represent Jag2, with both showing a gradient of expression.
Additionally, the graded expression of Atohl allows for greater plasticity of the second top-most
cell to quickly take on the hair cell fate if the top-most cell moves to another plane. If only the top cell
can express Atohl, it takes longer for the replacement cell to reach a high level of Atohl after the top
cell moves from CE (Figure 3.23). The mechanisms behind hair cell fate may recruit several cells to
express Atohl in ensure that a robust pattern of hair cells is produced after convergent extension. Our
results indicate that our model is robust to convergent extension, and furthers the concept that the
system may use a broader expression pattern of Atohl, coupled with the regulation via Notch pathway
signaling, to ensure the proper number of patterns of hair cells needed for a properly functioning
auditory system.
65
Atohi in
gradient
0
0z
Time
Atohi only
in top cell
Time
Figure 3.23 Plasticity of prosensory cells. These plots show
the response of the prosensory region when the top cell
moves to another plane (left schematic). When Atohl is
expressed in a stripe across the whole organ of Corti (top
panel), the second most cell can reach a high level of Atohl
(90% of maximum) quicker than if only the top cell expresses
Atohl (bottom panel).
3.4 Discussion
The results shown above demonstrate the use of smFISH to study a mechanism behind the
development of the auditory mammalian inner hair cell. The technique was first used to establish
expression patterns in the E16.5 cochlea. We established expression patterns for the fundamental
molecules involved in the Notch signaling pathway, including ligands, receptors, and effectors. The
Numb expression pattern we identified in our studies is consistent with the spatial and temporal
patterns of hair cell development, and indicates that in these regions Numb may be acting to promote
differentiation and possibly cell specification. Our results for the bHLH Atohl indicate that its initial
diffuse expression pattern may be used as an advantage by the system for precise cell fate decisions.
Since a set number of inner hair cells is required for a properly functioning auditory system, the
mechanism it might use is to create a pool of candidate cells and then refine this patterning. We found
a novel expression pattern for the ligand Jag2, which through a model may explain how this pattern is
refined.
The presence or absence of Atohl does not solely determine hair cell fate. To expand on this
work in the future, it would be interesting to perform smFISH in combination with
immunohistochemistry in mid-modular cochlear tissue sections include for additional hair cell
specification markers. The onset of these proteins may shed some more insight on the precise signaling
pattern required for the determination of a hair cell.
66
3.5 Conclusions
During mammalian hair cell development, prosensory cells acquire a spatial pattern of distinct
cell fates. This process is dependent upon the expression of the transcription factor Atohl. Previous
work has shown that the Notch signaling pathway plays a role in the regulation of Atohl and in hair cell
development. We use a single molecule RNA FISH technique to show that Atohl initially presents itself
in a graded fashion. Additionally, we demonstrate the same feature for Notch ligand Jag2. To elucidate
the quantitative implications of spatiotemporal expression patterns of Atohl and Jag2 for cell patterning
during mammalian hair cell development, we developed a mathematical model of Atohl/Jag2 mediated
signaling in the prosensory cell array with parameters set by our quantitative smFISH data. Our analysis
reveals that coupling Atohl expression and Notch signaling via Jag2 amplifies the Atohl-gradient,
leading to a more robust phenotype. Our results demonstrate a novel expression pattern for the Notch
ligand Jag2 and a model to demonstrate the impact on the phenotype. Elucidating high-resolution,
single-molecule quantifiable imaging methods for application to complex tissues will have profound
impacts on future investigations and promote a deeper comprehension of this and other developmental
systems.
3.6 References
Artavanis-Tsakonas S, Rand MD (1999) Notch signaling: cell fate control and signal integration in
development. Science 30(284):770-776.
Bermingham NA, et al. (1999) Mathi: an essential gene for the generation of inner ear hair cells. Science
284(5421):1837-1841.
Bok J, Bronner-Fraser M, Wu K (2005). Role of the hindbraind in dorso-ventral but not anteriorposterior
axial specification of the inner ear. Development 132:2115-2124.
Bosher SK, Warren RL (1986) Observations on the electrochemistry of the cochlear endolymph of the
rat: a quantitative study of its electrical potential and ionic composition determined by flame
spectrophotometry. Proc R Soc Lond B 171:227-247.
Braunstein EM, Crenshaw EBIII, Morrow BE, Adams JC (2008). Cooperative function of Tbxl and Brn4 in
the periotic mesenchyme is necessary for cochlea formation. J Assoc Res Otolaryngol 9:33-43.
Braunstein EM, Monks DC, Aggarwal VS, Arnold JS, Morrow BE (2009). Tbx1 ad Brn4 regulate retinoic
acid metabolic genes during cochlear morphogenesis. BMC Dev Biol 9:31.
Brooker R, Hozumi K, and Lewis J (2006) Notch ligands with contrasting functions: Jaggedi and Deltal in
the mouse inner ear. Development 133:1277-1286.
Chen N, Greenwald 1. The Lateral Signal for LIN-12/Notch in C. elegans Vulval Development Comprises
Redundant Secreted and Transmembrane DSL Proteins. Dev Cell. 2004;6:183-192
Chen P, Segil N (1999) p2 7 KiP1 links cell proliferation to morphogenesis in the developing organ of Corti.
Development 126: 1581-1590.
67
Chen P, Johnson JE, Zoghbi, HY and Segil N. (2002). The role of Mathi in inner ear development:
Uncoupling the establishment of the sensory primordium from hair cell fate determination.
Development 129, 2495-2505.
Dabdoub AC, et el. (2008) Sox2 signaling in prosensory domain specification and subsequent hair cell
differentiation in the developing cochlea. Proc Natl Acad Sci USA 105(47):18396-18401.
Dallos P (1992) The active cochlea. J Neurosci 12(12):4575-4585.
Driver EC, Sillers L, Coate TM, Rose MF, Kelley MW. The Atohl-lineage gives rise to hair cells and
supporting cells within the mammalian cochlea. Developmental Biology 376:86-98.
Fiuza UM and Arias AM (2007) Cell and molecular biology of Notch. J Endocrinol 194:459-474.
Giurumescu CA, Sternberg PW, Asthagiri AR (2006) Intercellular coupling amplifies fate segregation
during Caenorhabditis elegans vulval development. Proc NatlAcad Sci USA 103(5):1331-1336.
Groves AK and Fekete DM (2012) Shaping sound in space: the regulation of inner ear patterning.
Development 139, 245-257.
Gubbles, S.P., Woessner, D.W., Mitchell, J.C., Ricci, A.J., Brigande, J.V., 2008. Functional auditory hair
cells produced in the mammalian cochlea by in utero gene transfer. Nature 455, 537-541.
Hartman BH, Hayashi T, Nelson BR, Bermingham-McDonogh 0, Reh TA (2007) DLL3 is expressed in
developing hair cells in the mammalian cochlea. Dev Dyn 236(10):2875-2883.
Hume CR, Bratt DL, Oesterle EC (2007) Expression of LHX3 and SOX2 during mouse inner ear
development. Gene Expression Patterns, Mechanisms of Development 7:798-807.
Jones JM, Montcouquiol M, Dabdoub A, Woods C, Kelley MW (2006) Inhibitors of differentiation and
DNA binding (Ids) regulate Mathi and hair cell formation during the development of the organ of Corti. J
Neurosci 26:550-558.
Keller, R., (2002). Shaping the vertebrate body plan by poliarized embryonic cell movements. Science
298 1950-1954.
Keller, R., Davidson, L., Edlund, A., Elui, T., Ezin, M., Shook, D., and Skoglund, P., (2000). Mechanisms of
convergence and extension by cell intercalation. Philos. Trans. R. Soc. London B. Biol. Sci. 355, 897-922.
Kelley MW, Driver EC, Puligilla C (2009) Regulation of cell fate and patterning in the developing
mammalian cochlea. Curr Opin Otolaryngol Head Neck Surg 17(5):381-387.
Kelly MC, Chang Q, Pan A, Lin X, Chen P (2012) Atohi directs the formation of sensory mosaics and
induces cell proliferation in the postnatal mammalian cochlea in vivo. The Journal of Neuroscience
32(19):6699-6710.
Kiernan AE, et al. (2005) Sox2 is required for sensory organ development in the mammalian inner ear.
Nature 434(7036):1031-1035.
68
Kiernan AE, Cordes R, Kopan R, Gossler A, and Gridley T (2005) The Notch ligands DLLA and JAG2 act
synergistically to regulate hair cell development in the mammalian inner ear. Development 132:43534362.
McKenzie, E., Krupin, A. and Kelley M. W., (2004) Cellular growth and rearrangement during the
development of the mammalian organ of Corti. Dev. Dyn. 229, 802-812.
Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW (1999) Notch signaling
mediates hair cell development in mammalian cochlea. Nat Genetics 21:289-292.
Mak AC, Szeto IY, Fritzsch B, Cheah KS (2009) Differential and overlapping expression pattern of SOX2
and SOX9 in inner ear development. Gene Expr Patterns 9:444-453.
Montcouquiol, M. and Kelley M.W. (2003) Planar and vertical signals control cellular differentiation and
patterning in the mammalian cochlea. J. Neurosci. 23, 9469-9478.
Montcouquiol, M., Rachel, R.A., Lanford, P.J., Copeland, N.G., Jenkins N. A. and Kelley M.W. (2003)
Identification of Vangl2 and Scrbl as planar polarity genes in mammals. Nature 423, 173-177.
Morsli H, Choo D, Ryan A, Johnson R, Wu DK (1998) Development of the mouse inner ear and origin of
its sensory organs. J Neurosci 18:3327-3335.
Neves J, Uchikawa M, Bigas A, Giraldez (2012) The prosensory function of Sox2 in the chicken inner ear
relies on direct regulation of Atohi. PLoS One 7(1):e30871.
Norton JD (2000) ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci
113:3897-3905.
Pan W, Jin Y, Stanger B, Kiernan AE (2010) Notch signaling is required for the generation of hair cells and
supporting cells in the mammalian inner ear. Proc NatlAcadSci USA 107(36):15798-15803.
Phippard D, Lu L, Lee D, Saunders JC, Crenshaw EBIII (1999) Targeted mutagenesis of the POU-domain
gene Brn4/Pou3f4 causes developmental defects in the inner ear. J Neurosci 19:5980-5989.
Pujades C, Kamaid A, Alsina B, Giraldez F (2006) BMP-signaling regulates the generation of hair-cells. Dev
Biol 292(1):55-67.
Qian D, et al. (2007) Wnt5a functions in planar cell polarity regulation in mice. Dev Biol 306(1):121-133.
Riccomagno MM, Martinu L, Mulheisen M, Wu DK, Epstein DJ (2002) Specification of the mammalian
cochlea is dependent on Sonic hedgehog. Genes Dev 16:2365-2378.
Sellick PM, Patuzzi RB, Johnston BM (1982) Measurement of basilar membrane motion in the guinea-pig
using the Mossbauer technique. J Acoust Soc Am 72:131-141.
Shi F, Cheng YF, Wang XL, Edge AS (2010) Beta-catenin up-regulates Atohl expression in neural
progenitor cells by interaction with an Atohl 3' enhancer. J Biol Chem 285:392-400.
Sprinzak D et al (2010) Cis-interactions between Notch and Delta generate mutually exclusive signalling
states. Nature 465(7294):86-90.
69
Sprinzak D, Lakhanpal A, LeBon L, Garcia-Ojalvo J, Elowitz MB (2011) Mutual inactivation of Notch
receptors and ligands facilitates developmental patterning. PLoS Comput Biol 7(6):e1002069.
Wang, J., Mark, S., Zhang, X., Qian, D., Yoo, S.J., Radde-Gallwitz, K., Zhang, Y., Lin, X., Collazo, A.,
Wynshaw-Boris, A., et al. (2005) Regulation of polarized extension and planar cell polarity in the cochlea
by the vertebrate PCP pathway. Nat Genet. 37, 980-985.
Woods, C., Montcouquiol, M., Kelley, M.W., 2004. Mathi regulates development of the sensory
epithelium in the mammalian cochlea. Nature Neuroscience 7, 1310-1318.
Wu DK, Kelley MW (2012) Molecular mechanisms of inner ear development. Cold Spring Harb Perspect
Biol 4(8):a008409.
Yamamoto N, Tanigaki K, Tsuji M, Yabe D, Ito j, Honjo T (2006) Inhibition of Notch/RBP-J signaling
induces hair cell formation in neonate mouse cochleas. J Mol Med 84:37-45.
Yamamoto N, Okano T, Xuefie M, Aldestein RS, Kelley MW (2009) Mysoin 11 regulates extension, growth
and patterning in the mammalian cochlear duct. Development 136:1977-1986.
Yang J, Cong N, Zhao H, Huang Y and Chi F (2013) Ectopic hair cell-like cell induction by Mathi mainly
involves direct transdifferentiation in neonatal mammalian cochlea. Neuroscience Letters 549: 7-11.
Zheng, J.L., Gao, W.Q., 2000. Overexpression of Mathi induces robust production of extra hair cells in
postnatal rat inner ears. Nat. Neuroscience 3, 580-586.
Zine A, Aubert A, Qiu J, Theriasnos S, Guillemot F, Kadeyama R, de Ribaupierre F (2001). Hesi and Hes5
activities are required for the normal development of the hair cells in the mammalian inner ear. J
Neuroscience 21:4712-4720.
70
Chapter 4 Expression patterns of Wnt ligands during mammalian hair cell development
4.1 Introduction
The Wnt signaling pathway is known to play a significant role in the development of many
organs, including the chicken inner ear, and is expected to play a role of similar importance in the
developing mammalian auditory system (Wodarz and Nusse 1998; Sienknecht and Fekete 2008; Stevens,
Davies et al. 2003; MacDonald et al 2009). It is known that the Wnt pathway is involved in regulating
the size of the otic placode (Freyer and Morrow 2010; Ohyama, Mohamed et al. 2006) and controls
stereocilia bundle orientation; however, its role in hair cell specification is unknown (Groves and Fekete
2012; Qian, Jones et al. 2007; Wang, Guo et al. 2006; Xu, Wang et al. 2003; Dabdoub, Donohue et al.
2003). Several knockout mice for Wnt ligands or receptors have been investigated for inner ear defects.
The knockout mouse for Wnt5a has a shortened cochlea and additional outer hair cells (Qian, Jones et
al. 2007). A Fzd3-/-, Fzd6-/- mouse exhibited defects in the orientation of hair bundles and occasionally
has additional outer hair cells (Wang, Guo et al. 2006). The knockout mouse for the Fzd4 receptor is deaf
but does not exhibit any inner ear defects (Xu, Wang et al. 2003). However, a knockout mouse for the
Wnt7a ligand did not show a phenotype despite its expression in the developing cochlea (Dabdoub,
Donohue et al. 2003). These results indicate that further investigation into Wnt pathway signaling could
reveal mechanisms of hair cell specification.
Recent work investigating this role demonstrated that the activation of Wnt signaling in isolated
putatively post-mitotic non-hair cells to proliferate and express markers of differentiated hair cells (Shi,
Cheng et al. 2010). This occurs in a limited group of support cells from the postnatal organ of Corti that
express the marker and Wnt target gene Lgr5. These promising results have motivated additional work
to explore the role that the Wnt pathway might play during hair cell development in vivo. One recent
study of the role of the Wnt signaling pathway during hair cell differentiation and patterning
investigated the expression pattern of a TCF/Lef transgenic reporter mouse, a fluorescent reporter of
Wnt activity, during hair cell development (Jacques, Puligilla et al. 2012). This study shows that the
timing of the expression of the fluorescent construct occurs within an appropriate spatiotemporal
pattern that would suggest a role in hair cell differentiation. An expression map of all of the Wnt ligands
that could be activating Wnt during hair cell development has not yet made. To investigate the
additional molecular players that may be contributing to the initial expression pattern of Atohl and its
refinement, we have chosen to map the expression patterns of these ligands and visualize key players in
the Wnt signaling pathway with smFISH during hair cell development in embryonic cochleae.
4.2 Methods
4.2.1 Mouse model systems and PCR analysis
To obtain GFP-expressing mice, female homozygous mT/mG mice (Jackson Laboratory Stock#
007576) were bred with Sox2cre heterozygous male mice mT/mG mice (Jackson Laboratory Stock#
004783) to produce transgenic embryos. The breeding procedure and subsequent tissue processing and
preparation for smFISH imaging is detailed in the methods of Chapter 2.
4.2.2 Frozen tissue section preparation for single molecule RNA FISH
Frozen tissue blocks were sectioned into 6 - 10 micron sections and placed on Vectabond
treated #1 coverslips. Sections were then fixed to the coverslips with 4% paraformaldehyde for 15
71
minutes and washed with PBS. Sections were stored in 70% ethanol for 24 hours, and then washed in a
10% formamide wash buffer solution for 5 minutes before hybridizing to coupled probes at 30*C for 1620 hours. Coverslips were then washed with a 10% formamide solution and imaged immediately with a
100x objective on a widefield microscope setup.
4.2.3 Single molecule RNA FISH probes
Single molecule RNA FISH probes were designed using a custom algorithm to locate regions of
twenty oligonucleotides in length that had 45 - 55% GC content in the cDNA of the open reading frame
of the gene of interest. The 5' and 3' regions of the gene were included if the open reading frame did
not provide enough probe candidates. Probes were designed to be 45-55% in GC content and spaced 2
nucleotides apart. Selected probes were then BLASTed against the entire genome, and those with
significant hits were removed from the selection process. Forty-eight to ninety-six oligonucleotide
probes were synthesized by Biosearch with one coupling site for a single fluorophore per probe. Probes
were then coupled to a selected fluorophore and purified using HPLC. Probe concentration was then
measured using Nanodrop. After imagins of smFISH probes, the resulting images were analyzed with a
custom designed algorithm in MatLab named ImageM. ImageM allows for the localization of individual
mRNA transcripts and segmentation of individual cells.
4.3 Results
To investigate the additional molecular players that may be contributing to the initial expression
pattern of Atohl and its refinement, we investigated the Wnt signaling pathway. SmFISH was used to
generate spatially localized transcriptional profiles for the Wnt ligands in the developing cochlea and
map the gene expression profile for each in serial mid-modular sections of the E16.5 cochlea. This was
performed in combination with smFISH determination of Atohi expression to localize the position, of
future or specified hair cells. Three right ear cochleae taken from embryos of different pregnancies
were used to study the Wnt gene expression profiles. The resulting map demonstrated the presence
and position of each Wnt ligand during hair cell specification. In addition, we correlated the expression
level of MyoVi, a known hair cell marker (Xiang, Gao et al. 1998), with Atohl expression in the
developing cochlea to discover if a robust relationship between Atohl and MyoV expression level exists
on a single cell level.
Our first step was to determine the expression patterns of Wnt ligands. For initial studies we
chose to investigate the expression patterns of several Wnt ligands from a reported PCR screen
(Dabdoub 2003). We found expression of Wnt4, Wnt5a, Wnt7a, Wnt7b, Wnt8a and Wntll mRNA
transcripts inside the cochlear epithelium. Wntll ligands were found to be expressed directly above
developing hair cells in the lower and middle turns (Figure 4.1). Wnt4 is expressed in a mutually
exclusive pattern in cells medial to those expressing Wntll mRNA transcripts (Figure 4.2). In the base of
the developing cochlea, Wnt5a is expressed in almost every cell except the hair cells and the support
cells located directly below the hair cells (Figure 4.3). At earlier positions along the E16.5 cochlea,
Wnt5a overlaps the initial Atohl expression pattern that extends across the cochlear epithelium.
Wnt7a, Wnt7b and Wnt8a were found to be broadly expressed throughout the developing cochlea (data
not shown).
72
Figure 4.1 Expression of Wnt1l. In E16.5 mouse cochlear tissue, Wnt11 is found to be expressed in epithelial cells
neighboring the cochlear duct, but on the opposite side from the organ of corti in the lower (left panel) and middle
(right panel) turns using single molecule RNA FISH.
Wnt4 / Wnt1
Wnt4
Figure 4.2 Wnt ligands Wnt4 and Wnt11 are expressed in mutually exclusive regions in the developing cochlea.
Wnt ligand Wnt4 is expressed in a mutually exclusive region to Wnt ligand Wnt11 in the developing cochlea.
Images were obtained via single molecule RNA FISH. The left panel demonstrated the expression pattern of Wnt4,
and the right panel shows the expression pattern of both Wnt4 (green) and Wnt11 (red). The lines indicate where
putative hair cells will form.
73
Figure 4.3 Expression of Wnt ligand Wnt5a is found in regions
neighboring developing hair cells. Single molecule RNA FISH
demonstrates that the Wnt ligand Wnt5a is located in almost all regions
of the cochlear epithelium except for the region in which hair cells are
developing (outlined in white). In other systems, Wnt5a has been shown
to inhibit canonical Wnt signaling.
We did not detect expression of the Wnt ligands Wnt3, Wnt6, Wnt8b and WntlOb in the
cochlear epithelium in our preliminary explorations. We found the expression of Wnt2 transcripts to be
adjacent to the medial edge of the cochlear epithelium. We also explored the other Wnt ligands not
found in the initial PCR screen and found them not to be expressed. In addition to mapping Wnt ligands,
we performed preliminary studies of Wnt receptor distribution. We chose to investigate the receptors
Fzd6 and Fzd4 due to work from previous studies (Qian, Jones et al. 2007; Wang, Guo et al. 2006). We
found both Fzd4 and Fzd6 to be expressed in the developing cochlea. Fzd6 was expressed both in
developing hair cells, support cells and along the apical surface of cells along the lateral wall of the
cochlear duct (Figure 4.4). Fzd4 was expressed in hair cells and supporting cells neighboring the hair
cells (Figure 4.5). We also investigated the expression of additional genes associated with Wnt signaling
such as c-Myc and Axin2 (Jho, Zhang et al. 2002). We found Axin2 to be expressed throughout the
developing mouse cochlea (Figure 4.6) and c-Myc to be expressed in regions bordering the organ of
Corti and the cochlear duct.
74
Figure 4.4 Expression of Wnt receptor Fzd6 is localized to cells within the developing mouse E16.5 cochlea. Single
molecule RNA FISH reveals the mRNA expression pattern of Wnt receptor Fzd6. Here we show, using single
molecule RNA FISH, that Fzd6 is present within the region of developing hair cells. The right panel is a magnified
image of the left panel. The blue star indicates where the location of the hair cells.
Figure 4.5 Wnt receptor Fzd4 is found to be expressed in the mouse E16.5 cochlea. Here we use single molecule
RNA FISH to localize the expression of Fzd4 mRNA transcripts within the developing mouse cochlea. The right
panel is a magnified image of the left panel. The blue star indicates the location of the hair cells.
75
Figure 4.6 Axin2, a putative universal Wnt target, is expressed in the mouse E16.5 cochlea. Single
molecule RNA FISH is used to determine the expression patterns of Axin2 and Atohl in the developing
cochlea. The expression of Axin2 putatively is under the control of Wnt signaling. The panels on the
left demonstrate Axin2 expression in the middle and lower turn in the cochlea, respectively. The panels
on the right show expression of Atohl in the same tissue. In the lower turn (bottom images), Axin2
appears to be downregulated in areas of high Atohl expression. The tissue seen here was treated in
ethanol for more than 24 hours, in a first attempt to test the Axin2 probe, and has started to degrade.
4.4 Discussion
The smFISH-derived expression of several Wnt ligands and receptors during hair cell
specification suggests that Wnt signaling plays a role in hair cell development. SmFISH allows us to
determine the precise location of expression. In this case we found one molecule to be seen in a PCR
screen, but found outside of the cochlea with smFISH. This is a prime example of how smFISH
completes conventional molecular biology techniques, and helps to reduce the candidate list of
molecules that should be explored further.
One hypothesis is that Wnt ligands could cross the extracellular space and induce signaling in
the region of developing hair cells. When Atohl is expressed, Wnt signaling in the apical cell could
reinforce this cell's specification as an inner hair cell. This inner hair cell could use a lateral inhibition
mechanism via Notch signaling to deter its neighbors from choosing the same fate. Wnt ligands could
76
also define a region for Atohl expression in the organ of Corti via the induction, or oppression beyond
this region, of Atohl expression in the initial pattern described in Chapter 3. Wnt ligands Wnt7b and
Wnt8a, or ligands from other developmental pathways, could be putative candidates that play this role,
however additional exploration is needed to confirm the model.
To explore whether our suggested mechanism plays a role in hair cell development, it would be
interesting to investigate the phenotypes of the inner ear of Wntll, Wnt4, Wnt7b and Wnt8a knockout
mice, or in a molecule that would influence several of the Wnt signaling pathways such as a TCF/LeF
signaling molecule. It also would be interesting in studying the specific effects to particular exogenous
Wnt ligand effects in cochlea explants experiments similar to FGF experiments performed by Huh et al.
(2012) or Jacques et al. (2012). Huh et al. added exogenous FGF molecules to explants and observed
changes in the number of hair cells, whereas Jacques et al. activated or inactivated Wnt signaling with
Wnt activators or antagonists. Additionally, if our knockouts present with a phenotype, we could assay
knockout explants with exogenous Wnt molecules to see if hair cell specification could be recovered.
Both the Notch and Wnt pathways play a role in hair cell development. We added the known
lateral inhibition from Notch signaling to our simple mechanism to help explain how the two pathways
could work together to promote hair cell specification and patterning (Figure 4.8). The Wnt pathway
could reinforce the apical cell that expresses Atohl to become a hair cell, and lateral inhibition could
provide this cell with a mechanism to convert its neighbors into support cells.
4.5 Conclusions
Our results demonstrated the strengths of the smFISH technique to complement conventional
techniques and to provide additional information about the expression patterns of Wnt ligands during
hair cell development. Previous work has shown expression of seven ligands, which we confirmed and
demonstrated their precise locations. We found one of these ligands to be located outside of the region
of the organ of Corti. Additionally, we confirmed the lack of expression of the other Wnt ligands. This
qualitative data may be used to generate new hypothesis about cell proliferation and specification
during the development of the inner ear.
4.6 References
Dabdoub A, Donohue MJ et al. (2003) Wnt signaling mediates reorientation of outer hair cell
stereociliary bundles in the mammalian cochlea. Development. 130:2376-2384.
Freyer L and Morrow BE (2010) Canonical Wnt signaling modulates Tbxl, Eyal, and Six1 expression,
restricting neurogenesis in the otic vesicle. Dev Dyn 239:1708-1722.
Groves AK and Fekete DM (2012) Shaping sound in space: the regulation of inner ear patterning.
Development. 139, 245-257.
Huh SH, Jones J et al. (2012) Differentiation of the lateral compartment of the cochlea requires a
temporally restricted FGF20 signal. PloS Biology. 10(1).
Jacques BE, Pulgilla C et al. (2012) A dual function for canonical Wnt/p-catenin signaling in the
developing mammalian cochlea. Development. 139(23):4395-404.
77
Jho EH, Zhang T et al. (2002) Wnt/@-catenin/Tcf signaling induces the transcription of Axin2, a negative
regulator of the signaling pathway. Mol Cell Bio. 22: 1172-1183.
Lustig B, Jerchow B et al. (2002) Negative feedback loop of Wnt signaling through upregulation of
conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 22: 1184-1193.
MacDonald BT, Tamai K, He X (2009) Wnt/Beta catenin signaling: components, mechanisms, and
diseases. Developmental Cell 17:9-26.
Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK (2006) Wnt signals mediate a fate decision
between otic placode and epidermis. Development 133: 865-875.
Qian D, Jones C et al. (2007) Wnt5a functions in planar cell polarity regulation in mice. Developmental
Biology. 306: 121-133.
Shi F, Cheng YF et al. (2010) b-Catenin up-regulates Atohi expression in neural progenitor cells by
interaction with an Atohi 3' enhancer. J Biol Chem. 285: 392-400.
Sienknecht U and Fekete D (2008) Comprehensive Wnt-related gene expression during cochlear duct
development in chicken. J Comp Neurol. 510:378-395.
Stevens CB, Davies AL et al. (2003) Forced activation of Wnt signaling alters morphogenesis and sensory
organ identity in the chicken inner ear. Developmental Biology. 261:149-164.
Wang Y, Guo N et al. (2006) The role of Frizzled3 and Frizzled 6 in neural tube closure and in the planar
polarity of inner-ear sensory hair cells. J Neurosci. 26(8):2147-56.
Wodarz A and Nusse R (1998) Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Bio.
14:59-88.
Xiang M, Gao WQ et al. (1998) Requirement for Brn-3c in maturation and survival, but not in fate
determination of inner ear hair cells. Development. 125: 3935-3946.
Xu Q, Wang Y et al. (2003) Vascular development in the retina and inner ear: control by Norrin and
Frizzled-4, a high-affinity ligand-receptor pair. Cell. 116(6):883-95.
78
Chapter 5 Allele-specific detection of molecules during X Chromosome Inactivation
5.1 Introduction
Embryonic development is a time during which cells make lasting critical decisions. One of the
first major decisions made in mammalian female embryonic development is X chromosome inactivation
(Barakat and Gribnau 2010). Female cells receive both paternal and maternal X chromosomes (Nora and
Heard 2010). One of these two chromosomes must transform its chromatin structure and silence gene
activity so that the female organism develops normally (Nora and Heard 2010). The decision of which X
chromosome, maternal or paternal, will silence itself is stochastic, resulting in different patches of either
paternal or maternal X chromosome activation throughout the female organism, shown in Figure 5.1
(Lyon 1961; Migeon 2007). The phenotype for X-linked genes in these cells is then controlled by the
active X chromosome (Barakat and Gribnau 2010). The important decision of chromosome silencing is
influenced by non-coding RNAs (ncRNAs) that in turn are associated with changes in DNA topology and
mRNA expression (Wutz 2011; Qureshi and Mehler 2012). The X chromosome that randomly inactivates
produces a high level of the non-coding RNA Xist and dramatically changes its topology (Lee, Strauss et
al. 1996). The inactive chromosome forms a Barr body at the edge of the cell's nucleus and remains
inactive during the cell's lifetime, and in all of the cell's descendants, unless meiosis occurs (Clemson,
Hall et al. 2006). The topological changes of the inactive chromosome have yet to be precisely mapped
out.
Differentiation
*
I
Figure 5.1 Dynamics of X chromosome inactivation. During X-inactivation a randomly
chosen X-chromosome bundles up into a small barr body and attaches to the nuclear
envelope.
A standard system to study X chromosome inactivation is hybrid female mESCs (Panning,
Dausman et al. 1997). Female mESCs are useful due to the ability to induce differentiation and to
observe corresponding cellular changes. Hybrid mESC lines create a stage for allele-specific detections
due to the single nucleotide polymorphisms (SNPs) that exist between two different mouse strains
(Panning, Dausman et al. 1997; Keane, Goodstadt et al. 2011). The current techniques used to study X
chromosome inactivation include chromosome conformation capture, microarrays, DNA FISH and RNA
FISH (Nora, Lajoie et al. 2012). At present, methods of chromosome conformation capture are all
79
limited to the use of a large number of cells and the loss of single cell information (Dostie, Richmond et
al. 2006; Zhao, Tavoosidana et al. 2006; de Wit and de Laat 2012). As one X chromosome inactivates, it
will also silence the expression of many of its genes. We were interested in discovering if the topology
changes or the gene silencing occurs first on a single cell level. If the topology changes occur first, then
the remodeling of the chromatin structure could contribute to the silencing of gene expression. There
has been a recent report that indicates that the two processes are less related than previously
considered (Splinter et al. 2011). DNA and RNA FISH can complement chromosome conformation
capture or microarray assays with spatial information, yet allele-specific versions have not been used to
study X-inactivation (Pinter, Sadreyev et al. 2012).
Allele-specific smFISH is an extension of the smFISH technique that incorporates the design of
smFISH probes centered on SNPs that differ between each chromosome (Hansen and van Oudenaarden
2013), resulting in the creation of two X chromosome-specific probe sets (Figure 5.1). Allele-specific
smFISH would enable quantitative single-cell, single-chromosome detection of the presence and levels
of ncRNA, as well as detection of downstream topological and mRNA expression changes. Allele-specific
smFISH probes designed against introns tiling each chromosome will reveal the location and expression
pattern of transcription sites allowing for simultaneous detection of ncRNAs, transcription sites and
mRNAs for expression changes within cell. This information will provide a direct link between DNA
topology and mRNA expression changes in single cells. We can also combine RNA fish with DNA FISH, in
order to detect loci that are not currently expressing mRNA (Bienko et al. 2013; Beliveau et al. 2013). In
this chapter, we describe the testing of allele-specific tiling of introns along the X chromosome using
RNA smFISH and detection of DNA loci using DNA FISH.
For our studies on X chromosome inactivation, we altered both the probe design and biological
sample preparation steps developed in the mammalian cochlea studies described in other chapters.
Allele-specific RNA FISH probes were designed around the SNPs between the two different alleles.
Probes were designed to be SNP-centric and were 19-20 oligonucleotides in length. RNA FISH intron
probes were designed against the introns of the select gene of interest. DNA fish probes were designed
to be longer at ~200 nucleotides. We performed experiments using mESCs as described below.
5.2 Methods
5.2.2 BD Cell-Tak coating
Cell-Tak (BD Biosciences) was used to attach cells to coverslips. A standard solution was
prepared by mixing 197.5 microliters of fresh 0.1 M sodium bicarbonate with 1.7 microliters of Cell-tak
that had been diluted according to the manufacturer's instructions in 5% acetic-acid. To this solution,
0.85 microliters of 1N sodium hydroxide was immediately added. The solution was placed on coverslips
within 1 minute of the addition of sodium hydroxide. The coverslips was incubated with the solution for
20 minutes at room temperature, after which it was washed with water and either used immediately or
stored at 40 C.
5.2.3 Cell Culture for High Definition3-D DNA FISH
Cells were grown directly on gelatin or Cell-tak coated coverslips, washed with 1xPBS (+Ca, +Mg)
before being fixed with 4% paraformaldehyde for 10 minutes at room temperature. The coverslips were
then washed with 1xPBS (-Ca, -Mg)/0.05% Triton X-100 at room temperature before incubation in ix
PBS (-Ca, -Mg)/0.5% Triton X-100 for twenty minutes. Slides were incubated in 20% glycerol/1xPBS
overnight at room temperature.
The following day, the coverslips were processed through a
80
freeze/thaw cycle six times with liquid nitrogen, washed in 1xPBS (-Ca, -Mg)/0.05% Triton X-100 and
incubated in 0.1 N HCI for twenty minutes. The coverslips were again washed in 1xPBS (-Ca, -Mg)/0.05%
Triton X-100 and 2xSSC before being incubated in a 50% formamide/2xSSC (pH 7) solution overnight. In
the morning, the coverslips were transferred to +4*C.
The coverslips were equilibrated to room temperature before hybridization with DNA FISH
probes. A chamber was made with a glass slide, coverslip and the probes before sealing with Fixogum.
0
The chambers were denatured for 2-5 minutes at 75 C and incubated for 16-48 hours in a humidified
chamber at 37*C. The coverslips were washed in a series of saline-sodium citrate buffer solutions
containing Igepal at varying stringencies to remove unbound probes. The coverslips were incubated in a
buffer solution and treated with enzymes to prevent photobleaching before imaging was performed on
a widefield fluorescence microscopy setup.
5.2.4 Purification of DNA from mESCs
To create long DNA-fish probes, we used PCR to amplify complimentary oligos from purified
DNA (Bienko et al 2013). To purify DNA, five to ten million cells were obtained from mESCs grown in a
feeder-free environment. The cells were washed with 1xPBS, centrifuged and resuspended in
1xPBS/0.05% Triton X-100 (freshly added) at room temperature. Proteinase K (20 mg/ml) was added to
obtain a 200 pl/ml concentration. The cells were incubated for one hour at 65*C while shaking at 800
0
rpm. The cells were centrifuged for one minute at 4 C at maximum speed. Cells were immediately
placed on ice, and 60 pl of 5 M sodium acetate (pH 5.5) was added. Cells were vortexed briefly, treated
with 400 pl of ice-cold isopropanol and incubated for 15 minutes on ice. The cells were then spun for 15
minutes at 4 0C, and the supernatant was removed before repeating the washes with 70% ice cold
ethanol. After two washes with ethanol, the pellets were air dryed at room temperature and solubilized
in 200 pl of TE buffer (pH 8). The DNA pellets were rehydrated for several hours at 4C before being
shaken at 800 rpm at 55 0 C. Nanodrop was used to test the DNA concentration. If the ratio of
absorbance of DNA to protein was outside of the range of 1.7 - 1.9, then ethanol washes were
repeated.
5.2.5 High definition 3-D DNA FISH probe design, generation, labeling and purification
We designed long DNA-fish probes to test their potential to distinguish between alleles. Probe
length and SNP number were determined, and any primers containing SNPs were filtered out. For the
first set of primers targeted towards the high-SNP region of the X chromosome, we created 76 probes
with 5 or more SNPs of length of 200 - 210 base pairs each. The probes spanned the region 162.5 - 164
MB along the chromosome. Primers were ordered from IDT.
Double-stranded DNA probes were generated by PCR. The forward and reverse primers were
pooled and mixed with KAPA SYBER FAST qPCR Master Mix 2x (KapaBiosystems), TE buffer (pH 8),
nuclease-free water (Ambion) and purified mESC genomic DNA. The experimental plate was run in a
Light Cycler 480 instrument (Roche) and the following program was used:
81
Time
Temperature
0
Pre-incubation
95 C
5 min
Amplification (30 cycles)
95 0 C
10 sec
0
55 C
10 sec
72 0 C
10 sec
0
Cooling
40 C
10 sec
Table 5.1 PCR conditions for creating DNA FISH probes
Acquisition
None
None
None
Single
None
Samples were merged, treated with 3 M sodium acetate (pH 5.5) and 100% ice-cold ethanol and
incubated for two hours at -20 0 C. Probes were spun and treated with a set of ethanol washes at 40 C, air
dried and measured with Nanodrop before being stored at -20*C.
The DNA probes were dehydrated with a Speedvac before being labeled. Probes were dissolved
in TE and incubated for 15 minutes at 50"C while shaking at 800 rpm. The DNA probes were denatured
and placed on ice before 5 ptl of ULYSIS AF647 (Invitrogen) that was dissolved on the same was added.
The DNA probes were incubated for 15 minutes at 80 0 C and placed on ice. Unbound dye was removed
via a KREApure column system (see Kreatech Biotechnology website for protocol) before DNA and dye
concentrations were measured using Nanodrop.
Two hundred nanograms of DNA probes was purified via an ethanol precipitation containing
salmon 7.5 ptl ssDNA, 7.5 pl COT1, 300 pl nuclease-free water, 3 pl glycogen, 34 p sodium acetate and
900 pl 100% ethanol. The probes were incubated for two hours at -20 0 C, centrifuged for 20 minutes at
4*C, and treated to two 70% ethanol wash cycles. The pellets were then air dried and added to the 70%
formamide DNA hybridization buffer. The probes and hybridization buffer were heated to 50'C while
being shaken at 500 rpm to dissolve the probes and promote hybridization to the samples.
5.2.6 Allele-specificRNA FISH
All single molecule RNA FISH methods presented here were adapted from Raj et al. (2008). To
perform smFISH on cells in suspension, the cells were first mixed in ethanol and placed into a clean
Eppendorf tube. The cells were centrifuged, and the ethanol was aspirated. One millileter of 25% WB
(for allele-specific probes) was mixed with the cells, and the suspension was incubated at RT for three
minutes. The probe was prepared using 100 microliters of 25% formamide hybridization buffer and the
appropriate probes. The cells were centrifuged and and the WB was aspirate. The probes were added
and thoroughly mixed with the cells. Hybridization was performed at 30 0 C
For allele-specific smFISH, the probes were hybridized for two days, which provided time for probes
time to reach equilibrium. After hybridization, 1 ml of wash buffer (same percentage as was used for the
hybridization buffer) was added. The cells were centrifuged and the supernatant was aspirated. Another
1 ml of WB was added and incubated at 30 0 C for thirty minutes. A second wash was performed, but this
time 1 microliter of DAPI was added. The cells were centrifuged, and the supernatant was aspirated. A
total of 1 ml of 2xSSC was added, and the cells were centrifuged and the supernatant aspirated. Cells
were mixed with 100 microliters of Glox buffer that includes 1 microliter of catalase and 1 microliter of
glox enzyme. The cells were pipetted up and down approximately 20 times to mix. Then, two microliters
of the cell solution was placed on a small round glass coverslip and covered with a clean #1 square
coverslip. A Kim wipe or blotting paper was used to absorb the additional liquid so that the two
coverslips were as close as possible, leaving some liquid between the two.
82
5.2.7 Mouse ESCs
For experiments on X inactivation, three different mouse embryonic cell (mESC) lines were used.
Two of the cell lines contained cells that only had either the X chromosome from the 129 mouse, a
laboratory inbred mouse strain, or the castaneous mouse, a Southeast Asian house mouse. These cells
were used to generate genomic DNA to produce the DNA FISH probes and were for control experiments.
The third cell line was a hybrid female mESC line that contained two X chromosomes in each cell - one
from the 129 mouse and one from the castaneous mouse. There is a large amount of genomic diversity
between these two mouse strains, and thus a large number of SNPs to distinguish the two different X
chromosomes. The castaneous mESC and female hybrid (129/cas) mESCs were provided by Barbara
Panning (UCSF). The 129 mESC line was purchased from the Koch Institute and generated by the
Jaenisch Laboratory (MIT).
5.3 Results
X inactivation, the silencing of all but one cellular X chromosome during female embryonic
development, is associated with chromosome coating by non-coding RNA that induces topology changes
in chromosomal DNA and leads to different phenotypic outcomes (Migeon 2007; Nora and Heard
2010). The interaction between these topology changes and gene silencing on the inactivated
chromosome has not been well characterized. We tested probes for smFISH that can monitor topology
changes and gene expression in an allele-specific manner in order to investigate the sequence of events
involved in X inactivation. We targeted intron sites in order to track both expression and topology. In
preliminary experiments, we demonstrate the design and application of smFISH probes for allele-specific
single nucleotide polymorphisms (SNPs) in intron sites along each X chromosome of mouse embryonic
stem cells. Detection of RNA intron sites reveals both topological and expression information since they
occur near the site of DNA loci and decay quickly (Levesque and Raj 2013). Our tested probes can be
used to track X-chromosome expression and topology changes in mESC during a differentiation assay
involving retinoic acid.
To observe topological changes we must use a different method than the conventional DNA
FISH method for two reasons. First, the use of ethanol alters the morphology of the nucleus. Second, we
require a high resolution technique to visualize many loci along a chromosome. We chose a standard
model, mouse embryonic stem cells (mESCs), to study this process. The first stage of this project was to
demonstrate our ability to use 3-D DNA FISH as a potential tool to visualize loci. We performed 3-D DNA
FISH on human epithelial cells using a probe for the HER2 locus (Figure 5.2). We used this system
initially because there were additional independent experiments in the lab for data comparison and
technique refinement. Once this task was completed, we tested DNA fish on mESCs. We designed an
allele-specific 3-D DNA FISH probe for the one locus on the X-chromosome. We isolated DNA from
castaneous mESCs and produced 76 probes that targeted a region of many SNPs for the castaneous X
chromosome. These long probes detect the DNA-fish loci, but not in an allele-specific manner (Figure
5.3). For future studies, shorter probes may work better in allele-specific detection.
83
Figure 5.2 3D DNA FISH for HER2 in human epithelia cells. Here we show 3D DNA fish for
HER2, the materials and methods are adapted from Bienko et al. (2013) and described in
Chapter 5.
84
A
-
B
-------
to.8i
06
0.4
Detected 129 Pgk1 Transcription Sites
C
0.8
0.6
Detected Cas Pgkl Transcription Sites
Figure 5.3 RNA FISH for mESCs. (A) Allele
specific detection of Pgk1 transcription
using a colocalization of an identification
probe with allele-specific probes. (B) The
distribution
of detected
129 Pgk1
transcription sites in 2-1 mESCs (C) The
distribution of detected Cas transcription
sites in 2-1 mESCs.
In addition to testing 3-D DNA FISH probes, we also investigated the expression of RNA introns.
We selected the following eight genes along the X chromosome for investigation: Ubal, Gpc3, Hprt,
Nono, Rnf12, Pgk1, Rbbp7, and Midi (Figure 5.4). We selected these candidates from an RNAsequencing data set from V6.5 mESCs that indicated high expression levels before differentiation. We
applied additional filter sets to select candidates that contained many SNPs in intronic regions located in
chosen regions along the X chromosome. We isolated a set of probes that survived our unspecific
binding thresholds. In general, during RNA transcription introns are spliced and do not travel far from
the location of transcription, thus indicating the position of the DNA loci (Watson, Baker 2009). We
tested all probes and saw that Ubal, Gpc3, Hprt, Nono, Rnf12, Pgkl, Rbbp7 yielded bright dots, while
Midi resulted in only faint spots (Figure 5.5).
85
Gpc3
Gpc3
Ubal
Hprt
Rnf 12
Rnf 12
Pgkl
Non
1 1
Rbbp7 Midi
xist
Figure 5.4 Schematic of allele-specific smFISH intronic probes tiling X
chromosome.
Probe candidates were chosen due to position along X
chromosome, role during X chromosome inactivation, number of SNPs within
introns and high level of expression prior to X inactivation. NcRNA Xist indicates
the position of the X inactivation center.
Figure 5.5 Allele-specific smFISH in mESCs. Allele-specific detection of hprt (left panel)
and ubal (right panel) in mESCs. Cy5 is in red and TMR is in green, and Dapi is in blue.
Additionally, we tested how accurately our dot finding program can measure distances when
aligning between different fluorophore channels. We took 3D images of beads with wide-field
microscopy by taking a stack of sequential two-dimensional images. These beads fluoresce in every
channel, and therefore we can compare our predicted alignment between 2 channels and the actual
bead positions. Our results show that the error of localization in the z-direction is approximately double
the error in the x- and y-directions (Figure 5.6). This occurs because the stage of the microscope moves
in the z-direction. As a proof of principle experiments, we measured the distance between two intronic
probes at are designed against genes 374,187 bases apart (Figure 5.7).
86
(A
I
O 0.4
(A
0.4
.4
0.2-
-L -100
Z direction
Y direction
X direction
-50
K-
0
Error (nm)
50
00
4o
.-
0.2
.2
.80
-100
100
0
-50
50
SI-
0
500
U)
0.
U)
0. 2-
CL
0-
0
S
a
-500
Error (nm)
U)
0
-
100
Error (nm)
0
0
0.4[
0.4
-
.-
0 L-A
100
-50
0
Error (nm)
50
100
0.2
0
-100
0
-50
U
Error (nm)
50
100
aL
-500
U
Error (nm)
500
Figure 5.6 Filter shift alignment can be calculated with fluorescent beads. Here we show the predicted
bead position using our filter shift alignment algorithm as compared to the actual bead position for the x, y,
and z axes and also Cy5 (top row) and A594 (bottom row) channels.
87
L
E
z
0
S0
1000
1soo
2000
250
350
4000
So
"
3D distance in nm
Figure 5.7 Measurement between two positions. Here we show
a proof of principle experiment in which we measured the
distance between two positions in the TMR channel with smFISH
targeted towards introns. An example image is shown in the top
image, while the bottom graph shows the distribution of the 3D
distances for approximately 10 cells.
5.4 Discussion
This chapter focuses on adapting smFISH technology to investigate genes regulating X
chromosome inactivation. We have shown our ability to detect mRNA introns in an allele-specific
manner, as well as DNA loci. As the X chromosome inactivates, its genes will be silenced. Gene silencing
may complicate our topology measurements from transcription sites, and therefore DNA FISH can be
used in combination with allele-specific RNA FISH to mitigate this complication. Our probes are ready to
be used to measure topological and expression changes on the X-chromosome during differentiation.
88
5.5 References
Barakat TS and Gribnau J (2010). X Chromosome Inactivation and Embryonic Stem Cells. The Cell Biology
of Stem Cells. E. Meshorer and K. Plath, Landes Bioscience and Springer Science+Business Media.
Beliveau BJ et al. (2013) Versatile design and synthesis platform for visualizing genomes with Oligopaint
FISH probes. Proc Nati Acad Sci USA 109(52):21301-21306.
Bienko M et al. (2013) A versatile genome-scale PCR-based pipeline for high-definition DNA FISH. Nat
Methods 10(2):122-124.
Clemson CM, Hall LL et al. (2006). The X chromosome is organized into a gene-rich outer rim and an
internal core containing silenced nongenic sequences. Proc NatI Acad Sci U S A. 103(20): 7688-93.
de Wit E and de Laat W (2012). A decade of 3C technologies: insights into nuclear organization. Genes
Dev. 26(1): 11-24.
Dostie J, Richmond TA et al. (2006). Chromosome Conformation Capture Carbon Copy (5C): a massively
parallel solution for mapping interactions between genomic elements. Genome Res. 16(10): 1299-309.
Hansen CH, van Oudenaarden A (2013) Allele-specific detection of single mRNA molecules in situ. Nat
Methods 10(9):869-871.
Johnston Ri and Desplan C (2010). Stochastic mechanisms of cell fate specification that yield random or
robust outcomes. Annu Rev Cell Dev Biol 26: 689-719.
Jonkers I, Barakat TS et al. (2009). RNF12 is an X-Encoded dose-dependent activator of X chromosome
inactivation. Cell 139(5): 999-1011.
Keane TM, Goodstadt L et al. (2011). Mouse genomic variation and its effect on phenotypes and gene
regulation. Nature. 477(7364): 289-94.
Krietsch WK, Fundele R et al. (1982). The expression of X-linked phosphoglycerate kinase in the early
mouse embryo. Differentiation. 23(2): 141-4.
Lee JT, Strauss WM et al. (1996). A 450 kb transgene displays properties of the mammalian Xinactivation center. Cell. 861): 83-94.
Levesque MJ and Raj A (2013). Single chromosome transcriptional profiling reveals chromosome-level
regulation of gene expression. Nature Methods, epub Feb 17.
Lyon MF (1961). Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. 190:372-3.
Migeon BR (2007). Why females are mosaics, X-chromosome inactivation, and sex differences in disease.
Gend Med 4(2): 97-105.
Nora EP and Heard E (2010). Chromatin structure and nuclear organization dynamics during Xchromosome inactivation. Cold Spring Harb Symp Quant Biol. 75: 333-44.
89
Nora EP, Lajoie BR et al. (2012). Spatial partitioning of the regulatory landscape of the X-inactivation
centre. Nature. 485(7398): 381-5.
Panning B, Dausman BJ et al. (1997). X chromosome inactivation is mediated by Xist RNA stabilization.
Cell. 90(5): 907-16.
Pinter SF, Sadreyev RI et al. (2012). Spreading of X chromosome inactivation via a hierarchy of defined
Polycomb stations. Genome Res. 22(10): 1864-76.
Qureshi IA and Mehler MF (2012). Emerging roles of non-coding RNAs in brain evolution, development,
plasticity and disease. Nat Rev Neurosci. 13(8): 528-41.
Raj A, van den Bogaard P, et al. (2008). Imaging individual mRNA molecules using multiple singly labeled
probes. Nat Methods. 5(10): 877-9.
Splinter E et al. (2011) The inactive X chromosome adopts a unique three-dimensional conformation
that is dependent on Xist RNA. Genes Dev 25(13):1371-1383.
Watson JD, Baker TA et al. (2009). Molecular Biology of the Gene 6t ed. Cold Spring Harbor Laboratory
Press, New York, NY.
Wutz A (2011). Gene silencing in X-chromosome inactivation: advances in understanding facultative
heterochromatin formation. Nat Rev Genet. 12(8): 542-53.
Zhao Z, Tavoosidana G et al. (2006). Circular chromosome conformation capture (4C) uncovers extensive
networks of epigenetically regulated intra- and interchromosomal interactions. Nat Genet. 38(11):
1341-7.
90
Chapter 6 Final Conclusions
In summary, this thesis focused on adapting smFISH technology to investigate genes regulating
hair cell developing in the cochlea and X chromosome inactivation. Although these two systems use
different mechanisms to make decisions, regions of overlap exist. Both systems perform highly
structured biological decisions that result in cellular mosaics (Kelley 2006; Migeon 2007). When
operating normally, both X chromosome inactivation and hair cell specification lead to a concrete
decision of fate (Kelley 2006; Johnston and Desplan 2010). When an X chromosome is inactivated, it
remains inactive within that cell and its descendants (Barakat and Gribnau 2010). Once a hair cell is
specified, it remains a hair cell until its death (Groves 2010; Liu, Walters et al. 2012). Thus, there exists a
short period in which these biological decisions are made, which endure throughout the lifetime of the
organism. We believe that the results of this work represent an important step in extending gene
expression analysis to a variety of biological targets. Below we discuss some of ideas for possible future
directions that may result from our work.
6.1 Future Directions
Chapter 2 highlights some of the technical advances made to smFISH to approach studying
mammalian cochlear tissue. As mentioned in the discussion section, continued work extending these
techniques to study juvenile and adult mammalian cochlea would broaden the uses of smFISH to study
the peripheral inner ear. Additionally, it would also be useful to extend the use of smFISH to confocal
microscopy setups since they are currently widely available in molecular biology laboratories. These
advancements would allow smFISH to be used to precisely quantify transcriptional profiles in cells
beyond the onset of hearing in mice, as well as to study influences from perturbations to the auditory
system caused by aging and acoustic or pharmacological trauma.
More importantly, one would be able to determine the localization of transcripts, which might
reveal a new layer of information for studies within the peripheral inner ear. Planar cell polarity, the
orientation of specialized structures, plays a significant role for proper hair cell functioning (Deans
2013). Hair cell nuclei, stereocilia and patterning are all polarized in a particular fashion, and the
determination of the localization of the transcripts within the cells, stereocilia or tissue may provide
insight on mechanisms behind planar cell polarity processes. Also, the tectorial membrane, an acellular
gelatinous structure which sits above the inner and outer hair cells, is composed of collagen and
noncollagenous glyocproteins and proteogylcans (Thalmann et al. 1986). This unique structure plays an
important role in coupling the motions of the solids and fluids in the inner ear (Ghaffari et al. 2007).
Two glycoproteins which comprise the majority of the tectorial membrane are Tecta and Tectb, which
are specifically found in the inner ear (Moreno-Pelavo et al. 2008). SmFISH targeted towards the
expression of these molecules throughout the development and maintenance of the tectorial
membrane may reveal unique polarized expression patterns of these molecules excreted by cells to
form the membrane. It would be insightful to note if the location of transcripts are located in a
polarized or nonpolarized fashion within the cells. If one or both transcripts demonstrate polarized
patterning with a cell, this may reveal some insight towards translational mechanisms for these special
proteins that may occur towards cellular membranes or if they are disturbed all throughout the cell.
Finally, our work has shown gene expression patterns within tissues, however another critical
step would be to make the leap between the activation of a gene via its expression and translation of
this transcript into protein within individual cells. One approach would be to use smFISH combined with
immunohistochemistry, an approach to visualize proteins within cells or tissues, to identify which cells
contain both the transcripts and protein for a particular gene. We attempted preliminary work to join
91
smFISH and immunohistochemistry targeted at Atohl and MyoVI. Our initial results were promising;
however we found that complications can arise for immunohistochemistry from the availability of
robust antibodies. Since many molecules expressed during development do not have
Also, one must pay attention to the spacing of probes for proteins and transcripts across
fluororesecent channels. It is often the case that many fluorophores are used to tag proteins in cells,
especially in the cases where secondary antibodies are used to amplify the signal of a primary antibody.
The benefit of this approach is that the signal for the protein within the cells is very bright, however this
brightness can bleed through into neighboring channels. When combining this approach with an
approach such as smFISH, where the signal is detected slightly above the noise ratio, there is a potential
that the signal from the protein staining can bleed through into the channels with smFISH probes and
mask out the smFISH signal. Taken together, there are many future applications of smFISH to study the
auditory system, and the integration of this technique with conventional techniques will complement
future studies to understand its development, response to damage and
Chapter 3 focuses on the applications of smFISH to study the influences of Notch pathway
signaling on the developing mammalian cochlea. To follow up on our study in our transgenic mouse
model, one could perturb Notch signaling and assay the levels of Atohl and Jag2 expression in
prosensory cells. One way to perturb Notch signaling is to use knockout mice for genes such as Notchi
or a double knockout for Jag2 and DIll. Removing a receptor or ligand can reduce Notch signaling in the
system. Our model predicts that reducing Notch signaling will cause levels of Atohl to remain high in
cells underneath the putative inner hair cells. This would lead to potentially lead to the production of
additional hair cells, an observed phenotype (Kiernan et al. 2005). We would also predict that a pattern
in which the cell along the basement membrane expresses higher levels of Atohl than its direct
neighbor would no longer appear. This would be due to a lack of strong Notch signaling.
Another follow up study would be to include regulation of Atohl by additional pathways. It
would be interesting to couple putative impacts from the Wnt signaling pathway on hair cell
development. Also our model could include positive feedback of Atohl on itself, causing by binding to
its own promoter. Additionally, we could include additional Notch ligands in addition to Jag2, to see
how their slightly different expression patterns can effect hair cell specification.
We could also expand our model to include specification of outer hair cells by extending our
one-dimensional model to two-dimensions by including additional rows of cells. Additionally, since in
actuality the cochlea forms a spiral, we could extend our model to have prosensory cells extend in the
third dimension.
Chapter 4 focuses on the expression of genes involved in the Wnt pathway. Our expression data
predicts that[WNT] could be players in hair cell specification. We could test the role of these players by
performing smFISH for Atohl in mice that have knockouts of these genes. If these players effect the
expression level of Atohl and the number of hair cells that develop, we would then development a
model on the effect of Wnt signaling on hair cell specification. We could also compare Wnt expression
patterns in the mouse to expression patterns in the chicken, which have been done using traditional
RNA in situ (Sienknecht and Fekete 2008)
Chapter 5 explores the development of tools that can be used to study the relationship of X
chromosome silencing and conformation. The next step in this project would be to perform a
differentiation assay of embryonic stem cells and perform FISH using the probes we designed at several
time points. We would then look at the correlation between expression and distances between loci.
92
6.2 References
Barakat TS and Gribnau 1 (2010). X Chromosome Inactivation and Embryonic Stem Cells. The Cell Biology
of Stem Cells. E. Meshorer and K. Plath, Landes Bioscience and Springer Science+Business Media.
Deans MR (2013) A balance of form and function: planar polarity and development of the vestibular
maculae. Semin Cell Dev Biol 24(5):490-498.
Ghaffari R, Aranyosi AJ, Freeman DM (2007) Longitudinally propagating traveling waves of the
mammalian tectorial membrane. Proc Natl Acad Sci USA 104(42):16510-16515.
Groves AK (2010). The challenge of hair cell regeneration. Exp Biol Med (Maywood) 235(4): 434-46.
Johnston RJ, Desplan C (2010). Stochastic mechanisms of cell fate specification that yield random or
robust outcomes. Annu Rev Cell Dev Biol 26: 689-719.
Kelley MW (2006). Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci
7(11): 837-849.
Kiernan AE, et al. (2005) Sox2 is required for sensory organ development in the mammalian inner ear.
Nature 434(7036):1031-1035.
Liu Z, Walters BJ, et al. (2012). Regulation of p27Kipl by Sox2 maintains quiescence of inner pillar cells in
the murine auditory sensory epithelium. J Neurosci 32(31): 10530-10540.
Migeon BR (2007). Why females are mosaics, X-chromosome inactivation, and sex differences in disease.
Gend Med 4(2): 97-105.
Moreno-Pelayo MA et al. (2008) Characterization of a spontaneous, recessive, missense mutation arising
in the Tecta gene. J Assoc Res Otolaryngol 9(2):202-214.
Sienknecht UJ, Fekete DM (2008) Comprehensive Wnt-related gene expression during cochlear duct
development in chicken. J Comp Neurol 510(4):378-395.
Thalmann I, Thallinger G, Comegys TH, Thalmann R (1986) Collagen--the predominant protein of the
tectorial membrane. ORL J Otorhinolaryngol Relat Spec 48(2):107-115.
93