Supporting Information Haud et al. 10.1073/pnas.1009811107
advertisement

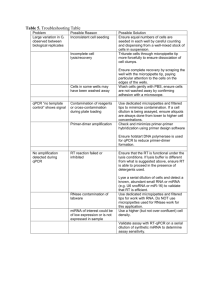
Supporting Information Haud et al. 10.1073/pnas.1009811107 SI Experimental Procedures Animal Husbandry, Mutagenesis, and Selection of AO127 Mutants. Zebrafish were raised and maintained at the University of Manchester Biological Services Unit as described elsewhere (1). ENU mutagenesis was performed on both AB and TL males as previously described (2). F1 families were generated and AB and TL progeny intercrossed to obtain F2 families of mixed lineage. Further inbreeding generated F3 embryos for screening and mapping. Day 5 postfertilization embryos were stained with 3 μg/μL of AO (Sigma) in embryo water for 15 min, then washed three times in embryo water. After anesthesia with Tricaine (Sigma), AO staining was visualized using a Zeiss stereo Lumar V12 fluorescent microscope fitted with filter set Lumar 38 and images were acquired using an AxioCam MRm camera and processed using AxioVision software (version 4.8; Image Associates). Subsequently, embryos were dissociated in trypsin and AO content measured by flow cytometry using a Beckman Coulter cyan ADP and data were analyzed using Summit software (Dako). All animal procedures were subject to local ethical review and performed under a Home Office license. Positional Cloning. We followed a previously described detailed protocol (3) (also available from http://zon.tchlab.org/). Genomic DNA samples were isolated from homozygous AO127 mutant and WT sibling embryos and pooled DNA samples from 20 embryos were used for the low-resolution mapping by bulk-segregant analysis. A total of 148 mutants were used for the high-resolution mapping. Simple sequence-length polymorphism markers (4, 5) were amplified by PCR and the products were analyzed on 4% agarose gels. SeqMan II software (DNASTAR) and Ensembl (www.ensembl.org/) were used to rebuild DNA contigs after a search for synteny with the human genome. Additional sequencelength polymorphism markers were designed to further narrow the critical region (primer sequences available on request). RNA Expression and Knockdown in Zebrafish. Zebrafish rnaset2 cDNA in the vector pDNR-LIB was obtained from Open Biosystems (clone zgc: 113369, catalog no. MDR1734-96866893). The T304A transversion was created using QuikChange sitedirected mutagenesis kit (Stratagene) with the following primers: T304A sense, 5′-CCCAAAATTTTGGAATTAAGAATGGACGAAACATGGGACC-3′ and T304A antisense, 5′-GGTCCCATGTTTCGTCCATTCTTAATTCCAAAATTTTGGG-3′. Capped mRNA was transcribed using the T7 mMessage mMachine kit (Ambion) and 1 nL of a 25 ng/μL stock of rnaset2 mRNA was injected into one to four cell stage mutant embryos using a PLI-90 Pico-Injector (Harvard Apparatus). An MO targeting the splicing of coding exon 3 of rnaset2 (rnaset2 MO, 5′-TGCACATCTGTCTTGAGAAACCCACCATAAT-3′), another targeting the splicing of exon 3 of rnaset2l (rnaset2l MO, 5′-TCCAATGTATATAAGGACATACTAT-3′), as well as a control MO (5′-GAGAGGAAATCCAATCAATCTGATG-3′), were obtained from GeneTools, and 1 nL of a 300 nM stock was injected into one to four cell stage heterozygous AO127 embryos. AO binding was analyzed at 3 d after fertilization by flow cytometry following disaggregation of AO-stained embryos using trypsin. Immunofluorescence. For RNASET2 subcellular localization, a 771-bp fragment encoding the full-length WT RNASET2 protein (1–256 aa) was PCR-amplified from control cDNA and cloned into the mammalian expression vector pcDNA3.1/myc (Invitrogen). C184R site-directed mutagenesis was performed using Haud et al. www.pnas.org/cgi/content/short/1009811107 the FlipFlop site-directed mutagenesis kit (Bioline) and the following primers: C184R sense, 5′-CCAAAATCCAGCGCCTTCCACCAAGC-3′; and C184R antisense, 5′-GCTTGGTGGAAGGCGCTGGATTTTGG-3′. HEK 293 cells growing on glass coverslips were stably transfected with WT or C184R mutant RNASET2 using the Effectene transfection reagent (Qiagen). Immunostaining was performed using standard methods and a polyclonal rabbit RNASET2-antiserum generated by conventional methods (Serunam) using His-fusion protein purified from cell culture supernatant as immunogen (dilution, 1:2,000). PDI (ER marker; Abcam), 58K Golgi protein (Abcam), and LAMP-1 (lysosome marker; BD Biosciences) antibodies were applied to detect subcellular colocalization. Alexa 488- and Cy3-conjugated anti-rabbit and anti-mouse secondary antibodies were used (Molecular Probes and Jackson ImmunoResearch; diluted 1:1,000). The cells were embedded in ProLongGold mounting medium with DAPI (Invitrogen). Fluorescence signals were detected by using an Axio-Imager M.1 fluorescence microscope (Zeiss). HEK 293 Culture and RNASET2 Knockdown. A control miRNA as well as a custom-designed miRNA targeting RNASET2, both based on the hairpin structure of mouse miR30a but with the targeting sequence replaced by sequence complementary to RNASET2 selected using BLOCK-iT RNAi Designer (https://rnaidesigner. invitrogen.com/rnaiexpress/), were generated by performing nine cycles of PCR using the following oligonucleotides (with italicized nucleotides corresponding to target sequence in RNASET2): Control miRNA forward: 5′-GCGGGGTACCGCGGGGATCCTGCTGTTGACAGTGAGCGA TTCTGGAAGCATGAGTGGGAA TAGTGAAGCCACAGATG-3′. Control miRNA reverse: 5′-CCGCGAATTCCCGCAGATCTCCTTGAAGTCCGAGGCAGTAGGCAG TTCTGGAAGCATGAGTGGGAA TACATCTGTGGCTTCAC-3′. RNASET2 miRNA forward: 5′-GCGGGGTACCGCGGGGATCCTGCTGTTGACAGTGAGCGA AAGCCAGGATGAGGAAGTACA TAGTGAAGCCACAGATG-3′. RNASET2 miRNA reverse: 5′- CCGCGAATTCCCGCAGATCTCCTTGAAGTCCGAGGCAGTAGGCAG AAGCCAGGATGAGGAAGTACA TACATCTGTGGCTTCAC-3′. The resultant PCR product was digested with KpnI and EcoRI and cloned into the intron of an EF1alpha-mCherry plasmid construct (comprising the promoter, noncoding exon I and intron I of the zebrafish EF1alpha gene spliced with cDNA encoding mCherry fluorescent protein) as described previously (6). HEK 293 cells were cultured in DMEM, supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 400 μg/mL Geneticin at 37 °C and 5% CO2. Equivalent numbers of cells in the wells of a six-well plate were transfected with 800 ng of control or RNASET2-targeting miRNA plasmid using Fugene HD (Roche) transfection reagent as instructed by the manufacturer. Western blotting with the rabbit polyclonal RNASET2 anti-serum described earlier was used to demonstrate RNASET2 protein knockdown. In parallel, cells were stained with 1 of 7 5 μM of the intravital dye LysoTracker Green DND-26 (Invitrogen) and analyzed immediately by flow cytometry using a Beckman Coulter cyan ADP and data analyzed using Summit software (Dako). Cells flow-sorted for mCherry fluorescence were fixed and prepared for EM analysis (as described as follows). TEM. TEM was carried out in the EM facility of the Faculty of Life Sciences at the University of Manchester. Embryos were sedated, fixed, and processed as previously described (7). HEK 293 cells were trypsinized, washed with PBS solution, pelleted, and fixed overnight at 4 °C in 5% glutaraldehyde in 0.2 M sodium cacodylate buffer, pH 7.4. Cells were washed three times in 0.1 M sodium cacodylate buffer, pH 7.4, and incubated in 0.1 M osmium tetroxide in 0.1 M sodium cacodylate buffer, pH 7.4, for 2 h. Cells were then washed three times in 0.1 M sodium cacodylate buffer, pH 7.4, and three times in double-distilled water. Cells were then incubated in 0.1% uranyl acetate in doubledistilled water for 1 h and washed again three times in doubledistilled water. This was followed by dehydration through an EtOH series (25%, 50%, 70%, 80%, 90%) for 10 min each and three times 10 min in 100% EtOH. The cell pellet was then quickly rinsed three times in propylene oxide and washed in increasing concentrations of low-viscosity resin medium: 25%, 50%, 75%. Finally, the pellet was washed three times with the resin and embedded overnight at 60 °C. For both zebrafish and human samples, ultrathin sections (70–80 nm) were cut using a Reichert Jung ultramicrotome. TEM observations were carried out using a FEI Tecnai12 BioTwin microscope and photographs were taken using a 4K × 4K CCD camera (Eagle; FEI) equipped with Kodak 4489 film. Images were acquired using an Imacon scanner and FlexColor software. In Situ Hybridization for 28S rRNA. Embryos were sedated in tricaine and fixed in 4% buffered paraformaldehyde for 2 h at room temperature. They were then dehydrated in methanol and stored at −20 °C. Embryos were rehydrated in descending concentrations of methanol in PBS solution. They were then embedded in 25% cold water fish gelatin (Sigma), 15% sucrose, at room temperature for 24 h. Samples were then frozen on dry ice and 8-μm sections were cut at −20 °C, collected on precoated polylysine slides (VWR), and stored at −80 °C. Frozen sections were allowed to dry and were immersed in acetone for 2 min before being rehydrated in PBS solution. Tissues samples were treated with 10 μg/mL protease K (Sigma) for 10 min, rinsed in PBS solution, then fixed in 4% PFA for 20 min. All samples were then washed with PBS solution and washed twice for 10 min with 2× SSC, 10% formamide. The in situ hybridization protocol was adapted from Rouquette et al. (8). Hybridization was performed at 37 °C for 3 h in the following buffer: 10% formamide, 10% dextran sulfate (Millipore), 2× SSC, 0.5 mg/mL tRNA, 50 mg/mL BSA, and 10 mM ribonucleoside–vanadyl complexes (NEB) containing 10 ng/mL of 28S rRNA Cy3 tagged antisense oligodeoxynucleotide probe (probe sequences, 28S antisense 5′-TATCTAGCGAAACCACAGCCAAGGGAACGGG-3′ and 28S sense 5′-CCCGTTCCCTTGGCTGTGGTTTCGCTAGATA-3′; Invitrogen). The slides were then washed twice with 2× SSC, 10% formamide, once with PBS solution, and then mounted with DAPI (Vector Labs). Images were collected on a Leica TCS SP5 AOBS inverted confocal using a 40× objective. When acquiring 3D optical stacks, the confocal software was used to determine the optimal number of Z sections. Only the maximum-intensity projections of these 3D stacks are shown. Reverse-Transcriptase Coupled PCR and Quantitative PCR Analysis. Total RNA was isolated from zebrafish larvae using RNAeasy Lipid Tissue mini kit (Qiagen) and from HEK293 cells using regular RNAeasy mini kit (Qiagen) and reverse-transcribed with Omniscript reverse transcription kit (Qiagen) to produce cDNA. Haud et al. www.pnas.org/cgi/content/short/1009811107 For direct visualization of amplification products, cDNA was amplified using standard PCR conditions (with amplification still in the linear phase) and the following primer pairs: rnaset2, forward, 5′-GGCCAGATCTGCTAGAACC-3′; and rnaset2Rv, 5′ATGCCAAGATGTGTTGCACC-3′ (used to analyze total rnaset2 message), rnaset2exon1, forward, 5′-CAGCCTTTACCCATCCTCG-3′; and rnaset2exon4, reverse, 5′-ATGCCAAGATGTGTTGCACC-3′ (used to detect disrupted splicing of exon 3 of rnaset2); rnaset2lexon2, forward, 5′-GGGCCTTTACCGCTGCTCTGG -3′; and rnaset2l exon10, reverse, 5′-CCTACGGGGTGTCTCTTCGTTC-3′ (used to detect disrupted splicing of exon 3 of rnaset2l); and β-Actin, forward, 5′-CTGACTACCTCATGAAGATCC-3′ and β-Actin, reverse, 5′-CTGTCCACCTTCCAGCAGATG-3′ (used to detect β-Actin mRNA as a control for RNA integrity). Quantitative real-time PCR was performed using the iQ SYBR Green Supermix (Bio-Rad) and the Chromo4 four-color real-time PCR detection system (Bio-Rad) according to the manufacturer’s instructions. Gene-specific primers were designed to generate a single amplicon of 75 to 100 nucleotides using Beacon Designer software. For evaluation of PCR efficiency, standard curves were generated using serial diluted cDNA samples (dilution factors of 1, 4, 16, and 64) and strong linear correlations between the CT values and the log of input cDNA amount were obtained with near 100% primer efficiency. Melting curve analyses were performed to verify that no primer dimers were amplified. All reactions were done in triplicate and the threshold cycle CT values were plotted against the log10 of the amount of cDNA by using Opticon Monitor 3.1 (BioRad) according to the manufacturer’s instructions. Data normalization was performed using the Genex macro provided by Bio-Rad and GAPDH was selected as the reference to normalize for input load of cDNA between samples. Primers were as follows: 28S, forward: 5′-CCTTGAAGCCTAGGGCGC-3′; 28S, reverse: 5′-GGGGCGAACCCATTCCAG-3′; GAPDH, forward: 5′-CTGTAATTGGAATGAGTC-3′; GAPDH, reverse: 5′-TTAATATACGCTATTGGAG-3′. MRI. MRI studies of the human brain were performed using a 3 T MRI system (Magnetom Trio; Siemens) with eight-channel phased-array head coils. Appropriate informed consent was obtained from the patients’ parents. Adult zebrafish were euthanized and were fixed in 4% buffered PFA for 2 d and subsequently embedded in Fomblin. All μMRI measurements were conducted on a 400 MHz (9.4 T) vertical bore system, using a transmit/receive birdcage radiofrequency coil with an inner diameter of 10 mm and a 1 Tm−1 gradient insert (Bruker). The system was interfaced to a Linux operating system running Topspin 2.0 and Para Vision 5.0 imaging software (Bruker Biospin). Before each measurement, the magnetic field homogeneity was optimized by shimming. Each session of measurements began with a multislice orthogonal gradient-echo sequence for position determination and selection of the desired region for subsequent experiments. For high resolution, T2-weighted MR images were acquired by a rapid acquisition with relaxation enhancement (RARE) sequence (9), which employs a single excitation step followed by the collection of multiple phase-encoded echoes. Basic measurement parameters used for the RARE sequence were as follows: echo time, 10.567 ms (22.45 ms effective); repetition time, 5 s; flip angle, 90°; averages, 64; and RARE factor (echo train length), 4. The field of view was 1.2 × 1.2 cm2, with an image matrix of 256 × 256. This yields an effective in-plane resolution of approximately 47 μm. Horizontal and sagittal image slices (n = 20) were acquired with slice thickness of 0.2 mm spaced 0.2 mm between slices. For T2 relaxation time measurement, a multislice multi echo sequence was used. Imaging parameters were as follows: field of view, 2.0 × 2.0 cm2; matrix size, 256 × 256; number of averages, 2; number of slices, 6; slice thickness, 0.5 mm; number of echoes, 12; echo 2 of 7 times, 8.5, 17.0, 25.5, 34.0, 42.5, 51.0, 59.5, 68.0, 76.5, 85.0, 93.5, and 102.0 ms; repetition time, 1.5 s. For calculation of T2 relaxation time, regions of interest were drawn at various locations within the brain. Means and SD for T2 relaxation times for each ROI were calculated. captured using a Zeiss Axioplan microscope and Axiocam MR camera and processed using Axiovision software. Paraffin Sections and Immunostaining. Adult fish euthanized by exposure to excess anesthesia were fixed in 4% buffered PFA and paraffin-embedded tissue sections (5 μm) were used for IHC. The primary antibodies used were mouse anti-APP (1:500 dilution; Zymed) and mouse anti-GFAP (1:300 dilution; Dako), incubated overnight at 4 °C. The secondary antibody used was Vectastain biotinylated horse anti–pan-Ig antibody. Following incubation with the secondary antibody, the sections were incubated with the ABC kit (Vectorlabs) and stained with DAB (Vectorlabs) before being mounted with Pertex. Images were RNase Activity Assay. Proteins were extracted from embryos following the method used by MacIntosh et al. (10). The protein extraction protocol was modified by eliminating β-mercaptoethanol and polyvinylpolypyrrolidone from the extraction buffer. Protein was quantified according to the Bradford method. RNase activity was determined by an in gel assay according to Yen and Green (11), using high molecular weight RNA purified from commercial torula (yeast) RNA (Sigma). A total of 50 μg of protein was run for each sample. Gels were incubated in 0.1 M Tris-HCl at pH 6.0. In parallel, SDS/PAGE gels were run using 50 μg of protein and subsequently stained with Coomassie blue to verify loading amounts and protein quality. All experiments were performed a minimum of three times and a representative sample was chosen for the figure. 1. Westerfield M (2000) The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio) (Univ of Oregon Press, Eugene, OR). 2. van Eeden FJ, Granato M, Odenthal J, Haffter P (1999) Developmental mutant screens in the zebrafish. Methods Cell Biol 60:21–41. 3. Bahary N, et al. (2004) The Zon laboratory guide to positional cloning in zebrafish. Methods Cell Biol 77:305–329. 4. Knapik EW, et al. (1998) A microsatellite genetic linkage map for zebrafish (Danio rerio). Nat Genet 18:338–343. 5. Shimoda N, et al. (1999) Zebrafish genetic map with 2000 microsatellite markers. Genomics 58:219–232. 6. Nicoli S, et al. (2010) MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature 464:1196–1200. 7. Michailidou C, et al. (2009) Dissecting the roles of Raf- and PI3K-signalling pathways in melanoma formation and progression in a zebrafish model. Dis Model Mech 2: 399–411. 8. Rouquette J, Choesmel V, Gleizes PE (2005) Nuclear export and cytoplasmic processing of precursors to the 40S ribosomal subunits in mammalian cells. EMBO J 24:2862–2872. 9. Hennig J, Nauerth A, Friedburg H (1986) RARE imaging: A fast imaging method for clinical MR. Magn Reson Med 3:823–833. 10. MacIntosh GC, Ulloa RM, Raices M, Tellez-Inon MT (1996) Changes in calciumdependent protein kinase activity during in vitro tuberization in potato. Plant Physiol 112:1541–1550. 11. Yen Y, Green PJ (1991) Identification and properties of the major ribonucleases of Arabidopsis thaliana. Plant Physiol 97:1487–1493. A B CAS I CAS II Fig. S1. Positional cloning of the mutated gene in AO127 mutants. (A) Low-resolution mapping and genome scanning suggested a region of interest in AO127 mutants surrounded by scaffold regions on chromosome 13 between the markers Z48615 and Z14311. A search for synteny using the genes CST7 and GinS1 revealed the true location of the mutated gene to be 50 Mbp away from the original interval. New markers (SSR2 and SSR5) were tested on critical recombinant mutant samples, resulting in one recombinant on each side of the critical region and narrowing the interval down to 140,850 bp within the BAC clone. Four genes were located within the interval and identified using Ensembl. (B) Alignment of protein sequences from multiple RNase T2 orthologues. The highly conserved catalytic active sites (CAS) I and II are high-lighted and the position of the nonsense mutation in AO127 mutants within the CASII domain indicated by an arrow. Haud et al. www.pnas.org/cgi/content/short/1009811107 3 of 7 intron 3 retained Cell count rnaset2 C M O lM O Co nt ro B rnaset2 MO rn as et 2 A Control MO in AO127 het rnaset2 MO in AO127 het 247 165 82 wild-type 0 100 101 102 103 AO fluorescence Fig. S2. Confirmation of the AO127 mutation by knockdown. (A) Schematic of the zebrafish rnaset2 gene mutated in AO127 mutants showing exon–intron structure and the position of a MO designed to interrupt splicing of coding exon 3. (B) RT-PCR revealed the presence of mRNA retaining intron 3 from animals injected with the rnaset2 MO. Residual WT mRNA is presumably maternal. β-Actin serves as a control for RNA integrity and equal input cDNA. (C) Injection of rnaset2 MO, but not control MO, in animals heterozygous for rnaset2 resulted in an increase in AO staining. brain eye jaw sense antisense Fig. S3. Specificity of the 28S rRNA probe. Frozen sections of WT embryos at 5 d after fertilization hybridized with a control sense 28S rRNA probe or antisense probe (green). Nuclei were counterstained with DAPI (blue). Note green autofluorescence in lens of section stained with sense probe. (Scale bar: 100 μm). Haud et al. www.pnas.org/cgi/content/short/1009811107 4 of 7 A B 7000 24h LysoTracker Fluorescence (AU) miRNA control 48h RNASET2 䃑-ACTIN * 6000 5000 4000 control miRNA 3000 2000 1000 0 D C 700 * * * * * control * * number of lysosomes 600 500 400 300 control miRNA 200 100 miRNA 0 E Relative normalized expression 28S rRNA expression 2 control miRNA 1.5 1 0.5 0 Fig. S4. RNASET2 depletion in HEK 293 cells results in increased lysosome biogenesis without rRNA accumulation. (A) RNASET2 protein levels in HEK 293 cells transfected with control construct (control) or specific miRNA targeting RNASET2 detected by immunoblotting. (B) Cells transfected as in A for 48 h were stained with LysoTracker and fluorescence intensity measured in mCherry-positive cells using a flow cytometer (n = 3 independent experiments; error bars represent SEM). (C) EM examination of mCherry-positive cells transfected as in A for 48 h revealed more numerous lysosomes following RNASET2 knockdown. (Scale bar: 1 μm.) (D) Quantification of lysosome numbers from C. Mean number of lysosomes per EM grid quadrant measuring 500 μm2 were enumerated (n = 3 independent experiments; error bars represent SEM; *P < 0.01, independent-samples t test). (E) Quantitative PCR was performed for 28S RNA: histogram depicts relative fold change in expression normalized to GAPDH ± SEM (n = 3). Haud et al. www.pnas.org/cgi/content/short/1009811107 5 of 7 A B A V D P 1 Area 3 2 4 5 T2 relaxation time (ms) 1 28.3㼼1.02 2 25.01㼼1.26 3 31.09㼼1.31 4 32.14㼼0.99 5 33.7㼼1.04 Fig. S5. Comparable MR presentations of RNase T2-deficient human and zebrafish. (A) Axial T2-weighted cerebral MR image of a patient with RNASET2deficient cystic leukoencephalopathy. The image indicates multifocal white matter abnormalities adjacent to the ventricles (black arrows) and in deep white matter (white arrows). (Scale bar: 1 cm). (B) T2 relaxation time measurement of the brain of a mutant zebrafish containing nullizygous mutation in rnaset2 gene. Sagittal MR image of the brain of an adult AO127 mutant zebrafish indicate the presence of lesions adjacent to the ventricles (white arrows) and were of similar intensity as that of CSF present in ventricles. (Boxed area, enlarged at right.) Areas were selected for T2 relaxation time measurements within healthy brain region (1, 2), in ventricles (4), and in lesion outside (3) or adjacent to ventricle (5). The lesions adjacent to ventricles have similar T2 relaxation time as that of ventricles, suggesting that these lesions may be filled with solution similar to CSF. Haud et al. www.pnas.org/cgi/content/short/1009811107 6 of 7 r Wi ldtyp e AO 12 7m uta nt Ma rke Ma rke r Wi ldtyp e AO 12 7m uta nt B Wi ldtyp e+ Wi ldtyp cont rol e+ MO rna s et2 AO lM 1 O M a 27 + rke rna r se t2l MO Wi ldtyp e+ co Wi nM ldtyp O e+ AO rna 12 se 7+ t2l MO rna Ma se rke t 2 r lM O A 250 35 130 100 70 27 55 35 27 17 C AO127 Key: Cell count Cell count Wild-type AO fluorescence 17 Control MO rnaset2l MO AO fluorescence rnaset2l MO D WT AO127 MO: Control rnaset2l Control rnaset2l rnaset2l intron 3 retained wild-type Actin Fig. S6. Decreased RNase T2 activity in AO127 mutants and redundancy with rnaset2l. (A) Left: In-gel activity assay was performed at pH 6.0 to analyze RNase T2 activity in protein extracts from WT and AO127 mutant embryos at 3 d after fertilization. White arrows indicate species present in only WT or greatly diminished in AO127 mutants. Note the different molecular weight species result from glycosylation (1) and/or possibly from proteolytic processing of RNase T2 (2). Right: SDS/PAGE gels run in parallel and stained with Coomassie blue to verify loading amounts and protein quality. (B) As in A, only samples prepared from embryos injected with a standard control oligonucleotide (control MO) or antisense oligonucleotide targeting rnaset2l (rnaset2l MO) are shown. (C) Embryos at 3 d after fertilization injected with control MO or rnaset2l MO were stained with AO, disaggregated, and subjected to flow cytometry. (D) Depiction of exon– intron structure of the rnaset2l gene and the position of a MO designed to interrupt splicing of exon 3. RT-PCR revealed the presence of mRNA retaining intron 3 from animals injected with the rnaset2l MO. Residual WT mRNA is presumably maternal. β-Actin serves as a control for RNA integrity and equal input cDNA. 1. Hillwig MS, et al. (2009) Zebrafish RNase T2 genes and the evolution of secretory ribonucleases in animals. BMC Evol Biol 9:170. 2. Campomenosi P, et al. (2006) Characterization of RNASET2, the first human member of the Rh/T2/S family of glycoproteins. Arch Biochem Biophys 449:17–26. Haud et al. www.pnas.org/cgi/content/short/1009811107 7 of 7