Mathematical Modeling of Capillary Formation and
advertisement

Bulletin of Mathematical Biology (2001) 63, 801–863
doi:10.1006/bulm.2001.0240
Available online at http://www.idealibrary.com on
Mathematical Modeling of Capillary Formation and
Development in Tumor Angiogenesis: Penetration into the
Stroma
HOWARD A. LEVINE
Department of Mathematics,
Iowa State University,
Ames,
Iowa 50011,
U.S.A.
E-mail: halevine@iastate.edu
SERDAL PAMUK
Matematik Bölümü,
Kocaeli Üniversitesi,
Kocaeli 41300,
Turkey
E-mail: spamuk@kou.edu.tr
BRIAN D. SLEEMAN
School of Mathematics,
University of Leeds,
Leeds LS2 9JT,
England, U.K.
E-mail: bds@amsta.leeds.ac.uk
MARIT NILSEN-HAMILTON
Department of Biochemistry, Biophysics and Molecular Biology,
Iowa State University,
Ames,
Iowa 50011,
U.S.A.
E-mail: marit@iastate.edu
The purpose of this paper is to present a mathematical model for the tumor vascularization theory of tumor growth proposed by Judah Folkman in the early 1970s
and subsequently established experimentally by him and his coworkers [Ausprunk,
D. H. and J. Folkman (1977) Migration and proliferation of endothelial cells in
performed and newly formed blood vessels during tumor angiogenesis, Microvasc
Res., 14, 53–65; Brem, S., B. A. Preis, ScD. Langer, B. A. Brem and J. Folkman
(1997) Inhibition of neovascularization by an extract derived from vitreous Am. J.
Opthalmol., 84, 323–328; Folkman, J. (1976) The vascularization of tumors, Sci.
Am., 234, 58–64; Gimbrone, M. A. Jr, R. S. Cotran, S. B. Leapman and J. Folkman
0092-8240/01/050801 + 63
$35.00/0
c 2001 Society for Mathematical Biology
802
H. A. Levine et al.
(1974) Tumor growth and neovascularization: an experimental model using the rabbit cornea, J. Nat. Cancer Inst., 52, 413–419]. In the simplest version of this model,
an avascular tumor secretes a tumor growth factor (TGF) which is transported
across an extracellular matrix (ECM) to a neighboring vasculature where it stimulates endothelial cells to produce a protease that acts as a catalyst to degrade the fibronectin of the capillary wall and the ECM. The endothelial cells then move up the
TGF gradient back to the tumor, proliferating and forming a new capillary network.
In the model presented here, we include two mechanisms for the action of angiostatin. In the first mechanism, substantiated experimentally, the angiostatin acts as
a protease inhibitor. A second mechanism for the production of protease inhibitor
from angiostatin by endothelial cells is proposed to be of Michaelis–Menten type.
Mathematically, this mechanism includes the former as a subcase.
Our model is different from other attempts to model the process of tumor angiogenesis in that it focuses (1) on the biochemistry of the process at the level of the
cell; (2) the movement of the cells is based on the theory of reinforced random
walks; (3) standard transport equations for the diffusion of molecular species in
porous media.
One consequence of our numerical simulations is that we obtain very good computational agreement with the time of the onset of vascularization and the rate of
capillary tip growth observed in rabbit cornea experiments [Ausprunk, D. H. and J.
Folkman (1977) Migration and proliferation of endothelial cells in performed and
newly formed blood vessels during tumor angiogenesis, Microvasc Res., 14, 73–65;
Brem, S., B. A. Preis, ScD. Langer, B. A. Brem and J. Folkman (1997) Inhibition of
neovascularization by an extract derived from vitreous Am. J. Opthalmol., 84, 323–
328; Folkman, J. (1976) The vascularization of tumors, Sci. Am., 234, 58–64; Gimbrone, M. A. Jr, R. S. Cotran, S. B. Leapman and J. Folkman (1974) Tumor growth
and neovascularization: An experimental model using the rabbit cornea, J. Nat.
Cancer Inst., 52, 413–419]. Furthermore, our numerical experiments agree with
the observation that the tip of a growing capillary accelerates as it approaches the
tumor [Folkman, J. (1976) The vascularization of tumors, Sci. Am., 234, 58–64].
c 2001 Society for Mathematical Biology
1.
I NTRODUCTION
The complex processes of angiogenesis as the outgrowth of new vessels from
a pre-existing vascular network is fundamental to the understanding of vascularization in many physiological and pathological processes. In normal situations,
angiogenesis is the process whereby new blood vessels are formed during embryogenesis, fetal development and placenta growth, for example. Under pathological
conditions, angiogenesis is basic to wound healing, rheumatoid disease and thromboses. In particular it is a key player during the initiation and progressive growth
of most types of solid tumors.
This last case is the focus of this paper.
During normal angiogenesis, the development and growth of blood vessels ceases
as soon as the fetus, for example, has become fully developed.
Modeling of Capillary Growth
803
However, in other situations, two outcomes are possible. In wound healing, angiogenesis ceases as soon as its purpose is fulfilled.
On the other hand, in the case of tumor growth, there may be periods of remission, angiogenesis never really ceases entirely. Indeed, it has been observed that
new vascular networks may collapse and remodel through new sprouting, looping
and anastomoses (Holash et al., 1998).
In order to explain the various events leading to tumor angiogenesis we begin
with a brief description of the central importance to angiogenesis of endothelial
cells lining normal vasculatures. Endothelial cells (EC) form the lining of all blood
vessels. In the interior of capillaries, they form a mono-layer of flattened and extended cells. The abluminal surface of the capillary, is covered by a collageneous
network intermingled with laminin and other proteins and carbohydrates. This is
called the basal lamina (BL). The BL serves as a scaffold or exoskeleton upon
which the EC can rest. The BL is mainly formed by EC while the layers of EC and
BL are sheathed by fibroblasts and possibly smooth muscle cells. In the neighborhood of the BL there are other cell types such as pericytes (Crocker et al., 1970),
platelets, macrophages and mast cells.
Among the several pathways by which tumor angiogenesis may be initiated is the
release of tumor angiogenic growth factors (TGF)† from an avascular tumor (Folkman, 1992). See Fig. 1 taken from Folkman (1992). This figure illustrates the variety of pathways by which tumors can induce angiogenesis. Here we are concerned
with pathway 1 → 4 and pathway 11. That is, we model the export of angiogenic
molecules from tumor cells; transport in the extra cellular matrix; endothelial cell
response to angiogenic molecules via basement membrane degradation and their
subsequent migration and proliferation as they form new capillaries. We are also
concerned with angiogenesis inhibition.
How angiogenic factor release is induced is not completely understood although
energy imbalance due to hypoxia may be a major factor. Other sources of angiogenic growth factors are also possible (Folkman, 1992). Indeed macrophage
cells can be chemotactically stimulated by the tumor to release angiogenic growth
factors.
Following TGF production by the tumor, EC in neighboring normal capillaries are activated to secrete proteases (Kabelic et al., 1983). These then degrade
the basal lamina and permit the EC to migrate into the extracellular matrix (ECM).
Small budding sprouts are subsequently formed that grow toward the tumor
following the chemotactic and haptotatic gradients initiated by the TGF and the
protease. This initial phase of the angiogenic process has been the subject of
previous studies in Levine et al. (2000, 2001).
In Levine et al. (2000, 2001), we laid the foundations for our modeling approach
by basing our ideas on the theory of reinforced random walks and a Michaelis–
Menten mechanism by which endothelial cells convert TGF into protease as
† We shall sometimes refer to these factors as VEGF or vascular endothelial cell growth factors.
804
H. A. Levine et al.
ANTIANGIOGENIC THERAPY: POINTS OF ATTACK
Basement
Membrane
Macrophage 6
Mast Cell
5
Chemotactic
Molecules
Tumor
1
4
Angiogenic
Molecules 2
Angiogenic Inhibitors 11
3
Fibrin 9
8
10
7 Plasminogen Activators
Collagenases
Heparanases
Figure 1. From Folkman (1992). Folkman’s concept of tumor vascularization. (By
permission)
described in Paweletz and Knierim (1989). That is, our model views the EC receptor as the catalyst for the transformation of TGF into proteolytic enzyme, which in
turn degrades fibronectin and destroys the basal lamina. The results of our modeling show that if sufficient angiogenic factor is supplied to the capillary wall, either
initially or at a steady rate (as might occur if it is being supplied from a remote
source such as a tumor or macrophage cells), there is a breakdown of the basal
lamina concurrent with a bimodal distribution of endothelial cells. That is, the EC
cell density, for a fixed time, exhibits two aggregated peaks of EC concentration
that lie just inside the walls of the fibronectin opening and form the lining of the
emerging sprout. The minimum of the EC density lies between these two peaks
and occurs at the center of the opening fibronectin channel.
In Levine et al. (2000) we developed the model further to include the effects
of haptotactic saturation of fibronectin and the role of pericytes and macrophages
in regulating angiogenesis. (This is the pathway 1 → 5 → 6 → 4 in Fig. 1.)
Furthermore, in this extended model, we included a mechanism for the action of
anti-angiogenic factors such as angiostatin.† However, unlike the present situation,
† Mathematical modelling of angiogenesis inhibition has been considered earlier in Orme and Chap-
lain (1997). The strategies proposed in Orme and Chaplain (1997) are primarily phenomenologically,
rather than biochemically based. Here we give two examples of biochemically based anti-angiogenic
strategies.
Modeling of Capillary Growth
805
in Levine et al. (2000) we were only concerned with the onset of sprout formation once chemotactic factors had reached the macrophage cells near the ‘mother’
capillary. Again, the simulations suggest that when the rate of supply of chemotactic factor was sufficiently large, the bimodal distribution of endothelial cells was
observed along the axis of the mother capillary. Additionally, the simulation predicted bimodal distributions of macrophage and pericyte cells. Interestingly, the
model ‘predicts’ that there should be three linings of the nascent capillary; an inner
lining of macrophage cells, an intermediate lining of endothelial cells and an outer
lining of pericyte cells. This seems to be consistent with what has been observed
in the laboratory (Schor et al., 1992) for endothelial cells and for epithelial cells
(Anderson, 1985).
In addition, in Levine et al. (2000), numerical simulations show that if angiostatin
is supplied at a constant rate, there is a marked inhibitory effect on the function of
the protease.
The models propose that angiostatin either acts as a protease inhibitor, or else, in
response to angiostatin, EC produce a protease inhibitor that impedes the action of
protease on fibronectin and its effect on endothelial cell movement. Experimental
justification for the first mechanism is to be found in Stack et al. (1999) and Nelson
(1998) and will be discussed in some detail below.
Our task in this paper is to extend the model in order to understand the angiogenic
process as the EC migrate into the ECM toward the tumor (or other remote source
of growth factor). Within the ECM behind the tip of the emerging capillary, the EC
proliferate to contribute to the number of migrating cells. These proliferating cells
then form solid strands of EC lining of the growing capillary in the ECM. Basal
lamina development continues with mitosis, fusion of sprouts, and the formation of
loops (anastomoses). The first signs of a micro blood circulation are observed. The
processes of mitosis, branching and looping etc. form a cascade of events as the EC
move along chemotactic and haptotactic gradients toward the tumor. Eventually the
tumor is penetrated by the micro vascular network. This network then provides a
direct supply of oxygen and other nutrients from the blood stream to the tumor,
thus permitting its rapid growth and local metastatic invasion.
Mathematical modeling of angiogenesis has been discussed by a number of authors (Balding and McElwain, 1985; Orme and Chaplain, 1996; Sleeman, 1996;
Chaplain and Anderson, 1999; Sherratt et al., 1999). These works have been
mainly devoted to modeling the macroscopic events of endothelial cell evolution
and migration characteristics within the ECM. The modeling ideas are based on
the principles of mass conservation and chemical kinetics. While there are some
formal similarities with the model we develop in this paper, there are several and
significant differences.
To begin with, we are modeling capillary formation at the level of the cell and its
receptors, i.e., at the boundary of cellular biology and biochemistry. See Kuwano
et al., 1994. That is, unlike previous work, we include the important mechanism
by which TGF activates the endothelial cells to secrete proteolytic enzymes that
806
H. A. Levine et al.
degrade the basal lamina and permit the EC to migrate into the ECM. Furthermore,
previous authors do not take into account mechanisms by which angiostatic agents
may inhibit angiogenesis. (Indeed, our model can be modified to treat the effects
of endostatins as well but we do not do this here.)
The biochemistry for this model is based on the rigorous use of MichaelisMenten (MM) kinetics for the chemical kinetics at every stage of its development
where appropriate, including use of the MM hypothesis for the action of protease
on fibronectin/collagen proteins.
Secondly, following on our previous work, (Levine et al., 2000, 2001), we base
the model for the chemotactic endothelial cell movement on the theory of reinforced random walk (Levine and Sleeman, 1997; Othmer and Stevens, 1997).† In
this model, the cells move in response to protease and by sensing a protease created cavity in the fibronectin/collagen into which they may move. The cells proliferate (to a point) in the ECM in response to protease and the rate of proliferation is
strongly affected by their location in the capillary. That is, the proliferation of EC
includes a tip curvature effect.
Thirdly, this is the first model we know of in which EC movement in a ‘mother
capillary’ is tied to EC movement and proliferation in the developing ‘daughter
capillary’. This naturally complicates the model but the pay off is that this is exactly what one observes in tissue morphology (Rakusan, 1995).
Finally, the ideas presented here are sufficiently broad to include other pathways
in Fig. 1. For example, in Levine et al. (in preparation) we are modifying the
model to include pathway 7, which is concerned with tumor emitted plasminogen
activators, collagenases and heparanases.
One consequence of our numerical simulations is that we obtain very good computational agreement with the time of the onset of vascularization and the rate of
capillary tip growth observed in rabbit cornea experiments (Gimbrone et al., 1974;
Folkman, 1976; Ausprunk and Folkman, 1977; Brem et al., 1997). Furthermore,
our numerical experiments agree with the observation that the tip of growing capillary accelerates as it approaches the tumor (Folkman, 1976).
The whole process of angiogenesis is extremely complex. Nevertheless we believe our ideas are a step forward in providing a logical and sound, biochemically
based modeling procedure. We hope this model will improve the current state of
the modeling art and provide some insight into how various growth inhibitory drugs
(e.g., angiostatins) may act to combat the progressively invasive disease of cancer.
† The theory of reinforced random walks allows us to model a number of important angiogenic
processes either from the point of view of individual cells as do Sleeman and Wallis, 2001 (for
a more restricted system than we consider here) or from the point of view of particle probability
density as we do here.
In either case, no rescalings need to be done at each time step either in the discrete master equation
or in any of the discretized equations obtained from the continuous equation using any of the standard
numerical methods. No artificial random numbers need to be selected in confidence intervals and then
the resultant equation renormalized. To within the limits of computational accuracy of the method,
the results we obtain here will be independent of the numerical method selected to solve the system.
Modeling of Capillary Growth
807
The outline of the paper is as follows:
1. Introduction
2. The geometry of the problem
3. The biochemistry of angiogenesis and its inhibition
3.1. The mechanism for the production of protease
3.2. Mechanisms for the production of protease inhibitors
3.3. The mechanism for the degradation of fibronectin
4. The equations of mass action
5. Chemical transport in the capillary and in the ECM
5.1. Chemical transport equations in the capillary
5.2. Chemical transport equations in the ECM
6. The principles of reinforced random walk applied to cell movement
6.1. Cell movement equations in the capillary
6.2. Cell movement equations in the ECM
7. Transmission, Boundary and Initial Conditions
7.1. Capillary-ECM transmission conditions
7.2. Boundary conditions
7.3. Initial conditions
8. Numerical experiments
8.1. Terminology
8.2. Expected properties of the solutions.
9.
10.
11.
12.
Summary and Conclusions
Appendix A. A brief discussion of the random walk equations
Appendix B. The choice of phenomenological constants
Appendix C. Code testing and convergence.
2.
T HE G EOMETRY OF THE P ROBLEM
Throughout this paper, we will use Fig. 2 as a basis for our simplified version
of Folkman’s tumor model. That is, in the x–y plane we envisage a capillary
segment of length L microns located along the x-axis on the interval [0, L] with
a tumor located ` microns above the x-axis as shown. A time-dependent function
defined on [0, L] × [0, `] will be denoted by an upper case letter, G, and will have
arguments (x, y, t) unless otherwise stated. A function defined inside the capillary
wall will be denoted by a lower case letter, g say, with argument list (x, t). In
general, g(x, t) 6= G(x, 0, t) i.e., the function designated by a lower case letter is
not the trace of the function designated by the upper case letter. This is necessary to
distinguish between quantities just outside the capillary wall and the corresponding
quantities just inside the capillary wall.
808
H. A. Levine et al.
Tumor colony
y= l
Extracellular Matrix
(ECM)
y=0
Basement Lamina
x=0
Capillary
(BL)
x=L
Figure 2. The geometry for the idealized Folkman model.
Although we imagine the capillary wall to be infinitely thin, we take as a measure
of its penetrability, the density of fibronectin f (x, t) defined on [0, L].
We shall sometimes refer to the capillary in Fig. 2 as the ‘mother’ capillary. Any
new capillaries branching from it will be called ‘daughter’ capillaries.
R EMARK 1. Notation. Throughout this paper we will have need of the Heaviside
function, H (x) which the reader will recall is defined as
(
1,
H (x) =
0,
if x ≥ 0,
if x < 0.
We will employ this function in what follows to serve as a switching mechanism for
our dynamical equations. In general, x might be the concentration of growth factor,
or protease, or the concentration of one of these variables over some threshold
value, in which case the argument will be of the form x − x0 where x0 is the
threshold value.
3.
T HE B IOCHEMISTRY OF A NGIOGENESIS AND ITS I NHIBITION
3.1. The mechanism for the production of protease. We propose to model this
process in the following manner.†
If V denotes a molecule of angiogenic factor (substrate) and R E denotes a receptor on the endothelial cell wall, they combine to produce an intermediate complex,
R E V which is an activated state of the receptor that results in the production and
† The discussion here is not unlike that in Edelstein-Keshet (1988, pp. 272–279) for the passage of
nutrients from the exterior to the interior of a bacterium.
Modeling of Capillary Growth
809
secretion of proteolytic enzyme, C, and a modified intermediate receptor R 0E . The
receptor R 0E is subsequently removed from the surface by a mechanism that is
presumed to be very fast in the time scale of the production of protease C. The
receptor R 0E is then either recycled back to the cell surface to again become R E or
it is degraded and new R E is synthesized which then moves to the cell surface to
replace the R E that had been removed.
The point of view is that the receptors at the surface of the cell function the same
way an enzyme functions in classical enzymatic catalysis. In symbols:
k1
V + R E [R E V ]
k−1
k2
[R E V ] −
→ C + RE .
(3.1.1)
3.2. Mechanisms for the production of protease inhibitors. There are several
ways in which angiostatic agents might inhibit angiogenesis (Nelsen, 1998). In
Han and Liu (1999), a table is provided which indicates several such mechanisms.
The model we present here allows for several of them, including the actions of
endostatins.
Here we restrict our attention to two such mechanisms, one of which has been
verified in the experimental literature (Stack et al., 1999). We begin with this
mechanism.
First, we consider angiostatin as a direct inhibitor of protease. When we do this,
A + CA CI .
(3.2.1)
Here C I denotes the proteolytic enzyme molecules which are inhibited by the inhibitor A from functioning as a catalyst for fibronectin degradation while C A denotes those molecules which degrade fibronectin. We refer to these species as
inhibited and active protease, respectively. In terms of concentrations, [C] =
[C A ] + [C I ]. Assuming that (3.2.1) is in equilibrium, we have [C I ] = νe [A][C A ]
where νe is the equilibrium constant for this step. In general, the reaction in (3.2.1)
is essentially complete. (That is, νe 1.) However, this is not always the case.
For example, consider the case where plasminogen activation is inhibited by angiostatin. The tissue plasminogen activator (tPA) is produced in response to a growth
factor such as VEGF. Then tPA cleaves plasminogen with the resultant product being plasmin (Pm). In the model proposed in Stack et al. (1999), the angiostatin
binds directly to the intermediate [tPA–Pg] complex to inhibit the production of
active plasmin protease. (The angiostatin here is a fragment of plasminogen with
a molecular weight of about 38 kDa.) The literature value in Stack et al. (1999)
given for νe−1 is of the order of 1 µM.
Another possibility is to involve the endothelial cells once more. In this more
complex mechanism, the angiostatin stimulates EC to produce an inhibitor I ac-
810
H. A. Levine et al.
cording to the mechanism
k3
A + R E A [A R E A ]
k−3
k4
[A R E A ] −
→ I + RE A
CA + I CI ,
(3.2.2)
where R E A is a receptor protein on the endothelial cell and [A R E A ] is the intermediate complex. Moreover, I is a protease inhibitor produced by the endothelial
cells in response to the angiostatic agent by an overall mechanism which we will
assume to be of Michaelis–Menten type also. Here C I denotes the proteolytic enzyme molecules that are inhibited by the inhibitor I from functioning as a catalyst
for fibronectin degradation. Assuming that the last step in (3.2.2) is in equilibrium,
we have that [C I ] = νe [I ][C A ] where νe is the equilibrium constant for this step.
Here too, the question of completeness must be considered. In general, the more
complete (the larger νe is) (3.2.2), the more efficacious will be the angiostatin in
the inhibition of angiogenesis.
One such mechanism is to be found in Stokes and Lauffenburger (1991). The
value for νe here is rather large, on the order of 103 (µM)−1 .†
3.3. The mechanism for the degradation of fibronectin. The decay of fibronectin via protease is assumed to satisfy a reaction mechanism of the form:
k5
C A + F [C A F]
k−5
k6
[C A F] −
→ C A + F 0.
4.
(3.3.1)
T HE E QUATIONS OF M ASS ACTION
Consider the case for which angiostatin is converted by endothelial cells into a
protease inhibitor. If we apply the law of mass action to the equations (3.1.1)–
(3.3.1) we obtain:
∂[V ]
= −k1 [V ][R E ] + k−1 [R E V ],
∂t
∂[R E ]
= −k1 [V ][R E ] + (k−1 + k2 )[R E V ],
∂t
† While we do not have rate constants involved in (3.2.2), we do have rate constants for the VEGF–
protease system (3.2.1). We shall assume that the rate constants for the former are roughly of the
same order of magnitude as those of the latter in our illustrative computations below.
Modeling of Capillary Growth
∂[R E V ]
∂t
∂[C]
∂t
∂[A]
∂t
∂[R E A ]
∂t
∂[R E A A]
∂t
∂[I ]
∂t
811
= k1 [V ][R E ] − (k−1 + k2 )[R E V ],
= k2 [R E V ] − µ[C],
= −k3 [A][R E A ] + k−3 [[R E A A],
= −k3 [A][R E A ] + (k−3 + k4 )[R E A A],
= k3 [A][R E A ] − (k−3 + k4 )[R E A A],
= k4 [R E A A].
(4.1)
For the case in which angiostatin acts as an inhibitor, the last four equations may
be omitted from (4.1).
The enzyme kinetics for (3.3.1) for the fibronectin decay leads to three additional
ordinary differential equations which we have not included in (4.1). However, it is
reasonable to assume that the kinetics for the degradation of fibronectin by protease
is of Michaelis–Menten type, i.e., the concentration of the intermediate complex
C A F may be assumed to be constant after a short time interval. That triple of
equations then reduces to
∂[F]
λ3 [C A ][F]
=−
.
∂t
1 + ν3 [F]
(4.2)
Here we have written [C A F] = ν3 [C A ][F] and
λ3 = ν3 k6 ,
ν3 = k5 /(k−5 + k6 )
whereas in the biochemical literature, one uses the notation
K cat = λ3 /ν3 ,
K m = 1/ν3 .
3
We shall denote these K cat , K m values by K cat
, K m3 since then the superscript will
refer to the third of the three enzyme kinetic mechanisms above.
R EMARK 2. In addition to the law of mass action we have included a term in the
protease equation (µ[C]) which reflects the decay rate of protease.
R EMARK 3. The fibronectin rate law above is incomplete as it stands. Endothelial cells are known to produce fibronectin (Jaffee and Mosher, 1978; Yamada and
Olden, 1978; Orme and Chaplain, 1996). We need to account for this in the capillary. Also, in the ECM, there will be background production of fibronectin. Additionally, in the ECM, there may be some diffusion of fibronectin. Therefore we
will need to complete this rate law in the discussion in the following sections.
812
H. A. Levine et al.
The system of equations (4.1) admit the following conservation laws (first integrals):
[R E ](t) + [R E V ](t) = [R E ](0) + [R E V ](0) = [R E ](0),
[R E A ](t) + [R E A A](t) = [R E A ](0) + [R E A A](0) = [R E A ](0)
(4.3)
where we have assumed that at the outset (t = 0) the concentrations of the intermediate ligand–receptor complexes R E V and R E A A vanish.
We assume the Michaelis–Menten hypothesis for (3.1.1) and (3.2.2) holds here,
i.e., the concentrations of the intermediate complexes, R E V, [R E A A] are assumed
to be constant (Murray, 1989). Thus we set ∂t [R E ]/k1 = ∂t [R E A ]/k3 = 0. If we
set ν1 = k1 /(k−1 + k2 ) ν2 = k3 /(k−3 + k4 ) and use (4.3) we obtain
[R E ](t) =
[R E ](0)
,
1 + ν1 [V ](t)
[R E V ](t) =
ν1 [R E ](0)[V ](t)
,
1 + ν1 [V ](t)
[R E A ](t) =
[R E A ](0)
,
1 + ν2 [A](t)
[R E A A](t) =
ν2 [R E A ](0)[A](t)
.
1 + ν2 [A](t)
(4.4)
Mathematically speaking, these equations cannot be correct as they stand. Consider for example, the first of (4.4). This cannot hold at t = 0 unless the initial concentration of the growth factor is zero. Of course, as pointed out in Murray (1989,
Chapter 5), this difficulty arises because the assumptions that 1/(k1 [R E ](0)) =
1/(k3 [R E A ](0)) = 0 are not consistent with the number of initial conditions for
the system (4.1). In other words, we are dealing with a singular perturbation problem here. The equations (4.4) are only valid in deriving the so-called ‘outer solution’. The outer solution is considered to be valid only for times t =
max{1/(k1 [R E ](0)), 1/(k3 [R E A ](0))} and must be matched with the so-called ‘inner solution.’ Murray does this in Murray (1989). If is very small, as we are
assuming here, we need only concern ourselves with the outer solution. In the
case of the system here, we discussed this hypothesis in Levine et al. (2000, 2001).
Other sources include Frenzen and Maini (1988), Segel (1988) and Segel and Slemrod (1989).
Murray refers to the outer solution as the ‘pseudo-steady state’. He also uses a
singular perturbation argument to give a set of circumstances under which (4.4)
may be justified. These conditions are met here for reaction mechanisms such
as (3.1.1) when the above assumptions on the rate constants are met and when
k1 [R E ](0) and k3 [R E A ](0) are very large.
Modeling of Capillary Growth
813
From now on, we make this assumption. Employing (4.4) in the equations (4.1)
for the time rates of change of [V ], [C], [A], [I ] we obtain
∂[V ]
−ν1 k2 [V ](t)[R E ](0)
=
,
∂t
1 + ν1 [V ](t)
∂[C]
ν1 k2 [V ](t)[R E ](0)
=
− µ[C](t),
∂t
1 + ν1 [V ](t)
−ν2 k4 [A](t)[R E A ](0)
∂[A]
=
∂t
1 + ν2 [A](t)
∂[I ]
ν2 k4 [A](t)[R E A ](0)
=
.
∂t
1 + ν2 [A](t)
Now, by a time scale argument, which we made reasonably precise in Levine
et al. (2000, 2001), we may replace the quantities [R E ](0), [R E A ](0) in these equations by [R E ](t), [R E A ](t). There results
∂[V ] −ν1 k2 [V ](t)[R E ](t)
=
,
∂t
1 + ν1 [V ](t)
∂[C] ν1 k2 [V ](t)[R E ](t)
=
− µ[C](t),
∂t
1 + ν1 [V ](t)
∂[A] −ν2 k4 [A](t)[R E A ](t)
=
,
∂t
1 + ν2 [A](t)
∂[I ] ν2 k4 [A](t)[R E A ](t)
=
.
∂t
1 + ν2 [A](t)
(4.5)
These equations, together with the conservation equation for protease and the
equilibrium equation of active protease,
[C] = [C A ] + [C I ] + [C A F]
[C I ] = νe [I ][C A ]
(4.6)
and the rate law for fibronectin, suitably corrected to allow for the contribution
of endothelial cell produced fibronectin, constitute the general chemical transport
equations to be used in what follows.
To complete our discussion we further exploit the relationship between cell density and receptor density. Suppose [EC] is the concentration of endothelial cells. In
general, the number of receptors per cell, [R E ]/[EC] (resp. [R[E A] /[EC]), which
we denote by δe or δa , is nearly constant although it may vary somewhat with [EC],
for example, if the cells are closely packed or after initiation of cell proliferation
and movement. We need to do this because, while we have good estimates of the
814
H. A. Levine et al.
number of receptors per cell, it is the number of cells per unit length that we can, in
principle, directly observe in sections of tissue under the microscope. In turn, this
linear cell density must be converted to a volumetric density expressed in cells per
liter.† To find the volumetric density, we imagine the cells to be small rectangular
parallelepipeds.
Since capillaries have a diameter of about 6–8 microns and red blood cells have
a diameter of 4–5 microns, we can estimate the thickness of an endothelial cell to
be about 1 micron with a width of about 7 microns × π/2 ≈ 10 microns. (The
thickness of the basement lamina itself is much smaller than that of an EC and
is neglected.) It is known that there are about 10–100 EC per millimeter so that
their length can be taken to be between 10 and 100 microns. This means that the
volumetric density of endothelial cells is roughly of the order of 1012 cells per
liter. [The dimensions of an endothelial cell are taken from Nerem et al. (1981).]
The number of receptors per cell is of the order of 105 (Waltenberger et al., 1994;
Ankoma-Sey et al., 1998). Therefore, the concentrations of receptor densities are
δe [EC](0) ≈ δa [EC](0) ≈ 1017 per liter or 10−6 M or one µM.
The import of this is that when we set
λ1 = ν1 k2 δe ≡
1
K cat
δe
,
1
Km
λ2 = ν2 k4 δe ≡
2
K cat
δe
2
Km
we may recast (4.5) in the form
∂[V ] −λ1 [V ](t)[EC](t)
=
,
∂t
1 + ν1 [V ](t)
∂[C] λ1 [V ](t)[EC](t)
=
− µ[C](t),
∂t
1 + ν1 [V ](t)
∂[A] −λ2 [A](t)[EC](t)
=
,
∂t
1 + ν2 [A](t)
∂[I ] λ2 [A](t)[EC](t)
=
.
∂t
1 + ν2 [A](t)
(4.7)
Then, when we re-normalize the variable [EC](t) in (4.7), the products λi [EC](0)
i
= K cat
/K mi and νi = 1/K mi and literature values for these kinetic parameters can
be inserted directly in the normalized versions of (4.7). We will do this renormalization below.
For the case in which angiostatin acts as an inhibitor, the last four equations may
be omitted from (4.7) and [A] plays the role of [I ] in (4.6).
In order to build the model problem, a version of each equation in (4.7) must
be written down twice, once as a transport equation in the capillary and once as a
† The issue of units is quite important. In order to relate the constants k to literature values where the
i
terminology, K cat , K m is used, the concentrations of the chemical species in (4.5) must be expressed
in volumetric units, say in micro moles per liter.
Modeling of Capillary Growth
815
transport equation in the ECM. Further, some additional transport terms must be
included in the resulting equations to account for diffusion and to link the two sets
of equations.
Moreover, these equations must also be coupled to the movement of endothelial
cells in the capillary wall and in the ECM.
This will naturally lead to a proliferation of variables and equations. But if we
organize our work carefully, no confusion should arise.
5.
C HEMICAL T RANSPORT IN THE C APILLARY AND IN THE ECM
We use the following notation for the concentrations of the various chemical
species along the capillary wall in µM: (micromoles per cubic liter):
v(x, t) = angiogenic factor, V ,
c(x, t) = proteolytic enzyme, C,
ca (x, t) = active proteolytic enzyme, C A ,
ci (x, t) = inhibited proteolytic enzyme, C I ,
ιa (x, t) = protease inhibitor I ,
f (x, t) = fibronectin, F,
a(x, t) = angiostatin, A,
η(x, t) = endothelial cell density, in cells/liter.
(5.1)
In the ECM, we have the corresponding variables (in µM):
V (x, y, t) = angiogenic factor, V ,
C(x, y, t) = proteolytic enzyme, C,
Ca (x, y, t) = active proteolytic enzyme, C A ,
Ci (x, y, t) = inhibited proteolytic enzyme, C I ,
Ia (x, y, t) = protease inhibitor, I ,
F(x, y, t) = fibronectin, F,
A(x, y, t) = angiostatin, A,
N (x, y, t) = endothelial cell density in cells/liter.
(5.2)
816
H. A. Levine et al.
5.1. Chemical transport equations in the capillary. In the capillary equations
in these variables, we must include two types of source terms. The first of these,
vr (x, t) represents the rate at which growth factor is being supplied to the capillary
from the tumor on the opposite side of the ECM (at y = L .) The precise choice
we make for this will be discussed in Section 7.1 below. The source term for
angiostatin, ar (x, t), will be taken to be a constant in the therapeutic case, i.e., in
the case that angiostatin is supplied to the patient intravenously.
Before writing down the equations for angiostatin, we need to take into account
that inhibitors have a natural rate of decay (which may be temperature dependent).†
Equations (4.5)–(4.6) now take the form‡§
λ1 v η
∂v
=−
+ vr (x, t),
∂t
1 + ν1 v η0
∂c
λ1 v η
=
− µc,
∂t
1 + ν1 v η0
4
f η
λ3 ca f
∂f
=
f 1−
−
,
∂t
Tf
f 0 η0 1 + ν3 f
∂a
λ2 a η
=−
+ ar (x, t),
∂t
1 + ν2 a η0
∂ιa
λ2 a η
ιa (x, t)
=
−
.
∂t
1 + ν2 a η0
Trel
c = ca ci + ν3 ca f,
ci = νe ιa ca .
(5.1.1)
† Suppose we know that angiostatin (when it functions directly as a protease inhibitor) has a relaxation time of Trel and we wish to maintain a concentration of a∞ micro moles per cubic millimeter
in the mother capillary. Suppose also that we supply Ar micromoles per cubic millimeter per hour
to the circulatory system. The rate equation for a will read
da
a
=−
+ Ar
dt
Trel
in the absence of any other source of sink for angiostatin. This equation has as its solution a(t) =
Trel Ar (1 − e−t/Trel ) if the blood stream is initially clear of angiostatin. The relaxation time is then
easily seen to be: Trel = a∞ /Ar . This equation obviously provides a means for the experimentalist
to determine the relaxation time of angiostatin in a healthy animal.
‡ As we remarked above, we have setη = [EC](0) and replaced η by η/η in order to be able to
0
0
express the λ0 s and ν 0 s directly in terms of the kinetic constants.
§ Consider the fibronectin rate equations in (5.1.1). In the absence of protease, we assume that the
EC generate fibronectin according to the logistic rate law: f t = β f ( f 0 − f )η0 . The constant β can
be rewritten as follows. Suppose that in T f hours, f 0 micromoles of fibronectin will be generated
by η0 endothelial cells. In the absence of protease we can write f 0 /T ≈ βη0 f 02 x(1 − x) where
x = f / f 0 . The maximum value of x(1 − x) on [0, 1] is 0.25. This gives a maximum possible value
β ≈ 4/(T f 0 η0 ).
Modeling of Capillary Growth
817
In the case that angiostatin itself acts as an inhibitor, these simplify to
∂v
λ1 v η
=−
+ vr (x, t),
∂t
1 + ν1 v η0
∂c
λ1 v η
=
− µc,
∂t
1 + ν1 v η0
∂f
4
f η
λ3 ca f
=
f 1−
−
,
∂t
Tf
f 0 η0 1 + ν3 f
∂a
a(x, t)
= ar (x, t) −
,
∂t
Trel
c = ci + ca + ν3 ca f,
ci = νe aca .
(5.1.2)
R EMARK 4. In principle, one should consider adding stabilizing terms such as
Dv vx x , Dc cx x , D f f x x to equations (5.1.1) or (5.1.2). However, this is not realistic for the biological situation we are considering here as we now argue. First,
fibronectin is not expected to diffuse much through the protein matrix along the
ablumenal capillary surface. Therefore we neglect its diffusion here.
Also, along this surface, the removal of v and the decay of c are on a much faster
exponential time scale than their diffusion along this surface. In particular, growth
factor is converted almost immediately into activated receptor complex upon arrival
at the capillary wall via the above reactions so that very little if any of it is left
to diffuse along the capillary lumen. Therefore, at the capillary wall, it seems
reasonable to neglect the diffusion of growth factor by comparison with its reaction
rate.†
The diffusion of growth factor cannot be neglected in the ECM in the full model
we are developing here since it is known that these proteins can diffuse through
tissues.
Mathematically, diffusion provides the transport mechanism in the model for
growth factor to move from the tumor to the capillary.
The situation here is also in marked contrast to that in the case of wound healing.
There, growth factors are released by damaged cells. Blood cells and platelets can
also generate growth factors. Thus growth factor diffusion in the plasma must be
considered at the outset.
We turn next to a discussion of protease movement. Protease movement is
viewed as being regulated by endothelial cell movement because the protease is
produced by the EC.
Secreted proteases are intimately involved in cellular migration through solid
tissues (Blasi, 1993; Chapman, 1997; Moerman, 1999; Murphy and Gavrilovic,
† This assumes that the rate of supply of the growth factor at the wall is insufficient to generate
quantities of growth factor to saturate all or nearly all the available EC receptors.
818
H. A. Levine et al.
1999). By degrading the ECM, proteins that impede cellular migration, extracellular proteases provide a space through which cells can move through the extracellular matrix. Examples of proteases with this role are the matrix metalloproteases
and the plasminogen activators. Matrix metalloproteases are responsible for the invasive behavior of the trophoblasts that embed the placenta in the uterus (Librach
et al., 1999). Invasion of cancer cells is also associated with large increases in
the production and secretion of proteases (Blasi, 1993; Curran and Murray, 1999).
Secreted proteases and proteases located on cell surfaces are responsible for invasion (Blasi, 1993; Sato et al., 1994). Proteases on the cell surface can be oriented
relative to the direction of cellular movement and used by the cell to cut through
the ECM much like an explorer cuts through the intertwined vines of the jungle
with a machete. The cell-surface associated proteases are the first step in a cascade that results in the activation of proteases in the ECM that might have been
secreted by cells in the surrounding environment. Thus, plasminogen activator
cleaves plasminogen to produce the active protease plasmin and plasmin cleaves
latent proteases such as pro-MMP2 to create the active proteases MMP2 type IV
collagenase (Saksela, 1985; Sato et al., 1994; Baramova et al., 1997). Current experimental evidence suggests that plasminogen activators and metalloproteinases
are likely to be involved in endothelial cell migration to form new capillaries. The
production and secretion of tissue type plasminogen activator (tPA) is increased by
VEGF (Mandriota et al., 1995; Olofsson et al., 1998). Endothelial cells that lack
the transmembrane metalloprotease, MT1-MMP, are incapable of invading tissues
to form capillaries (Hiraoka et al., 1998) and transgenic mice lacking MT1-MMP
have impaired angiogenesis (Zhou et al., 2000).
Proteases, once secreted, are rapidly either bound to the cell surface or bound by
key proteins in the ECM or they are inactivated by interaction with their specific
inhibitors, it also seems reasonable to neglect protease diffusion.
5.2. Chemical transport equations in the ECM. In this region, we must also
modify equations (4.5)–(4.6).
First, we assume that the background fibronectin production (by fibroblasts for
example) is in much greater excess than that of the endothelial cells so that now
the logistic term for fibronectin is independent of N . That is, we assume that the
cells in the ECM, such as fibroblasts, regulate fibronectin via the logistic rate law
Ft = β 0 F(F0 − F). [If F0 micromoles are produced in TF hours, then we may take
β 0 = 4/(TF F0 ).]
Secondly, we assume that, in so far as growth factor and angiostatin are concerned, the ECM is a porous medium through which these chemicals can diffuse.
We do not assume that the diffusivities for these species, namely DV , D A , are
constant.
Thirdly, we need to allow for the (inhomogeneous) diffusion of growth factor and
for angiostatin. We assume that molecules of either type see the ECM as a porous
medium (much like sand or soil) but with variable diffusivities, DV , D A which
Modeling of Capillary Growth
819
account for the inhomogeneity of the medium. On the other hand, extracellular
proteases tend to be associated with cell or near surfaces due to their interaction
with cell surface receptors and because they are secreted by cells and have an affinity for the proteins of the adjacent ECM. This localization of protease near cell
surface promotes cell migration in the ECM.†
Fourthly, we assume that the so-called porosity constant, m, is the same for both
species. This is reasonable because the sizes two proteins are about the same order of magnitude. For illustrative purposes, we have taken this to be unity in the
simulations.
Finally, we need to account for the ‘diffusion’ of fibronectin in the ECM. Generally, fibronectin diffuses very slowly. The classical diffusion equations used in
transport chemistry are usually based on Ficks’ law which states that the flux of
particles in a mixture is proportional to the gradient of the concentration of the
particles in the medium in which they find themselves. The assumption is that the
surrounding medium is homogeneous, the local concentration of the diffusing particle is small and the particles themselves are small. Fibronectin, on the other hand
is a high molecular weight protein in a highly heterogeneous region which is held
in the extracellular matrix by noncovalent linkages with other proteins. Therefore,
we cannot strictly apply classical diffusion theory to its diffusion.
Proteolytic action results in the reduction in size of proteins such as fibronectin
to smaller fragments that tend to have weaker interactions with other components
of the extracellular matrix. These smaller fragments are thus more mobile and their
tendency to diffuse is therefore greater than for intact fibronectin. Because the tip
curvature induces more EC proliferation at the tip, we see from the differential
equation for protease, that higher concentrations of proteases are also to be found
at or near the growing tip than relatively far behind it. Consequently, more small
protein fragments of fibronectin are to be expected at or near the tip. Thus, there
will be a greater propensity for diffusion of fibronectin at these sites rather than
farther back along the channel walls. To model this propensity for fibronectin diffusion to depend on curvature along the capillary wall, we will use diffusion by
mean curvature‡,§ of fibronectin. That is, the rate of fibronectin drift is assumed to
be proportional to the mean curvature of the level sets F(x, y, t) = constant for
† It is a major problem as to how to model the inhomogeneity of the ECM for two reasons. First,
it is not only a heterogenous matrix of various proteins and polysaccharides but it is also the home
of other cell types, fibroblasts, macrophages, mast cells etc., some of which are involved in ECM
synthesis, such as fibroblasts. See Alberts et al. (1994, p. 979, Figs 19–41). Therefore, in the
numerical simulations below, we have resigned ourselves to simply taking these diffusivities (as well
as Dη , D N ) to be constants. [An illustration of how complex the structure of the ECM can be, as
well as the mental image we have of it, may be found in (1994, p. 991, Figs 19–56).]
‡ If z = φ(x, y) then the curvature, for fixed z, of the level line is given by κ = ∇ ·
∇φ
|∇φ| . This is
also the mean curvature of the surface z = φ(x, y).
§ A second rationale for invoking motion by mean curvature is to be found in other fields such
as crystallography (Sethian, 1996). Here we are using it to model the the cellular biological phenomenon of contact inhibited cell growth and the changed condition of the cells behind the tip.
820
H. A. Levine et al.
each fixed time.†
∂V
λ1 V N
= ∇ · [DV (x, y)∇(V m )] −
+ Vr (x, y, t)
∂t
1 + ν1 V η0
∂C
λ1 V N
=
− µC
∂t
1 + ν1 V η0
∂F
4
F
λ3 Ca F
= D F κ(x, y)|∇ F| +
F 1−
−
∂t
TF
F0
1 + ν3 F
λ2 A N
F
∂A
= ∇ · [D A (x, y)∇(Am )] −
+ ar (x, t) 1 −
∂t
1 + ν2 A η0
F0
∂ Ia
λ2 AN
Ia
=
−
∂t
1 + ν2 A Trel
C = Ci + Ca + ν3 Ca F,
Ci = νe Ia Ca .
(5.2.1)
Again, in the case that angiostatin is itself the inhibitor, these are to be replaced by
λ1 V N
∂V
= ∇ · [DV (x, y)∇(V m )] −
+ Vr (x, y, t)
∂t
1 + ν1 V η0
∂C
λ1 V N
=
− µC
∂t
1 + ν1 V η0
4
F
λ3 Ca F
∂F
= D F κ(x, y)|∇ F| +
F 1−
−
∂t
TF
F0
1 + ν3 F
∂A
F
A
m
= ∇ · [D A (x, y)∇(A )] + ar (x, t) 1 −
−
∂t
F0
Trel
C = Ca + Ci + Ca f,
Ci = νe ACa .
(5.2.2)
† In mathematical terms, this amounts to writing the flux in the form J = −D ∇ F/|∇ F|. When
F
this is done, the continuity equation must be written in the form Ft + ∇ 0 · J = 0 where now ∇ 0 · J =
|∇ F|∇ · J where ∇ 0 · is the divergence operator which is dual to the ‘gradient operator’ (|∇ F|)−1 ∇.
The quantity κ(x, y)|∇ F| can be written in the form
κ(x, y)|∇ F| =
Fx x Fy2 − 2Fx y Fy Fx + Fyy Fx2
Fx2 + Fy2
where it is understood that |∇ F|2 = Fx2 + Fy2 > 0. In the case of ordinary diffusion, this term would
be replaced by Fx x + Fyy . which is a good approximation to mean curvature diffusion when Fx y ≈ 0
and the components of ∇ F are nearly equal constants. This will not be the case here. Hence we
have resorted to a more general form of diffusion.
Modeling of Capillary Growth
821
Thus, if κ < 0, the growth rate for fibronectin (Ft ) is diminished while if κ > 0
the growth rate is increased.
We have included a source term Vr (x, y, t) to allow for the situation in which
VEGF may be generated at certain sites in the ECM. We have also added a source term, ar (x, t) 1 − FF0 . This term will allow us to
introduce angiostatin in every region of the new capillary network for which the
fibronectin density is below its background value F0 at a rate which is proportional
to the the fibronectin deficit in the ECM.
R EMARK 5. The above equations can be modified to include naturally occurring
angiogenesis. It is has been suggested (Hanahan and Folkman, 1996) that endothelial cells produce both growth factors and angiostatins in normal tissues in such a
way that under normal circumstances, the action of the one regulates the action of
the other. In the model above, this observation may be expressed by incorporating
terms of the form σ1 η, and σ2 η in the first and fourth of equations (5.1.1) [respectively (5.1.2)] respectively and corresponding terms of the form σ1 N , and σ2 N in
the first and fourth of equations (5.2.1) [respectively (5.2.2)]. Likewise, one must
also account for inhibitor decay. This is the reason for including the relaxation
time terms −ιa /Trel in the fifth of equations (5.1.1) [respectively −a/Trel in the
fifth of equations (5.1.2)] and −Ia /Trel in the fifth of equations (5.2.1) [respectively −A/Trel in the fifth of equations (5.2.2)]. Unfortunately, we were unable
to locate any of the constants σi , Trel for the relevant proteases in tissues. Therefore, in our computations, we took the relaxation time to be infinite in the case that
angiostatin generates inhibitor from EC and assigned it a reasonable value when
angiostatin acts as an inhibitor. We also took the σ 0 s to be zero.
R EMARK 6. Clearly, from the point of view of inhibiting angiogenesis, it is better
to use an inhibitor around with a long ‘shelf life’ or high relaxation time than one
with a small relaxation time.
6.
T HE P RINCIPLES OF R EINFORCED R ANDOM WALK A PPLIED TO
C ELL M OVEMENT
In Appendix A we have given a brief discussion of the form of the cell transport
(chemotactic) equations we use in this model. While the transport equation has
several features in common with the standard equations of chemotactic transport,
this particular model, developed using the theory of reinforced random walk derived by Davis (1990), was used recently by Othmer and Stevens (1997) to model
fruiting bodies such as Myxococcus fulvus and Dictyostelium discoideum amoeba.
6.1. Cell movement equations in the capillary. Our discussion begins with the
governing equations of cell movement along the capillary. The primary equation
822
H. A. Levine et al.
governing the motion of endothelial cells is
∂η
∂
∂
η
= Dη
η
ln
∂t
∂x
∂x
τ
(6.1.1)
where τ is the so-called transition probability function which in turn depends on
one or more of the variables listed above. This function has the effect of biasing the
random walk of endothelial cells. In this case, we know that this walk is influenced
by the active proteolytic enzyme it produces in response to angiogenic factor that
has made its way to the cell receptors and by the fibronectin in the BL, i.e., we write
τ = τ (ca , f ).
(6.1.2)
A simple transition probability which reflects the influence of enzyme and fiγ
bronectin on the motion of endothelial cells is τ (ca , f ) = ca1 f −γ2 for positive
constants γi . The probabilistic interpretation of this choice is that endothelial cells
prefer to move into regions where c is large or where f is small, facts which have
basis in biological observations.
These factors are chosen in order to provide a measure of how responsive endothelial cells are to protease and to fibronectin. It is known that proteases promote the movement of endothelial cells, (Schleef and Birdwell, 1982; Roberts and
Forrester, 1990; Morimoto et al., 1993; Gordon and DeMoss, 1999).
It is also reasonable to suppose that τ is a decreasing function of f . That is, endothelial cells are attracted to sites of low fibronectin or collagen density (Dekker
et al., 1991; Gamble et al., 1993; Nicosia et al., 1993; Bourdoulous et al., 1998;
Soldi et al., 1999). For example, in Nicosia et al. (1993), the authors conclude
that the data from their experiments ‘. . . support the hypothesis that fibronectin
promotes angiogenesis and suggest that developing micro-vessels elongate in response to fibronectin as a result of an adhesion-dependent migratory recruitment
of endothelial cells that does not require increased cell proliferation.’
In order to avoid singularities in ln τ and its derivatives in this equation it is useful
to take
τ (ca , f ) =
ca + α1
ca + α2
γ1 f + β1
f + β2
γ2
,
(6.1.3)
where the αi , βi are empirical constants such that 0 < α1 1 < α2 and β1 > 1 β2 > 0. Clearly then τ is not singular for small or large values of c, f and will
approximate cγ1 f −γ2 reasonably well over a considerable range of these variables.
(The choice we make for τ is somewhat analogous to the assumption of a linear
relation between stress and strain that is made in the classical theory of Newtonian
fluid flow. This is an ad hoc postulate, not derivable on the basis of statistical
mechanics or any other ‘first principle.’ But as an assumption about the nature of
Modeling of Capillary Growth
823
Newtonian fluids, its validity is unquestioned. Our view here is that the choice we
make for τ here will have similar descriptive and predictive success.)
The reader will notice that in (6.1.1) we have not included any proliferation terms
as we shall do in the ECM transport equation given in the next subsection. In
Paweletz and Knierim (1989), the observations contained in Cliff (1963), Schoefl
(1963), Schoefl and Majno (1964), Warren (1970) and Sholley et al. (1984) in this
regard were summarized as follows: ‘the first events of angiogenesis are rearrangements of EC rather than induction of cell division. . . . Mitotic figures can only be
found when the sprout is already growing out.’ Indeed, according to Paweletz and
Knierim (1989) and Sholley et al. (1984), it has clearly demonstrated that sprouting
can occur without any cell division.
6.2. Cell movement equations in the ECM. In the ECM, we need to allow for
the birth (proliferation) and death of cells. Since cells may die once they reach the
ECM, and since they will proliferate due to the stimulus of the active enzyme, we
modify the random walk equations as follows:
∂N
N
= D N ∇ · N ∇ ln
+ Q(κ)
∂t
T (Ca , F)
∂Ca
N θ (1 − N /η0 ) + G(Ca )
H (Ca − Ca,0 ) − µ1 N (6.2.1)
∂t
We take the probability transition rate function, T , to be of the same form (although
not necessarily with the same values of the constants) as τ .
It is worth discussing the source terms on the right in some detail.
The factor in curly braces is the proliferation term which is in turn the difference
between cell birth and cell death rates. The birth rate consists of two terms, θ N (1−
N /η0 ) and N G(Ca )∂t Ca , both of which are multiplied by the factor H (Ca − Ca,0 ).
The role of this factor is to serve as a switch. If the active protease concentration
in the ECM is below a threshold value Ca,0 , then there is no proliferation of any
ambient endothelial cells which may be present in the ECM. The term θ N (1 −
N /η0 ) represents the natural or background birth rate for endothelial cells.
In order to understand the inclusion of second term, N G(Ca )∂t Ca , in the birth
rate we argue as follows. It is known that cell proliferation responds to growth
factor in the following manner. As one increases the concentration of growth factor,
the proliferation response percentage, (N − N0 )/N0 , first increases to a maximum
value and then decreases to zero (Unemori et al., 1992).
It is also to be expected that EC proliferation depends on protease concentration
in the same manner because proteases have two opposing effects on cell function.
The first, that is observed at low protease concentrations, is to stimulate proliferation either directly (Carney and Cunningham, 1977; Rochefort et al., 2000) or
824
H. A. Levine et al.
indirectly by creating an open space to relieve contact inhibition of growth (Gospodarowicz et al., 1978). The second effect of proteases is to cause cell death and
disintegration. Whereas low concentrations of protease are required to stimulate
cell proliferation, much higher concentrations are needed for destruction of the
cells. Thus, the result these two effects is again on the proliferation response percentage is that it should first increase and then decrease with increasing protease
concentration.
That is, the proliferation response function G is of the form
G(X ) =
20 (X )
1 + 2(X )
(6.2.2)
where 2(z) has a graph of the form in Fig. 3.† The precise form we take is 2(z) =
Az exp(−λz m 1 ) where A, λ, m 1 are found by curve fitting. See Table 2 and the
discussion following it.
It is known that proliferation of EC occurs just behind the tips of growing capillaries.‡ A contributing factor may be that near the tip, the ratio of tip surface area
to the volume of the tip is very large.§ Consequently, there is greater exposure of
endothelial cells in the tip to growth factors per unit area than elsewhere along the
growing capillary. To model this, we must include a factor that accounts for this
effect. To do this, we include a factor that is small when tip curvature is small and
large when the tip curvature is large. The coefficient Q(κ) is a curvature sensitivity
factor, i.e., some non negative, strictly increasing, function of the curvature κ with
the property that Q(X ) = 0 if X ≤ 0. The precise nature of this function has yet
to be established experimentally. Thus we regard it as a free parameter.
This proliferation function plays a critical role in determining how the cells line
the ECM. We note that if Ca is small, then the EC proliferation increases to a maximum rate at some value Camax say, after which, the EC proliferation rate decreases.
On the other hand, we see from (5.2.1) and (5.2.2) that when no angiostatin is
present (so that C = Ca ) C cannot become too large. As it increases, the natural
decay term −µC prevents it from becoming too large. As more angiogenic factor is supplied, C again increases and the EC proliferation rate again decreases.
This feedback looping mechanism may play a critical role in the growth of new
capillaries from existing capillaries.
† If we write N (t) = N + N θ (C ), then N 0 (t) = N θ 0 (C )∂ C . Here N is some reference
a
a t a
0
0
0
0
concentration of EC. Eliminating N0 between these, we obtain N 0 (t) = N G(Ca )∂t Ca as the birth
rate response term to protease where G is given in (6.2.2).
‡ More precisely, about one cell length behind the tip front, the tip itself inhibiting the proliferation
of the lead cell.
§ The use of mean curvature in the EC equation is motived by an idea taken from the theory of
dendritic crystal growth. There, growth of dendrites occurs only at the tip of the dendrite where the
local ratio of surface area to volume is largest. This ratio is proportional to the mean curvature, i.e.,
to the reciprocal of the radius of curvature.
Proliferation response fraction, (N-N_0)/N_0
Modeling of Capillary Growth
825
Proliferation Response Curve
Growth factor or active protease concentration
Figure 3. Generic form of active enzyme—proliferation rate response curve.
7.
T RANSMISSION , B OUNDARY AND I NITIAL C ONDITIONS
In this section we present the various transmission, boundary and initial conditions we will use for this problem.
7.1. Capillary–ECM transmission conditions. As remarked above, one needs
to link the ECM transport equations with the transport capillary equations. One
such linkage is the equation for the source term of angiogenic factor in the rate
equation in the first of equations (5.1.1).
In order to do this, we suppose that the rate of supply of growth factor depends
on (a) the concentration of growth factor arriving from the tumor at the capillary
wall and (b) the rate at which it is arriving at the capillary wall. That is, we take
vr (x, t) = A1
∂ V (x, 0, t)
+ B1 V (x, 0, t)
∂t
(7.1.1)
where the A1 , B1 are non-negative constants.†
R EMARK 7. A word of caution in the use of (7.1.1) for computational purposes
is needed here. An attempt to solve our system numerically by replacing the coefficient of A1 in (7.1.1) by the differential equation for Vt (x, 0, t) will lead to
numerical difficulties since the resulting expression involves second derivatives of
V (through DV 1V m ) on the boundary of the rectangle. These derivatives need not
exist. However, these derivatives can be avoided if we replace the first of equa† In Pamuk (2000), the author used a slight variant of this, taking A = 0 and replacing the second
1
term by a term of the form B1 (x, 0, t)H (V (x, 0, t) − V0 ) where V0 is a small threshold constant.
826
H. A. Levine et al.
tions (5.1.1) by
v(x, t) = v(x, 0) +
Z t
0
λ1 v(x, s)η(x, s)
B1 V (x, 0, s) −
ds +
1 + ν1 v(x, s)
A1 (V (x, 0, t) − V (x, 0, 0)).
(7.1.2)
In this form, the equation will not involve 1V m (x, 0, t). Moreover, v(x, 0),
V (x, 0, 0) are data for our problem. (We shall specify them later.)
In order to introduce angiostatin into the system via the fourth of equations (5.1.1)
or (5.1.2), we take
ar (x, t) = Ar H (t − Tiv )
(7.1.3)
where Ar is the rate at which angiostatin is being supplied intravenously in micro
moles per liter per hour and where Tiv is the elapsed time (in hours) since the tumor
began to secrete growth factor into the ECM. (That is, it is the total elapsed time
since the begining of the experiment to the point at which we introduce angiostatin
into the blood stream.) We also assume that endothelial cells in the capillary cannot
move into the ECM until the fibronectin density in the capillary wall falls below a
certain threshold level, f < f 1 say. That is, we take
N (x, 0, t) = ψ1 H ( f 1 − f (x, t))η(x, t).
(7.1.4)
The constant ψ1 ∈ (0, 1] is taken as a measure of the fraction of EC lining the
lumen that are able to penetrate into the ECM when the lumen fibronectin density
has fallen below f 1 .
Finally, we need boundary conditions for the growth factor and angiostatin partial
differential equations. Standard considerations from transport theory suggest that
we take them of the form
− DV (x, 0, t)
−D A (x, 0, t)
∂ V m (x, 0, t)
+ ψ(V (x, 0, t) − v(x, t)) = 0,
∂y
∂ Am (x, 0, t)
+ ψ 0 (A(x, 0, t) − a(x, t)) = 0,
∂y
DF
∂ F(x, 0, t)
= 0.
∂y
(7.1.5)
Again, the flux constants ψ, ψ 0 need to be found empirically.
R EMARK 8. In addition to being bound by specific cell surface signaling receptors that mediate the cellular response such as we have modeled here for the VEGF
receptor, growth factors such as VEGF are rapidly trapped in tissues by additional
Modeling of Capillary Growth
827
proteins and proteoglycans on the cell surface and in the extracellular matrix. These
molecules effectively immobilize the growth factors and, in some cases, present the
growth factor to the signaling receptor. For example, glypican binds to VEGF165
and potentiates VEGF165 binding to its signaling receptor, KDR/flk-1 (Gengrinovitch et al., 1999). The result is that there is likely to be little if any back flow of
the growth factor. This is in marked contrast to oxygen exchange across capillaries
for which flow in both directions must be considered.† It should also be noted that
the diffusivity of oxygen in blood and in tissue is of order 10−5 cm2 s−1 (Thews,
1960; Lagelund and Low, 1987) which is 10–1000 times larger than that for VEGF
in tissue.
7.2. Boundary conditions. At the tumor side of the ECM, we take the following
boundary conditions:
DV (x, `, t)
∂ V m (x, `, t)
− V` (x, t) = 0,
∂y
D A (x, `, t)
∂ Am (x, `, t)
=0
∂y
DF
DN N
∂
∂y
ln
N
T
∂ F(x, `, t)
= 0,
∂y
(x, `, t) + θ 0 N = 0.
(7.2.1)
In other words, the first equation says that the tumor is supplying a prescribed flux
of TGF which may depend on time. For example, one choice of V` might be
2π x m 0 −δt
σ
V` (x, t) = v0 1 − cos
e
L
L
(7.2.2)
where σ is a fixed constant, selected so that the mean value of the flux of TGF is
normalized to v0 e−δt , i.e., so that
L
Z
V` (x, t) d x = v0 e−δt .
0
The larger m 0 is, the more we can think of V` eδt /v0 as a unit impulse function. We
can also think of it as a measure of how localized the tumor secretion is.
Similarly, the second of these equations says that the flux of angiostatin into the
tumor is proportional to the quantity of angiostatin at the tumor wall. The fourth
equation says that the flux of EC into the tumor region is proportional to the density
of EC at the tumor wall, the proportionality constant being θ 0 .
† The authors thank Helen Byrne for bringing the Krogh cylinder model to their attention.
828
H. A. Levine et al.
To close off the problem at the ends of the capillary, x = 0, L, we use the
boundary conditions
∂ V m (0, y, t)
∂ V m (L , y, t)
=
=0
∂x
∂x
∂ Am (0, y, t)
∂ Am (L , y, t)
=
=0
∂x
∂x
∂ F(0, y, t)
∂ F(L , y, t)
=
=0
∂x
∂x
∂
N
∂
N
N
ln
(0, y, t) = N
ln
(L , y, t) = 0
∂x
T
∂x
T
η
∂
η
∂
η
ln
(0, t) − η
ln
(L , t) = 0
∂x
τ
∂x
τ
(7.2.3)
[Actually we used the slightly stronger condition:
∂
η
∂ η
η
ln
(0, t) = η
ln
(L , t) = 0
∂x
τ
∂x
τ
in our computations below.]
7.3. Initial conditions. One also has the following initial conditions for the densities and concentrations along the capillary wall. (We have normalized the EC and
fibronectin densities to unity.)
η(x, 0) = 1,
v(x, 0) = 0,
f (x, 0) = 1,
c(x, 0) = 0,
a(x, 0) = 0,
ιa (x, 0) = 0,
(7.3.1)
since the capillary is initially in a rest state. In so far as the initial state of the ECM
is concerned, we take
N (x, y, 0) = 0,
V (x, y, 0) = 0,
C(x, y, 0) = 0,
F(x, y, 0) = 1,
A(x, y, 0) = 0,
Ia (x, y, 0) = 0.
(7.3.2)
In the case that angiostatin is an inhibitor, the initial condition for ιa and for Ia is
to be omitted.
8.
N UMERICAL E XPERIMENTS
In Section B we have recorded the data constants and specific functions which
we used in our computations. (Fig. sets 5.1–7.5.) The figures were generated from
Modeling of Capillary Growth
829
Matlab code which was developed in Pamuk (2000).† Here we discuss some of
the particulars as to how these were selected. We also discuss some aspects of the
computation itself.
8.1. Terminology. We use the phrase ‘onset of angiogenesis’ to mean that a new
capillary sprout has begun to form from the existing capillary. We use the phrase
‘onset of vascularization ‘to mean that the newly formed capillary has just reached
the tumor.
8.2. Expected properties of the solutions. In vivo one might expect that tumor
stimulated rate of growth of new capillaries from existing capillaries will depend on
several variables. (For example, tumors grow much more slowly in some parts of
the body than in others.) Among these will be the chemical properties of the growth
factors and the proteases themselves. Also the distance from tumor to existing
capillary and the protein structure of the intermediate ECM will affect this. We
might also expect that the rate of supply growth factor as well as how localized
secretion from the tumor to the ECM is to play a role in the growth of a capillary
from a single bud.
The model we have written down above to some degree reflects this variability.
For example, lowering the value of f 1 , or decreasing the dosage rate v0 will
certainly slow the time of onset of angiogenesis. Furthermore, changing the percentage of endothelial cells, ψ1 , which are transferred from the capillary into the
ECM when this threshold is reached, will also influence the tip growth rate.
However, lowering the dosage rate v0 will also decrease the tip speed of the new
capillary as it crosses the ECM whereas f 1 cannot affect the tip speed. Lowering
the dosage rate also has the effect of causing more aggregation of EC along the
center line (Levine et al., 2001).
Moreover, raising the localization power, m 0 will narrow the capillary width
somewhat. There is a limiting value to the capillary width corresponding to the
value one would obtain if one used a delta function (unit impulse function) for v`
(Levine et al., 2001).
One parameter that can seriously affect the numerical results is the tip sensitivity.
We have discussed this in Appendix C.
In the computations below, we fixed the tumor–capillary distance. Then we adjusted some of the parameters above in order to achieve an EC density profile that
looked reasonable, i.e., that appeared to correspond to the observation that there is
a higher concentration of EC near the tip than at some distances further back.
We were most concerned with the effect of tip sensitivity on this distribution.
When we found values of these parameters that led us to EC and fibronectin
† The code developed in Pamuk (2000) is very general. It allows us to consider the case for which
the diffusivities DV , DC depend on the location in the ECM. This is a more realistic view of the
situation than we have developed here. Unfortunately it relies on having precise a priori information
on the protein structure in the ECM.
830
H. A. Levine et al.
(b)
(a)
AVASCULAR
TUMOR IMPLANT
GROWING TUMOR PLATE
CORNEA
(c)
VASCULARIZED
TUMOR NODULE
IRIS
LENS
NEW
CAPILLARIES
LIMBUS
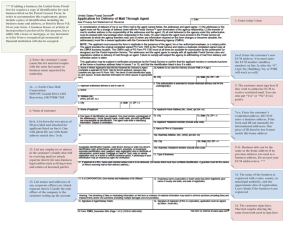
Figure 4. The rabbit cornea experiment. From Folkman (1976). (By permission)
profiles that were in agreement with what one might expect in a growing capillary, before vascularization has set in, we then computed the mean tip speeds and
the widths of the forming capillary. We describe how these computational results
compare with experimental data in the next section.
9.
S UMMARY AND C ONCLUSIONS
The computations in this model give very good agreement with the experimental observations reported in Ausprunk and Folkman (1977), Folkman (1976) and
Gimbrone et al. (1974).
Let us first recall the results of the corneal rabbit eye experiments reported in
Ausprunk and Folkman (1977), Folkman (1976) and Gimbrone et al. (1974) which
are relevant to our model.
In one set of experiments, (Gimbrone et al., 1974), tumor implants were placed
in corneal pockets approximately one to two millimeters from the limbus of the
rabbit eye. [Figure 4, from Folkman (1976).] The authors report that new capillary sprouts began to form as early as four days after implantation and that the
prevascular state was limited to the first week after implantation (Gimbrone et al.,
1974). There they report the growth of advancing hairpin vessel loops which grew
at a rate of approximately 0.50 mm per day. (They also report on the ‘brush border
effect’ wherein it was observed that there was tip splitting and branching as one
approaches the tumor.)
Centrally placed tumor fragments planted about 6 mm from the limbus grew
avascularly at a rate of 0.1–0.2 mm per day toward the limbus. It was observed,
(Folkman, 1976), that the initial rate of capillary growth accelerated toward the
tumor to rates of up to 1.0 mm per day.
In the first numerical experiment, we implanted a ‘tumor’ 25 microns from an
existing ‘capillary’. In Fig. 5.1, we see the advance of endothelial cells across the
Modeling of Capillary Growth
831
Figs 5.1. to 5.6.—Capillary growth in the absence of angiostatin.
Figure 5.1. Time course for EC propagation in the ECM. (The units on the z-axis are multiples of η0 , the cell density in a normal capillary expressed in cells mm−3 . We refer to this
as relative EC density.) Notice that the maximum EC density occurs near the leading edge.
ECM (expressed as cell density) while in Fig. 5.2 we see how the EC concentration
changes in the capillary wall itself. In Fig. 5.3, we see the advance of a fibronectin
hole in the ECM while in Fig. 5.4, the time course for fibronectin density in the
capillary wall is illustrated.
Notice that in Fig. 5.1, the EC density is quite high near the tip of the capillary.
This reflects the fact that we have built in EC tip proliferation into the model. Naturally, since precise values of EC density near the tip do not seem to be available,
we can only view this as a qualitative approximation of reality.
In Table 1, we give the travel times to various points in the artificial ECM after
the onset of ‘tumor implantation.’ For example, it takes 3.49 hours for growth
factor to diffuse across the ECM from a tumor 25 microns away from a ‘mother’
capillary and then induce a ‘daughter’ capillary to grow towards the tumor (onset
time). It takes another 3.74 − 3.49 = 0.25 hours for the daughter capillary to move
2.5 microns toward the tumor. The mean tip speed is 0.242 mm/day. The daughter
advances a further 2.5 microns at a mean tip speed of 0.436 mm/day and so on.
832
H. A. Levine et al.
Figure 5.2. Time course for EC propagation in the capillary. The model suggests that there
is a tendency for the EC to aggregate near the edge of the opening of the mother capillary.
The vertical scale is relative EC density as in the previous figure.
In the last two columns, we have extrapolated these times to a ‘tumor’ implanted
one millimeter and two millimeters from the mother capillary. The speeds and the
times are quite comparable to those reported experimentally.
In particular, notice that the tip speeds increase as the capillary tip approaches
the tumor. The reason for this is not surprising. As the tip moves toward the tumor,
the concentration of growth factor that it encounters is higher. This causes more
protease production and this in turn induces more rapid degradation of the ECM.
In Figs 5.3 and 5.4, we observe that the opening of the fibronectin channel is
about 6–8 microns. This too is in good agreement with the diameter of a capillary.
Finally, in Figs 5.5 and 5.6, we have plotted the growth factor and protease concentrations in the ECM for a few times. We see that near the tip, the protease
concentration is quite high, as it should be. On the other hand, the VEGF is nearly
all consumed in the capillary.
In the set of Figs 6.1–6.4, we have given time courses for the cell density and
fibronectin density when angiostatin produces a protease inhibitor via a mechanism
Modeling of Capillary Growth
833
Figure 5.3. Time course for fibronectin propagation in the ECM. Here we see the advancing
channel in the ECM. The x-axis scale indicates that the capillary width is about 8 microns.
The units on the z-axis are in (fractional) multiples of F0 , the mean fibronectin density in
the ECM expressed in micromolarity.
analogous to that for the EC production of protease from VEGF.†
† After this paper went to press, we discovered that we had made a slight error in our computations
involving the angiostatin cases. In our paper (Levine et al., 2000) we had neglected to include the
term [C A F] involving the concentration of the intermediate in the conservation law for protease, an
error we perpetuated in our computations here. However, the situation is not as serious as would first
appear. The exact expression for the active enzyme is
[C A ] =
[C]
l + νe [I ] + ν3 [F]
while the approximate expression we used in the computations here is
[C A ] =
[C]
.
l + νe [I ]
However, the product ν3 F is positive and generally small when compared with max{l, νe [I ]}. Had
we used the exact expression, the (numerical) efficacy of the angiostatin and its inhibitor would
have been improved only somewhat since the omitted term has a value of no more than 1.28 ×
0.01 = 0.0128 which is small when compared to unity. It is theoretically possible that the space
834
H. A. Levine et al.
Figure 5.4. Time course for fibronectin propagation in the capillary. Along the lumen of
the mother capillary, there appears to be a wide band of fibronectin degradation. However,
the actual percentage of fibronectin loss is greatest only in a small interval of no more than
7–8 microns near the center. If we had chosen a lower threshold of penetration than 0.6, we
would have seen a deeper degradation of the basal lamina at this point. Here vertical scale
(The units on the y-axis are multiples of f 0 , the mean fibronectin density in the lumen
expressed in micromolarity.)
Angiostatin is activated in the capillary system when the daughter is nearly fully
developed. For the data above, we introduced angiostatin after 4.45 hours.
Notice that the maximum cell density is shrinking while the fibronectin channel
narrows and shrinks also. Note further that the daughter capillary is retreating as
the opening to it in the mother capillary closes. Mathematically, the situation is
as follows: as the inhibitor concentration increases, the concentration of active
protease falls. This in turn allows the fibronectin density to recover to normal
values via the logistic and diffusion terms in the fibronectin equation in (5.2.1).
As this happens, the proliferation term in (6.2.1) is driven toward zero while the
surviving death term (µ1 N ) is left to reduce the EC density to zero in the ECM.
and time derivatives of ν3 [F] might lead to larger gradients in [C A ]. However, simulations with
the dimensional problems show no appreciable difference in the numerical values for any of the
components of the solution.
Modeling of Capillary Growth
835
Table 1. Travel times and tip speeds.
Distance from
mother capillary
to tip of daughter
(` = 25 µmm)
Time
in
hours
Mean
velocity
(mm/day)
Extrapolated
time
in days for
` = 1 mm
Extrapolated
time
in days for
` = 2 mm
0.00
2.50
5.00
7.50
10.00
12.50
15.00
17.50
20.00
22.50
3.49
3.74
3.88
3.99
4.09
4.18
4.25
4.32
4.38
4.43
0.242
0.436
0.545
0.580
0.703
0.831
0.914
1.015
1.015
5.817
6.817
7.317
7.650
7.900
8.100
8.267
8.410
8.535
8.646
11.633
13.633
14.633
15.300
15.800
16.200
16.533
16.819
17.069
17.291
( = 1.125, f 1 = 0.60, v0 = 4.0 µM mm h−1 , L = 50 µmm).
However, relative to the capillary formation, it takes quite a long time to close
the fibronectin channel. Notice that the EC density has dropped rather markedly
(compare Figs 5.1 and 6.1) and eventually disappears from the channel after 12
hours from the introduction of angiostatin. However, the fibronectin density is still
rather low in the channel. The mathematical explanation for this is again to be
found in the fibronectin equation in (5.2.1). Once there is very little active protease
in the channel region, fibronectin diffusion and the logistic term in the fibronectin
equation are left to do the work of repairing the ECM. (Compare Figs 6.4 and 6.6.)
When F(x1 , y1 , t1 ) is zero at a fixed point (x1 , y1 ) and time t1 , the logistic term
contributes nothing to the repair. Only diffusion can contribute to the tissue repair
at this point. (Of course in the biological system, collagen/fibronectin repair occurs
much more rapidly due to cells such as fibroblasts and endothelial cells themselves,
effects which we have not included in this model.)
Observe also (Figs 6.6 and 6.7) that while angiostatin has diffused fairly well
throughout the ECM, the inhibitor remains fairly localized in the capillary.
Similar conclusions are to be drawn from the alternate case for which angiostatin acts directly as an inhibitor. The results of the simulations are recorded in
Figs 7.1 to 7.5. However, here the equilibrium constant νe ≈ 1/µM for the equilibrium (3.2.1) is smaller by a factor of 103 than the equilibrium constant for the
third reaction in (3.2.2).
In order to achieve the same inhibiting effect on protease in this case, we started
with the requirement that Ca ≤ 10−4 C. This in turn leads to the requirement that
νe A∞ ≥ 104 . A reasonable hypothesis is that we supply angiostatin to the patient
sufficiently rapidly so that after one hour, the concentration in the blood stream is
at a steady state value of A∞ ≈ 500 µM. This in turn leads to the requirement that
Ar ≈ 250 µM h−1 .
836
H. A. Levine et al.
Figure 5.5. Time course for VEGF propagation in the ECM. We see that the growth factor
is consumed only in a neighborhood of the forming capillary, although it is to be found
throughout the ECM. The capillary follows the VEGF along the path of steepest ascent.
(The units on the z-axis are expressed in micromolarity.)
Simulations, which we do not show here due to considerations of length, also
indicate that if we introduce an inhibitor with such a small equilibrium constant
when the tip is nearly at the tumor, the tip will reach the tumor in spite of a relatively
high dosage rate.
These observations suggest the biologically obvious, namely, the quicker and
more efficaciously one can tie up the protease, the more rapidly can one inhibit
angiogenesis.
Several anti-angiogenic agents alone or in combination with conventional therapies are now in clinical trials.† These trials are based on strategies that (1) interfere
with angiogenic ligands; (2) upregulate or deliver endogenous inhibitors; or (3) directly target the vasculature. However there are a number of potential problems
as discussed in Carmeliet and Jain (1970) that warrant caution in clinical trials in
† J. Folkman as quoted in ‘Cancer Medicine’ (eds Holland J. F. et al.) Decker, Ontario, Canada,
132–152, (2000).
Modeling of Capillary Growth
837
Figure 5.6. Time course for protease propagation in the ECM. Here we see the protease
concentrations. Notice that the concentration of protease is highest near the tip as it should
be. (The units on the z-axis are expressed in micromolarity.)
humans. Never the less, the ideas outlined in this paper relating to the action of
angiostatin inhibitors are suggestive of experiment.
Anti-cancer therapy is currently a subject of considerable controversy.† While
it offers new hope for the successful treatment of cancer, a degree of caution is
necessary. We believe our work will make a positive contribution to the debate by
putting the possible mechanisms on a quantitative footing.
ACKNOWLEDGEMENTS
The work of Levine, Pamuk and Sleeman was supported by NSF grant DMS-9803992.
† See N. Wade, New gains cited in anti-cancer therapy, New York Times, September 17, 1999.
838
H. A. Levine et al.
Figs 6.1. to 6.9.—Capillary growth in the presence of angiostatin, angiostatin as an inhibitor generator. Angiostatin was introduced at T1 = 4.45 hours at the dosage described in
Table 2. When T = 11.93 hours, we accelerated the diffusion of fibronectin artificially in
order to verify numerically the comments we made in Section 9. That is, at T = 11.93
hours we replaced D F by D 0F = 103 D F . This amounts to a change of time scale for
fibronectin diffusion. Thus, in real time, the fourth graph in each of the figures corresponds
to a time of 16.67 days with fibronectin diffusion constant D F as in Table 2 or 12.33 hours
with the accelerated constant D 0F . This was done for illustrative purposes only.
Figure 6.1. Time course for EC propagation in the ECM after introduction of angiostatin.
Here we observe the drop in EC density. Compare the vertical scales.
A PPENDIX A:
A B RIEF D ISCUSSION OF THE R ANDOM WALK
E QUATIONS
The motion of endothelial cells in the capillary and in the ECM governed
by (6.1.1) and (6.2.1) is based on the idea of reinforced random walks as introduced in Davis (1990) and used in Othmer and Stevens (1997) to model the movement of living organisms that deposit a non-diffusible substance which modifies
the local environment for subsequent movement. In Levine and Sleeman (1997), a
detailed rigorous and semi-rigorous discussion was given to support the numerical
experiments reported in Othmer and Stevens (1997).
Modeling of Capillary Growth
839
Figure 6.2. Time course for EC propagation in the capillary after introduction of angiostatin. Notice the initial bimodal distribution of EC.
In order to understand the idea behind the notion of reinforced random walks,
we consider the capillary wall to be a one dimensional lattice with endothelial cells
(assumed to be a mono-layer of equal size and shape) equally spaced and in nonoverlapping contact located at reference points nh along the x-axis.
At first we imagine a distribution of endothelial cells at lattice points xn = nh
on the real axis. We let τ̂n± (·) be the transition probability rate per unit time for a
one-step move of an endothelial cell at site n to site n + 1 or n − 1 respectively. Let
ηn (t) be the probability density distribution of the endothelial cells at position nh
at time t. Then the time rate of change of ηn (t) is governed by the master equation
∂ηn (t)
+
−
= τ̂n−1
(W )ηn−1 + τ̂n+1
(W )ηn+1 − (τ̂n+ (W ) + τ̂n− (W ))ηn .
∂t
(A1)
That is, ηn will be augmented by cells moving from the positions (n±1)h to nh and
diminished by cells moving from nh to either (n + 1)h or (n − 1)h. The quantity
(τ̂n+ (W ) + τ̂n− (W ))−1 is the mean waiting time at site n.
It is convenient to think of this conditional probability density as the density of
endothelial cells. The point of view adopted here is analogous to that in quantum
840
H. A. Levine et al.
Figure 6.3. Time course for fibronectin propagation in the ECM after introduction of angiostatin. The first three figures show the gradual closing of the fibronectin channel. Notice
that the channel tip is backing away from the tumor. The fourth was obtained as explained
above.
mechanics where an electron can be thought of as a probability density or else as a
‘cloud of negative charge.’
The transition probability rates τ̂n± (·) depend on control substances which we
have denoted by W and are defined on the lattice at 12 -step size. The control substances W are very general and can include all the enzymes which are generated
in response to the growth factors described above i.e., FGF, VEGF, TGFα, TGFβ
etc. As remarked above, in this paper, we concentrate on the important role of
secreted fibronectin, an ECM component, and proteolytic enzyme which degrades
the ECM and ruptures the basement membrane. Denote the density of fibronectin
by f and the concentration of proteolytic enzyme by c. The control substance W
is represented as the vector of components
W = (. . . Wn− 1 , Wn , Wn+ 1 , . . . ),
2
2
(A2)
(Here the index n in the argument list runs over all integers, 0, ±1, ±2, . . . .)
While the basic model (A1) can be exploited (Othmer and Stevens, 1997) to de-
Modeling of Capillary Growth
841
Figure 6.4. Time course for fibronectin propagation in the capillary after introduction of
angiostatin. Also notice that the opening of the channel at the mother capillary closes quite
rapidly.
scribe many aspects of organism dynamics it gives no restriction on the transition
rates at a site and there is no correlation between transition rates to right or left.
Suppose, as suggested in Othmer and Stevens (1997), that the decision of ‘when
to move’ is independent of the decision of ‘where to move’. Then the mean waiting
time of the process is constant across the lattice. Hence the transitions τ̂n± must be
suitably scaled and normalized so that
τ̂n+ (W ) + τ̂n− (W ) = 2λ,
(A3)
where λ is a scaling parameter. Let the transition rates depend on W only at the
nearest neighbors, Wn± 1 . In order to achieve (A3) define the new jump process
2
τ by
τ̂n± (W ) = 2λ
τ (Wn± 1 )
2
τ (Wn+ 1 ) + τ (Wn− 1 )
2
2
≡ 2λN ± (W ).
(A4)
842
H. A. Levine et al.
Figure 6.5. Time course for VEGF propagation in the ECM after the introduction of angiostatin. Notice the gradual elimination of growth factor from most of the ECM.
The master equation now reads
1 ∂ηn
= N + (Wn− 1 , Wn− 3 )ηn−1 + N − (Wn+ 1 , Wn+ 3 )ηn+1
2
2
2
2
2λ ∂t
−[N + (Wn+ 1 , Wn− 1 ) + N − (Wn− 1 , Wn+ 1 )]ηn .
2
2
2
2
(A5)
We now proceed to the continuous limit by letting h → 0 and λ → +∞ in such a
way that
D=
1
lim
λh 2 .
2 h→0,λ→+∞
(A6)
[See Levine and Sleeman (1997), Othmer and Stevens (1997) for details] to obtain
the primary equation, which we shall refer to as the continuous limit of master
equation (CLME):
∂
∂
η
∂η
=D
η
ln
(A7)
∂t
∂x
∂x
τ
Modeling of Capillary Growth
843
Figure 6.6. Time course for angiostatin in the ECM. As time runs on, all the angiostatin is
converted to inhibitor (The units on the z-axis are expressed in micromolarity.) just outside
the capillary region.
which is precisely (6.1.1) provided we take D = Dη and τ (w) = τ (ca , f ).
In more or less the same manner, one can write a cell movement equation on a
two dimensional lattice with grid points (nk, mk) as follows:
i±
We define τ̂n,m
to be the transition probability for leaving position (nk, mk) to
the left or right when i = h, or up or down when i = v. The time rate of change of
Nn,m (t) is governed by:
∂ Nn,m (t)
h+
h−
= τ̂n−1,m
(W )Nn−1,m + τ̂n+1,m
(W )Nn+1,m
∂t
h+
h−
− (τ̂n,m
(W ) + τ̂n,m
(W ))Nn,m
v
v
+
−
+ τ̂n,m−1
(W )Nn,m−1 + τ̂n,m+1
(W )Nn,m+1
v+
v−
− (τ̂n,m
(W ) + τ̂n,m
(W ))Nn,m
(A8)
where W is defined on the two dimensional lattice with half step sizes in each
844
H. A. Levine et al.
Figure 6.7. Time course for inhibitor in the ECM. The region formerly occupied by a high
concentration of endothelial cells is now full of protease inhibitor. (The units on the z-axis
are expressed in micromolarity.).
direction, viz.
W = (. . . Wn− 1 ,m , Wn+ 1 ,m , Wn,m , Wn,m− 1 , Wn,m+ 1 · · · ).
2
2
2
(A9)
2
(Here the indices n, m in the argument list runs over all integers, 0, ±1, ±2, . . . .)
We assume that for movement in any direction, the mean waiting time in each
direction (left, right, up, down) is constant and is given by
h+
h−
v+
v−
τ̂n,m
(W ) + τ̂n,m
(W ) + τ̂n,m
(W ) + τ̂n,m
(W ) = 4λ.
(A10)
Next we suppose that the normalized transition probabilities are defined only by
nearest neighbors as
h±
τ̂n,m
= 4λ
τ (Wn± 1 ,m )
2
τ (Wn− 1 ,m ) + τ (Wn+ 1 ,m ) + τ (Wn,m− 1 ) + τ (Wn,m+ 1 )
2
≡ 4λN
2
h±
(W )
2
2
Modeling of Capillary Growth
845
Figure 6.8. Time course for protease in the ECM after addition of angiostatin. Protease
concentration remains high in the neighborhood of the tumor.
v±
τ̂n,m
= 4λ
τ (Wn,m± 1 )
2
τ (Wn− 1 ,m ) + τ (Wn+ 1 ,m ) + τ (Wn,m− 1 ) + τ (Wn,m+ 1 )
2
2
≡ 4λN
v±
2
2
(W )
(A11)
The master equation now reads
1 ∂ Nn,m
= N h+ (Wn− 1 ,m , Wn− 3 ,m , Wn−1,m− 1 , Wn−1,m+ 1 )Nn−1,m
2
2
2
2
4λ ∂t
+N h− (Wn+ 1 ,m , Wn+ 3 ,m , Wn+1,m− 1 , Wn+1,m+ 1 )Nn+1,m
2
−[N
+N
+N
h+
h+
2
2
(Wn+ 1 ,m , Wn− 1 ,m , Wn,m− 1 , Wn,m+ 1 )
2
2
2
2
(Wn+ 1 ,m , Wn− 1 ,m , Wn,m− 1 , Wn,m+ 1 )]Nn,m
2
v+
2
2
2
2
(Wn,m− 1 , Wn,m− 3 , Wn− 1 ,m−1 , Wn+ 1 ,m−1 )Nn,m−1
2
2
2
2
846
H. A. Levine et al.
Figure 6.9. Time course for active protease in the ECM after introduction of angiostatin.
The concentration of active protease has fallen to quite a low level. (The first figure here is
the same as the first figure in Fig. 6.8.).
+N v− (Wn,m+ 1 , Wn,m+ 3 , Wn− 1 ,m+1 , Wn+ 1 ,m+1 )Nn,m+1
2
2
2
2
−[N v+ (Wn,m+ 1 , Wn,m− 1 , Wn− 1 ,m , Wn+ 1 ,m )
2
+N
v+
2
2
2
(Wn,m+ 1 , Wn,m− 1 , Wn− 1 ,m , Wn+ 1 ,m )]Nn,m .
2
2
2
2
(A12)
We then proceed as before, letting k → 0, and λ → +∞ in such a way that
D N = λk 2 is constant, we obtain (using Taylor’s theorem in two variables)
N
∂N
= D N ∇ · N ∇ ln
.
(A13)
∂t
τ (W )
This will lead to (6.2.1) (without the proliferation and death terms) when we take
τ (W ) = T (Ca , F).
Sleeman and Wallis (2001) have carried out a computation using equation (A8)
and its three-dimensional analog, assuming a given distribution of fibronectin and
growth factor in the ECM, in order to simulate the movement of individual cells.
They used growth factor dependent probability rates.
Modeling of Capillary Growth
847
Figs 7.1. to 7.5.—Capillary growth in the presence of angiostatin, angiostatin as an inhibitor. Here we give only the initial and final time plots.
Figure 7.1a–d. Time course for EC propagation in the ECM. We give the the cross sections
in Fig. 7.1(a,b.).
A PPENDIX B:
T HE C HOICE OF P HENOMENOLOGICAL PARAMETERS
Finding good estimates for the constants we used in our model was not a trivial
task. Fortunately, with the aid of the dedicated and thorough help of Mr. John E.
Hinrichsen, we were able to locate some constants and obtain order of magnitudes
for some others. The entries in Table 2 include those he found as well as the others
we used for our computations. Table 2 and the explanatory material which follow
it are based on the commentary in Levine et al. (in press) and Pamuk (2000).
Notes on the tabular entries
(0) Remark. The natural length scale for our computations is millimeters (mm)
while the natural time scale is hours. Therefore, before entering the values
discussed below into Table 2, we converted, where necessary, the literature
values to these units.
848
H. A. Levine et al.
Figure 7.2.a–d. Time course for fibronectin propagation in the ECM after introduction
of angiostatin as inhibitor. These figures are self explanatory. Notice that in Fig. 7.1(b),
the fibronectin density is larger at the capillary opening and near the tip than in the same
positions in Fig. 7.1(a). Thus the channel is closing here also.
(1) Cell densities η0 . In Haas and Duling (1997), the length of an endothelial
cell was estimated to be in the range 94–141 microns while in Nerem et al.
(1981) an approximate width was given in the range of 10–18 microns. It is
generally known that an endothelial cell has a thickness of about 1 micron.
Using these dimensions, we estimate a volumetric density for endothelial
cells to be about 1012 cells per liter. As we noted earlier, the kinetic equations
may be rewritten so that the λ0 s, ν 0 s can be expressed directly in terms of the
0
K cat
s and K m0 s.
The capillary EC equation (6.1.1), is linear in η. Therefore earlier, if we renormalize η, η = η0 η̂, we may take η0 = 1, i.e., in the computations below,
the capillary cell density is expressed as a fraction of the equilibrium cell
density in the capillary and is therefore a dimensionless quantity. Likewise,
the EC equation (6.2.1) can also be rescaled in a similar manner and the
kinetic equations correspondingly rewritten since the ratio N /η0 appears in
the logistic factor.
Modeling of Capillary Growth
849
Figure 7.3.a–d. Time courses in the capillary after introduction of angiostatin as inhibitor.
7.3(a): EC time course. 7.3(b): fibronectin time course. 7.3(c): Protease time course.
7.3(d): Active protease time course.
(2) Length scales. In Fig. 2 we have taken ` = 25 microns = 2.5×10−2 mm and
L = 50 microns = 5.0 × 10−2 mm. Therefore, in the figures which illustrate
our computations, along the capillary, the scale is 0.1 = 10 microns while
the scale from the capillary to the VEGF source, 0.1 = 2.0 microns.
(3) Cell movement and diffusion constants. We took the ‘porosity’ power m = 1.
It is well known that the time of travel across the ECM (the time interval from
tumor activation to the onset of sprouting) will increase with m. [We took
m = 1.5 in another simulation (not shown) to verify this and to test the code
in the case m > 1.]
In Sherratt and Murray (1990) the authors used values in the range 6.9 ×
10−11 cm2 s−1 −3.5×10−10 cm2 s−1 for the cell movement constant D N . (The
reader is cautioned that in Table 2, we have converted these cell movement
constants as well as the diffusion constants DV , D A in units of mm2 / h.)
They also used values for DV in the range 3.1 × 10−7 cm2 s−1 –5.9 ×
10−6 cm2 s−1 . However, these values are not appropriate for growth factor
diffusion in the ECM since there the authors were modeling wound heal-
850
H. A. Levine et al.
Figure 7.4.a–d. Figure 7.4(a, b): Angiostatin time courses. Figure 7.4(c, d): Growth factor
time courses.
ing and diffusion was presumed to be taking place in the fluids that fill the
wound after injury. Molecular diffusion is presumably much slower in the
ECM which can be viewed as a porous medium. [The image of the ECM
we have in mind can be found on page 973 of Alberts et al. (1994) for the
cornea of a rat or the cartoon on page 991 of Alberts et al. (1994) for the
basel lamina.]
In order to obtain growth factor diffusion coefficients, we argued as follows. If one assumes that a protein is spherical, then its diffusion coefficient
should be inversely proportional to the two-thirds power of its volume and
consequently of its molecular weight The molecular weight of VEGF is of
the order of 1.65 × 105 . In the literature, (Hicks et al., 1998), the authors
give the value DT r = 7.4 × 10−7 cm2 s−1 for tirapazamine (3-amino-1,2,4benzotriazine-1,4-dioxide) which has a molecular weight of 168 daltons as
one easily calculates from its structure given in Cahill et al. (1993). Thus
DV ≈ 7.4 × 10−7 × (168/165000)2/3 ≈ 7.4 × 10−9 cm2 s−1 .† Because pro† The ratio of diffusion coefficients for small molecules is inversely proportional to the one-third
power of its volume and consequently of its molecular weight. This follows from the Stokes formula
Modeling of Capillary Growth
851
Figure 7.5.a–d. Figure 7.5(a, b): Protease time courses after introduction of angiostatin as
inhibitor. Figure 7.5(c, d): Active protease time courses.
teins are not spheres and the ECM is not a homogeneous fluid, we have been
somewhat more conservative than this and used DV ≈ 1.0 × 10−9 cm2 s−1 .
In order to estimate the diffusion coefficient D A for angiostatin, note that
the molecular weight of some angiostatins may be taken to be of the order
3.8 × 104 daltons. Therefore, D A ≈ DV × (16.5/3.8)2/3 ≈ 10−8 cm2 s−1 .
In Yamada and Olden (1978), the authors estimated the diffusion coefficient
of fibronectin, D F to be smaller than 5 × 10−12 cm2 s−1 .
(4) Proliferation and death rate constants, θ, µ1 . The proliferation rate, θ , was
given (Sherratt and Murray, 1990) as 0.04 h−1 and in Stokes and Lauffenburger (1991) as 0.056 h−1 . For the death rate, the value 0.5%/day was given
in Cho et al. (1997) and as 0.12%/day in Araki et al. (1990). We took the
latter value in the above table.
for the drag on spheres moving through a fluid and the Einstein formula D = kT u where k is the
Boltzman constant, T is the Kelvin temperature and u is the particle mobility. (Landau and Lifschitz,
1982). However, proteins are large molecules and the ECM is not a fluid. Hence our assumption
seems more reasonable. If we use the Stokes–Einstein relationship, the diffusion constant would be
larger by a factor of ten, i.e., DV ≈ 7.4 × 10−8 cm2 s−1 which is at the lower limit of the range of
values used in Sherratt and Murray (1990).
852
H. A. Levine et al.
(5) Proliferation response function, 2. We took this function to be of the form
m
2(C) = A2 Ce−λC 1 where we used the proliferation response data given in
Unemori et al. (1992). The data there gives the proliferation response as a
function of growth factor.
For the curvature sensitivity factor we took the function
κ
.
Q(κ) = √
1 + 2κ 2
(6)
(7)
(8)
(9)
(10)
This choice was made not only because we wanted the curvature sensitivity
to be dimensionless, but also because we wanted to control the sensitivity to
proliferation. (With this choice and with > 0 the maximum sensitivity is
1/.) It was found that for small the solutions of (6.2.1) attempted to blow
up in finite time.†
−1
Enzyme and inhibitor decay rates µ, Trel
. We took µ = 4.56 h−1 for illustrative purposes based on our reading of Boffa et al. (1998). As remarked earlier
we were not able to find in vivo values for the relaxation times. Therefore,
−1
in order to test angiostatin efficacy we took Trel
= +∞ when angiostatin
−1
generates an inhibitor and Trel = 1 hour when angiostatin acts directly as an
inhibitor. (In the latter case, a decay term in angiostatin must be included in
the model, otherwise the model will be computationally unstable.)
Initial densities fibronectin, f 0 , F0 . The density of fibronectin has been
estimated to be about 10−2 µM (Terranova et al., 1985). We took the density
of the lumen, f 0 to be the same as the background density of fibronectin, F0
in the ECM, for want of better information.
The number of angiogenic response receptors r0 , ra0 . The number of receptors per endothelial cell, δ has been variously estimated as 150 000 (Waltenberger et al., 1994) and 175 000 (Terman et al., 1992). We took δ ≈ 105
as an order of magnitude estimate based on these two numbers. Then r0 =
δη0 ≈ 1 µM. We also took ra0 = 1.7δη0 ≈ 1.7 µM.
The kinetic parameters λ1 , ν1 for VEGF. In Kendall et al. (1999), the followV
V
ing values are given for kcat
, and K mV respectively, namely kcat
= 162 min−1
V
V
and K m = 130 µM. Using these, we easily determine λ1 = kcat
/K mV and
V
ν1 = 1/K m .
The kinetic parameters λ2 , ν2 for angiostatin. The mechanism for the conversion of angiostatin to protease inhibitor which we propose here has yet to
† This is to be expected. If we eliminate C between (5.2.1) and (6.2.1) we see that the resulting
t
differential equation for N takes the form, for large V, N ,
Nt ≈ D N ∇ · N ∇ ln
N
T (Ca , F)
+ M N 2 Q(κ)
where M = M(V, C) will be positive if max G(C)λ1 /ν1 > θ. Thus if Q(κ) is large, we might expect
finite time blow up. (Differential equations of the much simpler variety such as Nt = D1N + N 2
are well known to possess solutions which form singularities in finite time.)
Modeling of Capillary Growth
(11)
(12)
(13)
(14)
(15)
853
be documented in the literature. Therefore, the values we have taken are for
illustrative purposes only. We took λ2 = λ1 and ν2 = 2ν1 .
Protease inhibitor equilibrium constant νe . In Takahashi et al. (1992), the
authors give values for 1/νe in the range 0.59 nmol l−1 to 2.4 nmol l−1 . We
took 1/νe = 1.0 nmol l−1 = 10−3 µM. The value used in the computations
has to be non-dimensionalized. This means νe = 1000 × ra0 = 1700.
In the case for which angiostatin is itself an inhibitor, we took νe = 1(µM)−1 .
This is based on the reported value for plasminogen derived angiostatin
which is an inhibitor of tPA. See Terranova et al. (1985).
The kinetic parameters λ3 , ν3 for fibronectin. For ‘fibronectin’, we have
taken values for λ3 , ν3 from Fields et al. (1990). We took, K cat = 16 per
hour and K m = 0.83 µM for the hydrolysis of type I collagen (rat tendon)
by HFC (human fibroblast collagenase).
The fibronectin production times T f , TF . The value T f = 18 hours has been
reported in Yamada and Olden (1978) and Orme and Chaplain (1996). We
shall take TF = T f = 18 hours and F0 = f 0 .
The tumor source term V` (x, t). The form of V` which was used in Pamuk (2000) was somewhat different than that used here in (7.2.2). We took
δ = 0.0 as the worst case scenario. The constant m 0 is representative of the
localization of the tumor source, the larger m 0 is, the more localized is the
TGF source.
The angiostatin source term ar (x, t). Here we took Ar as in the table. We
took Tiv = T1 where T1 is the time from the initiation of the tumor secretion
into the ECM to the time the responding capillary has crossed the ECM back
to the tumor. Since we do not as yet have a good mathematical model for the
penetration of the capillary into the tumor region, we have made this choice
for Tiv in (7.1.3) for illustrative purposes only. We took Tiv = 4.5 hours.
Other constants.
(i) Sensitivities, αi , βi , γi . These constants do not affect the travel time
across the ECM nor do they affect the width of the nascent capillary
opening as was demonstrated numerically in Levine et al. (in press).
However, they do control the distribution of endothelial cell density
within the forming capillary. We have taken them so that the endothelial cells are somewhat more responsive to protease and fibronectin
changes in the capillary than they are in the ECM. The values in Table
2 are for illustrative purposes only.
(ii) Threshold constants f 1 , ψ1 , Ca0 , F1 . These were selected for illustrative
purposes only. The threshold f 1 is a measure of the percentage of the
lumen that must be destroyed before endothelial cells can escape into
the ECM while ψ1 represents the percentage of endothelial cells that
are able to cross this barrier into the ECM.
(iii) Transport velocities ψ, ψ 0 . These constants were also selected for illustrative purposes only.
(7.2.1)
(7.2.2)
(7.1.1)
(7.1.3)
(7.1.3)
(7.1.4)
(7.1.5)
δ = 0.0
f 1 = 0.60 µM
ν1 = 0.007 µM−1
T f = 18.0 h
ν2 = 0.014 µM−1
Trel = 1.0 h
λ1 = 73.0 µM−1 h−1
ν2 = 0.014 µM−1
λ2 = 146.0 µ M−1 h−1
Trel = 1.0 h
λ3 = 19.0 µM−1 h−1
F0 = 1.0 × 10−2 µM
α1 = 0.1 µM
β1 = 1.0 µM
α1 = 0.1 µM
β1 = 1.0 µM
m1 = 2
Ca0 = 10−4 µM
B1 = 1.0/ h
Tiv = T1
v0 = 4.0 µM mm h−1
ν3 = 1.28 µM−1
γ1 = 4.0
γ2 = 4.0
γ1 = 2.0
γ2 = 1.5
θ = 0.056 h−1
Trel = +∞
νe = 1.7 × 103 µM−1
ν3 = 1.28 µM−1
λ3 = 19.0 µM−1 h−1
α2 = 1.0 µM
β2 = 0.1 µM
α2 = 1.0 µM
β2 = 0.5 µM
A2 = 44.13 µM−1
µ = 4.56 h−1
Trel = +∞
ν1 = 0.007 µM−1
νe = 1.7 × 103 µM−1
µ = 4.56 h−1
Explanatory notes are given in Appendix B.
Some of the constants used in Table 2 differ slightly from those used in Tables 3–6.
D N = 3.6 × 10−6 mm2 h−1
= 1.40
λ = 1.1 × 10−9 µM−2
µ1 = 0.005 h−1
A1 = 0.0
Ar = 10.0 µM h−1
Ar = 250.0 µM h−1
ψ1 = 0.3
ψ = 2.0 mm h−1
ψ 0 = 2.0 mm h−1
θ 0 = 0.0 mm h−1
m 0 = 12
(6.2.1)
(6.1.1)
(5.2.2)
(5.1.2)
(5.2.1)
λ1 = 73.0 µM−1 h−1
f 0 = 1.0 × 10−2 µM
λ2 = 146.0 µM−1 h−1
νe = 1.0 µM−1
DV = 3.6 × 10−3 mm2 h−1
Vr (x, y, t) = 0.0 µM h−1
D A = 6.5 × 10−3 mm2 h−1
νe = 1.0 µM−1
TF = 18.0 h
D F = 3.6 × 10−8 mm2 h−1
Dη = 3.6 × 10−6 mm2 h−1
(5.1.1)
Table 2. Physiological and kinetic constants.
854
H. A. Levine et al.
Modeling of Capillary Growth
A PPENDIX C:
855
C ODE T ESTING AND C ONVERGENCE
The hyperbolic nature of the master equations requires that we pay close attention
to how the numerical solution behaves with respect to parameters such as grid size
as well as how it behaves relative to the biological parameters in the model itself.
Here we discuss the code testing.†
The major computational difficulty here can be illustrated by the simple initial
value problem y 0 (t) = −ay(t) + y 2 (t) with y(0) given and a > 0. If we wish
to search for the stationary solution ys (t) = a numerically by varying y0 , we will
only find solutions which either decay to zero, the rate of decay converging to zero
as y0 increases to a from below or else which become singular in finite time, the
blow up time increasing to +∞ as y0 decreases to a from above.
The systems were solved by the method of lines using Matlab v5.3. Since DV D N , the system of partial differential equations in the ECM is said to be ‘stiff’. In
order to solve the system efficiently, therefore, we used one of the stiff ordinary
differential equation solvers found in the Matlab package.
In order to insure that our results were not merely coincidental, we tested the code
in three ways. [Here and in the tables below, Tα for α ∈ [0, 1] is the time, in hours,
from the onset of ‘tumor’ implantation to the time the capillary tip has crossed the
fraction α of the distance ` from the given capillary to the tumor.‡ Thus T0 is the
time elapsed from the implantation of the tumor to the onset of angiogenesis in the
ECM.]
(i) To insure that the travel time from the tumor across the ‘ECM’ to the ‘capillary’ and back was not a function of mesh size,§ at least for the optimal
choices we used above, we fixed such an and varied the mesh size. We
found that for low dosages of growth factor, (v0 ≈ 3 µM mm h−1 ) the travel
time across the ECM increased with decreasing mesh size but appeared to
approach a limiting value as the mesh size approached zero.
† The constants we used for the simulations below are somewhat different than those recorded in
Table 2. Also, we used the function
V` (x, t) =
(
h
i
L) m 0
v0 σ 1 − cos 2π(x−a
,
(b−a)L
if x ∈ [a L , bL] ⊂ [0, L]
0,
otherwise
(C1)
where again σ is chosen such that
Z L
0
V` (x, t)d x = v0 .
We took m 0 = 10.0, a = 0.45, b = 0.55, v0 = 3.0 µM mm h−1 , L = 100 microns and ` =
17 microns.
‡ That is, T is the time that it has taken some endothelial cells to have moved a distance α` from
α
the capillary located along the x-axis.
§ The mesh size, M × N , refers to the division of [0, L] into equal subintervals intervals of length
L/M and [0, `] into equal subintervals intervals of length `/N .
856
H. A. Levine et al.
Table 3. Mesh size sensitivity in the capillary direction.
Mesh size →
10 × 40
20 × 40
30 × 40
40 × 40
T0
T1
0.92 h
1.02 h
0.95 h
1.05 h
0.97 h
1.06 h
0.97 h
1.06 h
T1
1.12 h
1.11 h
1.09 h
1.08 h
2
T3
4
1.15 h
1.12 h
1.11 h
1.10 h
T1
1.18 h
1.14 h
1.12 h
1.11 h
4
( = 1.0, v0 = 3.0 µM mm h−1 , f 1 = 0.40, ` = 17µmm).
Table 4. Mesh size sensitivity in tumor direction.
Mesh size → 50 × 10 50 × 20 50 × 30 50 × 40
T0
T1
0.57 h
0.61 h
0.71 h
0.76 h
0.86 h
0.91 h
0.97 h
1.04 h
0.65 h
0.78 h
0.94 h
1.06 h
4
T1
2
T3
4
0.69 h
0.80 h
0.96 h
1.08 h
T1
0.74 h
0.82 h
0.97 h
1.10 h
( = 1.0, v0 = 3.0 µM mm h−1 , f 1 = 0.40, ` = 17 µmm).
In Table 3 we vary the mesh size along the capillary and fix the mesh size in the
direction from the capillary to the ‘tumor’.
In Table 4 we fix the mesh size along the capillary and vary the mesh size in
the direction from the capillary to the ‘tumor’. We see from the table that as we
refine the grid along the capillary–tumor axis, the travel times increase. However,
the conclusion of this and other experiments we have made indicates that as the
mesh size becomes finer, the travel times, Tα , for fixed sensitivity , approach a
limiting value.
(ii) To investigate how the travel time across the ‘ECM’ to the ‘capillary’ and
back to the tumor depended on the capillary sensitivity, we varied for a
fixed grid size. This experiment is quite difficult to do computationally at
fine grid sizes so we conducted the experiment with a relatively coarse grid
size taking 30 mesh points the capillary direction (x-axis) and 20 mesh points
in the tumor direction y-axis. The results are recorded in Table 5.
At high growth factor fluxes (v0 1) or small , (high curvature sensitivity)
the peak in EC concentration bifurcates into two peaks and the computation
grinds to a halt for the reasons we have explained in the Notes in Appendix B
(Section B). Lowering the dosage v0 , increasing the cell sensitivities (γ1 , γ2 )
and lowering the the threshold level at which EC can penetrate the ECM (increasing f 1 ) will prevent this from occurring numerically. [The observations
concerning the cell sensitivities and the dosage were made in Levine et al.
(in press).]
Modeling of Capillary Growth
857
Table 5. Variable , curvature sensitivity.
→ 0.900
1.00
1.10
1.20
1.30
1.40
1.50
1.60
1.18 h
1.247 h
1.18 h
1.254 h
1.18 h
1.28 h
1.18 h
1.29 h
1.18 h
1.31 h
1.18 h
1.34 h
1.18 h
1.38 h
1.18 h
1.40 h
T1
1.28 h
1.31 h
1.36 h
1.40 h
1.44 h
1.48 h
1.52 h
1.57 h
2
T3
4
1.29 h
1.32 h
1.38 h
1.45 h
1.51 h
1.59 h
1.66 h
1.78 h
T0
T1
4
T1 1.31 h
1.34 h
1.41 h
1.51 h
1.61 h
1.71 h
1.82 h
1.92 h
v 3.13 mm/d 2.55 mm/d 1.77 mm/d 1.16 mm/d 0.95 mm/d 0.77 mm/d 0.64 mm/d 0.55 mm/d
Mean tip speed is computed as v = 0.017/(T1 − T0 ) in millimeters per day.
(v0 = 3.0 µM mm h−1 , f 1 = 0.40, ` = 17 µmm).
Table 6. Tumor/capillary distance sensitivity (variable `).
`→
mesh size →
17.0 µm
30 × 20
34.0 µm
30 × 40
51.0 µm
30 × 60
68.0 µm
60 × 80
T0
T1
1.18 h
1.25 h
2.26 h
2.43 h
3.67 h
3.94 h
5.37 h
5.80 h
1.31 h
2.53 h
4.10 h
5.98 h
1.32 h
2.57 h
4.14 h
6.05 h
4
T1
2
T3
4
T1
1.34 h
2.59 h
4.18 h
6.13 h
Mean tip speed 2.55 mm/day 2.47 mm/day 2.40 mm/day 2.15 mm/day
v0 = 3.0 µM mm h−1 , f 1 = 0.40, = 1.0.
On the other hand, for large , cell travel across the ECM slows as one
might expect if the curvature dependence on proliferation is weaker. This
will clearly affect the rate of enzyme degradation of fibronectin since the
rate of protease growth is proportional to EC concentration. Consequently,
if protease concentration does not increase, the rate of fibronectin decay will
be lessened. For example, when = 5.0, we found that v = 0.24 mm/day.
However, when is large, then the diffusion term so dominates the proliferation term that we no longer observe a peak in EC density profile at the tip
of the capillary. The capillary also becomes wider. This is also due to the
increased effect of EC diffusion.
Below = 0.8 we observed instability for this mesh. This is due to the blow
up phenomenon we discussed earlier. Thus, an optimal choice of seems to
be in the range of 1.1–1.4 for v0 = 3.0 µM mm h−1 .
(iii) To investigate how the travel time across the ‘ECM’ to the ‘capillary’ and
back to the tumor depended on the distance from the tumor to the ECM, the
number `, we fixed the sensitivity and the mesh size and varied `. The results are recorded in Table 6. Clearly, one expects that as the distance to the
858
H. A. Levine et al.
tumor increases, the travel time will also increase but the issue here is how
it increases with `.
We see from Table 6 that our assumption that we can scale the tip travel times
for short distances up to distances of the order of 1.0 cm using linear extrapolation
is only approximate, for grid sizes this coarse. Since we do not have a specific
example for this mechanism, we shall test the model by selecting a large value
for νe .
R EFERENCES
Alberts, B., D. Bray, J. Lewis, M. Raff, K. Roberts and J. D. Watson (1994). Molecular
Biology of the Cell, 3rd edn, NY and London: Garland Pub. Inc.
Anderson, R. (1985). Mammary gland, in Lactation, Bruce Larson (Ed.), Ames: Iowa State
University Press, pp. 1–38.
Ankoma-Sey, V., M. Matli, K. B. Chang, A. Lalazar, D. B. Donner, L. Wong, R. S. Warren
and S. L. Friedman (1998). Coordinated induction of VEGF receptors in mesenchymal cell types during rat hepatic wound healing. Oncogene 17, 115–121.
Araki, S., Y. Shimada, K. Kaji and H. Hayashi (1990). Apoptosis of vascular endothelial
cells by fibroblast growth factor deprivation. Biochem. Biophys. Res. Commun. 168,
1194–1200.
Ausprunk, D. H. and J. Folkman (1977). Migration and Proliferation of endothelial cells
in preformed and newly formed blood vessels during tumor angiogenesis. Microvasc.
Res. 14, 53–65.
Balding, D. and D. L. McElwain (1985). A mathematical model of tumour-induced capillary growth. J. Theor. Biol. 114, 53–73.
Baramova, E. N., K. Bajou, A. Remacle, C. L’Hoir, H. W. Krell, U. H. Weidle, A. Noel
and J. M. Foidart (1997). Involvement of PA/plasmin system in the processing of proMMP-9 and in the second step of pro-MMP-2 activation. FEBS Lett. 405, 157–162.
Blasi, F. (1993). Urokinase and urokinase receptor: a paracrine/autocrine system regulating
cell migration and invasiveness. Bioessays 15, 105–111.
Boffa, M. B., W. Wang, L. Bajzar and M. E. Nesheim (1998). Plasma and recombinant
thrombin-activable fibrinolysis inhibitor (TAFI) and activated TAFI compared with respect to glycosylation, thrombin/thrombomodulin-dependent activation, thermal stability, and enzymatic properties. J. Biol. Chem. 273, 2127–2135.
Bourdoulous, S., G. Orend, D. A. MacKenna, R. Pasqualini and E. Ruoslahti (1998). Fibronectin matrix regulates activation of RHO and CDC42 GTPases and cell cycle progression. J. Cell Biol. 143, 267–276.
Brem, H. and J. Folkman (1975). Inhibition of tumor angiogenesis mediated by cartilage.
J. Exp. Med. 141, 427–439.
Brem, S., B. A. Preis, ScD. Langer, B. A. Brem and J. Folkman (1997). Inhibition of
neovascularization by an extract derived from vitreous. Am. J. Opthalmol. 84, 323–328.
Modeling of Capillary Growth
859
Cahill, A., T. C. Jenkins and I. N. H. White (1993). Metabolism of 3-amino-1,2,4benzotriazine-1,4-dioxide (SR 4233) by purified DT-diaporase unde aerobic and anaerobic conditions. Biochem. Pharmacol. 45, 321–329.
Carmeliet, P. and R. K. Jain (2000). Angiogenesis in cancer and other diseases. Nature
407, 249–257.
Carney, D. H. and D. D. Cunningham (1977). Initiation of check cell division by trypsin
action at the cell surface. Nature 268, 602–606.
Chaplain, M. A. J. and A. R. A. Anderson (1999). Modelling the growth and form of
capillary networks, in On Growth and Form: Spatio-Temporal Pattern Formation in
Biology, New York: Wiley, pp. 225–249.
Chapman, H. A. (1997). Plasminogen activators, integrins, and the coordinated regulation
of cell adhesion and migration. Curr. Opin. Cell Biol. 9, 714–724.
Cho, A., L. Mitchell, D. Koopmans and B. L. Langille (1997). Effects of changes in blood
flow rate on cell death and cell proliferation in carotid arteries of immature rabbits. Circ.
Res. 81, 328–337.
Cliff, W. J. (1963). Observations on healing tissue: a combined light and electron microscopic investigation. Philos. Trans. R. Soc. London B 246, 305–ff.
Crocker, D. J., T. M. Murad and J. C. Geer (1970). The role of the pericyte in wound
healing: an ultrastructural study. Exp. Mol. Pathol 13, 51–65.
Curran, S. and G. I. Murray (1999). Matrix metalloproteinases in tumour invasion
and metastasis. J. Pathol. 189, 300–308.
Davis, B. (1990). Reinforced random walks. Probability Theory Related Fields 84,
203–229.
Dekker, A., A. A. Poot, J. A. van Mourik, M. P. Workel, T. Beugeling, A. Bantjes, J. Feijen
and W. G. van Aken (1991). Improved adhesion and proliferation of human endothelial cells on polyethylene precoated with monoclonal antibodies directed against cell
membrane antigens and extracellular matrix proteins. Thromb. Haemost. 66, 715–724.
Edelstein-Keshet, L. (1988). Mathematical Models in Biology, Boston: McGraw-Hill.
Fields, G., S. J. Netzewl-Arnett, L. J. Windsor, J. A. Engler, H. Berkedal-Hansen and H.
E. van Wart (1990). Proteolytic activities of human fibroblast collagenase; hydrolysis of
a broad range of substrates at a single active site. Biochemistry 29, 6600–6677.
Folkman, J. (1976). The vascularization of tumors. Sci. Am. 234, 58–64.
Folkman, J. (1992). Angiogenesis-retrospect and outlook, in Angiogenesis: Key PrinciplesScience-Technology-Medicine, R. Steiner, P. B. Weisz and R. Langer (Eds), Basel:
Birkhäuser.
Frenzen, C. L. and P. K. Maini (1988). Enzyme kinetics for a two-step enzymic reaction
with comparable initial enzyme-substrate ratios. J. Math. Biol. 26, 689–703.
Gamble, J. R., L. J. Matthias, G. Meyer, P. Kaur, G. Russ, R. Faull, M. C. Berndt and M. A.
Vadas (1993). Regulation of in vitro capillary tube formation by anti-integrin antibodies.
J. Cell Biol. 121, 931–943.
Gengrinovitch, S., B. Berman, G. David, L. Witte, G. Neufeld and D. Ron (1999).
Glypican-1 is a VEGF165 binding proteoglycan that acts as an extracellular chaperone
for VEGF165. J. Biol. Chem. 274, 10816–10822.
860
H. A. Levine et al.
Gimbrone, M. A. Jr., R. S. Cotran, S. B. Leapman and J. Folkman (1974). Tumor growth
and neovascularization: an experimental model using the rabbit cornea. J. Natl. Cancer
Inst. 52, 413–419.
Gordon, S. R. and J. DeMoss (1999). Exposure to lysosomotropic amines and protease inhibitors retard corneal endothelial cell migration along the natural basement membrane
during wound repair. Exp. Cell Res. 246, 233–242.
Gospodarowicz, D., G. Greenburg, H. Bialecki and B. R. Zetter (1978). Factors involved
in the modulation of cell proliferation in vivo and in vitro: the role of fibroblast and
epidermal growth factors in the proliferative response of mammalian cells. In Vitro 14,
85–118.
Haas, T. L. and B. R. Duling (1997). Morphology favors an endothelial cell pathway
for longitudinal conduction within arterioles. Microvasc Res. 53, 113–120.
Han, Z. C. and Y. Liu (1999). Angiogenesis: state of the art. Int. J. Hematol. 70, 68–82.
Hanahan, D. and J. Folkman (1996). Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 86, 353–364.
Hicks, K. O., Y. Fleming, B. G. Siim, C. J. Koch and W. R. Wilson (1998). Extravascular
diffusion of tirapazamine: effect of metabolic consumption assessed using the multicellular layer model. Int. J. Radiat. Oncol. Biol. Phy. 42, 641–649.
Hiraoka, N., E. Allen, I. J. Apel, M. R. Gyetko and S. J. Weiss (1998). Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell 95,
365–377.
Holash, J., P. C. Maisonpierre, D. Compton, P. Boland, C. R. Alexander, D. Zagzag, G. D.
Yancopoulos and S. J. Wiegand (1998). Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science 284, 1994–1998.
Jaffee, E. A. and D. F. Mosher (1978). Synthesis of fibronectin by cultured human endothelial cells. J. Exp. Med. 147, 1779–1791.
Kabelic, T., S. Ganbisa, B. Glaser and L. A. Liotta (1983). Basement membrane collagen:
degradation by migrating endothelial cells. Science 221, 281–283.
Kendall, R. L., R. Z. Rutledge, X. Mao, A. J. Tebben, R. W. Hungate and K. A. Thomas
(1999). Vascular endothelial growth factor receptor KDR tyrosine kinase activity is increased by autophosphorylation of two activation loop tyrosine residues. J. Biol. Chem.
274, 6453–6460.
Kuwano, M., S. Ushiro, M. Ryuto, K. Samoto, H. Izumi, K. Ito, T. Abe, T. Nakamura,
M. Ono and K. Kohno (1994). Regulation of angiogenesis by growth factors. GANN
Monograph on Cancer Research 42, 113–125.
Lagelund, T. D. and P. A. Low (1987). A mathematical simulation of oxygen delivery in
rat peripheral nerve. Microvascular Research 34, 211–222.
Landau, L. D. and E. M. Lifschitz (1982). Course of Theoretical Physics, Vol. 6, Fluid
Mechanics, Oxford, UK: Pergamon Press.
Levine, H. A. and B. D. Sleeman (1997). A system of reaction diffusion equations arising
in the theory of reinforced random walks. SIAM J. Appl. Math. 57, 683–730.
Levine, H. A., B. D. Sleeman and M. Nilsen-Hamilton. Mathematical modeling of the
onset of capillary formation initiating angiogenesis. J. Math. Biol. (in press).
Modeling of Capillary Growth
861
Levine, H. A., B. D. Sleeman and M. Nilsen-Hamilton. A mathematical model for the
roles of plasminogen activators, collagenases and heparanase on tumor angiogenesis,
(in preparation).
Levine, H. A., B. D. Sleeman and M. Nilsen-Hamilton (2000). A Mathematical model
for the roles of pericytes and macrophages in the onset of angiogenesis: I. The role
of protease inhibitors in preventing angiogenesis. Math. Biosci. 168, 77–115.
Librach, C. L., Z. Werb, M. L. Fitzgerald, K. Chiu, N. M. Corwin, R. A. Esteves, D.
Grobelny, R. Galardy, C. H. Damsky and S. J. Fisher (1999). 92-kD type IV collagenase
mediates invasion of human cytotrophoblasts. J. Cell Biol. 189, 300–308.
Mandriota, S. J., G. Seghezzi, J. D. Vassalli, N. Ferrara, S. Wasi, R. Mazzieri, P. Mignatti
and M. S. Pepper (1995). Vascular endothelial growth factor increases urokinase receptor expression in vascular endothelial cells. J. Biol. Chem. 270, 9709–9716.
Moerman, D. G. (1999). A metalloprotease prepares the way. Curr. Biol. 9, R701–R703.
Morimoto, K., H. Mishima, T. Nishida and T. Otori (1993). Role of urokinase type
plasminogen activator (u-PA) in corneal epithelial migration. Thromb. Haemost. 69,
387–391.
Murphy, G. and J. Gavrilovic (1999). Proteolysis and cell migration: creating a path? Curr.
Opin. Cell Biol. 11, 614–621.
Murray, J. D. (1989). Mathematical Biology, Biomathematics Texts, Springer-Verlag.
Nelsen, N. J. (1998). Inhibitors of angiogenesis enter phase III testing. J. Natl. Cancer Inst.
90, 960–962.
Nerem, R. M., M. J. Levesque and J. F. Cornhill (1981). Vasuclar endothelial cell morphology as an indicator of the pattern of blood flow. J. Biomech. Eng. 103, 172–176.
Nicosia, R. F., E. Bonanno and M. Smith (1993). Fibronectin promotes the elongation of
microvessels during angiogenesis in vitro. J. Cell Physiol. 154, 654–661.
Olofsson, B. et al. (1998). Vascular endothelial growth factor B (VEGF-B) binds to VEGF
receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl.
Acad. Sci. U. S. A. 95, 11709–11714.
Orme, M. E. and M. A. J. Chaplain (1996). A mathematical model of the first steps of tumour related angiogenesis: capillary sprout formation and secondary branching. I.M.A.
J. Math. Appl. Med. Biol. 13, 73–98.
Orme, M. E. and M. A. J. Chaplain (1997). Two-dimensional models of tumour angiogenesis and anti-angiogenesis strategies. I.M.A. J. Math. Appl. Med. Biol. 14, 189–205.
Othmer, H. G. and A. Stevens (1997). Aggregation, blow up and collapse: the ABC’s of
taxis and reinforced random walks. SIAM J. Appl. Math. 51.
Pamuk, S. (May, 2000). Two dimensional models of tumor angiogenesis, PhD Thesis, Iowa
State University.
Paweletz, N. and M. Knierim (1989). Tumor related angiogenesis. Crit. Rev. Oncol. Hematol. 9, 197–242.
Rakusan, K. (1995). Coronary Angiogenesis. From morphology to molecular biology and
back. Ann. Ny Acad. Sci. 752, 257–266.
Roberts, J. M. and J. V. Forrester (1990). Factors affecting the migration and growth of endothelial cells from microvessels of bovine retina. Exp. Eye Res. 50, 165–172.
862
H. A. Levine et al.
Rochefort, H., M. Garcia, M. Glondu, V. Laurent, E. Liaudet, J. M. Rey and P. Roger
(2000). Cathepsin D in breast cancer: mechanisms and clinical applications, a 1999
overview. Clin. Chim. Acta. 291, 85–118.
Saksela, O. (1985). Plasminogen activation and regulation of pericellular proteolysis.
Biochim. Biophys. Acta 823, 35–65.
Sato, H., T. Takino, Y. Okada, J. Cao, A. Shinagawa, E. Yamamoto and M. Seiki (1994). A
matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 370,
61–65.
Schleef, R. R. and C. R. Birdwell (1982). The effect of proteases on endothelial cell migration in vitro. Exp. Cell Res. 141, 503–508.
Schoefl, G. I. (1963). Studies on inflammation. III. Growing capillaries: their structure and
permeability. Virchows Arch. Pathol. Anat. 337, 97–ff.
Schoefl, G. I. and G. Majno (1964). Regeneration of blood vessels in wound healing. Adv.
Biol. Skin 5, 173–ff.
Schor, A. M., A. E. Canfield, A. B. Sutton, T. D. Allen, P. Sloan and S. L. Schor (1992).
The behavior of pericytes in vitro: relevance to angiogenesis and differentiation, in
Angiogenesis: Key Principles-Science-Technology-Medicine, R. Steiner, P. B. Weisz
and R. Langer (Eds), Basel: Birkhäuser.
Segel, L. A. (1988). On the validity of the steady state assumption of enzyme kinetics.
Bull. Math. Biol. 50, 579–593.
Segel, L. A. and M. Slemrod (1989). The quasi steady state assumption: a case study in
perturbation. SIAM Rev. 31, 446–477.
Sethian, J. A. (1996). Level Set Methods and Fast Marching Methods, Cambridge, U.K.:
Cambridge University Press.
Sherratt, J. A. and J. D. Murray (1990). Models of epidermal wound healing. Proc. R. Soc.
Lond. B. 241, 29–36.
Sherratt, J. A., A. J. Perumpanani and M. R. Owen (1999). Pattern formation in cancer,
in On Growth and Form: Spatio-Temporal Pattern Formation in Biology, New York:
Wiley, pp. 47–73.
Sholley, M. M., G. P. Ferguson, H. R. Seibel, J. L. Montour and J. D. Wilson (1984).
Mechanisms of neovascularization. Vascular sprouting can occur without proliferation
of endothelial cells. Lab. Invest. 51, 624–634.
Sleeman, B. D. (1996). Solid tumor growth: a case study in mathematical biology. Nonlin.
Math. Appl., Ed. P. J. Aston, C.U. P 237–256.
Sleeman, B. D. and I. P. Wallis (2001). Tumor induced angiogenesis as a reinforced random walk: modelling capillary network formation without endothelial cell proliferation,
Math. Copmp. Mod., (in press).
Soldi, R., S. Mitola, M. Strasly, P. Defilippi, G. Tarone and F. Bussolino (1999). Role of
alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2.
EMBO J. 18, 882–892.
Stack, M. S., S. Gately, L. M. Bafetti, J. Enghild, J. Soff and G. A. Soff (1999). Angiostatin inhibits endothelial and melanoma cellular invasion by blocking matrix-enhanced
plasminogen activation. Biochem J. 340, 77–84.
Modeling of Capillary Growth
863
Stokes, C. L. and D. A. Lauffenburger (1991). Analysis of the roles of microvessel endothelial cell random motility and chemotaxis in angiogenesis. J. Theor. Biol. 152, 377–403.
Takahashi, K., H. C. Kwaan, E. Koh and M. Tanabe (1992). Enzymatic properties of the
phosphorylated urokinase-type plasminogen activator isolated from a human carcinomatous cell line. Biochem. Biophys. Res. Commun. 182, 1473–1481.
Terman, B. I., M. Dougher-Vermazen, M. E. Carrion, D. Dimitrov, D. C. Armellino, D.
Gospodarowicz and P. Bohlen (1992). Identification of the KDR tyrosine kinase as a
receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun.
187, 1579–1586.
Terranova, V. P., R. DiFlorio, R. M. Lyall, S. Hic, R. Friesel and T. Maciag (1985). Endothelial cells are chemotactic to endothelial cell growth factor and heparin. J. Cell.
Biol. 101, 2330–2334.
Thews, G. (1960). Dei sauerstoffdiffusion im gehirn: Ein beitrag tur frage der Saurstoffversorung der organe. Plugers Arch. 271, 197–226.
Unemori, E. N., N. Ferrara, E. A. Bauer and E. P. Amento (1992). Vascular endothelial
growth factor induces interstitial collagenase expression in human endothelial cells. J.
Cell Physiol. 153, 557–562.
Waltenberger, J., L. Claesson-Welsh, A. Siegbahn, M. Shibuya and C.H. Heldin (1994).
Different signal transduction properties of KDR and Flt1, two receptors for vascular
endothelial cell growth factor. J. Biol. Chem. 269, 26988–26995.
Warren, B. A. (1970). The ultrastructure of the microcirculation at the advancing edge of
Walker256 carcinoma. Microvasc. Res. 2, 443–453.
Yamada, K. M. and K. Olden (1978). Fibronectins—adhesive glycoproteins of cell surface and blood. Nature 275, 179–184.
Zhou, Z., S. S. Apte, R. Soininen, R. Cao, G. Y. Baaklini, R. W. Rauser, J. Wang, Y.
Cao and K. Tryggvason (2000). Impaired endochondral ossification and angiogenesis
in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci.
U.S.A. 97, 4052–4057.
Received 30 October 2000 and accepted 11 March 2001