Review Dysfunctional or Hyperfunctional? The Amygdala in Posttraumatic Stress Disorder

Journal of Neuroscience Research 00:00–00 (2015)
Review
Dysfunctional or Hyperfunctional? The
Amygdala in Posttraumatic Stress Disorder
Is the Bull in the Evolutionary China Shop
David M. Diamond
1,2,3,4
* and Phillip R. Zoladz
5
1
Medical Research Service, Veterans Administration Hospital, Tampa, Florida
2
Department of Psychology, University of South Florida, Tampa, Florida
3
Department of Molecular Pharmacology and Physiology, University of South Florida, Tampa, Florida
4
Center for Preclinical and Clinical Research on PTSD, University of South Florida, Tampa, Florida
5
Department of Psychology, Sociology, and Criminal Justice, Ohio Northern University, Ada, Ohio
Our motivation in writing this Review arose not only from the great value in contributing to this special issue of the
Journal of Neuroscience Research but also from the desire to express our opinion that the description of the amygdala as “dysfunctional” in posttraumatic stress disorder (PTSD) might not be appropriate. We acknowledge that excessive activation of the amygdala contributes to the cluster of PTSD symptoms, including hypervigilance, intrusive memories, and impaired sleep, that underlies the devastating mental and physical outcomes in trauma victims. The issue that we address is whether the symptoms of PTSD represent an impaired (dysfunctional) or sensitized (hyperfunctional) amygdala status. We propose that the amygdala in PTSD is hyperfunctional rather than dysfunctional in recognition of the fact that the individual has already survived one life-threatening attack and that another may be forthcoming. We therefore consider
PTSD to be a state in which the amygdala is functioning optimally if the goal is to ensure a person’s survival. The misery caused by a hyperfunctional amygdala in PTSD is the cost of inheriting an evolutionarily primitive mechanism that considers survival more important than the quality of one’s life.
V
2015 Wiley Periodicals, Inc.
Key words: PTSD; amygdala; evolution; hippocampus; hyperfunctioning addressed here is whether the brain, specifically the amygdala, should be considered to be dysfunctional in a person who has developed PTSD.
In this Review, we address the issue of amygdala functioning in PTSD at two levels. First, we describe the consensus on the routine use of the descriptor
“dysfunctional” in terms of brain structure functioning. It is potentially of benefit to understand the distinction between a dysfunctional and a hyperfunctional state of amygdala activity in PTSD. Second, we speculate on the relevance of amygdala functioning in PTSD in terms of its evolutionary fitness. Our view is that, in people with
PTSD, the amygdala is so protective of the individual’s
SIGNIFICANCE:
Posttraumatic stress disorder (PTSD) is a unique psychiatric disorder in which a person has a horrific and potentially life-threatening experience that produces devastating mental and physical outcomes. The issue that we address is whether the symptoms in PTSD reflect an impaired (dysfunctional) amygdala or one that has become overly sensitized (hyperfunctional). Our opinion is that, in PTSD, evolutionarily primitive mechanisms have been activated to produce a hyperfunctional rather than a dysfunctional amygdala. We further suggest that a remittance of symptoms can be accomplished in a person with PTSD with therapy that returns the amygdala to its normative level of functioning.
The editors of this special issue of the Journal of Neuroscience Research have taken on an admirable task in addressing how amygdala dysfunction contributes to psychiatric conditions such as posttraumatic stress disorder (PTSD).
Although we applaud their efforts, we question whether the term “dysfunction” is an appropriate description of the status of amygdala functioning in PTSD. We recognize that PTSD is a condition that describes devastating mental and physical outcomes in trauma victims involving unrelenting symptoms that include hypervigilance, intrusive memories, and impaired sleep and cognition. The issue
Contract grant sponsor: Department of Veterans Affairs (to D.M.D.).
*Correspondence to: David M. Diamond, PhD, Department of Psychology, PCD 4118G, University of South Florida, Tampa, FL 33620.
E-mail: ddiamond@mail.usf.edu
Received 2 August 2015; Revised 26 September 2015; Accepted 11
October 2015
Published online 00 Month 2015 in Wiley Online Library
(wileyonlinelibrary.com). DOI: 10.1002/jnr.23684
2015 Wiley Periodicals, Inc.
2 Diamond and Zoladz survival against all potential threats that it acts in a hyperfunctional albeit self-destructive manner.
WHAT CONDITIONS DESCRIBE A
DYSFUNCTIONAL BRAIN STATE?
The first task is to define the term “dysfunctional” in the context of routinely described forms of abnormal physiology and health. A typical example of the use of the term dysfunction in a neurological context is “motor dysfunction,” in which impaired functioning of a brain motor area, as a result of a stroke or disease, interferes with an individual’s ability to make fine or gross movements, control tremors, or initiate voluntary movements
(Thobois et al., 2005; Cortes et al., 2012; Ross et al.,
2014; Finney, 2015). Another example of dysfunction in common usage is “cognitive dysfunction,” in which impairment of intellectual functions, including processes involved in strategizing, memory storage and recall, and reasoning, is of sufficient severity to interfere with aspects of cognition. Cognitive dysfunction describes various forms of dementia in which brain structures are damaged or diseased, such as in Alzheimer’s disease, aphasia, agnosia, and vascular dementia, all of which are recognized as failures of brain structures to accomplish their functions
(Monza et al., 1998; Lanzino et al., 2002; Chen et al.,
2013; Neha et al., 2014; Choi and Park, 2015; Schultz et al., 2015). One specific and widely used subset of cognitive dysfunction is “hippocampal dysfunction,” which describes memory impairments that develop as a result of adverse effects on the hippocampus caused by drugs, disease, damage, or stress (Schott, 2008; Roth et al., 2011;
Small et al., 2011; Acheson et al., 2012; Pitman et al.,
2012; Son et al., 2014; Lucchi et al., 2015; Pirnia et al.,
2015).
A stark contrast to conditions of cognitive dysfunction is hypermnesia, which is the enhancement of memory produced by repetition or emotion (Lanza, 1881;
Stratton, 1919; Roediger and Payne, 1982; Mulligan,
2005; Hurlemann, 2006; Otani et al., 2008), as well as hyperthymesia, which is the abnormal and extraordinary capacity of some individuals to exhibit great accuracy in the retrieval of their autobiographical memories (Parker et al., 2006; Leport et al., 2012; Ally et al., 2013; Patihis et al., 2013). In one case in which memory testing and brain imaging were conducted for a person with hyperthymesia, the only brain structure with increased volume was the amygdala (Ally et al., 2013). Moreover, in the subject with hyperthymesia, the amygdala displayed significantly greater connectivity with the hippocampus than was found in controls. The authors conjectured that, in this individual, the amygdala “ . . .
is hyperactive, resulting in emotionally benign information being processed in a self-relevant affective manner.” Thus, evidence of an abnormally strong memory and a greater linkage between the amygdala and the hippocampus was interpreted as a form of amygdala hyperfunction that produced extraordinarily accurate retrieval of autobiographical memories.
It is worth noting that enhanced memory processing by individuals with hypermnesia or hyperthymesia has never been characterized as a form of dysfunctional brain processing. Across studies, evidence of greater than normal functioning of a brain structure is routinely referred to as a hyperactive or hyperfunctional state. By contrast, the term “dysfunctional” is routinely used to describe a condition or disease in which a brain structure fails to perform its function.
WHAT IS AMYGDALA FUNCTION AND
DYSFUNCTION?
We can now summarize the normative function of the amygdala within the context of the routinely used characterizations of hyperfunction and dysfunction described previously. Decades of research with humans and animals have thoroughly described amygdala functioning under control (i.e., imaging) and experimental (i.e., localized damage) conditions as well as in brain disease states. In general, the amygdala is described as being an essential brain structure for the formation of emotional memories, particularly in the processing of fear-provoking experiences (Fanselow and Gale, 2003; Rauch et al., 2003;
McGaugh, 2004; Phelps and LeDoux, 2005; Sigurdsson et al., 2007; Kim et al., 2011; Pare and Duvarci, 2012).
More broadly, the amygdala is a central component of a brain pathway devoted to predator-based (instinctual, lifethreatening) responses and conditioned fear (LeDoux,
1998; Rosen, 2004; Rosen et al., 2008; Canteras et al.,
2012). Thus, the amygdala is critically involved in different forms of instinctual and learned fear and in modulating attention to fear-related stimuli as well as fear recognition and perception. Although the scope of information that is processed by the amygdala is not limited to fear-provoking cues (Burgdorf and Panksepp, 2006; Berridge et al., 2010; Pessoa, 2010; Markowitsch and Staniloiu, 2011; Chau and Galvez, 2012; Fernando et al.,
2013; Hermans et al., 2014), extensive research suggests that fear-provoking stimuli are prominently featured in the domain of amygdala functioning (Phillips et al., 1998;
Phelps and LeDoux, 2005; Johansen et al., 2012).
In addition to research on fear conditioning, primarily in rodents with amygdala damage or inactivation (Fanselow and Gale, 2003; Maren, 2008; Chau and Galvez,
2012; Johansen et al., 2012; Hermans et al., 2014), naturalistic disease conditions have provided insight into the consequences of a failure of the amygdala to function properly (i.e., dysfunction). Research on people with
Urbach-Wiethe disease, in which there is a selective atrophy of the amygdala, has provided strong evidence that the loss of amygdala functioning leads to impaired fear responses and emotional memory processing (Markowitsch et al., 1994; Cahill et al., 1995; Hurlemann et al.,
2007; Klumpers et al., 2014). It is notable that emotion per se can be experienced by individuals with atrophy of their amygdala (Markowitsch and Staniloiu, 2011).
Indeed, people with Urbach-Wiethe disease can express enjoyment and even a thrill at experiencing stimuli that
Journal of Neuroscience Research
Amygdala: Dysfunctional or Hyperfunctional in PTSD?
3 would be viewed as terrifying by amygdala-intact people
(Feinstein et al., 2011). For example, the person with
Urbach-Wiethe disease described by Feinstein et al.
(2011) was so fearless that she put herself in harm’s way by approaching live venomous snakes and spiders. Therefore, we suggest that a core feature of amygdala functioning is to activate a fear-based sensory and cognitive system that enhances one’s survival by hindering an individual from engaging in unnecessary exposure to life-threatening stimuli.
In a very different but complementary approach, researchers examined the effects of Toxoplasma gondii on the brain, with an emphasis on assessing amygdala functioning and behavior in infected rats and people (Berenreiterova et al., 2011; Mitra et al., 2013; Hari Dass and
Vyas, 2014). This protozoan parasite forms cysts in the brains of warm-blooded animals, including rodents and humans (Innes, 2010). Extensive research has demonstrated that rodents infected with T. gondii show a reduction in their innate aversion of cats and their odors.
Moreover, T. gondii -induced changes in behavior appear to be specific to the loss of fear of a predator, such as a cat, because fear responses and memory for nonpredator forms of aversive cues (e.g., paw shock) remain intact
(Vyas et al., 2007). It is intriguing that T. gondii not only suppresses predator-based fear responses but also appears to increase the appeal of the predator to the rat (Berdoy et al., 2000; House et al., 2011). The basis of the shift from fear expressed in response to a predator to an appetitive (i.e., sexual) response by a rat to a cat appears to involve the targeting of the amygdala by T. gondii (Berenreiterova et al., 2011; House et al., 2011; Mitra et al.,
2013; Hari Dass and Vyas, 2014). Thus, there is strong evidence that T. gondii produces a dysfunctional response in the amygdala that is manifested as an increase in the likelihood that a rat will be killed by a cat.
The evolutionary basis for the “rewiring” of the brains of infected hosts is that T. gondii has a life cycle that includes reproducing asexually within any warm-blooded animal, but it must return to the digestive system of the cat to undergo sexual reproduction (Carruthers and
Suzuki, 2007; Flegr and Markos, 2014). Infection from the cat is transmitted to a host (e.g., a rodent or human) that ingests cysts in undercooked meat or in oocysts in cat feces, which results in the formation of cysts in the brain
(and other organs) of the host. Hence, when T. gondii reduces a rat’s fear of a cat, it increases rat predation rates, thereby facilitating the completion of the life cycle of T.
gondii because the cysts in the rat tissue return to the digestive system of the cat (Carruthers and Suzuki, 2007;
Innes, 2010; Flegr and Markos, 2014).
There is strong evidence that the effects of T. gondii that have been described in rats are present in infected humans as well. In perhaps the clearest translational evidence of T. gondii infection effects on behavior and brain,
Flegr et al. (2011) reported that infected men found the odor of cat urine significantly more pleasant than uninfected men. Related work has shown that people who test positive for T. gondii in serological assays exhibit personality characteristics different from noninfected individuals, including higher reactive aggression and impulsive sensation seeking (Carruthers and Suzuki, 2007;
Cook et al., 2015). In a real-life assay of the consequences of T. gondii infection on human behavior, numerous studies have shown that people who have been infected with
T. gondii are as much as six times more likely to have had a traffic accident than those who were uninfected (Flegr et al., 2002, 2009; Yereli et al., 2006; Galvan-Ramirez et al., 2013). One implication of this finding is that T.
gondii infection increases a person’s recklessness or at least the ability to respond efficiently in a life-threatening situation. Overall, the T. gondii findings from rodent and human subjects support the hypothesis that amygdala dysfunction (i.e., failure to respond appropriately in a lifethreatening situation) results in behaviors that put an individual in greater peril of loss of life.
The three examples of amygdala dysfunction described here (i.e., damage or suppression of amygdala activity in animal fear conditioning research, Urbach-
Wiethe disease, and infection by T. gondii ) are all comparable in terms of the consequences of their effects on cognitive and behavioral responses to life-threatening stimuli.
In all three cases, affected individuals exhibit insufficient amygdala activation in conjunction with reduced innate
(and life-protective) fear responses as well as impaired memory for these experiences. Thus, one component of the normative functioning of the amygdala can be seen as exhibiting a level of activity that optimizes the interplay between curiosity and exploratory behavior to obtain appetitive reward (e.g., foraging for food) and the avoidance of situations that can potentially cause harm and the necessary maintenance of a memory trace for the experience. The optimal functioning of the amygdala in this balance between curiosity and fear has been summarized by Choi and Kim (2010), who postulated that the amygdala “ . . .
regulates predation risk–foraging behavior in a dynamic fear environment. Without the amygdala and consequently devoid of fear, the animal’s foraging behavior becomes perilously maladaptive.”
It is also important to note in this context that some people diagnosed with PTSD appear to exhibit insufficient activation (perhaps even an active suppression) of the amygdala at the time of trauma as well as during symptom expression. Specifically, some traumatized people are impaired at managing their emotional control in response to trauma, exhibiting alternating periods of hyperarousal with emotional numbness, which, in its extreme form, is referred to as “dissociation” (Feeny et al., 2000; Frewen and Lanius, 2006). The dissociative subtype of PTSD (American Psychiatric Association,
2013) involves the fragmentation of consciousness, memory, identity, body awareness, and perception and is characterized by blunted emotional and physiological responses to trauma-related stimuli (Lanius et al., 2010;
Wolf et al., 2012; Armour et al., 2014b). Whereas subjects with the hyperarousal subtype of PTSD tend to exhibit increased amygdala activity (Rauch et al., 2000,
2003; Pissiota et al., 2002; Shin et al., 2006; Pitman et al.,
Journal of Neuroscience Research
4 Diamond and Zoladz
2012; Cisler et al., 2015), imaging studies have shown an absence of increased amygdala activity and blunted autonomic activity in people with the dissociative subtype of
PTSD (Lanius et al., 2001, 2002, 2003, 2005). Nevertheless, individuals with the dissociative subtype of PTSD exhibit exaggerated features of amygdala hyperactivity, including heightened symptoms of depression, hostility, and sleeping difficulties (Armour et al., 2014a).
The dissociative subtype of PTSD has been theorized to involve the excessive activation (overmodulation) by the prefrontal cortex to inhibit transiently the amygdala’s response to traumatic stress and its reminders (Hopper et al., 2007; Lanius et al., 2010). The observation of an overly inhibited amygdala during emotional numbing or dissociation is consistent with the hypothesis that traumainduced psychopathology is produced by an imbalance between amygdala and frontal corticex activity rather than pathology residing solely in the amygdala.
CONSEQUENCES OF A HYPERFUNCTIONAL
AMYGDALA IN PTSD
PTSD is commonly induced by a horrific, lifethreatening experience (Hou et al., 2005; Bar-Haim et al., 2010; Wald et al., 2011; Edmondson, 2014). The amygdala bears much of the responsibility for ensuring that an individual survives the life-threatening attack as well as preparing for future attacks. Therefore, in those individuals who develop PTSD, amygdala functioning becomes maximally activated (sensitized) to increase the likelihood of surviving future threats. The amygdala accomplishes this task with two different strategies that we will discuss in turn.
First, as we have discussed previously, the amygdala orchestrates the formation of memories of fear-provoking experiences. Thus, plasticity intrinsic to the amygdala
(Sears et al., 2014) as well as amygdala-mediated enhancement of memory-related plasticity in other brain structures (Roozendaal and McGaugh, 2011) generates memories of life-threatening experiences that can last for a lifetime. The great value of having a structure that provides a readily accessible reference system for lifethreatening events with a rapid reactivation in case of reexposure to related cues is obvious; one’s future survival, even decades later, may depend on the rapid recognition of cues that have been associated with a prior lifethreatening event.
An adverse consequence of a hyperactive amygdala that overconsolidates the trauma memory is a pathologically intense and intrusive memory. It is notable that there are features of the intrusive memory in common with the memories described by individuals with hyperthymesia.
Hyperthymesia is ordinarily considered to be a beneficial state of amygdala (and hippocampus) hyperfunctioning because the memories that are probed are typically neutral or positive (Parker et al., 2006; Leport et al., 2012; Ally et al., 2013). We suggest, therefore, that enhanced autobiographical memories in nontraumatized and traumatized people share the common feature that they both involve a hyperfunctional state of the amygdala that forms an abnormally strong linkage to the hippocampus (Diamond et al., 2007).
It is important to point out that, in PTSD, the memories are intrusive, pathologically intense, and typically composed of fragmented sensory features of the ordeal that may be accompanied by impaired retrieval of key aspects of the trauma experience (Van der Kolk and
Van der Hart, 1991; Van der Kolk, 1994; Brewin and
Holmes, 2003; Diamond et al., 2007; for discussion of the amnesia issue see Berntsen and Rubin, 2014). In previous work, we theorized that the fragmented features of traumatic memories are based on a hyperactive state of the amygdala in conjunction with brief but intense synaptic plasticity in the hippocampus during the traumatic experience (Diamond et al., 2007). We proposed that the hyperconsolidation of fragments of the memory by the hippocampus and amygdala focusing primarily on isolated cue-based features of traumatic experiences (Huff and
Rudy, 2004; Marschner et al., 2008; Lipka et al., 2011) would explain why avoidance of all cues associated with the trauma and the panic that develop from trauma reminders is a hallmark feature of PTSD symptoms.
Related research on memory and PTSD has demonstrated that the dreams in people with PTSD are different from dreams in nontraumatized people. Ordinarily, dreams are in the form of stories that are not accurate portrayals of daily events. That is, neutral episodic memories
“are almost never replayed veridically in dreams,” but, in those with PTSD, “traumatic episodic memories are repetitively (and accurately) replayed in sleep” (Stickgold,
2002). van der Kolk et al. (1984) provided similar observations of people with PTSD, noting that their dreams were aberrant and disturbing because they were exact replicas of the actual (traumatic) events. Thus, under nontrauma conditions, dreams appear to integrate episodic experiences into a more global categorical (semantic, neocortical) network. In the process of categorizing information, however, the accuracy of the episodic experience can be compromised (Hardt et al., 2013; Lane et al.,
2015). In theory, the hyperfunctioning amygdala in
PTSD interferes with the semantic categorization of the trauma memory, even in sleep, to maximize the veracity of the memory of a life-threatening experience.
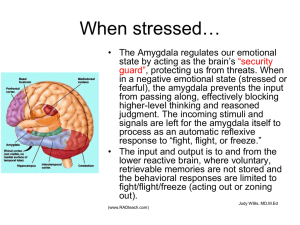
Second, the amygdala is intimately connected with cortical and subcortical structures that allow it to be sensitive to sensory cues as well as to cognitive features of fear to activate the fight, flight, or freeze (FFF) response
(Mobbs et al., 2015). The FFF response is evolutionarily adapted to optimize survival in advance of or in response to an attack. There are multiple levels of the FFF response that are relevant to amygdala functioning and, ultimately, to PTSD. Activation of the amygdala in response to a real or potential threat enhances long-term memory processing (discussed above) and, furthermore, produces a rapid mobilization of energy, including an increase in blood glucose and cardiovascular activity (increased heart rate and blood pressure; Stock et al., 1981; Seto et al., 1983; al
Maskati and Zbrozyna, 1989; Ohta et al., 1991) and an
Journal of Neuroscience Research
Amygdala: Dysfunctional or Hyperfunctional in PTSD?
5 increase in platelet aggregation and immune activation
(Shandra and Glukhov, 1992; Von Kanel et al., 2001;
Raison and Miller, 2003). All of these stress responses are adaptive from an evolutionary perspective because they allow an animal under attack to mobilize the necessary cognitive and physiological resources to respond to the threat as well as to prepare for hemorrhage, tissue damage, and pathogen invasion in case a wound is inflicted (Sapolsky, 1994).
These adaptive responses to trauma, which include enhanced attention, sensory, and cognitive processing in conjunction with activation of the FFF response, have great adaptive value if one truly is at risk for a lifethreatening attack at any moment. In PTSD, however, the repeated unbidden activation of intrusive memories and the FFF response underlies brain and somatic pathology, including amygdala hypertrophy and impaired hippocampal and prefrontal cortex functioning (McEwen,
2007; Zoladz and Diamond, 2013) as well as an increased incidence of cardiovascular, metabolic, and immunological disorders (Altemus et al., 2006; Violanti et al., 2006;
Boscarino, 2008; Gill et al., 2009; Brudey et al., 2015;
Williamson et al., 2015). Thus, amygdala-mediated heightened vigilance in PTSD maximizes the likelihood that a traumatized person will not be caught off guard by a predator; however, the loss of sleep, intrusive memories, traumatic nightmares, impaired cognition, and increased susceptibility to a multitude of diseases are devastating to quality of life. The misery caused by a hyperfunctional amygdala in PTSD is the cost of inheriting an evolutionarily primitive mechanism that considers survival more important than the quality of one’s life.
CONCLUSION: THE AMYGDALA IN
PTSD IS A BULL IN THE EVOLUTIONARY
CHINA SHOP
We consider the metaphor of the bull in a china shop to be of heuristic value in understanding the hyperfunctional status of the amygdala in PTSD. A raging bull confined within a china shop can cause immense destruction as it attempts to escape, but we do not consider the bull or its behavior to be dysfunctional. The collateral damage that the bull causes is incidental to its attempt to achieve the primary goal of escaping from a threatening environment.
Similarly, we propose that the amygdala in PTSD is excessively protective (i.e., hyperfunctional) in recognition of the fact that the individual has already survived one life-threatening attack and another may be forthcoming. From the amygdala’s perspective, a state of hypervigilance is justified because the traumatized person appears to be living in an unpredictable, hostile, and threatening world.
Just as a raging bull wreaking havoc in a china shop is acting in an adaptive manner, we consider that the amygdala and overall brain function in PTSD are responding to trauma in an evolutionarily adaptive manner as well. What is abnormal in PTSD are intrusive, pathologically intense, trauma memories acting on a
Journal of Neuroscience Research background of insufficient control from the prefrontal cortex and parasympathetic nervous system over a hyperactive amygdala and sympathetic nervous system (Hamner et al., 1999; Semple et al., 2000; Shin et al., 2006; Morris and Rao, 2013; Zoladz and Diamond, 2013). In theory, recovery of normative brain function may be accomplished in a person with PTSD in response to therapy that restores an appropriate level of prefrontal cortex control over amygdala hyperactivity (Levin et al., 1999; Osuch et al., 2009; Novakovic et al., 2011; King et al., 2013;
Kip et al., 2013a,b; Bormann et al., 2014; Marin et al.,
2014). Successful therapy for PTSD, therefore, is analogous to calming the bull in the china shop and bringing it to an environment it perceives as safe, thereby allowing balance among brain structures and hormonal systems to be restored.
ACKNOWLEDGMENTS
The views expressed in this paper are those of the authors and not of the Veterans Administration or the U.S.
government.
REFERENCES
Acheson DT, Gresack JE, Risbrough VB. 2012. Hippocampal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology 62:674–685.
al Maskati HA, Zbrozyna AW. 1989. Cardiovascular and motor components of the defence reaction elicited in rats by electrical and chemical stimulation in amygdala. J Auton Nerv Syst 28:127–131.
Ally BA, Hussey EP, Donahue MJ. 2013. A case of hyperthymesia: rethinking the role of the amygdala in autobiographical memory. Neurocase 19:166–181.
Altemus M, Dhabhar FS, Yang R. 2006. Immune function in PTSD.
Ann N Y Acad Sci 1071:167–183.
American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders.
Washington, DC: American Psychiatric
Association.
Armour C, Elklit A, Lauterbach D, Elhai JD. 2014a. The DSM-5 dissociative-PTSD subtype: can levels of depression, anxiety, hostility, and sleeping difficulties differentiate between dissociative-PTSD and PTSD in rape and sexual assault victims? J Anxiety Disord 28:418–426.
Armour C, Karstoft KI, Richardson JD. 2014b. The co-occurrence of
PTSD and dissociation: differentiating severe PTSD from dissociative-
PTSD. Soc Psychiatry Psychiatr Epidemiol 49:1297–1306.
Bar-Haim Y, Holoshitz Y, Eldar S, Frenkel TI, Muller D, Charney DS,
Pine DS, Fox NA, Wald I. 2010. Life-threatening danger and suppression of attention bias to threat. Am J Psychiatry 167:694–698.
Berdoy M, Webster JP, Macdonald DW. 2000. Fatal attraction in rats infected with Toxoplasma gondii . Proc Biol Sci 267:1591–1594.
Berenreiterova M, Flegr J, Kubena AA, Nemec P. 2011. The distribution of Toxoplasma gondii cysts in the brain of a mouse with latent toxoplasmosis: implications for the behavioral manipulation hypothesis. PLoS
One 6:e28925.
Berntsen D, Rubin DC. 2014. Involuntary memories and dissociative amnesia: assessing key assumptions in PTSD research. Clin Psychol Sci
2:174–186.
Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. 2010. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 1350:43–64.
Bormann JE, Oman D, Walter KH, Johnson BD. 2014. Mindful attention increases and mediates psychological outcomes following mantram
6 Diamond and Zoladz repetition practice in veterans with posttraumatic stress disorder. Med
Care 52:S13–S18.
Boscarino JA. 2008. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med 70:668–676.
Brewin CR, Holmes EA. 2003. Psychological theories of posttraumatic stress disorder. Clin Psychol Rev 23:339–376.
Brudey C, Park J, Wiaderkiewicz J, Kobayashi I, Mellman TA, Marvar
PJ. 2015. Autonomic and inflammatory consequences of posttraumatic stress disorder (PTSD) and the link to cardiovascular disease. Am J
Physiol Regul Integr Comp Physiol 309:R315–R321.
Burgdorf J, Panksepp J. 2006. The neurobiology of positive emotions.
Neurosci Biobehav Rev 30:173–187.
Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. 1995. The amygdala and emotional memory [letter]. Nature 377:295–296.
Canteras NS, Mota-Ortiz SR, Motta SC. 2012. What ethologically based models have taught us about the neural systems underlying fear and anxiety. Braz J Med Biol Res 45:321–327.
Carruthers VB, Suzuki Y. 2007. Effects of Toxoplasma gondii infection on the brain. Schizophr Bull 33:745–751.
Chau LS, Galvez R. 2012. Amygdala’s involvement in facilitating associative learning-induced plasticity: a promiscuous role for the amygdala in memory acquisition. Front Integr Neurosci 6:92.
Chen YF, Li YF, Chen X, Sun QF. 2013. Neuropsychiatric disorders and cognitive dysfunction in patients with Cushing’s disease. Chin Med
J 126:3156–3160.
Choi JS, Kim JJ. 2010. Amygdala regulates risk of predation in rats foraging in a dynamic fear environment. Proc Natl Acad Sci U S A 107:
21773–21777.
Choi SH, Park MH. 2015. Three screening methods for cognitive dysfunction using the Mini-Mental State Examination and Korean Dementia
Screening Questionnaire. Geriatr Gerontol Int [E-pub ahead of print].
Cisler JM, Bush K, James GA, Smitherman S, Kilts CD. 2015. Decoding the traumatic memory among women with PTSD: implications for neurocircuitry models of PTSD and real-time fMRI neurofeedback.
PLoS One 10:e0134717.
Cook TB, Brenner LA, Cloninger CR, Langenberg P, Igbide A,
Giegling I, Hartmann AM, Konte B, Friedl M, Brundin L, Groer MW,
Can A, Rujescu D, Postolache TT. 2015. “Latent” infection with Toxoplasma gondii : association with trait aggression and impulsivity in healthy adults. J Psychiatr Res 60:87–94.
Cortes M, Black-Schaffer RM, Edwards DJ. 2012. Transcranial magnetic stimulation as an investigative tool for motor dysfunction and recovery in stroke: an overview for neurorehabilitation clinicians. Neuromodulation 15:316–325.
Diamond DM, Campbell AM, Park CR, Halonen J, Zoladz PR. 2007.
The temporal dynamics model of emotional memory processing: a synthesis on the neurobiological basis of stress-induced amnesia, flashbulb and traumatic memories, and the Yerkes-Dodson law. Neural Plast
2007:60803.
Edmondson D. 2014. An enduring somatic threat model of posttraumatic stress disorder due to acute life-threatening medical events. Soc Personal
Psychol Compass 8:118–134.
Fanselow MS, Gale GD. 2003. The amygdala, fear, and memory. Ann N
Y Acad Sci 985:125–134.
Feeny NC, Zoellner LA, Fitzgibbons LA, Foa EB. 2000. Exploring the roles of emotional numbing, depression, and dissociation in PTSD.
J Trauma Stress 13:489–498.
Feinstein JS, Adolphs R, Damasio A, Tranel D. 2011. The human amygdala and the induction and experience of fear. Curr Biol 21:34–38.
Fernando AB, Murray JE, Milton AL. 2013. The amygdala: securing pleasure and avoiding pain. Front Behav Neurosci 7:190.
Finney GR. 2015. Perceptual-motor dysfunction. Continuum 21:678–
689.
Flegr J, Markos A. 2014. Masterpiece of epigenetic engineering: how
Toxoplasma gondii reprogrammes host brains to change fear to sexual attraction. Mol Ecol 23:5934–5936.
Flegr J, Havlicek J, Kodym P, Maly M, Smahel Z. 2002. Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study. BMC Infect Dis 2:11.
Flegr J, Klose J, Novotna M, Berenreitterova M, Havlicek J. 2009.
Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis 9:72.
Flegr J, Lenochova P, Hodny Z, Vondrova M. 2011. Fatal attraction phenomenon in humans: cat odour attractiveness increased for toxoplasma-infected men while decreased for infected women. PLoS
Negl Trop Dis 5:e1389.
Frewen PA, Lanius RA. 2006. Toward a psychobiology of posttraumatic self-dysregulation: reexperiencing, hyperarousal, dissociation, and emotional numbing. Ann N Y Acad Sci 1071:110–124.
Galvan-Ramirez ML, Sanchez-Orozco LV, Rodriguez LR, Rodriguez S,
Roig-Melo E, Troyo SR, Chiquete E, Armendariz-Borunda J. 2013.
Seroepidemiology of Toxoplasma gondii infection in drivers involved in road traffic accidents in the metropolitan area of Guadalajara, Jalisco,
Mexico. Parasit Vectors 6:294.
Gill JM, Saligan L, Woods S, Page G. 2009. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care 45:
262–277.
Hamner MB, Lorberbaum JP, George MS. 1999. Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress
Anxiety 9:1–14.
Hardt O, Nader K, Nadel L. 2013. Decay happens: the role of active forgetting in memory. Trends Cogn Sci 17:111–120.
Hari Dass SA, Vyas A. 2014.
Toxoplasma gondii infection reduces predator aversion in rats through epigenetic modulation in the host medial amygdala. Mol Ecol 23:6114–6122.
Hermans EJ, Battaglia FP, Atsak P, de Voogd LD, Fernandez G,
Roozendaal B. 2014. How the amygdala affects emotional memory by altering brain network properties. Neurobiol Learn Mem 112:2–16.
Hopper JW, Frewen PA, Van der Kolk BA, Lanius RA. 2007. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to scriptdriven trauma imagery. J Trauma Stress 20:713–725.
Hou WL, Wang HH, Chung HH. 2005. Domestic violence against women in Taiwan: their life-threatening situations, posttraumatic responses, and psychophysiological symptoms: an interview study. Int J
Nurs Stud 42:629–636.
House PK, Vyas A, Sapolsky R. 2011. Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PLoS One 6:e23277.
Huff NC, Rudy JW. 2004. The amygdala modulates hippocampusdependent context memory formation and stores cue-shock associations.
Behav Neurosci 118:53–62.
Hurlemann R. 2006. Noradrenergic control of emotion-induced amnesia and hypermnesia. Rev Neurosci 17:525–532.
Hurlemann R, Wagner M, Hawellek B, Reich H, Pieperhoff P, Amunts
K, Oros-Peusquens AM, Shah NJ, Maier W, Dolan RJ. 2007. Amygdala control of emotion-induced forgetting and remembering: evidence from Urbach-Wiethe disease. Neuropsychologia 45:877–884.
Innes EA. 2010. A brief history and overview of Toxoplasma gondii . Zoonoses Public Health 57:1–7.
Johansen JP, Wolff SB, Luthi A, LeDoux JE. 2012. Controlling the elements: an optogenetic approach to understanding the neural circuits of fear. Biol Psychiatry 71:1053–1060.
Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante
AN, Whalen PJ. 2011. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav
Brain Res 223:403–410.
Journal of Neuroscience Research
Amygdala: Dysfunctional or Hyperfunctional in PTSD?
7
King AP, Erickson TM, Giardino ND, Favorite T, Rauch SA, Robinson
E, Kulkarni M, Liberzon I. 2013. A pilot study of group mindfulnessbased cognitive therapy (MBCT) for combat veterans with posttraumatic stress disorder (PTSD). Depress Anxiety 30:638–645.
Kip KE, Rosenzweig L, Hernandez DF, Shuman A, Sullivan KL, Long
CJ, Taylor J, Mcghee S, Girling SA, Wittenberg T, Sahebzamani FM,
Lengacher CA, Kadel R, Diamond DM. 2013a. Randomized controlled trial of accelerated resolution therapy (ART) for symptoms of combat-related posttraumatic stress disorder (PTSD). Mil Med 178:
1298–1309.
Kip KE, Sullivan KL, Lengacher CA, Rosenzweig L, Hernandez DF,
Kadel R, Kozel FA, Shuman A, Girling SA, Hardwick MJ, Diamond
DM. 2013b. Brief treatment of co-occurring posttraumatic stress and depressive symptoms by use of accelerated resolution therapy. Front
Psychiatry 4:11.
Klumpers F, Morgan B, Terburg D, Stein DJ, van Honk J. 2014.
Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Soc
Cogn Affect Neurosci 10:1161–1168.
Lane RD, Ryan L, Nadel L, Greenberg L. 2015. Memory reconsolidation, emotional arousal, and the process of change in psychotherapy: new insights from brain science. Behav Brain Sci 38:e1.
Lanius RA, Williamson PC, Densmore M, Boksman K, Gupta MA,
Neufeld RW, Gati JS, Menon RS. 2001. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry 158:1920–1922.
Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M,
Neufeld RW, Gati JS, Menon RS. 2002. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry 52:
305–311.
Lanius RA, Hopper JW, Menon RS. 2003. Individual differences in a husband and wife who developed PTSD after a motor vehicle accident: a functional MRI case study. Am J Psychiatry 160:667–669.
Lanius RA, Williamson PC, Bluhm RL, Densmore M, Boksman K,
Neufeld RW, Gati JS, Menon RS. 2005. Functional connectivity of dissociative responses in posttraumatic stress disorder: a functional magnetic resonance imaging investigation. Biol Psychiatry 57:873–884.
Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C,
Bremner JD, Spiegel D. 2010. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatry
167:640–647.
Lanza C. 1881. Hypermnesia or exaltations of memory. Science 2:450–453.
Lanzino G, Lanzino DJ, Wang D. 2002. Cerebrovascular disease and cognitive dysfunction. Neurol Res 24:331–336.
LeDoux J. 1998. Fear and the brain: where have we been, and where are we going? Biol Psychiatry 44:1229–1238.
Leport AK, Mattfeld AT, Dickinson-Anson H, Fallon JH, Stark CE,
Kruggel F, Cahill L, McGaugh JL. 2012. Behavioral and neuroanatomical investigation of highly superior autobiographical memory (HSAM).
Neurobiol Learn Mem 98:78–92.
Levin P, Lazrove S, van der KB. 1999. What psychological testing and neuroimaging tell us about the treatment of posttraumatic stress disorder by eye movement desensitization and reprocessing. J Anxiety Disord
13:159–172.
Lipka J, Miltner WH, Straube T. 2011. Vigilance for threat interacts with amygdala responses to subliminal threat cues in specific phobia.
Biol Psychiatry 70:472–478.
Lucchi C, Vinet J, Meletti S, Biagini G. 2015. Ischemic–hypoxic mechanisms leading to hippocampal dysfunction as a consequence of status epilepticus. Epilepsy Behav 49:47–54.
Maren S. 2008. Pavlovian fear conditioning as a behavioral assay for hippocampus and amygdala function: cautions and caveats. Eur J Neurosci
28:1661–1666.
Marin MF, Camprodon JA, Dougherty DD, Milad MR. 2014. Devicebased brain stimulation to augment fear extinction: implications for
PTSD treatment and beyond. Depress Anxiety 31:269–278.
Markowitsch HJ, Staniloiu A. 2011. Amygdala in action: relaying biological and social significance to autobiographical memory. Neuropsychologia 49:718–733.
Markowitsch HJ, Calabrese P, Wurker M, Durwen HF, Kessler J,
Babinsky R, Brechtelsbauer D, Heuser L, Gehlen W. 1994. The amygdala’s contribution to memory—a study on two patients with Urbach-
Wiethe disease. Neuroreport 5:1349–1352.
Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, Buchel C. 2008.
Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. J Neurosci 28:9030–9036.
McEwen BS. 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 87:873–904.
McGaugh JL. 2004. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28.
Mitra R, Sapolsky RM, Vyas A. 2013.
Toxoplasma gondii infection induces dendritic retraction in basolateral amygdala accompanied by reduced corticosterone secretion. Dis Model Mech 6:516–520.
Mobbs D, Hagan CC, Dalgleish T, Silston B, Prevost C. 2015. The ecology of human fear: survival optimization and the nervous system.
Front Neurosci 9:55.
Monza D, Soliveri P, Radice D, Fetoni V, Testa D, Caffarra P, Caraceni
T, Girotti F. 1998. Cognitive dysfunction and impaired organization of complex motility in degenerative parkinsonian syndromes. Arch Neurol
55:372–378.
Morris MC, Rao U. 2013. Psychobiology of PTSD in the acute aftermath of trauma: integrating research on coping, HPA function, and sympathetic nervous system activity. Asian J Psychiatr 6:3–21.
Mulligan NW. 2005. Total retrieval time and hypermnesia: investigating the benefits of multiple recall tests. Psychol Res 69:272–284.
Neha Sodhi RK, Jaggi AS, Singh N. 2014. Animal models of dementia and cognitive dysfunction. Life Sci 109:73–86.
Novakovic V, Sher L, Lapidus KA, Mindes J, Golier A, Yehuda R.
2011. Brain stimulation in posttraumatic stress disorder. Eur J Psychotraumatol 2.
Ohta H, Watanabe S, Ueki S. 1991. Cardiovascular changes induced by chemical stimulation of the amygdala in rats. Brain Res Bull 26:575–
581.
Osuch EA, Benson BE, Luckenbaugh DA, Geraci M, Post RM,
McCann U. 2009. Repetitive TMS combined with exposure therapy for PTSD: a preliminary study. J Anxiety Disord 23:54–59.
Otani H, Kato K, Von Glahn NR, Nelson ME, Widner RL Jr, Goernert
PN. 2008. Hypermnesia: a further examination of age differences between young and older adults. Br J Psychol 99:265–278.
Pare D, Duvarci S. 2012. Amygdala microcircuits mediating fear expression and extinction. Curr Opin Neurobiol 22:717–723.
Parker ES, Cahill L, McGaugh JL. 2006. A case of unusual autobiographical remembering. Neurocase 12:35–49.
Patihis L, Frenda SJ, Leport AK, Petersen N, Nichols RM, Stark CE,
McGaugh JL, Loftus EF. 2013. False memories in highly superior autobiographical memory individuals. Proc Natl Acad Sci U S A 110:
20947–20952.
Pessoa L. 2010. Emotion and cognition and the amygdala: from “what is it?” to “what’s to be done?” Neuropsychologia 48:3416–3429.
Phelps EA, LeDoux JE. 2005. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48:175–187.
Phillips ML, Young AW, Scott SK, Calder AJ, Andrew C, Giampietro
V, Williams SC, Bullmore ET, Brammer M, Gray JA. 1998. Neural responses to facial and vocal expressions of fear and disgust. Proc Biol
Sci 265:1809–1817.
Pirnia T, Woods RP, Hamilton LS, Lyden H, Joshi SH, Asarnow RF,
Nuechterlein KH, Narr KL. 2015. Hippocampal dysfunction during
Journal of Neuroscience Research
8 Diamond and Zoladz declarative memory encoding in schizophrenia and effects of genetic liability. Schizophr Res 161:357–366.
Pissiota A, Frans O, Fernandez M, von Knorring L, Fischer H,
Fredrikson M. 2002. Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin
Neurosci 252:68–75.
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson
MW, Milad MR, Liberzon I. 2012. Biological studies of posttraumatic stress disorder. Nat Rev Neurosci 13:769–787.
Raison CL, Miller AH. 2003. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stressrelated disorders. Am J Psychiatry 160:1554–1565.
Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko
NB, Orr SP, Pitman RK. 2000. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47:769–776.
Rauch SL, Shin LM, Wright CI. 2003. Neuroimaging studies of amygdala function in anxiety disorders. Ann N Y Acad Sci 985:389–410.
Roediger HL, Payne DG. 1982. Hypermnesia: the role of repeated testing. J Exp Psychol Learn Mem Cogn 8:66–72.
Roozendaal B, McGaugh JL. 2011. Memory modulation. Behav Neurosci 125:797–824.
Rosen JB. 2004. The neurobiology of conditioned and unconditioned fear: a neurobehavioral system analysis of the amygdala. Behav Cogn
Neurosci Rev 3:23–41.
Rosen JB, Pagani JH, Rolla KL, Davis C. 2008. Analysis of behavioral constraints and the neuroanatomy of fear to the predator odor trimethylthiazoline: a model for animal phobias. Neurosci Biobehav Rev 32:
1267–1276.
Ross CA, Pantelyat A, Kogan J, Brandt J. 2014. Determinants of functional disability in Huntington’s disease: role of cognitive and motor dysfunction. Mov Disord 29:1351–1358.
Roth TL, Zoladz PR, Sweatt JD, Diamond DM. 2011. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of posttraumatic stress disorder. J Psychiatr Res 45:919–926.
Sapolsky RM. 1994. Why zebras don’t get ulcers. New York: St. Martin’s Press.
Schott JM. 2008. Ischemic bilateral hippocampal dysfunction during transient global amnesia. Neurology 70:1940–1941.
Schultz SA, Oh JM, Koscik RL, Dowling NM, Gallagher CL, Carlsson
CM, Bendlin BB, LaRue A, Hermann BP, Rowley HA, Asthana S,
Sager MA, Johnson SC, Okonkwo OC. 2015. Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-aged adults at risk for AD. Alzheimers Dement 1:33–40.
Sears RM, Schiff HC, LeDoux JE. 2014. Molecular mechanisms of threat learning in the lateral nucleus of the amygdala. Prog Mol Biol Transl
Sci 122:263–304.
Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF Jr,
Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik
K, Semple MA, Flener CR, Schulz SC. 2000. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry
63:65–74.
Seto K, Saito H, Otsuka K, Kawakami M. 1983. Influence of electrical stimulation of the limbic structure on insulin level in rabbit’s plasma.
Exp Clin Endocrinol 81:347–349.
Shandra AA, Glukhov VP. 1992. Role of adrenoreactive structures of the amygdaloid complex in the regulation of blood coagulation. Neurosci
Behav Physiol 22:430–435.
Shin LM, Rauch SL, Pitman RK. 2006. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 1071:
67–79.
Sigurdsson T, Doyere V, Cain CK, LeDoux JE. 2007. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology 52:215–227.
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. 2011. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12:585–601.
Son Y, Yang M, Kim JS, Kim J, Kim SH, Kim JC, Shin T, Wang H, Jo
SK, Jung U, Moon C. 2014. Hippocampal dysfunction during the chronic phase following a single exposure to cranial irradiation. Exp
Neurol 254:134–144.
Stickgold R. 2002. EMDR: a putative neurobiological mechanism of action. J Clin Psychol 58:61–75.
Stock G, Rupprecht U, Stumpf H, Schlor KH. 1981. Cardiovascular changes during arousal elicited by stimulation of amygdala, hypothalamus, and locus coeruleus. J Auton Nerv Syst 3:503–510.
Stratton GM. 1919. Retroactive hypermnesia and other emotional effects on memory. Psychol Rev 26:474–486.
Thobois S, Delamarre-Damier F, Derkinderen P. 2005. Treatment of motor dysfunction in Parkinson’s disease: an overview. Clin Neurol
Neurosurg 107:269–281.
van der Kolk BA. 1994. The body keeps the score: memory and the evolving psychobiology of posttraumatic stress. Harv Rev Psychiatry 1:
253–265.
van der Kolk BA, Van der Hart O. 1991. The intrusive past: the flexibility of memory and the engraving of trauma. Am Imago 48:425–454.
van der Kolk B, Blitz R, Burr W, Sherry S, Hartmann E. 1984. Nightmares and trauma: a comparison of nightmares after combat with lifelong nightmares in veterans. Am J Psychiatry 141:187–190.
Violanti JM, Fekedulegn D, Hartley TA, Andrew ME, Charles LE,
Mnatsakanova A, Burchfiel CM. 2006. Police trauma and cardiovascular disease: association between PTSD symptoms and metabolic syndrome. Int J Emerg Ment Health 8:227–237.
Von Kanel R, Mills PJ, Fainman C, Dimsdale JE. 2001. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosom Med 63:531–544.
Vyas A, Kim SK, Giacomini N, Boothroyd JC, Sapolsky RM. 2007.
Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A 104:
6442–6447.
Wald I, Shechner T, Bitton S, Holoshitz Y, Charney DS, Muller D, Fox
NA, Pine DS, Bar-Haim Y. 2011. Attention bias away from threat during life threatening danger predicts PTSD symptoms at one-year follow-up. Depress Anxiety 28:406–411.
Williamson JB, Porges EC, Lamb DG, Porges SW. 2015. Maladaptive autonomic regulation in PTSD accelerates physiological aging. Front
Psychol 5:1571.
Wolf EJ, Miller MW, Reardon AF, Ryabchenko KA, Castillo D, Freund
R. 2012. A latent class analysis of dissociation and posttraumatic stress disorder: evidence for a dissociative subtype. Arch Gen Psychiatry 69:
698–705.
Yereli K, Balcioglu IC, Ozbilgin A. 2006. Is Toxoplasma gondii a potential risk for traffic accidents in Turkey? Forensic Sci Int 163:34–37.
Zoladz PR, Diamond DM. 2013. Current status on behavioral and biological markers of PTSD: a search for clarity in a conflicting literature.
Neurosci Biobehav Rev 37:860–895.
Journal of Neuroscience Research