Document 10579543
advertisement

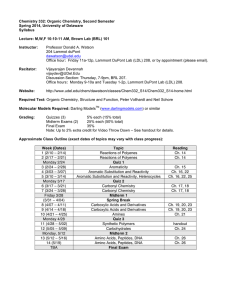
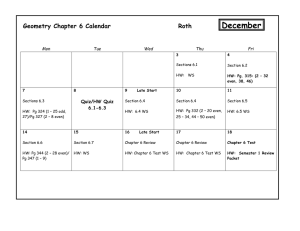
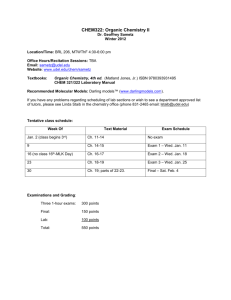
CHEM 322: Lecture Section 011 nd Organic Chemistry, 2 Semester University of Delaware Spring 2014 Instructor: Prof. Mary Watson (237 BRL, mpwatson@udel.edu) Office Hours: Wed, 11am–12noon, 237 BRL Thurs, 2–3pm, 237 BRL (Or by appointment) Lectures: MWF 10:10–11am, 101 BRL TA’s: Andrew Cinderella acinder@udel.edu (Office Hour: Mon 2:30-3:30pm, 220 BRL) Jennie Liao jenniel@udel.edu (Office Hour: Wed 9–10am, 220 BRL) Website: http://www.udel.edu/chem/mpwatson/mpwatson/Chem_332.html UD Capture: To be announced Textbooks: Organic Chemistry, 5 Edition By Maitland Jones & Steven Fleming Publisher: W. W. Norton & Co. th Molecular Models: You may use any reasonable ball and stick model set. Recommended: HGS 1003 Organic Chemistry C-Set or similar. (http://www.maruzen.info/hgs/catalog/product_info.php?cPath=4&products_id=4) These should be available in the bookstore. Grading: Quizzes (6) Problem Sets (6, done check only) Discussion Section Attendance Midterm Exams (2) Final Exam Music Video 3% each (18% total) 2% each (12% total) 10% 15% each (30% total) 30% 1% (extra credit, see handout) Quizzes: There will be six short quizzes given in class on the days indicated in the table below. These will be closed book, closed note. Model sets may be allowed and will be determined on a quiz-by-quiz basis. Make-up quizzes will be handled according to university policy. Problem Sets: There will be six problem sets posted on the website approximately on the days indicated in the table below. These will consist of both assigned problems from the text, as well as other problems. These problem sets will be turned in during class on the due date. Late problem sets will not be accepted. Your answers will only be graded for completeness (2 points for all answers attempted, 1 point for ~50–90% completion, 0 points for <50% completion). No half points will be awarded. Please note: these are easy points that are in place to encourage you to study… please take advantage of this! A key will be posted on the course website after problem sets have been turned in. You are responsible for making sure that you understand the correct answers once the key is posted. Answers may also be discussed in discussion sections. You are free to work in study groups when working on the problem sets, but each person must turn in a hand-written answer set of their own creation. Discussion Sections: There will be discussion sections for this class. These TA-led sections will allow time to work additional problems, ask questions and get more help. Attendance of your assigned discussion section is required! Attendance will be taken. You are allowed two unexcused absences from discussions without penalty. Beyond that, there will be a 1% deduction from your final course grade (up to 10% total). Again, there are very easy points that are designed to help you maximize your success… please take advantage. Also, be aware that signing in a classmate who is not present will be considered academic dishonesty and will be treated accordingly. Exams: Exams will be held in class and will be closed book, closed note. Models will be allowed. Exams will cover lecture material, problem sets, and assigned reading. Because organic chemistry is cumulative, exams will also be cumulative! 1 You are expected to know material from this semester, as well as from Chem 331 and 333. Make-up exams will be handled according to university policy. Regrades: All requests for regrades must be submitted in writing within 24 hours of the material being returned. Please note, the entire quiz or exam will be regraded. If grading errors are found, the final grades may be higher or lower than the original score. Also note, photocopies may be made prior to returning exams. If answers are altered, it will be obvious and provable (see below). Academic Dishonesty: Academic dishonesty will not be tolerated. Not only is such behavior unethical, but it also results in you not learning material that will be critical to your chosen career path. Any student who commits academic dishonesty will be punished according to the University of Delaware’s guidelines (http://www.udel.edu/stuguide/0910/code.html#honesty). Plagiarism is using someone else’s words or ideas without acknowledgement and most often results from uncited quoting or paraphrasing. Plagarism is a serious form of academic dishonesty. Approximate Class Outline Week (Dates) 1 (2/9 – 2/13) 2 (2/16 – 2/20) Mon, 2/23 3 (2/23 – 2/27) 4 (3/2 – 3/6) Mon, 3/9 5 (3/9 – 3/13) 6 (3/16 – 3/20) Mon, 3/16 7 (3/23 – 3/27) Fri, 3/27 (3/30 – 4/3) 8 (4/6– 4/10) Mon, 4/13 9 (4/13 – 4/17) 10 (4/20 – 4/24) Fri, 4/24 11 (4/27 – 5/1) Mon, 5/4 12 (5/4– 5/8) Mon, 5/11 13 (5/11 – 5/15) Mon, 5/18 14 (5/18) TBA Topic Conjugated π-Systems Aromaticity Quiz 1 Substitution of Aromatic Compounds Substitution of Aromatic Compounds Quiz 2 Carbonyl Chemistry Carbonyl Chemistry Quiz 3 Carboxylic Acids and Derivatives Midterm 1 Spring Break Carboxylic Acids and Derivatives Quiz 4 Enols and Enolates Enols and Enolates Midterm 2 Carbohydrates Reading Ch. 13 Ch. 14 Amino Acids, Peptides, and DNA Quiz 5 Pericyclic Reactions Quiz 6 Pericyclic Reactions Final Exam Ch. 22 Ch. 15 Ch. 15 Ch. 16 Ch. 16 Ch. 17 Problem Set PS 1 assigned 2/13 PS 1 Due PS 2 assigned 2/23 PS 2 Due PS 3 assigned 3/9 PS 3 Due PS 4 3/23 Ch. 17 Ch. 19 Ch. 19 PS 4 Due PS 5 assigned 4/13 Ch. 20 PS 5 Due PS 6 assigned 5/4 Ch. 22 PS 6 Due Ch. 23 Additional Resources: Tutoring: The Department of Chemistry & Biochemistry maintains a list of chemistry tutors. Please see Ms. Linda Staib (lstaib@udel.edu, 102 BRL) if you are looking for a chemistry tutor. The Office of Academic Enrichment also offers drop-in group tutoring sessions. For a schedule, check their website: http://ae.udel.edu. Course Learning Goals: After successful completion of this course, a student should be able to: 1. Describe the frontier molecular orbitals for conjugated π-systems and carbonyl compounds. (1)* 2. Understand the stabilizing effect of conjugation on electrons in π-bonds. (1) 3. Predict products, propose reaction conditions, and draw arrow-pushing mechanisms for reactions of dienes and allyl systems. (1) 2 4. Understand the concept of aromaticity. (1) 5. Predict products, propose reaction conditions, and draw arrow-pushing mechanisms for reactions of aromatic systems. (1) 6. Determine the identity of a chemical compound based on spectroscopic data. (1, 6) 7. Predict products, propose reaction conditions, and draw arrow-pushing mechanisms for reactions of carbonyl compounds. (1) 8. Predict products, propose reaction conditions, and draw arrow-pushing mechanisms for reactions of carboxylic acids and their derivatives. (1) 9. Understand the biological importance, structure and reactivity of carbohydrates and sugars. (1) 10. Understand the biological importance, structure and reactivity of amino acids. (1) 11. Safely perform a chemical reaction in a laboratory, making qualitative and quantitative observations of the experiment. (2, 6, 7, 8) 12. Prepare a laboratory report of an experiment they have performed. (10) (*Numbers in parentheses indicate the departmental learning goals with which each course goal is aligned. Please see: http://www.udel.edu/chem/goals.html.) 3