SCH 4U Day 5 Assignment: Section Page 37 # 1-3
advertisement

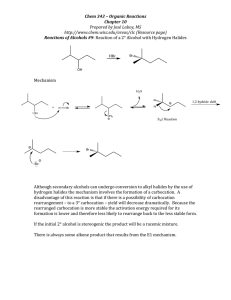
SCH 4U Day 5 Organic Halides Assignment: Page 33 # 1, 2;35 #5; 37 #6 Section Page 37 # 1-3 1.4 Organic Halides P Naming organic halides P Properties of organic halides P Cost of Air Conditioning P Preparing Organic Halides P Preparing Alkenes from Organic Halides Naming Organic Halides P Name as substitutions to main carbon chain P Replace “ine” ending with “o” P Fluoro-, chloro-, bromo-, iodoP Number the main chain and use prefixes Nomenclature of Alkyl Halides P Name alkyl halides as substituents on the main carbon chain Common names of Alkyl Halides P Common names end with the name of the halide Properties of Organic Halides P Halogens are more polar than H P C-X bonds are more polar than C-H bonds P Dipole-dipole attractions lead to higher BP P Dipole-dipole attractions lead to higher solubility in polar solvents (but not water) P Mixtures are produced during halogenation of hydrocarbons The Cost of Air Conditioning P Chlorofluorocarbons replaced toxic coolants in 1920's P CF2Cl2 or CFC-12 is known as Freon P CFC’s are inert in the lower atmosphere P CFC’s decompose in the upper atmosphere P Highly reactive Cl atoms catalyze ozone decompostion P Halons (Bromoalkanes) destroy ozone more effectively than CFC’s Radical Reactions The Ozone Layer and CFCs Halon Alternatives to CFC’s P Hydrochlorofluorocarbons HCFC’s P Hydrofluorocarbons HFC’s P H atoms react with OH- ions in atmosphere to decompose HCFC and HFC P No threat to ozone but powerful greenhouse gases P “Puron” Industrial uses of Organic Halides Cl P Solvents: CH2Cl2, CHCl3, CCl4 P Refrigerants: Cl C Cl < CF2Cl2 - Freon-12 (decomposes O3) < HCF2Cl - Freon-22 (destroyed at lower altitudes) P Pesticides: DDT < introduced 1939 < banned 1972 (Rachael Carson) CH Cl Cl Industrial Applications of Organic Halides P Chlordane -termites < banned 1995 Cl Cl Cl Cl H H Cl H H Cl Cl P Capacitors, etc. Cl < PCB (polychlorinated biphenyl) banned 1985 Cl Cl Cl Cl Cl Cl Cl Cl Cl Cl Industrial applications of Organic Halides P Anaesthetics < CHCl3 - chloroform carcinogenic < CH3CH2Cl - ethyl chloride topical use < CF3CHBr-Cl - halothane - general P Polymers < Polyvinylchloride (PVC) < Teflon Preparing Organic Halides P Halogenation of alkanes (slow) P Addition of halogens or hydrohalogens to alkenes and alkynes P Markovnikov’s rule for hydro halogens P Benzene derivatives are formed by substitution reaction with halogens (not addition reactions) Halogenation H H H C C H H H + Heat or Light D or hv Br2 H Ethane C Heat or Light D or hv H H Methane + H C C H H Br + HBr Bromoethane H H H Cl2 H H C Cl + HCl H chloromethane CH2Cl2 and CHCl3 may be observed Substitution R eaction – a reaction in which part of a small reacting molecule replaces an atom or a group of atoms on the organic molecule (usually very slowly) Preparing Alkenes from Alkyl Halides P Eliminate H and adjacent halide to form alkene P Hydroxide ions are required P Elimination reactions are the most commonly used method of preparing alkenes ELIMINATION REACTIONS An elimination reaction is one where starting material loses the elements of a small molecule such as HCl or H 2O or Cl2 during the course of the reaction to form the product. C C H Cl - HCl C C MAKING ALKYNES “DOUBLE ELIMINATION” COMPOUNDS WITH TWO HALOGENS If you have a compound with two halogens it can react twice (two E2 eliminations). If both halogens are on the same carbon, an alkyne is produced. The second elimination is more difficult than the first one - it requires a stronger base. H Cl H3 C H3 C C C CH3 H H Cl most bases will work Cl C C H3 C C C CH3 CH3 more difficult, requires a stronger base like NH 2 - COMPOUNDS WITH TWO HALOGENS If you have a compound with two halogens it can react twice (two E2 eliminations). If the halogens are on different carbons, a diene is usually produced. Cl Cl KOH EtOH Br Br 2 CCl4 D Br NaOEt EtOH D