Volatile Thiols in Wine: The Role of Sulfur in Wine... ABSTRACT
advertisement

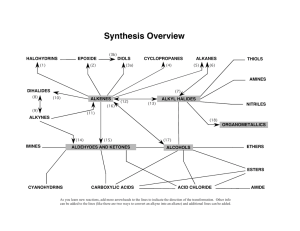
Volatile Thiols in Wine: The Role of Sulfur in Wine Aromas Lauren Musumeci, Graduate Student, Cornell University ABSTRACT Thiols are sulfur containing compounds that contribute significantly to the aroma of many different foods and beverages due in part to their extremely low sensory detection thresholds. They are of particular interest in wine as they are the most potent compounds in this beverage. Being highly unstable molecules and present at low concentrations, thiols pose a challenge when attempting to accurately measure their concentration in a complex wine matrix. An improved method for the measurement of three thiols that are known to be significant contributors in wine aroma (3mercaptohexanol, 4-mercapto-4-methyl-2-pentanone, and 3-mercaptohexyl acetate) was developed. This method was used for the measurement of thiol concentrations in wines produced in the Finger Lakes. BIOGRAPHY Lauren Musumeci is originally from Annandale, New Jersey. In 2011, she received her Bachelor of Science degree from Rider University, majoring in Chemistry and minoring in Mathematics. At Rider University, she performed research in the area of organic synthesis, working toward the development of a novel synthetic pathway for stereospecific cytotoxic natural products. In August 2013, she will receive her Master of Science degree from Cornell University in Food Science and Technology. As graduate student at Cornell University, her research is in the area of analytical aroma chemistry, developing an improved quantification method for volatile thiols in wine. She presented her research at the American Chemical Society National Meeting held in April 2013.