Enhanced In Situ Biodegradation of Trichloroethene/1,1,1 – Trichloroethane in Fractured Rock Groundwater
advertisement

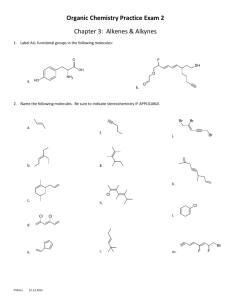
Enhanced In Situ Biodegradation of Trichloroethene/1,1,1 – Trichloroethane in Fractured Rock Groundwater Michael S. Kozar, PG © 2011 O’Brien & Gere Introduction Michael Kozar, PG, LSRP | Vice President, O’Brien & Gere 22 years of professional experience 2 including 20 years with O’Brien & Gere Provide client–focused solutions, technical consulting and project management services throughout the U.S., focusing on environmental site characterizations, soil and groundwater investigations and subsurface remediation Conducted environmental programs for industrial/federal sites under CERCLA programs including several USEPA Regions and various state regulatory programs Managed complex environmental investigation and subsurface remediation projects including in situ technologies, such as enhanced in situ bioremediation for hydrocarbons and chlorinated solvents in groundwater © 2011 O’Brien & Gere File Location BS in Geological Sciences from Pennsylvania State University Registered Professional Geologist in Pennsylvania Licensed Site Remediation Professional in New Jersey Bioremediation Consulting Inc bioremediation@bciLabs.com, www.bcilabs.com Lab-Based ConsultingTM Microcosm Experiments Remediation Monitoring Analysis Designer BacteriaTM Bioaugmentation Cultures © 2011 O’Brien & Gere Enhanced In Situ Biodegradation (EISB*) of Chlorinated Organics in Groundwater *also commonly called Accelerated In Situ Bioremediation (AISB) Optimizing groundwater redox conditions to sustain the microorganisms that facilitate biodegradation © 2011 O’Brien & Gere Biodegradation Mechanisms Reductive dechlorination The sequential replacement of chlorine atoms on the alkene molecule with hydrogen atoms Trichloroethene (TCE) 1,1,1-Trichloroethane (TCA) Aerobic cometabolism The process by which certain VOCs (e.g., TCE or its daughter products) are degraded by an enzyme or cofactor produced during microbial metabolism of another compound Cometabolism is limited to chlorinated solvents that have at least one hydrogen atom attached to the carbon (i.e., PCE cannot be co-metabolized) Direct oxidation cis-1,2-dichloroethene (DCE) and vinyl chloride serve as electron donors by bacteria © 2011 O’Brien & Gere PCE / TCE Degradation Pathways 6 © 2011 O’Brien & Gere File Location 1,1,1 – TCA Degradation Pathways 7 © 2011 O’Brien & Gere File Location Groundwater Contaminants: EPA MCLs/RSLs Contaminant Drinking Water Level - µg/L (ppb) 1,1,1-Trichloroethane (TCA) 200 1,1-Dichloroethane (DCA) 2.4 1 21,000 1 Chloroethane (CA) Tetrachloroethene (PCE) 5 Trichloroethene (TCE) 5 cis-1,2-Dichloroethene (DCE) 70 Vinyl Chloride (VC) 2 Ethene NS MCL – drinking water Maximum Contaminant Level 1 No specified MCL, value is EPA’s 2010 Regional Screening Level (RSL) for Tap Water 8 © 2011 O’Brien & Gere File Location Hydrogen Generation from Different Electron Donors OH CH3CHCOOH + H2O (lactate) HO CH3COOH + CO2 + 2H2 (acetate) OH CH3CHCH2 + 2H2O (propylene glycol) CH3COOH + CO2 + 4H2 (acetate) PCE / TCE / TCA: Electron Acceptors © 2011 O’Brien & Gere Dechlorination of Chlorinated Ethenes Various anaerobes PCE TCE DCE Dehalococcoides ethenogenes (Dhc) TCA to DCA to CA: Dehalobacter (Dhb) © 2011 O’Brien & Gere VC Ethene Microbial Community in Groundwater Physiological Group ORP mv Aerobes + 800 Denitrifiers + 650 Mn Reducers + 500 Iron Reducers +0 Fermentors - 100 Sulfate Reducers - 180 Acetogens - 200 Dehalococcoides - 200 Methanogens - 300 © 2011 O’Brien & Gere Anaerobic Microbial Community Supports Dhc Dechlorination Nitrate Reducers NO3 + Organics N2 Sulfate Reducers SO4 + Organics SH Lower ORP Organic Acids H2 Fermentors + Organics Methanogens (Low ORP) B12 D. Ethenogenes + DCE +B12 + H2 + Low ORP © 2011 O’Brien & Gere Ethene Dhc © 2011 O’Brien & Gere Example Biological Reductive Dechlorination: Chloroethenes Will accumulate if requisite bacteria like certain strains of Dehalococcoides are absent Anaerobic process that can be stimulated by injection of: Nutrients Alcohols, sugars, edible oils biostimulation Naturally occurring bacteria (e.g., Dehalococcoides culture) © 2011 O’Brien & Gere Or bioaugmentation EISB Case Study Superfund Site, Southeast Pennsylvania USEPA Region 3 © 2011 O’Brien & Gere Solvent Recycling Facility (Carbonate Valley, PA) 16 © 2011 O’Brien & Gere File Location Site Geology 17 © 2011 O’Brien & Gere File Location The Challenge Aquifer Characteristics Fractured dolomite setting Depth to groundwater: ~ 90 ft Source area aquifer thickness ~ 35 ft Travel time ~ 2.7 ft/day (bromide tracer) Groundwater pH < 6 (source area) to > 8 (downgradient groundwater) ~ 80,000 ppb VOCs © 2011 O’Brien & Gere The Challenge (2004 – 2005) Jump start stalled AISB field pilot test to dechlorinate TCE and TCA, after two prior bioaugmentation attempts and the following conditions: Dechlorination stalled at DCE DCE 67 ppm VC 0.01 ppm ethene 0.003 ppm Dechlorination stalled at TCA TCA 6 ppm DCA 0.3 ppm chloroethane < 0.01 ppm Toxic Donor Levels methanol 15000 ppm © 2011 O’Brien & Gere ethanol 27000 ppm organic acids 300 ppm Bioaugmentation Inhibition Defined Duhamel, M., S.D. Wehr, L.Yu, H. Rizvi, D. Seepersad, S. Dworatzek, E.E. Cox, and E.A. Edwards. 2002. “Comparison of anaerobic dechlorinating enrichment cultures maintained on tetrachloroethene, trichloroethene, cisdichloroethene, and vinyl chloride.” Wat. Res. 36:4193-4202 Co-Contaminant Inhibition Dehalococcoides cultures reported to be inhibited by: TCA > 0.7 ppm Chloroform > 50 ppb slows dechlorination Complete inhibition with chloroform > 300 ppb No commercially available culture that degrades TCE and TCA concurrently (2004 – 2005) © 2011 O’Brien & Gere Laboratory Approach Objective: Culture a TCA tolerant TCE degrading consortium using Site groundwater – capable of degrading methanol and ethanol and TCE/TCA Culturing Process - Bioremediation Consulting, Inc. (BCI) Site ground water amended with ammonia, phosphate, and minor and trace elements and vitamin B12 pH maintained at 6.9 Added Bacterial consortium was initially inhibited by methanol but eventually degraded the methanol and ethanol Sodium lactate was added as donor after the culture utilized organic acid already present in groundwater TCE, cDCE, VC, 1,1,1-TCA & 1,1-DCA were spiked in after these VOCs were dechlorinated and methanol was degraded © 2011 O’Brien & Gere Laboratory Microcosms Killed control Live – native bacteria donor: lactate Live – bioaugmented donor: lactate Headspace Contaminated site groundwater + native bacteria 22 © 2011 O’Brien & Gere File Location Soil / Groundwater Microcosm Set-Up in Anaerobic Glove Box Atmosphere: 96% N2 4% H2 Sieve and weigh soil Add to microcosms H2 + O2 + catalyst --> H2O © 2011 O’Brien & Gere Microcosm Results Summary 24 Conducted with Site groundwater and TCE, cDCE, and TCA concentrations much higher than in field (residual source concentrations) Bacteria culture added - contained 1,1,1-TCA-tolerant D. ethenogenes, Dehalobacter, fermenters, acetogens, sulfate-reducing bacteria, and methanogens Complete degradation of TCE/cDCE and TCA (concurrently) demonstrated using lactate and BCI culture © 2011 O’Brien & Gere File Location Microcosm Study (2005) 25 © 2011 O’Brien & Gere File Location Culturing for AISB Pilot and Full-Scale 26 © 2011 O’Brien & Gere File Location Culture Delivery (“Kegs”) © 2011 O’Brien & Gere Bioaugmentation Culture 28 © 2011 O’Brien & Gere File Location AISB Pilot Testing (2006 – 2010) Biostimulation/REDOX & Donor Management AISB pilot test operated in a manual, daily batch delivery mode Daily anoxic sodium lactate (Wilclear) injections in GW-7 ranged from ~ 3,000 to 5,000 mg/L 50 mg Vitamin B12 was added on daily basis Amendments adjusted based on monthly redox, organic acid, hydrogen, pH and nutrient groundwater results Ammonia and phosphate additions to maintain 1 mg/L each Later expanded injections to 2 additional wells © 2011 O’Brien & Gere AISB Pilot Test (“Phase 2”) GW-7 Anoxic Batch Injection System © 2011 O’Brien & Gere AISB Pilot System (“Phase 3”) GW-7 Direct Injection System metering pump sodium lactate flow meter/valves © 2011 O’Brien & Gere AISB Full-Scale Design (2009 – 2010) Pumping to control and facilitate distribution of donor / nutrients for source treatment through AISB USEPA issues remedy change document (August 2009) based on laboratory/pilot results! Accelerated in situ biological treatment (AISB) to degrade chlorinated VOCs throughout the source zone (and enhance dissolution) Groundwater extracted and amended with nutrients and re-injected in the source area creating some groundwater recirculation / treatment “cells” Groundwater to be bioaugmented with TCE/TCA degrading culture initially and as necessary Natural Attenuation to address downgradient VOC plume beyond AISB treatment zone © 2011 O’Brien & Gere AISB Injection and Extraction Wells 33 © 2011 O’Brien & Gere File Location Remediation Well Cross Section 34 © 2011 O’Brien & Gere File Location Installation of Injection / Extraction Wells 35 © 2011 O’Brien & Gere File Location Extraction Vault 36 © 2011 O’Brien & Gere File Location AISB Full-Scale: Year 1 Amendments (3/2010 – 2/2011) 3 to 5 gallons per minute (gpm) extraction/injection system Donor: Sodium Lactate (60%) Target concentration 825 mg/L Injection rate 22 mL/min Daily usage: ~6.5 gal/day Usage in 1 year ~ 1500 gallons Nutrients: Miracle Gro™ and Potassium Tri-poly Phosphate (source of ammonia and phosphate) Target concentrations: 1 mg/L Injection rate 22 mL/min Usage in 1 year: Miracle Grow ~ 525 gallons; KTPP ~ 330 gallons Vitamin B12 ~ 260 grams 76L of bacteria culture © 2011 O’Brien & Gere AISB Target Concentrations Target concentrations in extraction wells 100 ppm (mg/L) of total organic acids 1 ppm each of nitrogen and phosphate Geochemical conditions for anaerobic dechlorination pH ~ 6.9 S.U. (acceptable range: 6.3* – 8.5 S.U.) ORP ~ -200 mV DO ~ 0 ppm (<0.2 ppm) * 2011: new low-pH-tolerant culture available (pH 5.5) © 2011 O’Brien & Gere AISB Full Scale Remediation © 2011 O’Brien & Gere Full-Scale Treatment Building 40 © 2011 O’Brien & Gere File Location Total Organic Acids (1 Year Full-Scale) 41 © 2011 O’Brien & Gere File Location Organic Acid Concentration Table 1: Organic Acid Concentration in ppm Tank 1 IW-1 IW-2 IW-3 IW-4 IW-5 Lactate 704 361 129 18 395 87 Acetate 76 322 267 202 132 193 Propionate 146 659 512 394 257 391 0 0 0 0 0 0 Butyrate 42 © 2011 O’Brien & Gere File Location Microbial Community in Groundwater Physiological Group ORP mv Aerobes + 800 Denitrifiers + 650 Mn Reducers + 500 Iron Reducers +0 Fermentors - 100 Sulfate Reducers - 180 Acetogens - 200 Dehalococcoides - 200 Methanogens - 300 © 2011 O’Brien & Gere Oxidation-Reduction Potential (ORP) 44 © 2011 O’Brien & Gere File Location PCE/TCE Reductive Dechlorination and Daughter Products molar and ppm / ppb concentrations 1 mM PCE → 1 mM TCE→ → 1 mM DCE → 1 mM VC → 1 mM ethene 166 ppm PCE→ →131.5 ppm TCE→ →97 ppm DCE→ →62.5 ppm VC→ →28 ppm ethene 1 µM PCE → 1 µM TCE→ → 1 µM DCE → 1 µM VC → 1 µM ethene 166 ppb PCE→ →131.5 ppb TCE→ →97 ppb DCE→ →62.5 ppb VC→ →28 ppb ethene EXAMPLES: 100 µM TCE = 13,150 ppb 5,000 pbb total CVOCs = 38 µM TCE = 51 µM DCE = 79 µM VC © 2011 O’Brien & Gere PCE + TCE + DCE / VC + Ethene in Extraction & Monitoring Wells 46 © 2011 O’Brien & Gere File Location Ethene Production from PCE/TCE/DCE/VC 2006-2011 47 © 2011 O’Brien & Gere File Location AISB Groundwater Data – GW-1 (Downgradient/Source Area Well) Chlorinated Ethenes © 2011 O’Brien & Gere AISB Groundwater Data – GW-1 (Downgradient/Source Area Well) Chlorinated Ethenes © 2011 O’Brien & Gere AISB Groundwater Data – GW-1 (Downgradient/Source Area Well) Chlorinated Ethanes © 2011 O’Brien & Gere Source Area Extraction Well – GW-10 Chlorinated Ethenes © 2011 O’Brien & Gere Source Area Extraction Well – GW-10 Chlorinated Ethanes © 2011 O’Brien & Gere Downgradient Monitoring Well GW-17 - Chlorinated Ethenes © 2011 O’Brien & Gere Upcoming Webinar REMEDIATION ROUNDTABLE Wednesday June 8th | 2pm webinars@bnpmedia.com 54 © 2011 O’Brien & Gere File Location © 2011 O’Brien & Gere Microcosm Test MW-11 plus Donor. No N&P 90 TCE Effect of N and P 60 µM 30 PCE IESI Site 0 0 30 60 Donor, no N&P no dechlorination days 90 120 150 MW-11 with Donor and N&P Donor and N&P PCE -> ETHENE DCE 90 VC 60 µM TCE DCE 30 VC PCE ETHENE 0 0 30 © 2011 O’Brien & Gere 60 days 90 120 150 Requirement for B-12 Microcosm: GW7 Well Sample During Remediation. pH 6.6, donor present, add N & P Microcosm: GW7 Well Sample During Remediation. pH 6.6, donor present, add N & P & B12 150 150 VC DCE 100 100 VC µM µM DCE 50 50 0 0 0 20 40 60 0 20 40 days days © 2011 O’Brien & Gere 60 AISB Groundwater Data – GW-7(Source Area Well) Chlorinated Ethenes © 2011 O’Brien & Gere Source Area / Downgradient Extraction Well – GW-18 Chlorinated Ethenes © 2011 O’Brien & Gere Downgradient Extraction Well – GW-12A Chlorinated Ethenes © 2011 O’Brien & Gere AISB Groundwater Data – GW-12A pH vs GWE © 2011 O’Brien & Gere Bioaugmentation Inhibition Defined Low pH Microbial Inhibition – acid production processes lactic acid degradation results in acid production H+ + lactate ---> acetate - 2H+ + 2 H2 + HCO3Methanol and ethanol degradation result in acid production 2 methanol ---> acetate- + H+ + 2 H2 ethanol + H2O ---> acetate - + H+ + 2 H2 Methanogens can remove CO2 and acetate CO2 + 4 H2 ---> CH4 + 2 H2O acetate- + H+ ---> CH4 + CO2 methanogens are inhibited by high DCE © 2011 O’Brien & Gere Bioremediation Produces Acid © 2011 O’Brien & Gere Effect of pH… …on dechlorination and methanogenesis in groundwater microcosms Site M Three microcosms, Three different pH (1) pH 6.9 (2) pH 6.6 (3) pH 6.3 Ground water contains TCE, TCA, methanol BCI culture contains Dehalococcoides Dehalobacter Methanol utilizers and methanogens Analyzed by removing 100 uL headspace samples through septa and injecting into gas chromatograph © 2011 O’Brien & Gere © 2011 O’Brien & Gere Three Diagnostic Microcosms for site A #1 #2 #3 Conclusions from microcosm #3: Adjusting pH and amendments restored bioremediation in 60 days © 2011 O’Brien & Gere BCI Designer Bacteria™ Low-pH-Tolerant Cultures BCI Culture # 1 TCE to Ethene BCI Culture # 2 PCE to Ethene and TCA to CA © 2011 O’Brien & Gere BCI’s Solution to Low pH Problems 2009 • Initiated work on low-pH-tolerant Dhc with a grant from the National Science Foundation 2010 • Developed 2 low-pH-tolerant Dhc cultures 2011 • Started commercial production © 2011 O’Brien & Gere CC-7 Aerobic 69 © 2011 O’Brien & Gere File Location