resolved velocity and pH mapping in T-shaped microchannel Time- chiyanagi ato

13 th
Int. Symp on Appl. Laser Techniques to Fluid Mechanics, Lisbon, Portugal, June 26 – 29, 2006
Time-
r
esolved
v
elocity and pH
m
apping in T-shaped
m
icrochannel
Mitsuhisa I chiyanagi
1
, Yohei S ato
2
, Koichi H ishida
3
1: Dept. of System Design Engineering, Keio University, Japan, ichiyanagi@mh.sd.keio.ac.jp
2: Dept. of System Design Engineering, Keio University, Japan, yohei@sd.keio.ac.jp
3: Dept. of System Design Engineering, Keio University, Japan, hishida@sd.keio.ac.jp
Keywords : Microscale, Simultaneous measurement, Micro-PIV, LIF, Confocal microscope
In microfluidic devices, an unsteady mixing flow field is formed by chemical reactions which are controlled by the fluid velocity and the diffusion of ion concentration, especially protons. A simultaneous measurement for the velocity and pH distribution will extend our knowledge about mass transport phenomena and contribute to control
Blue
Green
Ar laser
Objective lens
3CCD camera
Prism
Dichroic mirror
Pin-hole disk
Microchannel the mixing or chemical reaction accurately. The objective of the present study is to establish a simultaneous measurement technique in time series by utilizing spatially averaged time-resolved particle tracking velocimetry (SAT-PTV) [1] and laser induced fluorescence (LIF)
[2]
.
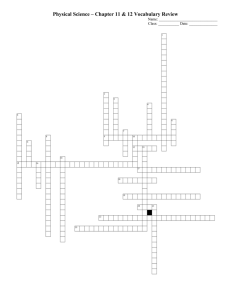
The present study employed the particles and dye. The emission wavelength of the particles differs from that of the dye. Figure 1 illustrates a schematic of the measurement
Red
Fig. 1
Confocal scanner
. Schematic of measurement system.
100
80
60
40
3CCD (B)
3CCD (G)
3CCD (R)
Dye
Particles system comprising of an inverted microscope, a confocal scanner, an Ar laser and a 3CCD camera. The depth of field for the present system is one fifth of the depth of field for a conventional microscope, because out-of-focus fluorescence was excluded from the captured images by the pin-hole in the confocal scanner. The 3CCD camera has the ability to separate RGB colors by the prism. Figure 2 summarizes the spectral response of the 3CCD camera and the spectra of the particles and dye. The present system enables to separate the fluorescence of the particles from that of the dye.
The experiments of the steady-state flow were conducted by injecting aqueous solutions with pH 6.0 and 7.2 into a
T-shaped microchannel. For the validation of the present technique, the proton concentration profiles from the experiments were compared with those from the numerical simulation as plotted in Figure 3 . The profiles from the experiments were estimated from pH data by LIF, while the profiles from the numerical simulation were evaluated by using the velocity data given by SAT-PTV. Figure 3 indicates that the profiles from the experiments agreed with those from the numerical simulation.
The present technique was applied to a pulsing flow, formed by using a syringe pump operated at 0.1 Hz. Figures
4 and 5 exhibit the time-evolution of the velocity vector map and that of the two-dimensional pH distribution, respectively.
It is obvious that a pH gradient in the y -direction at t = t
0
+5 s
(when the bulk velocity was increasing) became larger than that at t = t
0
s (when the bulk velocity was decreasing), because the formation process of a pH gradient depends on the balance between the diffusion and the convection.
It is concluded that the present technique enables to evaluate an unsteady mixing flow field quantitatively for further investigation of the mass transport in a microfluidic device.
References
[1] Sato et al ., Exp. Fluids, 35 , pp. 167 − 177, (2003).
[2] Sato et al ., Meas. Sci. Technol., 14 , pp. 114 − 121, (2003).
500
600
700
20
0
300 400 500 600 700 800
Wavelength [nm]
Fig. 2 . Spectral response of the 3CCD camera and spectra of the fluorescent particles and dye.
50
40
30
Exp. z = 05 μ m
Exp. z = 15 μ m
Sim. z = 05 μ m
Sim. z = 15 μ m
20
10
Fig. 3 . Proton concentration profiles obtained from the
( a ) 400
0
0 100 y [
200 m]
300 400 experiments and the numerical simulation at
( b ) x = 100 μ m.
500
μ m/s
Fig. 4 . Time-evolution of the velocity vector map at ( a ) t
= t
0
( a ) 400 y
200
[ μ m]
300 400
s and ( a ) t = t
0
+ 5 s.
( b ) y
200
[ μ m]
300 400 pH [-] and ( a
0
) t = t
0 y
200 400 0 y
200 400
Fig. 5 . Time-evolution of pH distribution at ( a ) t = t
0
+ 5 s.
s
6.5
6.6
6.7
6.8
6.9
7.0
7.1
7.2
23.4