NZIC 2006 CHEMISTRY - 2.7 (Describe oxidation-reduction reactions) ASSESSMENT SCHEDULE
advertisement
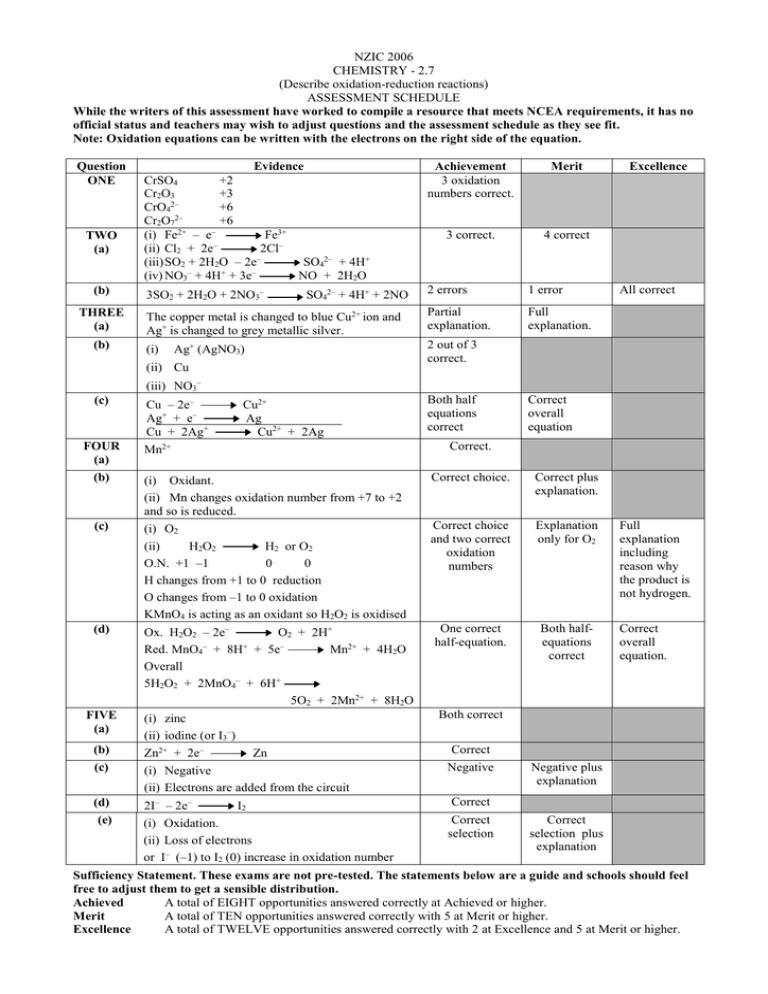
NZIC 2006 CHEMISTRY - 2.7 (Describe oxidation-reduction reactions) ASSESSMENT SCHEDULE While the writers of this assessment have worked to compile a resource that meets NCEA requirements, it has no official status and teachers may wish to adjust questions and the assessment schedule as they see fit. Note: Oxidation equations can be written with the electrons on the right side of the equation. Question ONE TWO (a) (b) THREE (a) (b) Evidence CrSO4 +2 Cr2O3 +3 CrO42– +6 Cr2O72– +6 (i) Fe2+ – e– Fe3+ – (ii) Cl2 + 2e 2Cl– – (iii) SO2 + 2H2O – 2e SO42– + 4H+ – + – (iv) NO3 + 4H + 3e NO + 2H2O Achievement 3 oxidation numbers correct. Merit 3 correct. 4 correct 3SO2 + 2H2O + 2NO3– 2 errors 1 error Partial explanation. Full explanation. SO42– + 4H+ + 2NO The copper metal is changed to blue Cu2+ ion and Ag+ is changed to grey metallic silver. (i) Excellence All correct 2 out of 3 correct. Ag+ (AgNO3) (ii) Cu (iii) NO3– (c) FOUR (a) Cu – 2e– Ag+ + e– Cu + 2Ag+ Cu2+ Ag Cu2+ + 2Ag Both half equations correct Correct overall equation Correct. Mn2+ (b) (i) Oxidant. (ii) Mn changes oxidation number from +7 to +2 and so is reduced. Correct choice. Correct plus explanation. (c) (i) O2 (ii) H 2O 2 H2 or O2 O.N. +1 –1 0 0 H changes from +1 to 0 reduction O changes from –1 to 0 oxidation KMnO4 is acting as an oxidant so H2O2 is oxidised Correct choice and two correct oxidation numbers Explanation only for O2 (d) Ox. H2O2 – 2e– O2 + 2H+ Red. MnO4– + 8H+ + 5e– Mn2+ + 4H2O Overall 5H2O2 + 2MnO4– + 6H+ 5O2 + 2Mn2+ + 8H2O One correct half-equation. Both halfequations correct FIVE (a) Correct overall equation. Both correct (i) zinc (ii) iodine (or I3–) (b) Zn2+ + 2e– (c) (i) Negative (ii) Electrons are added from the circuit (d) 2I– – 2e– (e) Full explanation including reason why the product is not hydrogen. Zn I2 (i) Oxidation. (ii) Loss of electrons or I– (–1) to I2 (0) increase in oxidation number Correct Negative Negative plus explanation Correct Correct selection Correct selection plus explanation Sufficiency Statement. These exams are not pre-tested. The statements below are a guide and schools should feel free to adjust them to get a sensible distribution. Achieved A total of EIGHT opportunities answered correctly at Achieved or higher. Merit A total of TEN opportunities answered correctly with 5 at Merit or higher. Excellence A total of TWELVE opportunities answered correctly with 2 at Excellence and 5 at Merit or higher.











