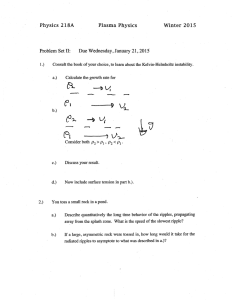
∂ =
advertisement

Homework G PHY3513 Due: In class Nov. 16, 2009 1. (16.1) Try without looking up the derivations in your textbook. ∂U 2. (16.2) (a) Use the cyclic relation and we derived = ? in the class. ∂V T 3. (16.2) (b) ∂Q ∂U ∂U 4. (16.3) dU = dQ − pdV = dT + dV and C p = and ∂T V ∂V T ∂T V 1 ∂V β p = . V ∂T p 5. (16.4) We already worked on the part (a). For the part (b), you have to first show 1 ∂U ∂ p ∂U ∂p = T − p from dU(T,V). Then, show 2 = . T ∂V T ∂T V T ∂V T ∂T V 6. Derived the entropy of an ideal gas in two different forms: S (T , V ) = S o + CV ln T + Nk B ln V S (T , p ) = S o′ + C p ln T − Nk B ln p Derive above expressions again and show that they are identical within a constant. Ex.16.6 shows you how to derive the first expression S(T,V). Follow a similar path to derive the second expression by choosing S = S(T, p).