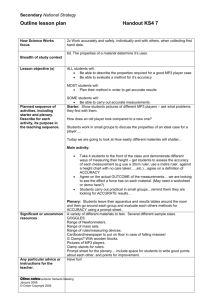
Figure 1: Completed fuel cell assembly Figure 2: Electrode and membrane 1
advertisement

Fuel Cell for mp3 Player Josh Kortas Figure 1: Completed fuel cell assembly Figure 2: Electrode and membrane 1 Summary: The idea behind this design project is to develop a fuel cell system for use in an mp3 player. It is true that used batteries can be harmful to the environment, and should be avoided, but the main point of the project is to demonstrate a technology in its development stages. The specific mp3 player in this example runs off of one AAA battery and is usually attached to an elastic, Velcro armband for use while working out. Tests run on the mp3 player have determined the peak draw on a battery to be 0.16 amps at 1.5 Volts. After the power requirements have been satisfied, the most important requirements are being small enough and light enough to fit on an armband. Durability and ease of use were also considered to make the apparatus suitable as a battery replacement. Different components of the system were designed independently. For the fuel cell portion, two separate cells had to be used since the operating voltage of a single cell was 0.8 V. Two pre-assembled cells were considered, but would have been too bulky. The reversible nature of the cells required extra components. Instead the fuel cell was to be constructed in a direct stack where the cathode of one cell was in direct contact with the anode from another cell. Graphite board was considered because it was both light and thin, but a previous student had difficulty getting them to seal due to their flexibility. A few options were considered for the hydrogen storage portion. A piston and cylinder option was rejected due to the bulkiness of the design and the difficulties it would cause while filling. The water displacement method used in the reversible fuel cell kits only works well without significant movement. The electrical connection had two main designs. Alligator clips were dismissed because the connection is not very strong and solder were dismissed because the connection would be too permanent. The more complicated idea involved a spring that changed the length of the “battery” so that the same apparatus could be used to connect to AAA and AA batteries with little effort. The final design employed three solid sheets of graphite as the electrodes. The middle sheet served as the anode of one cell and the cathode of the other cell to save space and time. A piece of circuit board was used to insulate one side. Aluminum with a groove served as the endplate and formed a loop for connecting to the armband. The whole device was held together with nylon screws for insulation. Grooves made with a 1/8” ball end-mill were used to channel gasses over the active sites. The oxygen electrode (cathode) had channels to the atmosphere to both obtain atmospheric oxygen and to remove of the water product of the reaction. Silicone gaskets served to electrically insulate the anode from the cathode and to seal in the hydrogen. The hydrogen traveled from a custom fitting through the electrodes to both of the anodes through drilled holes. The hydrogen was stored in a bladder recovered from an infant-sized blood pressure cuff. It was pressure fitted to the barbs on the pipe fitting and was attached to the armband via Velcro. The wires are connected with wooden dowel template the size of a AAA battery with screws as electrodes. The cell measured 6 cm by 6 cm by 4 cm. The fuel cell was able to produce in excess of 1.5V and the current necessary to run the mp3 player for over a minute. The pressure on the oxygen side had to be adjusted with the sphygmomanometer bulb to work. The cell was able to consistently produce 0.6 V with a draw. Hydrogen leaked from the bladder, probably due to diffusion through the walls, so a different storage method should be chosen. The total cost was $113 and the weight of the cell was 2/3 of a pound. 2 3