Johnson Group Research Summary
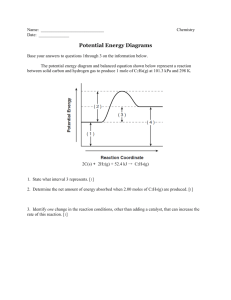
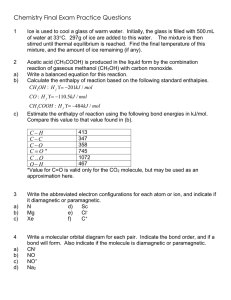
advertisement

Johnson Group Research Summary (updated November 2013) Work in the Johnson lab focuses on the mechanistic studies of known reactions, and the development of new reaction methodology. These two areas feed one another, with the results of mechanistic studies informing the development of new reactions, and new reactions providing a subject for mechanistic investigations. O N R' O O + R2Zn [Ni] N H R' N R' R O O + R2Zn [Ni] Decarbonylative Alkylation N R' HO R O Alkylative Desymmetrization Mechanistic Studies O OH [Pd] Ar-Br N + Ar-Ph β-aryl Elimination and Coupling N [Rh] O O O O Alkene Carboacylation Projects Involving Carbon-Carbon Single Bond Activation Many of the major products in the Johnson lab focus on the understanding and development of methodologies utilizing carbon-carbon single bond activation. Although still in relatively early stages of development, the process of C-C bond activation is slowly expanding beyond esoteric reactions limited to strained rings and highly specialized substrates. Through mechanistic studies, efforts are underway to better understand this transformation and to focus on the functionalization of organometallic intermediates. In time this transformation has the promise to become a common synthetic tool such as its related reaction, C-H bond activation. For our previous work in this area, see (undergraduate coauthors are underlined): Rathbun, C. M.; Johnson, J. B. J. Am. Chem. Soc. 2011, 133, 2031. Lutz, J. P.; Rathbun, C. M.; Stevenson, S. M.; Powell, B. M.; Boman, T. S.; Baxter, C. E.; Zona, J. M.; Johnson, J. B. J. Am. Chem. Soc. 2012, 134, 715. Bour, J. R.; Green, J. C.; Winton, V. J.; Johnson, J. B. J. Org. Chem. 2013, 78, 1665. Johnson Research Summary – Page 2 New Methodology Using information gathered from previous mechanistic studies, our group is currently developing new methodology that utilizes transition metal-catalyzed carbon-carbon single bond activation to generate nucleophiles for cross-coupling reactions. F O [Rh(C2H4)2Cl]2 (5 mol%) O + OMe N F O OMe 130 oC, 16h excess H N N O O O (cat) [Rh(C2H4)2Cl]2 (cat) N O [Rh(C2H4)2Cl]2 (cat) O O OMe N -CO OMe O Investigation of Directing Groups To achieve C-C bond activation, a directing group is necessary to promote the desired reactivity. Our group is currently exploring potential directing groups and evaluating their relative abilities to promote C-C bond activation in aryl ketones. N O [Rh] N N = N N N -CO O N N O NH2 Mechanism of Ketone Decarbonylation Aryl ketones containing benzoquinoline or 2-pyridyl directing groups have been demonstrated to undergo rhodium-catalyzed decarbonylation. There are two probable metalacyclic intermediates for this process, shown below. Mechanistic investigations are underway to determine the relatively likelihood of each pathway. N O [Rh] N O [Rh] N - [Rh] [Rh] CO N [Rh] O - CO N Johnson Research Summary – Page 3 Projects Involving Nickel-Catalyzed Alkylation Methodology For our previous work in this area, see (undergraduate coauthors are underlined): Dennis, J. M.; Calyore, C. M.; Sjoholm, J. M.; Lutz, J. P.; Gair, J. J.; Johnson, J. B. Synlett eFirst (October, 2013). Havlik, S. E.; Simmons, J. M.; Winton, V. J.; Johnson, J. B. J. Org. Chem. 2011, 76, 3588. Mechanism of Decarbonylative Coupling Our initial work achieved the nickel-mediated decarbonylative cross-coupling of imides with diorganozinc reagents. Efforts are ongoing to understand the mechanism of this process. Investigations include kinetic work, intermediate analysis with in situ IR and NMR spectroscopy, and the analysis of product mixtures. O + Et2Zn (3 equiv) N R O 100 mol% Ni(acac)2 110 mol% bipy N H dioxane, 95 oC Et O 13 C NMR R in situ IR Development of Catalytic Decarbonylative Coupling Although it uses inexpensive nickel species, our early methodology is limited by the requirement of stoichiometric metal. Several approaches are being explored to develop methods that are catalytic in the transition metal necessary for reactivity. These include changing variables such as the solvent, the ligand, and the temperature, but also exploring substrate-based attributes such as the electronics of the N-substitution. O N R + Et2Zn (1.1 equiv) O 10 mol% Ni(COD)2 11 mol% ligand N H R Et O Exploration of Regioselectivity We continue to explore our earlier methodology through the investigation of the regioselectivity of decarbonylative alkylation using unsymmetrically substituted phthalimide substrates. O R' N R O + Et2Zn (3 equiv) 100 mol% Ni(acac)2 110 mol% bipy O R' R' N H dioxane, 95 oC Et Et R H N and/or O R Johnson Research Summary – Page 4 Use of Saturated Backbones Efforts are ongoing to expand the substrate scope to include non-aromatic backbones. With the decarbonylative methodology, this could lead to the construction of new Csp3-Csp3 bonds as well the formation of β-substituted chiral amides with contiguous stereocenters from achiral precursors. O N R O + Et2Zn (1.1 equiv) 10 mol% Ni(COD)2 11 mol% ligand -CO O Et N H R