A (very) Little Organic Chemistry
advertisement

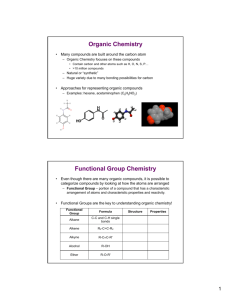
A (very) Little Organic Chemistry Carbon Chemistry • Electronic structure leads to a variety of bonding possibilities – Infinite number of p possible combinations! Organic Chemistry Rule of thumb: Carbon typically forms four bonds! – Four possible combinations of single, double & triple – Stability and movement of bonds in carbon compounds. Allotropic forms of carbon: • Diamond - sp3 • Graphite - sp2 • Fullerenes (buckyballs & buckytubes) - "sp2" 1 Classes of Carbon Compounds Organic compounds are classified by functional group – Group of atoms that has a characteristic structure and reactivity. Class Alkane Formula C-C and C-H single bonds Alkene R2-C=C-R2 Alkyne R-CC-R' Alcohol R-OH Ether R-O-R' Structure Properties 2 1 More Functional Groups Class Formula R-NH2, R NH R' R-NH-R' Amine Aldehyde R-CHO Carboxylic Acid R-COOH Ester R-COO-R' Amide RCON-R’ Structure Properties • Because of common reactivity of functional groups, it is possible to predict reactivity of organic compounds 3 Functional Group Reactivity EXAMPLE: Ester formation • Esters can be formed by the dehydration reaction of an alcohol and a carboxylic acid as shown below: O R C O OH + R' OH H+ R C O R' H OH + • “R” can be any group O O H3 C OH C OH H3C OH + H+ C O CH3 + H OH OH O C H3 C OH + H3C OH H+ O C O CH3 + H OH 4 2 Functional Group Reactivity So, we can build larger, more complicated molecules by taking advantage of functional group reactivity! – Regardless of whether we we're re talking about small molecules like methanol or huge molecules like proteins, behavior typically boils down to functional group reactivity! 5 Identification and Naming of Organic Compounds Two key criteria 1. Types of functional groups present 2. Length g of carbon backbone • Prefix tells length of carbon chain • Virtually any organic compound can be named based on rules developed from these criteria. Name Molecular Formula Structural Formula Isomers methane CH4 CH4 1 ethane C2H6 CH3CH3 1 propane p p C3H8 CH3CH2CH3 1 butane C4H10 CH3CH2CH2CH3 2 pentane C5H12 CH3(CH2)3CH3 3 hexane C6H14 CH3(CH2)4CH3 5 CH3(CH2)5CH3 9 heptane C7H16 octane C8H18 CH3(CH2)6CH3 18 nonane C9H20 CH3(CH2)7CH3 35 CH3(CH2)8CH3 75 decane C10H22 6 3 Organic Nomenclature: IUPAC Rules for Alkane Nomenclature: 1. Find and name the longest continuous carbon chain. 2 Identify and name groups attached to this chain. 2. chain 3. Number the chain consecutively, starting at the end nearest a substituent group. 4. Designate the location of each substituent group by an appropriate number and name. 5. Assemble the name, listing groups in alphabetical order. The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing. Alkyl Substituents: Group CH3– C2H5– CH3CH2CH2– (CH3)2CH– Name Methyl Ethyl Propyl Isopropyl Group CH3CH2CH2CH2– (CH3)2CHCH2– CH3CH2CH(CH3)– (CH3)3C– Name Butyl Isobutyl sec-Butyl tert-Butyl 7 Means of representing organic compounds: 1. Molecular Formula: Identifies only number and types of atoms present, no structural information. Ex: C4H10 2. Structural Formula: Also gives structural information (Lewis structure). Allows us to distinguish structural isomers. – Structural isomers (aka constitutional isomers): Same molecular formula, different structural formula (arrangements of atoms). Ex: C4H10 has two isomers TWO DIFFERENT COMPOUNDS! 3 Condensed 3. C d d Formula: F l Hybrid H b id off molecular l l and d structural t t l fformulae. l Attempts to provide some structural information. 4. Skeletal Formula: Shows carbon backbone and any non-H atoms 8 4 Isomers Isomers: different compounds with same molecular formula Stereoisomers: isomers where connections are same, but arrangement in space different Constitutional Isomers: isomers where atoms are connected differently Enantiomers: stereoisomers that are mirror images Diastereomers: stereoisomers that are not mirror images EXAMPLE: C2H2Cl2 has 3 possible structures, only 2 are diastereomers 9 Enantiomers Molecules that have Enantiomers are Chiral – Amino Acids are one Enantiomer – Some bacteria use the amino acids of the other chirality to trick their hosts Enantiomers have similar physical properties (nearly identical) – Interact differently with polarized light – May have dramatically different reactivity • Thalidomide 10 5 Polymer Chemistry “Big” molecules, but the result of several functional group reactions •Monomer A •+ •Monomer B •Repeat Unit •Repeat Unit •Repeat Unit •Repeat Unit •Polymer Polymer properties depend on several factors, including: – the chemical composition of the monomer units, – length of the chain, chain – the three dimensional arrangement of the chains in the solid, – the branching in the chain, – the bonding/interaction between chains, Plasticizers: Crosslinking: 11 Polymerization Reactions Two major classes of polymerization reactions: Addition and Condensation Addition Polymerization – no other products are formed Examples: polyethylene and polystyrene H n H C H C H H H H C C H H H n H n H C H C H H H C C H H n 12 6 Polymerization Reactions Condensation Polymerization – small molecules are produced as byproducts Example: Polytehylene Terephthalate (PETE) O HO CH2 CH2 OH + O HO C C OH O O H O CH2 CH2 O C C O H + n H2O n • More condensation polymers – Polyamides (Nylon and Proteins) OH O O + NH2 H2N OH O NH2 O O OH R O H2N R + CH C OH N + H2O n H H2N C R CH OH R O O CH C NH2 CH NH O C + H2O 13 OH The Big Six 14 7