Western Illinois University 1 University Circle, Macomb IL 61455 Chemistry Department
advertisement

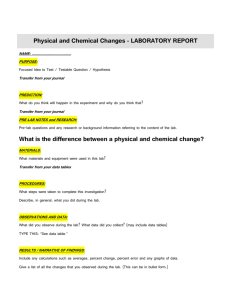
Western Illinois University 1 University Circle, Macomb IL 61455 Chemistry Department General Chemistry I (Gen Ed/Natural Sciences– IAI P1 902 L) CHEM101 Laboratory Syllabus, Spring 2016 Laboratory Location: Currens 327 Lab Book is required: Chem 101 Laboratory Manual, Western Illinois University 1st edition by Pearson Solutions 2011. ISBN 10: 0-558-88734-1; ISBN 13: 978-0-558-88734-6 Students enrolled in this course are levied a non-refundable laboratory usage fee of $35 to cover the cost of consumable supplies utilized during the semester. Lab Coordinator: Dr. Mai-Lei Chen, Office: Currens 519-B Phone: 298-2578; Fax: 298-2180 Email: m-chen2@wiu.edu Laboratories: Coordinated by Dr. Mai-Lei Chen and taught by the graduate teaching assistants in the following sessions:(TA assignments may be changed) Sections Time 021 Tuesday 8 am - 9:50 am TA Taiwo Esan Email te-esan@wiu.edu 022 Tuesday 10 am - 11:50 am Nakoa Kemp nl-kemp@wiu.edu 023 Tuesday 1 pm - 2:50 pm Rebecca Corbett rn-corbett@wiu.edu 024 Tuesday 3 pm - 4:50 pm Nakoa Kemp nl-kemp@wiu.edu 025 Thursday 8 am - 9:50 am David Vanderway dr-vanderway@wiu.edu 026 Thursday 10 am - 11:50 am Rebecca Corbett 1 rn-corbett@wiu.edu Experiments: Each student is expected to successfully complete all 10 experiments. Lab Reports: Each laboratory report consists of 3 parts: pre-lab questions, experiment’s report sheets and post-lab questions. Each lab is total of 15 points. Pre-lab Questions: The questions preceding each experiment are to be answered before coming to lab and submitted to the TA at the beginning of each lab period. Report sheets and Post-lab Questions: The report sheets, which include experimental data and calculations as well as answers to the post-lab questions, should be submitted at the beginning of the next lab period. All questions should be answered unless otherwise indicated by your instructor. Late report policy: If lab reports are turned in 1 day late or within the same week of scheduled deadlines, students only earn 90% of their original lab grade. If lab reports are turned in one week late, students only earn 50% of their original lab grade. After one week, no lab reports will be accepted and students will get a zero for that lab report. Lab Quizzes: Five Lab Quizzes will be posted in WesternOnline under “Assessment”. Each quiz is 10 points. You have two attempts for each lab quiz, and the highest score will be counted. If students miss the deadline for submitting their lab quiz on WesternOnline, no quiz makeup will be offered. Lab Makeup: Only one lab makeup for the excused absence will be allowed. The makeup lab is scheduled in Week 15 (04/26-04/28). * You must earn at least 60% (120 points) of the maximum points attainable (200) In the lab part or you automatically fail the course. * If a student misses three labs including both excused and non-excused absences, the student will fail the course immediately. Lab Grading System: 10 Labs (15 points each) = 150 points 5 Lab Quizzes (10 points each) = 50 points Total: 200 points Attendance: Each student is expected to attend and complete each scheduled laboratory. Excused absences will be allowed due to illness and certain other mitigating circumstances. In such cases, it is the student’s responsibility to contact the instructor. Additionally, it is the student’s responsibility to ensure that the instructor is informed of his or her presence, so that he or she will not be marked absent from the assigned laboratory. Personal protective eye wear is required. The student must purchase safety goggles and lab manual. Students are not allowed to participate lab if they don’t have safety goggles. Students are expected to wear clothing that completely covers the feet and legs in lab. (Sandals, skirts, dresses and shorts are not allowed in lab). 2 CHEM101 Laboratory Learning Outcomes: Lab 1 Density and Specific Gravity Outcomes: After students complete this laboratory session, they will be able to: 1. calculate the density of a substance from measuring its mass and volume. 2. calculate the specific gravity of a liquid from its density. 3. determine the specific gravity of a liquid using a hydrometer. Assignments: 1. Reading Lab 1 (Lab manual p. 9-15) 2. Pre-Lab 1 Study Questions (p. 17), Lab 1 Report Sheet, and Questions and Problems (p. 19-23). Lab 2 Separation of the components of a Mixture Outcomes: After students complete this laboratory session, they will be able to: 1. employ decantation, extraction, and sublimation techniques to separate substances from one another. Assignments: 1. Reading Lab 2 (Lab manual p. 25-29) 2. Pre-Lab 2 Questions ( p. 29), Lab 2 Report Sheet (p. 31-32), and Questions 1-5 (p. 32) * Lab Quiz 1 (Lab 1 and Lab 2): Due 02/22 at 8:00 pm Lab 3 Empirical Formulas of Compounds Outcomes: After students complete this laboratory session, they will be able to: 1. determine the empirical formula for magnesium oxide. 2. determine the empirical formula for copper(II) sulfide. 3. learn techniques for using a crucible. Assignments: 1. Reading Lab 3 (Lab manual p. 35-38) 2. Pre-Lab 3 Assignment Q. 1-9 (p. 39-40), Lab 3 Report Sheet (p. 41-42), and Post-Lab 3 Assignment Q. 1-7 (p. 43-44). Lab 4 Chemical Reactions and Equations Outcomes: After students complete this laboratory session, they will be able to: 1. describe physical and chemical properties associated with chemical changes. 2. give evidence for the occurrence of chemical reactions. 3. write a balanced equation for a chemical reaction. 4. identify a reaction as a combination, decomposition, replacement, or combustion reaction. Assignments: 1. Reading Lab 4 (Lab manual p. 47-53) 2. Pre-Lab 4 Study questions 1-4 (p. 55), Lab 4 Report Sheet (p. 57-59) and Lab 4 Questions and Problems Q. 1-5 (p. 59-60) * Lab Quiz 2 (Lab 3 and Lab 4): Due 03/07 at 8:00 pm 3 Lab 5 Chemicals in Everyday Life Outcomes: After students complete this laboratory session, they will be able to: 1. observe some reactions of common substances found around the home. 2. identify these substances. Assignments: 1. Reading Lab 5 (Lab manual p. 61-65) 2. Pre-Lab 5 Questions 1-12 (p. 65-66), Lab 5 Report Sheet (p. 67-68), and Questions 1-8 (p. 68-69). Lab 6 Behavior of Gases: Molar Mass of a Vapor Outcomes: After students complete this laboratory session, they will be able to: 1. observe how changes in temperature and pressure affect the volume of a fixed amount of a gas. 2. determine the molar mass of a gas from a knowledge of its mass, temperature, pressure, and volume. Assignments: 1. Reading Lab 6 (Lab manual p. 73-78) 2. Pre-Lab 6 Questions 1-12 (p. 78-79) , Lab 6 Report Sheet & Questions 1-4 (p. 81-82), and Gas-Law Problems 1-9 (p. 83-84). * Lab Quiz 3 (Lab 5 and Lab 6): Due 03/28 at 8:00 pm Lab 7 Soluble and Insoluble Salts Outcomes: After students complete this laboratory session, they will be able to: 1. predict the formation of an insoluble salt. 2. observe the effect of temperature on solubility. 3. measure the solubility of KNO3 at various temperatures, and graph a solubility curve. 4. test a variety of water samples for water hardness. 5. use water treatment techniques to purify water. Assignments: 1. Reading Lab 7 (Lab manual p. 87-92) 2. Pre-Lab 7 Study Questions 1-4 (p. 93), Lab 7 Report Sheet, Questions and Problems 14 (p. 95-98). Lab 8 Analysis of Alum Outcomes: After students complete this laboratory session, they will be able to: 1. determine the percentage of water in alum hydrate. 2. determine the percentage of water in an unknown hydrate 3. calculate the water crystallization for an unknown hydrate 4. develop the laboratory skills for analyzing a hydrate. Assignments: 1. Reading Lab 8 (Lab manual p. 99-102) 2. Pre-Lab 8 Assignment Q. 1-8 (p. 103-104), Lab 8 Report Sheet (p. 105-106), and PostLab 8 Assignment Q. 1-6 (p. 107-108). * Lab Quiz 4 (Lab 7 and Lab 8): Due 04/11 at 8:00 pm 4 Lab 9 Reaction Rates and Equilibrium Outcomes: After students complete this laboratory session, they will be able to: 1. identify a reaction as exothermic or endothermic. 2. identify the factors that affect the rate of reaction. 3. observe that chemical reactions are reversible. 4. identify factors that cause a shift in equilibrium. Assignments: 1. Reading Lab 9 (Lab manual p. 111-118) 2. Pre-Lab 9 Study Questions 1-4 (p. 119), Lab 9 Report Sheet, Questions and Problems 1-9 (p. 121-125). Lab 10 Acid-Base Titration Outcomes: After students complete this laboratory session, they will be able to: 1. prepare a sample for titration with a base. 2. set up a buret and perform a titration. 3. calculate the molar concentration and percentage of acetic acid in vinegar. 4. determine the acid-absorbing capacity of a commercial antacid. Assignments: 1. Reading Lab 10 (Lab manual p. 127-134) 2. Pre-Lab 10 Study Questions 1-5 (p. 135), Lab 10 Report Sheet, Questions and Problems 1-2 (p. 137-139). * Lab Quiz 5 (Lab 9 and Lab 10): Due 04/25 at 8:00 pm 5 CHEM101 Lab schedule Week of / 2016 1 (01/19-01/21) Spring 2016 Expt. # -- Experiment No Lab 2 (01/26-01/28) -- 3 (02/02-02/04) -- 4 (02/09-02/11) 1 Density and Specific Gravity (p. 9-23) 5 (02/16-02/18) 2 Separation of the Components of a Mixture (p. 25-33) 6 (02/23-02/25) 3 7 (03/01-03/03) 4 No Lab Check–in and Safety Rules (p. 1-8) *Bring safety goggles and Lab Manual Quiz 1 (Lab 1 & 2) is due on Mon. 2/22 at 8:00 pm Empirical Formulas of Compounds (p. 35-45) Chemical Reactions and Equations (p. 47-60) Quiz 2 (Lab 3 & 4) is due on Mon. 3/7 at 8:00 pm 8 (03/08-03/10) 5 9 (03/15-03/17) -- No Lab (Spring Break) 10 (03/22-03/24) 6 Behavior of Gases: Molar Mass of a Vapor (p. 73-86) 11 (03/29-03/31) 7 12 (04/05-04/07) 8 13 (04/12-04/14) 9 14 (04/19-04/21) 10 Chemicals in Everyday Life (p. 61-71) Quiz 3 (Lab 5 & 6) is due on Mon. 3/28 at 8:00 pm Soluble and Insoluble Salts (p. 87-98) Analysis of Alum (p. 99-109) Quiz 4 (Lab 7 & 8) is due on Mon. 4/11 at 8:00 pm Reaction Rates and Equilibrium (p.111-125) Acid-Base Titration (p. 127-139) Quiz 5 (Lab 9 & 10) is due on Mon. 4/25 at 8:00 pm 15 (04/26-04/28) Check out/Lab Makeup 6