Mitigating the impact of oil-palm monoculture Contributed Paper
advertisement

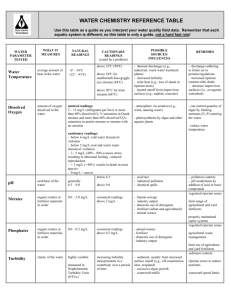
Contributed Paper Mitigating the impact of oil-palm monoculture on freshwater fishes in Southeast Asia Xingli Giam,∗ † ‡‡§§ Renny K. Hadiaty,‡ Heok Hui Tan,† Lynne R. Parenti,§ Daisy Wowor,‡ Sopian Sauri,‡ Kwek Yan Chong,∗∗ Darren C. J. Yeo,∗∗ and David S. Wilcove∗ †† §§ ∗ Department of Ecology and Evolutionary Biology, Princeton University, Princeton, NJ 08544, U.S.A. †Lee Kong Chian Natural History Museum, National University of Singapore, S117546, Singapore ‡Museum Zoologicum Bogoriense, Research Center for Biology, Indonesian Institute of Sciences, Cibinong 16911, Indonesia §Division of Fishes, Department of Vertebrate Zoology, National Museum of Natural History, P.O. Box 37012, MRC 159, Smithsonian Institution, Washington, D.C. 20013, U.S.A. ∗∗ Department of Biological Sciences, National University of Singapore, S117543, Singapore ††Woodrow Wilson School of Public and International Affairs, Princeton University, Princeton, NJ 08544, U.S.A. Abstract: Anthropogenic land-cover change is driving biodiversity loss worldwide. At the epicenter of this crisis lies Southeast Asia, where biodiversity-rich forests are being converted to oil-palm monocultures. As demand for palm oil increases, there is an urgent need to find strategies that maintain biodiversity in plantations. Previous studies found that retaining forest patches within plantations benefited some terrestrial taxa but not others. However, no study has focused on aquatic taxa such as fishes, despite their importance to human well-being. We assessed the efficacy of forested riparian reserves in conserving freshwater fish biodiversity in oil-palm monoculture by sampling stream fish communities in an oil-palm plantation in Central Kalimantan, Indonesia. Forested riparian reserves maintained preconversion local fish species richness and functional diversity. In contrast, local and total species richness, biomass, and functional diversity declined markedly in streams without riparian reserves. Mechanistically, riparian reserves appeared to increase local species richness by increasing leaf litter cover and maintaining coarse substrate. The loss of fishes specializing in leaf litter and coarse substrate decreased functional diversity and altered community composition in oilpalm plantation streams that lacked riparian reserves. Thus, a land-sharing strategy that incorporates the retention of forested riparian reserves may maintain the ecological integrity of fish communities in oil-palm plantations. We urge policy makers and growers to make retention of riparian reserves in oil-palm plantations standard practice, and we encourage palm-oil purchasers to source only palm oil from plantations that employ this practice. Keywords: agriculture, buffers, fishes, functional diversity, land sharing, riparian reserves, stream, structural equation model Mitigación del Impacto del Monocultivo de Palma de Aceite sobre los Peces de Agua Dulce en el Sureste Asiático Resumen: El cambio en la cobertura antropogénica del suelo está causando una pérdida mundial de biodiversidad. En el epicentro de la crisis se encuentra el sureste asiático, en donde los bosques ricos en biodiversidad se convierten a monocultivos de palma de aceite. Junto con el incremento de la demanda por la palma de aceite hay una necesidad urgente para encontrar estrategias que mantengan la biodiversidad dentro las plantaciones. Estudios previos encontraron que la retención de fragmentos de bosque dentro de las plantaciones benefició a algunos taxones terrestres pero no a los demás. Sin embargo, ningún estudio se ha enfocado en los taxones acuáticos, como los peces, a pesar de su importancia para el bienestar humano. Evaluamos la eficacia de las reservas ribereñas de bosque en la conservación de la diversidad de peces de agua dulce en los monocultivos de palma de aceite al muestrear a las comunidades de peces en una plantación ‡‡Current address: School of Aquatic and Fishery Sciences, University of Washington, Seattle, WA 98105, U.S.A. §§Address correspondence to: Xingli Giam, email giamxingli@gmail.com or David S. Wilcove, email dwilcove@princeton.edu Paper submitted August 14, 2014; revised manuscript accepted December 14, 2014. 1357 Conservation Biology, Volume 29, No. 5, 1357–1367 C 2015 Society for Conservation Biology. DOI: 10.1111/cobi.12483 1358 Conserving Fishes in Oil-Palm Monocultures de palma de aceite en Kalimantan Central, Indonesia. Las reservas ribereñas de bosque mantuvieron la diversidad funcional y la riqueza local de especies de peces previa a la conversión. En contraste, la riqueza total y local de especies, la biomasa y la diversidad funcional declinaron marcadamente en los arroyos sin reservas ribereñas. Mecánicamente, pareció que las reservas ribereñas incrementaron la riqueza local de especies al incrementar la cobertura de hojas caı́das y mantener el sustrato áspero. La pérdida de peces especializados en la cobertura de hojas caı́das y el sustrato áspero disminuyó la diversidad funcional y alteró la composición comunitaria en los arroyos de plantaciones de palma de aceite que carecı́an de reservas ribereñas. Ası́, una estrategia de tierras compartidas que incorpora la retención de las reservas ribereñas de bosque puede mantener la integridad ecológica de las comunidades de peces en las plantaciones de palma de aceite. Instamos a quienes hacen las polı́ticas y a los cultivadores a que hagan de la retención de reservas ribereñas en las plantaciones de palma de aceite una práctica estandarizada, y alentamos a los compradores del aceite de palma a que lo hagan sólo de las plantaciones que empleen esta práctica. Palabras Clave: agricultura, amortiguadores, arroyo, diversidad funcional, modelo de la ecuación estructural, peces, reservas, ribereño, tierras compartidas Introduction Anthropogenic land-cover change is the major driver of biodiversity loss worldwide (Millennium Ecosystem Assessment 2005). The destruction of tropical forests deserves particular attention for at least 3 reasons: tropical forests hold the majority of the world’s species (Wilson 1988); biodiversity declines markedly when tropical forests are converted to other cover types (e.g., cropland) (Gibson et al. 2011); and tropical deforestation continues unabated due to increasing demands for food, timber, and other commodities (Balmford et al. 2012; Putz & Romero 2014). One prominent approach to balancing biodiversity conservation with commodity production is to identify strategies that reduce biodiversity losses in production landscapes. Understanding the efficacy of such strategies is imperative for making informed decisions regarding managing production landscapes for conservation (land-sharing approach) versus managing them to maximize commodity yields while preserving intact forests outside production lands (land-sparing approach) (Phalan et al. 2011). The need to quantify the efficacy of measures to protect biodiversity in working landscapes is especially acute in Southeast Asia, where both species richness and deforestation rates are among the highest globally (Sodhi et al. 2004). A major threat to Southeast Asia’s biodiversity is the conversion of forests (mostly selectively logged) to monoculture crops, especially oil-palm (Elaeis guineensis) (Wilcove et al. 2013). Conversion of forests to oilpalm monoculture reduces species richness and changes the composition of both terrestrial and aquatic communities (e.g., Peh et al. 2006; Fayle et al. 2010; Mercer et al. 2014). Thus far, measures to maintain biodiversity within oil-palm plantations (e.g., by leaving patches of forest) have been ineffective for birds (Edwards et al. 2010) and butterflies (Koh 2008), effective for riparian ants (Gray et al. 2015), and partially effective for riparian dung beetles (Gray et al. 2014). Taken together, these results suggest that the land-sparing versus land-sharing Conservation Biology Volume 29, No. 5, 2015 debate remains unresolved. However, no one has assessed the efficacy of a land-sharing strategy to mitigate the impact of oil-palm monoculture on freshwater biota, despite the fact that Southeast Asia is a global hotspot for freshwater fish biodiversity (WWF & TNC 2013) and that many of its people depend on freshwater fishes for protein (MacKinnon et al. 1997). If oil-palm agriculture indeed threatens fish species and if these impacts are not mitigated, consequences could be dire for both biodiversity and humans. The most common strategy used to protect water quality in production landscapes is retaining forests along streams (Allan & Flecker 1993; Naiman & Décamps 1997). Forested riparian reserves (hereafter, riparian reserves) reduce sedimentation, chemical runoff, and maintain temperature regimes in streams within temperate production landscapes (Naiman & Décamps 1997; Sweeney & Newbold 2014); their efficacy in the tropics is less well-studied. RR also maintain terrestrial subsidies to the freshwater system (e.g., inputs of leaf litter, wood, and terrestrial insects) (Dudgeon 2000). However, the degree to which riparian reserves protect aquatic biodiversity seems to vary depending on geography and land-use context. For example, riparian reserves effectively protect fishes in Brazilian (Casatti et al. 2012) and Costa Rican (Lorion & Kennedy 2009) pastures, but do not protect fishes in pine monoculture in New Zealand (Rowe et al. 2002). More studies are needed, especially from the beleaguered forests of Southeast Asia. In Indonesia and Malaysia—the world’s 2 largest palmoil-producing countries—retention of riparian reserves is required by law (Government of Malaysia 1965; Republic of Indonesia 1990). Riparian reserves are also mandatory for growers who join the Roundtable for Sustainable Palm Oil (RSPO) certification program (www.rspo.org), a voluntary program whereby growers agree to employ best management practices aimed at improving environmental and social outcomes in exchange for recognition of the environmental sustainability of their products. But whether riparian reserves actually conserve Giam et al. freshwater biota in tropical oil-palm monocultures is unknown. We attempted to bridge this crucial knowledge gap by asking whether freshwater fish biodiversity can be conserved by retaining forested riparian reserves in oilpalm plantations. We compared freshwater fish species richness, community composition, functional diversity, and biomass in streams located in riparian reserves against streams without riparian reserves in an oil-palm plantation in Central Kalimantan, Indonesia. By comparing our contemporary field data with historical data, we examined whether riparian reserves effectively retained pre-conversion species richness and functional diversity. We also examined causal ecological mechanisms through which riparian reserves affect species richness via the instream environment. Methods Study Landscape We conducted research in a 14,445-ha oil-palm dominated landscape in Central Kalimantan Province, Indonesia, on the island of Borneo. The oil-palm plantation (PT Unggul Lestari; 10,838 ha) was established in 2007 on lands previously covered by logged and secondary forests, community forest gardens, and shrublands. Forested conservation areas and community-owned lands (3607 ha) are embedded within the plantation. The landscape is drained by tributaries of the Mentaya River, which empties into the Java Sea. Riparian reserves comprise selectively logged and secondary forests that have been retained along tributaries. Riparian reserves (>50 m wide) were present along almost all large tributaries (>15 m wide) in the estate. For small tributaries (3–10 m wide), however, the riparian reserves were irregular in shape and width (from 10 to >100 m wide) and were present along some stream lengths but not others, thereby providing an opportunity to test their efficacy. Where riparian reserves were not retained, oilpalms were planted to the stream edge. Pesticides and herbicides were not applied within 50 m of any stream (including streams lacking riparian reserves), which allowed a fair comparison of biodiversity values across sampling sites. Site Description and Fish Sampling Fieldwork was conducted in June 2013. We established 12, 100 m long sampling sites in small streams (3.5–8 m wide, 1 m deep) within the plantation (Fig. 1). Eight sites had forested riparian reserves (RR sites) and 4 sites lacked riparian reserves (i.e., streams surrounded by oil palms [OP sites]). The 8 RR sites were further classified into 2 habitat types: logged forest (RRLF ) (3 sites) and 1359 community forest (RRCF ) (5 sites). Both were dominated by selectively logged or secondary forest, but RRCF sites were continuously disturbed by local people through cultivation of rubber (Hevea brasiliensis), tapioca (Manihot esculenta), banana (Musa cultivars), rattan, or subsistence logging. Each sampling site was in an independent tributary to ensure spatial independence of data. We sampled fishes on clear-weather days using 3 capture methods, performed in the following order, at each site: (1) electrofishing, (2) push-kick netting, and (3) seine netting. We employed these methods to target all major fish microhabitats so as to obtain comprehensive and unbiased fish communities (Supporting Information). Captured fishes were euthanized with MS-222, fixed in 10% formalin, and transferred to 75% ethanol for storage. We used an analytical balance to measure the preserved wet weight of each specimen and calculated the total fish biomass in each sampling site. We identified all fish to species (Kottelat 2013) and deposited specimens in Museum Zoologicum Bogoriense, Indonesia, and Lee Kong Chian Natural History Museum, National University of Singapore. Instream Environmental Variables In each site, we recorded 13 instream environmental variables that could explain fish community responses: littoral leaf litter cover, littoral root cover, volume of large woody debris, substrate size, overstorey canopy cover, stream width, stream depth, stream velocity, dissolved oxygen concentration, water conductivity, dissolved nitrate concentration, dissolved iron concentration, and water pH (Supporting Information). Site- and Meso-Scale Forest Cover We quantified forest cover in riparian zones of multiple widths at 2 scales (site and meso-scale) by visually interpreting a true-color WorldView 2 satellite image at 0.5 m resolution. Site scale was defined as the area immediately adjacent to each 100 m sampling site. Meso-scale was defined as the area adjacent to the 1000 m stream section extending upstream from the downstream boundary of each sampling site. We used ArcGIS 10.2 (ESRI, Redlands, California) to measure site- and meso-scale forest cover at different widths (10, 25, 50, 100, and 200 m) extending outward from both sides of the stream. Preconversion Species Richness in Continuous Forests We were unable to sample continuous forest streams because our study landscape (and much of lowland Central Kalimantan) is deforested. We therefore identified 5 streams that were described as “forest” or “forested” (but otherwise physically similar to our RR and OP sites in Conservation Biology Volume 29, No. 5, 2015 Conserving Fishes in Oil-Palm Monocultures 1360 Figure 1. (a) Study site and fish sampling locations in Central Kalimantan Province, Indonesia, on the island of Borneo (inset: black dot, location of study sites; solid gray shading, Mentaya drainage; gray shading with lines, Kapuas drainage, for which the baseline fish community was reconstructed [see Methods]). Examples of stream habitat types: (b) community forest riparian reserve (RRCF ) (Sungai Karuk); (c) logged forest riparian reserve (RRLF ) (Sungai Samai); and (d) oil-palm (OP) (Sungai Balai). terms of stream width, depth, substrate, and water color or pH) in Tyson R. Roberts’ 1976 survey of the Kapuas drainage, West Kalimantan (Roberts 1989), as preconversion controls (Supporting Information). These forest or forested streams were likely embedded in the typical preconversion forest landscape—a continuous forest matrix of largely logged or secondary forest, community forest gardens, and primary forest patches—because the Kapuas survey predated large-scale oil-palm cultivation in the province (Potter 2008). Sampling lengths of the Kapuas sites were estimated to be at least 100 m (and more likely 200 m) by the researcher who performed the field work (T. R. Roberts, personal communication). To ensure that the Kapuas sites were comparable to our sites, we scaled species richness values to the length of our sampling sites Conservation Biology Volume 29, No. 5, 2015 (100 m) by applying a richness versus sampling length model developed for small lowland forest streams (dos Anjos & Zuanon 2007) (Supporting Information). To assess the sensitivity of our results to the uncertainty in sampling length, we conducted the analyses for a wide range of values (100, 110, 125, 150, 200, and 300 m). Because the Kapuas fish communities were sampled using ichthyocide, these sites were likely to be as (or more) comprehensively sampled as our RR and OP sites and were therefore adequate (or conservative) controls. Functional Diversity Functional diversity can be defined as the variation in species traits that influence ecosystem function (Petchey & Gaston 2006). We focused on one particular aspect Giam et al. of ecosystem function: energy flow (Violle et al. 2007). For each species, we compiled 9 species traits hypothesized to affect energy flow in stream ecosystems through their influence on a species’ life history, feeding strategy, habitat use, and locomotion. The traits compiled from the taxonomic literature (e.g., Roberts 1989), FishBase (www.fishbase.org), and field observations were body size, body shape, trophic position, mouth position, presence of jaw teeth, gregariousness, presence of barbels, vertical position in water column, and air-breathing capability (Supporting Information). We quantified functional diversity with 4 complementary metrics: functional group richness (FGR) (Petchey & Gaston 2006), functional dispersion (FDis) (Laliberté & Legendre 2010), functional evenness (FEve) (Villéger et al. 2008), and Rao’s quadratic entropy (Rao’s Q) (BottaDukát 2005). FGR is calculated by delimiting ecologically relevant functional groups following hierarchical clustering of species based on functional traits (Supporting Information). FDis is the average distance of individual species to the community centroid in trait space (as projected by principal coordinates analysis). FEve quantifies community evenness in trait space. Rao’s Q is similar to FDis in that it describes community dispersion in trait space by measuring the mean pairwise functional distances between species. We used the FD package (Laliberté & Shipley 2011) in R 3.1.0 (R Core Team 2014) to calculate these metrics. Data Analyses We fitted and analyzed generalized linear models (GLMs) in a multimodel inferential framework (Burnham & Anderson 2002) to examine whether local species richness differed among continuous forest, RRCF , RRLF , and OP sites (Supporting Information). We then investigated the mechanisms controlling local species richness. We first identified the best instream environmental correlates of local species richness by comparing univariate GLMs (Supporting Information). Following that we assessed possible mechanisms linking presence of RR to local species richness via instream environmental variables (Supporting Information) by comparing relative support across candidate structural equation models (SEMs). For the best-supported model, we assessed model adequacy with Shipley’s (2009) d-sep test and calculated standardized path coefficients to quantify relative signal strengths across paths (Grace et al. 2012). In addition, we explored the spatial scale at which forest cover best predicts local species richness (Supporting Information). We examined whether biomass differed among RRCF , RRLF , and OP sites by comparing candidate GLMs (Supporting Information). To determine whether RRCF , RRLF , and OP sites supported distinct species communities, 1361 we used the vegan package (Oksanen et al. 2014) in R to perform nonmetric multidimensional scaling (NMDS) and permutational multivariate analysis of variance (perMANOVA) (Anderson 2001). We performed sample-based rarefaction in EstimateS (Colwell 2013) to compare the total species richness supported by each stream type. Finally, we examined whether functional diversity (FGR, FDis, FEve, and Rao’s Q) differed among continuous forest, RR, and OP sites by comparing candidate GLMs (Supporting Information). In all GLM and SEM analyses, we included models with stream width as a covariate to control for its potential effect on species richness, biomass, and functional diversity. The sample size in our study (12–17 sites) was low relative to terrestrial studies (e.g., Peh et al. 2006; Koh 2008) but typical of riparian studies (Martin-Smith 1998; Jones III et al. 1999; Mercer et al. 2014) because sample sizes in the latter are generally limited by the number of independent yet comparable streams in a study landscape. To make accurate inferences from a limited sample size, we fitted Bayesian GLMs and SEMs (Grace et al. 2012), from which inferences on model coefficients and effect sizes are exact and free from large-sample assumptions (Kéry 2010) (Supporting Information). In each GLM and SEM analysis, we identified the bestsupported model with modified deviance information criterion (DIC∗ ) which incorporates a larger penalty for model complexity (Spiegelhalter et al. 2014) because conventional DIC tends to overfit models when sample:parameter size ratio is low (Plummer 2008; Spiegelhalter et al. 2014). To assess goodness of fit of each GLM and SEM submodel, we calculated R2 between observed and predicted values (Grace et al. 2012). To assess model adequacy, we checked observed values against posterior means and 95% prediction intervals. Data and example R code are in Supporting Information. Results Local Species Richness The best-supported GLM indicated that values for local species richness in RRLF , RRCF , and continuous forest sites were similar to each another and higher than values for OP sites after controlling for stream width, assuming the sampling length of the Kapuas continuous forest sites was 200 m (R2 = 0.50) (Fig. 2 & Supporting Information). Changing the sampling length assumption for the Kapuas sites from 110–300 m did not change our results; the bestsupported model remained the same (R2 = 0.47–0.52) (Supporting Information). Conservation Biology Volume 29, No. 5, 2015 Conserving Fishes in Oil-Palm Monocultures 1362 site to meso-scale) and riparian zone width (from 10 to 200 m) increased (Supporting Information). Biomass, Community Composition, and Total Species Richness Figure 2. (a) Relationship between mean fish species richness and stream width in continuous forest (forest) and riparian reserve (RR) sites (solid line) versus oil-palm plantation sites lacking riparian reserves (OP) (dashed line) as indicated by the best-supported model. (b) Model-fitted mean fish biomass in forest and RR sites versus OP sites (diamonds). Shaded areas are 95% prediction intervals. The best-supported GLM indicated that fish biomass was similar in RRLF and RRCF sites and higher in RRLF and RRCF sites than in OP sites (R2 = 0.39) (Fig. 2 & Supporting Information). Community composition was similar in RRLF and RRCF sites (perMANOVA R2 = 0.13, p = 0.151). However, RR sites (consisting of RRLF and RRCF sites pooled together) were distinct from OP sites (perMANOVA R2 = 0.24, p = 0.009). The NMDS ordination supported this result (Fig. 4). Total species richness (rarified to n = 3 sites) was higher in RRLF (mean [SD] = 42 [2.4]) and RRCF sites (40.8 [2.6]) than in OP sites (26.5 [2.7]) (Fig. 5). Functional Diversity For all functional diversity metrics, the best-supported model indicated that functional diversity was similar in RR sites and continuous forest sites and higher in RR sites and continuous forest sites than in OP sites (Table 1 & Supporting Information). Mechanisms Maintaining Local Species Richness Among instream environmental variables, species richness was positively correlated with substrate size, littoral leaf litter cover, canopy cover, and stream depth and negatively correlated with dissolved nitrate concentration and water conductivity (Supporting Information). Leaf litter cover and substrate size were the 2 best predictors of richness both before and after controlling for stream width. The best-supported SEM indicated that riparian reserves increased local species richness by increasing leaf litter cover and maintaining substrate size; leaf litter cover exerted a stronger effect on species richness than substrate size (standardized path coefficients = 0.55 and 0.30, respectively) when stream width was controlled (Fig. 3 & Supporting Information). After accounting for indirect effects of substrate size and leaf litter cover, riparian reserves had no direct effect on species richness. The d-sep test confirmed that the best-supported SEM was adequate (Supporting Information). Forest Cover, Local Species Richness, and Spatial Scales Percent forest cover in the 10 m riparian zone directly adjacent to the sampling site (site scale) best predicted species richness. Predictive power of forest cover (R2 and DIC∗ ) showed a general decline as spatial scale (from Conservation Biology Volume 29, No. 5, 2015 Discussion Our study is the first to indicate that retention of forest patches along streams is effective in conserving aquatic biodiversity in oil-palm plantations. At the local scale, streams within forested riparian reserves in oil-palm plantations supported as many fish species as continuous forests, but streams lacking forested riparian reserves supported far fewer species. For example, our results showed that a 5 m wide OP stream is expected to support, on average, 42% fewer species than a stream of the same size in riparian reserves or continuous forests. At the landscape scale, total species richness was 36% lower in streams lacking riparian reserves than in streams with riparian reserves. How then does retention of riparian reserves in oilpalm plantations maintain fish species richness? From our best-supported SEM, we inferred that littoral leaf litter cover and substrate size increased with the presence of riparian reserves and that these 2 instream environmental variables in turn increased fish species richness. The presence of riparian forest likely maintained quantity and quality of leaf litter inputs at preconversion levels (Pringle et al. 1988). Leaf litter supports fish communities by enhancing and spatially concentrating their food resources—invertebrates, biofilm, and algae (Pringle et al. 1988; Wallace et al. 1997). Leaf litter also provides fishes with a cool, dark microhabitat and shelters them Giam et al. 1363 Figure 3. Best-supported structural equation model of how riparian reserves drive local fish species richness via instream environmental variables. (a) Causal diagram showing standardized path coefficients. Effect of riparian reserves on (b) littoral leaf litter cover and (c) substrate size. Effects of (d) littoral leaf litter cover and (e) substrate size on local species richness (diamonds, model-fitted means; shaded bars, 95% prediction intervals; black lines, relationship between species richness versus [d] littoral leaf litter cover and [c] substrate size when all other variables are constant at their mean values; data points for substrate size are jittered for clarity). Abbreviations: RRLF , logged forest riparian reserve sites; RRCF , community forest riparian reserve sites; OP, oil-palm plantation sites lacking riparian reserves. from predators (Sazima et al. 2006). Fine niche partitioning in terms of space and food among species present in leaf litter may also contribute to high species richness in RR streams (Henderson & Walker 1990). Besides providing leaf litter inputs, riparian reserves likely reduce soil erosion from adjacent uplands and therefore minimize fine substrate deposition into streams (Jones III et al. 1999). Increased siltation and sedimentation reduce fish spawning grounds and zoobenthic food resources (Berkman & Rabeni 1987; Jones III et al. 1999), thereby reducing fish species richness. Our field observations corroborated this: species occurring in streams with gravel (or larger) substrates (mostly benthic or benthopelagic species, for example, Glyptothorax spp., Nemacheilus spp., Acantopsis dialuzona) did not usually occur in clayey or silty streams. Given the importance of riparian reserves for fish, the scale at which forest cover influences species richness needs to be determined. Forest cover in the 10 m zone directly adjacent to the sampling sites best predicted richness patterns, most probably because this is the scale at which terrestrial leaf litter enters the stream. However, species richness remained positively correlated with forest cover up to the maximum spatial scale investigated (200 m riparian zone extending to 1 km upstream of sampling site). Thus, while maintaining forest cover in the first 10 m of the riparian zone is arguably the most important step in conserving fish in oil-palm plantations, retaining forest cover over larger spatial scales appears to also increase species richness, likely by decreasing sedimentation and chemical runoff and maintaining stream temperatures. The community composition of RR streams was distinct from that of OP streams, which is consistent with our finding that removing riparian reserves decreased species richness through reduction of leaf litter and coarse substrate microhabitats. Species that specialize in foraging or hiding in these microhabitats (e.g., loaches, small catfishes) were predictably extirpated in the absence of RR. Pelagic species that do not appear to specialize in particular benthic microhabitats occurred in both OP and RR streams (e.g., Puntigrus navjotsodhii and Rasbora elegans) or only in OP streams (Trigonopoma pauciperforatum). Streams protected by riparian reserves maintained functional diversity comparable to the level observed in Conservation Biology Volume 29, No. 5, 2015 Conserving Fishes in Oil-Palm Monocultures 1364 Figure 4. Fish community composition in logged forest riparian reserve sites (RRLF ), community forest riparian reserve sites (RRCF ), and oil-palm plantation sites lacking riparian reserves (OP) as described by nonmetric dimensional scaling ordination of sites. See Supporting Information for corresponding ordination of fish species. continuous forest, whereas OP streams lacking RR exhibited a reduction in functional diversity. FGR was higher in RR than in OP streams. We obtained the same result with richness-independent metrics, FDis and Rao’s Q, which suggests higher functional diversity in RR streams did not arise just from having more species. Functional groups rare or absent in OP streams included surface-dwelling terrestrial insectivores (e.g., Hemirhamphodon phaiosoma) and species associated with specific benthic microhabitats such as gregarious benthic invertivores (e.g., Pangio spp. associated with leaf litter; Acanthoposoides spp. associated with coarse substrate). The removal of riparian reserves also reduced FEve as pelagic, schooling cyprinids dominated the species assemblage alongside a few functionally different species (e.g., barbelled [Hemibagrus capitulum] and air-breathing [Channa Figure 5. Total fish species richness in logged forest riparian reserve sites (RRLF ), community forest riparian reserve sites (RRCF ), and oil-palm plantation sites lacking riparian reserves (OP). Shaded areas are 95% confidence intervals. striata] predators). Together, these results suggest that functional diversity losses in OP streams were driven by the loss of benthic microhabitats and terrestrial food resources. If these functional groups do not recover as riparian forest regenerates, terrestrial energy inputs (via benthic food chains and consumption of terrestrial insects by fish) may be attenuated, potentially decreasing secondary fish production. Fish biomass was similar in logged forest and community forest RR streams and higher at these streams than in OP streams. This likely resulted from a combination of greater food (energy) availability and a greater diversity of species with complementary niches, conditions that maximized energy transfer and increased fish production in riparian reserves sites relative to OP sites. By retaining riparian reserves land managers can ensure that fish for subsistence use, an important ecosystem service, is maintained at high levels. Across the multiple biodiversity metrics we investigated, riparian reserves comprising in community forests Table 1. Relationship between functional diversity metrics of fish communities and stream types (i.e., continuous forest streams, oil-palm plantation streams located within forested riparian reserves, and oil-palm plantation streams lacking forested riparian reserves) based on the best-supported generalized linear model. Stream type, mean (SD) Functional diversity metricsa FGR FDis Rao’s Q FEve a Abbreviations: b Abbreviations: Continuous forest Forested riparian reserve Oil-palm Inferenceb R2 9.20 (1.92) 0.32 (0.01) 0.11 (0.01) 0.95 (0.02) 11.38 (3.62) 0.33 (0.02) 0.11 (0.01) 0.95 (0.02) 6.75 (3.30) 0.27 (0.08) 0.09 (0.04) 0.90 (0.04) (forest = RR) > OP (forest = RR) > OP (forest = RR) > OP (forest = RR) > OP 0.63 0.28 0.23 0.48 FGR, functional group richness; FDis, functional dispersion; FEve, functional evenness; Rao’s Q, Rao’s quadratic entropy. RR, forested riparian reserve sites; OP, oil-palm plantation sites lacking riparian reserves. Conservation Biology Volume 29, No. 5, 2015 Giam et al. with active human use performed as well as riparian reserves comprising currently unused but previously logged forests. This suggests limited amounts of extractive use do not compromise the utility of riparian reserves for protecting fish, which lowers the opportunity cost of creating such reserves. However, we do not yet know if the efficacy of riparian reserves will persist over time. Given that small tropical freshwater fishes have a typical lifespan of 2–5 years (Ng & Tan 1997) and that we captured juveniles as well as adults of many species across all sites (X.G., unpublished data), fish communities were likely stabilized when we conducted our surveys (6 years after plantation establishment). Nevertheless, our results provide a much-needed baseline for future studies on long-term efficacy of maintaining or restoring riparian reserves. Our study would have benefitted from a larger number of sampling sites. Unfortunately, this was not possible because we sampled the maximum number of comparable and independent sites in our study landscape. However, to ensure that the limited replication did not invalidate our analyses, we fitted Bayesian models that are free from large-sample assumptions. Moreover, the effect sizes in all analyses were medium to large (R2 of best models = 0.23–0.87), giving us confidence in our findings. A potential pitfall is that the model selection metric, DIC, becomes less accurate and tends to overfit models when sample sizes are low. We alleviated this problem by using a modified metric (DIC∗ ) that assigns a larger penalty for model complexity (Spiegelhalter et al. 2014). Overall, we are confident that our model inferences are robust; however, further research, ideally incorporating a larger number of sampling sites over multiple drainages and regions (e.g., Borneo, Sumatra, and Peninsular Malaysia), would help confirm our findings as well as examine alternative mechanisms by which RR can conserve fish communities (e.g., by reducing chemical runoff from pesticides). Although the retention of riparian reserves is mandated by law in Indonesia and Malaysia, noncompliance is common owing to poor enforcement (Lajiun 2013) and, possibly, legal ambiguity. For example, in Indonesia, small and large rivers are mandated to have 50 and 100 m buffers of natural vegetation, respectively (Republic of Indonesia 1990). Yet, definitions of small and large rivers and natural vegetation are lacking; thus, oil-palm growers have wiggle room. Relevant governmental agencies should clarify legislative language pertaining to river width, vegetation type, and type of production landscapes in which riparian reserves are required. Beyond better definition and enforcement of laws by governments, international and national multistakeholder regulatory groups (e.g., RSPO and Indonesia Sustainable Palm Oil) should ensure that member companies maintain riparian reserves. Downstream participants in the palm-oil supply chain such as purchasers and end 1365 users could drive changes in environmental performance of upstream growers. By purchasing only palm oil originating from plantations that retain riparian reserves, downstream participants can create market-based incentives to preserving riparian forests. Our study suggests that a land-sharing strategy that involves retaining forests along streams is effective in conserving freshwater fish biodiversity in oil-palm plantations. Streams with forested riparian reserves do not just hold more fish species than those that lack riparian reserves, they also support higher fish biomass and functional diversity, which are important for sustaining human livelihoods and ecosystem function, respectively. Our results are globally relevant because oil-palm monoculture is expanding in both the Neotropics (Lees & Vieira 2013) and Afrotropics (Wich et al. 2014). We therefore urge governments, regulatory groups, growers, and downstream participants in the oil-palm supply chain to make conservation of forested riparian reserves a key policy objective. Acknowledgments This research was principally funded by a Wildlife Reserves Singapore grant and the Mary and Randall Hack ’69 Award to X.G., and a High Meadows Foundation grant to D.S.W. Rufford Foundation and Idea Wild provided additional funding. The National Museum of Natural History, Smithsonian Institution, supported the publication of this paper. We are grateful to T.R. Roberts for providing information on the Kapuas sites. We thank L.T. Gan, T.S. Chock, management, and staff of Musim Mas Group for research permission and field support, RISTEK for granting a research permit (No: 20/EXT/SIP/FRP/SM/III/2013), and LIPI for coordinating research. We benefitted from discussions with J. Green and A. Yee. We also thank the handling editor, M. Jones, and 4 anonymous reviewers whose comments greatly improved this manuscript. This research is approved by Princeton University IACUC (Protocol 1861). Supporting Information Dendrogram of functional groups (Appendix S1), relationship between instream environmental variables and species richness (Appendix S2), relationship between forest cover and species richness (Appendix S3), species ordination (Appendix S4), summary of instream environmental variables (Appendix S5), description of functional traits (Appendix S6), functional trait values (Appendix S7), functional groups (Appendix S8), candidate model sets (Appendices S9–S14), model rankings (Appendices S15–S17, S19–21), d-sep test (Appendix S18), physical characteristics and species richness of continuous forest Conservation Biology Volume 29, No. 5, 2015 1366 sites (Appendix S22), fish sampling methods (Appendix S23), instream environmental variables (Appendix S24), continuous forest sites: description and richness scaling (Appendix S25), Bayesian model settings (Appendix S26), and data and code (Appendix S27) are available online. The authors are solely responsible for the content and functionality of these materials. Queries (other than absence of the material) should be directed to the corresponding author. Literature Cited Allan JD, Flecker AS. 1993. Biodiversity conservation in running waters. BioScience 43:32–43. Anderson MJ. 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46. Balmford A, Green R, Phalan B. 2012. What conservationists need to know about farming. Proceedings of the Royal Society B 279:2714– 2724. Berkman HE, Rabeni CF. 1987. Effect of siltation on stream fish communities. Environmental Biology of Fishes 18:285–294. Botta-Dukát Z. 2005. Rao’s quadratic entropy as a measure of functional diversity based on multiple traits. Journal of Vegetation Science 16:533–540. Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York. Casatti L, Teresa TB, Gonçalves-Souza T, Bessa E, Manzotti AR, Gonçalves CdS, Zeni JdO. 2012. From forests to cattail: How does the riparian zone influence stream fish? Neotropical Ichthyology 10:205–214. Colwell RK. 2013. EstimateS: Statistical estimation of species richness and shared species from samples. Version 9.1. Core R Team. 2014. R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing. dos Anjos MD, Zuanon J. 2007. Sampling effort and fish species richness in small terra firme forest streams of central Amazonia, Brazil. Neotropical Ichthyology 5:45–52. Dudgeon D. 2000. The ecology of tropical Asian rivers and streams in relation to biodiversity conservation. Annual Review of Ecology and Systematics 31:239–263. Edwards DP, Hodgson JA, Hamer KC, Mitchell SL, Ahmad AH, Cornell SJ, Wilcove DS. 2010. Wildlife-friendly oil palm plantations fail to protect biodiversity effectively. Conservation Letters 3:236– 242. Fayle TM, Turner EC, Snaddon JL, Chey VK, Chung AYC, Eggleton P, Foster WA. 2010. Oil palm expansion into rain forest greatly reduces ant biodiversity in canopy, epiphytes and leaf-litter. Basic and Applied Ecology 11:337–345. Gibson L, et al. 2011. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478:378–381. Government of Malaysia. 1965. National Land Code (No. 56 of 1965). Grace JB, Schoolmaster Jr DR, Guntenspergen GR, Little AM, Mitchell BR, Miller KM, Schweiger EW. 2012. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3, art73. doi: http://dx.doi.org/10.1890/ES12-00048.1. Gray CL, Lewis OT, Chung AYC, Fayle TM. 2015. Riparian reserves within oil palm plantations conserve logged forest leaf litter ant communities and maintain associated scavenging rates. Journal of Applied Ecology 52:31–40. Gray CL, Slade EM, Mann DJ, Lewis OT. 2014. Do riparian reserves support dung beetle biodiversity and ecosystem services in oil palmdominated tropical landscapes? Ecology and Evolution 4:1049– 1060. Conservation Biology Volume 29, No. 5, 2015 Conserving Fishes in Oil-Palm Monocultures Henderson PA, Walker I. 1990. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. Journal of Fish Biology 37:401–411. Jones III EBD, Helfman GS, Harper JO, Bolstad PV. 1999. Effects of riparian forest removal on fish assemblages in Southern Appalachian streams. Conservation Biology 13:1454–1465. Kéry M. 2010. Introduction to WinBUGS for ecologists. Academic Press, Burlington. Koh LP. 2008. Can oil palm plantations be made more hospitable for forest butterflies and birds? Journal of Applied Ecology 45:1002– 1009. Kottelat M. 2013. The fishes of the inland waters of Southeast Asia: a catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bulletin of Zoology Supplement 27:1–663. Lajiun J. 2013. Enforce law on oil palm plantation encroachment. The Borneo Post Online. Available from http://www. theborneopost.com/2013/11/12/enforce-law-on-oil-palmplantation-encroachment-masidi/ (accessed April 2014). Laliberté E, Legendre P. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91:299–305. Laliberté E, Shipley B. 2011. FD: measuring functional diversity from multiple traits, and other tools for functional ecology. R package v.1.0–11. Lees AC, Vieira IC. 2013. Forests: oil palm concerns in Brazilian Amazon. Nature 497:188. Lorion CM, Kennedy BP. 2009. Riparian forest buffers mitigate the effects of deforestation on fish assemblages in tropical headwater streams. Ecological Applications 19:468–479. MacKinnon K, Hatta G, Halim H, Mangalik A. 1997. The ecology of Kalimantan. Periplus Editions, Hong Kong. Martin-Smith KM. 1998. Relationships between fishes and habitat in rainforest streams in Sabah, Malaysia. Journal of Fish Biology 52:458– 482. Mercer EV, Mercer TG, Sayok AK. 2014. Effects of forest conversion to oil palm on freshwater macroinvertebrates: a case study from Sarawak, Malaysia. Journal of Land Use Science 9:260–277. Millennium Ecosystem Assessment. 2005. Current state and trends assessment. Island Press, Washington D.C. Naiman RJ, Décamps H. 1997. The ecology of interfaces: riparian zones. Annual Review of Ecology and Systematics 28:621–658. Ng PKL, Tan HH. 1997. Freshwater fishes of Southeast Asia: potential for the aquarium fish trade and conservation issues. Aquarium Sciences and Conservation 1:79–90. Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H. 2014. Vegan: community ecology package. R package v. 2.0–10. Peh KS-H, Sodhi NS, De Jong J, Sekercioglu CH, Yap CA-M, Lim SL-H. 2006. Conservation value of degraded habitats for forest birds in southern Peninsular Malaysia. Diversity and Distributions 12:572– 581. Petchey OL, Gaston KJ. 2006. Functional diversity: back to basics and looking forward. Ecology Letters 9:741–758. Phalan B, Onial M, Balmford A, Green RE. 2011. Reconciling food production and biodiversity conservation: land sharing and land sparing compared. Science 333:1289–1291. Plummer M. 2008. Penalized loss functions for Bayesian model comparison. Biostatistics 9:523–539. Potter L. 2008. Dayak resistance to oil palm plantations in West Kalimantan, Indonesia. 17th Biennial Conference of the Asian Studies Association of Australia. Available from http://artsonline. monash.edu.au/mai/files/2012/07/lesleypotter.pdf (accessed April 2014). Pringle CM, Naiman RJ, Bretschko G, Karr JR, Oswood MW, Webster JR, Welcomme RL, Winterbourn MJ. 1988. Patch dynamics in lotic systems: the stream as a mosaic. Journal of the North Americal Benthological Society 7:503–524. Giam et al. Putz PE, Romero C. 2014. Futures of tropical forests (sensu lato). Biotropica 46:495–505. R Core Team. 2014. R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing. Republic of Indonesia. 1990. Keputusan Presiden No. 32 Tahun 1990. Roberts TR. 1989. The freshwater fishes of Western Borneo (Kalimantan Barat, Indonesia). Memoirs of the California Academy of Sciences 14:1–210. Rowe RK, Smith J, Quinn J, Boothroyd I. 2002. Effects of logging with and without riparian strips on fish species abundance, mean size, and the structure of native fish assemblages in Coromandel, New Zealand, streams. New Zealand Journal of Marine and Freshwater Research 36:67–79. Sazima I, Carvalho LN, Mendonça FP, Zuanon J. 2006. Fallen leaves on the water-bed: diurnal camouflage of three night active fish species in an Amazonian streamlet. Neotropical Ichthyology 4:119–122. Shipley B. 2009. Confirmatory path analysis in a generalized multilevel context. Ecology 90:363–368. Sodhi NS, Koh LP, Brook BW, Ng PKL. 2004. Southeast Asian biodiversity: an impending disaster. Trends in Ecology and Evolution 19:654–660. Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. 2014. The deviance information criterion: 12 years on. Journal of the Royal Statistical Society B 76:485–493. 1367 Sweeney BW, Newbold JD. 2014. Streamside forest buffer width needed to protect stream water quality, habitat, and organisms: a literature review. Journal of the American Water Resources Association 50:560–584. Villéger S, Mason NW, Mouillot D. 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89:2290–2301. Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116:882–892. Wallace JB, Eggert SL, Meyer JL, Webster JR. 1997. Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104. Wich SA, Garcia-Ulloa J, Kuhl HS, Humle T, Lee JSH, Koh LP. 2014. Will oil palm’s homecoming spell doom for Africa’s great apes. Current Biology 24:1–5. Wilson EO. 1988. The current state of biological diversity. Pages 1-18 in Wilson EO, editor. Biodiversity. National Academy Press, Washington, D.C. Wilcove, DS, Giam X, Edwards DP, Fisher B, Koh LP. 2013. Navjot’s nightmare revisited: logging, agriculture, and biodiversity in Southeast Asia. Trends in Ecology & Evolution 28:531–540. WWF and TNC (World Wildlife Fund and The Nature Conservancy). 2013. Freshwater ecoregions of the world (FEOW). Available from www.feow.org (accessed January 2014). Conservation Biology Volume 29, No. 5, 2015