Organic Model Exercise: An Adventure in Structure and Bonding Introduction
advertisement

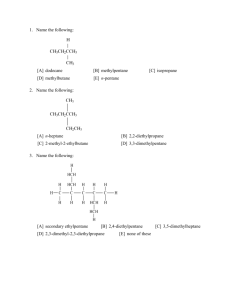
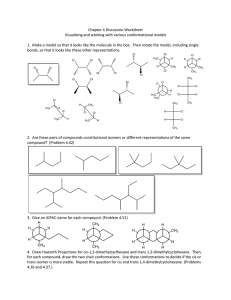
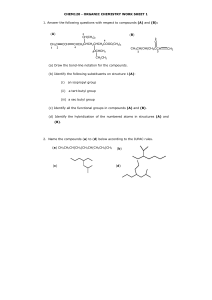
Organic Model Exercise: An Adventure in Structure and Bonding Dr. Gergens - Mesa College Introduction Organic chemistry is the study of compounds that contain the element of carbon; compounds that do not contain carbon are termed inorganic. Carbon is singled out as a branch of chemistry because of the tremendous number of compounds it forms. While there are about 200,000 known inorganic compounds, there are over 6 million known compounds of carbon. While organic chemistry is the study of the compounds of carbon, biochemistry is the study of the chemistry of living organisms. Organic compounds are found in all living organisms, foods (fats, proteins and carbohydrates), fuels (petroleum), wood, paper, fabrics, plastics, dyes, paints, cosmetics, drugs, medicines, insecticides, herbicides, soaps, and detergents. Organic compounds can be classified according to their structural features. The structural features that make it possible to classify compounds by reactivity are called functional groups. In this experiment you will familiarize yourself with the structures of functional groups shown in Chapter 3 of the McMurry text. The use hand-held models will aid you in this task. Many studies have shown that tactile (touch) learning far outweighs visual absorption of this sort of information. Getting Started: Start by reviewing Chapter 3 in your McMurry text. For this exercise, pay particular attention to the naming of the general classification of functional groups. Your instructor will discuss structural isomerism, geometric isomerism, and chirality for aliphatic and cyclic compounds covered in Chapter 3 in laboratory. This project is composed of three parts: Part A - covalencey; Part B - functional group classification and their threedimensional representations through the use of hand held models; and Part C - working with structural, geometric, and optical isomerism. While working on this exercise, you may hand draw the structures and answer questions in pencil. However, your may be required to turn in your answers to the following questions as a word processed document. Data sheets to word process this assignment are available for download from the web site at http://homework.sdmesa.edu/dgergens/chem231L/structure_bonding/ You will use a model kit to build each functional group. This will greatly assist you in visually seeing the threedimensional perspective of organic compounds drawn on a two-dimensional piece of paper. For example, dimethylether drawn below can be drawn with a 2-D and 3-D perspective. If drawn in 2-D, the molecule appears to be linear. In actuality, the ether has a bent structure about the oxygen atom. If your were to make a molecular model of this ether, you would quickly recognize the central oxygen atom as being bent in geometry and the carbons as tetrahedral. To represent this molecule as having a three-dimensional structure, the tetrahedral methyl carbons are drawn with a solid black wedge, , and a hashed wedge to represent a 3-D perspective of a bond in three-dimensional space. In the 3-D structure, each black wedge shows bonded atoms pointing towards you, coming out of the page, whereas each hashed wedge represents bonded atoms pointing away from you, going into the page. Each solid line represents bonds lying in the plane of the page. Being able to look at compounds drawn on a two-dimensional piece of paper and convert it to a 3-D perspective structure will be an important outcome of this exercise. A. H H C H O 2-D H CH H Functional Group: ether H H H O H H H 3-D 49 Part A - Covalencey The atoms in organic compounds are generally held together by covalent bonding. In contrast, inorganic compounds are usually ionic, though they can have covalent bonds. Since carbon has four electrons in its outer shell, it forms four covalent (shared) bonds. These may be single, double or triple bonds as long as the total number of bonds to carbon equals four. Other elements, such as hydrogen and oxygen, are found in organic compounds, making the possibilities for covalent combinations to become enormous. In the table below, are the most stable bonding modes for carbon, nitrogen, and oxygen. For each bonding mode, give the angle, geometric name, and hybridization about the central atom. Word process this image by using the drawing tool in Microsoft Word to add angles, geometric names, and hybridizations. VSEPR = ________ ________ ________ ________ ________ Determine the angles between bonds, name the geometry about the central atom and give the its hybridization. Ideal Geometries H H H bond angles C H H H C C H H C C H : N N O C O H geometric name hybridization Non-Ideal Geometries : : : N H H N N : H bond angles H H geometric name hybridization : H : bond angles : O H : H O C H geometric name hybridization On the next page, we will be using ball and stick models to demonstrate the versatility of bonding and therefore structure in organic compounds. In general, the color correlation of the plastic spheres to elements is as follows: C - carbon H - hydrogen O - oxygen N - nitrogen Cl - chlorine Br - bromine black white red blue green red 50 Part B - Functional Group Classification Structural features make it possible to classify compounds by functional groups and the methods for the writing chemical bonds are highly descriptive and useful. In this exercise, you will be asked to identify and use of a model kit to build each functional group for the compounds below. Identify each functional group. Construct molecular models for the compounds below. This will greatly assist you in visually seeing the three-dimensional perspective of organic compounds. Notice the tetrahedral carbon has a three dimensional array of atoms. For the moment, sketch a hand drawn 3D-chemical structure for each on this handout. Afterwards, use a computer assisted chemical drawing program (i.e. ISIS) to redraw a three-dimensional representation for each molecule and paste its structure into the table below. A. B. H H C H O H CH H Functional Group: ether H H D. C Functional Group: H CH H Functional Group: O C H I. H H C H NH2 Functional Group: OH OH Functional Group: J. Functional Group: K. H HC H O C Functional Group: H O CH H C H Functional Group: H. O C OH Functional Group: G. C H F. H H C H Functional Group: H H C H C H E. H H C H C H H O H H Cl H O H C. H H C H L. H H C H Functional Group: NH2 H H C H O C H Functional Group: 51 Part C - Structural, Geometric, and Optical Isomerism. In this laboratory activity, you will be examining molecular models of various organic compounds. You will pay particular attention to the existence of isomers. Isomers are prevalent in organic compounds due primarily to carbon's ability to make 4 bonds. Constitutional isomerism. Two molecules with the same molecular formula but different structures are called isomers. Using your model kit, construct a model of the requested isomer. Using either ISIS or ChemDraw, draw a threedimensional representation and paste its figure into the table below. 1. an alcohol of compound A. 2. an aldehyde of compound D 3. an ester of compound E. Geometric cis- trans isomerism is a type of geometric isomerism that arises when two species have the same molecular formulas but different structures. For example, square planar complexes, [PtBr 2 Cl 2 ]2– . Br 2- Cl 2- cis & trans alkenes H Cl Pt Br Br Pt Br Cl Cl cis chlorine atoms next to each other on the same side trans C CH3 chlorine atoms opposite to each other on opposite sides H C CH3 cis H CH3 C CH3 C H trans Alkenes have rigid double bonds that prevent rotation, giving rise to cis- and trans-isomers. Construct the cis and trans 2-butenes above. Notice the restrictive rotation about a double bond can result in cis-trans geometric isomerism in organic molecules; the methyl groups cannot interconvert. Construct a molecular model and draw the three-dimensional representation for the three isomers of dibromoethene. Using either ISIS or ChemDraw, draw each isomer and paste its figure into the table below. cis trans neither cis or trans 52 Geometric isomerism is also possible when there is a ring present. A cycloalkane has two distinct faces. If substituents on a cyclic ring point toward the same side, they are cis. If they point toward opposite sides of the ring they are trans. Note the geometric isomers of 1,2-dimethylcylcohexane cannot inter convert without breaking and reforming bonds. a. Below are cis- and trans-isomers of 1,2-dimethylcylcohexane. Make models of these compounds to convince yourself that cis- and trans-1,2-dimethylcylcohexane cannot inter convert by simple rotations about the bonds. Also convince yourself that all three forms draw for cis and for trans CH3 CH3 CH3 CH3 CH3 CH3 cis trans CH3 cis Cl CH3 Cl trans Cl Cl trans cis b. Construct a molecular model for each isomer of 1,2-dibromocyclohexane. As seen for 1,2-dimethylcylcohexane above, duplicate the three perspectives for 1,2-dibromocyclohexane and paste their images into each box. c. Can you construct a molecular model for an isomer of dibromocyclohexane the is neither cis or trans? same three perspectives for this structural isomer and paste them into the box below. Draw the neither cis or trans 53 Optical Isomerism - The Optically Active Tetrahedral Carbon Optical isomerism is a type of isomerism that is frequently encounter throughout organic chemistry. It occurs because of the tetrahedral nature of the bonding around a carbon atom. Molecules may be chiral, or handed; think of your left and right hand. Chiral is derived from the Greek word cheir, meaning hand. A carbon atom with four different atoms or groups attached to it is referred to as a chiral center, meaning the carbon is without a plane of symmetry. A chiral center is asymmetric, just like your left and right hand. A molecule containing such a carbon atom may show optical isomerism. Optical activity is exhibited by molecules that have a nonsuperimposable mirror image. Your hands are nonsuperimposable mirror images, and a pair of nonsuperimposable mirror images are called enantiomers. a. Construct a molecule of 2-butanol. Notice the number two carbon, C2 , has four different groups attached to it—a methyl, ethyl, hydroxyl, and hydrogen—thus a chiral carbon. Construct the mirror image of 2-butanol. Note that both are isomers—actually stereoisomers (stereo meaning in space). Me Me H H HO b. OH Et Et Draw the three-dimensional representation using ISIS draw for each optical isomer of 2-butanol and 2-bromobutane and paste the structure into box at the right. Note these molecules are nonsuperimposable. CH3 CH3 CH3 CH2 HO H CH3 H CH2 Br c. What are the tests or observations you can make on the structure of a molecule to determine whether it is chiral? d. Using your models, construct the optical isomers of carvone. You may have to look up the structure using Chemfinder on the computer. Smell the authentic (-)- and (+)-carvones that are in the hood. What do they smell like? Can you smell a difference between the odors spearmint and caraway? In the space below, draw the isomers of carvone using ISIS Draw and identify which carvone corresponds to which scent. 54 Name:_______________________________ Isomers of Octane Experiment Chemistry 231L/232L Dr. Gergens - SD Mesa College Section:_____________ Date:____________ Write out the line-angle formula for all 18 isomers with the formula C8 H18 . Organize the isomers from decreasing parent chain length starting with octane. The first three are done for you. Give all isomers an I.U.P.A.C. name. Identify by letter, all equivalent carbon atoms. Use the first three examples as a guideline. Note, 3-methylheptane has no equivalent carbon atoms because it is asymmetric due to its chiral center marked '* '. Mark all asymmetric centers with a '* '. Finally, answer the questions on the next page. a * a b Name: c d octane d c b a a Name: 2-methylheptane Name: Name: Name: Name: Name: Name: Name: Name: Name: Name: Name: Name: Name: Name: Name: Name: 3-methylheptane 55 Isomers of Octane - Additional Questions Dr. Gergens - SD Mesa College Answer the following questions. Use a molecular model kit to assist you. 1. Give the total number of each isomer having the following parent chain name. octane ____, heptane ____, hexane ____, pentane ____, butane ____, propane ____? 2. How many alkanes have the ethyl— branch in its name ____. Give their IUPAC name: 3. How many alkanes have the propyl— branch in its name ____. Give their IUPAC name: 4. How many alkanes have the isopropyl— branch in its name ____. Give their IUPAC name: 5. How many alkanes have the prefix di in their IUPAC name ____. 6. How many alkanes have the prefix tri in their IUPAC name ____. 7. How many alkanes have the prefix tetra in their IUPAC name ____. 8. How many alkanes have only one chiral carbon center ____ . A tetrahedral carbon atom that bears four different substituents is called a chiral center. Give their IUPAC name: 9. How many alkanes have two chiral centers ____. Give their IUPAC name: 10. Which alkane appears to have six equivalent methyl branches its structure? Give its IUPAC name: 11. Which alkane appears to have three equivalent ethyl branches in its structure. Give its IUPAC name: 12. How many alkanes contain both two equivalent methyl and two equivalent ethyl branches in its structure? Give their IUPAC name: 56 13. Which alkane appears to have two equivalent sec-butyl branches? (Hint: It is the one with two chiral centers as well). Give its IUPAC name: 14. Which alkane appears to have two equivalent n-butyl branches in its structure? Give its IUPAC name: 15. Which alkane appears to have two equivalent isobutyl branches in its structure? Give its IUPAC name: 16. Which alkane appears to have two equivalent t-butyl branches in its structure? Give its IUPAC name: 17. Which alkane appears to have two equivalent n-propyl branches in its structure? Give its IUPAC name: 18. Which alkane appears to have two equivalent isopropyl branches in its structure? Give its IUPAC name: 19. Which chiral molecule appears to have a methyl, ethyl, n-butyl branch about its chiral center? Give its IUPAC name: 20. Which chiral molecule appears to have a methyl, ethyl, t-butyl branch about its chiral center? Give its IUPAC name: 21. Which chiral molecule appears to have a methyl, propyl, isopropyl branch about its chiral center? Give its IUPAC name: 22. IUPAC stands for_________________________________________________________________ 57