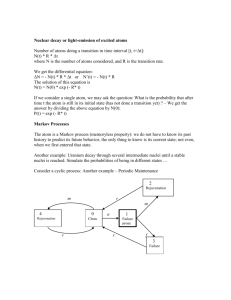
(A) van der Waals-London Interaction - present between - short range,
advertisement

(A) van der Waals-London Interaction - present between all atoms - short range, weak - responsible for condensation (gas-liquid) and freezing (liquid-solid) if no stronger forces present No interaction if charge distribution were rigid. Ar Ar Charge on real atoms fluctuates, induces dipole moments in each other, and causes an attractive interaction. + Ar - + Ar - A simple argument for van der Waals-London Interaction <p>=0 p2=E1 ~ p1/R3 p1 At time t average + - R atom 1 + - is the polarizability of the atom Electric Field: E1~ p1/R3 atom 2 p2 p p I t Interactio ti n energy p2 E1 ~ 31 13 ~ 16 R R R The time-averaged interaction energy of the two atoms: U c p12 A 6 6 R R p12 0, U 0 Van der Waals force: - Always attractive. - A quantum mechanical effect. This is called van der Waals interaction, also called the London interaction or induced dipole-dipole interaction. 1 (B) The Repulsive Interaction When two atoms are brought sufficiently closer, the interaction energy becomes repulsive due to Pauli exclusion principle. R atom1 atom2 electron charge distribution Charge distribution overlap Two electrons cannot occupy the same quantum state. For inert gases, the repulsive energy can be fitted well to the form: U= B/R12. Therefore , the total potential energy of two atoms is: U(R) V (R) 4 12 6 6 =A B A U ( R) 12 6 4 where 4 2 R R R R and 4 = B . 6 2 R R 1/ 12 U rep ( R4) 4 e R / This is the Lennard-Jones potential. Sometimes, the repulsive energy is written as R (C) Equilibrium Lattice Constants j In an inert gas crystal, the interaction energy between atom i and atom j is: U ij 4 Rij 12 R ij 6 Rij= pijR 12 6 4 pij R pij R R i where R is the nearest-neighbor distance. With N atoms, the total cohesive energy is: U total 6 12 1 ' ' N (4 ) 2 j pij R j pij R i is the reference atom, ( j ≠ i). pij is a pure number determined by the lattice type. For an FCC lattice: 1 p j 1 12 ij 12.13188 1 p j 1 6 14.45392 ij 2 For an FCC lattice: U total 1 N ( 4 ) 2 j ' p R ij 12 6 ' p R j ij 12 6 2 N 12 . 13 14 . 45 R R Rij= pijR R i When a crystal is in equilibrium, the energy is at a minimum: dUtotal 12 6 0 2N (12)(12.13) 13 (6)(14.45) 7 dR R R Therefore the equilibrium nearest neighbor atomic spacing is (R0/) = 1.09 For all inert gas elements with an FCC structure at temperature = 0 k and pressure = 0. Measured R0/ values: Ne: R0/=1.14; Ar: (40): R0/=1.11; Kr: R0/=1.10; Xe: R0/=1.09. The slight differences are due to zero point quantum effects. (D) Cohesive Energy From the equilibrium inter atomic spacing, we can calculate the cohesive energy of an inert gas crystal: 12 6 U total 2 N 12 . 13 14 . 45 R R Using: We get: (R0/) = 1.09 12 6 1 U total ( R0 ) N (4 ) (12.13) (14.45) (2.15)(4 N ) 2 R0 R0 same for all inert gases, (at 0 K and 0 pressure) Quantum mechanical corrections (KE contributions) to the cohesive energy of inert gases: Ne (AW=4): -28%; Ar (40): -10%; Kr (84): -6%; Xe (131): -4%. 3 2. Ionic Crystals - electrostatic interaction Ionic crystals => made up of positive and negative ions. Ionic bonds => electrostatic interaction between the ions. Complication – long range interaction U ~1/r NaCl Simplicity – Coulomb interaction dominates. Ionic crystals form between low ionization energy atoms (e.g. alkalis: Li, Na) and high electron affinity atoms (e.g. halogens: F, Cl) + 5.14 eV +electron + electron + Requires energy (ionization energy) + 3.61 eV Releases energy (electron affinity) + 7.9 eV Releases energy (cohesive energy) Net energy released per NaCl unit = 7.9 eV + 3.6 eV - 5.1 eV = 6.4 eV 4 The binding energy of ionic crystals is predominantly electrostatic, and is called the Madelung energy, which is defined relative to the energy of the ions at infinite separation. (A) The Madelung Energy If Uij is interaction energy between ion i and j, then the total energy involving ion i is: rij= pij R j U i 'U ij j i For each pair: U ij e Repulsive (short range) For each ion pair: i rij / q2 rij R (in CGS units) Repulsive or attractive (long range) U ij e rij / rij= pij R q2 rij j i If we measure distances in R (nearest neighbor distance), and consider the repulsive interaction only for the nearest neighbors, then q2 R / , e R U ij 2 q 1 , pij R R (otherwise , where rij pij R ) (nearestt neighbors i hb ) For a crystal y of N molecules ((2N ions), ), the total lattice energy gy is: q2 U total NU i N U ij N e R / R j j ( n.n.) 2 1 q 2 N z e R / q pij R R j other where z = number of nearest neighbors of an ion, and is called the madelung constant. ' j pij The sum includes nearest-neighbor contributions. 5 The madelung constants of several lattices ' j NaCl: CsCl: z=6 z=8 z=4 =1.747 =1.762 =1.638 pij ZnS: 2 The total lattice energy U total NU i N ze R / q R At the equilibrium inter-ionic separation, We have: R02 e R 0 At equilibrium: / q 2 z dU total 0 dR where R0 is the equilibrium nearestneighbor distance. q 2 N q 2 U total N ze R0 / R0 R0 1 R0 The attractive component, -Naq2/R0, is called the Madelung energy. The repulsive interaction is short- ranged. Calculation of the Madelung constant pij 2 For a crystal to be stable, the total energy must U N ze R / q total R contain both attractive and repulsive terms j i Thus must be positive ! rij= pij R Assume that ion i is negative, then in the definition of j rj i q2 U total NU i N e R / R j ( n.n.) R 2 1 q 2 N z e R / q p R R j other ij + is for a positive ion (j) and – for a negative ion (j). we can write R j i rj => distance of ion j to the reference ion i. R => nearest neighbor distance. rj Example: find the Madelung constant for a 1-d ionic crystal. + - + - + - + - + - R + - + Reference ion R j i rj 1 1 1 2 ln 2 1 2 R R 2 R 3R 4 R - + - For 3-d lattices, can be calculated from the structure in a similar fashion. 6 3. Covalent Crystals Energy The covalent bond between two atoms is usually formed by two electrons, one from each atom. The electrons forming the bond tend to be partly localized in the region between the two atoms. 1S 1S H atom H atom H2 molecule Features of covalent crystals: - Quantum mechanics is needed to calculate the binding energy. - Covalent bonds are highly directional (low APF, low density). - Only few crystals are covalently bound (diamond, Si, Ge, SiC) - Covalent bonds are strong (hardest materials, high melting points, insoluble). - All covalent crystals have tetrahedral (diamond) structure. - There is a continuous range of crystals between the ionic and covalent limits. APF = 0.34 7