COLLIGATIVE PROPERTIES
advertisement
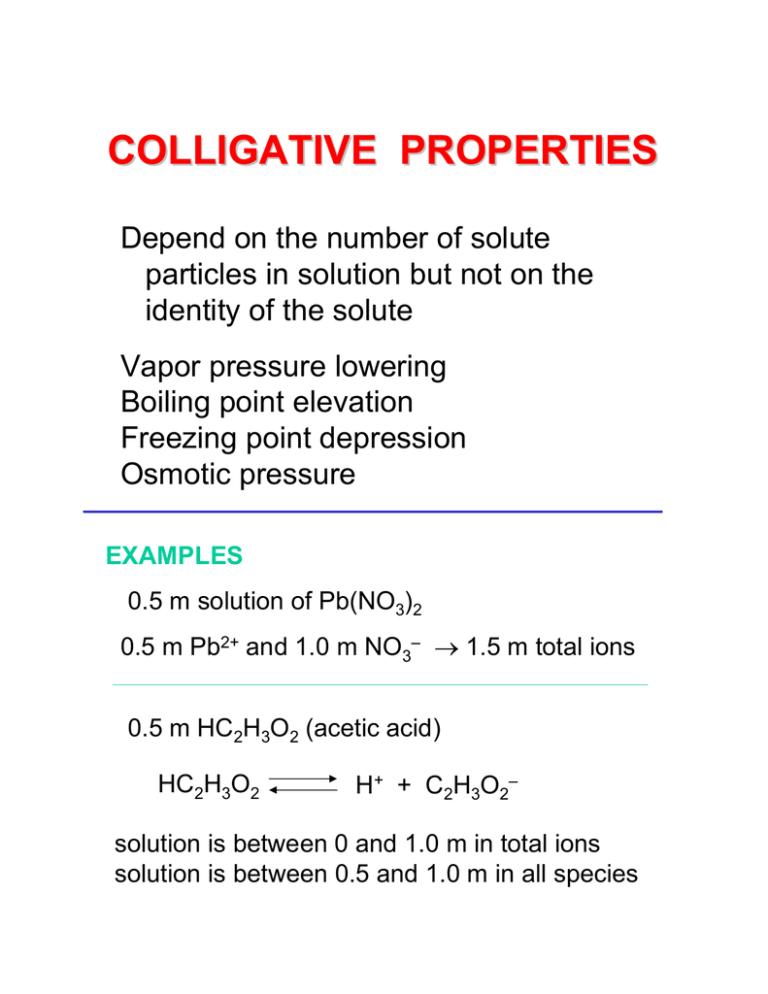
COLLIGATIVE PROPERTIES Depend on the number of solute particles in solution but not on the identity of the solute Vapor pressure lowering Boiling point elevation Freezing point depression Osmotic pressure EXAMPLES 0.5 m solution of Pb(NO3)2 0.5 m Pb2+ and 1.0 m NO3– → 1.5 m total ions 0.5 m HC2H3O2 (acetic acid) HC2H3O2 H+ + C2H3O2– solution is between 0 and 1.0 m in total ions solution is between 0.5 and 1.0 m in all species EXPRESSING CONCENTRATION weight percent = mass component x 100 total mass mole fraction moles component = of component total moles molarity = moles solute liters solution moles solute molality = mass solvent (kg) (%) (fraction) (M) (m) ELECTROLYTES A substance that yields ions when dissolved in water is an electrolyte Strong electrolytes completely ionized in solution good conductors Weak electrolytes partially ionized in solution poor conductors Nonelectrolytes not ionized in solution non-conductors STRONG & WEAK ELECTROLYTES STRONG NaCl(s) + H2O → Na+(aq) + Cl–(aq) + H2O salt completely ionized HCl(aq) + H2O → H3O+(aq) + Cl–(aq) complete ionization of strong acid WEAK NH4+(aq) + OH–(aq) NH3(aq) + H2O partial ionization of weak base or weak acid H3O+(aq) + C2H3O2–(aq) HC2H3O2(aq) + H2O NON C6H12O6(s) + H2O → C6H12O6(aq) + H2O glucose no ionization Flowchart for identifying electrolytes note: ionic compounds are strong electrolytes but they could be insoluble(!!!) • memorize strong acids and bases (BLB Table 4.2); If a compound is an acid or a base, but NOT one of the strong acids or bases, then it MUST be a weak electrolyte • Common misconception: electrolytes are ionic compounds: this is NOT TRUE (e.g., HCl) Sheets Page 3 Lecture 18 Which of these aqueous solutions has the greatest total concentration of ions? Which has the least? 1. 2. 3. 4. 0.4 M NH4NO3 0.2 M Pb(NO3)2 0.3 M Na2SO4 0.2 M AlPO4 5. 0.5 M C6H12O6 (sugar) VAPOR PRESSURE LOWERING Raoult’s Law vapor pressure of solution PA = XA PAo vapor pressure pure solvent mole fraction of solvent vapor pressure lowering is a colligative property — its depends on the conc but not on the nature of the solute RAOULT’S LAW EXAMPLE 1 Calculate total vapor pressure of a liquid at room temperature that is composed of a mixture of benzene and toluene. The mole fractions of benzene and toluene are Xben = 0.33 and Xtol = 0.67. o Benzene: Pben = 75 torr Toluene: Ptolo = 22 torr PA = XA PAo RAOULT’S LAW EXAMPLE 2 Calculate the vapor pressure at 25 oC of a solution made by adding 50.00 mL of glycerin (C3H8O3, a nonvolatile nonelectrolyte with a density of 1.26 g/mL) to 500.0 g of water. The vapor pressure of pure water at 25 oC is 23.8 torr. PA = XA PAo BOILING POINT ELEVATION FREEZING POINT DEPRESSION Shift in vapor pressure, and shift in phase diagram explains ΔTf and ΔTb ΔTb = Kb m molal BP elevation const ΔTf = Kf m molal FP depression const molality of the solution (solute particles) FREEZING POINT DEPRESSION SEAWATER Ocean salinity ~ 35 g salt / 1 kg seawater Cl– SO42– Mg2+ Na+ Ca2+ K+ molar mass of NaCl is 58.5 g/mol 35 g = 0.60 mol NaCl 58.5 g/mol x2 1.2 mol = 1.2 molal m= 1000 g seawater Kf = 1.86 °C/m for H2O ΔTf = Kf m = (1.86)(1.2) = 2.23 °C Tf = – 2.23 °C FREEZING POINT DEPRESSION EXAMPLE Choose the solute which would decrease the freezing point to -5 oC of a solution that is made by dissolving 0.0538 moles of the substance in 100 g of water. The freezing point depression constant of water is 1.86 oC/m A. B. C. D. E. NaF CaCl2 Al(NO3)3 Ca3(PO4)2 C6H12O6 OSMOTIC PRESSURE dilute solution concentrated solution semipermeable membrane movement continues until osmotic pressure builds up to stop it π = MRT M is molarity of particles Example: cucumber in brine loses water by osmosis to make pickle Osmosis • flow of molecules through a semi-permeable membrane; NET movement of is toward solution with higher solute concentration; movement of solvent continues until osmotic pressure builds up to stop it • osmotic pressure ( ): pressure needed to of a molecule through a membrane n = RT = MRT V • is osmotic pressure (what units will this be in???) • R is gas constant in (L atm)/(mol K) • T is temperature in K • M is concentration in molarity (mol/L) • what does this equation remind you of??? Sheets Page 10 Lecture 20 Consequence of osmotic pressure • red blood cells: the cell membrane of red blood cells is a semi-permeable membrane cell in hypertonic soln cell in hypotonic soln Sheets Page 12 Lecture 20 OSMOTIC PRESSURE EXAMPLE Honey is 82% sugar by mass. Most of the rest is water. Bacteria love sugar, so why don’t they grow in honey? Calculate the osmotic pressure of honey to find out. Osmotic pressure of bacteria ~ 8 - 30 atm. Composition of sugars: 70% fructose and glucose (C6H12O6) 30% maltose and sucrose (C12H22O11) Density of honey: 1.40 g/mL Example A 25 mL aqueous solution containing 0.420 g of hemoglobin has an osmotic pressure of 4.6 torr at 27°C. What is the molar mass of hemoglobin? 1atm = 6.05 10 3 atm = ( 4.6torr ) 760torr 1L = 0.025mL V = ( 25mL ) 1000mL T = 300K n = RT V V n= RT 6.05 10 3 atm mol K n= ( 0.025L ) 300 K 0.08206 L atm n= 0.420g molar mass of hemoglobin = 6.14 10 6 mol molar mass of hemoglobin = Answer: 6.84 104 g/mol Sheets Page 13 Lecture 20 COLLOIDS small true solution molecules SIZE colloidal suspension 2–2000 nm MILK (fat particles) FOG (water droplets) Phases mutually insoluble hydrophillic vs. hydrophobic colloids large mixture particles influenced by gravity river silt WHY IS THE SKY BLUE? Light passes thru solns without scattering Light passes thru colloidal suspensions with scattering (milk, fog) Tyndall Effect - particles scatter light of λ about the same as their size atoms molecules ~ nm, scatter x-rays colloids up to ~hundreds of nm’s (visible light is 400-700 nm) So…colloids scatter light Scatter blue more effectively than red LIGHT SCATTERING …or… WHY IS THE SKY BLUE? sun s un colloids scatter more blue than red Hydrophilic vs. hydrophobic colloids • hydrophilic: water-loving • hydrophobic: water-fearing • (water-soluble) proteins: hydrophobic core with hydrophilic surface • detergents: hydrophobic tail with hydrophilic head Sheets Page 16 Lecture 20