•Reading: •Homework: –Ch. 5 –None
advertisement

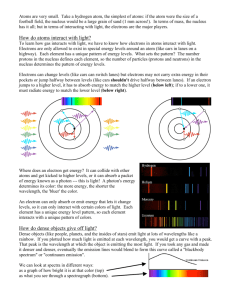
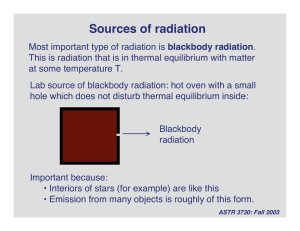
•Reading: –Ch. 5 •Homework: –None 1 Electromagnetic Spectrum Radio (0.4 GHz) Atomic Hydrogen Radio (2.7 GHz) Molecular Hydrogen Infrared Mid-Infrared Near-Infrared Visible X-Ray Gamma ray http://adc.gsfc.nasa.gov/mw/mmw_images.html 2 Nature of Light • The exact nature of light was an enigma to scientists for many centuries • Light has properties similar to waves –Young’s double-slit experiment • Light sometimes behaves like a particle –Photoelectric effect 3 Photoelectric Effect • Light shining on a piece of metal can cause electrons to be ejected from the surface – only happens if the light has a wavelength shorter than some value (depends on the metal) • As intensity of light increases, number of electrons increases, but not their energy • As the wavelength of light decreases, number of electrons remains the same but their energy increases 4 Quantization of Light • Electromagnetic radiation is quantized hc E= λ E = energy of photon h = Plank� s constant = 6.625 × 10−34 J s c = speed of light λ = wavelength of light • Einstein used the idea of the quantization of light to explain the photoelectric effect –light is quantized into packets of energy (called photons) –energy of each photon is determined by its wavelength –only photons with enough energy are able to eject electrons –higher intensity light means more photons, not more energy per photon –won Nobel prize in physics in 1921 5 Electromagnetic Spectrum http://www.lbl.gov/MicroWorlds/ALSTool/EMSpec/EMSpec2.html 6 How Light is Produced 7 Kirchhoff’s Laws 1.A hot, opaque body, such as a perfect blackbody or a hot, dense gas, produces a continuous spectrum of radiation • a complete rainbow of colors without any spectral lines 2.A hot, transparent gas produces an emission line spectrum • a series of bright lines against a dark background 3.A cool, transparent gas in front of a source of continuous spectrum produces an absorption line spectrum • a series of dark lines among the colors of a continuous spectrum 8 Kirchhoff’s Laws • http://bcs.whfreeman.com/universe9e –Animation 5-1 9 Blackbody • An idealized object –Does not exist in nature • A perfect blackbody absorbs all of the radiation that falls on it. –Does not reflect any light • A blackbody emits radiation only according to its temperature 10 Temperature • Measure of relative heat contained in 2 objects • Three common temperature scales Fahrenheit (° F) Celsius (° C) Kelvin (K) Ice point 32 0 273 Steam point 212 100 373 Note: A change of 1° C is the same as a change of 1 K 11 Temperature 12 Temperature • Temperature is a measure of the average random motion of atoms or molecules in a substance • The atoms & molecules in all substances are moving –gas molecules in the air –water molecules in a cup of water –even the molecules in a solid are vibrating with some amount of energy 13 Blackbody Radiation • Any hot opaque body produces a continuous spectrum of radiation –All such objects emit radiation at all wavelengths (gamma rays to radio waves) –Such objects do not emit at the same intensity at all wavelengths –Peak wavelength depends on the temperature • Sun emits mostly in the visible • Room-temperature objects emit mostly in the infrared 14 Blackbody Curve •Hotter blackbody –higher intensity at all wavelengths –peak intensity at shorter wavelength • hotter blackbody is bluer •Wien’s Law 15 Sun as a Blackbody • Stars, such as the Sun, are approximate blackbodies • Using Wien’s law, can estimate the surface temperature of a star just by observing its blackbody spectrum • Anyone notice anything curious about the Sun’s intensity curve? 16 Sun as a Blackbody • Wien’s law does not depend on the chemical composition of the blackbody • All perfect blackbodies at a given temperature produce the same spectrum 17